Prostaglandins and the Reproductive Cycle
Authors
INTRODUCTION
Prostaglandins are a group of endogenously occurring acidic lipids that appear to be involved in a large number of reproductive processes. Kurzrok and Leib first reported that a substance in human semen altered the contractile activity of human uterine strips.1 In 1935, Von Euler named this substance prostaglandin because he believed that it was produced in the prostate gland; however, it was later shown to be from the seminal vesicles.2 Subsequent studies showed that semen contained a mixture of more than 14 polyunsaturated fatty acids, each composed of 20 carbons.
It was not until 1957 that the first prostaglandins (PGE1 and PGF2α) were isolated, and their structures were not determined until 1963.3,4 In the late 1960s, the first research samples of prostaglandins became available. These samples allowed the first investigations of the role of these lipids in physiologic and pathophysiologic states. The 1970s brought two new discoveries that further clarified the mechanism by which prostaglandins exert their effects on tissues. The first observation was made by Vane, who reported that anti-inflammatory drugs such as aspirin exert their effects by inhibition of the cyclooxygenase enzyme system, which converts the prostaglandin precursor arachidonic acid to endoperoxides, thus giving rise to the prostaglandins.5 This discovery allowed investigators the first opportunity to block endogenous prostaglandin synthesis. The second discovery was made by Pace-Asciak and Wolfe, who found a novel derivative of arachidonic acid in rat stomach homogenates.6 This compound later was named prostacyclin (PGI2) by Vane and coworkers, who showed that it was important in preventing both platelet aggregation and thrombus formation on vascular walls.7,8
Like steroids, prostaglandins are a group of compounds that have somewhat similar structures (Fig. 1) but may have totally different effects. Prostaglandins appear to be autocrine or paracrine regulators rather than true hormones because they are both produced and act locally. These compounds are metabolized locally in many tissues, but if not degraded locally, they enter the circulatory system and are neutralized effectively by the action of the degradative enzyme prostaglandin dehydrogenase in the lungs.
Formation and Nomenclature
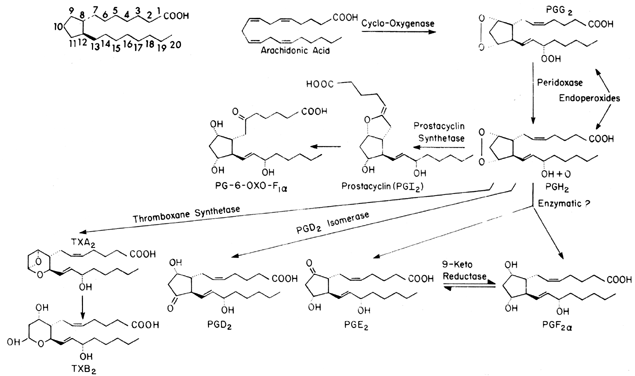
Prostaglandins are formed from one of three fatty acids that give rise to different families of compounds depending on the number of unsaturated carbon-to-carbon double bonds that are left in the final molecule; dihomogamma linolenic acid has one double bond, arachidonic (eicosatetraenoic) acid has two, and eicosapentaenoic acid has three. Each series of prostaglandins then is identified by a subscript (i.e., 1, 2, or 3). In the case of arachidonic acid, the subscript would be 2, thus PGE2. In addition to the numeric subscript, prostaglandins may have an α or β subscript, which is used to denote the steric configuration of the hydroxyl group at the C9 position: PGF2α. The majority of endogenous prostaglandins formed in mammals are the result of metabolism of arachidonic acid, which is available both in the diet and after anabolic formation from linolenic acid.
Arachidonic acid is transported in plasma in the unesterified form and is linked noncovalently to plasma proteins. In the cell, arachidonic acid is stored in the phospholipid pools in the esterified form. However, only free unesterified fatty acids can be converted to prostaglandins by the cyclooxygenase enzyme system. Thus, availability of unesterified arachidonic acid generally is believed to be the rate-limiting step in prostaglandin biosynthesis. The release of arachidonic acid is controlled by the activity of an acylhydrolase enzyme, phospholipase A2, which acts on the sn-2 position of the phospholipid to free arachidonic acid. On release, the cyclooxygenase enzyme places molecular oxygen at the C15 position (carbons are numbered from the carboxylic acid [see Fig. 1]) to form a peroxide and a second molecule across C9 and C11 to form an endoperoxide. Simultaneously, a bond forms between C8 and C12, forming a cyclopentane ring, thus resulting in PGG2. PGG2 is converted to PGH2 by a peroxidase acting at C15. Both PGG2 and PGH2 have relatively short half-lives (t 1/2 = 5 minutes). As can be seen in Figure 1, once it is formed, PGH2 can undergo a number of transformations to form the stable prostaglandin PGD2, PGE2, or PGF2α. These changes occur either nonenzymatically or by reductase or isomerase enzymes that occur in a number of tissues. The distribution of these enzyme systems differs for each organ and species studied.
Two other prostanoids, thromboxane A2 (TxA2) and prostacyclin (PGI2), are formed from the endoperoxides PGG2 and PGH2. TxA2, which is formed by thromboxane synthetase, has a relatively short half-life (30 seconds) and has been implicated in both platelet aggregation and arterial vasoconstriction.9 TxA2 breaks down spontaneously to TxB2. PGI2 (formed by prostacyclin synthetase) appears to be important in preventing platelet aggregation and is a potent vasodilator.10 PGI2 also has a relatively short half-life (t 1/2 = 2 minutes) at physiologic pH and temperature and undergoes spontaneous hydrolysis to form the biologically inactive 6-keto PGF1α. Reports suggest that, at least in the liver, 6-keto-PGF1α can be converted enzymatically to the biologically active 6-keto-PGE1, but the importance of this compound in reproductive medicine is unclear.11 One other pathway requires discussion, the conversion of PGE2 to PGF2α by the enzyme 9-ketoreductase. Because in most tissues these two prostaglandins have opposite actions, this interconversion could lead to local modulation of the final biologic action.
Metabolism
Metabolism of prostaglandins PGD2, PGE2, and PGF2α involves a series of enzymic steps. The first is oxidation of the C15 hydroxyl group to a ketone by the enzyme 15-prostaglandin dehydrogenase (15 PGDH). This step can occur both locally (i.e., placenta, lung, spleen, kidney) and after release into the systemic circulation in the lungs, spleen, and liver. The second step is reduction of the double bond between C13 and C14 by the enzyme prostaglandin-13-reductase. It is the combination of steps one and two that gives rise to the major circulating metabolites 13, 14-dihydro-15-ketoprostaglandins, which are measured by radioimmunoassay (RIA). The third step in metabolism is beta oxidation, which occurs both in the liver and the kidney and which can remove two or four carbons, giving dinor or tetranor metabolites, respectively (see Fig. 1). The final step occurs in the liver and removes two carbons (from C20 and C19) by omega oxidation. These last two steps produce more water-soluble metabolites, which are excreted in the urine.
In contrast, the metabolism of PGI2 is influenced further by its nonenzymatic hydrolysis to 6-keto-PGF1α as the major plasma metabolite. Both this compound and 6-keto-PGF1α are candidates for beta and omega oxidation, leading to a range of urinary metabolites. 2,3 dinor 6-keto-PGF1α and 6-keto-PGF1α usually are measured in urine as indices of prostacyclin production. Thromboxane A2 also is hydrolyzed spontaneously, yielding the inactive metabolite TxB2, itself a target for beta oxidation, yielding the urinary metabolite of thromboxane 2,3 dinor TxB2. However, TxB2 also may be dehydrogenated at C-11 to give 11-dehydro TxB2, which has a longer plasma half-life, making it a suitable candidate for measurement. The 11-dehydro TxB2 metabolite also is found in urine.
Isoprostanes
Isoprostanes are prostaglandin-like compounds that are produced by a nonenzymatic mechanism involving free radical catalyzed peroxidation of arachidonic acid. The major compounds formed contain the F-type prostane ring and are isomeric to PGF2α and thus are termed F2-isoprostanes, although E2- and D2-prostanes and isothromboxanes also can be formed.12 Isoprostanes are formed initially by peroxidation of arachidonic acid esterified to tissue lipids and thus are markers for lipid peroxidation. Hydrolysis of isoprostanes from phospholipids in vivo is catalyzed by phospholipases. Measurement of 8-isoprostane within plasma and urine currently is being used as a reliable noninvasive approach to assess oxidative stress status (balance of pro-oxidants vs. antioxidant defenses) in humans. Increased urinary 8-isoprostane has been reported in chronic smokers,13 and treatment with antioxidant vitamin C significantly reduced 8-isoprostane excretion. A recent report14 found plasma 8-isoprostane to be increased significantly in preeclamptic women versus controls, which is consistent with the concept of preeclampsia being a state of oxidative stress. The 8-iso-PGF2α compound is a potent vasoconstrictor of the renal and pulmonary vasculature and a bronchoconstrictor probably acting by means of a novel isoprostane receptor.
Measurement
Several techniques have been used to measure prostaglandins. These include bioassays, gas chromatography combined with mass spectrometry (GC-MS), RIA, and enzyme immunoassay (EIA). Of these methods, GC-MS probably is the most accurate and specific. However, this technique is expensive, difficult to perform, and time consuming. The bioassay methods generally require the presence of large concentrations of the prostaglandins and carefully treated bioassay tissues. These techniques, however, do give a continuous measurement, are invaluable when studying release of prostaglandins from tissues, and also are the only assays of biological activity. RIA and EIA have been the methods of choice for biologic investigation because they are easy to perform, provide rapid results, and allow measurement in a large number of samples. These techniques are not without problems, however, and care must be taken to ensure the specificity and accuracy of measurements.15 Over the past 20 years, the levels of prostaglandins measured by RIA have consistently become lower and approached levels measured by GC-MS. Although this suggests that the RIA methods have improved, it also suggests that earlier studies must be interpreted with care.
RIAs and EIAs for prostaglandins are generally preceded by extraction and chromatographic techniques, but recently several assays have been reported that utilize direct plasma measurements.16,17 This may be desirable in some cases, since extraction may lead to breakdowns of the prostanoid to be measured. RIAs and EIAs have been described for most primary prostaglandins (PGD2, PGE2, PGF2α), hydrolysis products (6-keto-PGF1α, TXB2), the circulating plasma metabolites (6, 15-diketo-PGF1α; 13, 14-dihydro-15-keto-PGE2; and 13, 14-dihydro-15-keto-PGF2α), and urinary metabolites (dinor compounds).
When investigating physiologic phenomena in humans by measurement of peripheral (antecubital vein) prostaglandin levels, remember that concentrations of PGE2 and PGF2α are very low (1–2 pg/mL), that PGE2 and PGF2α are metabolized quickly to their respective 13, 14-dihydro-15-keto-metabolite (see Metabolism) and that measurement of the primary prostaglandins (PGE2 or PGF2α) does not reflect ongoing changes. Unfortunately, although the metabolite 13, 14-dihydro-15-keto-PGF2α (PGFM) is stable, the PGE2 counterpart is unstable and bicyclizes to form 11-deoxy-15-keto-13, 14-dihydro-11, 16-cyclo-PGE2, also called bicyclic PGE2.18 An assay for this compound has been described, but very little information exists regarding circulating levels of the PGE metabolite.18 Measurement of these plasma metabolites, which have a larger half-life than the present compound, does appear to be adequate for measuring PGE2 and PGF2α output.
