Authors
INTRODUCTION
Urinary incontinence is a condition that impacts approximately 30% to 40% of older American women,1 with the majority afflicted with stress urinary incontinence.2 This is a socially disruptive condition that has a significant impact on health care costs, with one population study suggesting that an American woman has up to an 11% lifetime chance of undergoing a procedure for correction of incontinence or prolapse.3 This chapter addresses the etiology, diagnosis, pretreatment evaluation, and treatment of stress urinary incontinence. Also reviewed are the new terminology related to this condition, evolving medical and surgical therapies, and complications of these therapies.
DEFINITIONS
Stress urinary incontinence (SUI) is the symptom of any involuntary leakage of urine on effort or exertion, or on sneezing or coughing.4 The sign of SUI is the involuntary leakage observed from the urethra seen at the exact time of exertion/effort, or sneezing or coughing.4 The International Continence Society (ICS) has replaced the term genuine stress incontinence with urodynamic stress incontinence. This condition is observed during urodynamic testing and is defined as the involuntary leakage of urine with increased intra-abdominal pressure in the absence of a detrusor contraction. Previously, stress incontinence had been divided into hypermobile stress incontinence caused by anatomic defects, and intrinsic sphincter deficiency (ISD) with incontinence resulting from a poorly functioning urethra. Most patients with stress incontinence have a continuum of anatomical and sphincteric components which lead to a wide spectrum of varying degrees of severity.
ETIOLOGY
There are several factors believed to cause or promote SUI or lead to decompensation resulting in SUI (Table 1). The primary factors resulting in SUI are believed to be related to the effects of childbearing, aging, genetics, conditions causing chronic straining or coughing, iatrogenic causes, and obesity. Injury to the pudendal and perineal nerves, musculofascial attachments, and increased pelvic relaxation as measured by the pelvic organ prolapse quantitation (POP-Q) examination has been documented to occur after parturition, even if the delivery was uneventful and without excess overt perineal damage.5, 6, 7 One review by Thom and Brown8 demonstrated an odds ratio (OR) range of 1.3 to 4.6 for any incontinence (by varied definitions) for parous patients as compared with nulliparous controls in a series of retrospective reviews. A triphasic occurrence of incontinence after parturition can be seen in some patients. Most pregnancy-induced and puerperal incontinence will resolve within months of delivery as a functional recovery ensues in the younger patient. At some point, the effects of age-related nerve degeneration, in combination with the original nerve damage, tissue damage, and other factors, results in loss of compensatory mechanisms and stress incontinence.
Table 1. Factors contributing to stress urinary incontinence
| Factor | Possible Mechanisms |
| Childbirth | Disruption of normal anatomy, pelvic nerve injury |
| Aging | Estrogen deficiency, peripheral neuropathy |
| Iatrogenic after surgery | Disruption of normal anatomy, pelvic nerve injury, loss of tissue elasticity, loss of vascular cushion |
| COPD, chronic heavy lifting, chronic constipation | Straining causing stresses on normal anatomy and pelvic nerve injury |
| Iatrogenic- medication side effect | Loss of urethral tone |
| Pelvic radiation | Peripheral neuropathy, loss of tissue elasticity, loss of vascular cushion |
| Obesity | Increased intra-abdominal pressure causing stresses on normal anatomy and pelvic nerve injury |
| Neurogenic disease | Loss of normal reflexes, pelvic floor tone, and urethral tone |
| Congenitally poor tissues: | Poor anatomic integrity |
| Connective tissue disorder | Loss of tissue elasticity |
| Genetic predisposition | Conditions favoring any of the above factors |
PATHOPHYSIOLOGY
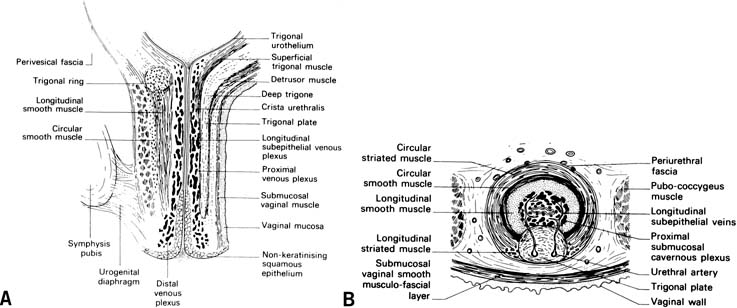
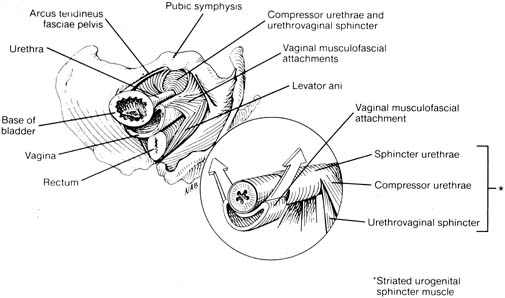
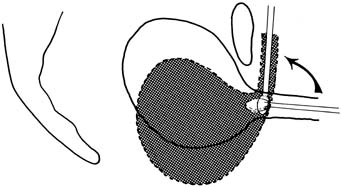
The continence mechanism of the lower urinary tract relies on a complex interaction of anatomy, tissue integrity, and nerve reflexes. The purpose of the urethra is to provide a conduit from the bladder to the outside. Essential anatomic elements to continence include proper mucosal apposition, constant urethral tone, and maintenance of the bladder neck/proximal urethra in the retropubic position. The mucosa and submucosa of the urethra contain an extensive submucosal venous plexus that forms a cushion to keep the urethra closed at rest (Fig. 1). Two layers of smooth muscle, an inner longitudinal and outer circular layer, surround the mucosa. The longitudinal layer serves primarily to shorten the urethra during micturition, but the circular layer adds further to the urethral tone. The striated urethral sphincter, also termed the urogenital sphincter, surrounds the inner layers and is composed of three elements located in the mid part of the urethra: the rhabdosphincter, the compressor urethrae, and the urethrovaginal sphincter (Fig. 2). These striated muscles are under voluntary control but are subject to reflex arcs, with the three portions acting as a functional unit. This sphincter contributes to the resting tone of the urethra as well as to continence during stress, acting primarily as a back-up mechanism in those with hypermobility of the urethrovesical junction. With a sudden increase in intra-abdominal pressure (i.e., coughing, sneezing), reflex contraction of this sphincter aids in continence. Pelvic floor training (Kegel exercises) can increase the efficacy of these muscles both in strength and duration of contraction.
The muscular and fascial attachments of the urethrovaginal junction (UVJ) or bladder neck keep this crucial area in an intra-abdominal position. The proximal two thirds of the urethra and the UVJ are fused to the anterior vagina, with the underlying endopelvic fascia forming a hammock of support. This hammock under the UVJ helps proximal urethral closure during increased abdominal pressure and extends laterally to the arcus tendineus fascia pelvis and the levator muscles.9 The tone of the levator ani pelvic muscles maintains the UVJ in the retropubic intra-abdominal position at rest and allows descent and funneling during relaxation for micturition. Contraction of the levator ani during stress prevents movement of the supportive hammock which acts as a backstop for urethral closure (see Fig. 2).
