Steroid Receptors and Selective Estrogen Receptor Modulation in Mammary and Gynecologic Malignancy
Authors
INTRODUCTION
Malignancy of the breast, ovary, and endometrium is linked to endogenous hormones. Observations over the past 100 years have established this causal relationship. In 1900, Beatson and Boyd independently established that the removal of the ovaries from premenopausal women with metastatic breast cancer would, roughly one third of the time, cause regression of disease with improved prognosis.1, 2 Therefore, an ovarian factor was implied in the promotion of carcinogenesis.
Allen and Doisey identified the “estrus stimulating principle”, endogenous estrogens that could activate tumors of the breast, and subsequent research in synthetic chemistry identified nonsteroidal estrogens.3 Dodds and associates described an extremely potent estrogen in animals, diethylstilbestrol (DES),4 but Haddow and colleagues5 first tested high-dose DES as a treatment for advanced breast cancer in postmenopausal women. Use of high-dose estrogen treatment is somewhat paradoxical, but effective in one third of patients. The mechanism of action is unknown. Alternatively, reducing endogenous steroids by adrenalectomy improves prognosis of some postmenopausal women in similar proportions to oophorectomy.6 It is known that adrenal androstenedione and testosterone are converted to estrone and estradiol by peripheral aromatase enzyme systems.
Numerous studies have linked breast cancer risk to prolonged, unopposed exposure to estrogen as in early menarche,7 late menopause,8, 9 and increased age at first pregnancy.10 Other hormonal risk factors are less well established. Reports on abortion and the risk of breast cancer have been equivocal.11, 12, 13, 14 Studies of the effect of lactation on breast cancer risk have suggested that a longer duration of lactation reduces risk in premenopausal women.15 Postmenopausal obesity has been shown to increase risk.16, 17
Similarly, obesity18 is a well-defined risk factor for endometrial cancer stemming from increased availability of peripheral estrogens especially in postmenopausal women with a compounding lack of progesterone.19, 20 Estrogen is a proliferative hormone, whereas progesterone is a differentiating hormone in the uterus. Perhaps most important, periodic withdrawal bleeding sheds any malignant epithelial cells. Analogous factors related to high or unopposed estrogen levels also elevate the risk of endometrial cancer; these include early menarche, late menopause,21, 22 nulliparity,23 and unopposed estrogen replacement therapy.23, 24
The effects of exogenous hormones in the form of oral contraceptives and hormone replacement therapy (HRT) on breast cancer have been studied extensively. Some studies suggest that the long-term use of oral contraceptives in young women before their first pregnancy may increase breast cancer risk.25, 26 Two meta-analyses of this effect demonstrate small but statistically significant increases in risk for HRT users.27, 28 Steinberg and coworkers27 noted that after five years of estrogen use there is a proportional yearly increase in risk, whereas Sillero-Arenas and associates28 did not observe a significant association between duration of HRT and breast cancer risk. The Iowa Women's Health Study found a longer duration of HRT was associated with increased risk of invasive carcinoma with a favorable histology, with a relative risk (RR) of 1.81 for HRT use of less than five years duration versus a RR of 2.65 for use longer than five years.29 Even so, women who develop breast cancer while using HRT at the time of diagnosis have a similar prognosis in terms of type, size, or grade of tumor as that of nonusers.30 In breast epithelia, progesterone, like estradiol, also has a strong proliferative effect. Progesterone accelerates the appearance, growth rate, and number of tumors in carcinogen-induced rat mammary cancers.31 Furthermore, use of progestins in combination with estradiol for HRT has been linked with a higher risk of developing breast cancer than use of estradiol alone.32
DISCOVERY OF THE ESTROGEN RECEPTOR
In 1962, Jensen and Jacobson33 demonstrated that [3H] estradiol bound to and was retained by estrogen target tissues including the uterus, vagina, and pituitary gland in the female rat. In contrast, tissues that did not respond to estrogen did not retain [3H] estradiol. Jensen33 proposed that an estrogen receptor (ER) in estrogen target tissues must capture circulating steroids and initiate the cascade of biochemical events associated with estrogen action in that particular tissue. Toft and respective coworkers in two studies34, 35 first identified the ER as an extractable protein from the rat uterus. Subsequently, Gorski and associates36 and Jensen and coworkers37 independently proposed subcellular models of estrogen action in target tissues. Jensen and associates38 then proposed the clinical ER assay to predict hormone responsive breast cancer. This established a mechanistic link between estrogen action and the growth of breast cancer.
Monoclonal antibodies to the ER subsequently established that the ER is a nuclear protein,39 and the technology of immunocytochemistry is now standard for the determination of receptor status in breast biopsies.40, 41 The ER status of the patient is highly predictive of a treatment response to endocrine ablative surgery42 or antiestrogen therapy.43
Similar experimental techniques have been used with human endometrial tissue. The uptake of radiolabeled estrogen is highest during the first two weeks of the menstrual cycle,44 that is, during the time of unopposed estrogen action. In addition, quantitation of the human ER protein revealed that concentrations are markedly higher during the proliferative phase than in the secretory phase.45, 46, 47, 48 In contrast, uptake of radiolabeled progesterone is higher in the secretory phase than in the proliferative phase of the menstrual cycle.49 The progesterone receptor (PgR) level is low during the proliferative phase, peaks at midcycle, and becomes lower again during the secretory phase.46, 48
Endometrial cancers contain ERs and an inverse relationship exists between ER levels and tumor grade.50 However, cytosol receptor assays are inherently inaccurate in endometrial carcinoma because of the contribution from normal stromal and myometrial cells, which also contain receptors.51, 52, 53 Development of monoclonal antibodies to the PgRs allowed the quantitation of hormone receptor levels in endometrial cancer.54
Molecular cloning of the cDNAs for the estrogen55 and progesterone receptors56, 57 led to the realization that these proteins are members of the steroid and thyroid hormone receptor superfamily of ligand-responsive transcription factors.58 Both receptors are encoded by eight exons, which correspond to domains of functional significance.59, 60, 61
Biologic effects of estrogen are mediated by two receptors, ERα and ERβ.62, 63 These two ERs share a conserved structure with six functional domains, A to F. ERβ is homologous to ERα at the ligand-binding domain (58%) and DNA-binding domain (95%). The remaining domains are not well conserved.64 Discovery of ERβ has advanced our understanding of estrogen signaling and may explain tissue responses to estrogen in which ERα is undetectable.65 Furthermore, the existence of ERα and β subtypes provides a possible explanation for the tissue selectivity of selective ER modulators (SERMs).
CLINICAL VALUE OF RECEPTOR STATUS
The responsiveness of neoplastic tissues to steroid hormones and antiestrogen therapy correlates with hormone receptor concentration in target cells. Neoplastic cells that retain a full or partial amount of steroid receptors are expected to respond to endocrine therapy whereas cells lacking receptors do not. Response to endocrine therapy in breast cancer correlates with levels of ER and PgR in the tumor,43 which makes receptor status useful in the management of breast cancer. In general, women who are ER- and PgR-positive have a longer disease-free interval and longer survival time than women with receptor-negative tumors.66, 67
More than three fourths of endometrial carcinomas contain ER and PgR.50, 68, 69, 70, 71, 72, 73 ER levels in hyperplastic and neoplastic tissues are comparable with those found in early proliferative endometrium.74 Ehrlich and coworkers71, 75 found a correlation between the response of patients with advanced endometrial cancer to progestin therapy and the presence of PgR. Early stage endometrial cancers are more likely to be receptor-positive and have higher receptor levels than more advanced tumors.68 Receptor concentrations are greater in well or moderately differentiated tumors than in anaplastic carcinomas. Receptor-poor tumors are generally more aggressive with decreased five-year survival rates.68 Positive hormone receptor status is associated with improved disease-free and overall survival rates.13, 76, 77 When considered separately, positive PgR status in endometrial cancer appears to represent a more reliable predictor of prognosis than positive ER status.71, 72, 78, 79 These studies suggest that there is a tendency for PgR-positive status to correlate with early stage tumors, that is, type I endometrial carcinoma. Receptor status does not correlate with invasion and metastasis found in advanced stages of disease.