It is abundantly clear that PGI2 is present in such low concentrations (1 pg/mL) in the peripheral circulation that it does not function as a circulating vasodilator. The concentrations of the metabolite 6-keto-PGF1α in plasma also are too low to measure reliably by RIA and EIA; therefore, GC-MS or high-pressure liquid chromatography (HPLC)-RIA measurement of 2,3 dinor 6-keto-PGF1α in urine currently is performed as an index of whole-body PGI2 production. Although 6-keto-PGF1α also is measured in urine, this may arise from local kidney production and may escape beta oxidation and therefore is believed solely to represent renal synthesis.
The major problem in plasma thromboxane measurements is the synthesis of TxA2 by platelets activated during venipuncture and blood clotting, despite the use of prostaglandin synthesis inhibitors during the collection procedure. Measurement of the hydrolysis product TxB2 in plasma is therefore subject to sampling artifact. TxB2 undergoes metabolism by two major pathways in humans either by beta oxidation, which forms the 2,3 dinor TxB2 metabolite, or by dehydrogenation of the hemiacetal alcohol at C-11 to give a 11-dehydro TxB2 metabolite. Being less polar, 11-dehydro-TxB2 has a longer plasma half-life and can be measured by RIA.19 However, 11-dehydro-TxB2 is as an abundant urinary metabolite as 2,3 dinor TxB2, and measurement of either of these compounds in urine may represent changes in whole-body TxA2 production.20
Because of the plethora of urinary metabolites of both PGI2 and TxA2 that are produced (and the potential for cross-reactivity of the antisera), a HPLC separation of these metabolites before RIA of the 2,3 dinor metabolites is essential.
Prostaglandin Receptors
Previously, prostaglandin-binding sites primarily were characterized by the relative affinities for various prostaglandins,21 and this led to the pharmacologic description of different prostanoid receptor classes.22 The prostanoid receptors now have been cloned and sequenced, including thromboxane (TP), prostacyclin (IP), prostaglandin F (FP), and several subtypes of the prostaglandin E (EP) receptor (EP1, EP2, EP3, and EP4). These subtypes have been defined on the basis of different pharmacologic profiles and linkage to different signal transduction pathways.23 The prostanoid receptors show common features with the G protein-coupled receptor superfamily, with seven transmembrane spanning domains. Several isoforms of the EP3 receptor have been described, which arise through alternative splicing of mRNA and are coupled to different signalling pathways. A variety of receptors are present on myometrium.
PROSTAGLANDINS AND THE REPRODUCTIVE PROCESS
The aforementioned background allows us to discuss the role of prostaglandins in the reproductive cycle. Although it is clear that prostaglandins play an important role in a number of reproductive processes, our understanding of the precise mechanism of action remains very basic. Additionally, the interrelationships between prostaglandins and other vasoactive substances such as nitric oxide have added to the complexity of this area. In many cases, only experimental data from isolated human tissue and experimental animals are available. However, it is clear that endogenously occurring compounds are important in clinical medicine.
The Menstrual Cycle
Peripheral plasma levels of PGF2α and 13, 14dihydro-15-keto-PGF2α have been measured throughout the menstrual cycle.24,25,26,27 However, none of these studies demonstrated a cyclic change. Koullapis and Collins reported that although extremely variable, levels of 13, 14-dihydro-15-keto-PGF2α consistently showed two peaks: one preovulatory, which was associated with increasing estrogen, and one premenstrual, which was associated with rapid decreases in estrogen and progesterone levels.28 The failure to measure cyclic changes in the peripheral plasma in PGF2α most likely reflects the fact that PGF2α is converted quickly to 13, 14-dihydro-15-keto-PGF2α in the lungs and other organs. The failure to routinely measure cyclic changes in the PGF2α metabolite may reflect a simple dilutional problem. By the time uterine PGF or its metabolites reach the peripheral circulation, they have been diluted into a large blood volume.
Cyclic changes in PGE and PGF levels do occur in the uterus, as determined by endometrial and myometrial biopsies.29,30,31,32 In the endometrium, PGE and PGF increased sequentially throughout the menstrual cycle. The endometrium of the late secretory phase contained considerably elevated PGF levels and a reversal of the ratio of PGF to PGE. These increases in PGF levels in the endometrium appear to be caused by progesterone withdrawal and have been reported for the postovulatory period.30,32
In the myometrium, the concentration of PGF varied throughout the menstrual cycle, characterized by two peaks, one occurring during the late proliferative phase and the other in the late secretory phase. In contrast, PGE peaked in the late proliferative stage (higher levels than PGF) but then decreased in the later stages of the cycle.33 The 13, 14-dihydro-15-keto metabolites of PGE and PGE did not show any cyclic changes in the myometrium, suggesting no cyclic alteration in PGF and PGE metabolism. The elevated levels of prostaglandins in the periovulatory period may provide a mechanism to facilitate migration of ova and spermatozoa, but this has not been proven. The change in the PGE-to-PGF ratio in both endometrium and myometrium in the late secretory phase may be important in the onset of menstruation.
Menstruation and Dysmenorrhea
The postovulatory period is associated with progesterone withdrawal and increased PGF-to-PGE ratios in the endometrium.29,30,31 PGF2α has been shown to be a vasoconstrictor in the uterus and endometrial blood vessels, and the local endometrial vasoconstriction that occurs during menstruation is believed to be due in part to prostaglandins.34,35,36,37,38
In 1957, Pickles proposed that a substance produced by the menstruating uterus might be responsible for stimulation of uterine contractility and that this “menstrual stimulant” might be responsible for primary dysmenorrhea.39 Pickles observed that menstrual fluid of patients with primary dysmenorrhea had higher PGF2α concentration than that of nondysmenorrheic patients and that these patients had an increased PGF-to-PGE ratio.29 This study was later confirmed by Lundstrom and Green and substantiated by the observation that long-term infusions of PGF2α resulted in symptoms similar to dysmenorrhea.40,41
Csapo and associates measured the intrauterine pressures during menstruation in healthy and dysmenorrheic patients.42 In contrast to the normal resting pressure of 10 mmHg, the dysmenorrheic patient had levels of 60 mmHg. This increased tone could result in vascular compression and, thus, reduced uterine blood flow. This would further compromise a uterine circulation already reduced by the direct action of PGF2α on blood vessels in the uterus and endometrium. It is this reduced blood flow and resultant anoxia of uterine tissue that is believed to produce the pain of dysmenorrhea.29 In the study by Csapo and coworkers,42 treatment of dysmenorrheic patients with the prostaglandin-synthesis inhibitor, naproxen sodium, reduced the resting pressure as well as the amplitude and frequency of cyclic intrauterine pressure. Naproxen and ibuprofen both are associated with a reduction of prostaglandins in menstrual blood and decreased clinical symptoms of dysmenorrhea.43,44 Additional information is available in a recent review on nonsteroidal anti-inflammatory drugs and reproduction.45
Treatment of dysmenorrhea with prostaglandin-synthesis inhibitors is an excellent example of integration of basic research and clinical medicine. This therapeutic method of treatment appears to be effective in 70% to 80% of all clinical cases of dysmenorrhea.
Ovulation
Ovulation has three major phases in which prostaglandins may be involved: (1) follicular maturation; (2) rupture of the follicle, leading to oocyte release; and (3) formation of the corpus luteum. The actions of prostaglandins in hypothalamic and pituitary function, which can influence follicular development, are not discussed in this chapter. In rabbits, LeMaire and associates showed that isolated follicles could be stimulated with human chorionic gonadotropin (HCG) to increase their PGE and PGF2α levels.46 This increase only occurred in those follicles destined to be ovulated.47 Pretreatment of animals with prostaglandin synthesis inhibitors blocks ovulation,48,49 although attempts to do this in humans have not been successful. It is known that rat, bovine, and human follicles predominantly synthesize PGI2 and lesser amounts of PGE and PGF2α and that follicular fluid concentrations increase as ovulation approaches. Addition of luteinizing hormone (LH) increases granulosa cell conversion of arachidonic acid to PGI2 and perfusion of rat ovaries with PGI2 induces rupture of mature follicles, although less efficiently than HCG.50 The majority of ova ovulated by PGI2 were immature and would not undergo germinal vesicle breakdown and maturation, thereby drawing a distinction between oocyte maturation and follicular rupture.
Prostaglandins may be intermediates in the cyclic adenosine monophosphate (cAMP) signalling pathway because ovulation induced by forskolin or dibutyryl cAMP in PMSG-primed rats could be inhibited by indomethacin, an effect itself reversed by the addition of PGE2.51 There is a progressive increase in dispersion of cells of the mural granulosa and cumulus oophorus of preovulatory ovine follicles 12 hours after initiation of the LH surge that can be negated by indomethacin. The effect of indomethacin itself can be counteracted by PGF2α.52 This suggests that prostaglandins may be involved in reduction of junctional complexes that adhere granulosa cells to each other and the oocyte before ovulation. Yoshimura and colleagues50 found that perfusion of ovaries with PGI2 produced vasodilation, extravasation, and filling defects at the apical region of preovulatory follicles, findings similar to those with HCG treatment, and suggested that follicular rupture may be secondary to increased capillary permeability and vasodilation due to PGE2. Although HCG stimulated collagenase in PMSG-primed rats, which may break down the follicular wall, indomethacin had no effect on collagenase, although it reduced prostaglandin synthesis.53 More recent biochemical and molecular advances in this field are reviewed elsewhere.54
Luteinization does not appear to involve prostaglandins because pretreatment of animals with indomethacin at concentrations that block ovulation do not alter luteinization.48
Luteolysis
Progesterone, which initially is produced in the corpus luteum, is necessary to prepare the uterus for implantation of a fertilized ova and to monitor the pregnancy until the placenta can begin progesterone production.55 Interruption of progesterone production by the corpus luteum then leads to termination of pregnancy. In 1969, it was shown that PGF2α caused luteolysis in rats,56 and in guinea pigs, the uterus was shown to be the source of a luteolysin identified as PGF2α.57 In sheep and cows, PGF2α of uterine origin appears to be a physiologic luteolysin.58 The PGF2α is transferred to the uterus by a local counter current mechanism59 between uterine vein and ovarian artery or from uterine lymphatic vessels to the adjacent ovary.60 In nonhuman primates and women, PGF2α also appears to be a luteolysin.61 Only transient decreases in circulating progesterone are seen after systemic administration of PGF2α62,63 perhaps because of the rapid systemic metabolism of PGF2α. Because hysterectomy in primates or women does not alter ovarian cyclicity, it was postulated that the luteolysin was generated locally within the ovary or corpus luteum.64 However, recently, this concept has been questioned.65
The luteolytic action of PGF2α may be directly on luteal cells or may be indirect by directing blood flow away from the corpus luteum. PGF2α appears to interfere with gonadotropin effects on luteal cells, being a potent antagonist of LH or HCG action in rat luteal cells and blocking LH-stimulated cAMP production,66 although not interfering with gonadotropin binding.67 PGF2α inhibits basal and LH-influenced progesterone production by granulosa cells from preovulatory follicles that spontaneously luteinize in culture.68 Further, PGF2α has an antigonadotropic effect on LH-HCG-stimulated progesterone and cAMP formation from human luteal tissue in the mid to late luteal phase.69
Pharriss and Wyndarden70 first postulated that the luteolytic action of PGF2α may be due to its vasoconstrictor action, decreasing blood flow to the corpus-luteum-bearing ovary. Subsequent work has not proven this observation but does not preclude local effects of PGF2α causing shunting of blood away from the corpus luteum.71
Riley and Carlson72 demonstrated that one of the early events in PGF2α-induced luteolysis is membrane breakdown or decrease in fluidity. This was associated with a decrease in HCG binding and HCG receptor concentrations. Further, addition of phospholipase A2 gave a similar increased effect on membrane rigidity, suggesting that a product of phospholipase action (e.g., free fatty acid or lysophosphatidic acid) may be responsible for this ridigification during luteolysis. Interestingly, addition of prolactin, which is luteotropic in the rat, was able to attenuate PGF2α-induced increases in membrane rigidity and decreases in plasma progesterone.