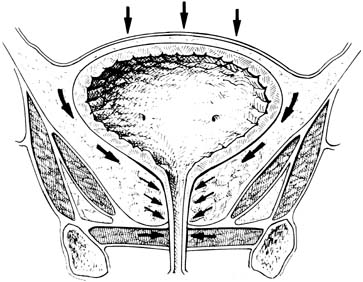
Alterations in pelvic innervation can affect the internal smooth muscle sphincter and the striated sphincter, as well as the levator tone and normal reflexes. Disruption of the normal anatomic supports of the hammock can allow excess rotation of the UVJ with physical stress, resulting in impaired transmission of pressure to this area. The increased pressure on fluid in the bladder overcomes the lower pressure in the urethra, allowing urine to escape (Fig. 3). Additionally, abnormal anatomy and hypermobility may compromise the effectiveness of compensatory mechanisms such as the striated urethral sphincter and/or put further stretch on branches of the pudendal nerve, further impairing function.
A poorly functioning urethra can also contribute to or be the primary cause of SUI in patients. Patients with this condition were previously described as having intrinsic sphincteric deficiency (ISD) or type III incontinence. An atrophic urethral mucosa, a scarred or fibrotic submucosal layer, a poor venous plexus, and/or impaired smooth muscle function can prevent normal apposition of the urethral mucosa. This loss of the normal tissue elastic properties from multiple surgeries, aging, estrogen deficiency, or radiation can impair normal coaptation and pressure transmission in the urethra, resulting in urine loss with minimal exertion. Importantly, patients may have some degree of a poorly functioning urethra working in concert with deranged anatomy or nerve injury, with all factors contributing to stress incontinence. When these urethral characteristics manifest in a severe form, descriptive terms such as low-pressure urethra, lead-pipe urethra, or patulous urethra have been used. Although the condition of ISD with defining diagnostic criteria is still debated, a urethra with these characteristics but with intact support (i.e., immobile) helps determine specific therapies as discussed later.
DIAGNOSIS
The Agency for Health Care Policy and Research (AHCPR) published its consensus guidelines for the evaluation and management of urinary incontinence in 1996.10 These recommendations from a panel of experts include the following: a thorough history (including voiding diary), physical examination, postvoid residual, and urinalysis. Other recognized groups also indicated that a basic evaluation was all that was required for initial management of incontinence. These included the Society of Obstetricians and Gynaecologists of Canada (SOGC),11 International Consultation on Incontinence,12 and the American College of Obstetricians and Gynecologists.13, 14 Described here are these basic elements with other potential tools that may also help determine the best mode of treatment.
History
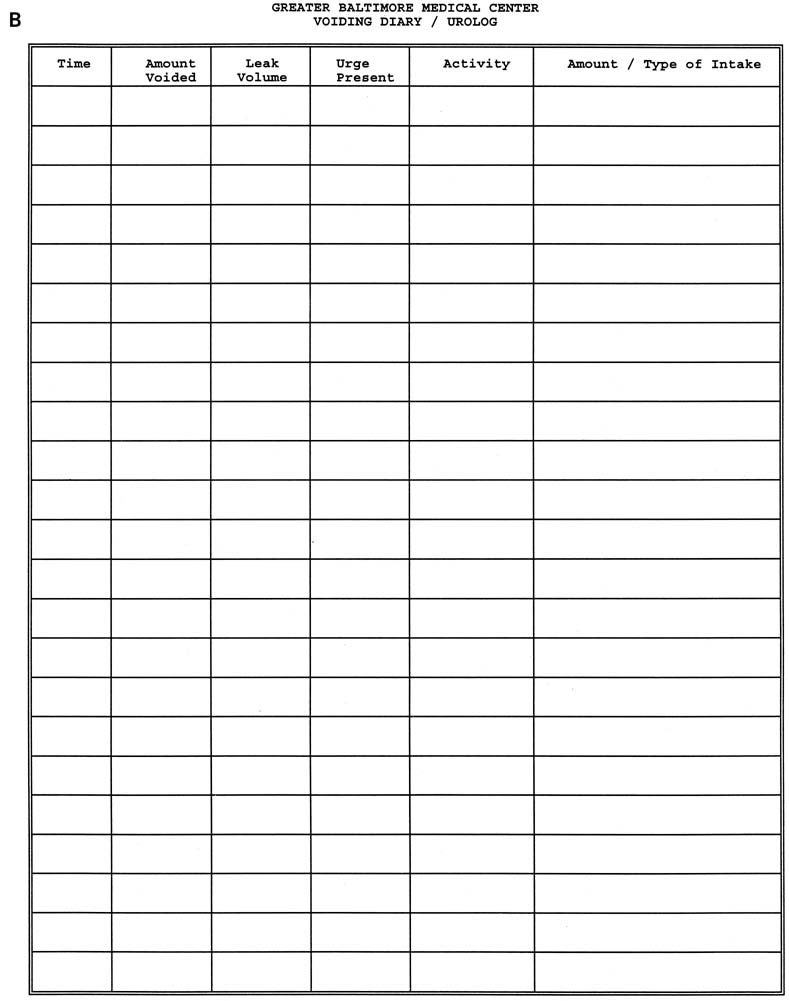
Patients with stress urinary incontinence have a history of urine loss during physical stress such as coughing, laughing, sneezing, running, and lifting. Other features important to ascertain include the time of onset, progression or remission of symptoms (especially in relation to parturition), and frequency of leakage. Indications of severity can be garnered from number of pads used per day and interference or cessation of both physical and social activities. Determination of urinary frequency, nocturia, and presence or absence of urgency, urge incontinence, and nocturnal enuresis are essential to exclude overactive bladder or mixed incontinence. Previous surgeries, medical conditions, obstetric history, and a list of medications should be elucidated. Completion of a 24-hour or, more ideally, a 72-hour urolog (urinary diary) gives extremely valuable information validating symptoms. This urinary diary should contain micturition frequency, leak episodes, pad use, voided volume, and volume of fluid intake (Fig. 4).
|
Examination
The general physical examination, including pelvic examination, may not confirm the diagnosis of SUI but is important in the assessment of specific pelvic support defects and prolapse severity. After the patient empties her bladder, a general abdominal examination and focused neurologic examination of the lower extremities should be performed. The neurologic assessment of the sacral reflex arc is accomplished by stroking over the labia majora parallel to the labia minora or tapping the clitoris with a cotton swab. A normal response is contraction of the anal sphincter. During pelvic examination, a Sims' speculum or the posterior half of a Graves's speculum may be used for direct observation of the anterior vaginal wall, and then reversed for examination of the posterior vaginal wall. The POP-Q can be performed at this point to assess severity of support defects.15 Palpation of the anterior vaginal wall can help assess for a suburethral mass such as a urethral diverticulum. Before bimanual examination, a vaginal examination to assess voluntary pelvic floor muscle strength (pubococcygeus squeeze) is performed by asking the patient to hold her urine and/or squeeze her rectum.
Before assessment of bladder neck mobility, have the patient empty her bladder and perform the empty supine stress test by asking her to cough and strain down. A positive test may indicate severe incontinence. Checking a postvoid residual is also prudent to help exclude chronic retention or overflow incontinence. A standard sterile cotton swab lubricated with anesthetic jelly is passed through the urethra just to the level of the bladder neck. The axis of the applicator is observed at rest and with coughing or Valsalva maneuver. A positive straining angle of 30 degrees or greater from the horizontal indicates urethrovesical junction (UVJ) hypermobility (Fig. 5). A straining angle of less than 30 degrees indicates adequate UVJ support, or hypomobility.
