Primary Surgery for Ovarian Carcinoma
Authors
INTRODUCTION
The diagnosis and management of ovarian cancer is the most important challenge facing the gynecologic oncologist today, and it is likely to remain so for the foreseeable future. In 2010, the American Cancer Society estimated that ovarian cancer would be diagnosed in 21,880 women in the United States and that 13,850 would die of their disease.1 Although it accounts for only 26% of new cases of cancer of the female genitalia, it causes 50% of the deaths caused by these malignancies. These grim figures result from the lack of an effective means of early diagnosis for ovarian cancer and the fact that most patients with advanced disease will eventually die of it.
Surgery is a cornerstone of the management of ovarian cancer and plays a crucial role in diagnosis and treatment. However, major advances have been made in chemotherapy for ovarian cancer during the past 10–15 years, and the optimal treatment of a patient with this disease usually requires surgery and chemotherapy. Ovarian cancer is an important example of a condition that requires the skilled application of multimodal therapy.
This chapter provides an overview of the role of primary surgery in the diagnosis and treatment of ovarian cancer. Discussion is limited to the management of epithelial tumors of the ovary, which constitute approximately 90% of all ovarian malignancies. Discussions of the rarer germ cell and stromal malignancies appear elsewhere on this website.
NATURAL HISTORY AND PATTERNS OF SPREAD
The surgeon who manages ovarian carcinoma must understand as much as possible about the natural history and patterns of spread of the disease. This understanding is as important as a thorough knowledge of anatomy and good surgical technique. The aggressiveness of treatment and the potential for conservation of reproductive function are dependent on the natural history of the disease, and the search for metastatic disease is based on its patterns of spread.
The ovary develops from the genital ridge in the embryo. The genital ridge is composed of thickened coelomic epithelium, which is thought by some authors to have the potential for developing into ovarian-like tumors. This thickened coelomic epithelium forms the mesothelial (epithelial) covering of the ovary and gives rise to epithelial tumors. The germ cells originate in the primitive streak of the embryo and migrate to the gonad, where they proliferate into a component of the ovarian cortex. The mesenchyma of the medulla is the origin of the ovarian stromal cells. These three cell types are thought to give rise to the three types of malignant ovarian neoplasms: epithelial, germ cell, and stromal.2, 3
Epithelial carcinomas, which constitute approximately 90% of malignant ovarian neoplasms,2 spread by direct extension to adjacent organs, via lymphatics, and by dissemination of clonogenic cells into the peritoneal cavity. Blood-borne metastases are infrequent. The incidence of bilateral ovarian involvement is highly dependent on the stage of disease, varying from 6% to 13% in apparent stage IA disease to as high as 70% in stage III disease.2, 4 Direct extension to the uterus or fallopian tubes occurs in only 5% of patients thought to have stage IA disease, but involvement is frequent in more advanced stages.4
The most significant extraovarian spread of epithelial tumors takes place by direct dissemination of clonogenic cells into the peritoneal cavity. Although this dissemination is usually thought to occur when the tumor penetrates the ovarian capsule, the presence of malignant cells in the peritoneal cavity has been documented even with an intact ovarian capsule.5 Once the malignant cells enter the peritoneal cavity, they follow the normal circulation of the peritoneal fluid up the right paracolic gutter to the diaphragm. This clockwise flow of peritoneal fluid is the result of the respiratory motion of the diaphragm, and it accounts for the fact that the right hemidiaphragm is more frequently involved than the left. Exfoliated cells are further distributed by peristaltic movements of the intestine, which can result in implantation on any of the peritoneal surfaces, leading to micrometastases that eventually become clinically evident tumor nodules.
Free malignant cells are cleared into lymphatic channels in the diaphragm (especially the right diaphragm) and enter the pleural space and finally the mediastinal lymphatics.6 Clinical studies have found occult involvement of the undersurface of the diaphragm in a significant proportion of patients who appeared to have disease confined to the pelvis.7, 8
The omentum is almost always involved in advanced ovarian cancer. Occult omental involvement is seen in 3–11% of patients thought at exploration to have localized disease.7, 8, 9 In many cases, the omentum is the only site of extraovarian spread.
The lymphatic vessels of the ovary drain via the ovarian hilus to the subovarian plexus. From the subovarian plexus, the lymphatic drainage follows the course of the ovarian blood supply in the infundibulopelvic ligament to terminate in the high para-aortic lymph nodes. The most frequent site of para-aortic nodal involvement is between the inferior mesenteric artery and the renal vessels. Collateral drainage occurs via the broad ligament to the external iliac and hypogastric lymph nodes and via the round ligament to the inguinal lymph nodes.10, 11 The incidence of lymphatic involvement in epithelial ovarian carcinoma appears to be approximately 10–20% in apparent stage I and stage II diseases, and between 60% and 70% in stage III and stage IV diseases.9, 12, 13
In summary, the spread pattern of ovarian carcinoma is characterized by local extension, lymphatic metastases, and intraperitoneal dissemination of tumor cells. These unique routes of spread require that the entire peritoneal cavity be evaluated in any staging procedure for ovarian carcinoma. The potential for intraperitoneal spread must also be taken into account in the treatment plan. Finally, the relatively high incidence of lymphatic spread requires that the retroperitoneum be evaluated, particularly in cases in which the tumor appears clinically to be confined to the ovaries. Nodal sampling must include the high para-aortic lymph nodes that lie between the inferior mesenteric artery and the renal vessels. Both the right and left para-aortic lymph nodes must be evaluated. As initial therapies for ovarian cancer become more effective and patients live longer because of improved control of intraperitoneal disease, distant metastases may be seen more often and may take on greater clinical significance.
CANCER STAGING OF THE OVARY
Staging systems for human malignancies are designed to provide a standardized assessment of the extent of cancer at the time of diagnosis. The stage of a patient's tumor, assigned at the time of diagnosis, does not change if the disease recurs or progresses. Proper determination of cancer stage is a crucial element in the management of ovarian cancer, because treatment and prognosis are both strongly determined by stage.
In 1964, the General Assembly of the International Federation of Gynecology and Obstetrics (FIGO) approved a staging system for ovarian cancer based on findings at exploratory laparotomy. The staging systems for many other malignancies rely on the results of less invasive diagnostic procedures, such as physical examination and radiographic studies, but diagnostic procedures short of laparotomy are wholly inaccurate in determining the extent of spread of ovarian cancer.
In 1970, the Cancer Committee of FIGO recommended a definitive stage grouping of ovarian cancer based on surgical and pathologic assessment of the extent of disease. This system used four stages, which indicated whether the tumor involved only the ovaries (stage I), other pelvic organs (stage II), abdominal organs (stage III), or distant organs (stage IV). This system incorporated several factors that had recently been recognized to have prognostic significance, including the presence of tumor on the external surface of the ovary, the presence of ascites or peritoneal washings containing malignant cells, and the involvement of retroperitoneal lymph nodes.