The rate-limiting step for all steroidogenic tissues is believed to be the conversion of cholesterol to progesterone. Rajkumer and coworkers73 presented evidence in rats that PGF2 decreases the ability of luteal cells to mobilize endogenous cholesterol at relatively short time intervals (4 hours) after PGF2α. At longer time periods (24 hours), there appear to be decreased ability of these luteal cells to convert cholesterol to progesterone, which may contribute to the decreased progesterone synthesis. Cholesterol is imported into luteal cells as a lipoprotein complex by a membrane receptor-mediated process. However, luteal cells from PGF2α-treated cows74 and PGF2α-treated rats73 do not show decreased utilization of lipoproteins.
Thus, although we have a reasonable understanding of how prostaglandins are involved in luteolysis in some species, we still do not have a clear indication of how they work in humans.75
Fallopian Tube Motility
PGE and PGF have opposite effects on the oviduct; PGE2 causes relaxation, whereas PGF2 induces contractions.76,77 The final response of the oviduct to these prostanoids appears to depend on the steroid levels associated with the reproductive cycle. In the rhesus monkey, PGF2α has no effect on the oviduct during the follicular phase but produces contractions just before ovulation. PGE2 also has no effect during the follicular phase but suppresses activity during and after ovulation.78,79 It is known that the oviduct can synthesize PGE and PGF2α locally, but endogenous prostaglandins do not appear to be involved in regulation of spontaneous activity.80,81 The effects of PGI2 and 6-keto-PGF1α have been determined in the fallopian tubes of nonpregnant women. PGI2 is similar to PGE2 and relaxes the fallopian tube, whereas 6-keto-PGF1α causes contractions similar to those seen with PGF2α.82 Although the role of prostaglandins in regulation of fallopian tube motility is not completely understood, it is thought that prostaglandin-increased contractile activity is important for ovum transport to the uterus.
Implantation
Before the arrival of the blastocyst, the endometrium has undergone profound changes that are regulated by estrogen and progesterone. In most mammals, these endometrial changes occur after each ovulation. The information available about the mechanism of implantation relies heavily on data acquired from laboratory animals. When the blastocyst arrives at the uterine wall, changes occur in the underlying endometrium. The endometrial vessels dilate and exhibit increased vascular permeability. The trophoblast begin to invade the endometrium, and the blastocyst undergoes apposition, attachment, adhesion and fusion (known together as implantation).
It currently is unclear whether and how the blastocyst controls the implantation process. It is clear that changes occur only in the areas of blastocyst attachment. Prostaglandins have been implicated as the mediator of the vascular permeability changes that occur at the implantation site. Prostaglandin levels have been shown to increase significantly at the site of implantation.83,84,85 The rate-limiting enzyme for prostaglandins synthesis in some situations may be prostaglandin H synthase (cyclooxygenase). Two isoforms of cyclooxygenase exist, the constitutive COX-1 and the inducible COX-2 isoforms, which are regulated differently. Recent studies in the mouse have shown that the COX-1 gene is expressed in the uterine epithelium during the peri-implantation period but is down-regulated at the initiation of attachment.86 This corresponds to the generalized edema in the uterus. In contrast, the COX-2 gene is expressed in the luminal epithelium and subepithelial stromal cells surrounding the blastocyst at the time of implantation. Thus, the COX-1 gene is involved with decidualization or localized endometrial changes in vascular permeability, whereas the COX-2 gene is involved with angiogenesis for the establishment of the placenta.86 As these changes occur, expression of prostaglandin receptors EP3 and FP occur simultaneously in the circular muscle of the myometrium on days 3 to 5 of pregnancy, which probably is important for embryo transport, spacing, or accommodation in the uterus. In contrast, the expression of EP3 in a subpopulation of stromal cells on the mesometrium side and EP4 receptors in the epithelium and stroma suggest that PGE2 prepares the uterus for implantation.87 The source of the prostaglandins that are generated locally at the site of implantation still is not clear but is believed to be the endometrium because the blastocyst has not been shown to produce prostaglandins.83
Indomethacin pretreatment prevents the normal increases in prostaglandins as well as the changes in vascular permeability in mice,88 rats,83,89 hamsters,84 and rabbits.90 Indomethacin also is effective in blocking implantation in mice,91 rats,92 and rabbits.93 Further evidence for a role of prostaglandins is supported by the fact that intraluminal administration of PGE2 in rats pretreated with indomethacin results in implantation-like alterations in vascular permeability,91 but this is not true for PGF2α or PGI2.94,95 Although blockade of implantation would provide a potential method of contraception, it currently is not known whether similar implantation mechanisms occur in humans. A recent detailed review on prostaglandins and implantation appears elsewhere.96
Circulating Levels During Pregnancy
Prostaglandins are produced in large quantities in a number of reproductive tissues during pregnancy, and this production may be affected by the changing hormonal milieu of pregnancy. A more detailed discussion of the site of synthesis of these compounds can be found elsewhere.97,98,99 Studies in chronically catheterized animals and measurement of prostaglandins in uterine and placental tissue or umbilical cord blood suggest that peripheral levels of prostaglandins should be elevated significantly in pregnancy. Several attempts have been made to measure circulating levels of prostaglandins during pregnancy in humans.100,101 Because the primary prostaglandins PGE and PGF are converted quickly to 13, 14-dihydro-15-keto-metabolites (see previous section on metabolism and measurement), it was believed that these metabolites would more accurately reflect gestation changes. Measurements of 13, 14-dihydro-15-keto-PGE2 have not been reliable because this compound is unstable.18 Measurements of the circulating levels of 13, 14-dihydro-15-keto-PGF2α also have failed to show any gestationally related change except during parturition, when they are increased significantly.101,102,103
The discovery of the potent vasodilator PGI2 and the development of RIAs to measure its metabolite, 6-keto-PGF1α, renewed interest in measuring circulating levels of prostaglandins in pregnancy. PGI2 is produced in vascular tissue and appears to be important in local vasodilation. During pregnancy, there is a profound decrease in peripheral vascular resistance, and the uterine vasculature is almost totally dilated. It was expected that PGI2 levels (measured as 6-keto-PGF1α) would be increased significantly, reaching a maximum in the third trimester. However, measurements of circulating levels of 6-keto-PGF1α have not shown any gestationally related alterations,99,104,105 probably because of the low circulating concentrations and interference problems in direct plasma assays. Measurement of the 2,3 dinor 6-keto-PGF1α metabolites in urine have shown an 8- to 10-fold increase in PGI2 synthesis during pregnancy,106 although whether this increase is generalized throughout the whole vascular tree or confined to certain vascular beds (e.g., uterus and kidney) remains to be established.
It was further speculated that low production rates of PGI2 in disease states associated with reduced uterine blood flow (i.e., hypertension, preeclampsia) might be the cause of reduced uteroplacental perfusion. Again, measurements of peripheral circulating levels of 6-keto-PGF1α were made in patients with a number of diseases associated with pregnancy, and no differences have been observed.104 In contrast, measurements have shown the ability of umbilical and placental blood vessels from preeclamptic, chronic hypertensive, and intrauterine growth retardation (IUGR) pregnancies to produce PGI2 to be reduced considerably.107,108 Again, urinary 2,3 dinor 6-keto-PGF1α measurement confirmed this reduction in PGI2 synthesis in hypertensive or IUGR pregnancies. Thromboxane synthesis also increases during pregnancy (as measured by urinary metabolites); however, the ratio of vasodilator PGI2 to vasoconstrictor TxA2 favors PGI2, giving an antithrombotic tendency in normal pregnancy. Whereas, PGI2 synthesis is reduced in hypertensive pregnancy, urinary TxB2 metabolites appear unchanged.106 The net result, however, is an alteration in the PGI2/TxA2 ratio toward TxA2 and a more vasoconstrictive and prothrombotic state. Recently, most of the interest in the regulation of uterine and systemic vascular resistance in normal and hypertensive pregnancies has moved away from prostaglandins (with the exception of prostacyclin) and focused on nitric oxide. Preeclampsia is believed to be caused by endothelial cell dysfunction, and this may include reductions in both endothelial cell production of prostacyclin and nitric oxide synthesis (see discussion elswhere in these volumes).
Uterine Blood Flow
Prostaglandins can affect uterine blood flow in three different ways. First, prostaglandins can have a direct effect on the vascular smooth muscle, acting through receptors to produce either uterine vasodilation or vasoconstriction. Second, prostaglandins can increase uterine tone or contractile activity, resulting in a significant reduction in uterine blood flow by mechanical compression of the uterine vasculature. The third potential mechanism is by potentiating or by depressing adrenergic neurotransmission. This is accomplished by altering the local neurogenic release of norepinephrine from the presynaptic nerve terminal or by modifying the responsiveness of the postsynaptic nerve terminal to norepinephrine.
