Specific Bacterial Infections: Group B Streptococcus
Authors
INTRODUCTION
Invasive group B streptococcus (GBS, Streptococcus agalactiae) infection in adults is being identified with increased frequency. The infection originates from soft-tissue infections, bacteremia and pneumonia.1 Those with reduced immunity from diabetes or cancer have a 10- to 15-fold increased rate of GBS infection.1 In adults, GBS infection has a high case-fatality rate; it is also one of the most important causes of neonatal infection. Reports since the 1930s had linked GBS with neonatal meningitis, but the scope of perinatal and neonatal GBS infection did not become evident until the 1960s, when associations were made between maternal genital GBS colonization and spontaneous abortions, stillbirths, and preterm deliveries.2 GBS became the most common cause of symptomatic neonatal sepsis, replacing Escherichia coli as the predominant pathogen.3 The neonatal sepsis attack rate for E. coli and other Enterobacteriaceae species has remained stable since the 1960s, and the emergence of GBS as the predominant pathogen represents an absolute increase in the total incidence of serious neonatal infections. GBS infection has more recently been reported in antenatal complications, including urinary tract infection, premature rupture of the membranes (PROM), and amniotic fluid (AF) infection, and in postpartum maternal infection.
EPIDEMIOLOGY OF INFECTION
The Organism
GBS is one of many serologically distinct species within the genus Streptococcus. Streptococci are facultatively anaerobic, gram-positive cocci, usually found on Gram's stain to be arranged in chains. The most important pathogenic streptococcal species for humans include group A streptococcus (Streptococcus pyogenes), GBS, group D streptococcus (enterococci), Streptococcus pneumoniae, and Streptococcus viridans.
GBS can be recovered from sterile sites on nonselective blood agar. However, use of a selective broth medium (SBM) (e.g., Todd-Hewitt, or Lim broth) that contains antibiotics such as nalidixic acid and colistin or gentamicin to inhibit other bacteria is now the standard for isolation of GBS from genital or rectal sites.4 The swab is placed in the liquid broth; after being incubated overnight, the broth is placed on blood agar.
Most GBS colonies appear on blood plates as 1- to 2-mm, gray-white colonies surrounded by a zone of β-hemolysis, although 2% of strains are nonhemolytic. Preliminary identification includes βhemolytic colonies or, for ones that are nonhemolytic, a gram-positive, catalase-negative reaction. Distinction of GBS from other streptococci can be based on GBS antigen detection or on biochemical reactions including resistance to bacitracin, hydrolysis of sodium hippurate, and the production of a soluble hemolysin that acts synergistically with β-lysin of Staphylococcus aureus to produce β-hemolysis (CAMP test). Definitive identification is based on the presence of a polysaccharide group—specific antigen common to all GBS strains determined by serologic testing.
A major limitation of cultures is the length of time necessary for growth and identification of GBS. A number of more rapid diagnostic tests are available to directly detect group- or type-specific GBS antigens. Examples of these tests include immunoassays and latex particle agglutination. Commercial kits have a greater than 90% sensitivity and specificity in the diagnosis of meningitis or bacteremia attributed to GBS; however, these kits identify only large concentrations of GBS. Although the attack rate of neonatal GBS sepsis is highest in neonates of mothers with large concentrations of GBS, approximately 30% of neonatal GBS sepsis occurs in pregnancies with low concentrations of GBS. These rapid identification tests should not be used to detect GBS in the genital tract because they are too insensitive to identify the 30% of women with a low concentration of GBS.4
GBS can be further subdivided into types Ia, Ib, Ic, II, and III on the basis of distinctive type-specific polysaccharide antigens. Approximately 99% of strains can be typed into one of these five antigen types.
Maternal Colonization
The prevalence and natural history of GBS colonization of the female lower genital tract have been extensively studied during pregnancy. GBS was recovered from the vagina and cervix in approximately 19% (range, 9% to 26%) of 8000 pregnant women using selective culture medium.5 The gastrointestinal tract is the primary reservoir of GBS, and vaginal colonization occurs secondarily from the gastrointestinal source. The prevalence of GBS isolation is highest in the rectum, intermediate in the vagina, and lowest in the cervix. A combination vaginal-rectal culture is now recommended to detect GBS in pregnant women.4 Approximately 20% (range, 10% to 30%) of pregnant women have GBS in vaginal and rectal cultures in studies using selective media.6,7,8,9,10 Colonization rates can vary by age, ethnicity, and different geographic locations. GBS can also be recovered from the urethra of 45% to 63% of the male partners of female carriers, indicating that sexual transmission may also occur.10,11
Pregnant women have the same prevalence of GBS as nonpregnant women. The rate of colonization does not vary with gestational age.12,13 The distribution of GBS serotypes also remains stable throughout gestation and the puerperium. Approximately one third of isolates are type I, one third type II, and one third type III. Although the rate of GBS remains constant, throughout pregnancy either intermittent or transient carriage occurs in 35% to 40% of pregnant GBS carriers. Persistent GBS carriage with multiple consecutive positive cultures occurs in only 30% to 50% of pregnant GBS carriers. Approximately 30% of pregnant GBS carriers do not fall into these categories.7,13,14
Maternal cultures performed close to delivery are better predictors of GBS in the vaginal-rectal culture at delivery than maternal culture performed at 26 to 28 weeks' gestation.6,15 The positive predictive value of a culture in the late second trimester is approximately 72%6,15,16 compared to 82% at 36 weeks.6,17,18 The negative predictive value is approximately 94% for a second-trimester maternal culture and 97% for a 36-week culture.18
Neonatal Colonization
Neonatal infection is divided into early-onset infection, occurring at less than 7 days of age; and late-onset infection, occurring at or beyond 7 days of age. Early GBS infection develops in approximately 1% to 2% of neonates born to colonized mothers. The GBS that causes early-onset infection is usually vertically transmitted to the neonate due to AF infection or from the birth canal of a colonized mother. As expected, the highest rate of neonatal colonization occurs among infants born to mothers with GBS. Between 40% and 70% of neonates born to GBS-colonized mothers become colonized, usually with the same serotype present in the mother.6,7,13,15,16,17,18,19 In contrast, only 1% to 12% of neonates born to noncolonized mothers become culture-positive.6,16 The neonatal colonization rates of abdominal delivery versus vaginal delivery are similar.