In 1985, the Cancer Committee of FIGO14 revised the surgical staging system for ovarian cancer to reflect not only the results of a thorough exploration of the areas at risk of spread but also a more precise determination of the extent of spread. In this current system, ascites must be cytologically positive to affect the stage. The presence of tumor on the surface of the ovary or the rupture of a localized ovarian tumor, whether spontaneous or during surgery, affects the stage in the same way as positive ascites. Stage III, indicating abdominal spread, has been subdivided into three substages to reflect the size of tumor nodules found in the upper abdomen and the presence of retroperitoneal or inguinal nodal metastases. A pleural effusion must be cytologically positive to permit a diagnosis of stage IV disease. The complete FIGO staging system for ovarian cancer is given in Table 1. Table 2, taken from the FIGO 1991 Annual Report, shows the distribution by stage of more than 8000 patients with epithelial cancers of the ovary reported to FIGO from 95 participating institutions around the world.15 It can be seen that approximately one quarter of these patients had stage I, and more than half did not have disease diagnosed until stage III or IV was present. The small number of patients listed as “unstaged” includes those who did not undergo exploration because of medical problems or other reasons.
Table 1. FIGO Staging System for Ovarian Cancer14
| Stage I: Growth limited to the ovaries |
| IA: Growth limited to one ovary, no ascites, no tumor on the external surfaces, capsule intact |
| IB: Growth limited to both ovaries, no ascites, no tumor on the external surfaces, capsule intact |
| IC: Tumor stage IA or stage IB but with tumor on the surface of one or both ovaries, or with capsule ruptured, or with ascites present containing malignant cells, or with positive peritoneal washings |
| Stage II: Growth involving one or both ovaries with pelvic extension |
| IIA: Extension and/or metastases to the uterus and/or tubes |
| IIB: Extension to other pelvic tissues |
| IIC: Tumor stage IIA or IIB but with tumor on the surface of one or both ovaries, or with capsule (s) ruptured, or with ascites present containing malignant cells; or with positive peritoneal washings |
| Stage III: Tumor involving one or both ovaries with peritoneal implants outside the pelvis and/or positive retroperitoneal or inguinal nodes: superficial liver metastases equals stage III, tumor is limited to the true pelvis but with histologically verified malignant extension to small bowel or omentum |
| IIIA: Tumor grossly limited to the true pelvis with negative nodes but with histologically confirmed microscopic seeding of abdominal peritoneal surfaces |
| IIIB: Tumor of one or both ovaries, histologically confirmed implants of abdominal peritoneal surfaces, none exceeding 2 cm in diameter, nodes negative |
| IIIC: Abdominal implants greater than 2 cm in diameter and/or positive retroperitoneal or inguinal nodes |
| Stage IV: Growth involving one or both ovaries with distant metastases, if pleural effusion is present, there must be positive cytologic test results to allot a case to stage IV, parenchymal liver metastases equals stage |
Table 2. Distribution by Stage of Ovarian Cancer Patients15
| Stage | Patients (N) | Percentage |
| I | 2549 | 23.4 |
| II | 1409 | 12.9 |
| III | 5170 | 47.4 |
| IV | 1784 | 16.3 |
| Total | 10,912 | 100.0 |
SURGICAL MANAGEMENT OF EARLY OVARIAN CANCER
As outlined in the section on surgical staging, approximately 40% of patients with epithelial cancer have early-stage disease diagnosed.16 Of these patients, approximately 25% have stage I disease and 15% have stage II disease. In any reported series of ovarian cancer patients, the proportion of women listed as having early-stage disease depends strongly on the adequacy of surgical staging. When full surgical staging is conducted, fewer patients are recorded as having early-stage disease.
A review of the literature reveals a marked variation in reported survival rates for early-stage epithelial ovarian carcinoma. This variability is caused by two factors. First, unless thorough surgical staging is performed, patients with occult advanced disease will be included among early stage patients, resulting in a falsely low survival rate. Second, if patients with stage I and II epithelial ovarian cancer are grouped together, significant variation will occur in the population of patients from one institution to another. Such a discrepancy would result in divergent survival rates that depend on the composition of the various groups within the report.
For the purpose of study design, the Gynecologic Oncology Group (GOG) has divided patients into two early-disease categories (Table 3). It should be emphasized that this classification is applicable only to patients who have undergone rigorous surgical staging. Low-risk disease group includes patients whose tumors are stage IA and stage IB, grade 1 and grade 2. Patients with grade 3 or stage IC carcinoma are grouped into a high-risk category with all patients with stage II who have no residual disease. Patients with residual disease after primary cytoreductive surgery, even if the disease is confined to the pelvis (stage II), are considered to have advanced disease and are not included in either the low-risk or the high-risk early disease categories.
Table 3. Gynecologic Oncology Group Classification of Early-Stage Epithelial Ovarian Carcinoma
| Low-Risk Disease | High-Risk Disease |
| Stage IA,IB; grade 1,2 | Stage IA,IB; grade 3 |
| Stage IC | |
| Stage IIA, IIB, IIC, with no residual disease* |
*Stage II patients with residual disease at the conclusion of primary cytoreductive surgery are classified as having advanced disease.
In a study reported by Young and co-workers17 in 1990, the results of two randomized trials of the management of early-stage ovarian cancer by the GOG seem to justify this classification of patients into low-risk and high-risk categories. In this study, all patients underwent complete surgical staging, and most patients underwent second-look laparotomy at the conclusion of therapy. Low-risk patients (randomized to either observation or treatment with oral melphalan) had 5-year survival rates of approximately 95%, with no difference in the treatment arms. High-risk patients (randomized to either intraperitoneal 32P or oral melphalan) had 5-year survival rates of approximately 85%, with no difference in the treatment arms. Thus, the classification of risk categories seemed valid based on thorough surgical staging and previously published survival data.
Surgical therapy for apparent early ovarian cancer begins with a thorough surgical staging (Table 4) to determine the true extent of disease. Staging may be accomplished via laparotomy with adequate exposure, or via minimally invasive techniques such as laparoscopy. Several retrospective studies have compared different routes for staging and have generally noted comparable adequacy and accuracy between laparoscopy and laparotomy, with surgical outcomes in favor of a minimally invasive approach.18, 19, 20, 21
Ultimately, management depends on the stage of the disease, the histologic grade of the tumor, and the patient's age and reproductive plans. For those who have completed childbearing, any diagnosis of ovarian cancer warrants a total hysterectomy and bilateral salpingo-oophorectomy. The benefit of retaining the contralateral ovary for the purpose of preserving ovarian function is outweighed by the risk that carcinoma may subsequently develop or that an occult bilateral carcinoma may be missed in the retained ovary.
Table 4. Surgical Staging Procedure for Apparent Early Ovarian Cancer
| Multiple cytologic washings |
| Intact tumor removal |
| Complete abdominal exploration |
| Removal of remaining ovary, uterus, tubes* |
| Omentectomy |
| Lymph node sampling |
| Biopsy specimens of random peritoneal areas, including diaphragm |
*May be preserved in selected patients.
For the patient who wants to retain reproductive potential, however, conservative surgery may be feasible. If the patient has low-risk disease (histologic grades 1 and 2) confined to one ovary, removal of the involved ovary and ipsilateral fallopian tube is sufficient if a full surgical staging procedure is performed. These patients do not require adjunctive therapy, but close observation is necessary because of the increased risk for subsequent development of ovarian carcinoma in the contralateral ovary. Most authorities recommend removal of the remaining reproductive organs when childbearing is complete, because of the increased risk for carcinoma in the retained ovary.