The uterine vasculature effects of prostaglandins have been investigated mainly in experimental animals. Clark and coworkers have shown that PGE1 and PGE2 produce vasodilation in the uterine vasculature of the anesthetized nonpregnant dog, whereas PGF2α does not significantly alter uterine blood flow.109,110 Subsequent studies in unanesthetized chronically catheterized nonpregnant sheep have shown similar responses for PGE1 and PGE2, whereas PGF2α produced dose-related decreases in uterine blood flow.111,112 Similar studies have shown that PGI2 and PGD2 are potent uterine vasodilators in nonpregnant sheep.37,113 In contrast, the PGI2 breakdown product (6-keto-PGF1α) and TxB2 were very weak vasoconstrictors.37
The ability of prostaglandins to reduce uterine blood flow in nonpregnant sheep by increasing uterine tone and contractile activity (mechanical compression) has been documented for PGE2 and PGF2α.37,111,112 In addition, PGE and PGF also have been shown to alter both presynaptic and postsynaptic sympathetic nerve transmission, thus affecting the vascular response to uterine sympathetic nerve stimulation and circulating norepinephrine.109,111,114 The local release of prostaglandins does not appear to be under neurogenic control because levels of PGE and PGF in uterine venous effluent were not altered by sympathetic nerve stimulation.114
Prostaglandins act by means of receptors, and, at least in the canine uterus, PGF2α is able to competitively antagonize vasodilation produced by other prostaglandins.115 This is important because the final vascular effects of endogenous prostaglandin production are dependent on the summation of the individual prostanoids. Finally, the nonpregnant uterus is composed of two separate tissue layers, the myometrium and the endometrium. Studies that evaluate uterine vascular effects of prostaglandins generally combine changes in both tissue layers. Prostaglandins have been shown to act differently in the myometrium and the endometrium.27 However, specific tissue changes would not be detected if only total uterine blood flow were measured. An example of this has been reported in the dog uterus, in which PGE1 preferentially dilates the myometrium, PGF2α preferentially constricts the endometrium, and PGE2 acts equally on both tissue layers.36 Whether this occurs in other species or during pregnancy has not been investigated carefully.
During the ovarian cycle, estrogen levels increase just before ovulation. This elevation in estrogen is associated with a significant increase in uterine blood flow.116,117 Prostaglandins have been implicated in this response because of their uterine vasoactivity.118,119 Ryan and coworkers have shown that prostaglandin-synthesis inhibitors, meclofenamate and indomethacin, decrease the uterine responses to estrogen in rats.120 In contrast, studies in sheep have shown that although estrogen increases PGI2 levels, inhibition of prostaglandin synthesis does not alter the uterine vascular response to estrogen.118 These studies strongly suggest that estrogen-induced decreases in uterine blood flow are not mediated by prostaglandins. More recently, Van Buren and associates121 showed that estrogen-induced increases in uterine blood flow are mediated by nitric oxide and are prevented by inhibitors of nitric oxide synthetase.
Pregnancy creates a unique cardiovascular challenge for the maternal organism. Blood volume and cardiac output increase by as much as 40%, yet arterial blood pressure is not altered significantly. The reason for this is the development of the uteroplacental vasculature and the fact that the maternal vasculature dilates. The resistance in the uteroplacental vasculature is very low. This is due to the development of a new uteroplacental vascular bed and vasodilating hormonal factors that appear to play a major role in regulating uteroplacental blood flow during pregnancy. Prostaglandins are known to be produced in the uterus and placenta in large quantities and may be important in regulating both uterine and umbilical blood flow.97,99,122,123
Early studies by Terragno and coworkers124 and Venuto and coworkers125 demonstrated that the pregnant uterus of dogs and rabbits produced large quantities of PGEs and that after blockade of prostaglandin synthesis, uterine vascular resistance significantly increased. In contrast, in animals that recovered from surgery for a period of 5 to 7 days, uterine blood flow initially decreased but then returned to control levels within 15 minutes.126,127 In these studies, uterine prostaglandin synthesis was inhibited significantly by 15 minutes and remained low for the next 4 hours, even though blood flow returned to control levels.126 These studies suggest that endogenous vasodilator prostaglandins may be important in maintaining uterine blood flow during stressful situations but may be less important in regulation of blood flow during normal pregnancy. When interpreting these results, remember that prostaglandin-synthesis inhibitors block the formation of all prostaglandins (i.e., vasodilators and vasoconstrictors) and that a complete understanding of the role of endogenous prostaglandins in regulating uteroplacental blood flow requires specific inhibition. Also, recent studies discussed elsewhere strongly suggest that nitric oxide may play a more critical role in regulating the uterine and systemic vascular resistance.
The uterine vascular effects of prostaglandins in pregnancy have been evaluated mainly in the unanesthetized chronically catheterized pregnant sheep. PGE2 (a vasodilator in the nonpregnant animal) produced dose-related reductions in uterine blood flow in pregnant sheep but after administration to the fetus caused uterine vasodilation.38,128,129 Whether this uterine effect (vasodilation) seen after fetal administration is the result of PGE2 or PGE2 metabolite is not known. PGE2 did produce a significant increase in myometrial tone after maternal administration, and thus, decreased blood flow partially may reflect myometrial compression of the vasculature.38,128,129 PGF2α produced dose-related reductions in uterine blood flow and, at the highest doses tested, also increased uterine contractile activity and tone.38,129 The initial PGF2α-induced changes in uterine blood flow appear mainly to be the result of vasoconstriction.38 In contrast, PGI2 produced dose-related increases in uterine blood flow in late term pregnant sheep, whereas its breakdown product, 6-keto PGF1α, was a weak vasoconstrictor.38 The PGI2-induced increases in uterine blood flow in this species, which were observed during intra-arterial infusions of PGI2, were largely the result of increases in endometrial blood flow rather than increases in placental blood flow.130 Studies evaluating PGD2 also have shown it to be a potent vasodilator of the uterine vasculature in late-term pregnant sheep, but whether it is able to dilate the placental vasculature is unknown. In pregnant rabbits, PGD2 had a biphasic response, producing vasodilation in the myometrium and vasoconstriction in the placenta.38,131 Similar studies have not been conducted in the sheep or other species. To date, very little data exist that suggest that prostaglandins can dilate the maternal placental vasculature. This most likely is because the maternal placental vasculature is dilated maximally. If prostaglandins have a role in regulation of uteroplacental blood flow, PGI2 would be the most likely candidate, but it would share this role with nitric oxide.
Umbilical Blood Flow
Until recently, the effect of prostaglandins on the umbilical vasculature in the intact animal has been limited to PGE2 and PGF2α, both of which were shown to be vasoconstrictors. PGE2 was by far the more potent vasoconstrictor.129,132 It is widely accepted in the clinical literature that prostacyclin is a potent vasodilator of the uterine, umbilical, and placental vasculature and that reduction of PGI2 production is associated with preeclampsia. The decrease in PGI2 is thought to be associated with reduced uterine and umbilical blood flow. Recently, several studies have evaluated the umbilical vascular response to PGI2.133,134 In both of these studies, PGI2 produced dose-related decreases in fetal arterial pressure, increases in fetal heart rate, and decreases in umbilical blood flow that were associated with increases in umbilical vascular resistance. These data are interpreted to suggest that systemic prostacyclin administration leads to peripheral vasodilation and shunting of blood away from the placenta, thus leading to decreases in umbilical blood flow. Additionally, it is believed that because the placental vasculature is dilated maximally and has no vascular tone, prostacyclin could not produce any further relaxation. This hypothesis however was tested in two separate studies134,135 by increasing umbilical vascular tone by simultaneous infusion of the thromboxane A2-like compound U46619. Even under the condition of increased tone, prostacyclin (PGI2) could not dilate the umbilical circulation. Thus, to date, there is no evidence in favor of an active prostaglandin dilator system in the umbilical circulation, although several members of the prostaglandins are effective vasoconstrictors in the umbilical circulation. Thus, the proof that prostaglandins modulate umbilical blood flow does not currently exist, and further investigation is required.
Prostaglandins and Preeclampsia
Although the aforementioned studies suggest that PGI2 is not a dilator in the umbilical circulation, no studies have ruled out the possibility that PGI2 produced in the vessel walls is not important in regulating vascular tone. It is clear that umbilical blood vessels from patients with preeclampsia, hypertension, and IUGR all show significantly depressed PGI2 synthetic capabilities.107 These disease states also are associated with reduced uterine or umbilical blood flow. Because of this, many investigators have made an association between the two phenomena. The hypothesis, which is not proven, is that decreased PGI2 production in the uterine and umbilical vasculature results in reduced blood flow in these vascular beds. Preeclampsia is thought to be caused by endothelial dysfunction, which results in altered production of several vasoactive substances such as PGI2 and nitric oxide. A second reasonable interpretation that could be made, based on the current state of our knowledge, is that reduced uterine and umbilical synthesis of PGI2 and nitric oxide leads to increased platelet aggregation, which in turn leads to increased release of thromboxane A2 and serotonin. Thus, the increasing levels of thromboxane A2 or serotonin could be responsible for the significant decreases in uterine and umbilical blood flow. Thromboxane A2 (evaluated as the thromboxane mimetic U46619) is a potent vasoconstrictor of the uterine (unpublished data) and umbilical vasculature.134,135 Serotonin is also a potent vasoconstrictor in both the uterine and umbilical vasculature.136,137
Based on the accepted belief that preeclampsia and reduced uteroplacental blood flow are caused by inadequate production of vasoactive substances such as prostacyclin, Fidler and colleagues138 attempted to treat preeclamptic patients with intravenous PGI2. In this study, the patient's diastolic blood pressure was decreased from 140 to 80 mmHg. PGI2 was used only when other therapeutic agents failed to control the blood pressure. It should be stressed that the safety of infusing this endogenous prostanoid into pregnant women currently is not known. Uteroplacental blood flow is extremely dependent on perfusion pressure, and PGI2 infusions that decrease mean arterial pressure significantly may lead to significant reductions in uteroplacental blood flow, which could lead to fetal hypoxia.139
Pregnancy also is associated with a reduced vascular responsiveness to angiotensin II, both in humans and in sheep.140,141 Before the development of pregnancy-induced hypertension (PIH), angiotensin II refractoriness is lost.140 This increase in vascular responsiveness probably is not specific for angiotensin II but rather reflects increased vascular reactivity to all vasoconstrictors.142 It originally was believed that angiotensin II increased uterine blood flow and released PGEs from the uterus, which modulated uterine and systemic pressor response to angiotensin II in pregnancy.124,125,143 This possibility has been addressed in two studies, one that showed release of prostaglandins (PGE2 and PGI2 measured as 6-keto PGF1a) from the uteroplacental vasculature at high doses of angiotensin II144 and one that did not show any change.145 Neither study showed sufficient uterine release of prostaglandins to alter systemic responses to angiotensin II or systemic vascular resistance. However, angiotensin II did produce uterine vasoconstriction.141,144,145 In the study that found no uterine release of prostaglandins, bradykinin released large quantities of both PGE and 6-keto-PGF1α, serving as a positive control.145 Because prostaglandins are metabolized quickly or undergo nonenzymatic breakdown, the likelihood of a circulating vasodilator prostaglandin released from the pregnant uterus being responsible for reduced systemic vascular resistance or responsiveness does not seem likely. If prostaglandins are responsible for regulating vascular responsiveness of blood vessels, this would have to occur by local vessel synthesis.