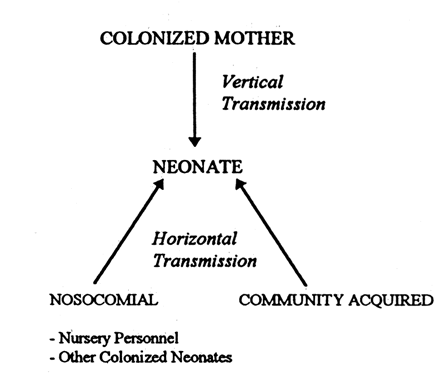
Late-onset GBS infection most commonly results from horizontal transmission through nosocomial spread in the nursery by colonized nursery personnel or other colonized neonates, or by acquisition from community sources (Fig. 1). Of all neonates, 3% to 12% are colonized by GBS within the first week of life. The impact of vertical transmission at birth on the total pool of colonized infants at one week is lessened because of the large number of pregnant women without GBS. Approximately one third of neonates colonized at 4 days of age are born to noncolonized mothers.19 Only approximately 45% of all neonatal colonization is directly attributable to vertical transmission,20 and nosocomial spread is an important route of transmission, particularly in late-onset infection. Further, up to 35% of neonates initially colonized at birth are culture-negative by the fourth day of life.19
Several factors modify the risk of vertical transmission of GBS. Higher neonatal transmission rates occur from women persistently culture-positive and from women with a high concentration of GBS (Table 1).6,7,21 The site of maternal carriage is also important: the rate of vertical neonatal transmission is higher when maternal GBS infection originates from the cervix (89%) compared to the rectum only (65%).5,7
TABLE 1. The Relationship Between Neonatal Colonization and the Semiqauntitative Level of Maternal Colonization: Percentage of GBS-Colonized Neonates
| Semiquantitative Level of Growth Among GBS-Colonized Mothers | |||
Author (Red) | None (0) | Light (1+) | Moderate (2+) | Heavy (3+) |
Boyer et al | 1 | 17 | 23 | 65 |
Hoogkamp-Korstanje et al | 1 | 30 | 50 | 87 |
Anocona et al | - | 31 | 43 | 67 |
GBS = group B streptococcus
PATHOGENESIS OF INFECTION
The low attack rate of serious neonatal GBS infection, despite a high prevalence of maternal and neonatal GBS colonization, suggests considerable protection against invasive infection. GBS has been associated with both AF infection and neonatal sepsis diagnosed at birth, indicating that infection occurs before birth. In fact, approximately 70% of infants with early-onset GBS infection are bacteremic at birth.22 This indicates that bacteremia develops in utero as a result of aspiration of infected AF, or contamination of umbilical blood due to a GBS-infected placenta. The chorioamnion provides an anatomic barrier against infection. AF also contains several factors that are antibacterial, including peroxidase, lysozyme, transferrin, immunoglobulins, complement, and a zinc-dependent bactericidal polypeptide. The attack rate of GBS infection, however, is increased in the setting of preterm labor,22 and GBS can be isolated from the AF of patients in preterm labor with intact membranes, suggesting that GBS can cross the intact chorioamnion. Rupture of membranes (ROM) allows vaginal bacteria to enter into AF, and as expected, the attack rate of GBS infection increases with prolonged ROM. The risk of clinical AF infection is increased in the presence of the following: GBS colonization, ROM lasting more than 6 hours, internal fetal monitoring lasting more than 12 hours, and more than six vaginal examinations.23
A potentially effective deterrent to invasive infection may be maternal antibodies directed against the capsular polysaccharide antigens of GBS. Immunity to GBS is mediated by antibody-dependent phagocytosis. Mothers of infants with type III GBS sepsis have lower serum levels of type-specific antibodies than women giving birth to asymptomatically colonized infants.24 The type III IgG antibody has some broad reactivity to all GBS, and it readily crosses the placenta. When measured in mother-infant pairs, an excellent correlation exists between maternal and cord antibody levels. More than 73% of GBS-colonized mothers with healthy neonates were found to have high type III serum antibody in contrast to only 19% of GBS-colonized mothers whose neonates acquired early-onset septicemia or meningitis (p 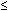 0.001).25 However, GBS acts as a poor immunogen. GBS colonization and even invasive GBS infection in the neonate often fails to produce serum antibody against GBS in the mother or the neonate. This explains why neonatal GBS infection can occur in subsequent pregnancies. Often, antibody production is stimulated in none of the neonates with early-onset disease and in only a proportion of infants with late-onset disease. A detectable increase in antibody in convalescent sera is present in none of the neonatal early-onset GBS-disease survivors and in only 35% of late-onset disease survivors.
0.001).25 However, GBS acts as a poor immunogen. GBS colonization and even invasive GBS infection in the neonate often fails to produce serum antibody against GBS in the mother or the neonate. This explains why neonatal GBS infection can occur in subsequent pregnancies. Often, antibody production is stimulated in none of the neonates with early-onset disease and in only a proportion of infants with late-onset disease. A detectable increase in antibody in convalescent sera is present in none of the neonatal early-onset GBS-disease survivors and in only 35% of late-onset disease survivors.
Not all infants without antibody become affected with invasive disease, and not all infants with antibody remain well. Research by Vogel and colleagues found that although nearly 50% of pregnant women studied had specific IgG type III antibody, only 5% of these women had protective levels of antibody to a lethal inoculum of GBS into chick embryos.26 These data suggest that most newborns are susceptible to GBS infection and that factors other than humoral immunity also play a role in explaining the wide discrepancy between high rates of asymptomatic colonization and low rates of invasive infection. Both the attack rate and death rate of invasive GBS infections are increased in low-birth-weight (low-gestational-age) neonates, as shown in Table 2. This indicates reduced immunity to invasive GBS infections because of many factors present in a premature delivery, including an immature neonatal immune system and a relative resistance to delivery in the face of infection. Other factors that influence GBS infection include differences in virulence between strains, differences in inoculum size, and the length of exposure to the microorganisms after ROM.