Ancillary Testing
A provocative cough stress test or office cystometry can be performed as an extension to the basic examination to confirm the diagnosis of SUI. Leakage of urine/water from the urethra coincident with coughing or straining after retrograde filling of the bladder with a known amount (usually 250–300 mL) of fluid demonstrates a positive stress test. This can be attempted first in the lithotomy position, but standing may be necessary to demonstrate leakage. Bladder filling can take the form of office cystometry, which should be performed in the standing or upright sitting position after placement of a catheter. Placement of the catheter can also drain the bladder for determination of postvoid residual. Attachment of a 60-mL catheter tip syringe to the catheter aids in filling, which should be performed in 50- to 60-mL increments with sterile water. First sensation, fullness, and maximum cystometric capacity can be approximately determined during the filling phase. The syringe should be elevated 10–15 cm above the symphysis pubis, with close observation of the meniscus. Rising of the meniscus, sometimes associated with the patient's urge to void or loss of fluid around the catheter from the urethra, indicates a detrusor contraction, confirming urge incontinence. After filling to maximum capacity, the catheter is removed and a cough test is performed to assess for SUI.
Multichannel cystometry can also be performed with pressure transducer catheters in the bladder to detect bladder pressure and in the vagina or rectum to assess intra-abdominal pressure. The subtraction of the two values (bladder pressure minus abdominal pressure) reveals the true detrusor pressure and may show contractions in patients with detrusor overactivity (formerly termed detrusor instability).4 This more exact study allows calculation of Valsalva leak-point pressures (VLLP). During the filling phase, at predetermined volumes (usually 150 and/or 200 mL), the patient is asked to gradually strain down and the point of fluid leakage is marked. The VLLP is calculated by subtracting the bladder pressure value at the moment of leakage from the baseline bladder pressure. Assessment of urethral closure pressures may also be performed as part of the urodynamic evaluation. The urethral pressure catheter, which may have dual leads for the bladder and urethra, is slowly withdrawn from the bladder by a puller. In addition to assessing the maximum urethral closure pressure (MUCP), functional urethral length, evaluation of the continence zone, and pressure transmission profiles can be obtained.
Multichannel urodynamics can be helpful in the exclusion of detrusor overactivity incontinence, determination of normal bladder capacity (usually more than 250–300 mL), and evaluation of voiding dysfunction before any planned procedure for suspected SUI. Occult incontinence in patients with significant POP can be determined by cystometry or stress test. Reduction of prolapse with tampons, a pessary, speculum, or sponge stick can straighten out a previously kinked urethra that may now show fluid loss with stress. Additionally, determinations of VLPP and MUCP in patients with SUI have helped to guide therapy, especially in patients suspected of having a poorly functioning urethra. Specifically, a MUCP of less than 20 cmH2O has been correlated with an increased risk of failure of retropubic suspension in patients with SUI.16, 17 When this value has been used as an indicator for sling surgery in SUI patients, the success rates have been excellent.16, 18 Other researchers have proposed that a VLPP of less than 60 cmH2O is indicative of a weak sphincter19 and mandates sling surgery for repair. There continues to be debate within the research community regarding the diagnosis of a poorly functioning sphincter and labeling patients with the condition of ISD. Using a combination of historic and clinical criteria appears to be the most prudent when evaluating the urethra. Table 2 lists historic and clinical characteristics for classic incontinence caused by hypermobility in contrast to incontinence caused by a poorly functioning urethra.
Table 2. Clinical and diagnostic findings commonly seen in hypermobile SUI and ISD
Hypermobile SUI | Poorly Functioning Urethra (ISD) | |
| Incontinence history | Urine loss with activity, after child-bearing age, usually parous | Severe urine loss with minimal activity, elderly, may be nulliparous |
| Surgical history | No previous surgery | Failed previous continence surgery, previous radical pelvic surgery or radiation |
| Examination | Normal anterior vagina, positive cotton >30º ± positive ESST | Scarred anterior vagina, cotton swab may be <30º or >30°, positive ESST |
| Urodynamics | Normal CMG, VLPP One >60 cmH2O, MUCP >20 cmH2O | Normal CMG, VLPP <60 cmH2O, MUCP <20 cmH2O |
SUI, stress urinary incontinence; ISD, intrinsic sphincteric deficiency; ESST, empty supine stress test; CMG, cystometrogram; VLPP, Valsalva leak point pressure; MUCP, maximum urethral closure pressure.
Perineal pad testing is another means of assessing the severity of incontinence, and it has been used as an objective measure of improvement or cure.20, 21 A 20-minute pad test is performed by ensuring that the patient has a relatively full bladder (or placing a known quantity of fluid in the bladder by catheter) and then asking her to perform a series of exercises intended to produce stress incontinence. A preweighed pad is placed in position and weighed immediately after the exercises. An increase of 2 g or more is considered significant. The test may also be performed as a 12-hour pad test at home. The patient is given six pre-weighed, sequentially numbered pads to wear for 2 hours each. During this time, she keeps a voiding diary to indicate amount of fluid intake, time of intake, amount voided, activities performed, and leak episodes. The test is clearer if she also administers phenazopyridine tablets three times during the day to stain the urine dark orange. Each pad is contained within a sealed plastic storage bag, on which is written the number of the pad and the weight. The patient removes the backing from the pad and places the backing back in the bag; after wearing the pad for 2 hours, she places it in the bag and seals it. All bags are kept refrigerated overnight and returned to the clinician for weighing the next day. A significant loss is greater than a 2 g increase during any 2-hour period, or 1 g per hour. This is an excellent test for determining severity of leakage as viewed by the patient. Not infrequently, there are very small losses that the patient may consider a major problem. This patient should be counseled regarding expectations for any therapeutic intervention.
NONSURGICAL MANAGEMENT
Conservative treatment should always be considered and offered first to patients presenting with symptoms of SUI. Most patients who present with uncomplicated SUI can be evaluated by the guidelines delineated by the AHCPR: a thorough history with voiding diary, general physical and pelvic examination, postvoid residual determination, and urinalysis. Primary treatments should center on lifestyle alterations, pelvic muscle rehabilitation, pessaries, and, perhaps, trials of pharmacologic therapy before any more advanced work-up or referral to a specialist.22, 23, 24
Lifestyle Alterations
Most patients have already altered many of their usual activities before seeking medical help. Obvious alterations include having the patient empty her bladder before physical activity, regulation of caffeinated beverage and fluid intake, and wearing protective pads. Job or activity alteration is seldom a reasonable avenue, because the patient usually has discontinued the activity or has considered and rejected this option.
Patients with chronic obstructive pulmonary disease, asthma, and other related lung diseases should seek optimal control of these conditions. Although one study of more than 600 women, most referred to a specialist for lower urinary tract symptoms, did show an OR of 2.2 for SUI in former and current smokers,25 no studies have been performed to demonstrate that cessation of smoking will alleviate symptoms. Needless to say, cessation of smoking and weight loss should always be encouraged, but reliance on these changes can lead to frustration. Of note, though, two small studies did show subjective improvement in SUI in women after weight reduction induced by bariatric surgery for morbid obesity.26, 27
Mechanical Devices
Numerous devices aimed at nonsurgical correction of stress urinary incontinence have been developed over the years. Almost all of them have been removed from the United States market for various reasons, usually infectious or compliance issues. The primary devices used now for correction of SUI are pessaries (see Fig. 6), some of which have been designed with added incontinence knobs. These devices are fitted in the office but can be removed and replaced by patients when they expect to be in situations that aggravate or result in incontinence. Alternatively, in the elderly or less agile patient, the pessary can be fitted and the patient may return periodically for removal and cleaning (i.e., every 3–4 months). Great vigilance to assess for urinary retention should be taken during the initial trial period if the patient is not removing it herself. These devices primarily act to help support the bladder neck or partially occlude the urethra during times of increased physical stress, acting as a mechanical object restricting excess mobility and keeping pelvic anatomy in a normal position.