Parturition
The role of prostaglandins in parturition has been investigated extensively both in humans and in sheep. The similarities and differences in endocrine changes that occur at the time of parturition in these two species have been reviewed.146,147,148 Although peripheral levels of most prostaglandins do not change throughout gestation, the levels of 13, 14-dihydro-15-keto-PGF2α, the PGF2α metabolite, are elevated during the last days of pregnancy and during labor in both species.99,101,102,103 In contrast, the levels of PGE2, PGF2α, and 6-keto-PGF1α all are elevated in amniotic fluid during labor,102,149 and a recent report suggests that increased amniotic fluid prostaglandins precede the onset of labor.150 Increasing amounts of data strongly suggest that prostaglandins play a major role in the mechanism of parturition in both species. The observation that PGE2 and PGF2α, as well as their synthetic analogues (15-methyl and 16, 16-dimethyl), are effective in induction of abortions and labor and the fact that prostaglandin-synthesis inhibitors can block premature labor and delay parturition add further support to this concept.
All the intrauterine tissues produce prostaglandins; however, the tissues that produce these specific oxytocic prostaglandins that cause uterine contractility at parturition and the signals that initiate this increased production are not firmly identified. In sheep, the placenta appears to be the tissue that produces prostaglandins at parturition as a result of a signal from the fetal hypothalamic-pituitary-adrenal-placental axis.147 No similar signal route occurs in human pregnancy at term, so current attention is focused on paracrine mechanisms that operate in the fetal membranes to initiate prostaglandin synthesis and stimulate labor.151 The amnion has been recognized as a major site of PGE2 synthesis at the time of labor in humans.152,153 The amnion is an extremely rich source of arachidonic acid, the precursor of prostaglandins esterified in membrane phosphatidylethanolamine or phosphatidylinositol. Whereas before labor, amnion synthesizes mainly lipoxygenase products of arachidonic acid,154 during labor, PGE2 synthesis predominates with release of substrate arachidonic acid from membrane phospholipids under the control of phospholipase A2 being seen.155 To reach the myometrium and thus cause contraction, PGE2 first has to cross the chorion, a rich tissue source of prostaglandin dehydrogenase. However, in vitro transfer experiments have shown that PGE2 can traverse amnion-chorion-decidua path intact, apparently by means of an extracellular route,156 although the relative amount transferred is hotly debated.157,158,159 Therefore, PGE2 produced by the amnion could mediate uterine contractility. However, measurements of peripheral plasma prostaglandin metabolites in labor showed that PGF2α metabolites are increased,160 with little or no data available for increase in the corresponding PGE2 metabolites. This PGF2α does not appear to arise by reduction of PGE2 by fetal tissues; rather, it may represent true PGF2α synthesis. Wilson and associates161 recently noted that the decidua produces increased concentrations of PGF2α during labor and that this PGF2α is oxytocic.
In the ovine model, changes in placental estrogen/progesterone synthesis in favor of estrogen, under the influence of increased fetal adrenal cortisol, appear to regulate prostaglandin synthesis. Such changes in peripheral plasma estrogen or progesterone have not been demonstrated in humans, giving rise to the concept of local changes in estrogen/progesterone synthesis in the fetal membranes, which then locally regulate prostaglandin synthesis.162 In addition, a variety of peptide hormones (cytokines,163 epidermal growth factor,164 transforming growth factor alpha,165 relaxin166) have been shown to stimulate fetal membrane prostaglandin synthesis in vitro and have been suggested variously as signals for parturition. To increase prostaglandin production, arachidonic acid must be liberated from membrane phospholipids by phospholipases and the activity of PGH synthase must increase (perhaps under the influence of peptide hormones) to convert that arachidonic acid to prostaglandin. Activity of phospholipase may be regulated directly or indirectly. Gravidin, a 58,000 molecular weight protein produced by the chorion and found in amniotic fluid, recently has been described as an inhibitor of phospholipase activity.167 The activity of gravidin was reported to decrease during labor, thereby paradoxically promoting an increase in phospholipase activity. Gravidin may be a member of the annexin family of calcium-phospholipid-binding proteins that inhibit phospholipase activity indirectly by binding to the substrate phospholipid. Activity of annexin I in human fetal membranes has been found to decrease at the time of parturition168 consistent with an increase in phospholipase activity. Two isoforms of the PGH synthase (cyclooxygenase) enzyme have been described, the constitutively expressed PGHS-1 (COX-1) isoform and the PGHS-2 (COX-2) isoform, which is inducible by a variety of cytokines and growth factors. The cytokine interleukin-1 (IL-1) and growth factors such as epidermal growth factor (EGF), transforming growth factor (TGF), and tumor necrosis factor (TNF) all can rapidly induce PGHS-2 and stimulate prostaglandin synthesis in human amnion cells.169 Whereas the specific activity of PGHS enzyme in the human amnion increases sharply before the onset of labor,170 the abundance of PGHS-1 mRNA in the amnion, the choriodecidua, and the placenta does not change with labor and delivery.171 However, expression of PGHS-2 is increased significantly in both the amnion and the chorion at the time of labor,172 suggesting that upregulation of PGHS-2 mediates the increased prostaglandin synthesis. The use of indomethacin—a nonselective PGHS inhibitor—as a tocolytic is limited because it causes oliguria, suggesting that PGHS-2-specific inhibitors may be more useful tocolytics.
Arachidonic acid for prostaglandin synthesis by PGHS enzyme is liberated from cell membrane phospholipids by phospholipase enzymes. Recently, several isoforms of the phospholipase A2 enzyme have been described, including the 85-kDa cytosolic phospholipase A2 (cPLA2), which has a preference for substrate phospholipid-containing arachidonic acid in the SN-2 position.173 Cytokines and growth factors also rapidly induce cPLA2 expression and activity in human fetal membranes, and IL-1β coordinately induces both cPLA2 and PGHS-2 in amnion-derived epithelial cells for sustained PGE2 synthesis.169 Activity of cPLA2 in the human amnion increases throughout gestation and is highest before the onset of labor,174 suggesting that it plays a role in prostaglandin production at labor.
Since the first studies of Karim and coworkers and Bygdeman and coworkers in 1968, it has been clear that PGE2 and PGF2α can induce labor both in term pregnancies and in the first and second trimesters.175,176 The problem with these agents was not their effectiveness but rather the persistent side-effects of vomiting and diarrhea. Attempts were made to produce analogues of these primary prostaglandins that would reduce side-effects. In the original studies, large quantities of PGE2 or PGF2α had to be used because of the efficient metabolism of primary prostaglandins after intravenous infusion. Thus, one of the major objectives in producing synthetic analogues was to generate compounds that were not metabolized quickly, allowing a lowering of the therapeutic dose. These synthetic attempts gave rise to the 15-methyl and 16, 16-dimethyl analogues, which have greatly improved effectiveness but still can produce some nonuterine side-effects.
Hundreds of articles have appeared that discuss the benefits and disadvantages of different routes of administration (oral, intravenous, vaginal, intra-amniotic), dosages to be used, and types of prostaglandins to be used (PGE2, PGF2α, or an analogue) for the induction both of labor and abortions. This area of research is still quite active because pharmaceutical corporations continue to seek the most effective agent with the fewest side-effects. Several reviews of the use of prostaglandins for induction of labor and abortion have appeared; the reader is referred to them for additional information.177,178 In addition to normal labor, prostaglandins also have proved to be very effective in inducing labor in cases of intrauterine fetal death, anencephaly, and hydatidiform mole.179,180,181 Reports also exist in which 15-methyl-PGF2α has been used effectively to treat postpartum or postabortion bleeding due to uterine atony.182,183
The final clinical use of prostaglandins to be discussed is that of cervical ripening. In early studies in which prostaglandins were used to induce abortion and labor, it was noted that the cervix seemed to dilate, soften, and efface even before strong uterine contractions. Tissue samples taken from the human cervix in the first trimester have been shown to produce PGE, PGF, the PGF2α metabolite 13, 14-dihydro-15-keto-PGF2α, and 6-keto-PGF1α. This production is enhanced at term.99 Using an animal model, Stys and coworkers have shown that intracervical administration of PGE2 results in significant changes in compliance of the cervix of the pregnant sheep at a time when no uterine contractile activity is observed.184 This study clearly demonstrates that PGE2 can effectively change cervical compliance. In recent years, PGE2-impregnated gels have been used effectively to ripen the cervix before induction of labor in all three trimesters. These gels are applied directly to the cervix by means of a transcervical catheter (12 hours before induction) and produce ripening of the cervix in a large percentage of primigravidas and multigravidas. Once the cervix is ripe, the outcome of labor is improved significantly. A further discussion of this clinical use can be found elsewhere.185,186