MOLECULAR MECHANISMS OF THE ESTROGEN RECEPTOR
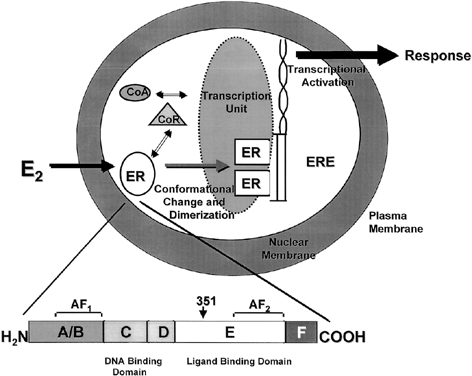
Both ERα and ERβ contain activation functions (AFs) that contribute to the ER's transcriptional activity. AF-1 is located in the amino-terminal region within the A/B region and is believed to be constitutively active and ligand-independent in ERα80, 81 (Fig. 1). AF-2 is present at the carboxy-terminus, and is believed to be ligand dependent. Mutational analyses demonstrate the importance of this region for ER transcription because AF-2 can interact with a number of transcriptional coactivators in a ligand-dependent manner.82, 83 AF-1 and AF-2 can each activate transcription independently but in most cases they synergize with one another as promoters within the specific cell context. The ER activates gene expression through binding to estrogen response elements (EREs) in responsive genes through the action of AF-1 and AF-2.84 ERβ also activates transcription of target genes through EREs.85 Additionally, estrogen can induce an AP-1 site in a reporter construct through ERα, but it is inactive through ERβ. Interestingly, ER antagonists activate ERβ to induce activity through an AP-1 site.86 These two receptors, ERα and ERβ, form functional heterodimers on DNA and stimulate transcription of a target gene. The existence of two rather than one ER portends a more complex mechanism of action than previously thought and remains a primary therapeutic target for new and existing SERMs.
One of the functional areas of the ER, the E region, is the steroid-binding domain that undergoes a conformational change on binding with estrogen, locking the ligand into the hydrophobic pocket of the receptor. This allows the ER to dissociate from heat shock proteins, dimerize, and bind to specific DNA sequences of estrogen responsive genes. Changes in conformation of the ER allow for interaction with coregulatory proteins (coactivators or corepressors) that act as signaling intermediates between the ER and the transcriptional machinery.87 The crystal structure of the ligand binding domain of ERα was determined with estradiol88 and DES.89 A key feature of the agonist–receptor complex is the availability of a portion of the ligand binding site of ERα, helix 12, to lock the steroid in the hydrophobic pocket and allow for recruitment of coactivators to the AF2 site. The repositioning of helix 12 after ligand binding is an important mechanism for full estrogen action at ERα.90, 91
The nuclear steroid receptors must associate with other nuclear proteins to form a transcription complex. Several coactivators, such as ERAP16092 and RIP140,93 have been identified based on their ability to interact with the agonist-bound receptor and not to the antagonist-bound receptor at AF-2. However, the SRC-1 protein has been identified and it also interacts with AF-194 and with ERb through phosphorylation of AF-1 by the MAP-kinase signaling cascade.95 It has been demonstrated that SRC-1 and another protein, p300/CBP, contain intrinsic acetyltransferase activity and can interact with other histone acetyl transferases (HATs). Acetylation by SRC-1 of histones bound at specific promoters could be a mechanism by which the AFs of ER and associated coactivators activate transcription of specific genes by enhancing formation of a stable preinitiation complex.96
Antiestrogens are competitive inhibitors of estrogen action; the shape of the ligand is essential to reducing the estrogenic efficacy. The side chain of the antiestrogen prevents helix 12 from closing so that activation cannot occur.88, 97, 98 It is known that tamoxifen silences AF-2 whereas AF-1 remains constituitively activated.81, 99 Other antiestrogens, such as raloxifene, silence both AF-1 and AF-2.100, 101 This is a possible explanation for the promiscuous estrogen-like actions of tamoxifen compared with raloxifene.
TAMOXIFEN
Lerner and coworkers102 described the pharmacologic properties of a low potency nonsteroidal antiestrogen, ethamoxytriphetol (MER 25). In laboratory animals, MER 25 and the related compound MRL 37 demonstrated antifertility actions103, 104, 105stimulating a search for more potent agents for clinical applications. Clomiphene nafoxidine,106, 107, 108 nitromifene109and tamoxifen110, 111 were all the result of that search, but clinical application as postcoital contraceptives was found to be inappropriate. The drugs stimulate ovulation in subfertile women.112 Clomiphene remains available as a profertility agent, inducing ovulation in subfertile women.
Further study revealed that tamoxifen is a SERM. It has both pro-estrogenic and anti-estrogenic effects on different tissues. In the breast, it serves as a competitive inhibitor of ER receptor activity, and therefore inhibits growth of estrogen receptor-positive breast cancers. In the uterus, it has partial agonist effects and therefore is associated with a two to fourfold increase in the incidence of uterine cancer.113, 114, 115, 116, 117
In the early 1970s, tamoxifen was tested successfully as an antiestrogen for the treatment of advanced breast cancer in postmenopausal women.118, 119 Tamoxifen is now the endocrine therapy of choice for the treatment of all stages of ER-positive breast cancer. A five-year course is currently considered to be optimal adjuvant therapy and produces a profound increase in disease-free and overall survival.43 The 1998 Oxford Overview Analysis43 combined 55 randomized trials that began before 1990, comparing adjuvant tamoxifen therapy to no tamoxifen before recurrence. The study population, of 37,000 women with node-positive and node-negative breast cancer, comprised 87% of the known randomized clinical trials with tamoxifen. Of these women, fewer than 8,000 had a very low or zero level of ER, and 18,000 were classified as ER-positive. The ER status of the remaining nearly 12,000 women was unknown. Based on the normal distribution of ER in random populations, the authors estimated that two thirds would be ER-positive.
The recurrence reductions with tamoxifen in ER-positive patients are all significant whereas the therapeutic effect on ER-negative patients is minimal. In five-year trials of tamoxifen treatment, the reductions of recurrence were 43% and 60% for patients with less than and greater than 100 fmol/mg cytosol of ER protein, respectively, translating to reductions in mortality rates of 23% and 36%.
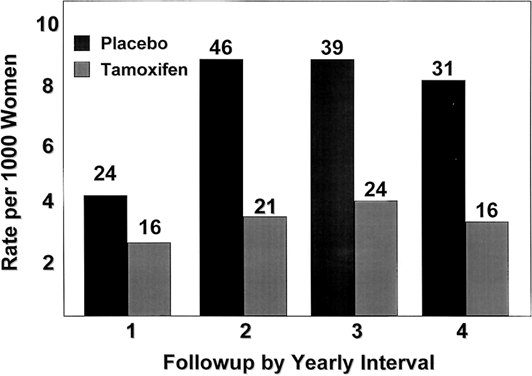
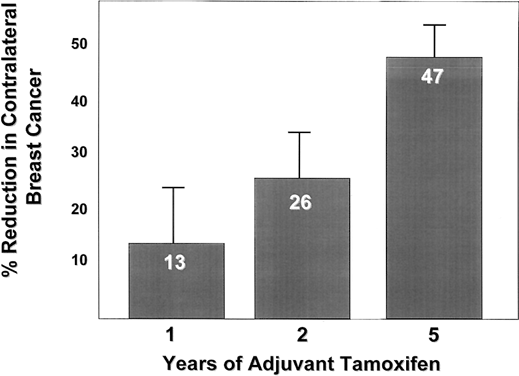
The Overview Analysis also provided unequivocal proof of the laboratory principle that longer tamoxifen adjuvant therapy is more beneficial.120, 121, 122 Duration of therapy is extremely important for the ER-positive premenopausal woman with large amounts of circulating estrogen that can rapidly reverse the effect of short-term tamoxifen treatment (Fig. 2). The benefit of one year of tamoxifen in the premenopausal woman is virtually nonexistent, with a 20-fold increase in effectiveness with five years of therapy. In contrast, the effect of tamoxifen duration on women over the age of 60 is less dramatic because one year of tamoxifen is much more effective in postmenopausal women. The principle that longer therapy is more beneficial than shorter therapy is also demonstrated in the control of contralateral breast cancer (Fig. 3). Tamoxifen has no significant effect if one year of treatment is used but extending therapy to five years produces a 47% reduction in breast cancer incidence. This is a powerful demonstration of the effect of tamoxifen as a chemopreventive.
