Syphilis
Authors
INTRODUCTION
Although syphilis rates have seen an overall decline since syphilis was first reported in the 1940s, only a few decades ago this disease was as widespread as chlamydial infection is today. During the first half of the 20th century, autopsy series indicated that syphilis affected 5–10% of the population.1, 2 After World War II, with the introduction of penicillin and aggressive public health efforts led by Surgeon General Thomas Parran, syphilis rates fell almost 95% from 66 cases per 100,000 persons in 1946 to 4 cases per 100,000 in 1956.3 Cutbacks in federal funding for syphilis control in the mid-1950s colluded with profound changes in sexual and substance use behaviors beginning in the 1960s to reverse this precipitous decline. The resulting syphilis epidemic hit the United States in a series of waves of increasing magnitude that culminated in a 1990 peak rate of 20.3 per 100,000 (Fig. 1). Although the rate of primary and secondary (P&S) or infectious syphilis in the United States declined 89.7% between 1990 and 2000, the rate of infectious syphilis increased between 2001 and 2006.4 Due to the historically low rates of syphilitic disease in 1999, the Centers for Disease Control and Prevention (CDC) launched the National Plan to Eliminate Syphilis in 1999.5, 6 However, recent resurgence of syphilis and other STDs in several parts of the country present a serious challenge to the national elimination effort that is underway.5, 7, 8
Overall increases in rates between 2001 and 2006 were observed primarily among men.9 After persistent declines since 1990, infectious syphilis rates among women increased from 0.8 cases per 100,000 population in 2004 to 0.9 cases per 100,000 population in 2005 to 1.0 case per 100,000 population in 2006. From the early 1960s to 1980, the syphilis epidemic was concentrated among homosexual men, but by the late 1980s, reported cases came largely from ethnic minority heterosexuals, particularly marginalized African Americans living in the South and in large cities. In both cases, the explosive spread of syphilis probably contributed to emerging HIV epidemics and may in turn have been accelerated by unrecognized HIV infection.10, 11
The biologic and behavioral relationships between ulcerative STDs and transmission and acquisition of HIV have been examined in multiple studies from many parts of the world.12, 13 Several prospective studies have documented increased incidence of HIV infection among patients with syphilis and, conversely, increased incidence of syphilis among HIV-infected persons.14, 15, 16, 17, 18, 19 A recent review of US studies with HIV seroprevalence data among syphilis patients highlights the high HIV seroprevalence in this population.20 Therefore, all patients with syphilis represent a critical HIV prevention opportunity and should be counseled and encouraged to undergo HIV testing. Equally important, risk assessment and screening for syphilis should be offered routinely to all patients who are HIV infected or at increased risk for HIV infection.20, 21
Syphilis remains a common cause of morbidity and mortality during pregnancy, despite the continued sensitivity of T. pallidum to penicillin, the widespread availability of inexpensive, accurate tests, and substantial efforts to encourage routine screening through early prenatal care. Surveillance data show that 85-90% of women reported to have primary and secondary syphilis in the United States are in the reproductive age group of 15–44 years.4 Prevalence rates of primary and secondary syphilis among pregnant women receiving prenatal care in urban teaching hospitals range from 2.2–3.9%.22, 23
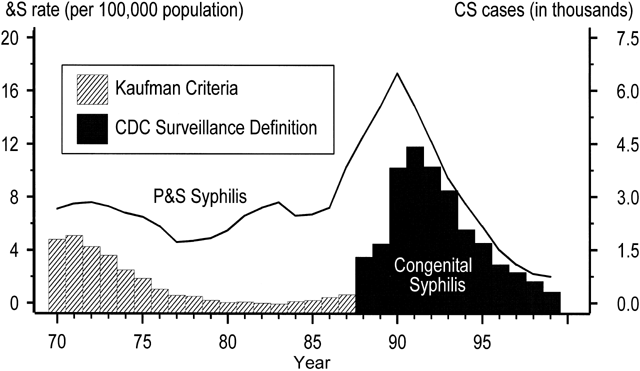
National surveillance data for congenital syphilis (CS) span five decades and roughly parallel trends among adult women (Fig. 2). The data reveal a dramatic increase in CS in the early 1990s both because of a revision in the CS case definition and because of the syphilis epidemic of the late 1980s in adults.24, 25, 26 Before 1989, criteria for reporting CS cases were based on a clinical case definition, such that only infants who had clinical disease or CS laboratory findings were reported as cases.24, 25 These criteria were specific, but infected liveborn infants without stigmata of CS often were missed, and the definition relied heavily on follow-up serologic data, which often could not be obtained.27, 28 After 1989, the sensitivity of the surveillance case definition was markedly increased by including all infants with clinical signs of CS, as well as clinically normal neonates and stillbirths delivered to women with untreated or inadequately treated syphilis. The attendant drop in specificity resulted in substantially more reported CS cases being identified in infants who were uninfected.24, 29 As Figure 2 shows, the number of reported CS cases increased by fourfold to fivefold after the case definition was changed.25, 30, 31
Adjustment of CS trends for changes in case definition reveals true increases that resulted from the second contributory factor, the dramatic increase in infectious syphilis among heterosexual adults. Trends in congenital syphilis usually follow trends in P&S syphilis among women, with a lag of one to two years. As expected, an upsurge in reported CS cases followed the adult epidemic of the late 1980s by about 12 to 18 months, with a peak CS rate of 107.3 per 100,00 live births in 19914 (see Fig. 2). CS rates declined by 92.4% to 8.2 cases per 100,000 live births in 2005.9, 32 (see Fig. 2 and Fig. 3). The rate of P&S syphilis among women declined 94.8% (from 17.3 to 0.9 cases per 100,000 females) during 1990–2005, and then increased to 0.9 and 1.0 cases per 100,000 population in 2005 and 2006, respectively.4 (Fig. 3).
After 14 years of decline in the United States, the rate of CS increased 3.7% between 2005 and 2006 (from 8.2 to 8.5 cases per 100,000 live births). Between 2005 and 2006, the overall rate of CS increased 3.7% in the United States, from 8.2 to 8.5 cases per 100,000 live births. Compared with other pregnant women, mothers delivering babies with CS are more likely to be adolescent, unmarried, and of lower socioeconomic status.33, 34 However, the most important risk factor for CS appears to be inadequate prenatal care,30, 34, 35 with data from the late 1970s showing mothers of babies with CS to have had, on average, significantly fewer prenatal visits (three to six visits)36 compared with all pregnant women in the United States (11 visits).37 Recent studies show that mothers affected by CS also are more likely to have had late or no prenatal care, or to have been a victim of missed screening opportunities by health care providers.33, 34 An evaluation of a recent, large CS epidemic in a US city reveals that 90% of cases of CS would have been preventable had adequate prenatal care and timely screening and treatment been in place.38
THE ORGANISM
Treponema pallidum subspecies pallidum is the motile, flagellated spirochete that causes syphilis. T. pallidum is a member of the order Spirochaetales, which contains two other genera of helical pathogenic bacteria: Borrelia and Leptospira.39 In addition to T. pallidum subspecies pallidum, which causes venereal syphilis, three other Treponema species are human pathogens: T. pallidum subspecies endemicum, which causes endemic syphilis (bejel), T. pallidum subspecies pertenue, which causes yaws, and Treponema carateum, which causes pinta.27 At least six other human nonpathogenic species comprise the normal flora of the mouth, genitalia, and gastrointestinal mucosal surfaces.39 Early studies suggest that the four pathogenic species had over 95% DNA homology.40, 41 However, recent genetic analyses have defined a region that distinguishes T. pallidum subspecies pallidum from T. pallidum subspecies pertenue and endemicum.42 Unfortunately, current serologic tests remain unable to distinguish among these subspecies.