Patients with high-risk disease confined to one ovary (stage IA, grade 3, or stage IC, confined to one ovary) have the option of conservative surgery followed by chemotherapy. However, only a small number of cases have been managed in this manner. Schilder and colleagues22 conducted a multi-institutional retrospective study of 52 patients with stage IA (42 patients) or IC (10 patients) epithelial ovarian cancer treated with conservative surgery. With median follow-up of 68 months, five patients had recurrence. Overall, 50 patients were alive without evidence of disease, and two died of disease 13 and 97 months after initial treatment, yielding an estimated 5-year survival of 98%. Both patients who died of disease had IA disease diagnosed initially, one with grade 1 histology, and the other with grade 2 histology. Of 24 patients who attempted pregnancy, 17 (71%) conceived and had 26 term deliveries and five spontaneous abortions. Park et al.23 reported on 59 patients with stage I disease (36 IA, 2 IB, 21 IC). Recurrence was noted in 14% of those with IA disease, and 19% of those with IC disease; 5-year overall survival was 95% and 42%, respectively. Given that 5-year survival for stage I epithelial ovarian cancer is as high as 76−93%,24, 25 the reported 42% survival among the IC patients in this study is somewhat alarming. Zanetta and colleagues26 reported on 56 women (32 stage IA, 2 stage IB, and 22 stage IC) undergoing fertility-sparing surgery for epithelial ovarian cancer. At 7 years median follow-up, 27 pregnancies had occurred in 20 patients, with five patients experiencing recurrent disease.
In most other cases of high-risk, early-stage disease, preservation of reproductive functioning is impossible because the uterus or both ovaries are involved. Because of the rapid advancement in reproductive techniques, however, partial conservation of the reproductive organs should be considered in any young patient who desires further childbearing. A patient with an intact uterus who has had her ovaries removed can still carry a pregnancy from a donor's egg and her partner's sperm by in vitro fertilization. A woman with a single ovary can produce her own genetic offspring by using her ova and her partner's sperm through in vitro fertilization and the use of a surrogate mother. All of these options must be explained to the patient preoperatively if any possibility of early-stage ovarian cancer exists.
Although most cases of ovarian cancer are considered to be sporadic (i.e., with no discernible familial tendency), it is now recognized that approximately 10% of ovarian cancers occur in association with germline mutations in one of several known cancer predisposition genes.27 In 1994, the BRCA1 gene was identified and cloned.28 In that same year, BRCA2 was localized to chromosome 13q.29 BRCA1 and 2 are involved in homologous recombination in DNA damage repair. The frequency of BRCA1 mutations in the general population is approximately 1 in 833; in Jewish women of Eastern European descent, it is approximately 1 in 100.30
Women carrying a germline mutation of BRCA1 have a significantly increased risk of breast and ovarian cancer compared with the general population. Brose and associates31 examined 483 BRCA1 mutation carriers from 147 families and noted the risk for ovarian cancer to be 41% and the risk for female breast cancer to be 73% by age 70. The lifetime probability for ovarian cancer associated with BRCA2 mutations ranges from 15 to 40%. Hereditary ovarian cancer not related to mutations in the BRCA genes may be related to the hereditary nonpolyposis colorectal cancer (HNPCC) genes.27
Risk-reducing bilateral salpingo-oophorectomy should be considered for women with documented mutations in cancer susceptibility genes. Rebbeck and associates32 compared 259 women with BRCA1 or BRCA2 mutations undergoing prophylactic salpingo-oophorectomy with 292 matched controls who did not have the procedure and noted a marked reduction in the risk of primary peritoneal cancer. After excluding six patients with early ovarian cancer diagnosed at the time of prophylactic surgery, only two women (0.8%) had subsequent primary peritoneal carcinoma in the group undergoing oophorectomy compared with 58 women (19.9%) having subsequent ovarian cancer in the control group.
A later meta-analysis demonstrated an 80% risk reduction after bilateral salpingo-oophorectomy in BRCA mutation carriers.33 A decision analysis by Kurain et al. suggests age 40 as the most effective age for prophylactic surgery.34 Given that up to 12% of women undergoing risk-reducing surgery have occult disease detected, care must be taken during the procedure to examine all abdominal and pelvic peritoneal surfaces, and to biopsy any suspicious lesions. Given the risk of fallopian tube carcinoma, complete resection of all visible fallopian tube should be performed.
SURGICAL MANAGEMENT OF ADVANCED OVARIAN CANCER
Despite decades of research directed at the problem of early diagnosis of ovarian cancer, most cases are still not detected before metastasis. Although the development of serum tumor markers35 and sensitive imaging techniques such as vaginal color-flow sonography36 have focused attention on the problem of early detection, neither method has been proved effective as a screening tool for the general population or even for a high-risk population.
As a result of the lack of effective methods for early diagnosis and the fact that ovarian cancer produces no specific symptoms in its early stages, patients often present with obvious ascites and palpable tumor masses in the pelvis and upper abdomen. On exploration, it is often not possible to resect all gross tumor, and such disease certainly cannot be cured by surgery alone. The surgeon confronted with such a situation must decide what level of surgical aggressiveness is appropriate. For many human tumors, aggressive surgery can be justified only if all known tumor can be removed, thus making the operation at least potentially curative. For epithelial cancer of the ovary, however, there is considerable theoretic and clinical evidence that cytoreduction, or debulking, of large tumor masses may be beneficial to the patient even if all gross tumor cannot be removed.
The theoretic evidence in support of debulking comes largely from concepts of the kinetics of tumor cell growth and the mechanisms of tumor cell destruction by cytotoxic chemotherapy. For diseases in which chemotherapy is essentially ineffective, such as certain gastrointestinal malignancies, the concepts of cytoreduction do not hold. In ovarian cancer, however, the cancer cells are usually sensitive to chemotherapy, and the removal of large tumor masses may improve the response to subsequent chemotherapy. The blood supply of these masses may be relatively poor, especially centrally. Although poor blood supply can ultimately cause central necrosis of the tumor mass, it can also lead to the development of adjacent areas of viable tumor that cannot be reached by adequate concentrations of chemotherapeutic agents. Additionally, cells in masses with a poor blood supply tend to have a low growth fraction and a higher proportion of cells in the nonproliferating (Go) phase of the cell cycle, making them relatively insensitive to the effects of chemotherapy.37 The removal of large tumor masses may actually decrease the number of cycles of chemotherapy required to eliminate all remaining tumor cells.
Skipper and associates38 demonstrated the phenomenon of fractional cell kill, which applies to tumors that grow in an exponential fashion. In this phenomenon, a given dose of a cytotoxic agent will kill a constant fraction of the tumor, rather than a constant number of tumor cells, regardless of the initial cell population. Although solid tumors such as ovarian cancer do not grow in a true exponential fashion, it is still likely that surgical reduction of tumor burden may reduce the number of cycles of chemotherapy that are needed. The likelihood of acquired chemoresistance caused by exposure of the tumor cells to the cytotoxic agent will therefore be decreased, as will the risk for chemotherapy-related toxicity. According to the mathematical model of Goldie and Coldman,39 in addition to increasing the response to chemotherapy, effective cytoreduction may improve the patient's comfort by improving or restoring intestinal function and decreasing the formation of ascites. The patient will be better able to maintain adequate nutrition and reduce the adverse metabolic consequences of the tumor, which in turn can increase her ability to withstand intensive combination chemotherapy. Blythe and Wahl40 reported improvement in the quality of life of patients who had undergone successful cytoreductive surgery. These patients were more likely to be able to continue their normal activities, including employment, compared with the patients who did not undergo aggressive cytoreduction. Nearly 80% of the patients who underwent optimal cytoreduction were able to tolerate a regular diet, compared with only 40% of patients who had suboptimal surgery. Morton41 proposed that debulking might be beneficial in terms of decreasing the immunosuppression caused by the tumor.