TABLE 2. Attack Rate and Death Rate of Early-Onset GBS Infection In High-Risk Groups
|
|
|
| Death Rate |
|
| No with | Early-Onset | Among Those |
| Percentage | GBS | GBS Infection | With GBS |
| of Births | Infection | Attack Rate* | Infection |
Birth Weight (g) |
|
|
|
|
500–1000 | 1% | 10 | 26 | 90% |
1001–2000 | 4% | 11 | 8.5 | 27% |
2001–2500 | 6.5% | 9 | 4 | 33% |
>2500 | 89% | 31 | 1.1 | 3% |
Rupture of Membranes (h) |
|
|
|
|
<6 | 61% | 15 | 1 | 33% |
7–18 | 27% | 15 | 2 | 20% |
19–24 | 6% | 11 | 6 | 27% |
25–48 | 4% | 11 | 9 | 18% |
>48 | 3% | 9 | 11 | 33% |
Peak Labor Temperature |
|
|
|
|
| 95% | 49 | 1.5 | 29% |
>37.5°C | 6% | 12 | 6.5 | 17% |
Any of three risk factors † |
|
|
|
|
Present | 18% | 45 | 7.6 | 33% |
Absent | 82% | 16 | 0.6 | 6% |
*Per 1000 live births
†Birth weight <2500 g, rupture of membranes >18 hours, and fever in labor >37.5°C
GBS = group B streptococcal
(Boyer KM, Gadzala CA, Burd LI et al: Selective intrapartum chemoprophylaxis of neonatal group B streptococcal early-onset disease: 1. Epidemiologic rationale. J Infect Dis 148:795, 1983)
Strain virulence also influences GBS infection. Type III strains of GBS represent approximately one third of isolates from asymptomatically colonized infants, but they account for more than 85% of the isolates from early-onset meningitis or late-onset infection and 60% of isolates from all varieties of invasive GBS infection. It is of interest that an extracellular toxin produced by a virulent strain of type III GBS, when injected into sheep models, produces a biphasic response characterized initially by an increase in the pulmonary artery pressure, a decrease in the arterial partial pressure of oxygen (PaO2), and a temperature elevation; and later by granulocytopenia and an increase in the pulmonary vascular permeability.27 These effects closely parallel the clinical infection present during early neonatal septicemia.
Neonatal Infection
GBS causes neonatal pneumonia, sepsis, and meningitis. It has become the leading cause of septicemia and meningitis in the first 2 months of life. The reasons for the increased GBS infection rate during this time remain speculative. The early-onset neonatal GBS attack rate is 1 to 3 in 1000 live births.4 In 1990, the incidence of GBS infection was 1.8 in 1000 live births in neonates (up to 90 days of age).28 Early-onset infection accounts for 80% of neonatal GBS infection. This rate was estimated from a multistate surveillance, which reported 7600 episodes of neonatal GBS infection and 310 deaths annually.28 Up to 30% of survivors of GBS meningitis will have neurologic sequelae.29
Several pregnancy and fetal factors increase the chance of early-onset GBS infection. The concept of high-risk factors for GBS neonatal sepsis was initially based on 61 cases of early-onset GBS infection among more than 32,000 pregnancies studied by Boyer and colleagues.6,22 This constitutes the largest and most complete set of data on early-onset GBS infection. The attack rate for early-onset GBS sepsis was increased among the following three groups (see Table 2): birth weight less than 2500 g, greater than 18 hours' duration of ROM, or maternal fever greater than 37.5°C. Attack rates were linear for birth weight and duration of ROM and were especially high for very low birth weight and prolonged ROM. The perinatal death rate was also highly related to birth weight, but not to ROM or maternal fever. Approximately 18% of the pregnancies were in the high-risk group. Pregnancies with preterm ROM, which is also a risk factor, were included in the group with low birth weight less than 2500 g. As seen in Table 2, approximately 11% of pregnancies are at increased risk for GBS on the basis of low birth weight. A study by Baker and Barrett determined that approximately 7% of term pregnancies have ROM lasting greater than 18 hours or maternal fever during labor.18 The early GBS sepsis rate per 1000 live births for the high-risk group (7.6) compared to the low-risk group (0.6) was increased nearly 13-fold. Boyer and associates22 estimated that there was a 70% chance that an infected infant would have one of these risk factors.
The risk of neonatal GBS sepsis is increased in neonates born to mothers with GBS bacteriuria.30 Neonates born to mothers who previously delivered a child with GBS sepsis are also at increased risk for GBS sepsis.31 Thus, there are six factors that cause an increased rate of neonatal GBS sepsis. In addition, some studies link age less than 20, black ethnicity, and diabetes with GBS infection.
CLINICAL SYNDROME.
Two distinct clinical syndromes occur among neonates with GBS infections. These differ in the age at onset, pathogenesis, and outcome (Table 3). Early-onset infection occurs within the first 7 days of life. The mean age of clinical onset is the first few hours of life. A significant portion of these infections are apparent at birth (14%) or become symptomatic within the first 90 minutes of life (29%), indicating that in utero GBS exposure and infection often occur.32 In fact, approximately 70% of blood cultures are positive at birth in early-onset GBS infection.22 Approximately 70% of early-onset GBS neonatal infections occur under the following conditions: low birth weight (less than 2500 g), greater than 18 hours' duration of ROM, and/or intrapartum fever.