T. pallidum is characterized by its long, thin, spiral morphologic characteristic and corkscrew motility. Its 6- to 20-μm length and 0.10- to 0.18-μm diameter, combined with its resistance to staining with commonly used bacterial stains, make the organism invisible through the light microscope.43 This requires the use of darkfield microscopy (Fig. 3) or immunofluorescent staining for visualization in clinical practice. Its shape and flagella-driven motility play critical roles in pathogen invasion and dissemination.44
T. pallidum is similar to gram-negative bacteria in that it has an outer membrane and a thin peptidoglycan cell wall; it differs from gram-negative bacteria in that its outer membrane lacks lipopolysaccharide and is more susceptible to disruption by physical or chemical manipulation.45, 46, 47, 48 The outer membrane of this organism also contains few transmembrane proteins. The paucity of these treponemal rare outer membrane proteins may partially explain the limited antigenicity of T. pallidum.49, 50
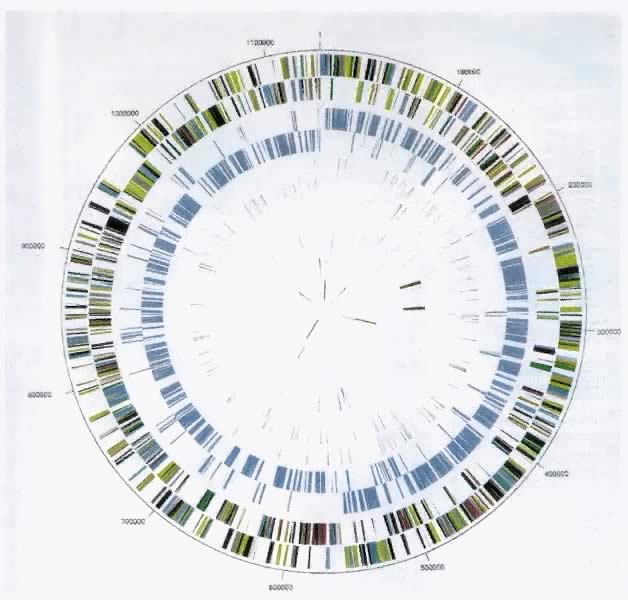
T. pallidum is unusual among human pathogens in that it can be cultured in vitro only after extended periods of laboratory incubation. Therefore, until recently, most of our understanding of the structure and function of T. pallidum was derived from studies of T. pallidum grown in animal models (usually rabbit testicles), from antigenic analysis, and, subsequently, from the cloning and expression of treponemal genes in Escherichia coli.51, 52, 53, 54, 55, 56, 57 However, sequencing of the entire genome of T. pallidum subspecies pallidum was completed recently.58 This scientific advance has lead to new insights about the physiologic features of T. pallidum subspecies pallidum, as well as molecular approaches to identifying distinct subtypes of the organism. It also holds great promise for development of vaccines, alternative diagnostic and therapeutic approaches, and in vitro culture methods. The genome is a circular chromosome containing 1,138,006 base pairs and 1041 open reading frames58 (Fig. 4). Detailed study of the genome has confirmed or extended many prior findings. For example, T. pallidum subspecies pallidum appears to have limited biosynthetic capacity and depends on requiring multiple nutrients from the host.58 Consistent with its preference for a microaerophilic environment, the organism has no genes encoding enzymes that protect against oxygen toxicity.59 Motility-associated genes appear to be highly conserved because of the importance of motility to pathogen invasion.58 Numerous genes encoding proteins similar to bacterial hemolysins and potential virulence factors also have been identified.58
Sequencing the genome for T. pallidum subspecies pallidum has lead to development of molecular subtyping of the organism. One newly developed polymerase chain reaction (PCR)-based T. pallidum subtyping technique detects heterogeneity in T. pallidum clinical subtypes using PCR amplification of a gene that codes for a putative acidic repeat protein (arp) and restriction fragment length polymorphism analysis of the T. pallidum repeat (tpr) gene.60 Subtyping could be an important adjunct to prevention and control of syphilis by distinguishing patients who are not epidemiologically related and by helping to identify the source of new cases.61
These advances also will facilitate vaccine development efforts. Although data from humans and animals clearly indicate that syphilis results in some degree of acquired immunity,62, 63, 64 previous attempts at complete, long-term protection have been achieved using only protracted immunization regimens (of as many as 60 injections) with inactivated T. pallidum organisms.65 Several recombinant antigens have been demonstrated to be promising vaccine candidates, including the enzyme glycerophosphodiester phosphodiesterase, members of the tpr family of proteins, and a 92-kd T. pallidum protein called Tp92.66, 67, 68
TRANSMISSION AND PATHOGENESIS
Syphilis is transmitted primarily by sexual exposure to a partner with an active, infectious lesion.69 The next most common mode of transmission is by transfer across the placenta from mother to an unborn infant.69 No published studies document transmission of syphilis through breast milk. Nonsexual transmission by accidental inoculation in laboratory or health care settings by exposure to infected blood has been described.70, 71, 72, 73
For a susceptible sexual partner or a fetus, transmission risk is related to the stage of disease of the infected person, which likely correlates with the number of organisms present in lesions and blood. An individual is most infectious during primary and secondary syphilis and probably is significantly less infectious after the first year, when early latent syphilis ends and late latent infection begins.74 Most studies estimate that approximately 30–60% of identified sexual contacts to persons with primary, secondary, or early latent syphilis will develop clinical or serologic evidence of infection.70, 71, 74, 75
Although vertical transmission is more efficient, with rates of at least 80% in the presence of primary and secondary or early latent syphilis, the same stage-related pattern is observed.35, 76 For example, Fiumara and colleagues found that approximately 50% of pregnancies in mothers with primary or secondary syphilis resulted in infants who were premature, stillborn, or who died in the neonatal period, whereas the remainder developed CS. With early latent syphilis, approximately 40% of pregnancies terminated in premature births or perinatal death, and 40% of infants developed CS. In contrast, 70% of the infants born to mothers with late latent syphilis were healthy, approximately 10% had CS, and roughly 20% were either born prematurely or died perinatally35 (Table 1). According to Kassowitz's law, the longer the interval between infection and pregnancy, the more benign is the outcome in the infant.33 Nevertheless, despite the substantial decrease in mother-to-child transmission seen in late-stage disease, any stage of maternal syphilis can result in fetal infection.77, 78
Table 1. Pregnancy outcomes among women with early or late syphilis compared with women without syphilis
Stage of maternal syphilis | |||
Pregnancy outcome | Untreated early syphilis | Untreated late syphilis | No syphilis |
Normal full-term live infant | 19% | 72% | 88% |
Premature infant without syphilis | 23% | 6% | 9% |
Congenital syphilis | 43% | 6% | — |
Stillbirths and neonatal deaths | 20% | 12% | 2% |
Based on data from Ingraham76 and Fiumara et al.35
In the United States and other industrialized countries, acquisition of syphilis through blood transfusion is rare. Early studies documented survival of treponemal organisms for up to 5 days in refrigerated blood, making this a viable route of transmission.79, 80 However, routine serologic testing of all blood donors and a shift from transfusion of fresh blood to transfusion of refrigerated blood components in modern blood banks have minimized transfusion-related transmission.81 The importance of continuing to screen transfusion products was underscored by a study demonstrating that T. pallidum DNA could be amplified from whole blood of persons known to have untreated syphilis (from incubation to latency) or a significant exposure to a person with infectious syphilis.82 Reports of transfusion-related syphilis being transmitted from patients with latent syphilis of several years' duration suggest that even these patients may intermittently seed the bloodstream with T. pallidum.82, 83, 84, 85, 86
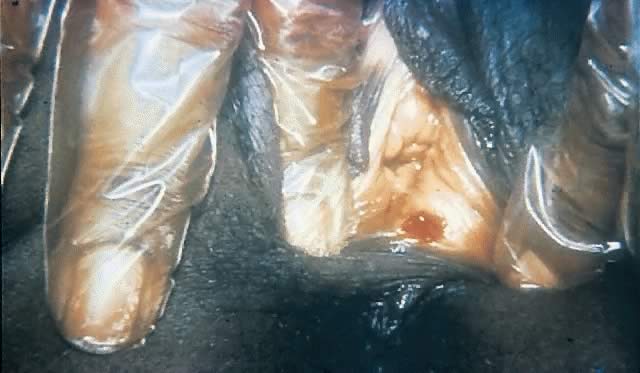
The pathogenesis of syphilis is not completely understood but is characterized across all stages by vasculitis (endarteritis and periarteritis) and, in gummatous lesions (see later), by granulomas. After sexual exposure, T. pallidum subspecies pallidum invades the body through mucous membranes or abraded skin.87, 88 It rapidly attaches to host cells and begins to multiply.69 Within hours, organisms appear within regional lymph nodes and disseminate to multiple organs through the circulation using their corkscrew motility to escape through the tight junctions of vascular endothelium.69, 89, 90 The primary ulcerative lesion, or chancre (Figs. 5 and 6), develops at the site of inoculation with the recruitment of a dense perivascular infiltrate of mononuclear cells, which includes HIV target cells such as CD4+ lymphocytes, as well as plasma cells and histiocytes.91, 92 This usually occurs within 2–6 weeks after infection but is dependent on inoculum size, with large inocula (e.g., 107 organisms) resulting in chancre formation within a week.62, 63 Studies of intracutaneous inoculation in human volunteers indicate that for T. pallidum subspecies pallidum, the dose at which 50% of individuals will become infected may be less than 60 organisms,62 and the median incubation period of 3 weeks suggests an average inoculum of 500–1000 organisms.91 However, chancres often go unnoticed by patients, and many patients present after this stage has passed.