Clinical studies that support debulking now span more than three decades and include patients treated with radiotherapy, nonplatinum-based chemotherapy, and platinum-based combination regimens, suggesting that all of these treatments are more effective in patients with small tumor burdens. In 1968, Munnell,42 who developed the concept of “maximal surgical effort” for ovarian cancer, reported that patients who underwent a “definitive operation” had an improved survival rate compared with patients who had “partial removal” or “biopsy only.” In a 1969 report, Elclos and Quinlan43 reported that 25% of patients with stage II ovarian cancer whose disease was surgically reduced to “nonpalpable” survived, compared with 9% of those left with “palpable” disease.
The measures of cytoreduction and residual disease used in these early studies were obviously quite crude. Griffiths,44 in a seminal report published in 1975, was the first to quantify accurately the amount of residual disease after the primary operation for ovarian cancer and to attempt to correlate this with outcome after chemotherapy. Reporting on 102 patients who received alkylating agent chemotherapy for stage II or stage III ovarian cancer, he demonstrated with the use of a linear regression model that survival duration was significantly related to the size of the residual tumor at the completion of primary debulking. In his report, patients with no residual tumor had a median survival of 39 months, compared with 12.7 months for patients with residual tumor of greater than 1.5 cm in maximum diameter. Griffiths also made the important observation that aggressive resection of bulk tumor without removal of all tumor masses greater than 1.5 cm in diameter did not improve survival.
Subsequent reports of clinical trials of both nonplatinum-based combination regimens45 and platinum-based regimens46 have supported the concept of debulking. Reports from many institutions have evaluated the effect of surgical cytoreduction in ovarian cancer. Patients undergoing optimal cytoreduction are generally shown to have an increased chance of negative surgical reexploration after chemotherapy,47 an increase in median survival,48 and a decrease in the rate of recurrence after a negative second-look operation.49Table 5 shows a compilation of 10 reports,50, 51, 52, 53, 54, 55, 56, 57, 58, 59 comprising a total of 1012 patients, that demonstrates the effect of the amount of residual disease after primary cytoreduction on the frequency of negative surgical reexploration after chemotherapy (second-look laparotomy). Table 6, based on data from nine reports,60, 61, 62, 63, 64, 65, 66, 67, 68 shows the effect of the amount of residual disease on median survival in patients with advanced ovarian cancer treated with chemotherapy.
Table 5. Effect of Amount of Residual Disease After Primary Cytoreduction on Findings at Second-Look Laparotomy
| Percent Negative by Initial Residual | |||||
| First Author | Year | Patients (N) | None | Optimal* | Suboptimal* |
| Barnhill50 | 1984 | 96 | 67 | 61 | 14 |
| Berek51 | 1984 | 56 | – | 41 | 12 |
| Podratz58 | 1985 | 135 | 82 | 44 | 39 |
| Smirz59 | 1985 | 88 | 75 | 37 | 19 |
| Cain52 | 1986 | 177 | 76 | 50 | 28 |
| Dauplat54 | 1986 | 51 | 85 | 73 | 19 |
| Gallup55 | 1987 | 65 | 71 | 63 | 13 |
| Carmichael53 | 1987 | 146 | 62 | 30 | 25 |
| Lippman56 | 1988 | 67 | – | 49 | 07 |
| Lund57 | 1990 | 131 | 77 | 55 | 25 |
| Total | 1012 | ||||
| Weighted Mean | 75 | 48 | 22 | ||
Table 6. Survival of Patients With Advanced Ovarian Cancer According to the Amount of Residual Tumor After Primary Cytoreduction
| Median Survival (in Months) According to Amount of Residual Tumor | |||
| First Author | Year | Optimal* | Suboptimal* |
| Pohl66 | 1984 | 450 | 16 |
| Conte60 | 1985 | 25+ | 14 |
| Posada65 | 1985 | 30+ | 18 |
| Louie62 | 1986 | 240 | 15 |
| Redman67 | 1986 | 370 | 26 |
| Neijt63 | 1987 | 400 | 21 |
| Hainsworth61 | 1988 | 720 | 13 |
| Piver64 | 1988 | 480 | 21 |
| Sutton68 | 1989 | 450 | 23 |
| Mean | 410 | 18 | |
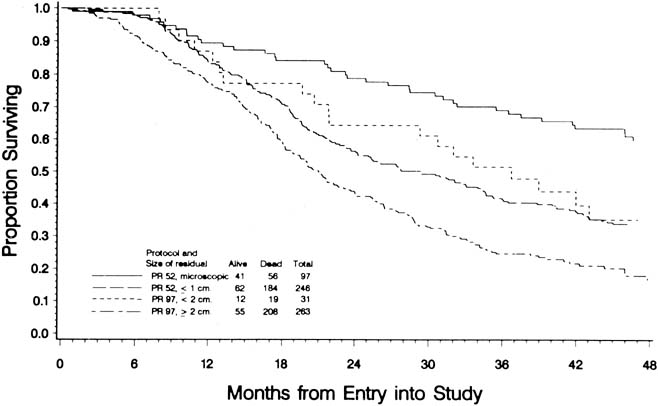
To evaluate the effect of residual disease on survival, Hoskins and colleagues69 reviewed data on 458 patients treated in accordance with a GOG protocol that evaluated standard versus high-dose chemotherapy with cisplatin and cyclophosphamide in suboptimal (residual disease greater than 1 cm) stage III and stage IV epithelial ovarian cancer. They found that patients with residual disease of 1–2 cm had better survival than patients with disease greater than 2 cm (Fig. 1). They found that cytoreduction that did not achieve residual disease of less than 2 cm had little effect on survival. When they combined the results of this analysis with an analysis of a similar GOG protocol (cisplatin and cyclophosphamide versus a cisplatin, cyclophosphamide, and doxorubicin combination) that enrolled patients with optimal (residual disease less than 1 cm) stage III epithelial ovarian cancer, they found that survival at 48 months was 58% for microscopic disease, 34% for residual gross disease up to 2 cm in diameter, and 16% for patients with residual disease greater than 2 cm (Fig. 2).
|
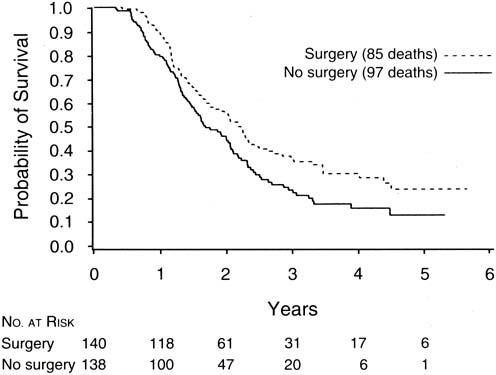
The finding of Hoskins and co-workers that disease that could not be cytoreduced to less than 2 cm in diameter was associated with uniformly poor survival and should be viewed in relation to a study of interval cytoreductive surgery by the European Organization for Research and Treatment of Cancer reported in 1995 by Van der Burg and associates.70
These authors randomized patients with suboptimal disease that could not be cytoreduced to six cycles of cisplatin and cyclophosphamide versus three cycles of the same chemotherapy followed by interval cytoreductive surgery. The surgical patients then received an additional three cycles of cisplatin and cyclophosphamide. They found a median survival of 26 months for patients undergoing interval cytoreduction versus 20 months for those who did not (p = 0.012) (Fig. 3).
|
Patients who present with suspected advanced ovarian cancer should undergo the routine preoperative tests before any major operation, including a complete blood count, biochemical profile, chest radiograph, and electrocardiogram. In addition, special radiographic studies such as computerized tomography, ultrasonography, or magnetic resonance imaging may be helpful in confirming the presence of a mass and identifying ascites or other evidence of metastatic disease. Intestinal imaging studies or upper and lower endoscopy is performed if there is clinical suspicion of a gastrointestinal primary tumor. Most gynecologic oncologists use a regimen of mechanical and antibiotic bowel preparation to cleanse the intestine in case intestinal resection is necessary or the bowel is entered inadvertently. Many gynecologic oncologists also use a regimen of low-dose subcutaneous heparin or intermittent calf compression devices to prevent deep vein thrombosis and perioperative intravenous antibiotics to decrease infections.