Pelvic Floor Rehabilitation
Kegel described a pelvic muscle exercise program in 1948 that has formed the basis for many techniques of exercising the appropriate muscles.28, 29 Pelvic floor muscle training (PFMT) is best taught by a therapist who can instruct the patient on which muscles to exercise. Many formulas have been used for instruction, but the successful ones include use of maximum strength contractions for 10 seconds with 15 seconds of rest between contractions. One program starts with 15 contractions per day, gradually increasing to 45 contractions per day. The patient should keep a record of the number of times the exercises are performed, and she should be seen for follow-up weekly as part of pelvic rehabilitation program. Maximum effect should be seen at 12 weeks, although benefit may continue to increase up to 24 weeks, especially in the elderly. Of patients who adhere to a pelvic muscle exercise program, approximately one-quarter at most will be cured; however, importantly, 40% to 60% achieve significant improvement in symptoms and may avoid surgery. Other methods of patient instruction include pressure-monitoring devices (perineometers), which show the patient a response based on the effectiveness of her contraction and may include auditory signals. Vaginal weighted cones (StepFree; Timm Medical Technologies, www.timmmedical.com; Kegel Exercise Kones; Milex, www.milexproducts.com) may also be used to strengthen the pelvic floor to aid in continence. The patient must contract the pelvic floor to retain the cone, which is used twice daily for 15 minutes. As pelvic floor strength increases, higher weighted cones may be used in stepwise fashion from 20 to 100 g.30 A recent Cochrane Database review reaffirmed the usefulness of PFMT for SUI with some suggestion that it may be more effective than electrical stimulation (discussed later) or vaginal cones.31
Functional Electrical Stimulation
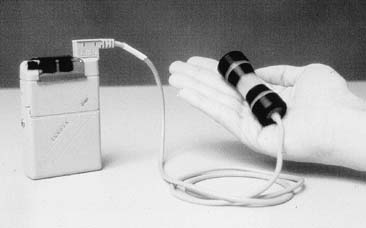
Functional electrical stimulation (FES) is another form of conservative therapy that results in a passive Kegel contraction. FES has been used in Europe for more than 30 years for both stress and urge incontinence, with some reporting improvement rates as high as 60%, a figure confirmed by a double-blinded placebo controlled trial in the United States.32 FES is delivered to the patient through a vaginal probe or conducting pads connected to a small, portable, battery-operated unit (Fig. 7). The unit delivers an electrical charge to the nerves, which innervate the surrounding pelvic muscles; these muscles, in turn, contract in response to the stimulus. The settings are in the range of 65 milliamperes, 5 seconds on and 5–10 seconds off, 50-Hz frequency, and duration of 15–30 minutes twice per day. Treatment is continued for 3–6 months to achieve maximum benefit.
Pharmacologic Treatment for Stress Incontinence
Drug therapy for SUI has shown poor results in the past. Because of the historical link of increasing incontinence after menopause, estrogen has been proposed as a possible treatment. Some studies have shown estrogen to increase urethral blood supply33 and improvement in some urodynamic parameters in baboons,34 but the only large randomized clinical trial did not show improvement in SUI symptoms in menopausal women receiving estrogen replacement as compared with those not using the therapy.35 Although not indicated for treatment of SUI, estrogen, administered locally 2 to 3 times per week or systemically in those women with no contraindications, may nonetheless help some women with lower urinary tract symptoms.
Drugs with alpha-adrenergic action (phenylpropanolamine, phenylephrine, ephedrine, pseudoephedrine) have been used in the past for the treatment of mild SUI. This off-label use to increase the tone of the rhabdosphincter has decreased because of cardiovascular and cerebrovascular concerns. Because of the lack of specific action on the urethra, elevation in blood pressure, headaches, and tremors can occur with effective doses of these drugs. These and more serious concerns, such as stroke and heart attacks, have moved the FDA to recommend removal of phenylpropanolamine from the market. Imipramine, a tricyclic antidepressant with anticholinergic and alpha-adrenergic properties, has been shown to improve SUI symptoms in a few small, nonplacebo, controlled trials.36, 37 The usual dose for SUI is 25 mg one to three times per day. It is notable that the drug does have an indication for nocturnal enuresis in children at doses up to 25 mg at night (maximum 2.5 mg/kg per day).
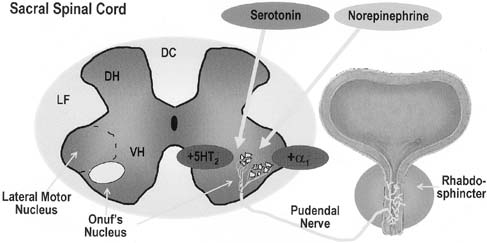
Newer pharmacologic studies have focused on the central nervous system's influence on the bladder and continence mechanism. Onuf's nucleus, located in the ventral (anterior) horn of the sacral spinal column, contains the nuclei of the lower motor neurons (LMNs) that feed the external striated sphincter of the urethra. These cell bodies in Onuf's nucleus can be influenced not only by upper motor neuron pathways but also from input from the hypothalamus and autonomic regions. Stimulation of these specialized LMN nuclei can occur from these surrounding norepinephrine (NE) and serotonin (5-HT) nerve terminals. The impulse is carried through the pudendal nerve resulting in contraction of the striated urethral sphincter. The novel drug, duloxetine, is a 5-HT and NE re-uptake inhibitor aimed to promote continence by acting at Onuf's nucleus, causing increased impulses along this pathway (Fig. 8). In the double-blind phase III clinical trial, duloxetine demonstrated a significant reduction in incontinence episodes (64% compared with 41%) and increase in quality of life as compared with placebo.38 In spite of many studies, including favorable ones, this drug remains available in Europe but is an unlikely product for use in North America.
SURGICAL INTERVENTIONS FOR SUI
The general principles of any surgical procedure are to relieve suffering, restore normal anatomy, and maintain function. The function of the lower urinary tract is to store urine until volitional emptying can occur. Hence, relief of suffering by resolving untimely and socially embarrassing urine loss by procedures that compensate for abnormal anatomy should not overcorrect, causing impairment of storage (diminished bladder capacity or urge incontinence) or release of urine (retention or voiding dysfunction). Proper counseling and preoperative evaluation can be crucial in the avoidance of these problems.
The American College of Obstetricians and Gynecologists (ACOG) has (Table 3) recommended that a number of steps be taken before surgical intervention, much of which has been outlined.13 The presence of stress incontinence and absence of urge incontinence symptoms should be confirmed in the history. The transient causes of urinary incontinence, including delirium, infection, hypoestrogenism, pharmaceutical causes, psychological causes, excessive urine production, restricted mobility, and stool impaction, should be identified and managed before other intervention is considered. The basic preoperative evaluation should include a history and physical examination, local neurologic examination, pelvic examination, 24-hour voiding diary, stress test demonstrating leakage, cotton swab test to confirm urethral hypermobility, residual urine determination, urinalysis or urine culture, and a screening cystometrogram.
Table 3. ACOG guidelines for primary surgery for SUI
| Confirmation of Indication | Actions to the Procedure |
| Documentation of stress incontinence | Document normal voiding habits |
| Identify and manage transient causes of stress incontinence | Document normal neurological examination |
| Demonstrate stress loss and confirm low-residual urine | Document absence of previous incontinence or radical surgery |
| Document absence of pregnancy | |
| Counsel patient regarding alternative therapy |
More than 200 procedures have been described in the literature for the treatment of stress incontinence. This extraordinary number reflects a combination of the alteration of techniques and approaches of established and effective procedures, the introduction of newer technologies and materials, as well as the discarding of procedures shown to be less efficacious. In today's surgical practice, it is well-established that performing an anterior repair or Kelly plication for the treatment of SUI is substandard compared to more effective procedures.39 Because of significant recurrence rates at even 1 and 2 years of follow-up, long-needle procedures such as the Peyera, Stamey, or Raz procedures, and their other modifications are no longer recommended. ACOG,14 the Society of Obstetricians and Gynaecologists of Canada (SOGC),40 and the International Consultation on Incontinence 41 have affirmed the above recommendations. They recommend retropubic urethropexy and sling procedures as the two primary surgical interventions. Injection of urethral bulking agents also is performed as a less invasive intervention for SUI in certain patients.