The signs and symptoms of secondary syphilis are caused by the dissemination and proliferation of treponemes in affected tissues. This dissemination and deposition of lymphocytes and plasma cells in the dermis, which characterizes secondary syphilis, begins during the formation of the primary chancre, but the manifestations appear approximately 4–12 weeks after initial infection.93, 94 The deposition of circulating immune complexes in affected organs, such as the liver or kidneys, also is a major factor in the pathogenesis of the lesions of secondary syphilis.95, 96
Both the latent and tertiary stages of syphilis are characterized by the presence of treponemes in tissues. However, the pathogenesis of these stages is not completely understood, and the pathogenic trigger that causes progression from the latent to tertiary stage continues to be investigated. During the latent stage of syphilis, treponemes present in tissues exhibit little to no replication.83 This period of quiescence results from a relative equilibrium between treponemal activity and host response that does not invoke acute disease. A waning of the host immune response may break this equilibrium and lead to the increased treponemal activity of tertiary syphilis.83 The pathogenesis of tertiary syphilis is characterized by the inflammatory invasion of treponemes in major organs, such as the brain, heart, eyes, and skin, and activation of a delayed-type hypersensitivity response.83
As in adults, the pathogenesis of CS results from an obliterative endarteritis characterized by perivascular mononuclear and plasma cell infiltrates, intimal hyperplasia, and bloated, hyperplastic endothelial cells.97, 98 Both treponemal lipoproteins and intact, motile organisms activate vascular endothelium.99 In contrast to the distinct histopathologic characteristics that are associated with different stages of adult disease, however, fibrosis and gummas may accompany acute, inflammatory changes in CS. Fulminant vascular inflammation, stromal hyperplasia, and gumma also involve the placenta, frequently resulting in gross enlargement with weights as much as one third that of the fetus.100, 101, 102 Necrotizing funisitis, an aggressive inflammatory process of the umbilical cord marked by a distinctive, swollen, “barber pole” appearance of the cord, is highly suggestive of CS, occurring in 33% of umbilical cords of women with untreated syphilis.103, 104 It also is frequently associated with a poor perinatal outcome, especially in preterm infants.
Most infants with CS are infected transplacentally in utero, but the newborn also can be infected by contact with an active genital lesion.35, 71, 72 Although mother-to-infant transmission theoretically may occur as a result of contact with infected blood at delivery, no published data document this occurrence. Previously, many believed that fetal infection does not occur before 18 weeks of gestation because of protection by the Langhans' cell layer of the cytotrophoblast early in pregnancy. However, recent research indicates that vertical transmission may occur at any point during pregnancy in an infected mother, with the risk of fetal infection increasing with duration of gestation.105, 106, 107, 108 Typical histopathologic evidence of fetal infection may, however, be muted before 18 weeks of gestation because of the immaturity of the fetal immune system at that stage.109
CLINICAL MANIFESTATIONS AND NATURAL HISTORY
Syphilis is classified in sequential clinical stages to guide patient treatment, management of sexual partners, and, in the case of pregnant women, newborn care. Despite their clinical and public health utility, these stages are neither precise nor mutually exclusive. Symptoms and signs of early syphilis often are missed or confused with manifestations of other diseases. The temporal distinction between early and late latent infection is somewhat arbitrary. Furthermore, in individual patients, stages may overlap (Fig. 7). However, the stages discussed in this section offer a critical conceptual framework for clinical management decisions.
Primary Syphilis
The ulcer, or “chancre” (see Figs. 5 and 6), which is the defining manifestation of primary syphilis, usually develops from a painless papule about 3 weeks after exposure, with the range of the 10- to 90-day incubation period being directly related to the size of the T. pallidum inoculum.62, 110 Even in the absence of therapy, syphilitic chancres resolve spontaneously in 2–6 weeks.
Although traditionally characterized as a single, painless (unless secondarily infected), indurated, clean-based ulcer that often is associated with regional adenopathy, syphilitic chancres probably occur as multiple lesions at least as frequently and meet this classic description in less than half of cases.1 In one study, the classic constellation of chancre signs was 98% specific but only 31% sensitive for a diagnosis of syphilis in men.111 Ulcers from syphilis usually range from 0.3 to 3.0 cm in diameter and occur at the site of infectious contact.1 Chancre location and degree of associated discomfort are important determinants of whether and when ulcers are detected by patients, thus triggering them to seek care. In female patients, the most common chancre locations are the labia majora, labia minora, fourchette, cervix, and perineum.69, 112, 113 In pregnant women, cervical chancres are common, with deposition of treponemes on the often-friable cervix.114 As a result, signs of primary syphilis often go unrecognized in women. Extragenital lesions also may occur on the lips, oropharynx, nipples, and fingers, although these are uncommon, occurring in approximately 2% of patients in one large series.112, 113 A history of oral or anal intercourse always should prompt examination for chancres in these locations.
In the United States, genital ulcers are most commonly caused by infection with herpes simplex virus (HSV), followed by syphilis and chancroid.115 In different areas and clinical settings, however, the relative importance of specific etiologies varies substantially.111 Both because of the limited sensitivity of clinical diagnosis of genital ulcer disease for distinct infectious etiologies and because mixed infections (e.g., HSV and syphilis) are common, all genital ulcers should be tested for T. pallidum infection, regardless of ulcer morphologic features.111, 115 Other considerations in the differential diagnosis of genital ulcers are summarized in Table 2.
Table 2. Differential diagnosis of genital ulcers
Infectious agent | Classic ulcer characteristics | Laboratory diagnosis | Treatment | |
Genital herpes | Herpes simplex virus type I or type II | Multiple pustules and vescicles coalesce to form painful ulcers | Culture of lesion or herpes simplex virus serology | Acyclovir or famciclovir or valacyclovir |
Syphilis | Treponema pallidum | Single, painless papule, which necroses to an indurated, clean-based chancre | Darkfield examination or direct immunofluorescence test for T. pallidum; serology | Benzathine penicillin |