Before surgery, the patient and her family should be counseled thoroughly about the risk of malignancy and the surgical procedures that might be necessary. If there is a suspicion of rectal involvement, the possibility of low rectal anastomosis or colostomy should be mentioned. The patient should also understand that although every attempt will be made to remove as much tumor as possible, advanced ovarian cancer cannot be cured by surgery alone, and chemotherapy will be necessary.
The abdomen should be opened through a vertical incision extending from the pubic symphysis to well above the umbilicus to allow access to the upper abdominal structures. Before undertaking any pelvic operation, a complete exploration of the upper abdomen should be performed, with particular attention to the hemidiaphragms and omentum. The stomach and the small and large intestines should be evaluated, as should the retroperitoneal structures, including the lymph nodes, pancreas, and kidneys. An estimate of the resectability of upper abdominal disease may be important in determining the appropriate procedure for dealing with the pelvic tumor. In evaluating the pelvis, the surgeon must consider whether the primary tumor in the ovaries can be completely removed, the extent to which hysterectomy would contribute to debulking, and the resectability of any areas of pelvic spread. An experienced gynecologic oncologist should be able to assess the extent of possible tumor removal and to decide which operative procedures would be most appropriate for cytoreduction.
In some cases, an omental tumor can be removed adequately by an infracolic omentectomy with division of the omental attachments to the transverse colon. If the tumor involves the gastrocolic omentum, the dissection will have to be carried upward to detach the omentum from the greater curvature of the stomach. Care must be taken to avoid injury to the mesentery of the transverse colon, to which the omentum is often adherent, and to the gastroepiploic vessels along the greater curvature of the stomach wall. Omental tumors often extend toward the spleen. An appropriately large incision usually allows resection of the tumor without the need for a splenectomy; however, a splenectomy is occasionally indicated. If the omental tumor infiltrates into the wall of the transverse colon, resection of the colon may be necessary to allow optimal debulking. Bulky tumor nodules on the diaphragm are often the limiting factor in achieving optimal cytoreduction, although to some extent they can be debulked sharply or with the use of the ultrasonic aspirator71 or argon beam coagulator.72 If resection of small areas of residual disease on the diaphragm will render the patient's disease optimal, small areas of the diaphragm can be resected with primary repair. It is usually necessary to mobilize the liver to perform this procedure. Although a chest tube may be required after resection of a portion of the diaphragm, one can often insert a catheter into the chest before tying the last stitch; with the end of the tube under water, the anesthesiologist can expel the air in the chest with a deep inspiration. Isolated nodules on the small intestine can be resected.
In the pelvis, bulky tumor often distorts or eliminates normal intraperitoneal tissue planes and anatomic landmarks. The retroperitoneal tissue planes, however, are usually well preserved and should be used as primary landmarks during the pelvic operation. The retroperitoneal space can usually be entered via incision of the peritoneum lateral to the infundibulopelvic ligament or the colon. After development of the space and direct identification of the ureter, the infundibulopelvic ligament can be ligated and transected. Continuing the retroperitoneal dissection, the uterine artery can also be ligated. After this is repeated contralaterally, mobilization of the ovarian tumors can proceed with less blood loss. In the case of a large tumor adherent to the pelvic sidewall, removal may require dissection of the ureter from the mass. If the tumor involves the lateral aspect of the uterus, one can perform the type of dissection used in a modified radical hysterectomy, unroofing the ureter as it passes through the parametrial tunnel and ligating the uterine vessels at this point. Extensive involvement of the rectosigmoid may require en bloc resection along with hysterectomy, as is performed in a posterior exenteration. Intestinal continuity can usually be reestablished by low anastomosis using intestinal stapling instruments. A colostomy is rarely indicated. Keeping in mind the observation first made by Griffiths that unless “optimal” cytoreduction can be accomplished the outcome is not improved, the surgeon should be reasonably certain that all bulky disease can be resected before undertaking an aggressive procedure.
Management of the pelvic and aortic lymph nodes in advanced ovarian cancer is not well defined. Although most patients with advanced ovarian cancer have involvement of pelvic or paraaortic nodes,73, 74 most gynecologic oncologists agree that there is no need for routine diagnostic node sampling in patients with obvious intraperitoneal dissemination, because the information gained will have no bearing on treatment or prognosis. Whether an extensive lymphadenectomy has any effect on survival is unknown. One group has reported a retrospective series in which patients undergoing lymphadenectomy had an improved survival rate compared with historical controls.75 These data await confirmation. If the resection of bulky nodal disease allows optimal cytoreduction, it should be performed. It is our practice to remove enlarged lymph nodes in patients able to undergo optimal resection of intra-abdominal tumor.
The closure of the abdominal incision in advanced ovarian cancer patients deserves special attention. These patients are often elderly, obese, and nutritionally depleted, and they may have concomitant medical problems, such as diabetes, that interfere with wound healing and increase the risk of dehiscence. This closure may be performed with interrupted sutures or with a continuous suture using nonabsorbable or delayed absorbable material.76, 77 Most studies show improved results with continuous mass closure techniques.
A number of centers have reported their experiences with the primary debulking of advanced ovarian cancer. Table 7 shows data from 11 reports,64, 67, 78, 79, 80, 81, 82, 83, 84, 85 published since 1979, on a total of 2282 patients. The great variation among these individual studies in the ability to achieve optimal cytoreduction (range 17–87%) must in part be explained by patient selection, varying degrees of surgical skill and aggressiveness, and different definitions of optimal cytoreduction. In general, skilled and experienced gynecologic oncologists should be able to accomplish optimal debulking in at least one-half of their patients with advanced ovarian cancer.
Table 7. Optimal Cytoreduction at Primary Surgery in Stage III And Stage IV Ovarian Cancer
| First Author | Year | Patients (N) | Optimal Number | Cytoreduction Percentage |
| Smith78 | 1979 | 792 | 190 | 24 |
| Delgado80 | 1984 | 75 | 13 | 17 |
| Neijt83 | 1984 | 186 | 76 | 41 |
| Wharton85 | 1984 | 395 | 154 | 39 |
| Redman67 | 1986 | 86 | 34 | 40 |
| Heintz82 | 1986 | 70 | 49 | 70 |
| Neijt86 | 1987 | 191 | 94 | 49 |
| Piver64 | 1988 | 40 | 35 | 87 |
| Potter84 | 1991 | 185 | 119 | 64 |
| Eisenkop81 | 1992 | 126 | 103 | 82 |
| Baker79 | 1994 | 136 | 113 | 83 |
| Total | 2282 | 980 | 43 |
Although some authors have questioned the value of debulking surgery in patients with stage IV ovarian cancer, reports from several institutions, including Memorial Sloan-Kettering and the University of Pennsylvania,87, 88, 89, 90 indicate a significant survival benefit to optimal cytoreduction in stage IV patients. Data from these studies are summarized in Table 8. These authors concluded that attempted cytoreduction was indicated in all patients with advanced ovarian cancer, even in patients with stage IV disease.