In 1996, Fischer et al published a study which demonstrated that tamoxifen treatment longer than five years did not appear to improve outcomes.123 In that trial, when therapy was continued beyond five years, there was a decrease in DFS and DDFS. Overall survival was unchanged. There was a higher incidence of thrombembolic events and endometrial cancer in patients who took tamoxifen longer than five years. Since that trial, five years of adjuvant tamoxifen has been considered the optimal duration of therapy. New and contradictory data were recently presented at the 2007 San Antonio Breast Cancer Symposium. The ATLAS trial (Adjuvant Tamoxifen, Longer Against Shorter) found a small, but significant lower annual rate of recurrence in breast cancer patients who took tamoxifen for 10 years (2.9% per year, versus 3.4% per year).124 There was no difference in overall survival. The optimal duration of antiestrogen therapy remains controversial.
SIDE EFFECTS OF TAMOXIFEN
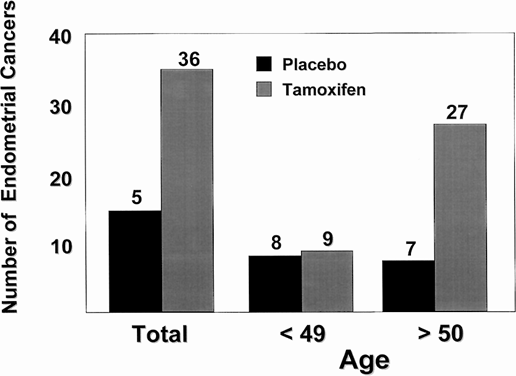
It is well known that tamoxifen produces a partial agonist action in the rat uterus,110 but until recently, little information was available about the actions of tamoxifen in the normal human uterus. Although tamoxifen has been used to treat endometrial cancer,125 the drug causes stromal thickening in the uterus126, 127 and would be expected to encourage growth of preexisting endometrial cancer.128 Tamoxifen treatment is associated with a fourfold increase in the incidence of endometrial carcinoma in postmenopausal women126, 127, 129, 130, 131 (Fig. 4). Most important, the stage and grade of endometrial cancers observed in women taking tamoxifen are the same as those in the general population.129 It is known that the uterus harbors five times the amount of occult disease as is reported clinically.132 Any 'estrogen-like' molecule enhances growth and promotes selection of hormone responsive disease. A recent report by Bernstein and colleagues133 analyzed the incidence of endometrial cancer associated with tamoxifen therapy with known risk factors for the disease. This population of women warrant careful evaluation before the use of tamoxifen. The women most likely to develop endometrial cancer with tamoxifen therapy have previously used HRT and have known risk factors, such as obesity. There is no reported increase in risk of endometrial cancer in premenopausal women133 inasmuch as menstrual cycles continue during tamoxifen treatment for most women.
Another life-threatening side effect of tamoxifen is thromboembolic disease. Women taking tamoxifen have more strokes, pulmonary embolisms, and deep-vein thrombosis than age-matched controls. According to the NSABP B-14, women taking tamoxifen had a 0.9% incidence of thromboembolism, compared to 0.2% in the placebo group.113 One of those patients died of a pulmonary embolism.
In terms of non life-threatening side effects, tamoxifen use results in an elevated risk of cataracts (RR=1.14, 95% CI=1.01–1.29), which is associated with an increased rate of cataract surgery.129 Women taking tamoxifen also have more hot flashes, vaginal dryness, and vaginal discharge.
AROMATASE INHIBITORS
Within the past decade, aromatase inhibitors (AI) have become an option for post-menopausal women with hormone receptor-positive breast cancer. After menopause, a woman's major source of estrogen is through the peripheral conversion of adrenal steroid hormones to estrogen. The AIs inhibit the aromatase enzyme responsible for this conversion, which facilitates a very complete suppression of estrogen.
Over 25 years ago, the first AI, aminoglutethimide, became available. The drug had significant toxicity which limited its clinical use.114 Third generation AIs were evaluated in Phase I and II trials for women with metastatic breast cancer. Once these drugs were shown to be effective in the metastatic setting, they were evaluated in the adjuvant setting. The three AIs currently being used in clinical practice are anastrozole, letrazole, and exemestane.
The landmark trial which established the superiority of AIs over tamoxifen was the ATAC trial (Arimidex, Tamoxifen, Alone or in Combination) published in 2003. This study was a prospective randomized controlled trial that compared adjuvant therapy with tamoxifen versus arimidex, and was limited to postmenopausal women with hormone receptor-positive breast cancer.134 Patients were randomized to either tamoxifen, arimidex, or a combination of both. The combination arm was stopped early because it did not demonstrate any benefit over tamoxifen alone. At four years, arimidex was superior to tamoxifen in terms of DFS (86.9% vs. 84.5% with a HR= 0.86, 95% CI 0.76–0.99, p=0.03). Arimidex was also superior in terms of time to recurrence (TTR), with a HR=0.83 (95% CI 0.71–0.96; p=0.015). There was no statistically significant difference between treatments regarding overall survival (OS).
In 2005, an updated analysis of these data was released with 68 months follow-up.135 Treatment with anastrozole continued to demonstrate a significant improvement in DFS with a HR = 0.87 (95% CI 0.73–0.94) and TTR with a HR = 0.74 (95% CI = 0.64–0.87). There was still no statistically significant difference in overall survival. Fewer patients withdrew from anastrozole treatment than from tamoxifen treatment. The conclusion of the updated analysis was that anastrozole was the treatment of choice for post-menopausal hormone receptor-positive breast cancer patients.
Since the ATAC was published, several other trials have been initiated to study the role of AIs in the treatment of breast cancer. The BIG 1-98 trial compared another AI, letrozole, with tamoxifen and also demonstrated an improved DFS.115 Other trials have been initiated to determine the benefit of adding an AI after an abbreviated course of tamoxifen. Sequential trials are looking at a five-year course of tamoxifen, followed by treatment with an AI. These trials will help to determine the optimal use and sequencing of aromatase inhibitor therapy.
SIDE EFFECTS OF ANASTROZOLE
Arimidex (anastrozole) has a very different side effect profile to tamoxifen. The ATAC trial provided a useful comparison of drug side effects amongst similar groups of patients. Since anastrozole does not have partial agonist effects on the uterus, it is not associated with an increased risk of endometrial cancer. In the ATAC trial, the risk of endometrial cancer with anastrozole was 0.1%, which is comparable to age-matched controls. Accordingly, anastrozole offers a significant risk reduction for this co-morbidity. Anastrozole is associated with a lower risk of thromboembolic events compared to tamoxifen (2.1% vs. 3.5%, p=0.0006). There were also fewer strokes and pulmonary embolisms in the anastrozole group.
Anastrozole was associated with an increased risk of musculoskeletal problems. Arthralgias were much more common and often led to discontinuation of the drug. Osteoporotic fractures were increased with anastrozole use (5.9% vs. 3.7%, p=<0.0001). Although the rate of hip fractures was equivalent, there were more spine and wrist fractures in the anastrozole group. In terms of quality of life, hot flashes, vaginal discharge, and vaginal bleeding were much less common in patients taking anastrozole.
HORMONAL THERAPY IN THE NEOADJUVANT SETTING
The role of neoadjuvant chemotherapy in the treatment of breast cancer was established by the NSABP B-18 and the EORTC 10902 trials.116, 117 The NSABP B-18 randomized patients with primary operable breast cancer to either preoperative or postoperative adriamycin and cytoxan. The conclusion of the study was that an additional 12% of lumpectomies could be performed after neoadjuvant chemotherapy due to downsizing of the tumor, with a 37% increase in the incidence of pathologically negative axillary lymph nodes. The EORTC trial randomized patients to preoperative or postoperative fluorouracil, epirubicin, and cyclophosphamide. The conclusion of this trial was that no difference in overall survival or time to local regional recurrence was found for patients treated in the neoadjuvant setting. Based on these two trials, and subsequently several others, patients with larger primary tumors are offered preoperative chemotherapy to potentially facilitate breast conservation as a surgical option.