REFERENCES
Kurzrok R, Leib CC: The action of semen on the human uterus. Proc Soc Exp Biol Med 28: 268, 1930 |
|
Von Euler US: A depressor substance in the vesicular gland. J Physiol (Lond) 84: 21, 1935 |
|
Bergstrom S, Sjovall J: The isolation of prostaglandin. Acta Chem Scand 11: 1086, 1957 |
|
Bergstrom S, Ryhage R, Samuelsson B, Sjovall J: Prostaglandin and related factors: 15. The structures of prostaglandin E1, F1α and F2α. J Biol Chem 238: 3555, 1963 |
|
Vane JR: Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature 231: 234, 1971 |
|
Pace-Asciak C, Wolfe LS: A novel prostaglandin derivative formed from arachidonic acid by rat stomach homogenates. Biochemistry 10: 3657, 1971 |
|
Gryglewski R, Bunting S, Moncada S et al: Arterial walls are protected against deposition of platelet thrombi by a substance (prostaglandin X) which they make from prostaglandin endoperoxides. Prostaglandins 12: 685, 1976 |
|
Johnson RA, Morton DR, Kinner JH et al: The chemical structure of prostaglandin X (prostacyclin). Prostaglandins 12: 915, 1976 |
|
Hamberg M, Svensson J, Samuelsson B: Thromboxanes: A new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci USA 72: 2994, 1975 |
|
Moncada S, Vane JR: The discovery of prostacyclin: A fresh insight into arachidonic acid metabolism. In Vane JR, Bergstrom S (eds): Prostacyclin, p 155. New York, Academic Press, 1977 |
|
Wong PYK, Malik K, Desiderio D et al: Hepatic metabolism of prostacyclin (PGI2) in the rabbit: Formation of a potent novel inhibitor of platelet aggregation. Biochem Biophys Res Com 93: 486, 1980 |
|
Roberts LJ, Morrow JD: The generation and action of isoprostanes. Biochemica et Biophysica Acta 1345: 121, 1997 |
|
Reilly M, Delanty N, Lawson JA, Fitzgerald GA: Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation 94: 19, 1996 |
|
Barden A, Berlin LJ, Ritchie J et al: Plasma and urinary 8-iso-prostane as an indicator of lipid peroxidation in preeclampsia and normal pregnancy. Clin Sci 91: 711, 1996 |
|
Granstrom E: Radioimmunoassay of prostaglandins. Prostaglandins 15: 3, 1978 |
|
Gordon D, Myatt L, Gordon-Wright A et al: Radioimmunoassay of 15 keto-prostaglandin E2 in peripheral plasma after oral administration of prostaglandin E2 tablets used for induction of labour. Prostaglandins 13: 399, 1977 |
|
Morris HG, Sherman NA, Shepperdson FT: Variables associated with radioimmunoassay of prostaglandins in plasma. Prostaglandins 21: 771, 1981 |
|
Granstrom E, Hamberg M, Hansson G, Kindahl H: Chemical instability of 15-keto-13,14-dihydro-PGE2: The reason for low assay reliability. Prostaglandins 19: 933, 1980 |
|
Kumlin M, Granstrom E: Radioimmunoassay for 11-dehydro TxB2: A method for monitoring thromboxane production in vivo. Prostaglandins 32: 741, 1986 |
|
Ciabattoni G, Pugliese F, Davi G et al: Fractional conversion of thromboxane B2 to urinary 11-dehydrothromboxane B2 in man. Biochemica Biophysica Acta 992: 66, 1989 |
|
Rao CV: Eicosanoid biding sites in ovarian and uterine tissue. In Mitchell MD (ed): Eicosanoids in Reproduction, pp 39–45. Boca Rotan, CRC Press, 1990 |
|
Coleman RA, Kennedy I, Humphrey PPA et al: Prostanoids and their receptors. In Hansch C, Sammes PG, Taylor JB et al (eds): Comprehensive Medicinal Chemistry, Vol 3, pp 643–717. Oxford, Pergaman Press, 1990 |
|
Myatt L, Moore SD. Myometrium and preterm labor: Steroids and prostaglandins. In Mitchell MD (ed): Seminars in Reproductive Endocrinology, Preterm Labor, pp 298–313. New York, Thieme Medical Publishers, 1994 |
|
Harrison, RF, Walker E, Youssefnejadian E, Craft IL: Comparison of serial PGF2α concentrations in serum of normal women and in those receiving oral contraceptives. Prostaglandins 10: 729, 1975 |
|
Van Orden DE, Swanson JA, Clancey CJ, Farley DB: Plasma prostaglandins in the normal menstrual cycle. Obstet Gynecol 50: 639, 1977 |
|
Kindahl H, Granstrom E, Edquist LE, Eneroth P: Prostaglandin levels in peripheral plasma during the reproductive cycle. Adv Prostaglandin Thromboxane Leukotrienes Res 2: 667, 1976 |
|
Ghodgaonkar RB, Dubin NH, Blake DA, King TM: 13, 14 Dihydro-15-keto prostaglandin F2α concentrations in human plasma and amniotic fluid. Am J Obstet Gynecol 134: 265, 1979 |
|
Koullapis EN, Collins WP: The concentration of 13, 14-dihydro-15-oxo-prostaglandin F2α in peripheral venous plasma throughout the normal ovarian and menstrual cycle. Acta Endocrinol 93: 123, 1980 |
|
Pickles VR, Hall WJ, Best FA et al: Prostaglandins in endometrium and menstrual fluid from normal and dysmenorrheic subjects. Br J Obstet Gynaecol 72: 185, 1965 |
|
Downie J, Poyser NL, Wunderlich M: Levels of prostaglandins in human endometrium during the normal menstrual cycle. J Physiol (Lond) 236: 465, 1974 |
|
Singh EJ, Baccarini I, Zuspan F: Levels of prostaglandins F2α and E2 in human endometrium during the menstrual cycle. Am J Obstet Gynecol 121: 1003, 1975 |
|
Maathuis JB, Kelly RW: Concentrations of prostaglandins F2α and E2 in the endometrium throughout the human menstrual cycle, after administration of clomiphene or an oestrogen-progesterone pill and in early pregnancy. J Endocrinol Metab 77: 361, 1978 |
|
Vijayakumar R, Walters WAW: Myometrial prostaglandins during the human menstrual cycle. Am J Obstet Gynecol 141: 313, 1981 |
|
Csapo AI: The prospects of PGs in post-conceptional therapy. Prostaglandins 3: 245, 1973 |
|
Pulkkinen MO, Pitkanen Y, Ogala A, Hannelin H: Decrease of utero-placental blood flow during prostaglandin F2α induced abortion. Prostaglandins 9: 61, 1975 |
|
Clark KE, Brody MJ: Prostaglandins and uterine blood flow. In Greenburg S, Kadowitz PJ, Burks T (eds): Prostaglandins: Organ and Tissue Specific Actions, p 107. New York, Marcel Dekker, 1982 |
|
Clark KE, Austin JE, Stys SJ: Effect of bisenoic prostaglandins on the uterine vasculature of the nonpregnant sheep. Prostaglandins 22: 333, 1981 |
|
Clark KE, Austin JE, Seeds AE: Effect of bisenoic prostaglandins and arachidonic acid on the uterine vasculature of pregnant sheep. Am J Obstet Gynecol 142: 261, 1982 |
|
Pickles VR: A plain-muscle stimulant in the menstruum. Nature 180: 1198, 1957 |
|
Lundstrom V, Green K: Endogenous levels of prostaglandin F2α and its main metabolites in plasma and endometrium of normal and dysmenorrheic women. Am J Obstet Gynecol 130: 640, 1978 |
|
Roth-Brandel U, Bygdeman M, Wiquist N: The effect of intravenous administration of prostaglandin E1 and F2α on the contractility of the nonpregnant human uterus in vivo. Acta Obstet Gynecol Scand 49: 19, 1970 |
|
Csapo AI, Pulkkinen MO, Henzl MR: The effect of naproxen-sodium on the intrauterine pressure and menstrual pain of dysmenorrheic patients. Prostaglandins 13: 193, 1977 |
|
Pulkkinen MO, Henzl MR, Csapo AI: The effect of naproxen-sodium on the prostaglandin concentration of menstrual blood and uterine “jet washing” in dysmenorrheic women. Prostaglandins 15: 543, 1978 |
|
Chan WY, Dawood MY: Prostaglandin levels in menstrual fluid of nondysmenorrheic and dysmenorrheic subjects with and without oral contraceptive on buprofen therapy. Adv Prostaglandin Thromboxane Leukotrienes Res 8: 1443, 1980 |
|
Dawood MY. Nonsteroidal antiinflammatory drugs and reproduction. Am J Obstet Gynecol 169:1255, 1993 |
|
LeMaire WJ, Yang NST, Behrman HR, Marsh JM: Preovulatory changes in the concentration of prostaglandins in rabbit graafian follicles. Prostaglandins 3: 367, 1973 |
|
Yang NST, Marsh JM, LeMaire WJ: Postovulatory changes in the concentrations of prostaglandins in rabbit graafian follicles. Prostaglandins 6: 37, 1974 |
|
Armstrong DT, Grinwich DL: Blockade of spontaneous and LH induced ovulation in rats by indomethacin, an inhibitor of prostaglandin biosynthesis. Prostaglandins 1: 21, 1972 |
|
Zanagnolo V, Dharmarajan AM, Endo K, Wallach EE. Effects of acetylsalicylic acid (aspirin) and naproxen sodium (naproxen) on ovulation, prostaglandin, and progesterone production in the rabbit. Fertil Steril 65:1036, 1996 |
|
Yoshimura Y, Dharmarajan AM, Gips S et al: Effects of prostacyclin on ovulation and microvasculature of the in vitro perfused rabbit ovary. Am J Obstet Gynecol 159: 977, 1988 |
|
Brannstrom M, Koos RD, LeMaire WJ, Janson PO: Cyclic adenosine 3',5'-monophosphate-induced ovulation in the perfused rat ovary and its mediation by prostaglandins. Biol Reprod 37: 1047, 1987 |
|
Murdoch WJ: Disruption of cellular associations with the granulosal compartment of periovulatory ovine follicles: relationship to maturation of the oocyte and regulation by prostaglandins. Cell Tissue Res 252: 459, 1988 |
|
Curry TE Jr, Clark MR, Dean DD et al: The preovulatory increase in ovarian collagenase activity in the rat is independent of prostaglandin production. Endocrinology 118: 1823, 1986 |
|
Olofsson JI, Leung PC. Prostaglandins and their receptors: Implications for ovarian physiology. Biol Signals T 5: 90, 1996 |
|
Csapo AI, Pulkkinen M: Indispensability of the human corpus luteum in the maintenance of early pregnancy luteectomy evidence. Obstet Gynecol Surv 33: 69, 1978 |
|
Pharriss BB, Wyngarden LJ: The effect of prostaglandin F2α on the progestogen content of ovaries from pseudopregnant rats. Pro Soc Exp Biol Med 130: 92, 1969 |
|
Blatchley FR, Donovan BT: Luteolytic effects of prostaglandins in guinea-pigs. Nature 221: 1065, 1969 |
|
Horton EW, Poyser NL: Uterine luteolytic hormone: A physiological role for prostaglandin F2α. Physiol Rev 56: 595, 1976 |
|
Hixon JE, Hansel W: Evidence for preferential transfer of prostaglandin F2α to the ovarian artery following intrauterine administration in cattle. Biol Reprod 11: 543, 1974 |
|
Heap RB, Fleet IR, Hamon M: Prostaglandin F2α is transferred from the uterus to the ovary in the sheep by lymphatic and blood vascular pathways. J Reprod Fertil 74: 645, 1985 |
|
Auletta PJ, Flint APF: Mechanisms controlling corpus luteum function in sheep, cows, non-human primates and women especially in relation to the time of luteolysis. Endocrine Reviews 9: 88, 1988 |
|
Jones GS, Wentz AC: The effect of PGF2α infusion on corpus luteum function. Am J Obstet Gynecol 114: 393, 1972 |