Table 8. Effect of Debulking on Survival in Stage IV Ovarian Cancer
| First Author | Year | Surgical Result | Patients (N) | Optimal (%) | Median Survival | P |
| Curtin88 | 1997 | Optimal (<2 cm) | 41 | 45 | 40 | 0.0100 |
| Suboptimal | 51 | 18 | ||||
| Liu89 | 1997 | Optimal (<2 cm) | 14 | 30 | 37 | 0.0200 |
| Suboptimal | 33 | 17 | ||||
| Munkarah90 | 1997 | Optimal (<2 cm) | 31 | 34 | 25 | 0.0200 |
| Suboptimal | 61 | 15 | ||||
| Bristow87 | 1998 | Optimal (<1 cm) | 25 | 30 | 38 | 0.0004 |
| Suboptimal | 59 | 10 |
The morbidity of aggressive cytoreductive surgery appears to be within acceptable limits, based on publications from major centers. Heintz and co-workers82 reported that with increasing experience, improved cardiovascular monitoring, and the increased use of parenteral hyperalimentation, the risk of serious perioperative morbidity decreased to 10%, the most commonly encountered problem in their series being postoperative pneumonia.
Although patients with small-volume residual tumor at the initiation of primary chemotherapy have an improved prognosis, these patients may have had smaller, less aggressive tumors to begin with. Their improved prognosis may relate more to the biology of the tumor than to the extent of the surgery. Several authors have attempted to address this issue by retrospective studies that examine the prognostic significance of the extent of disease before and after cytoreduction. In a report by Hacker and co-workers,91 patients with large-volume metastatic disease (greater than 10 cm) were analyzed according to the success of cytoreduction. Of the patients with bulky disease, those for whom tumor was reduced to optimal status had a longer median survival than those for whom it was not. However, patients who started with smaller volume disease and achieved optimal status had an even longer survival. In this series, published in 1983, Hacker and associates used only a single alkylating agent as the chemotherapy after surgery. In a 1988 series, Heintz and colleagues92 reported on the effect of preoperative tumor size in patients treated with cisplatin-based combination chemotherapy. They observed that optimal tumor resection in patients with large-volume metastatic disease improved median survival by approximately 12 months. Once again, the survival of patients with optimal cytoreduction (smallest residual disease) who started with large-volume disease was not as good as that for their counterparts who started with smaller volume disease.
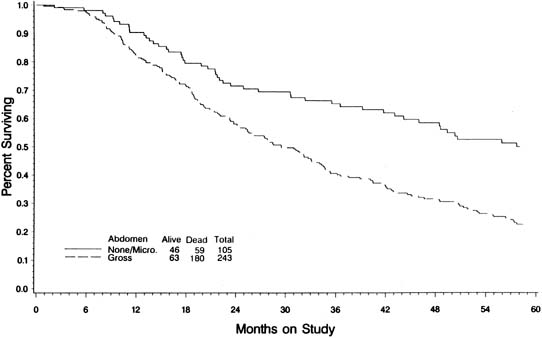
In an attempt to evaluate the relationship between cytoreductive surgery and tumor biology, Hoskins and colleagues93 reviewed 349 patients with stage III optimal (less than 1 cm) epithelial ovarian cancer. They divided patients into those found to have disease less than 1 cm in diameter on exploration and those who required cytoreductive surgery to achieve disease less than 1 cm. They theorized that if only surgery was important, survival would be equal between the two groups. They observed that patients found to have small-volume abdominal disease had a better survival than those who achieved small-volume residual disease via debulking (Fig. 4) (p = 0.0001). When evaluated by multivariate analysis, a variety of factors in addition to cytoreduction were found to be significant. The authors concluded that although cytoreduction was important, the biology of the tumor also influenced outcome. These studies suggest that although the finding of large-volume metastatic disease confers a poorer prognosis, and although the biology of the tumor is important, optimal tumor resection in these patients can improve median survival.
No prospective study randomizing ovarian cancer patients to aggressive cytoreduction versus less aggressive surgery has ever been performed, nor is such a study likely to be completed in the future. The GOG attempted such a trial with its protocol 80, but the trial came to a halt because of slow patient accrual; this failed attempt also was a reflection of the strong belief among gynecologic oncologists in the benefits of debulking.
The use of neoadjuvant chemotherapy is emerging as an attractive option in management of certain patients with either bulky unresectable disease, or those with medical morbidity. Patients initially undergo chemotherapy and then interval surgical cytoreduction. Retrospective analyses have demonstrated equivalent overall survivals when comparing patients treated with primary chemotherapy followed by interval debulking with conventionally treated patients.94, 95 In 2010, Vergote et al.96 reported results of the first randomized control trial of 670 patients assigned to either neoadjuvant chemotherapy followed by debulking or traditional debulking surgery followed by adjuvant platinum-based chemotherapy. As expected, postoperative morbidity and mortality were higher on the primary surgery arm. Overall and progression-free survival were statistically similar between the two groups. Notably, complete cytoreduction of all visible disease was the strongest independent factor influencing survival, whether performed at initial surgery or at interval surgery: optimal debulking was achieved in 80.6% and 41.6% of patients, respectively.
Few procedures offer as great a challenge to the skill and judgment of the surgeon as the cytoreduction of advanced ovarian cancer. Because optimal cytoreduction is such an important part of treatment, we believe that patients with suspected advanced ovarian cancer should be referred to a center where trained and experienced gynecologic oncologists are available.