TABLE 3 Characteristics of Early-Onset and Late-Onset Group B Streptococcal Infection in Neonates
Characteristic | Early-Onset | Late-Onset |
Onset | <7 days |
|
Mean age at onset | 20 hours | 24 days |
Obstetric complications | Yes | No |
Exposure (transmission) | Vertical | Horizontal nosocomial |
Predominant pathology | Septicemia | Meningitis (80%) |
| Pneumonia (40%) |
|
| Meningitis (30%) |
|
Serotype distribution | All types | Type III (90%) |
Disease incidence | 1.8 | 0.4 |
(per 1000 liveborn) |
|
|
Mortality rate | 12–15% | 20% |
In early-onset GBS, there is a direct relationship between the rate of neonatal attack and the size of the inoculum and number of colonized neonatal sites.8 The serotype distribution of early-onset neonatal infection reflects the serotype distribution of maternal colonization, and a 90% concordance is present between recovery of the same serotype from the infant and the mother. With early-onset meningitis, however, more than 80% of the neonatal isolates are serotype III. Early-onset infection usually manifests as rapid-onset septicemia or pneumonia. Approximately 30% of infected neonates have concomitant meningitis. Acute respiratory distress is the initial manifestation of virtually all neonatal pneumonia. The pulmonary infection may be radiographically indistinguishable from respiratory distress syndrome (RDS), and at least one half of infected neonates are initially diagnosed with RDS. The clinical manifestations that help distinguish GBS sepsis from RDS include neutropenia, unexplained severe apnea, poor peripheral vascular perfusion and shock, and lower peak inspiratory pressures on a respirator than are usually present with RDS. The identification of gram-positive cocci in gastric aspirate has not been a useful diagnostic test to distinguish GBS infection from RDS. GBS identification on blood or cerebrospinal fluid culture takes a minimum of 24 hours. Thus, the confirmation of infection may be delayed among infants with atypical clinical manifestations. A delay in diagnosis that leads to a delay in therapy further increases infant mortality. Recent estimates of the overall mortality rate from early-onset infection is 12% to 15%.4
Late-onset infections occur in infants after the first week of life. The mean age at onset is 24 days.32 The overall attack rate is estimated to be 0.4 in 1000 live births.4 In contrast to early-onset infection, horizontal transmission through nosocomial pathways appears to be an important factor in late-onset infection. The serotype distribution of strains recovered from late-onset infection does not reflect the serotypes present in the maternal genital tract; more than 90% of late-onset infection is caused by type III GBS.18 In more than 80% of neonates with late-onset infection, the disease manifests as meningitis, which has a mortality rate of approximately 20%.32 Between 15% and 30% of survivors have neurologic sequelae, including cortical blindness, diabetes insipidus, deafness or other cranial nerve deficits, and spasticity. Although the majority of late-onset infection occurs as meningitis, other manifestations include septic arthritis, osteomyelitis, empyema, endocarditis, cellulitis, and otitis media.
Maternal Infection
GBS is also an important pathogen in maternal intrapartum and postpartum infections. The incidence of puerperal septicemia due to GBS is approximately 1 to 2 in 1000 deliveries.33,34 In one study, GBS was isolated from 15% of positive blood cultures taken from postpartum patients.33 In a similar proportion of women with postpartum endometritis, GBS was isolated from the endometrium.35 Yet another study found that, despite the administration of antibiotic prophylaxis, endometritis usually developed in women who were initially found to have GBS in the endometrium during cesarean section.36
GBS is also associated with clinical AF infection following ROM. GBS was the most frequent pathogenic facultative isolate recovered from AF (12% of 67 total isolates), followed by E. coli (10% of 67 isolates).37 Bacteroides species and other anaerobes accounted for 58% of all isolates from infected patients, emphasizing the polymicrobial, mixed facultative-anaerobic microbiology of AF infection.
GBS has also been associated with preterm ROM and with preterm delivery before 32 weeks' gestation. In one prospective study, cervical GBS colonization was present in 24.6% of all patients with preterm ROM and in 38% of preterm deliveries before 32 weeks.38 However, differences in age, parity, and demographic factors, as well as the coexistence of other microorganisms associated with adverse pregnancy outcome between the groups, were not ascertained. A high concentration of GBS (3 to 4+ semiquantitative levels) found in 2% of pregnant women was associated with an increased rate of delivery at less than 37 weeks' gestation.5 GBS bacteriuria is also associated with high concentrations of GBS in the genital tract. GBS bacteriuria has been related to preterm delivery and preterm ROM.30,39 Treating GBS-positive pregnant women with erythromycin, however, has not reduced preterm delivery,40 and antepartum treatment of GBS to reduce preterm delivery or preterm ROM is not currently recommended. Antibiotics fail to eliminate GBS from the maternal genital tract, the impact of GBS on prematurity is probably small, and until better data are available on strategies to reduce GBS and preterm delivery, most attention should be directed toward the prevention of neonatal GBS sepsis.
Penicillin or ampicillin remains the drug of choice for GBS infections of the mother. Almost all strains of GBS are very sensitive to penicillin, with minimum inhibitory concentrations within the range of easily achievable serum and tissue levels. Both postpartum endometritis and AF infection, however, frequently represent a polymicrobial infection involving more antibiotic-resistant facultative or anaerobic bacteria. Thus, a broader spectrum antibiotic with anaerobic activity, or combination antimicrobial therapy, is often used to treat maternal GBS infection.
PREVENTION STRATEGIES
Prevention strategies have focused on early-onset GBS infection. Maternal immunization is an attractive strategy because maternal IgG is transferred across the placenta and appears to offer the neonate protection from GBS.24,25,37 The main defense against GBS is antibody-dependent phagocytosis. Vaccines that produce antibodies against the capsule of GBS have been made.41 Unfortunately vaccination to prevent GBS neonatal sepsis needs to overcome several obstacles to have a place in clinical practice. Antibody levels can be achieved in the majority of vaccines, but some vaccines do not induce antibody development.42 Important genetic determinants of the antibody response may limit the effectiveness of these polysaccharide vaccines. In a study of allotype frequencies in Sweden, some women were found to have a genetic deficiency in their ability to produce IgG antibodies against many bacterial polysaccharides.43 A large vaccination trial is needed to resolve this issue. In addition, the impact of protective antibody may be limited for premature infants because transport of antibody across the placenta is reduced before 32 weeks' gestation. Conversely, immunoprophylaxis appears to offer a rational approach to control GBS disease without the need for antibiotic prophylaxis.