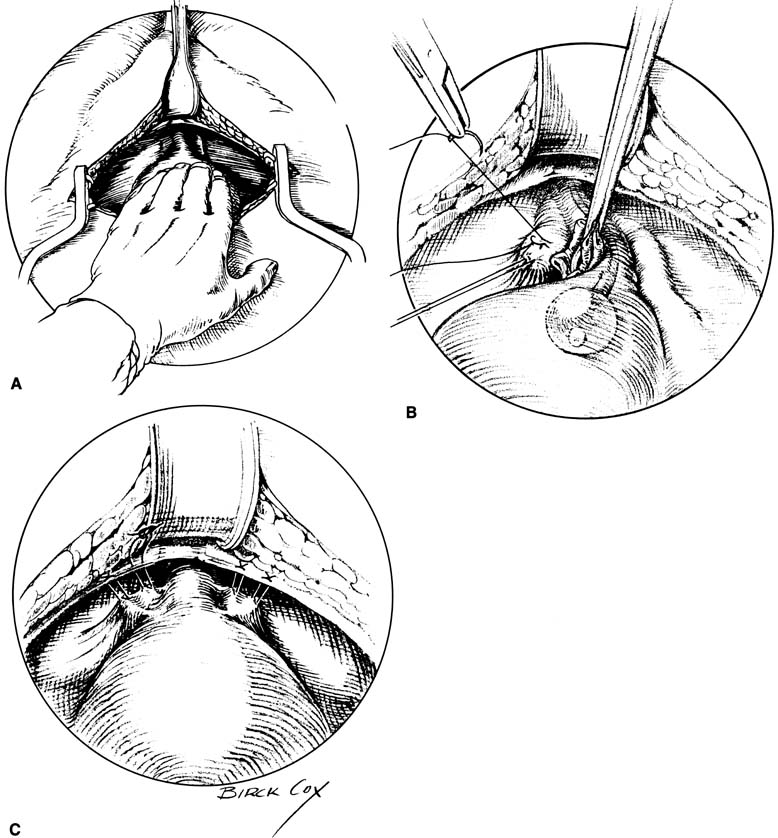
The gold standard surgical treatment of SUI in patients with a hypermobile bladder neck but otherwise normally functioning urethra has been accomplished through a retropubic approach (Fig. 9). The two procedures most studied are the Burch retropubic urethropexy and the Marshall-Marchetti-Krantz (MMK) procedure. In a Burch procedure, permanent sutures are placed lateral to both sides of the mid urethra and UVJ into the fibromuscular layer of the vagina (avoiding full thickness) and fixed to the ipsilateral Cooper's ligament for a total of four sutures. Attachment to the fibrocartilage of the symphysis pubis is performed for the MMK. Placement of these sutures is aimed at reestablishing the intra-abdominal location of the proximal urethra and UVJ and to minimize descent, thus allowing normal pressure transmission to this crucial area during times of stress. Cure rates for these procedures range from 85% to 90% at 1 to 5 years, and most 10-year data with more than a 70% cure rate.42, 43
Traditionally, this procedure can be performed in concert with an abdominal hysterectomy or alone, through a retropubic approach without opening the peritoneal cavity. With the increased trend toward minimally invasive surgery, methods for laparoscopic urethropexy have been developed. Besides either an intraabdominal or an extraperitoneal approach, numerous modifications have been suggested including using only two sutures, substituting mesh, or using tacks, anchors, and other tools to elevate the bladder neck. These variations significantly lower cure rates as compared with traditional open urethropexy. Placement of four permanent sutures identical to an open procedure, though, has yielded similar 1- and 2-year cure rates as an open Burch (93% and 89%, respectively),44 although no prospective trial between abdominal and laparoscopic Burch procedures has been published. Currently, it is our practice to place four sutures identical to the open procedure when using a laparoscopic approach for optimal results. Transvaginal placement of urethropexy sutures has also been reported in small numbers, but no RCTs demonstrating comparable efficacy have been performed.45
Placement of a sling under the bladder neck and proximal urethra had traditionally been used for patients with severe incontinence and poorly functioning urethras, and for those with recurrent stress incontinence. More often, though, suburethral slings (SUS) are being used as treatment for primary stress incontinence, an approach advocated by some urologists.46, 47 The debated increase in morbidity, specifically urinary retention and urge incontinence, as compared with urethropexy is the point of contention regarding primary use of this procedure for treatment of SUI.48 Intermediate (1–2 years) and long-term (2–10 years) cure rates of 85–90% and 70–85%, respectively, are cited in the literature for most slings.47, 49
In general, SUS can be considered full-length slings extending from rectus fascia all the way around the bladder neck and back up to the contralateral rectus fascia, or patch slings that use suture bridges that are tied over or sutured to the fascia. Full-length autologous slings can be obtained by harvesting fascia either from the rectus sheath or from the fascia lata of the leg. The sling is usually sutured into place with delayed absorbable sutures under the bladder neck. The tails of the full-length slings are sutured to the rectus fascia directly with good care to keep the sling loose. Similarly, the permanent suture bridge of patch slings is tied over the fascia or to the fascia loosely to protect against overcorrection and outlet occlusion.
The two slings described are the most common types of autologous slings cited in the literature. Other less-proven autologous materials include vaginal wall slings and rectus abdominus slings. Cadaveric fascia lata, dermal allograft, porcine dermis, and porcine small intestinal submucosa are other sources of nonsynthetic sling material. Synthetic slings, used as either full-length or patches, include Mersilene, Marlex, Prolene, Gore-tex, and silastic bands. Some of these novel materials have used bone anchors or other minimally invasive means to place a sling under the bladder neck, although long-term data regarding efficacy of these modifications, as compared with traditional slings, remain lacking.
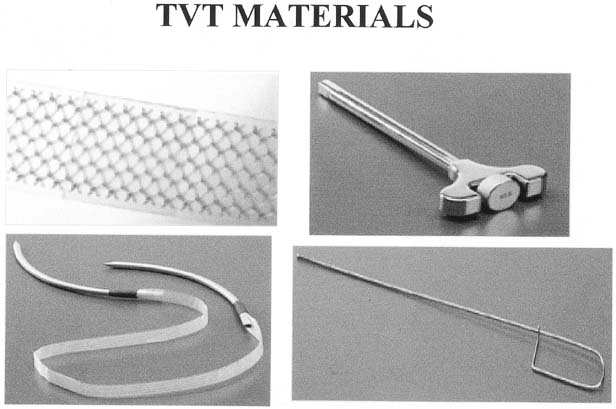
A variation of the classic sling was developed in Europe by Ulmsten and colleagues using a polypropylene mesh placed under the mid urethra (Fig. 10).50 The tension-free vaginal tape (TVT) and its subsequent forms were developed as a minimally invasive outpatient procedure that can be performed under intravenous sedation and local anesthesia. Placement of the mesh is accomplished through a small 1- to 2-cm incision under the mid urethra with blind passage of delivery trocar-needles up behind the pubic bone and through two small 5-mm supra-pubic skin incisions. The mesh is positioned loosely under the mid urethra, the protective plastic sheath removed, and the tails trimmed, which retract under the skin. The sling is not sutured in place, but rather the friction of the mesh pores keeps it in position initially until ingrowth of fibroblasts scars the sling in place. Most studies report an 85% cure rate with an additional 5–10% significantly improved.50, 51, 52 Because the needles are passed blindly, common complications of bladder perforation (4–9%) and, less commonly, hematoma formation (1%) can occur.50, 51, 52, 53 The low incidence of postoperative urge incontinence and obstructive voiding as well as the quick return to normal voiding has been attributed to the minimal peri-urethral dissection and loose application of the sling as directed by the developers. Although a lower incidence of erosion (<1%) has been seen with this synthetic mesh compared with historical numbers for synthetic slings, the blind passage of the needles has caused some notable trauma to bowel, iliac vessels, and epigastric vessels, resulting in life-threatening situations.