Subsequent to the success of neoadjuvant chemotherapy, trials were designed to evaluate the potential role of neoadjuvant hormonal therapy. A study by Eiermann et al compared neoadjuvant letrozole with tamoxifen in hormone receptor-positive patients who either required mastectomy or had locally advanced or inoperable breast cancers.136 Clinical objective response rates (ORR) were better with letrozole than with tamoxifen (55% vs. 36%, p<0.001). More patients in the letrozole group were treated with breast conserving surgery (45% vs. 35%, p<0.022). There have been two trials comparing neoadjuvant anastrozole with tamoxifen, the IMPACT and PROACT trials.137, 138 A combined analysis of these trials failed to demonstrate a significant difference in ORR between the different treatment groups. There was an improvement in ORR for patients treated with anastrozole who were either inoperable or were thought to require a mastectomy (47% vs. 35%, p=0.026). These trials would suggest that aromatase inhibitors may be more effective than SERMs in reducing tumor size. The American College of Surgeons Oncology Group (ACOSOG) and the Cancer Trials Support Unit are currently accruing patients for the Z1031 trial which will compare the effectiveness of letrozole, anastrozole, and exemestane in the neoadjuvant setting. Based on the available data, neoadjuvant endocrine therapy appears to be effective in regard to the ORR and the ability to downstage tumors with limited side effects.139
Neoadjuvant therapy also has significant implications for research. Tumor tissue can be studied prior, during, and after the completion of systemic therapy to evaluate the response of the tumor to specific treatments. Valuable data can be acquired using biomarkers, RNA profiling, p53 microarray analysis, and comparative genomic hybridization, which will hopefully help to identify patients who will have a better response to therapy.
PREVENTION
The decrease in contralateral breast cancers following adjuvant therapy and favorable toxicity profile in clinical practice suggested tamoxifen could play a role in breast cancer prevention. Furthermore, estrogenic hormones act over many years to promote a population of initiated cells. Given that the promotional stage is characterized by reversibility, much interest has been shown in preventing the consequences of prolonged, unopposed estrogenic stimulation of the breast.
The National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1129 opened in the United States and Canada in May 1992 with an accrual goal of 16,000 women from 100 North American sites. The specific aim was to test the utility of tamoxifen as a preventive for breast cancer. It closed after accruing 13,338 women in 1997. Women over the age of 60, or women between the ages of 35 and 59 whose 5-year risk of developing breast cancer, as predicted by the Gail model,140 was equal to that of a 60-year-old woman, were eligible for inclusion. Additionally, women over age 35 with a diagnosis of lobular carcinoma in situ (LCIS) treated by biopsy alone were eligible for entry. In the absence of LCIS, the risk factors necessary to enter the study varied with age, such that a 35-year-old woman needed an RR of 5.07, whereas the required RR for a 45-year-old woman was 1.79. Routine endometrial biopsies were performed in both arms of the study.
The breast cancer risk of women enrolled in the study was generally extremely high. Recruitment was also age balanced, with about one third younger than 50 years, one third between 50 and 60 years, and one third older than 60 years. Secondary endpoints of the study include the incidence of fractures and cardiovascular deaths.
The first results of the NSABP study were reported in September 1998, after a mean followup of 47.7 months. The data safety monitoring committee prematurely unblinded the study due to a significant reduction in the incidence of breast cancer among patients taking tamoxifen. In total, 363 invasive and noninvasive breast cancers occurred in the participants; 124 in the tamoxifen group and 239 in the placebo group. There was a 49% reduction in the risk of invasive breast cancer and a 50% reduction in the risk of noninvasive breast cancer in patients treated with tamoxifen. A subset analysis demonstrated that women with LCIS had a 56% reduction in risk. Women with atypical hyperplasia had an 86% reduction in risk.
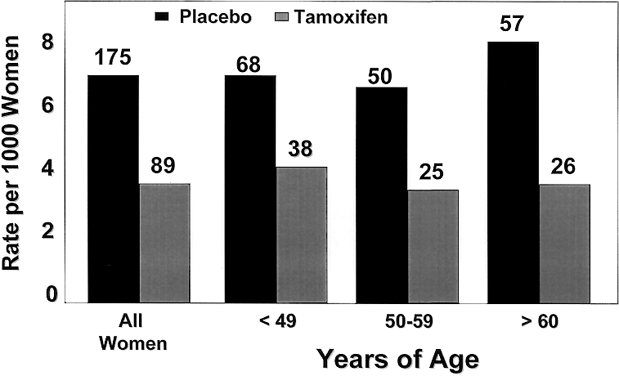
The benefits of tamoxifen were observed in all age groups, with an RR of breast cancer ranging from 0.45 in women aged 60 and older to 0.49 for those in the 50–59 year-old group, and 0.56 for women aged 49 and younger (Fig. 5). The benefit of tamoxifen was observed in all levels of breast cancer risk within the study and was not confined to a particular lower- or higher-risk subset.
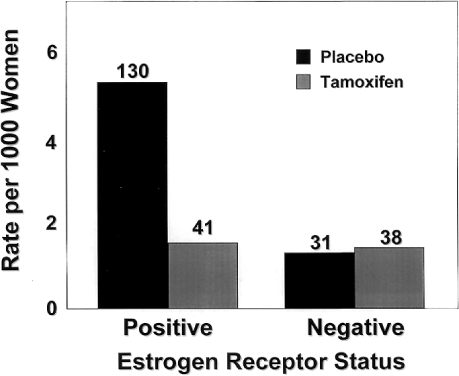
As expected, tamoxifen affected the incidence of ER-positive tumors, which were reduced by 69% per year. The rate of ER-negative tumors in the tamoxifen group (1.46 per 1,000 women) did not significantly differ from that of the placebo group (1.20 per 1,000 women) (Fig. 6). Tamoxifen reduced the rate of invasive cancers of all sizes, but the greatest reduction was in the incidence of tumors 2.0 cm or smaller. Tamoxifen also reduced the incidence of both node-positive and node-negative breast cancer. The beneficial effects of tamoxifen were observed for each year of follow-up in the study. After year 1, the risk was reduced by 33%, and in year 5, by 69%.
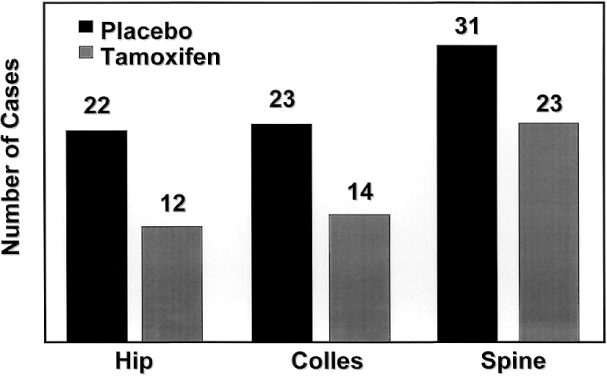
Tamoxifen also reduced the incidence of osteoporotic fractures of the hip, spine, and radius by 19% (Fig. 7),129 approaching, but not reaching statistical significance. This reduction was greatest in women who were 50 years and older at study entry. No differences in the risks of myocardial infarction, angina, coronary artery bypass grafting, or angioplasty were noted between groups.129 The prevention study also confirmed the association between tamoxifen and endometrial carcinoma.113 The relative risk of endometrial cancer in the tamoxifen group was 2.5, with women over age 50 having an RR of 4.01. All endometrial cancers in the tamoxifen group were grade 1 and none of the women receiving tamoxifen died as a result of endometrial cancer. One endometrial cancer death occurred in the placebo group. Although no doubt remains that tamoxifen increases the risk of endometrial cancer, it is important to recognize that this increase translates to an incidence of 2.3 women per 1,000 per year.129
More women in the tamoxifen group developed deep vein thrombosis (DVT),129 with the excess risk confined to women over 50 years. The RR of DVT in the older age group was 1.71 (95% confidence interval [CI], 0.85 to 3.58). An increase in pulmonary emboli was also seen in the older women taking tamoxifen, with an RR of approximately 3. Three deaths from pulmonary emboli occurred in the tamoxifen study arm, all in women with significant comorbidities. An increased incidence of stroke, RR 1.75, was also seen in the tamoxifen group, but this did not reach statistical significance.
An assessment of quality of life showed similar depression scores between groups. Hot flashes were reported in 81% of the women taking tamoxifen compared with 69% of the placebo group, and the tamoxifen-associated hot flashes appeared to be of comparable severity. Twenty-nine per cent of the women in the tamoxifen group and 13% in the placebo group reported moderate or severe vaginal discharge. No differences in the occurrence of irregular menses, nausea, fluid retention, skin changes, or weight gain or loss were reported.