Antibiotics given several weeks before labor has not reduced the maternal GBS colonization rate when labor begins,10,44 probably because the genital tract becomes recolonized with GBS from the rectal reservoir. Postnatal penicillin given to infants at birth has limitations because 70% of the neonates with early-onset infection are infected before birth.22 Preterm neonates are particularly likely to be infected before birth, and postnatal penicillin given to preterm infants did not reduce early-onset GBS infection.45 In another study, penicillin given at birth significantly reduced the rate of GBS sepsis, but the overall sepsis rate was not reduced in neonates who received penicillin because they had an increased rate of infection from penicillin-resistant bacteria.46,47
Thus, the focus in preventing early-onset GBS infection has been on giving intrapartum antibiotics after the onset of labor or ROM. Two randomized studies found that antibiotics significantly reduced early-onset GBS sepsis in high-risk groups with GBS48 and in heavily colonized neonates.49 Three other nonrandomized antibiotic trials also found a significant reduction in early-onset infection when intravenous ampicillin was given to GBS-colonized women with preterm labor or ROM lasting more than 12 hours.48,50,51 In the largest randomized trial, early-onset GBS neonatal infection was reduced in the treated compared to the control group (0% versus 6%, p < 0.02).48 Neonates given intrapartum ampicillin were continued on ampicillin after birth. It was estimated that at least 50% of early-onset GBS infection could be prevented by this strategy. For effective intrapartum antibiotic prophylaxis, however, the following steps are necessary: GBS identification, risk identification, and protocol institution.
In 1992, two strategies to help prevent neonatal GBS sepsis were published by the American College of Obstetrics and Gynecology (ACOG)52 and the American Academy of Pediatrics (AAP).53 The AAP strategy has not been widely accepted because cultures recommended at 26 to 28 weeks' gestation were poor predictors of exposure to GBS at term. Recently, a strategy was published by the Centers for Disease Control (CDC) that includes a culture at 35 to 37 weeks' gestation.4 These strategies are compared in Table 4.
TABLE 4. Comparison of Antibiotic Prophylaxis Strategies and Estimated Prevention of Early—Onset Neonatal GBS Sepsis
High—Risk Groups | |||||||||
|
|
|
|
| Prior |
| Weeks | Pregnancies | Early |
| Labor | ROM | ROM | Temperature | Pregnancy | GBS | Screen | Receiving | GBS |
Strategy | <37 wk | <37 wk | at Term | in Labor | with GBS | Bacteriuria | for GBS | Antibiotics* | Prevented* |
CDC4 | + | + |
|
| + | + | 35–37 | 27% | 86% |
ACOG53 56 57 | + | + |
|
| + | + | None | 18% | 69% |
AAP54 | + | + | >12 h |
| + | No (twins) | 26–28 | 3% | 51% |
*Estimated in Rouse et al.55
CDC = Centers for Disease Control; ACOG = American College of Obstetricians and Gynecologists;
AAP = Association of American Physicians; ROM = rupture of membranes; GBS = group B streptococcus.
Identification
As mentioned, GBS colonization during pregnancy is not stable. The closer to delivery the GBS culture is performed, the more predictive it will be as to the risk of neonatal exposure at delivery. The culture result, however, needs to be available by the time of labor. Thus, 35 to 37 weeks is advised to perform cultures to identify patients with GBS at term. This approach leaves unscreened those pregnancies that deliver prematurely.
The appropriate site to culture is the rectum and vaginal introitus because these sites are most likely to contain GBS.4 The vaginal introital sample should be obtained without a speculum examination, and the same swab can be introduced into the rectum and sent as a single culture. Commercially available selective broth media (SBM broth or Lim broth) should be used. The CDC has provided in detail the technique of culture and the laboratory methods to use.4
High-Risk Group Identification and Strategy
The AAP recommended that the following risk factors be identified because of results from the only randomized treatment trial that used risk factors48: labor or ROM occurring at less than 37 weeks' gestation, ROM occurring more than 12 hours before delivery, and maternal temperature greater than 37.5°C.53 In addition, intrapartum antibiotics were advocated for women with GBS-positive pregnancies with a prior neonate with GBS infection and for those with multiple gestations.
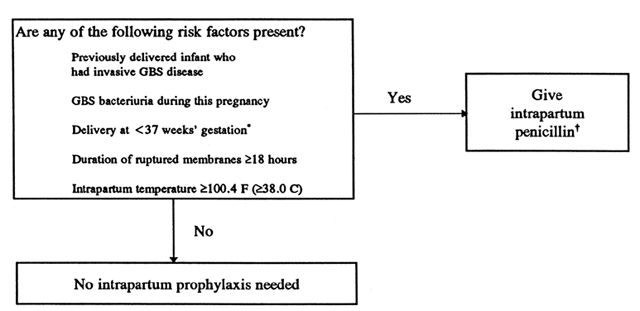
The ACOG guidelines were slightly different than those developed by the AAP. ACOG did not recommend routine GBS screening at any time during pregnancy. Intrapartum antibiotics were recommended for the following: preterm labor or ROM at less than 37 weeks, ROM lasting 18 hours or longer, fever equal to or greater than 38°C during labor, and a previous neonate with symptomatic GBS infection (Fig. 2).52 This strategy has not been tested for efficacy, and it does not include low-risk pregnancies with GBS at term.