In 2001, Delorme 41 developed the transobturator approach to placement of the tension-free tape. The material remained the same – a large pore light-weight, polypropylene mesh. The outside-in approach used a blind passage of the needle-trocar from just lateral to the labia minora, around the ischiopubic ramus, through the obturator foramen, and into the anterior vaginal wall at the level of the midurethra. The inside-out approach passes the needle-trocar from the vaginal incision, around the ischiopubic ramus and through the obturator foramen, to an incision on the inner thigh. The comparison of the retropubic and transobturator approaches has shown equal efficacy.54, 55 The advantage of the transobturator passage is avoiding potential injury to the bladder, bowel, and iliac vessels. There is also less voiding dysfunction. The disadvantage appears to be less efficacy in treating patients with hypomobility of the bladder neck.56 The transobturator procedure is a very common tension-free tape procedure with world-wide usage exceeding the original retropubic type tension-free tape. There is ongoing development of a mini sling which is placed through a vaginal incision only, and inserts and fixes to the tissue connected to the descending pubic rami.57
Injectable Treatments for Stress Incontinence
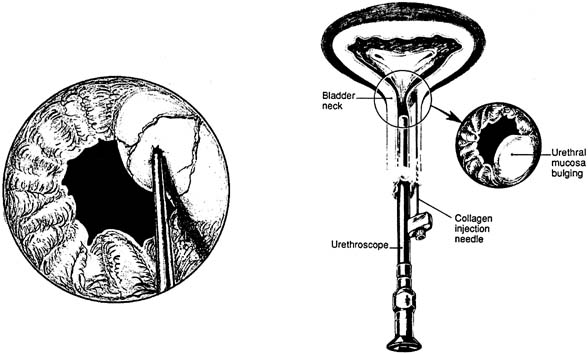
Injectable agents designed to bulk the proximal urethra and bladder neck have been developed as a minimally invasive, office-based procedure for the treatment of SUI. The numerous agents now in use and in development are primarily aimed for the treatment of hypomobile stress incontinence in patients with poorly functioning urethral sphincters. Cystoscopically guided placement of these agents, either transurethrally or periurethrally, under the urethral mucosa aids in proximal urethral and bladder neck closure during times of increased stress (Fig. 11). Although primarily aimed to treat patients with immobile bladder necks, patients with severe comorbidities precluding more extensive procedures for their stress incontinence may benefit from injections despite a hypermobile UVJ.58
Similar to the variations on slings and colposuspension, many agents have been used or are in trials as a urethral bulking substance (Table 4). The many substances reflects the desire for an agent that is easily injectable, is permanent and will not be degraded by the host, and does not migrate or cause a damaging host reaction. The most commonly used agent is sterile bovine dermal collagen that is cross-linked with glutaraldehyde (Contigen; Bard Collagen Implant). A skin test to detect an allergic response is required before injection. Cure rates generally range from 20% to 40% with up to 70–80% improved and satisfied at 1 year.59 Degradation of the foreign collagen, which may occur between 3 months and 3 years after placement, is the most common cause necessitating reinjection. Similar results have been seen with trials of carbon beads (Durasphere), the next most commonly used bulking agent, when compared with collagen.
Table 4. Periurethral bulking agents
Generic Name | Trade Name |
| Bovine cross-linked collagen | Contigen |
| Pyrolytic carbon coated beads suspended in a water-based gel | Durasphere |
| Silicone particles in a water-based carrier gel | Macroplastique |
| Dextranomer and stabilized hyaluronic acid gel | Zuidex |
| Spherical particles of hydroxylapatite in aqueous carrier gel | Coaptite |
Placement of artificial urinary sphincters tends to be performed by urologists in patients who have not had symptoms cured by other aforementioned interventions. Most of these devices involve an occlusive cuff placed around the proximal urethra that is fed by a reservoir situated in the labia majora for patient control. Consensus regarding suitable patients and efficacy is still in question because of small cohort studies and poor standardization. A success rate of between 80% and 90% is most quoted, but a significant rate of revision, explantation, or erosion of 5.9%60 to 17%61 has been seen.
A different, minimally invasive, treatment modality being developed involves radiofrequency thermal energy to cause tissue shrinkage at the bladder neck (SURx; Thermatrx). This procedure, performed as an outpatient procedure by a laparoscopic or vaginal approach, is designed to cause scarring at the bladder neck to restrict mobility. In a single small study of 94 women who underwent the laparoscopic procedure,62 subjective improvement, and cure rates, as well as objective cure (negative stress test) were seen in approximately 80% of patients. Further studies and longer follow-up are needed.
COMPLICATIONS OF INCONTINENCE SURGERY
Urge Incontinence
Although symptoms of urgency and frequency are not uncommon in the immediate postoperative period after any anti-incontinence procedure, patients must be warned before surgery regarding the possibility of persistence or de novo occurrence after any procedure. Furthermore, distinction between new or worsened urge incontinence and voiding dysfunction/outlet obstruction (discussed later) may be difficult in the postoperative period. Between 17% and 33% of patients presenting with SUI may have urge incontinence,63 with a good proportion having resolution of their symptoms after surgery. Unfortunately, approximately 15% (range: 5%–28%) will have de novo detrusor instability after a Burch or sling procedure.42, 47, 59 Fortunately, if symptoms are not too severe, treatment with anticholinergics and/or pelvic muscle rehabilitation may alleviate or resolve the condition in the majority.
Voiding Dysfunction
Voiding dysfunction may present in the postoperative period as the complete inability to void, only partial emptying, urgency-frequency syndrome, or other irritative voiding symptoms. It is not uncommon for patients to have some symptoms along this spectrum in the immediate postoperative period caused by bruising of tissues, hematomas, and pain. Persistence without improvement beyond 4 to 6 weeks may warrant intervention in patients with complete or almost complete obstruction, severe urgency-frequency or significant irritative symptoms not caused by infection and not responsive to conservative therapy. Urethrolysis is the term used when releasing an anti-incontinence procedure in this situation. This generally involves vaginal transection of a sling and release of periurethral scarring, or, in the case after a retropubic procedure, abdominal dissection of the space of Retzius and removal of sutures if possible. The majority of patients will have improvement or resolution of their symptoms after urethrolysis in the range of 60% to 88%. Unfortunately, there is a small group that will need to continue with self-catheterization, and approximately one-third will have recurrence of stress incontinence.
Surgical Failure
Failure of a surgical procedure usually results from one of four causes: (1) incorrect or incomplete diagnosis; (2) wrong procedure; (3) technical failure; and (4) new diagnosis or condition.