Chancroid | Haemophilus ducreyi | Ragged edges, without induration; purulent exudate at ulcer base | Culture for H. ducreyi | Azithromycin or ceftriaxone or ciprofloxacin or erythromycin base |
Granuloma inguinale | Calymmatobacterium granulomatis | Vascular, painless, progressive, ulcerative lesions without regional adenopathy | Visualization of dark-staining Donovan bodies on tissue crush preparation or biopsy | Trimethoprim-sulfamethoxazole or doxycycline |
Lymphogranuloma venereum | Chlamydia trachomatis serovars L1, L2, or L3 | Small, nonindurated, genital papule, which ulcerates quickly; associated with acute lymphadenitis | Complement-fixation test, with titer of | Doxycycline or erythromycin base |
Secondary Syphilis
Lesions of secondary syphilis result from systemic, hematogenous spread of organisms and usually follow by 4–10 weeks the development of primary chancres.103 Overlap between the two stages therefore occurs, with as many as a third of patients with secondary syphilis having a chancre present.112, 113 In untreated patients, the lesions of secondary syphilis usually resolve within 3–12 weeks but can persist for several months.69, 116
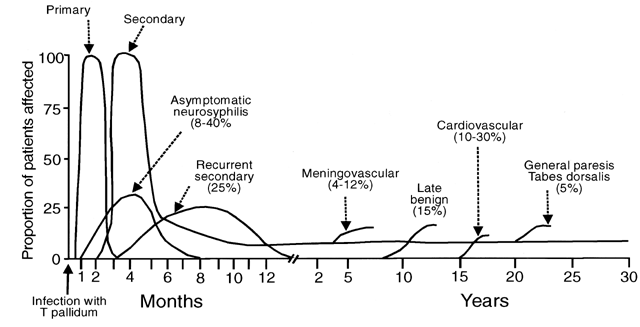
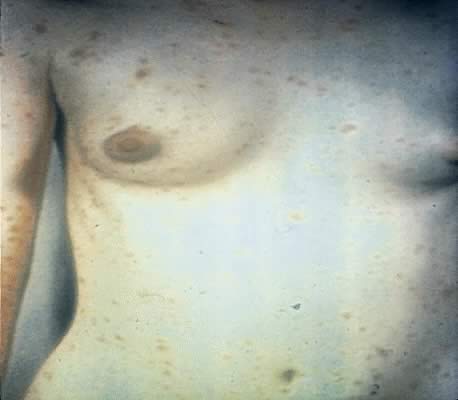
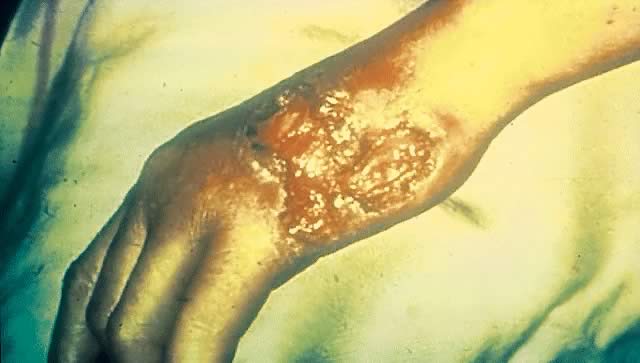
Secondary syphilis is manifested by highly variable skin and mucous membrane lesions, constitutional signs and symptoms, and local organ involvement.69, 110, 112, 116 An evanescent macular eruption, which evolves into a maculopapular or papular rash on the trunk and extremities (Fig. 8), is the most common finding in secondary syphilis, occurring in at least 90% of patients in some series.112, 113 Lesions often are particularly noticeable on the palms and soles and are traditionally described as resembling “copper pennies” because of their round, red, or reddish brown appearance112, 113 (Fig. 9). Papules vary from 0.5 to 2 cm in diameter, often cause pruritus, and can be confused with the lesions of psoriasis, pityriasis rosea, and drug eruptions.107 These skin lesions may heal with scarring or altered pigmentation, although most heal without any sequelae.112, 113
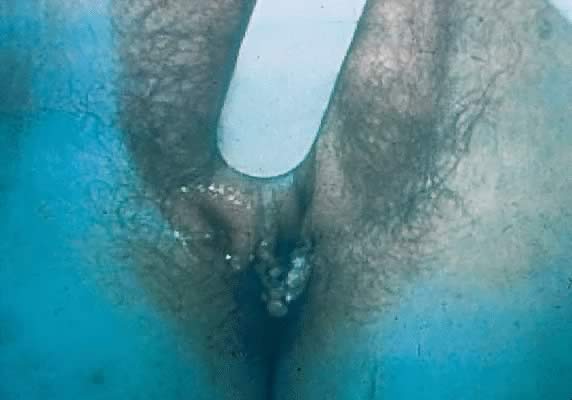
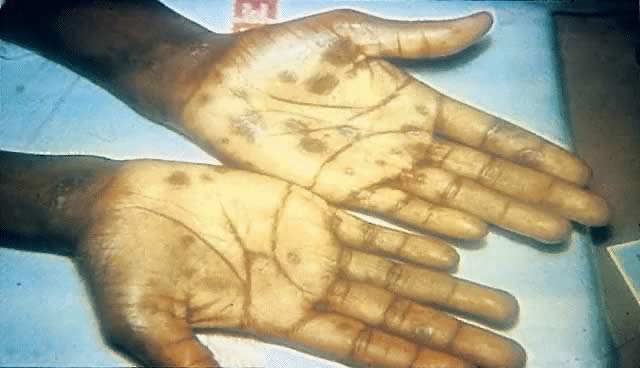
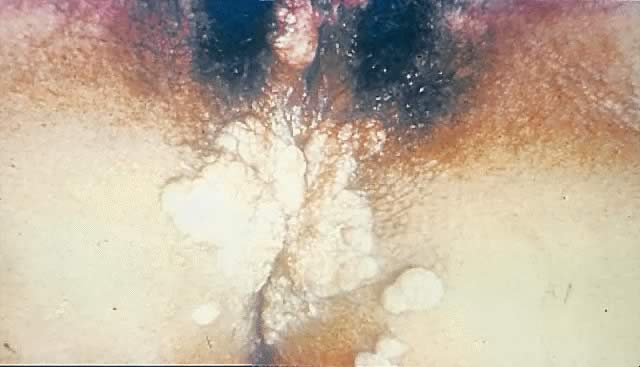
Other manifestations of secondary syphilis include condyloma lata, which occur in 10–15% of patients112 (Fig. 10). These large, raised, white or gray lesions are highly infectious, often occur in an area near a primary chancre, and may result from the direct spread of treponemes from the primary lesion in the anogenital region.116 Condyloma lata are readily confused with condyloma acuminatum, the warty genital lesions that are caused by human papillomavirus, making it important to screen for syphilis when any ambiguous condylomatous genital lesions are identified. Mucus patches—shallow, painless, aphthous ulcer-like plaques that occur in as many as one in five patients—typically are found on the tongue, buccal mucosa, lips, and cervix.112, 116 Secondary syphilis also is an important curable cause of alopecia.
Systemic signs and symptoms associated with secondary syphilis include fever, generalized lymphadenopathy, malaise, headache, weight loss, and myalgia.112, 113 Some lymph node enlargement is present in 70–85% of patients; the most commonly involved nodes are inguinal, axillary, posterior cervical, femoral, and epitrochlear.112, 113 Asymptomatic meningitis occurs in as many as 40% of secondary syphilis patients, subclinical hepatitis in roughly 20%, and jaundice, glomerulonephritis, ocular complications, and sensorineural deafness in smaller but significant proportions.116, 117, 118, 119
Latent Syphilis
After spontaneous resolution of secondary syphilis, the untreated patient, for a variable period of time, is completely free of symptoms and signs of the persistent infection being harbored. This state, in which only serologic evidence of infection can be found, is called latent syphilis. It is arbitrarily further classified into early and late latent stages to guide therapy in light of data indicating that 24% of patients with latent syphilis experience at least one relapse to infectious, secondary disease and that about 90% of these relapses occur within the first year of latency.110, 116, 120, 121 Patients with latent syphilis of less than 1 year's duration are, therefore, considered by the Centers for Disease Control and Prevention to have early latent infection and to be potentially infectious, whereas patients with latent syphilis of more than 1 year's duration carry a diagnosis of late latent infection and are deemed relatively noninfectious, as well as increasingly immune to reinfection. The World Health Organization uses a 2-year cutoff to distinguish between early and late latent infection. Roughly two-thirds of untreated patients with latent syphilis maintain this status for the rest of their lives, but patients often remain reactive to treponemal tests, even if nontreponemal test results become negative in the latent stage of syphilis.83
Tertiary Syphilis
The three late manifestations of untreated syphilis are called tertiary syphilis and include neurosyphilis, cardiovascular, and gummatous disease. In the preantibiotic era, tertiary syphilis was recognized in 15–40% of large cohorts of patients with untreated infection, resulting in estimates that almost a third of untreated patients may develop these potentially fatal complications and that about 20% died as a direct result of them.121, 122, 123, 124 Tertiary syphilis may occur from months to many years after initial infection, and patients frequently experience more than one of the three syndromes. However, with the advent of both antibiotics and HIV infection, the incidence and timing of the manifestations of tertiary syphilis appear to have changed.
Neurosyphilis currently is the most important of the tertiary complications of syphilis, both because it is probably the most common and because it is frequently severe, when it does occur. However, neurologic involvement from syphilis is not restricted to late-stage disease. Many patients with syphilis have cerebrospinal fluid (CSF) invasion by treponemes during the first year but remain asymptomatic, with CSF abnormalities such as elevated leukocyte count and protein, and reactive CSF Venereal Disease Research Laboratory (VDRL) test in the absence of clinical disease.125, 126, 127 In these patients, the degree of CSF abnormality correlates with the risk of subsequent development of symptomatic neurosyphilis.128, 129 Symptomatic disease ranges from meningitis, which generally occurs within the first year, to meningovascular and parenchymous involvement, which usually present 4–12 years and 15–20 years after initial infection, respectively.110, 128 Thus, neurosyphilis should be considered a continuum that includes tertiary manifestations but that can and does occur at any time during the life of a patient with untreated syphilis.
Syphilitic meningitis often presents with headache, nausea, vomiting, confusion, and nuchal rigidity.129, 130 Cranial nerve palsies are frequent and most commonly cause visual disturbances, sensorineural hearing loss, or facial weakness.