REFERENCES
Jemal A, Siegel R, Xu J et al: Cancer statistics. CA Cancer J Clin 60:5, 2010 |
|
Young RA, Fuks Z, Hoskins WJ: Cancer of the ovary. In DeVita VT, Jr, Hillman SRosenberg SA, (eds): Principles and Practice of Oncology. Philadelphia, JB Lippincott, 1995 |
|
Scully RE: Tumors of the ovary and maldeveloped gonads. Washington, D.C., Armed Forces Institute of Pathology, 1979 |
|
Fuks Z: Patterns of spread of ovarian carcinoma: Relation to therapeutic strategies. Adv Biosci 26:39, 1980 |
|
Keettel WC, Pixley EE: Diagnostic value of peritoneal washings. Clin Obstet Gynecol 1:592, 1958 |
|
Feldman GB, Knapp RC: Lymphatic drainage of the peritoneal cavity and its significance in ovarian cancer. Am J Obstet Gynecol 119:991, 1974 |
|
Bagley CM, Jr, Young RC, Schein PS et al: Ovarian carcinoma metastatic to the diaphragm--frequently undiagnosed at laparotomy. A preliminary report Am J Obstet Gynecol 116:397, 1973 |
|
Piver MS, Barlow JJ, Lele SB: Incidence of subclinical metastasis in stage I and II ovarian carcinoma. Obstet Gynecol 52:100, 1978 |
|
Buchsbaum HJ, Brady MF, Delgado G et al: Surgical staging of carcinoma of the ovaries. Surg Gynecol Obstet 169:226, 1989 |
|
Eichner E, Bove E: In vivo studies on the lymphatic drainage of the human ovary. Obstet Gynecol 3:287, 1954 |
|
Plentyl A, Friedman E: Lymphatic System of the Female Genitalia. Philadelphia, WB Saunders, 1971 |
|
Musumeci R, De Palo G, Kenda R et al: Retroperitoneal metastases from ovarian carcinoma: Reassessment of 365 patients studied with lymphography. AJR Am J Roentgenol 134:449, 1980 |
|
Burghardt E, Pickel H, Stettner H: Management of advanced ovarian cancer. Eur J Gynaecol Oncol 5:155, 1984 |
|
Staging announcement: FIGO Cancer Committee. Gynecol Oncol 25:383, 1986 |
|
Pettersson F: Annual report on the results of treatment in gynecological cancer. Stockholm, International Federation of Gynecology and Obstetrics, 1991 |
|
Landis SH, Murray T, Bolden S et al: Cancer statistics 1998. CA Cancer J Clin 48:6, 1998 |
|
Young RC, Walton LA, Ellenberg SS et al: Adjuvant therapy in stage I and stage II epithelial ovarian cancer. Results of two prospective randomized trials N Engl J Med 322:1021, 1990 |
|
Park JY et al. Comparison of laparoscopy and laparotomy in surgical staging of early-stage ovarian and fallopian tubal cancer. Ann Surg Oncol 2008; 15(7):2012-9; |
|
Park JY, et al. Laparoscopic and laparotomic staging in stage I epithelial ovarian cancer: a comparison of feasibility and safety. Int J Gynecol Cancer 2008; 18(6):1202-9; |
|
Lee M, et al. Comparisons of surgical outcomes, complications, and costs between laparotomy and laparoscopy in early-stage ovarian cancer. Int J Gynecol Cancer 2011; 21(2):251-6; |
|
Ghezzi F, et al. Laparoscopy staging of early ovarian cancer: our experience and review of the literature.Int J Gynecol Cancer 2009; 19 Suppl 2:S7-S13 |
|
Schilder JM, Thompson AM, DePriest PD et al: Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol Oncol 87:1, 2002 |
|
Park JY, et al. Outcomes of fertility-sparing surgery for invasive epithelial ovarian cancer: oncologic safety and reproductive outcomes. Gynecol Oncol 2008, 110(3):345-353 |
|
Voest EE, et al. A meta-analysis of prognostic factors in advanced ovarian cancer with median survival and overall survival (measured with the log (relative risk] as main objectives.Eur J Cancer Clin Oncol 1989 25:711-720 |
|
Heintz AP et al. Carcinoma of the ovary. Int J Gynaecol Obstet 2003, 83:135-166 |
|
Zanetta G, Chiari S, Rota S et al: Conservative surgery for stage I ovarian carcinoma in women of childbearing age. Br J Obstet Gynaecol 104:1030, 1997 |
|
Boyd J, Rubin SC: Hereditary ovarian cancer: molecular genetics and clinical implications. Gynecol Oncol 64:196, 1997 |
|
Miki Y, Swensen J, Shattuck-Eidens D et al: A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266:66, 1994 |
|
Wooster R, et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science 1994, 265(5181):2088-90 |
|
Ford D, Easton DF, Bishop DT et al: Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium Lancet 343:692, 1994 |
|
Brose MS, Rebbeck TR, Calzone KA et al: Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst 94:1365, 2002 |
|
Rebbeck TR, Lynch HT, Neuhausen SL et al: Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med 346:1616, 2002 |
|
Rebbeck TR, et al. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst 101:80-87. 2009 |
|
Kurain AW, et al. J Clin Oncol 2010, 2:222-231 |
|
Bast RC, Jr, Klug TL, St John E et al: A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 309:883, 1983 |
|
Bourne T, Campbell S, Steer C et al: Transvaginal colour flow imaging: A possible new screening technique for ovarian cancer. BMJ 299:1367, 1989 |
|
Skipper HE: Thoughts on cancer chemotherapy and combination modality therapy. JAMA 230:1033, 1974 |
|
Skipper HE, Schabel FJ, Wilcox W: Experimental evaluation of potential anticancer agents XII: On the criteria and kinetics associated with “curability” of experimental leukemia. Cancer Chemother Rep 35:1, 1979 |
|
Goldie, J. H. & Coldman, A. J. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat. Rep. 63, 1727–1733 (1979). |
|
Blythe JG, Wahl TP: Debulking surgery: does it increase the quality of survival. Gynecol Oncol 14:396, 1982 |
|
Morton DL: Changing concepts of cancer surgery: surgery as immunotherapy. Am J Surg 135:367, 1978 |
|
Munnell EW: The changing prognosis and treatment in cancer of the ovary. A report of 235 patients with primary ovarian carcinoma 1952-1961 Am J Obstet Gynecol 100:790, 1968 |
|
Elclos L, Quinlan EJ: Malignant tumors of the ovary managed with postoperative megavoltage irradiation. Radiology 93:659, 1969 |
|
Griffiths CT: Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr 42:101, 1975 |
|
Young RC, Chabner BA, Hubbard SP et al: Advanced ovarian adenocarcinoma. A prospective clinical trial of melphalan (L-PAM) versus combination chemotherapy N Engl J Med 299:1261, 1978 |
|
Omura GA, Bundy BN, Berek JS et al: Randomized trial of cyclophosphamide plus cisplatin with or without doxorubicin in ovarian carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol 7:457, 1989 |
|
Rubin SC, Lewis JL, Jr: Second-look surgery in ovarian carcinoma. Crit Rev Oncol Hematol 8:75, 1988 |
|
Hoskins WJ: The influence of cytoreductive surgery on progression-free interval and survival in epithelial ovarian cancer. Baillieres Clin Obstet Gynaecol 3:59, 1989 |
|
Rubin SC, Hoskins WJ, Saigo PE et al: Prognostic factors for recurrence following negative second-look laparotomy in ovarian cancer patients treated with platinum-based chemotherapy. Gynecol Oncol 42:137, 1991 |
|
Barnhill DR, Hoskins WJ, Heller PB et al: The second-look surgical reassessment for epithelial ovarian carcinoma. Gynecol Oncol 19:148, 1984 |
|
Berek JS, Hacker NF, Lagasse LD et al: Second-look laparotomy in stage III epithelial ovarian cancer: Clinical variables associated with disease status. Obstet Gynecol 64:207, 1984 |
|
Cain JM, Saigo PE, Pierce VK et al: A review of second-look laparotomy for ovarian cancer. Gynecol Oncol 23:14, 1986 |
|