Urge incontinence as a primary diagnosis is unlikely to be cured by a procedure to correct stress incontinence. If a diagnosis of ISD is missed, an incorrect procedure may have been performed. Technical failures include incorrect suture placement, incorrect suture selection, tissue fragility, infection or hematoma, suture pull-through or dislodgment, and damage to surrounding structures. New symptoms may develop postoperatively, such as urge incontinence, detrusor instability, or a sphincter problem resulting in incontinence. To form an exact diagnosis and plan for therapy, a complete evaluation of the patient presenting with surgical failure should be instituted.
Coital Incontinence
Incontinence occurring with intercourse is a result of either stress or urge incontinence. In the former case, the patient has urine loss associated with penetration and/or thrusting. The increased intra-abdominal pressure results in a small but persistent loss of urine. Corrective actions include precoital voiding and altering coital positions. The effect of surgery is variable. Some patients may have the problem after surgery, while others have resolution after surgery. Although there are no studies to support the various theories, there seems to be a higher frequency of this problem after sling surgery than after other surgeries. Persistent problems are managed by pelvic floor muscle exercises and any other symptom-relieving maneuvers.
The second kind of intercourse-related incontinence occurs when detrusor activity is stimulated by physical contact near the bladder or by orgasm, which triggers a detrusor contraction. These patients often empty larger amounts of urine during intercourse compared with those with stress incontinence, and voiding immediately before intercourse only partly relieves the problem. Behavioral intervention and pharmacotherapy do not offer much improvement. It would be prudent to discuss these difficulties before surgical intervention.
Modifiable Risk Factors
Obesity and smoking are two modifiable factors that may put patients at risk for stress incontinence. As mentioned previously, altering these characteristics of the patient may be difficult, and no studies have demonstrated that smoking cessation or moderate weight loss will aid continence. That being said, certain aspects inherent to these patients must be considered when proceeding to surgery. Firstly, there may be an increased risk of venous-thromboembolic events and poor tissue healing with risk of wound breakdown. Increased abdominal pressure from excess coughing or abdominal girth always makes one concerned about suture disruption or pull-through. Because of this last concern, some surgeons prefer to use primary slings in these patients with the thought that they are more durable, although no data support this methodology. Either way, these patients must be counseled regarding increased surgical risk, whether from technical difficulties from excess soft tissue or from possible pulmonary compromise caused by underlying lung disease.
SUMMARY
Although some of the terminology has changed, the basic diagnosis of SUI remains the same. A deeper understanding of the anatomy and neurophysiology of the lower urinary tract has led to viewing sphincter dysfunction and hypermobile bladder neck on an overlapping spectrum of incontinence. Well-established conservative therapies such as pelvic muscle exercise programs and behavioral therapy are the cornerstone of conservative therapy. Surgery may be indicated for SUI after the proper work-up as outlined. The choice of a suburethral sling or retropubic Burch for incontinence are essentially the same in short-term and long-term success. The development of the new mid urethral slings (TVT) are a minimally invasive alternative for all stress incontinence with some mobility. In patients with a fixed urethra and SUI, urethral bulking agents continue to evolve from the tested, but temporary, bovine collagen to longer-lasting synthetics and other biomaterials.
Stress urinary incontinence is a condition that generalist physicians need be more cognizant of as life expectancy lengthens and the proportion of the elderly within the population increases. Patients suspected of having stress incontinence need to be evaluated with a history and physical, residual urine determination, urinalysis, 24-hour voiding diary, stress test, and cotton swab test. If preliminary evaluation reveals SUI, conservative therapy may be attempted without further testing. Patients who refuse or do not respond to conservative therapy should be evaluated, at the minimum, as dictated by the ACOG guidelines before consideration for surgery. Patients with recurrent stress incontinence or other complicated medical or surgical history, or those suspected of having a weak urethral sphincter, may benefit from ancillary testing such as multichannel urodynamics. All patients should be counseled regarding the risks of anti-incontinence surgery, and those with complications and voiding problems should be identified and evaluated accordingly.
REFERENCES
Bump RC, Norton PA: Epidemiology and Natural History of Pelvic Floor Dysfunction. Obstet Gynecol Clin 25:(4):723-746, 1998 |
|
Cundiff GW, Harris RL, Coates KW et al: Clinical predictors of urinary incontinence in women. Am J Obstet Gynecol 177:262-267, 1997 |
|
Olsen AL, Smith VJ et al: Epidemiology of Surgically Managed Pelvic Organ Prolapse and Urinary Incontinence. Obstet Gynecol 89:501-506, 1997 |
|
Abrams P, Cardozo L, Fall M et al: The standardization of terminology of lower urinary tract function: Report from the standardization sub-committee of the international continence society. Am J Obstet Gynecol 187:(1):116-126, 2002 |
|
Smith ARB, Hosker GL, Warrell DW: The role of patial denervation of the pelvic floor in the aetology of genitourinary prolapse and stress incontinence of urine: A nerurophysiological study. Br J Obstet Gynecol 96:24-28, 1989 |
|
DeLancey JOL, Kearney R, Chou Q et al: The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol 101:46-53, 2003 |
|
Boyle AL, Woodman PJ, O'Boyle JD et al: Pelvic organ support in nulliparous pregnant and nonpregnant women: a case control study. Am J Obstet Gynecol 187:99-102, 2002 |
|
Thom DH, Brown JS: Reproductive and Hormonal Risk Factors for Urinary Incontinence in Later Life: A Review of the Clinical and Epidemiologic Literature. J Am Geriatr Soc 46:(11):1411-1417, 1998 |
|
DeLancey JOL: Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. Am J Obstet Gynecol 70:1713-1723, 1994 |
|
Fantl JA, Newman DK, Colling J et al: Urinary incontinence in adults: acute and chronic management. Clinical Practice Guideline, No. 2, 1996 Update. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service, Agency for Health Care Policy and Research. AHCPR Publication No. 96-0682 March 1996 |
|
Farrell SA, Epp A, Flood C et al. The evaluation of stress incontinence prior to primary surgery. J Obstet Gynaecol Can 25:313-318, 2003 |
|
Abrams P, Andersson KE, Brubaker L et al. Recommendations of the International Scientific Committee. In Abrams P, Cardozo L, Khoury S, Wein A, eds. Incontinence. Health Publications Ltd, Paris, 2005, pp 1606-1609 |
|
American College of Obstetricians and Gynecologists, Committee on Quality Assessment, ACOG Criteria Set: Surgery for Genuine Stress Incontinence Due to Urethral Hypermobility. No. 4 1995 |
|
American College of Obstetricians and Gynecologists, Clinical Management Guidelines: Urinary Incontinence in Women. Number 63, June 2005 |
|
Bump RC, Mattiasson A, Bo K et al: The standardization of terminology of femal pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet. Gynecol 175:10-17, 1996 |