Meningovascular syphilis refers to central nervous system (CNS) ischemia or infarction as a result of syphilitic endarteritis. It occurs in approximately 3% of untreated syphilis patients and accounts for about 10% of neurosyphilis cases.121, 122, 123, 124, 125 Any part of the CNS may be involved, and symptoms reflect lesion location. They may include hemiparesis or hemiplegia, aphasia, seizures, headaches, impaired memory, apathy, vertigo, and insomnia.125, 128 Involvement of the third cranial nerve may result in a characteristic small, fixed Argyll Robertson pupil.128, 131
Parenchymous neurosyphilis includes both general paresis and tabes dorsalis. Older studies suggest that together these syndromes occurred in about 5% of individuals with untreated syphilis and that general paresis accounted for about 10% of neurosyphilis, whereas tabes dorsalis was diagnosed in about a third of neurosyphilis cases.121, 122, 123, 124, 125, 128 General paresis is a progressive dementia caused by treponemal invasion of the cerebrum and evidenced by gradual impairment of memory and cognitive functions, irritability, personality changes, psychosis, and, ultimately, death.130 Seizures, tremors, and impaired speech or handwriting may be the presenting complaint. Tabes dorsalis is characterized by demyelinization of the dorsal ganglia, dorsal roots, and posterior columns. Early clinical features include sensory ataxia, lightning pains of the lower extremities (75–90% of patients), autonomic disturbances, paresthesia, and altered pupillary light responses.125, 126, 130 Pains with hyperesthesia worsen as the disease progresses.121 Patients may experience severe epigastric pain, nausea, vomiting, ataxia, bladder disturbances, impotence, and impaired position and vibration sense.125, 126, 130 Argyll Robertson pupils and Charcot's joints (trophic degeneration) also are reported.130, 131
Cardiovascular syphilis is rarely diagnosed currently but may have accounted for 10–15% of all clinical cardiovascular disease in the preantibiotic era.69, 128, 132, 133 Studies from that period suggest that at least 10–30% of patients with untreated syphilis developed cardiovascular complications, with autopsy series producing estimates ranging from 30–80%.121, 122, 123, 124 Cardiovascular problems usually occur 15 to 30 years after primary infection and result from local inflammation caused by the multiplication of treponemes within the wall of the aorta.128, 134 The ascending aorta is most commonly involved in the aortitis of cardiovascular syphilis, with endarteritis obliterans of the vasa vasorum producing medial necrosis and resulting, most frequently, in aneurysms of the aorta or other large vessels, aortic insufficiency, and congestive heart failure.128, 132, 135, 136, 137 Although syphilitic aortitis often is uncomplicated and asymptomatic, about 20% of patients with aortitis have symptoms of substernal dull, aching pain, and 25% experience heart failure.69, 133
Like cardiovascular syphilis, the incidence of gummatous syphilis has declined substantially from as high as 15% of untreated syphilis patients in the preantibiotic era to a relatively rare diagnosis.121, 128, 134 The gummas of tertiary syphilis probably result from delayed-type hypersensitivity reactions to treponemal antigens and are locally destructive, recurrent lesions of varying size that usually occur in the skin and bones but also may involve mucous membranes of the oropharynx, visceral organs such as the liver, heart or brain, ocular structures, and muscles, with the symptoms and severity of the consequences being related to the location74, 138 (Fig. 11). Most gummas develop 2 to 45 years after initial infection, with most occurring after 10 to 15 years.139 Skeletal gummas commonly cause nocturnal pain and local swelling because of the associated periostitis.128 Because gummas contain few treponemes and respond rapidly to antibiotic treatment, this late manifestation of syphilis also is called late benign syphilis, despite the lethal consequences of myocardial and nervous system lesions.74, 134
Congenital Syphilis
Congenital syphilis manifestations are divided into two stages—early (less than 2 years), and late (more than 2 years). Early clinical signs may include lesions seen in cases of secondary syphilis, pneumonia, or failure to thrive.69, 72, 73 Most manifestations develop within the first 3 months of life and include persistent rhinitis (snuffles) consisting of a highly infectious, profuse, purulent or blood-tinged nasal discharge, hepatosplenomegaly, palmar and plantar bullae, glomerulonephritis, generalized lymphadenopathy, erythematous maculopapular rash, petechia, anemia, jaundice, abnormal bone radiographs, and pseudoparalysis.97, 140 CSF abnormalities in the absence of other symptoms occur in up to 80% of infected infants.141
Osteochondritis in the long bones affects more than 80% of infected infants142 and most often is diagnosed radiographically in the femur, tibia, and radius.143 It causes a painful, flaccid pseudoparalysis, usually within 8 months of life.143 Radiographic findings for osteochondritis are specific and require approximately 5 weeks to become demonstrable: a classic “celery stick” appearance is caused by proliferative and destructive changes in the affected bones, usually in areas of rapid bone growth such as the periosteum.144 Another bone lesion, diaphyseal periostitis, is asymptomatic, does not produce radiologic signs until after 12–16 weeks of age, and most commonly affects the tibias, the tubular bones of the hands and feet, and the clavicles and skull.76, 144
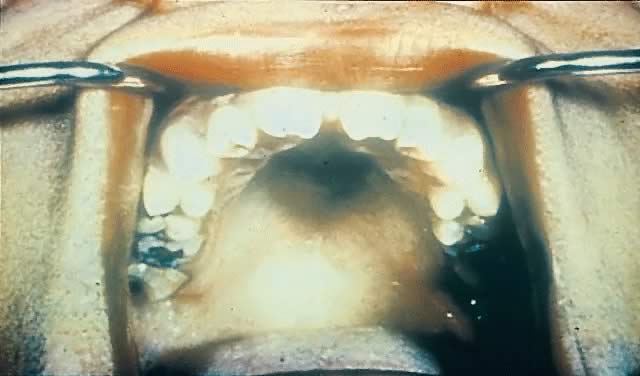
Late CS is preventable by diagnosis and treatment of early infection. Late manifestations of CS are not infectious because they are the delayed consequences of earlier treponemal inflammatory processes.145, 146 They often occur near puberty and include Hutchinson's triad of interstitial keratitis, peg-shaped upper incisors, and eighth cranial nerve deafness.147 Keratitis is the most common manifestation of the triad (20–50%)148 and involves unilateral photophobia, pain, excess tearing, and blurred vision.69, 148 Its onset usually is between 5 and 16 years of age and occurs more commonly in female than male patients.69 The peg-shaped upper incisors (Hutchinson's teeth) result from misshapen and hypoplastic permanent teeth in late CS149 (Fig. 12). Hearing loss, the least common symptom of Hutchinson's triad, often occurs by age 10 and results from osteochondritis causing cochlear degeneration.150
Other characteristic findings of late CS include frontal bossing, short maxillae, saddle nose, palatal deformations, bilateral knee effusions, sternoclavicular thickening, flaring scapulas, mental retardation, hydrocephalus, and neurosyphilis.141, 150, 151
Syphilis in HIV-Infected Persons
Clinical manifestations of syphilis are largely unchanged in persons with HIV infection. However, a few human and animal studies suggest more rapid and more severe manifestations of syphilis with concurrent HIV infection, likely because of compromise of immune function.152, 153, 154 Patients with HIV infection may be more likely to present in the secondary stage of syphilis.152 Those with secondary syphilis may more often have concurrent manifestations of primary disease, such as chancres or ulcerating skin lesions.153 In addition, an increased incidence of neurologic sequelae, such as meningitis and ocular syphilis, has been reported in patients coinfected with syphilis and HIV.155
CLINICAL AND LABORATORY DIAGNOSIS
Clinical Evaluation and Darkfield Examination
Everyone who has ever been in a nonmutually monogamous sexual relationship is potentially at risk for syphilis. The clinician must assess the magnitude of each individual's risk by taking a thorough sexual history that includes both patient's and partners' sexual and drug use behaviors, anatomic sites and timing of recent sexual exposure, contraceptive practices, prior STD diagnoses, and relevant symptoms. In light of the protean clinical manifestations with which syphilis may present, a comprehensive physical examination is critical, with particular attention to the orogenital areas, lymph nodes, trunk, and extremities. Laboratory screening in asymptomatic women without any signs of syphilis is particularly important during pregnancy because of the risk to the fetus but is also important for nonpregnant adults who live in communities with high syphilis rates or who are personally at high risk. However, laboratory tests always should complement, not replace, the history and clinical evaluation in establishing a diagnosis of syphilis and defining the stage of infection.
Direct detection methods such as darkfield microscopy or direct fluorescent antibody tests that identify treponemes in lesion exudate or tissue are the definitive diagnostic approaches for early syphilis.156 Darkfield microscopy is the most specific and rapid clinic-based method to diagnose primary and secondary syphilis in patients with chancres or other skin or mucous membrane lesions, particularly moist lesions such as condyloma lata or mucous patches. Direct examination of lesion exudate for motile spirochetes by darkfield microscopy may be particularly useful in early primary syphilis, before a serologic response can be detected. After lesions are gently cleaned and abraded with sterile gauze, exudate should be expressed from the lesion and collected on a coverslip or microscope slide for immediate examination.156 With an experienced laboratory technician or health care provider, the sensitivity of such an examination approaches 80%.156 However, darkfield microscopy by persons with limited experience may lead to false-positive or false-negative results, since other spiral organisms can resemble T. pallidum. Indeed, darkfield examination of oral lesions should not be performed because nonpathogenic, oral treponemes cannot be distinguished from T. pallidum.156 In evaluating oral lesions or when specimens cannot be examined immediately, the direct fluorescent antibody-T. pallidum (DFA-TP) test offers an alternative approach if a fluorescent microscope is available. This T. pallidum-specific test circumvents the need to distinguish among spirochetes on the basis of morphologic features and motility.156 A multiplex polymerase chain reaction assay that detects the three principal causes of genital ulcers—HSV, Haemophilus ducreyi, and T. pallidum (see Table 1)—is a third, new method to diagnose syphilis from lesion specimens.157 This test is not available for routine clinical use but appears to be more than 90% sensitive for each organism and highly specific.158 However, the clinician must remember that a negative result on any of these tests does not rule out the diagnosis of syphilis.