Carmichael JA, Shelley WE, Brown LB et al: A predictive index of cure versus no cure in advanced ovarian carcinoma patients—replacement of second-look laparotomy as a diagnostic test. Gynecol Oncol 27:269, 1987 |
|
Dauplat J, Ferriere JP, Gorbinet M et al: Second-look laparotomy in managing epithelial ovarian carcinoma. Cancer 57:1627, 1986 |
|
Gallup DG, Talledo OE, Dudzinski MR et al: Another look at the second-assessment procedure for ovarian epithelial carcinoma. Am J Obstet Gynecol 157:590, 1987 |
|
Lippman SM, Alberts DS, Slymen DJ et al: Second-look laparotomy in epithelial ovarian carcinoma. Prognostic factors associated with survival duration Cancer 61:2571, 1988 |
|
Lund B, Williamson P: Prognostic factors for outcome of and survival after second-look laparotomy in patients with advanced ovarian carcinoma. Obstet Gynecol 76:617, 1990 |
|
Podratz KC, Malkasian GD, Jr, Hilton JF et al: Second-look laparotomy in ovarian cancer: evaluation of pathologic variables. Am J Obstet Gynecol 152:230, 1985 |
|
Smirz LR, Stehman FB, Ulbright TM et al: Second-look laparotomy after chemotherapy in the management of ovarian malignancy. Am J Obstet Gynecol 152:661, 1985 |
|
Conte PF, Sertoli MR, Bruzzone M et al: Cisplatin, methotrexate, and 5-fluorouracil combination chemotherapy for advanced ovarian cancer. Gynecol Oncol 20:290, 1985 |
|
Hainsworth JD, Grosh WW, Burnett LS et al: Advanced ovarian cancer: Long-term results of treatment with intensive cisplatin-based chemotherapy of brief duration. Ann Intern Med 108:165, 1988 |
|
Louie KG, Ozols RF, Myers CE et al: Long-term results of a cisplatin-containing combination chemotherapy regimen for the treatment of advanced ovarian carcinoma. J Clin Oncol 4:1579, 1986 |
|
Neijt JP, ten Bokkel Huinink WW, van der Burg ME et al: Randomized trial comparing two combination chemotherapy regimens (CHAP-5 v CP) in advanced ovarian carcinoma. J Clin Oncol 5:1157, 1987 |
|
Piver MS, Lele SB, Marchetti DL et al: The impact of aggressive debulking surgery and cisplatin-based chemotherapy on progression-free survival in stage III and IV ovarian carcinoma. J Clin Oncol 6:983, 1988 |
|
Posada JG, Jr, Marantz AB, Yeung KY et al: The cyclophosphamide, hexamethylmelamine, 5 fluorouracil (CHF) regimen in the treatment of advanced and recurrent ovarian cancer. Gynecol Oncol 20:23, 1985 |
|
Pohl R, Dallenbach-Hellweg G, Plugge T et al: Prognostic parameters in patients with advanced ovarian malignant tumors. Eur J Gynaecol Oncol 5:160, 1984 |
|
Redman JR, Petroni GR, Saigo PE et al: Prognostic factors in advanced ovarian carcinoma. J Clin Oncol 4:515, 1986 |
|
Sutton GP, Stehman FB, Einhorn LH et al: Ten-year follow-up of patients receiving cisplatin, doxorubicin, and cyclophosphamide chemotherapy for advanced epithelial ovarian carcinoma. J Clin Oncol 7:223, 1989 |
|
Hoskins WJ, McGuire WP, Brady MF et al: The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol 170:974, 1994 |
|
van der Burg ME, van Lent M, Buyse M et al: The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer N Engl J Med 332:629, 1995 |
|
Adelson MD, Baggish MS, Seifer DB et al: Cytoreduction of ovarian cancer with the Cavitron ultrasonic surgical aspirator. Obstet Gynecol 72:140, 1988 |
|
Brand E, Pearlman N: Electrosurgical debulking of ovarian cancer: A new technique using the argon beam coagulator. Gynecol Oncol 39:115, 1990 |
|
Burghardt E, Pickel H, Lahousen M et al: Pelvic lymphadenectomy in operative treatment of ovarian cancer. Am J Obstet Gynecol 155:315, 1986 |
|
Wu PC, Qu JY, Lang JH et al: Lymph node metastasis of ovarian cancer: A preliminary survey of 74 cases of lymphadenectomy. Am J Obstet Gynecol 155:1103, 1986 |
|
Burghardt E, Lahousen M, Stettner H: The significance of pelvic and para-aortic lymphadenectomy in the operative treatment of ovarian cancer. Baillieres Clin Obstet Gynaecol 3:157, 1989 |
|
Archie JP, Feldtman RW: Primary abdominal wound closure with permanent, continuous running monofilament sutures. Surg Gynecol Obstet 153:721, 1981 |
|
Knight CD, Griffen FD: Abdominal wound closure with a continuous monofilament polypropylene suture. Experience with 1,000 consecutive cases Arch Surg 118:1305, 1983 |
|
Smith JP, Day TG, Jr: Review of ovarian cancer at the University of Texas Systems Cancer Center, M.D. Anderson Hospital and Tumor Institute Am J Obstet Gynecol 135:984, 1979 |
|
Baker T, Piver MS, Hempling RE: Improved long-term survival by cytoreductive surgery to less than 1 cm, induction cisplatin and monthly cisplatin, Adriamycin, cyclophosphamide in advanced ovarian adenocarcinoma. Proc Ann Meet Am Soc Clin Oncol 13:A861, 1994 |
|
Delgado G, Oram DH, Petrilli ES: Stage III epithelial ovarian cancer: The role of maximal surgical reduction. Gynecol Oncol 18:293, 1984 |
|
Eisenkop SM, Spirtos NM, Montag TW et al: The impact of subspecialty training on the management of advanced ovarian cancer. Gynecol Oncol 47:203, 1992 |
|
Heintz AP, Hacker NF, Berek JS et al: Cytoreductive surgery in ovarian carcinoma: Feasibility and morbidity. Obstet Gynecol 67:783, 1986 |
|
Neijt JP, ten Bokkel Huinink WW, van der Burg ME et al: Randomised trial comparing two combination chemotherapy regimens (Hexa-CAF vs CHAP-5) in advanced ovarian carcinoma. Lancet 2:594, 1984 |
|
Potter ME, Partridge EE, Hatch KD et al: Primary surgical therapy of ovarian cancer: How much and when. Gynecol Oncol 40:195, 1991 |
|
Wharton JT, Edwards CL: Cytoreductive surgery for common epithelial tumours of the ovary. Clin Obstet Gynaecol 10:235, 1983 |
|
J.P. Neijt, W.W. ten Bokkel Huinink, M.E.L. van der Burg, et al. Randomized Trial Comparing Two Combination Chemotherapy Regimens (CHAP-5 v CP) in Advanced Ovarian Carcinoma. Journal of Clinical Oncology 1987;5:1157-1168 |
|
Bristow RE, Montz FJ, Lagasse LD et al: Survival impact of surgical cytoreduction in stage IV epithelial ovarian cancer. Gynecol Oncol 72:278, 1999 |
|
Curtin JP, Malik R, Venkatraman ES et al: Stage IV ovarian cancer: Impact of surgical debulking. Gynecol Oncol 64:9, 1997 |
|
Liu PC, Benjamin I, Morgan MA et al: Effect of surgical debulking on survival in stage IV ovarian cancer. Gynecol Oncol 64:4, 1997 |
|
Munkarah AR, Hallum AV, 3rd, Morris M et al: Prognostic significance of residual disease in patients with stage IV epithelial ovarian cancer. Gynecol Oncol 64:13, 1997 |
|
Hacker NF, Berek JS, Lagasse LD et al: Primary cytoreductive surgery for epithelial ovarian cancer. Obstet Gynecol 61:413, 1983 |
|
Heintz AP: Surgery in advanced ovarian carcinoma: is there proof to show the benefit. Eur J Surg Oncol 14:91, 1988 |
|
Hoskins WJ, Bundy BN, Thigpen JT et al: The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: A Gynecologic Oncology Group study. Gynecol Oncol 47:159, 1992 |
|
Schwartz PE, Rutherford TJ, Chambers JT et al: Neoadjuvant chemotherapy for advanced ovarian cancer: long-term survival. Gynecol Oncol 72:93, 1999 |
|
Kayikcioglu F, Kose MF, Boran N et al: Neoadjuvant chemotherapy or primary surgery in advanced epithelial ovarian carcinoma. Int J Gynecol Cancer 11:466, 2001 |
|
Vergote et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010; 363:943-53 |