|
Sand PK, Bowen LW, Panganiban R et al: The low pressure urethra as a factor in failed retropubic urethropexy. Obstet Gynecol 69:399, 1987 |
|
Koonings PP, Bergman A, Ballard CA: Low pressure urethra and stress urinary incontinence in women: Risk factor for failed retropubic surgical procedure. Urology 36:245, 1990 |
|
Horbach NS, Blanco JS, Ostergard DR et al: A suburethral sling procedure with polytetrafluoroethylene for the treatment of genuine stress incontinence in patients with low urethral pressure. Obstet Gynecol 71:648, 1988 |
|
McGuire EJ, Fitzpatrick CC, Wan J et al: Clinical assessment of urethral sphincter function. J Urol 150:1452-1454, 1993 |
|
Sutherst J, Brown M, Shawer M: Assessing the severity of urinary incontinence in women by weighing perineal pads. Lancet 23:1128, 1981 |
|
Thind P, Gerstenberg TC: One-hour ward test vs 24-hour home pad weighing test in the diagnosis of urinary incontinence. Neurourol Urodyn 10:241, 1991 |
|
Hay-Smith J, Nygaard I, Wyman J et al. Adult conservative management. In Abrams P, Cardozo L, Khoury S, Wein A, eds. Incontinence. Health Publications Ltd, Paris, 2005, pp 855-964 |
|
Scheufele L, Abraham K. Conservative therapy for stress incontinence. Bent AE, Cundiff GW, Swift SE, eds. Ostergards Urogynecology and Pelvic Floor Dysfunction. 6th ed. Philadelphia. Wolters Kluwer, Lippincott Williams Wilkins. 2008. Pages 206-224 |
|
Magali R, Ross S. Conservative management of urinary incontinence. J Obstet Gynaecol Can 28:1113-1118, 2006 |
|
Bump RC, McClish DK: Cigarette smoking and urinary incontinence. Am J Obstet Gynecol 17:1213-1218, 1992 |
|
Deitel M, Stone E, Kassam HA et al: Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr 7:147-153, 1988 |
|
Bump RC, Sugerman HJ, Fantl JA et al: Obesity and lower urinary tract function in women: Effect of surgically induced weight loss. Am J Obstet Gynecol 166:392-329, 1992 |
|
Kegel AH: Progressive resistance exercises in the functional restoration of the perineal muscles. Am J Obstet Gynecol 56:238, 1948 |
|
Wells TJ, Brink CA, Diokno AC et al: Pelvic muscle exercise for stress urinary incontinence in elderly women. J Am Geriatr Soc 39:785, 1991 |
|
Peattie A, Plevnik S, Stanton S: Vaginal cones: A conservative method of treating genuine stress incontinence. Br J Obstet Gynecol 95:1049, 1988 |
|
Hay-Smith EJ, Herbison P, Morkved S: Physical therapies for prevention of incontinence in adults. Cochrane Protocol. 2001 |
|
Sand PK, Richardson DA, Staskin DR et al: Pelvic floor electrical stimulation in the treatment of genuine stress incontinence: A multicenter, placebo controlled trial. Am J Obstet Gynecol 173:72-79, 1995 |
|
Tsai EM, Yang CH, Chen HS et al: Bladder neck circulation by Doppler ultrasonography in postmenopausal women with urinary stress incontinence. Obstet Gynecol 98:52-56, 2001 |
|
Bump RC, Friedman CI: Intraluminal urethral pressure measurements in the female baboon: effects of hormonal manipulation. J Urol 136:508-511, 1986 |
|
Fantl JA, Bump RC, Elser DM et al: Efficacy of estrogen supplementation in the treatment of urinary incontinence. Obstet Gynecol 88:745-749, 1996 |
|
Gilgja I, Radeh M, Kovacic et al: Conservative treatment of female stress incontinence. J Urol 132:909, 1984 |
|
Lin HH, Sheu BC, Lo MC et al: Comparison of treatment outcomes for imipramine for female genuine stress incontinence. Br J Obstet Gynaecol 106:1089, 1999 |
|
Norton PA, Zinner NR, Yalcin I et al: Duloxetine versus placebo in the treatment of stress urinary incontinence. Am J Obstet Gynecol 187:40-48, 2002 |
|
Glazner CM: Anterior vaginal repair for urinary incontinence in women. Cochrane Database Syst Rev. January 2000 |
|
Magali R, Farrell SA. Choice of surgery for stress incontinence. J Obstet Gynaecol Can 27: 964-971, 2005 |
|
Delorme E. Transobturator urethral suspension: mini-invasive procedure in the treatment of stress urinary incontinence in women. Prog Urol 11: 1306-1313, 2001 |
|
Jarvis GJ: Surgery for genuine stress incontinence. Br J Obstet Gynecol 101:371-374, 1994 |
|
Alcalay M, Monga A, Stanton S: Burch colposuspension: a 10–20 year follow up. Br J Obstet Gynaecol 102:740-745, 1995 |
|
Ross JW: Multichannel urodynamic evaluation of laparoscopic Burch colposuspension for genuine stress incontinence. Obstet Gynecol 91:55-59, 1998 |
|
Nguyen JK: Transvaginal bladder neck suspension to Cooper's ligament: A review of the literature. Int Urogynecol J Pelvic Floor Dysfunct 11:(5):320-324, 2000 |
|
Appell RA: Primary slings for everyone with genuine stress incontinence? The argument for. Int Urogynecol J 9:249-251, 1998 |
|
Leach GE, Dmochowski RR, Appell RA et al: Female stress urinary incontinence clinical guidelines panel summary report on surgical management of female stress urinary incontinence. J Urol 158:875-880, 1997 |
|
Ostergard DR: Primary slings for everyone with genuine stress incontinence? The argument against. Int Urogynecol J 8:321-322, 1998 |
|
Morgan TO, Westney OL, McGuire EJ: Pubovaginal sling: 4 year outcome analysis and quality of life assessment. J Urol 163:1845-1848, 2000 |
|
Ulmsten U, Henricksson L, Johnson P et al: An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. Int Urogynecol J 7:81-86, 1996 |
|
Ulmsten U, Falconer C, Johnson P et al: A mulicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J 9:210-213, 1998 |
|
Olsson I, Kroon U: A three-year postoperative evaluation of tension-free vaginal tape. Gynecol Obstet Invest 48:267-269, 1999 |
|
Meschia M, Pifarotti P, Bernasconi F et al: Tension-free vaginal tape: analysis of outcomes and complications in 404 stress incontinent women. Int Urogynecol J Suppl 2:S24-S27, 2001 |
|
Sung VW, Schleinitz MD, Rardin CR et al. Comparison of retropubic vs transobturator approach to midurethral slings: a systematic review and meta-analysis. Am J Obstet Gynecol 197: 3-11, 2007 |
|
Barber MD, Kleeman SD, Karram MM et al. A multicenter randomized trial comparing the transobturator tape with tension free vaginal tape for the surgical treatment of stress urinary incontinence. J Pelvic Med Surg 13: 228, 2007 |
|
Schierlitz LHE, Dwyer PL, Rosamilia A et al. A randomized controlled study to compare tension-free vaginal tape (TVT) and Monarc trans-obturator tape in the treatment of women with urodynamics stress incontinence (USI) and instrinsic sphincter deficiency (ISD). Int Urogynecol J 18 (suppl 1): S19, 2007 |
|
Karram M, Lucente V, Khandwala S et al. An evaluation of the Gynecare TVT Secur system (tension-free support for incontinence) for the treatment of stress urinary incontinence. Int Urogynecol J 18 (Suppl 1): S3, 2007 |
|
Bent AE, Foote J, Siegle S et al: Collagen implant for treating stress urinary incontinence in women with urethral hypermobility. J Urol 166:1354-1357, 2001 |
|
Smith T, Daneshgari F, Dmochowski R et al: Surgical Treatment of Incontinence in Women. In: Abrams P, Cardozo L, Khoury S, et al (eds): Incontinence. pp 825-863, Plymouth, Health Publications Ltd, 2002 |
|
Costa P, Mottet N, Rabut B et al: The use of an artificial urinary sphincter in women with type III incontinence and a negative Marshall test. J Urol 165:1172-1176, 2001 |
|
Webster GD, Perez LM, Khoury JM et al: Management of type III stress urinary incontinence using artificial urinary sphincter. Urology 39:499, 1992 |
|
Fulmer BR, Sakamoto K, Turk TMT et al: Acute and long-term outcomes of radio frequency bladder neck suspension. J Urol 167:141-145, 2002 |
|
Bent AE: Etiology and management of detrusor instability and mixed incontinence. Obstet Gynecol Clin North Am 16:853, 1989 |