In 2005, an update of the NSABP P-1 trial was published with 7 years of follow-up.141 The results were very similar to the original data in terms of the decreased incidences of invasive and noninvasive breast cancer and osteoporotic fractures. There were comparable increases in endometrial cancer, thromboembolic events, and cataracts in women taking tamoxifen, which did not differ from the original statistics. Despite the increased length of follow-up, there was no survival benefit demonstrated. The authors contend that the study was not designed to detect a survival benefit, and that 15–20 years of follow-up would be needed.
The ATAC trial also demonstrated that in the adjuvant setting, anastrozole decreased the incidence of contralateral breast cancers. The more favorable side effect profile of AIs makes them a more appealing option for healthy high-risk women who are trying to modulate their breast cancer risk. The International Breast Cancer Intervention Study II (IBIS-II)142 is a trial which is currently accruing high-risk post-menopausal women and randomizing them to anastrozole versus placebo.
RALOXIFENE
Raloxifene is a SERM currently approved for the treatment of osteoporosis. Evaluation of 60-mg daily raloxifene on bone homeostasis in postmenopausal women found that although the suppression of remodeling was greater for estrogen, the remodeling balance was the same for the two agents.143 Raloxifen increases bone by 2.4% in the lumbar spine and hip, and has been shown to decrease spine fractures by 40%.144
Raloxifene inhibits the growth of 7,12-dimethylbenz[a]-anthracene (DMBA)-induced rat mammary carcinoma,145 and more important, for the proposed evaluation as a preventive therapy, raloxifene reduces the incidence of N-nitrosomethylurea-induced tumors if given after the carcinogen, but before the appearance of palpable tumors.146 As would be anticipated with a drug that has a short biologic half-life, raloxifene is not superior to tamoxifen at equivalent doses. Raloxifene and its analogs are clearly effective and potent inhibitors of the growth of breast cancer cells in culture,147, 148 but the complication of first pass metabolism in vivo reduces potency. For this reason, doses above 60 mg daily have been tested in clinical trials to prevent osteoporosis.
Based on the hypothesis that raloxifene reduces incidence of breast cancer as a beneficial side effect of the prevention of osteoporosis,149 placebo-controlled trials with raloxifene are ongoing. The Multiple Outcomes of Raloxifene Evaluation (MORE) randomized 7,704 postmenopausal women, mean age 66.5 years, with osteoporosis defined by hip or spine bone density at least 2.5 standard deviations (SDs) below normal or vertebral fractures, and no history of breast or endometrial cancer, to placebo, 60 mg, or 120 mg of raloxifene daily. Results at three years, with a total of 40 cases of breast cancer confirmed, indicate a 76% reduction in the risk of breast cancer.150 Another database pooled all placebo-controlled trials and included 10,553 women monitored for, on average, three years. In this group there was a 54% reduction in the incidence of breast cancer in the raloxifene-treated patients.151 Similar to tamoxifen, raloxifene reduces the incidence of ER-positive breast cancer only.
Raloxifene's effect in the human uterus is currently being evaluated. A study in women without preexisting endometrial abnormalities shows that raloxifene, unlike estrogen, does not increase endometrial thickness.152 Raloxifene is less estrogenic than tamoxifen and only increases the growth of human endometrial carcinomas under laboratory conditions by approximately 50% of tamoxifen.128 Raloxifene has not been found to increase incidence of endometrial cancer during trials of osteoporosis treatment.150
Preliminary data on the ability of raloxifene to decrease the risk of breast cancer and the risk of endometrial cancer led to the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial.153 This trial was a phase III, prospective, double-blind, randomized trial that assigned 19,747 postmenopausal high-risk women to either five years of tamoxifen or raloxifene. The results of this study were published in 2006 and demonstrated that raloxifene was as effective as tamoxifen in decreasing the incidence of invasive breast cancer (RR=1.02, 95% CI, 0.82–1.28). Tamoxifen appeared to be more effective at decreasing noninvasive breast cancers compared to raloxifene (RR=1.40, 95% CI, 0.98–2.00), although these results did not reach statistical significance. Raloxifene was associated with a lower risk of thromboembolic events, with a 30% decrease in pulmonary embolism and DVT (RR, 0.79; 95% CI, 0.54–0.91). There were fewer cataracts in the raloxifene group (RR=0.79, 95% CI, 0.68–0.92), which also resulted in fewer cataract surgeries. Although there was a trend towards a decrease in the risk of endometrial cancer and endometrial hyperplasia, this decrease did not reach statistical significance. The risk of developing other cancers, bone fractures, and ischemic heart disease was similar between the two treatment groups.
FUTURE DIRECTIONS
The central issue for research on hormone receptor pharmacology is the discovery of mechanisms of target site specificity for the modulation of estrogenic and antiestrogenic response. The model must encompass the sum of pharmacologic consequences of signal transduction through ERα and ERβ with the simultaneous competition from endogenous estrogens at both sites. Dissection of the underlying pathways responsible for this activity may permit the development of tissue-selective ER modulators.100
The development of ERβ monoclonal antibodies, the classification of target sites for the protein, and the continuing evaluation of ERβ and ERα,β knock-out mice will identify new therapeutic targets to modulate physiologic functions.154 Clearly, the successful crystallization of ERα with raloxifene88 has acted as a stimulus for the crystallization of ERβ with SERMs.155 However, the description of an agent that produces agonist or antagonist effects exclusively at ERα or ERβ will determine the therapeutic usefulness of ERβ as a target for disease prevention.
Hormonal therapy has made a major contribution to the treatment of breast cancer. Reduced recurrence rates and longer survival will continue to drive the need for newer agents to use after first-line hormonal therapy. Duration of therapy continues to be a key issue. Additional trials and longer follow-up of existing trials are needed to answer this question. The results of sequencing and switching trials will be essential to help determine optimal hormonal therapy regimens. Individualized treatment plans will continue to be important to minimize side effects and maximize benefit for patients. Additionally, resistance to tamoxifen has emerged as a clinical problem, requiring the development of novel agents to circumvent resistance in this patient population.
Major clinical questions remain regarding the best application of tamoxifen, raloxifene, or AIs as chemoprevention. The P-1 and P-2 trials have established the benefits of tamoxifen and raloxifene in high-risk patients. The results of the IBIS-II will help to define the role of AIs as chemopreventive agents. When the goal is prevention rather than cure, the balance between risk and benefit is particularly critical, necessitating greater efforts towards minimizing side effects.
The era of gene sequencing and molecular analysis has expanded our knowledge of breast cancer tremendously. Stratification of recurrence risk using the Oncotype Dx and other genomic assays will allow physicians to tailor therapy to the individual cancer patient. Cytotoxic chemotherapy will ultimately be reserved for those patients most likely to recur. Further research will continue to identify the differences in cancers which can then be exploited for therapeutic purposes.
Breast cancer mortality has declined slightly for the first time in 30 years.156, 157 Of particular impact has been the appropriate and systematic use of antiestrogens in more recently diagnosed patients. Hopefully, the development of new chemopreventives will reveal an even greater potential for improved patient outcomes.