Serologic Tests
In part because skin and mucosal lesions are not present in latent and tertiary disease, most cases of syphilis are diagnosed presumptively using serologic tests to detect antibodies to T. pallidum rather than the organism itself.156 Two types of serologic tests, nontreponemal (or reaginic) and treponemal tests, are used sequentially. Nontreponemal tests usually are used in initial screening because of their low cost, widespread availability, and ease of performance. Reactive specimens on nontreponemal tests should be titered and followed over time to give a quantitative measure of response to therapy. A fourfold or two-dilution change in titer (e.g., from 1:32 to 1:8) usually is the minimum change that is considered clinically significant.133 Treponemal tests are used to confirm the diagnosis, and in most of the successfully treated patients, results remain positive for many years, if not for the duration of the patient's life.110, 156
The most common nontreponemal tests include the VDRL and the rapid plasma reagin (RPR) tests. These tests detect antibodies directed against an antigen composed of cardiolipin, cholesterol, and purified lecithin—antibodies that may be produced not only in response to T. pallidum and other treponemal infections, but also in response to nontreponemal diseases that cause the release of lipid debris from damaged host cells. Although the nontreponemal tests exhibit specificities of approximately 98% and peak sensitivities in secondary and latent syphilis of 95–100%, their sensitivities may be poor during the primary (78–86%) and late (71–73%) stages.156 Even without therapy, nontreponemal titers often fall to relative low levels (less than 1:4) in patients with late latent infection. Roughly a quarter of patients undergoing the VDRL test eventually revert to being nonreactive.110
Because the antigens used in nontreponemal tests include normal components of host cells, biologic false-positive (BFP) reactions are common. Their frequency varies with the test being used and the population being tested. Nontreponemal BFP reactions usually are positive at low titers (less than 1:8) and are associated with a broad range of acute and chronic conditions such as viral hepatitis, mononucleosis, HIV infection, pneumococcal pneumonia, bacterial endocarditis, tuberculosis, malaria, increased age, pregnancy, intravenous drug use, malignancy, and autoimmune diseases.110, 156 Confirmatory testing with treponemal tests is, therefore, essential to establish a diagnosis of syphilis. Treponemal tests include the serum fluorescent treponemal antibody absorption test (FTA-ABS) and the microhemagglutination test for T. pallidum. These tests detect antibodies against treponemal antigens and have higher sensitivities than the nontreponemal tests, especially in late disease.156 False-positive results also may occur with treponemal tests in the context of diseases such as lupus, mononucleosis, Lyme's disease, relapsing fever, and leptospirosis.74 However, if both of these treponemal test are reactive, the probability of a BFP reaction is 5% or less.159
False-negative results or weakly reactive nontreponemal tests occur in 1–2% of patients with secondary syphilis and may result from prozone reactions.160, 161 This reaction occurs when nontreponemal antibody is present in high concentration or blocks the normal antigen-antibody reaction.156 Dilution of the serum sample reduces the high antibody concentration and gives an easily detectable positive test result.
Rapid serologic tests that are easily performed and allow point-of-care diagnosis and treatment in a single clinical visit are particularly important in field settings where the need for multiple return visits creates obstacles to timely and effective prevention and control measures.162, 163 Several tests are under development or commercially available in other countries, but none are on the market in the United States.162, 163, 164, 165 A recently developed, rapidly performed, on-site RPR test using plasma (rather than serum) offers a qualitative result within 10 minutes but risks undertreatment, since low titers (less than 1:16) may give a falsely negative result.162, 163 A newly developed, visually read, rapid, on-site, qualitative immunoassay can detect antibodies to T. pallidum in serum, plasma, and whole blood of infected individuals.164 Results are available within 15 minutes. However, as will all available treponemal tests, use of this test may result in overtreatment of patients, because previously treated syphilis cannot be distinguished from active syphilis using this test alone.
Decision to Perform a Lumbar Puncture
A lumbar puncture for examination of CSF should be performed in all patients with signs or symptoms of neurologic or ophthalmic involvement, evidence of active tertiary syphilis, treatment failures, and HIV infection with late latent syphilis or syphilis of unknown duration.21, 166 A pregnant woman meeting any of these criteria who declines lumbar puncture should be considered for empirical treatment of neurosyphilis. Infants believed to have CS also should undergo a CSF examination if the infant has an abnormal physical examination finding consistent with CS, a quantitative nontreponemal titer in serum that is fourfold greater than the mother's titer, or a positive darkfield or DFA-TP result on tests of body fluids.21, 167 CSF evaluation should include assessment of cell count, protein concentration, and reactivity to the VDRL test.21 The CSF VDRL test has low sensitivity (30–78%) in neurosyphilis but excellent specificity.156, 168 A false-positive CSF VDRL test result is rare but possible if the CSF is contaminated with blood.168 Although not widely used, FTA-ABS testing of CSF is recommended by some experts because its lower specificity is offset by high sensitivity, making a nonreactive result helpful in excluding a diagnosis of neurosyphilis.156, 168
Diagnosis of Syphilis in HIV-Infected Persons
Results of nontreponemal and treponemal tests usually are accurate and reliable in persons who are co-infected with syphilis and HIV.21 However, several studies suggest that manifestations of disease and serologic response may be altered in persons with HIV infection.169, 170, 171, 172, 173 For example, when compared with HIV-negative patients, HIV-infected patients who have early syphilis may be at increased risk for neurologic complications and may warrant a CSF examination based on presence of neurologic signs or symptoms.21 In addition, patients who are coinfected with syphilis and HIV may have unusually high titers of delayed reactivity on serologic testing. When clinical findings suggest that syphilis is present but serologic tests are nonreactive or unclear, alternative diagnostic tests include biopsy of a lesion, darkfield examination, or direct fluorescent antibody staining of lesion material.21
PREVENTION OF CONGENITAL SYPHILIS
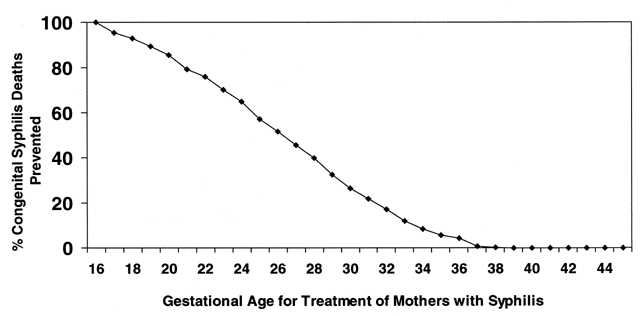
Congenital syphilis is preventable with appropriate screening and treatment programs. Furthermore, infant deaths from CS are significantly decreased when women are screened and treated early in pregnancy32 (Fig. 13). Among patients with no or late third trimester prenatal care, missed prevention opportunities often occur earlier in pregnancy during visits to health care providers, the emergency room, or social support agencies.38, 174 Practical measures to improve early detection in pregnant women include on-site testing for pregnancy as well as for syphilis in STD clinics and drug addiction programs. Early testing by prenatal programs and among women whose menstrual cycles are late in other clinical settings is encouraged.28 In STD clinics, routine RPR testing should occur regardless of pregnancy or menstrual status. In settings where the system for testing and early return visits are complicated, on-site, rapid testing and treatment for syphilis at the first prenatal visit is critical to ensure coverage for persons who otherwise may not return.162, 163
An estimated 48% of pregnant women diagnosed with syphilis are asymptomatic and are detected only by serologic testing.175 This makes the need for routine, early (first trimester or first prenatal visit) serologic screening of all pregnant women crucial. In addition, many clinicians and US state legislatures advocate a repeat serologic screening for syphilis in the third trimester to detect women who may have acquired syphilis after an initially negative result of a first trimester screen.176 The Centers for Disease Control and Prevention recommend serologic testing at the first prenatal visit, with repeat testing at the beginning of the third trimester (28 weeks) and at delivery for women in communities with high rates of syphilis, are previously untested, have positive serology in the first trimester or who are at high personal risk of acquiring an STD.177 Women who are at high risk for syphilis, live in areas of high syphilis morbidity, should be screened again early in the third trimester (28 weeks’ gestation) and at delivery. Some states require all women to be screened at delivery. Infants should not be discharged from the hospital unless the syphilis serologic status of the mother has been determined at least one time during pregnancy and preferably again at delivery. Any woman who delivers a stillborn infant should be tested for syphilis. The American College of Obstetricians and Gynecologists also recommends routine screening for syphilis at the first prenatal visit with a repeat test in the third trimester for women at risk of acquiring an STD.178 Midtrimester fetal losses and term fetal deaths always should prompt an evaluation for maternal syphilis.35
The signs and symptoms of the various stages of syphilis in the pregnant women are unchanged from what is seen in nonpregnant patients. Syphilis screening recommendations for the pregnant patient are more comprehensive than for nonpregnant women to detect and treat all patients during pregnancy so that CS can be prevented. As a routine part of quality prenatal care, all pregnant women also should be counseled and screened for other STDs including chlamydia, gonorrhea, and HIV infection.177, 178 Primary syphilis in late pregnancy can be missed if the mother shows no signs of infection, the infected newborn appears normal at birth, and if the serologic conversion to reactivity has not yet occurred.72, 73, 179 The devastating effects of untreated maternal syphilis have already been described (see Table 1).