GBS-positive pregnancies at term without risk factors are expected to represent approximately 30% of cases of neonatal GBS infection.54 A nonrandomized trial suggested the efficacy of treating all women screened and found positive for GBS.50 Thus, a CDC-appointed committee recommended a combined strategy of (1) treating all unscreened, preterm, high-risk pregnancies (preterm labor or ROM at less than 37 weeks, prior pregnancy with GBS infection, GBS bacteria in the current pregnancy); (2) screening at 35 to 37 weeks; and (3) treating all women in labor who were colonized with GBS at 35 to 37 weeks irrespective of the risk for GBS infection. This strategy has not been subjected to an antibiotic trial, but it is estimated to prevent the greatest number of GBS infections (see Table 4). It also results, however, in the largest percentage of pregnant women receiving antibiotics (see Table 4). Several nuisances exist in this strategy. All high-risk preterm pregnancies regardless of knowledge of GBS status, and all term pregnancies with GBS irrespective of high-risk factors, would be treated. Antibiotic prophylaxis for pregnancy with ROM at less than 37 weeks' gestation, in the absence of labor, could be started and continued until the GBS culture result is known or withheld until the culture result is known to be positive. This, of course, is also an option with the ACOG and AAP strategies, because antibiotics would be given to all women with GBS-positive term pregnancies at greater than 37 weeks who had a fever equal to or greater than 38°C during labor, or ROM lasting more than 18 hours. These three strategies are compared in Table 4.54,55,56
Although strategies differ, the adoption of one of these strategies to prevent early-onset GBS sepsis is advocated,4 preferably the CDC or ACOG strategy because they are estimated to prevent the greatest percentage of neonatal infections. All of these strategies are estimated to be cost-effective. A full discussion of these strategies is provided in the CDC recommendation.4
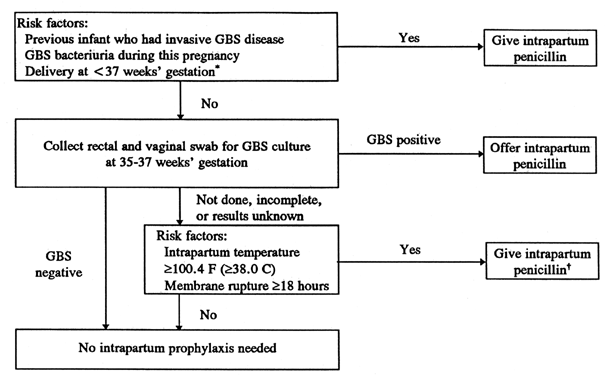
Women with GBS bacteriuria during pregnancy should be treated at the time of diagnosis, and because these women are usually heavily colonized with GBS, they should also be treated during labor without further screening. Likewise, women who previously delivered an infant with neonatal GBS infection should not be screened for GBS, but should be treated in labor (see Fig. 2; Fig. 3). Unscreened pregnancies or known GBS-positive pregnancies with the major risk factors (see Table 4) should also be treated (see Fig. 2 and Fig. 3). According to the CDC strategy, all pregnancies with a positive GBS culture at 35 to 37 weeks should also be treated at the onset of delivery, regardless of risk factors (see Fig. 3).
The recommended antibiotics and doses are listed in Table 5. Penicillin is recommended over ampicillin because it may cause less resistance in other bacteria. Penicillin-allergic patients should receive clindamycin or erythromycin. Patients with fever during labor with an unknown GBS status should receive gentamicin to inhibit Enterobacteriaceae, including E. coli, in addition to a penicillin.
TABLE 5 Antibiotic Protocol for Intrapartum GBS Prophylaxis
Recommended: penicillin G | 5 million IV load |
| 2.5 million units IV every 4 hours until delivery |
Alternative: ampicillin | 2 g IV load |
| 1 g IV every 4 hours until delivery |
Penicillin allergy: |
|
Recommended: clindamycin | 900 mg IV every 8 hours until delivery |
Alternative: erythromycin | 500 mg IV every 6 hours until delivery |
(Adapted from Schuchat A, Whitney C, Zangwell K: Prevention of perinatal group B streptococcal disease: A public health perspective. MMWR 45:1, 1996)
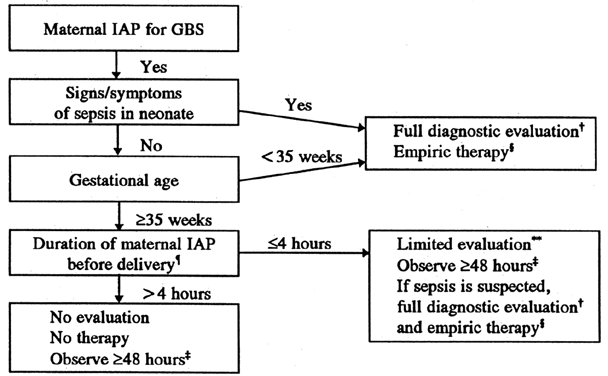
An important aspect of early-onset GBS infection prevention is the administration of antibiotics to premature and symptomatic neonates after delivery. Asymptomatic infants at 35 weeks' gestation or older whose mothers received antibiotic prophylaxis 4 hours before delivery can be observed for 48 hours and not evaluated or treated. If antibiotics were given to the mother 4 hours before delivery, a limited evaluation with a blood count and blood culture and observation for 48 hours would be recommended. Symptomatic neonates with suspected sepsis should receive a full evaluation (blood count, blood culture, chest x-ray, and perhaps a spinal tap) and antibiotic therapy (Fig. 4). A word of caution: this algorithm has not been field tested, because all neonates were treated after delivery in the one randomized antibiotic trial.48
Several issues about antibiotic prophylaxis require comment. First, none of the antibiotic prophylaxis strategies presented in Table 4 is going to be totally protective against early-onset GBS infection. Approximately 14% of GBS infection still would be expected with the CDC-recommended strategy. Some of this would be related to the fact that the culture method is not 100% effective and that approximately 3% of women cultured at 35 to 37 weeks are found to have GBS during labor. The impact of antibiotic prophylaxis in late-onset GBS disease is unknown, but because late-onset infection is usually acquired from nonmaternal horizontal GBS transmission, its impact may be small. The institution of these strategies requires complex communication for successful implementation. In one report, 37% of more than 2000 pregnancies had at least one risk factor, but 13% of women with high-risk pregnancies did not receive intrapartum antibiotics.57 This was similar to 20% of GBS-positive pregnant women who did not receive intrapartum antibiotics in a university practice setting.58 Patients who do not receive prenatal care or those who deliver rapidly before antibiotics can be administered are particularly likely to not receive antibiotics. However, failure to administer antibiotics despite the presence of risk factors and/or GBS colonization attests to the difficulty of implementing routine treatment to all eligible pregnant women.
A strategy that uses antepartum cultures requires additional coordination to ensure that cultures are performed and reported in a timely manner and that reports are available 24 hours/day.4 It was also found that the availability of culture results before delivery prompted up to one third of physicians to treat GBS in the genital tract of the antepartum patient before labor, even though they knew this treatment to be ineffective.59 This practice must be discouraged.