|
Bolognese RJ, Corson SL: The effect of vaginally administered PGF2α on corpus luteum function. Am J Obstet Gynecol 117: 240, 1973 |
|
Auletta FJ, Kamps DL, Pories S et al: An intra-corpus luteum site for the luteolytic action of PGF2α in the rhesus monkey. Prostaglandins 27: 285, 1984 |
|
Fisch B, Rose MP, Elder MG et al: Effects of oestrogen on progesterone synthesis and arachidonic acid metabolism in human luteal cells. Clin Endocrinol 40: 21, 1994 |
|
Lahav M, Freud A, Lindner HR: Abrogation of prostaglandin F2α of LH-stimulated cyclic AMP accumulation in isolated rat corpora lutea of pregnancy. Biochem Biophys Res Commun 68: 1294, 1976 |
|
Thomas JP, Dorflinger LJ, Behrman HR: Mechanism of the rapid antigonadotropic action of prostaglandins in the cultured luteal cells. Proc Natl Acad Sci USA 75: 1344, 1978 |
|
McNatty KP, Henderson KM, Sawers RS: Effects of prostaglandin F2α and E2 on the production of progesterone by human granulosa cells in tissue culture. J Endocrinol 67: 231, 1978 |
|
Dennefors BL, Sjogren A, Hamberger L: Progesterone and adenosine 3'5'-monophosphate formation by isolated human corpora lutea of different ages: Influence of human chorionic gonadotropin and prostaglandins. J Clin Endocrinol Metab 55: 102, 1982 |
|
Pharriss BB, Wyngarden LJ: The effects of prostaglandin F2α on the progestogen content of ovaries from pseudopregnant rats. Proc Soc Exp Biol Med 130: 92, 1969 |
|
Janson PO, Williams D, Petrucco OM et al: Blood flow in the ovary and adjacent structures of the non-pregnant sheet. Acta Endocrinol (Cophen) 103: 259, 1983 |
|
Riley JCM, Carlson JC: Impartment of gonadotropin binding occurs during membrane rigidification in plasma membrane samples prepared from regressing rat corpora lutea. Can J Physiol Pharmacol 66: 76, 1988 |
|
Rajkumar K, Ganguli S, Menor KMJ et al: Studies in the mechanism of action of PGF2α-induced luteolysis in rats. Prostaglandins 36: 547, 1988 |
|
Pate JL, Nephew KP: Effects of in vivo and in vitro administration of PGF2α on lipoprotein utilization in cultured bovine luteal cells. Biol Reprod 38: 568, 1988 |
|
Patton PE, Stouffer RL: Current understanding of the corpus luteum in women and nonhuman primates. Clin Obstet Gynecol 34: 127, 1991 |
|
Sundberg F, Ingleman-Sundberg L, Rydin G: The effect of prostaglandin E2 on the human uterus and the fallopian tubes in vitro. Acta Obstet Gynecol Scand 42: 269, 1963 |
|
Lindblom B, Hamberger L, Wiquist N: Differentiated contractile effects of prostaglandins E and F in the isolated circular and longitudinal smooth muscle of the human oviduct. Fertil Steril 30: 553, 1978 |
|
Spilman CH: Oviduct motility in the rhesus monkey: Spontaneous activity and response to prostaglandins. Fertil Steril 25: 935, 1974 |
|
Spilman CH: Oviduct response to prostaglandin influence of estradiol and progesterone. Prostaglandins 7: 465, 1974 |
|
Ogra SS, Kurton KT, Tomasi TB, Lippes J: Prostaglandins in the human fallopian tube. Fertil Steril 25: 250, 1974 |
|
Elder MG, Myatt L, Chaudhuri G: The role of prostaglandins in spontaneous motility of the fallopian tube. Fertil Steril 28: 86, 1977 |
|
Omini C, Pagargiklian R, Folco GC et al: Pharmacological activity of PGI2 and 6 keto PGF1α in the human uterus and fallopian tubes. Prostaglandin 15: 1045, 1978 |
|
Kennedy TG: Evidence for a role of prostaglandins in the initiation of blastocyst implantation in the rat. Biol Reprod 16: 286, 1977 |
|
Evans CA, Kennedy TG: The importance of prostaglandin synthesis for the initiation of blastocyst implantation in the hamster. J Reprod Fertil 54: 255, 1978 |
|
Kennedy TG, Zamecnik J: The concentration of 6-oxo-PGF1α is markedly elevated at the site of blastocyst implantation in the rat. Prostaglandins 16: 599, 1978 |
|
Chakraborty I, Das SK, Wang J, Dey SK. Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J Mol Endocrinol 16:107, 1996 |
|
Yang ZM, Das SK, Wang J et al: Potential sites of prostaglandin actions in the periimplantation mouse uterus: Differential expression and regulation of prostaglandin receptor genes. Biol Reprod 56: 368, 1997 |
|
Lundkvist O, Nilsson BO, Ultrastructural changes of the trophoblast-epithelial complex in mice subjected to implantation blocking tratment with indomethacin. Biol Reprod 22:719, 1980 |
|
Phillips CA, Poyser NL: Studies on the involvement of prostaglandins in implantation in the rat. J Reprod Fertil 62: 73, 1981 |
|
Hoffman LH, DiPetro DL, McKenna TJ: Effects of indomethacin on uterine capillary permeability and blastocyst development in rabbits. Prostaglandins 15: 823, 1978 |
|
Holmes PR, Gordashko BJ: Evidence of prostaglandin involvement in blastocyst implantation. J Embryol Exp Morphol 55: 109, 1980 |
|
Garg SK, Chaudhury RR: Evidence for a possible role of prostaglandins in implantation in rats. Arch Int Pharmacodyn Ther 262: 299, 1983 |
|
Cao ZD, Jones MA, Harper MJK. Progesterone and estradiol receptor concentration and translocation in uterine tissue of rabbits treated with indomethacin. J Endocrinol 107:197, 1985 |
|
Kennedy TG: Prostaglandins and increased vascular permeability resulting from the application of an artificial stimulus to the uterus of the rat sensitized for the decidual reaction. Biol Reprod 20: 560, 1979 |
|
Kennedy TG: Does prostaglandin I2 mediate the increased endometrial vascular permeability which results from application of the deciduogenic stimulus to the sensitized rat uterus? Biol Reprod 20 (Suppl 1): 99A, 1979 |
|
Psychoyos A, Nikas G, Gravanis A: The role of prostaglandins in blastocyst implantation. Human Reprod 10 (Suppl 2): 30, 1995 |
|
Challis JRG, Patrick JE: The production of prostaglandins and thromboxanes in the feto-placental unit and their effects on the developing fetus. Semin Perinatol 4: 23, 1980 |
|
Novy MJ, Liggins GC: Role of prostaglandins, prostacyclin and thromboxanes in the physiologic control of the uterus and in parturition. Semin Perinatol 4: 45, 1980 |
|
Mitchell MD: Prostaglandins during pregnancy and the perinatal period. J Reprod Fertil 62: 305, 1981 |
|
Whalen JB, Clancey CJ, Farley DB, Van Orden DE: Plasma prostaglandins in pregnancy. Obstet Gynecol 51: 52, 1978 |
|
Mitchell MD, Flint APF, Bibby JG et al: Plasma concentrations of prostaglandins during late human pregnancy; influence of normal and pre-term labour. J Clin Endocrinol Metab 46: 947, 1978 |
|
Satoh K, Yasumizu T, Fukuoka H et al: Prostaglandin F2α metabolite levels in plasma, amniotic fluid and urine during pregnancy and labor. Am J Obstet Gynecol 133: 886, 1979 |
|
Dubin NH, Johnson JWC, Calhoun S et al: Plasma prostaglandin in pregnant women with term and pre-term deliveries. Obstet Gynecol 57: 203, 1981 |
|
Ylikorkala O, Kirkinen P, Viinikka L: Maternal plasma prostacyclin concentration in preeclampsia and other pregnancy complications. Br J Obstet Gyanecol 88: 968, 1981 |
|
Ylikorkala O, Viinikka L: Maternal plasma levels of 6 keto prostaglandin F1α during pregnancy and puerperium. Prostaglandins Med 7: 95, 1981 |
|
Meagher EA, Fitzgerald GA: Disordered eicosanoid formation in pregnancy-induced hypertension. Circulation 88: 1324, 1993 |
|
Remuzzi G, Marchesi D, Zoja C et al: Reduced umbilical and placental vascular prostacyclin in severe preeclampsia. Prostaglandins 20: 105, 1980 |
|
Stuart MJ, Sunderji SG, Yambo T et al: Decreased prostacyclin production: A characteristic of chronic placental insufficiency syndromes. Lancet 1: 1126, 1981 |
|
Clark KE, Ryan MJ, Brody MJ: Effects of prostaglandins E1 and F2α on uterine hemodynamics and motility. Adv Biosci 9: 779, 1973 |
|
Clark KE, Ryan MJ, Brody MJ: Effect of prostaglandins on vascular resistance and adrenergic vasoconstriction responses in the canine uterus. Prostaglandins 12: 71, 1976 |
|
Still JG, Greiss FC: The effect of prostaglandins and other vasoactive substances on uterine blood flow and myometrial activity. Am J Obstet Gyencol 130: 1, 1978 |
|
Resnik R, Brink GW: Effects of prostaglandins E1, E2, and F2α on uterine blood flow in nonpregnant sheep. Am J Physiol 234: H557, 1978 |
|
Resnik R, Brink GW: Uterine vascular response to prostacyclin in nonpregnant sheep, Am J Obstet Gynecol 137: 267, 1980 |
|
Clark KE, Farley DB, Van Orden DE, Brody MJ: Role of endogenous prostaglandins in regulation of uterine blood flow and adrenergic neurotransmission. Am J Obstet Gynecol 127: 455, 1977 |
|
Clark KE, Brody MJ: Antagonism by PGF2α of the vasodilation induced by PGE1, PGE2 and PGA in the canine uterus. Blood Vessels 14: 204, 1977 |
|
Greiss FC, Anderson SG: Uterine vascular changes during the ovarian cycle. Am J Obstet Gynecol 103: 629, 1969 |
|
Resnik R, Killam AP, Battaglia FC et al: The stimulation of uterine blood flow by various estrogens. Endocrinology 94: 1192, 1974 |
|
Clark KE, Stys SJ, Austin JE, Golter M: Prostaglandins: Mediator of estrogen induced increases in uterine blood flow? (Meeting abstract) Society Gynecological Investigation, p 176, 1980 |
|
Resnik R: The endocrine regulation of uterine blood flow in the nonpregnant uterus: A review. Am J Obstet Gynecol 140: 151, 1981 |
|
Ryan MJ, Clark KE, Van Orden DE et al: Role of prostaglandins in estrogen induced uterine hyperemia. Prostaglandins 5: 257, 1974 |
|
Van Buren, GA, Yang DS, Clark KE: Estrogen-induced uterine vasodilatation is antagonized by L-nitroarginine methyl ester, an inhibitor of nitric oxide synthesis. Am J Obstet Gyencol 1992;167:828, 1992 |
|
Mitchell MD, Brunt J, Clover L, Walker DW: Prostaglandins in the umbilical and uterine circulation during late pregnancy in the ewe. J Reprod Fertil 58: 283, 1980 |
|
Tuvemo T: Role of prostaglandins, prostacyclin and thromboxanes in the control of the umbilical-placental circulation. Semin Perinatol 4: 91, 1980 |
|
Terragno NA, Terragno DA, Pacholczyk D, McGiff JC: Prostaglandins and the regulation of uterine blood flow in pregnancy. Nature 249: 57, 1974 |
|
Venuto RC, O'Dorisio T, Stein JH, Ferris TF: Uterine prostaglandin E secretion and uterine blood flow in the pregnant rabbit. J Clin Invest 55: 193, 1975 |