REFERENCES
Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment with illustrative cases. Lancet 1896;2:104–107 |
|
Boyd S. On oophorectomy in cancer of the breast. BMJ 1900;2:1161–1167 |
|
Allen E, Doisey DE: A ovarian hormone: Preliminary report on its localization, extraction, and partial purification and action in test animals. JAMA 81: 819-821, 1923 |
|
Dodds EC, Lawson W, Noble RL. Biological effects of the synthetic oestrogenic substance 4:4'-dihydroxy-alpha:beta-diethylstilbene. Lancet 1938;1:1389–1391 |
|
Haddow A, Watkinson JM, Paterson E. Influence of synthetic oestrogens upon advanced malignant disease. BMJ 1944;2:393–398 |
|
Kennedy BJ. Hormone therapy for advanced breast cancer. Cancer 1965;18:1551–1557 |
|
MacMahon B, Trichopoulos D, Brown J et al. Age at menarche, probability of ovulation and breast cancer risk. Int J Cancer 1982;29:13–16 |
|
Trichopoulos D, MacMahon B, Cole P. Menopause and breast-cancer risk. J Natl Cancer Inst 1972;48:605–613 |
|
Kvale G, Heuch I. Menstrual factors and breast cancer risk. Cancer 1988;62:1625–1631 |
|
MacMahon B, Purde M, Cramer D et al. Association of breast cancer risk with age at first and subsequent births: A study in the population of the Estonian Republic. J Natl Cancer Inst 1982;69:1035–1038 |
|
Harris BM, Eklund G, Meirik O et al. Risk of cancer of the breast after legal abortion during first trimester: A Swedish register study. BMJ 1989;299:1430–1432 |
|
Daling JR, Malone KE, Voigt LF et al. Risk of breast cancer among young women: Relationship to induced abortion. J Natl Cancer Inst 1994;86:1584–1592 |
|
Newcomb PA, Storer BE, Longnecker MP et al. Pregnancy termination in relation to risk of breast cancer. JAMA 1996;275:283–287 |
|
Melbye M, Wohlfahrt J, Olsen JH et al. Induced abortion and the risk of breast cancer. N Engl J Med 1997;336:81–85 |
|
Kvale G, Heuch I. Lactation and cancer risk: Is there a relation specific to breast cancer? J Epidemiol Community Health 1988;42:30–37 |
|
de Waard F, Baanders-van Halewijn EA. A prospective study in general practice on breast-cancer risk in postmenopausal women. Int J Cancer 1974;14:153–160 |
|
De Waard F, Cornelis JP, Aoki K et al. Breast cancer incidence according to weight and height in two cities of the Netherlands and in Aichi prefecture, Japan. Cancer 1977;40:1269–1275 |
|
Lawrence C, Tessaro I, Durgerian S et al. Smoking, body weight, and early-stage endometrial cancer. Cancer 1987;59:1665–1669 |
|
Siiteri PK. Steroid hormones and endometrial cancer. Cancer Res 1978;38:4360–4366 |
|
Enriori CL, Reforzo-Membrives J. Peripheral aromatization as a risk factor for breast and endometrial cancer in postmenopausal women: A review. Gynecol Oncol 1984;17:1–21 |
|
Elwood JM, Cole P, Rothman KJ et al. Epidemiology of endometrial cancer. J Natl Cancer Inst 1977;59:1055–1060 |
|
La Vecchia C, Franceschi S, Decarli A et al. Risk factors for endometrial cancer at different ages. J Natl Cancer Inst 1984;73:667–671 |
|
Horwitz RI, Feinstein AR. Case-control study of oral contraceptive pills and endometrial cancer. Ann Intern Med 1979;91:226–227 |
|
Parazzini F, Franceschi S, La Vecchia C et al. The epidemiology of ovarian cancer. Gynecol Oncol 1991;43:9–23 |
|
Meirik O, Lund E, Adami HO et al. Oral contraceptive use and breast cancer in young women. A joint national case-control study in Sweden and Norway. Lancet 1986;2:650–654 |
|
Oral contraceptive use and breast cancer risk in young women. UK National Case-Control Study Group. Lancet 1989;1:973–982 |
|
Steinberg KK, Thacker SB, Smith SJ et al. A meta-analysis of the effect of estrogen replacement therapy on the risk of breast cancer. JAMA 1991;265:1985-1990; published erratum appears in JAMA 1991;266:1362 |
|
Sillero-Arenas M, Delgado-Rodriguez M, Rodigues-Canteras R et al. Menopausal hormone replacement therapy and breast cancer: A meta- analysis. Obstet Gynecol 1992;79:286–294 |
|
Gapstur SM, Morrow M, Sellers TA. Hormone replacement therapy and risk of breast cancer with a favorable histology: Results of the Iowa Women's Health Study. JAMA 1999;281:2091–2097 |
|
Stallard S, Litherland JC, Cordiner CM et al. Effect of hormone replacement therapy on the pathological stage of breast cancer: Population based, cross sectional study. BMJ 2000;320:348–349 |
|
Huggins C. Two principles in endocrine therapy of cancers: Hormone deprival and hormone interference. Cancer Res 1965;25:1163–1167 |
|
Ross RK, Paganini-Hill A, Wan PC et al. Effect of hormone replacement therapy on breast cancer risk: Estrogen versus estrogen plus progestin. J Natl Cancer Inst 2000;92:328–332 |
|
Jensen EV, Jacobson HI. Basic guides to the mechanism of estrogen action. Rec Prog Horm Res 1962;18:387–414 |
|
Toft D, Gorski J. A receptor molecule for estrogens: Isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci USA 1966;55:1574–1581 |
|
Toft D, Shyamala G, Gorski J. A receptor molecule for estrogens: Studies using a cell-free system. Proc Natl Acad Sci USA 1967;57:1740–1743 |
|
Gorski J, Toft D, Shyamala G et al. Hormone receptors: Studies on the interaction of estrogen with the uterus. Recent Prog Horm Res 1968;24:45–80 |
|
Jensen EV, Suzuki T, Kawashima T et al. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci USA 1968;59:632–638 |
|
Jensen EV, Block GE, Smith S et al. Estrogen receptors and breast cancer response to adrenalectomy. Natl Cancer Inst Monogr 1971;34:55–70 |
|
King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature 1984;307:745–747 |
|
King WJ, DeSombre ER, Jensen EV et al. Comparison of immunocytochemical and steroid-binding assays for estrogen receptor in human breast tumors. Cancer Res 1985;45:293–304 |
|
DeSombre ER, Thorpe SM, Rose C et al. Prognostic usefulness of estrogen receptor immunocytochemical assays for human breast cancer. Cancer Res 1986;46:4256S–4264S |
|
McGuire WL, Chamness GC, Costlow ME et al. Steroids and human breast cancer. J Steroid Biochem 1975;6:723–727 |
|
Early Breast Cancer Trialists' Collaborative Group: Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet 1998;351:1451–1467 |
|
Brush MG, Taylor RW, King RJ. The uptake of [6,7–3H]oestradiol by the normal human female reproductive tract. J Endocrinol 1967;39:599–607 |
|
Hunter RE, Jordan VC. Detection of the 8 S oestrogen-binding component in human uterine endometrium during the menstrual cycle. J Endocrinol 1975;65:457–458 |
|
Bayard F, Damilano S, Robel P et al. Cytoplasmic and nuclear estradiol and progesterone receptors in human endometrium. J Clin Endocrinol Metab 1978;46:635–648 |
|
Janne O, Kauppila A, Kontula K et al. Female sex steroid receptors in normal, hyperplastic and carcinomatous endometrium. The relationship to serum steroid hormones and gonadotropins and changes during medroxyprogesterone acetate administration. Int J Cancer 1979;24:545–554 |
|
Levy C, Robel P, Gautray JP et al. Estradiol and progesterone receptors in human endometrium: Normal and abnormal menstrual cycles and early pregnancy. Am J Obstet Gynecol 1980;136:646–651 |
|
Edwards R, Brush MG, Taylor RW. The uptake and intracellular distribution of (1,2–3H) progesterone by human endometrium. J Endocrinol 1969;45(Suppl):3–4 |
|
Hunter RE, Longcope C, Jordan VC. Steroid hormone receptors in adenocarcinoma of the endometrium. Gynecol Oncol 1980;10:152–161 |
|
Bergeron C, Ferenczy A, Shyamala G. Distribution of estrogen receptors in various cell types of normal, hyperplastic, and neoplastic human endometrial tissues. Lab Invest 1988;58:338–345 |
|
Press MF, Udove JA, Greene GL. Progesterone receptor distribution in the human endometrium: Analysis using monoclonal antibodies to the human progesterone receptor. Am J Pathol 1988;131:112–124 |
|
Segreti EM, Novotny DB, Soper JT et al. Endometrial cancer: Histologic correlates of immunohistochemical localization of progesterone receptor and estrogen receptor. Obstet Gynecol 1989;989;73:780–785 |
|
Clark CL, Zaino RJ, Feil PD et al: Monoclonal antibodies to human progesterone receptor: Characterization by biochemical and immunohistochemical techniques. Endocrinology 121: 1123-1132, 1987 |