Penicillin would be expected to result in a mild allergic reaction in 10% of women, anaphylaxis in approximately 1 in 10,000,60 and death from anaphylaxis in 1 in 100,000.61 Although serious penicillin reactions are uncommon, a second direct problem of antibiotics is the potential for the production of drug-resistant bacterial strains. Antibiotic resistance would not be expected in GBS (which is exquisitely sensitive to penicillin), but it would be expected in other bacteria. The possible administration of even short-term antibiotics in up to 30% of all pregnancies4 (see Table 4) would be expected to produce resistance to bacteria that could colonize hospitals and eventually lead to clinically significant problems due to antibiotic-resistant sepsis.
Strategies aimed at GBS infection would have little impact on penicillin-resistant bacteria.47 In fact, the majority of sepsis cases in newborns are not caused by GBS, but rather by penicillin-resistant E. coli, other Enterobacteriaceae, Staphylococcus aureus, and anaerobic bacteria.
Finally, intrapartum antibiotic administration has a major impact on the clinical management of the newborn.57 In some instances, additional diagnostic tests or prolonged observation in the hospital is given to antibiotic-exposed neonates. These additional measures increase hospital costs. Although a reasonable guide is provided in the CDC recommendations to follow the neonate (see Fig. 4),4 this algorithm has not been extensively tested. The algorithm is subject to a variety of management interpretations, and it may take years to know the impact of these variations on the prevention of neonatal GBS sepsis.
REFERENCES
Schwartz B, Schucket A, Oxtoby MJ et al: Invasive group B streptococcal disease in adults: A population based study in metropolitan Atlanta. JAMA 266: 1112, 1991 |
|
Hood M, Janney A, Dameron G: Beta-hemolytic streptococcus group B associated with problems of the perinatal period. Am J Obstet Gynecol 82: 809, 1961 |
|
Eickhoff TC, Klein JO, Daly AK et al: Neonatal sepsis and other infections due to group B beta-hemolytic streptococci. N Engl J Med 271: 1221, 1964 |
|
Schuchat A, Whitney C, Zangwell K: Prevention of perinatal group B streptococcal disease: A public health perspective. MMWR 45: 1, 1996 |
|
Regan JA, Klebanoff MA, Nugent RP: The epidemiology of group B streptococcal colonization in pregnancy. Obstet Gynecol 77: 604, 1991 |
|
Boyer KM, Gadzala CA, Kelly PD et al: Selective intrapartum chemoprophylaxis of neonatal group B streptococcal early-onset disease: II. Predictive value of prenatal cultures. J Infect Dis 148: 802, 1983 |
|
Hoogkamp-Korstanje JAA, Gerards LJ, Cats BP: Maternal carriage and neonatal acquisition of group B streptococci. J Infect Dis 145: 800, 1982 |
|
Pass MA, Gray BM, Khare S et al: Prospective studies of group B streptococcal infections in infants. J Pediatr 95: 437, 1979 |
|
Merenstein GB, Todd WA, Brown G et al: Group B hemolytic streptococcus: Randomized controlled treatment study at term. Obstet Gynecol 55: 315, 1980 |
|
Gardner SE, Yow MD, Leeds LJ et al: Failure of penicillin to eradicate group B streptococcal colonization in the pregnant woman. Am J Obstet Gynecol 135: 1062, 1979 |
|
Franciosi RA, Knostman JD, Zimmerman RA: Group B streptococcal neonatal and infant infections. J Pediatr 82: 707, 1973 |
|
Dillon HC, Gray E, Pass MA et al: Anorectal and vaginal carriage of group B streptococci during pregnancy. J Infect Dis 195: 794, 1982 |
|
Yow MD, Leeds LJ, Thompson PK et al: The natural history of group B streptococcal colonization in the pregnant woman and her offspring. Am J Obstet Gynecol 137: 34, 1980 |
|
Anthony BF, Okada DM, Hobel CJ: Epidemiology of group B streptococcus: Longitudinal observations during pregnancy. J Infect Dis 137: 524, 1978 |
|
Allardice JG, Baskett TF, Seshia MMK et al: Perinatal group B streptococcal colonization and infection. Am J Obstet Gynecol 142: 617, 1982 |
|
Matorras R, Garcia-Perea A, Vsandizago JA, Ominaca F: Natural transmission of group B streptococcus during delivery. Int J Gynaecol Obstet 30: 99, 1989 |
|
Ferrieri P, Cleary PP, Seek AE: Epidemiology of group B streptococcal carriage in pregnant women and newborn infants. J Med Microbiol 10: 103, 1977 |
|
Baker CJ, Barrett FF: Transmission of group B streptococci among parturient women and their neonates. J Pediatr 83: 919, 1973 |
|
Christensen KK, Dahlander K, Ekstrom A et al: Colonization of newborns with group B streptococci: Relation to maternal urogenital carriage. Scand J Infect Dis 13: 23, 1981 |
|
Anthony BF, Okada DM, Hobel CJ: Epidemiology of the group B streptococcus: Maternal and nosocomial sources for infant acquisitions. J Pediatr 95: 431, 1979 |
|
Ancona RJ, Ferrieri P, Williams PP: Maternal factors that enhance the acquisition of group B streptococci by newborn infants. J Med Microbiol 13: 273, 1980 |
|
Boyer KM, Gadzala CA, Burd LI et al: Selective intrapartum chemoprophylaxis of neonatal group B streptococcal early-onset disease: I. Epidemiologic rationale. J Infect Dis 148: 795, 1983 |
|
Yancey MK, Duff P, Clark P et al: Peripartum infection associated with vaginal group B streptococcal colonization. Obstet Gynecol 84: 816, 1994 |
|
Baker CJ, Kasper DL: Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med 294: 753, 1976 |
|
Baker CJ, Edwards MS, Kasper DL: Role of antibody to native type III polysaccharide of group B streptococcus in infant infection. Pediatrics 68: 544, 1981 |
|
Vogel LC, Boyer KM, Gadzala CA et al: Prevalence of type-specific group B streptococcal antibody in pregnant women. J Pediatr 96: 1047, 1980 |
|