|
Clark KE, Stys SJ, Austin JE, Golter M: Post surgical effects of indomethacin on uterine blood flow. (Meeting abstract) Society Gynecologic Investigation, p 37, 1980 |
|
McLaughlin MK, Brennan SC, Chez RA: Effects of indomethacin on sheep uteroplacental circulations and sensitivity to angiotension II. Am J Obstet Gynecol 132: 430, 1978 |
|
Rankin JHG, Phernetton TM: Effect of prostaglandin E2 on ovine maternal placental blood flow. Am J Physiol 231: 754, 1976 |
|
McLaughlin MK, Brennan SC, Chez RA: Vasoconstrictive effects of prostaglandins in sheep placental circulation. Am J Obstet Gynecol 130: 408, 1978 |
|
Landauer M, Phernetton TM, Parisi VM et al: Ovine placental vascular response to the local application of prostacyclin. Am J Obstet Gynecol 151: 460, 1985 |
|
McLaughlin MK, Phernetton TM, Rankin JHG: Prostaglandin D2: Effects on the rabbit utero-placental circulation. Proc Soc Exp Biol Med 162:187, 179 |
|
Novy MJ, Piasecki G, Jackson BT: Effect of prostaglandins E2 and F2α on umbilical blood flow and fetal hemodynamics. Prostaglandins 5: 543, 1974 |
|
Parisi VM, Walsh SW: Fetal vascular response to prostacyclin. Am J Obstet Gynecol 160: 871, 1989 |
|
Clark KE, Mack CE: Umbilical responses to PGI2, U46619 (a TXA2 mimetic) and 6 keto PGE1 in the fetal lamb. Presented at the Ninth Annual Meeting of the Society of Perinatal Obstetricians, New Orleans, LA, Feb 1–4, 1989 |
|
Parisi VM, Walsh SW: Fetoplacental vascular responses to prostacyclin after thromboxane-induced vasoconstriction. Am J Obstet Gynecol 160: 502, 1989 |
|
Lange U, Prada J, Clark KE: Systemic and uterine vascular response to serotonin in third trimester pregnant ewes. Eur J Obstet Gynecol Reprod Biol 51: 131, 1993 |
|
Clark KE, Moore KE, Mack CE: Umbilical vascular response to angiotensin II, norepinephrine, and serotonin in pregnant sheep. Presented at the 35th Annual Meeting Society for Gynecologic Investigation. Baltimore, MD, Mar 17–20, 1988 (abstract 264) |
|
Fidler J, Bennett MJ, de Swiet M et al: Treatment of pregnancy hypertension with prostacyclin. Lancet 2: 31, 1980 |
|
Skillman CA, Plessinger MA, Woods JR Jr, Clark KE: Effects of graded reductions in uteroplacental blood flow on the fetal lamb. Am J Physiol 249: H1098, 1985 |
|
Gant NF, Daley GL, Chand S et al: A study of angiotension II pressor response throughout primigravid pregnancy. J Clin Invest 52: 2682, 1973 |
|
Rosenfeld CR, Gant NF: The chronically instrumented ewe: A model for studying vascular reactivity to angiotension II in pregnancy. J Clin Invest 67: 486, 1981 |
|
Chesley LC, Talledo OE, Bohler CS, Zuspan FP: Vascular reactivity to angiotension II and norepinephrine in pregnant and nonpregnant women. Am J Obstet Gynecol 91: 837, 1965 |
|
Franklin GO, Dowd AJ, Caldwell BV, Speroff L: The effect of angiotension II intravenous infusion on plasma renin activity and prostaglandins A, E and F levels in the uterine vein of the pregnant monkey. Prostaglandins 6: 271, 1974 |
|
Naden RP, Rosenfeld CR: Effect of angiotension II on uterine and systemic vasculature in pregnant sheep. J Clin Invest 6: 468, 1981 |
|
Clark KE, Austin JE, Golter M, Stys SJ: Prostaglandin modulation of vasoconstrictor responses in the uterine vasculature. (Meeting abstract) Society Gynecological Investigation, p 39, 1981 |
|
Nathanielsz PW: Endocrine mechanisms of parturition. Ann Rev Physiol 40: 411, 1978 |
|
Thorburn GD, Challis JRG: Endocrine control of parturition. Physiol Rev 59: 863, 1979 |
|
Challis JRG, Mitchell BF: Hormonal control of preterm and term parturition. Semin Perinatol 5: 192, 1981 |
|
Keirse MJ: Endogenous prostaglandins in human parturition. In Keirse MJW, Anderson ABM, Bennebroek-Gravenhorst J (eds): Human Parturition: New Concepts and Developments, p 101. Leiden, Leiden University Press, 1980 |
|
Romero R, Baumann P, Gonzalez R et al: Amniotic fluid prostanoid concentrations increase early during the course of spontaneous labor at term. Am J Obstet Gynecol 171: 1613, 1994 |
|
Challis JRG: Causes of parturition. Royal Society of Medicine Services, LTD. International Congress and Symposium Series, No. 92, p 5, 1985 |
|
Okazaki T, Sagawa N, Okita JR et al: Diacylglycerol metabolism and arachidonic acid release in human fetal membranes and decidua vera. J Biol Chem 256: 7316, 1981 |
|
Skinner KA, Challis JRG: Changes in the synthesis and metabolism of prostaglandins by human fetal membranes and decidua at labor. Am J Obstet Gynecol 151: 519, 1985 |
|
Myatt L, Rose MP, Elder MG: Lipoxygenase products of arachidonic acid in human fetal membranes. Presented at the 32nd Annual Meeting Society for Gynecologic Investigation, Phoenix, AZ, March 1985 (abstract 160) |
|
Bleasdale JE, Johnston JM: Protaglandins and human parturition: Regulation of arachidonic acid metabolism. Rev Perinat Med 5: 151, 1984 |
|
Bennett PR, Chamberlain GV, Patel L et al: Mechanisms of parturition: the transfer of prostaglandin E2 and 5-hydroxyeicosatetraenoic acid across fetal membranes. Am J Obstet Gynecol 162: 683, 1990 |
|
Nakla S, Skinner K, Mitchell BF, Challis JR: Changes in prostaglandin transfer across human fetal membranes obtained after spontaneous labor. Am J Obstet Gynecol 155: 1337, 1986 |
|
McCoshen JA, Johnson KA, Dubin NH, Ghodgaonkar RB: Prostaglandin E2 release on the fetal and maternal sides of the amnion and chorion-decidua before and after term labor. Am J Obstet Gynecol 156: 173, 1987 |
|
Roseblade CK, Sullivan MH, Khan H et al: Limited transfer of prostaglandin E2 across the fetal membrane before and after labor. Acta Obstet Gynecol Scand 69: 399, 1990 |
|
Green K, Bygdeman M, Toppozada M et al: The role of prostaglandin F2α in human parturition: Endogenous plasma levels of 15-keto-13-14-dehydroprostaglandin F2α during labor. Am J Obstet Gynecol 120: 25, 1974 |
|
Wilson T, Liggins GC, Whitaker DJ: Oxytocin stimulates the release of arachidonic acid and prostaglandin F2α from human decidual cells. Prostaglandins 35: 771, 1988 |
|
Challis JRG, Mitchell BF, Power SGA et al: Steroid and prostaglandin production by intrauterine tissues in the sheep and human in relation to parturition. In Albrecht E, Pepe GJ (eds): Perinatal Endocrinology, p 262. Ithaca, Perinatology Press, 1985 |
|
Romero R, Durum S, Dinarello CA et al: Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins 37: 13, 1989 |
|
Casey ML, Korte K, MacDonald PC: Epidermal growth factor stimulation of prostaglandin E2 biosynthesis in amnion cells. J Biol Chem 262: 7846, 1988 |
|
Mitchell MD: Actions of transforming growth factors of amnion cell prostaglandin biosynthesis. Prost Leuk Eicos 33: 159, 1988 |
|
Lopez Bernal A, Bryant-Greenwood GD, Hansell DJ: Effect of relaxin on prostaglandin E production by human amnion: Changes in relation to the onset of labor. Br J Obstet Gynecol 94: 1045, 1987 |
|
Wilson T, Liggins GC, Joe L: Purification and characterization of an uterine phospholipase inhibitor that loses activity after labor onset in women. Am J Obstet Gynecol 160: 602, 1989 |
|
Lynch-Salamon DI, Everson WV, Myatt L: Decrease in annexin I mRNA expression in human amnion with labor. Am J Obstet Gynecol 167: 1657, 1992 |
|
Xue S, Slater DM, Bennett PR, Myatt L: Induction of cytosolic phospholipase A2 and prostaglandin H synthase-2 by interleukin-1β in WISH cells is inhibited by dexamethasone. Prostaglandins 51: 107, 1996 |
|
Teixeira F, Zaker T, Hirst JJ et al: Prostaglandin endoperoxide-H synthase (PGHS) activity and immunoreactive PGHS-1 and PGHS-2 levels in human amnion throughout gestation, at term and during labor. J Clin Endocrin Metab 78: 1396, 1994 |
|
Freed KA, Aitken MA, Brennecke SP, Rice GE: Prostaglandin G/H synthase-1 messenger RNA relative abundance in human amnion, choriodecidua and placenta before, during and after spontaneous onset of labour at term. Gynecol Obstet Invest 39: 73, 1995 |
|
Slater DM, Berger LC, Newton R et al: Expression of cyclooxygenase types 1 and 2 in human fetal membranes at term. Am J Obstet Gynecol 172: 77, 1995 |
|
Mayer RJ, Marshall LA: New insights on mammalian phospholipase A2 (s): Comparison of arachidonyl selective and nonselective enzymes. FASEB J 7: 339, 1993 |
|
Skannal DG, Brockman D, Eis ALW et al: Alterations in activity of cytosolic phospholipase A2 in human amnion at parturition. Am J Obstet Gynecol 177: 179, 1997 |
|
Karim SMM, Trussell RR, Hillier K, Patel RC: Induction of labour with prostaglandin F2α. J Obstet Gynaecol Br Commonw 76: 769, 1969 |
|
Bygdeman M, Kuan SU, Mulcherjee T et al: Effect of intravenous infusion of prostaglandin E1 and E2 of motility of the pregnant human uterus: Prostaglandins and related factors. Am J Obstet Gynecol 102: 317, 1968 |
|
Bygdeman M: Clinical applications. Adv Prostaglandin Thromboxane Leukotrienes Res 6: 87, 1980 |
|
Bygdeman M, Green KK, Bremme K: Research in the use of prostaglandin analogues. In Zatuchni GI, Sciarra JJ, Speidel JJ (eds): Pregnancy Termination, p 234. New York, Harper & Row, 1978 |
|
Tricomi V, Kohl SG: Fetal death in utero. Obstet Gynecol 74: 1092, 1975 |
|
Schwartz ML, Brenner WE: Termination of pregnancy complicated by anencephaly with intra-amniotic prostaglandin F2α. Am J Obstet Gynecol 136: 203, 1980 |
|
Karim SMM, Amy JJ: Interruption of pregnancy with prostaglandin. In Karim SMM (ed): Prostaglandins and Reproduction, p 77. Lancaster, MTP, 1975 |
|
Corson SL, Bolognese RJ: Postpartum uterine atony treated with prostaglandins. Am J Obstet Gynecol 129: 918, 1977 |
|
Takagi S, Yoshida T, Togo Y et al: The effects of intramyometrial injection of prostaglandin F2α in severe postpartum hemorrhage. Prostaglandins 12: 565, 1976 |
|
Stys SJ, Dresser BL, Otte TE, Clark KE: Effect of prostaglandin E2 on cervical compliance in pregnant ewes. Am J Obstet Gynecol 140: 415, 1981 |
|
Calder AA: Prostaglandins and biological control of cervical function. Aust N Z J Obstet Gynaecol 34: 347, 1994 |
|
O'Brien WF. The role of strong prostaglandins in labor and delivery. Clin Perinatol 22:973, 1995 |