In pregnant women with a history of syphilis, an increase in treponemal titers may occur in the absence of new active infection.156, 177 However, reinfection must be ruled out if the treatment history is unclear, the titer is increased fourfold or more, or the woman has had recent sexual contact with a person who has syphilis.156
All infants born to women with reactive serologic tests for syphilis or darkfield-positive lesions should be examined thoroughly for signs and symptoms of CS.177 Pathologic examination of the placenta, umbilical cord, or body fluids also should be performed.177 In addition, all infants born to mothers with signs of syphilis should be evaluated by a quantitative nontreponemal serologic test within the first month of life.177 Serum from the infant is the preferred specimen, since cord blood can be contaminated and produce false-positive results.180
Diagnosing CS is complicated by the passive vertical transfer of maternal IgG antibodies, but most of these antibodies are undetectable in uninfected infants by 6–12 months of life.177, 181 The diagnosis of CS depends on multiple findings, including physical signs and symptoms, radiographic and serologic evidence, and direct microscopic criteria.28, 181 For reporting and surveillance purposes, a confirmed case is defined as an infant or stillbirth with T. pallidum identified by darkfield microscopy or specific stains in specimens from lesions, placenta, umbilical cord, or autopsy material.28, 182 A presumptive case occurs in any infant or child whose mother was untreated or inadequately treated at delivery or who has a reactive treponemal test for syphilis and one of the following: evidence of CS on physical examination or on long bone radiographs, abnormal CSF, reactive FTA-ABS immunoglobulin M antibody, a nontreponemal antibody titer fourfold higher than the mother's, a rising titer during follow-up, or a persistently reactive treponemal test after 18 months of age.28, 182 A syphilitic stillbirth is defined as a fetal death occurring after 20 weeks' gestation or weighing more than 500 g in which the mother had untreated or inadequately treated syphilis at delivery.28, 182
Regardless of maternal history of infection or treatment, if an infant has abnormal physical examination results that are consistent with CS, a serologic titer that is fourfold greater than the mother's titer, or a body fluid test that is positive for treponemes, the infant should undergo a CSF analysis, a complete blood count with differential and platelet count, and other tests as clinically indicated (e.g., long bone radiographs, liver function tests, eye examination).177
THERAPY AND FOLLOW-UP
In part because T. pallidum has yet to develop clinically significant antibiotic resistance, parenteral penicillin G continues to be the drug of choice for all stages of syphilis. It is the only drug with documented efficacy for syphilis during pregnancy and neurosyphilis.21 Doses and duration of therapy, as well as drug formulation, depend on clinical stage and disease manifestations. Because some of the available penicillin preparations with similar packaging, labeling, and proprietary names (e.g., Bicillin L-A and Bicillin C-R) differ with respect to benzathine and procaine penicillin G content, inadvertent errors have occurred and highlight the importance of using the appropriate penicillin formulation, which currently is Bicillin L-A.183
No data from randomized, controlled trials are available to guide the design of penicillin regimens, and data on the use of other antibiotics, such as tetracyclines and erythromycin, are more limited. However, current doses and strategies for use of parenteral penicillin G in the treatment of syphilis are based on decades of clinical experience and observational studies. In addition, both oral and parenteral treatment options are being expanded by the advent of new regimens that are promising and are being evaluated in ongoing clinical trials.
Any treatment regimen for syphilis can lead to the development of a Jarisch-Herxheimer reaction, which is an acute febrile reaction thought to caused by the release of T. pallidum lipoproteins and others with inflammatory constituents from dead or dying organisms. These reactions have been observed in one to two thirds of patients with early syphilis and approximately half of infants with CS, usually within the first 12 hours after treatment, and should be discussed with all patients.184, 185, 186 Symptoms often include fevers, chills, headache, myalgia, and arthralgia. These symptoms may be ameliorated by antipyretics. In pregnancy, premature labor or fetal distress also may occur. For example, in one study of 50 pregnant women treated between 1991 and 1996, 20 experienced the Jarisch-Herxheimer reaction, 13 developed regular uterine activity without delivery, and 12 developed recurrent variable decelerations on their fetal heart rate tracing.187 However, regardless of its severity, a Jarisch-Herxheimer reaction usually ends within 24 hours after syphilis therapy and is not an indication to discontinue or postpone treatment.
The following treatment recommendations are based on the 2006 Guidelines for Treatment of Sexually Transmitted Diseases by the Centers for Disease Control and Prevention177 and are summarized in Table 3.
Table 3. Treatment for syphilis by stage (for HIV-uninfected adults)
Nonpregnant adults (nonallergic) | Nonpregnant penicillin-allergic adults | Pregnant adults | |
Primary and secondary | Benzathine penicillin G 2.4 million units IM in a single dose | Doxycycline 100 mg PO bid for 2 wk or tetracycline 500 mg PO qid for 2 wk | Same as for nonpregnant adults (Allergic patients should be desensitized and treated with penicillin) |
Early latent | Benzathine penicillin G 2.4 million units in a single dose | Doxycycline 100 mg PO bid for 2 wk or tetracycline 500 mg PO qid for 2 wk | Same as for nonpregnant adults (Allergic patients should be desensitized and treated with penicillin) |
Late latent | Benzathine penicillin G 2.4 million units IM for 3 doses at weekly intervals, for a total of 7.2 million units | Doxycycline 100 mg PO bid for 28 days or tetracycline 500 mg PO qid for 28 days | Same as for nonpregnant adults (Allergic patients should be desensitized and treated with penicillin) |
Tertiary syphilis | Benzathine penicillin G 2.4 million units IM for 3 doses at weekly intervals, for a total of 7.2 million units | Doxycycline 100 mg PO bid for 2 wk or tetracycline 500 mg PO qid for 2 wk | Same as for nonpregnant adults (Allergic patients should be desensitized and treated with penicillin) |
Neurosyphilis | Aqueous crystalline penicillin G 18–24 million units daily, given as 3–4 million units IV q 4 hr for 10–14 days or Procaine penicillin 2.4 million units IM daily, plus probenicid 500 mg PO qid, both for 10–14 days or ceftriaxone 2 g IM or IV daily for 10–14 days | Ceftriaxone 2 g IM or IV daily for 10–14 days (cross-reactivity is 5–10%). Persons with allergies to beta-lactam should be desensitized and treated with penicillin or managed in consultation with an expert. | Same as for nonpregnant adults (Allergic patients should be desensitized and treated with penicillin) |
Congenital syphilis (<1 mo of age) | Aqueous crystalline penicillin G 100,000–150,000 units/kg/day, given as 50,000 units/kg/dose IV q 12 hr during the first 7 days of life, then q 8 hr for 10 days or procaine penicillin G 50,000 units/kg/dose IM a day in a single dose for 10 days | NA (Use of agents other than penicillin requires close serologic follow-up to assess adequacy of therapy) | NA |
Congenital syphilis (>1 mo of age) | Aqueous crystalline penicillin G 200,000–300,000 units/kg/day IV, given as 50,000 units/kg q 4–6 hr for 10 days | NA | NA |
HIV, human immunodeficiency virus; NA, not applicable.
Primary, Secondary, and Early Latent Syphilis
Patients who have primary, secondary, or early latent syphilis (collectively called early syphilis) should be treated with a single dose of benzathine penicillin G, 2.4 million U intramuscularly (IM) unless allergy to penicillin has been documented. This regimen is curative in 90–95% of cases.110, 188 Children with acquired early syphilis should be evaluated and treated using a pediatric single dose regimen of benzathine penicillin G, 50,000 U/kg IM, up to the adult dose of 2.4 million U. Child protective services should be consulted whenever acquired syphilis is diagnosed in a child.
Nonpregnant patients who are allergic to penicillin should be treated for 2 weeks with 100 mg of doxycycline orally, twice daily, or 500 mg of tetracycline orally, four times daily. Limited clinical studies suggest ceftriaxone should be effective for early-stage syphilis. The optimal dose and duration of therapy are undefined, but some experts recommend 1 g IM or IV daily for 8–10 days. Azithromycin may be effective as a single oral dose of 2 g. Because the response rate to these therapies is not well documented, they are not currently recommended by the CDC. If used, they should be used with caution, and close follow-up is essential. The efficacy of these alternatives in HIV-infected individuals has not been studied.
Serologic follow-up of patients with primary or secondary syphilis should be obtained at 6- and 12-month intervals, whereas in patients with early latent disease, an additional 24-month follow-up should be assured. More frequent re-evaluation should be considered if follow-up is likely to be difficult. Failure to achieve a fourfold or greater decline in nontreponemal test titer should prompt re-evaluation for possible treatment failure. These patients should be re-treated with 7.2 million U of benzathine penicillin G administered in three weekly doses IM of 2.4 million U. They also should be encouraged to undergo HIV testing, especially if they declined HIV testing at initial presentation. If reinfection with T. pallidum cannot be ruled out, a lumbar puncture should be performed to exclude neurosyphilis.
Late Latent Syphilis, Latent Syphilis of Unknown Duration, and Tertiary Syphilis (Excluding Neurosyphilis)
The duration of therapy is extended in late syphilis because treponemes are believed to divide more slowly during this stage than during early syphilis. Non-penicillin-allergic adults with late latent syphilis, latent syphilis of unknown duration, or tertiary syphilis other than neurosyphilis should be treated with 7.2 million U of benzathine penicillin G administered as three weekly doses of 2.4 million U IM. For children, benzathine penicillin G is given as three weekly doses of 50,000 U/kg IM, up to the adult dose of 2.4 million U. Some experts believe that persons who go more than 10 –14 days between any weekly doses should restart the regimen from the first dose. Missed doses are not acceptable for pregnant patients being treated for late latent syphilis or syphilis of unknown duration. Nonpregnant patients with these late stages of syphilis who are allergic to penicillin should be treated with the same alternative regimens described earlier for allergic patients with early syphilis, but treatment should be extended to 4 weeks. Serologic follow-up of patients with late latent syphilis, latent syphilis of unknown duration, or tertiary syphilis other than neurosyphilis should be obtained at a minimum of 6-, 12-, and 24-month intervals. The detailed management of cardiovascular or gummatous syphilis is complex and should be conducted in consultation with an expert.