Hellerqvist CG, Fojas J, Green RS et al: Studies on group B b-hemolytic streptococcus: I. Isolation and partial characterization of an extracellular toxin. Pediatr Res 15: 892, 1981 |
|
Zangwill KM, Schuchat A, Wenger JD: Group B streptococcal disease in the United States, 1990: Report from a multistate active surveillance system. In CDC surveillance summaries (November 20). MMWR 41 (SS-6):25, 1992 |
|
Baker CJ: From the National Institutes of Health: Summary of the workshop on perinatal infections due to group B streptococcus. J Infect Dis 136: 137, 1977 |
|
Moller M, Thomsen AC, Borch K et al: Rupture of fetal membranes and premature delivery associated with group B streptococci in urine of pregnant women. Lancet ii:69, 1984 |
|
Faxelius G, Bremme K, Christensen KK et al: Neonatal septicemia due to group B streptococci: Perinatal risk factors and outcome of subsequent pregnancies. J Perinat Med 16: 423, 1988 |
|
Baker CJ: Group B streptococcal infections in neonates. Pediatr Rev 1: 5, 1979 |
|
Blanco JD, Gibbs RS, Costaneda YS: Bacteremia in obstetrics: Clinical course. Obstet Gynecol 58: 621, 1981 |
|
Pass MA, Gray BM, Dillon HC Jr: Puerperal and perinatal infections with group B streptococci. Am J Obstet Gynecol 143: 147, 1982 |
|
Rosene KA, Eschenbach DA, Tompkins LS et al: Polymicrobial early postpartum endometritis with facultatively anaerobic and anaerobic bacteria, genital mycoplasmas and Chlamydia trachomatis: Treatment with piperacillin or cefoxitin. J Infect Dis 153: 1028, 1986 |
|
Watts DH, Hillier SL, Eschenbach DA. Upper genital tract isolates at delivery as predictors of post cesarean infections among women receiving antibiotic prophylaxis. Obstet Gynecol 77:287, 1991 |
|
Gibbs RS, Blanco JD, St Clair PJ et al: Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis 145: 1, 1982 |
|
Regan JA, Chao S, James LS: Premature rupture of membranes, preterm delivery, and group B streptococcal colonization of mothers. Am J Obstet Gynecol 141: 184, 1981 |
|
Thomsen AL, Morup L, Hansen KB: Antibiotic elimination of group B streptococci in urine in prevention of preterm labour. Lancet 1: 591, 1987 |
|
Regan JA, Klebanoff, Nugent RP et al: Colonization with group B streptococci in pregnancy and adverse outcomes. Am J Obstet Gynecol 174: 1354, 1996 |
|
Schuchat A, Wenger JD: Epidemiology of group B streptococcal disease: Risk factors, prevention strategies and vaccine development. Epidemiol Rev 16: 374, 1994 |
|
DeCueninck BJ, Eisenstein TK, McIntosh TS et al: Quantitation of in vitro opsonic activity of human antibody induced by a vaccine consisting of the type III-specific polysaccharide of group B streptococcus. Infect Immun 39: 1155, 1983 |
|
Christensen KK, Christensen P, Hdgerstrand I et al: The clinical significance of group B streptococci. J Perinat Med 10: 133, 1982 |
|
Hall RT, Barnes W, Krishnan L et al: Antibiotic treatment of parturient women colonized with group B streptococci. Am J Obstet Gynecol 124: 630, 1976 |
|
Pyati SP, Pildes RS, Jacobs NM et al: Penicillin in infants weighing two kilograms or less with early-onset group B streptococcal disease. N Engl J Med 308: 1383, 1983 |
|
Siegel JD, McCracken GHJ, Threlkeld N et al: Single dose penicillin prophylaxis against neonatal group B streptococcal infections: A controlled trial in 18,738 newborn infants. N Engl J Med 303: 769, 1980 |
|
Siegel JD, McCracken GHJ, Threlkeld N et al: Single-dose penicillin prophylaxis of neonatal group B streptococcal disease. Lancet i:1426, 1982 |
|
Boyer KM, Gotoff SP: Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med 314: 1665, 1986 |
|
Tuppurainen N, Hallman M: Prevention of neonatal group B streptococcal disease: Intrapartum detection and chemoprophylaxis of heavily colonized parturients. Obstet Gynecol 73: 583, 1989 |
|
Garland SM, Fliegner JR: Group B streptococcus and neonatal infections: The case for intrapartum chemoprophylaxis. Aust NZ J Obstet Gynaecol 31: 119, 1991 |
|
Morales WJ, Lim D: Reduction of group B streptococcal maternal and neonatal infections in preterm pregnancies with premature rupture of membranes through a rapid identification test. Am J Obstet Gynecol 157: 13, 1987 |
|
American College of Obstetrics and Gynecology: Group B streptococcal infection in pregnancy. ACOG Technical Bulletin no. 170. Washington, DC, ACOG, 1992 |
|
American Academy of Pediatrics: Guidelines for prevention of group B streptococcal infection by chemoprophylaxis. Pediatrics 90:775, 1992 |
|
Rouse DJ, Goldenberg RL, Cliver SP et al: Strategies for the prevention of early-onset neonatal group B streptococcal sepsis: A decision analysis. Obstet Gynecol 83: 483, 1994 |
|
Hankins GV, Chalas E: Group B streptococcal infections in pregnancy: ACOG's recommendations. ACOG Newsletter 37: 2, 1993 |
|
American College of Obstetricians and Gynecologists. Survey shows continued confusion over management of GBS in pregnancy. ACOG Newsletter 38:1, 1994 |
|
Pylipow M, Gaddis M, Kinney JS: Selective intrapartum prophylaxis for group B streptococcus colonization: Management and outcome of newborns. Pediatrics 93: 631, 1994 |
|
Gibbs RS, McDuffie RS, McNabb F et al: Neonatal group B streptococcal sepsis during 2 years of a universal screening program. Obstet Gynecol 84: 496, 1994 |
|
Jafari HS, Schuchat A, Hilsdon R et al: Barriers to prevention of perinatal group B streptococcal disease. Pediatr Infect Dis J 14: 662, 1995 |
|
Goodman LS, Gilman A, Gilman AG (eds): Goodman and Gilman's the Pharmacologic Basis of Therapeutics, 8th ed. New York, Pergamon, 1990 |
|
Schwartz B, Jackson L: Invasive group B streptococcal disease in adults. JAMA 266: 3284, 1991 |


 7 days
7 days