|
Green S, Walter P, Kumar V et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 1986;320:134–139 |
|
Misrahi M, Atger M, d'Auriol L et al. Complete amino acid sequence of the human progesterone receptor deduced from cloned cDNA. Biochem Biophys Res Commun 1987;143:740–748 |
|
Kastner P, Krust A, Turcotte B et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 1990;9:1603–1614 |
|
Evans RM. The steroid and thyroid hormone receptor superfamily. Science 1989;240:889–895 |
|
Ponglikitmongkol M, Green S, Chambon P. Genomic organization of the human oestrogen receptor gene. EMBO J 1988;7:3385–3388 |
|
Jeltsch JM, Turcotte B, Garnier JM et al: Characterization of multiple mRNAs originating from the chicken progesterone receptor gene. Evidence for a specific transcript encoding form A. J Biol Chem 1990;265:3967–3974 |
|
Misrahi M, Venencie PY, Saugier-Veber P et al. Structure of the human progesterone receptor gene. Biochim Biophys Acta 1993;1216:289–292 |
|
Mosselman S, Polman J, Dijkema R. ER beta: Identification and characterization of a novel human estrogen receptor. FEBS Lett 1996;392:49–53 |
|
Kuiper GG, Enmark E, Pelto-Huikko M. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 1996;93:5925–5930 |
|
Enmark E, Pelto-Huikko M, Grandien K et al. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab 1997;82:4258–4265 |
|
Couse JF, Curtis SW, Washburn TF et al. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol 1995;9:1441–1454 |
|
McGuire WL, Horwitz KB, Zava DT et al. Hormones in breast cancer: Update 1978. Metabolism 1978;27:487–501 |
|
Thorpe SM, Rose C, Rasmussen BB et al. Prognostic value of steroid hormone receptors: Multivariate analysis of systemically untreated patients with node-negative primary breast cancer. Cancer Res 1987;47:6126–6133 |
|
Kauppila AJ, Isotalo HE, Kivinen ST et al. Prediction of clinical outcome with estrogen and progestin receptor concentrations and their relationships to clinical and histopathological variables in endometrial cancer. Cancer Res 1986;46:5380–5384 |
|
Lindahl B, Alm P, Ferno M et al. Relapse of endometrial carcinoma related to steroid receptor concentration, staging, histologic grading and myometrial invasion. Anticancer Res 1986;6:1317–1320 |
|
Utaaker E, Iversen OE, Skaarland E. The distribution and prognostic implications of steroid receptors in endometrial carcinomas. Gynecol Oncol 1987;28:89–100 |
|
Ehrlich CE, Young PC, Stehman FB et al. Steroid receptors and clinical outcome in patients with adenocarcinoma of the endometrium. Am J Obstet Gynecol 1988;158:796–807 |
|
Palmer DC, Muir IM, Alexander AI et al. The prognostic importance of steroid receptors in endometrial carcinoma. Obstet Gynecol 1988;72:388–393 |
|
van der Putten HW, Baak JP, Koenders TJ et al. Prognostic value of quantitative pathologic features and DNA content in individual patients with stage I endometrial adenocarcinoma. Cancer 1989;63:1378–1387 |
|
Tseng L, Gusberg SB, Gurpide E. Estradiol receptor and 17 beta-dehydrogenase in normal and abnormal human endometrium. Ann NY Acad Sci 1977;286:190–198 |
|
Ehrlich CE, Young PC, Cleary RE. Cytoplasmic progesterone and estradiol receptors in normal, hyperplastic, and carcinomatous endometria: Therapeutic implications. Am J Obstet Gynecol 1981;141:539–546 |
|
Kauppila A. Oestrogen and progestin receptors as prognostic indicators in endometrial cancer: A review of the literature. Acta Oncol 1989;28:561–566 |
|
Soper JT, Christensen CW. Steroid receptors and endometrial cancer. Clin Obstet Gynaecol 1986;13:825–842 |
|
Creasman WT, Soper JT, McCarty KS Jr et al. Influence of cytoplasmic steroid receptor content on prognosis of early stage endometrial carcinoma. Am J Obstet Gynecol 1985;151:922–932 |
|
Kleine W, Maier T, Geyer H, Pfleiderer A. Estrogen and progesterone receptors in endometrial cancer and their prognostic relevance. Gynecol Oncol 1990;38:59–65 |
|
Kumar V, Green S, Stack G et al. Functional domains of the human estrogen receptor. Cell 1987;51:941–951 |
|
Metzger D, Ali S, Bornert JM et al. Characterization of the amino-terminal transcriptional activation function of the human estrogen receptor in animal and yeast cells. J Biol Chem 1995;270:9535–9542 |
|
Beato M, Sanchez-Pacheco A. Interaction of steroid hormone receptors with the transcription initiation complex. Endocr Rev 1996;17:587–609 |
|
L'Horset F, Dauvois S, Heery DM et al. RIP-140 interacts with multiple nuclear receptors by means of two distinct sites. Mol Cell Biol 1996;16:6029–6036 |
|
Kraus WL, McInerney EM, Katzenellenbogen BS. Ligand-dependent, transcriptionally productive association of the amino- and carboxyl-terminal regions of a steroid hormone nuclear receptor. Proc Natl Acad Sci USA 1995;92:12314–12318 |
|
Pace P, Taylor J, Suntharalingam S et al. Human estrogen receptor beta binds DNA in a manner similar to and dimerizes with estrogen receptor alpha. J Biol Chem 1997;272:25832–25838 |
|
Paech K, Webb P, Kuiper GG et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 1997;277:1508–1510 |
|
Horwitz KB, Jackson TA, Bain DL et al. Nuclear receptor coactivators and corepressors. Mol Endocrinol 1996;10:1167–1177 |
|
Brzozowski AM, Pike AC, Dauter Z et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997;389:753–758 |
|
Shiau AK, Barstad D, Loria PM et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 1998;95;927–937 |
|
Feng W, Ribeiro RC, Wagner RL et al. Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science 1998;280:1747–1749 |
|
Moras D, Gronemeyer H. The nuclear receptor ligand-binding domain: structure and function. Curr Opin Cell Biol 1998;10:384–391 |
|
Halachmi S, Marden E, Martin G et al. Estrogen receptor-associated proteins: Possible mediators of hormone-induced transcription. Science 1994;264:1455–1458 |
|
Cavailles V, Dauvois S, L'Horset F et al. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J 1995;14:3741–3751 |
|
Webb P, Nguyen P, Valentine C et al. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol 1999;13:1672–1685 |
|
Tremblay A, Tremblay GB, Labrie F et al. Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol Cell 1999;3:513–519 |
|
Spencer TE, Jenster G, Burcin MM et al. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 1997;389:194–198 |
|
Lieberman ME, Jordan VC, Fritsch M et al. Direct and reversible inhibition of estradiol-stimulated prolactin synthesis by antiestrogens in vitro. J Biol Chem 1983;258:4734–4740 |
|
Tate AC, Greene GL, DeSombre ER et al. Differences between estrogen- and antiestrogen–estrogen receptor complexes from human breast tumors identified with an antibody raised against the estrogen receptor. Cancer Res 1984;44:1012–1018 |
|
McInerney EM, Weis KE, Sun J et al. Transcription activation by the human estrogen receptor subtype beta (ER beta) studied with ER beta and ER alpha receptor chimeras. Endocrinology 1998;139:4513–4522 |
|
Levenson AS, Jordan VC. Selective oestrogen receptor modulation: Molecular pharmacology for the millennium. Eur J Cancer 1999;35:1628–1639 |
|
Schafer JI, Liu H, Tonetti DA et al. The interaction of raloxifene and the active metabolite of the antiestrogen EM-800 (SC 5705) with the human estrogen receptor. Cancer Res 1999;59:4308–4313 |
|
Lerner LJ, Holthaus JF, Thompson CR. A non-steroidal estrogen antagonist 1-(p-2-diethylaminoethoxyphenyl)1-phenyl-2-p-methoxyphenylethanol. Endocrinology 1958;63:295–318 |
|
SEgal JS, Nelson WO: An orally active compound with antifertility effects in rats. Proc Soc Exp Biol Med 98: 431-436,1958 |
|
Chang MC: Degeneration of ova in the rat and rabbit following oral administration of 1-(p-diethylaminoethoxyphenyl)-1-phenyl-2-p-anisylethanol. Endocrinology 65: 339-342, 1959 |
|
Barnes JE, Meyer RK: Effects of ethamoxytriphetol, MRL37 and clomophene or reproduction in rats. Fertil Steril 13: 472-480, 1962 |
|
Segal JS, Nelson WO. Antifertility action of ahloramiphene. Anat Rec 1961;139:273 |
|
Holtkamp DE, Greslin SC, Root CA et al. Gonadotropin inhibiting and antifecundity effects of chloramiphene. Proc Soc Exp Biol Med 1960;105:197–201 |
|
Duncan GW, Lyster SC, Clark JJ et al: Antifertility activities of two diphenyl-dihydromaphthalene derivatives. Proc Soc Exp Biol Med 112: 429-442, 1963 |
|
Callantine MR, Humphrey RR, Lee SL et al: Action of an estrogen antagonist on reproductive mechanisms in the rat. Endocrinology 79: 153-169, 1966 |
|
Harper MJ, Walpole AL. A new derivative of triphenylethylene: Effect on implantation and mode of action in rats. J Reprod Fertil 1967;13:101–119 |
|
Harper MJ, Walpole AL. Mode of action of I.C.I. 46,474 in preventing implantation in rats. J Endocrinol 1967;37:83–92 |
|
Greenblatt RB, Barfield WE, Jungck EC et al. Induction of ovulation with MRL-41: Preliminary report. JAMA 1961;178:101–104 |
|
Fisher B, Costantino JP, Redmond CK et al. Endometrial cancer in tamoxifen-treated breast cancer patients: Findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst 1994;86:527–537 |
|
Baum M. Adjuvant Endocrine Therapy in Postmenopausal Women with Early Breast Cancer: Where are we now? Eur J Can 2005;41(12):1667-1677 |
|
The Breast International Group 1-98 Collaborative Group. A comparison of Letrozole and Tamoxifen in postmenopausal women with early breast cancer. NEJM 2005;353:26:2747-2757 |
|
Fisher B, Borwn A, Mamounas E et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from the National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Onc 1997;15:2483-2493 |
|
van der Hage J, van de Velde C, Julien JP. Preoperative Chemotherapy in Primary Operable Breast Cancer: Results from the European Organization for Research and Treatment of Cancer Trial 10902 J Clin Onc 2001;19(22):4224-4237 |
|
Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer: An early clinical appraisal of ICI46474. Br J Cancer 1971;25:270–275 |
|
Ward HW. Anti-oestrogen therapy for breast cancer: A trial of tamoxifen at two dose levels. BMJ 1973;1:13–14 |
|
Jordan VC, Dix CJ, Allen KE: The effectiveness of long term tamoxifen treatment in a laboratory model for adjuvant hormone therapy of breast cancer. Vol2. In : Salmon SE,Jones SE(eds): Adjuvant Therapy of Cancer. New York, Grune & Stratton, 1979 |
|
Jordan VC, Allen KE: Evaluation of the antitumour activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma model. Eur J Cancer 16: 239-251, 1980 |
|
Jordan VC: Laboratory studies to develop general principles for the adjuvant treatment of breast cancer with antiestrogens: Problems and potential for future clinical applications. Breast Cancer Res Treat 3: S73-S86, 1983 |
|
Fisher B, Dignam J, Bryant J et al. Five versus more than five years of Tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. JNCI 1996;88(21):1529-1542 |
|
ATLAS trial (Adjuvant Tamoxifen, Longer Against Shorter), 30th Annual San Antonio Breast Cancer Symposium, Abstract 48, 2007 |
|
Bonte J, Ide P, Billiet G et al. Tamoxifen as a possible chemotherapeutic agent in endometrial adenocarcinoma. Gynecol Oncol 1981;11:140–161 |
|
Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol 1994;170:447–451 |
|
DeCensi A, Fontana V, Bruno S et al. Effect of tamoxifen on endometrial proliferation. J Clin Oncol 1996;14:434–440 |
|
Gottardis MM, Ricchio ME, Satyaswaroop PG et al. Effect of steroidal and nonsteroidal antiestrogens on the growth of a tamoxifen-stimulated human endometrial carcinoma (EnCa101) in athymic mice. Cancer Res 1990;50:3189–3192 |
|
Fisher B, Costantino JP, Wickerham DL et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998;90:1371–1388 |
|
Fornander T, Rutqvist LE, Cedermark B et al: Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet 1: 117-120, 1989 |
|
Fischer B, Costantino JP, Redmond CK et al: Endometrial cancer in tamoxifen-treated breast cancer patients: Findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst 91: 1654-1662, 1999 |
|
Horwitz RI, Feinstein AR, Horwitz SM et al. Necropsy diagnosis of endometrial cancer and detection-bias in case/control studies. Lancet 1982;2:66–68 |
|
Bernstein L, Deapen D, Cerhan JR et al. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst 1999;91:1654–1662 |
|
ATAC Trialists' Group. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer. Cancer 2003;98(9):1802-1810 |
|
ATAC Trialists' Group. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' of adjuvant treatment for breast cancer. Lancet 2005;365:60-62 |
|
Eiermann W, Paepke S, Appfelstaedt J et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 2001;12:1527-32 |
|
Smith I, Dowsett M, Ebbs SR et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Onc 2005;23:5108-5116 |
|
Cataliotti L, On behalf of PROACT trialists. Efficacy of preoperative anastrozole compared with tamoxifen in postmenopausal women with hormone receptor positive breast cancer. Germany: Presented at 4th European Breast Cancer Conference Hanburg, 2004 |
|
Macaskill EJ, Dixon JM. Neoadjuvant Use of Endocrine Therapy in Breast Cancer. Breast Journal 2007;13(3):243-250 |
|
Gail MH, Brinton LA, Byar DP et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989;81:1879–1886 |
|
Fischer B, Costantino JP, Wickerham DL. Tamoxifen for the prevention of breast cancer: current status of the national surgical adjuvant bowel and breast project P-1 study. JNCI 2005;97(22):1652-1662 |
|
IBIS-II (Prevention) Protocol. An international multi-centre study of anastrozole vs. placebo in postmenopausal women at increased risk of breast cancer. 2002 |
|
Heaney RP, Draper MW. Raloxifene and estrogen: Comparative bone-remodeling kinetics. J Clin Endocrinol Metab 1997;82:3425–3429 |
|
Delmas PD, Bjarnason NH, Mitlak BH et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med 1997;337:1641–1647 |
|
Clemens JA, Bennett DR, Black LJ et al. Effects of a new antiestrogen, keoxifene (LY156758), on growth of carcinogen-induced mammary tumors and on LH and prolactin levels. Life Sci 1983;32:2869–2875 |
|
Gottardis MM, Jordan VC. Antitumor actions of keoxifene and tamoxifen in the N-nitrosomethylurea-induced rat mammary carcinoma model. Cancer Res 1987;47:4020–4024 |
|
Jiang SY, Parker CJ, Jordan VC. A model to describe how a point mutation of the estrogen receptor alters the structure-function relationship of antiestrogens. Breast Cancer Res Treat 1993;26:139–147 |
|
Poulin R, Merand Y, Poirier D et al. Antiestrogenic properties of keoxifene, trans-4-hydroxytamoxifen, and ICI 164384, a new steroidal antiestrogen, in ZR-75-1 human breast cancer cells. Breast Cancer Res Treat 1989;14:65–76 |
|
Lerner LJ, Jordan VC. Development of antiestrogens and their use in breast cancer: Eighth Cain Memorial Award lecture. Cancer Res 1990;50:4177–4189 |
|
Cummings SR, Eckert S, Krueger KA et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: Results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999;281:2189–2197 |
|
Jordan VC, Glusman JE, Eckert S et al. Incident primary breast cancer are reduced by raloxifene: Integrated data from multicenter, double blind, randomized trials in 12,000 postmenopausal women. Breast Cancer Res Treat 1998;50:227 |
|
Boss SM, Huster WJ, Neild JA et al. Effects of raloxifene hydrochloride on the endometrium of postmenopausal women. Am J Obstet Gynecol 1997;177:1458–1464 |
|
Vogel VG, Costantino JP, Wickerham DL et al: Effects of Tamoxifen vs Raloxifene on the risk of developing invasive breast cancer and other disease outcomes (STAR) P-2 Trial, JAMA, 295(23), 2727-2741, 2006 |
|
Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 1999;20:358–417; published erratum appears in Endocr Rev 1999;20:459 |
|
Pike AC, Brzozowski AM, Hubbard RE et al. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J 1999;18:4608–4618 |
|
Byers T, Mouchawar J, Marks J et al. The American Cancer Society challenge goals. How far can cancer rates decline in the U.S. by the year 2015? Cancer 1999;86:715–727 |
|
Cole P, Rodu B. Declining cancer mortality in the United States. Cancer 1996;78:2045–2048 |