Neurosyphilis
In 1986, the recommended treatment regimen for patients with documented neurosyphilis was changed from that for other forms of tertiary syphilis because of data indicating that (1) benzathine penicillin G does not reliably achieve treponemicidal levels in CSF, and (2) viable organisms can be recovered from CSF, and neurologic symptoms may persist after benzathine penicillin G therapy.110 Instead, patients with neurosyphilis should receive a 10- to 14-day course of aqueous crystalline penicillin G, 18–24 million U intravenously (IV) daily in doses given every 4 hours of 3–4 million U. If compliance can be assured, patients alternatively may be treated with procaine penicillin, 2.4 million U IM daily, with probenecid, 500 mg orally four times daily, both for 10–14 days. The above regimens result in penicillin levels in CSF several-fold above the minimal treponemicidal concentration of 0.018 μg/mL during treatment.189 Still, some clinicians administer an additional 2.4 million U of benzathine penicillin IM after completion of these regimens to provide a total duration of therapy that is comparable with the 3-week total therapy given in cases of late disease.
A recent addition to the CDC-recommended regimens for neurosyphilis is ceftriaxone. Ceftriaxone, 2 g IM or IV daily for 10–14 days, can be used as an alternative in the treatment of the patient with neurosyphilis, although consideration should be given to the 5–10% incidence of cross-reactivity among penicillin-allergic patients. Data have not been collected systematically for evaluation of other therapeutic alternatives to penicillin for treatment of neurosyphilis. Where concerns regarding the safety of β-lactam antibiotics persist, patients who report being allergic to penicillin should either be desensitized to penicillin or be treated in consultation with an expert.
Patients treated for neurosyphilis should be followed with CSF examinations every 6 months until the cell count is normal. Improvement in CSF protein concentration and decline CSF VDRL titer may lag behind normalization of CSF pleocytosis. If the cell count has not fallen within 6 months or if the CSF profile is not entirely normal after 2 years, re-treatment should be considered, and the patient should be counseled regarding re-evaluation for HIV infection.
Syphilis During Pregnancy
If previous adequate treatment or appropriately declining titers cannot be documented in pregnant women, they should be considered infected and treated with the penicillin regimen appropriate for the stage of disease. Some experts recommend a second dose of 2.4 million U of benzathine penicillin IM, 1 week after the initial dose for women with early syphilis.177 However, no data indicate that adequacy of treatment is improved with that second dose.190 Ultrasonographic signs of fetal infection such as hydrops or hepatomegaly indicate an increased risk of fetal treatment failure and should be managed in consultation with perinatal and neonatal specialists.
There are no alternatives to penicillin of proven efficacy for treatment of syphilis during pregnancy. Pregnant women with penicillin allergy should be desensitized and treated with penicillin. Tetracycline and doxycycline should not be used because they may have adverse effects on long bone growth in the newborn and cause yellow-brown discoloration of teeth.122 Erythromycin is not recommended because it does not readily cross the placenta, so the fetus may develop congenital infection despite maternal therapy.177 Studies continue to explore azithromycin and ceftriaxone as possible alternatives for treatment during pregnancy. 191192
Follow-up of pregnant women should include serologic titers in the third trimester and at delivery, and the antibody response should be appropriate for the stage of disease. Many women deliver before their serologic response to treatment can be confirmed. Inadequate maternal treatment is likely if delivery occurs within 30 days of therapy.177
Congenital Syphilis
Infants should be treated for presumed CS if they were born to mothers with (1) untreated syphilis at delivery, (2) serologic evidence of relapse or reinfection after treatment (fourfold or greater increase in nontreponemal test titer), (3) treatment with nonpenicillin regimens for syphilis during pregnancy, (4) treatment for syphilis less than 4 weeks from delivery, (5) poor documentation of treatment for syphilis, (6) failure to achieve a fourfold decrease in nontreponemal test titer after therapy with an appropriate penicillin regimen, or (7) insufficient serologic follow-up during pregnancy to ensure an adequate treatment response or lack of current infection. These treatment criteria may not always be consistent with the case definition criteria discussed earlier.
The recommended treatment regimen for infants during the first month of life is as follows: (1) aqueous crystalline penicillin G, 100,000–150,000 U/k/day, in divided doses of 50,000 U/kg/dose IV every 12 hours during the first 7 days of life and every 8 hours thereafter for a total of 10 days, or (2) procaine penicillin G 50,000 U/kg/dose IM daily in a single dose for 10 days. If more than 1 day of therapy is omitted, the whole course should be restarted. Inadequate data are available to recommend the use of other antibiotics for treatment of CS. All seroreactive infants should receive careful follow-up testing every 2–3 months until tests become nonreactive or the titer has decreased fourfold. 177
For children who are identified as having reactive tests for syphilis after the neonatal period, maternal serologic testing should be performed and records reviewed to assess whether the child has congenital or acquired syphilis.
Syphilis in HIV-Infected Persons
Although data are limited and conflicting, several studies suggest that HIV-infected patients may be less likely than HIV-uninfected patients to meet standard criteria for serologic cure (at least two-dilution decline in nontreponemal titer) following standard therapy for syphilis.166, 169, 172, 173, 193, 194, 195, 196 There is little evidence for increased incidence of treatment failure by clinical criteria, however. Furthermore, the only published randomized clinical trial of enhanced therapy for early syphilis in patients with HIV infection showed no significant improvement in outcomes when compared with the standard regimen.196 In the absence of clear evidence for a correlation between serologic and clinical failure, or for the improved efficacy of an alternative regimen, currently recommended treatments for persons with HIV are the same, stage by stage, as for persons who are not infected with HIV.21
Careful follow-up is particularly critical after treatment of HIV-infected syphilis patients. It is important that HIV-infected patients be evaluated clinically and serologically for treatment failure at 3, 6, 9, 12, and 24 months after therapy. HIV-infected persons who fail treatment by serologic or clinical criteria should be managed in the same way as HIV-uninfected patients (i.e., a CSF examination and re-treatment).21
Ceftriaxone
Ceftriaxone, a broad-spectrum cephalosporin with a long serum half-life and good CSF penetration, is active against T. pallidum and is used by some clinicians as an alternative in their penicillin-allergic patients.196, 197 A recent survey of over 400 specialists in infectious disease found that 19% of respondents reported using ceftriaxone to treat syphilis in both HIV-infected and HIV-negative patients for various stages of disease.198 Several clinical studies support the efficacy of ceftriaxone in the treatment of syphilis.199, 200, 201 However, one study reports a 23% treatment failure rate in HIV-infected individuals with latent syphilis or asymptomatic neurosyphilis.202
Azithromycin
Azithromycin appears to offer another promising alternative to standard syphilis therapy. In HIV-negative patients, this azalide antibiotic provides curative therapy for chlamydia, gonorrhea, and chancroid in a single oral dose. Directly observed, oral therapy that simultaneously cures multiple STDs is attractive not only because it eliminates the painful injections required by benzathine penicillin G, but also because it facilitates more novel, community-based syphilis prevention and control methods. Limited studies with azithromycin are encouraging. It has been shown to be effective for treatment of experimental syphilis in laboratory rabbits203 and has been documented to cure 11 of 13 patients with primary and secondary syphilis in a small pilot study.204 A recent randomized trial compared azithromycin with benzathine penicillin G therapy in persons exposed to sex partners with infectious syphilis and found that a single, 1-g dose of azithromycin was as effective as penicillin for the treatment of incubating syphilis.205 Notice that in this study loss to follow-up was substantially higher among participants randomized to penicillin given IM than among those randomized to oral azithromycin. Research that further evaluates the use of azithromycin for treatment of early or incubating syphilis is ongoing. However, azithromycin treatment failures and strain resistance among high-risk groups in the United States and Ireland have raised concerns. 206, 207
Reporting Requirements
Just as clinicians must have clinical and laboratory information to diagnose and treat diseases in individual patients, health officials must have information on the number of people affected by a disease, the factors that place them at risk and the effectiveness of health services to reduce the impact of the disease on the community. For syphilis, this surveillance information depends on the timely and accurate reporting of all documented syphilis cases to the local health department by clinical and laboratory providers. Health department staff in turn have a responsibility to disseminate this information, in aggregate form without identifying information, back to providers to assist them in implementing appropriate detection, treatment, and prevention strategies.
In the United States, reporting of syphilis cases is required in all 50 states and in all the territories, but specific reporting procedures vary by state. Although laboratories usually report positive syphilis serologic test results, health departments may not consider these as active syphilis cases, particularly if titers are low, without additional information from the clinician. Therefore, it is important that clinicians also report every syphilis case. Health care providers are encouraged to consult with their local health department or, in large facilities, the designated liaison with the health department about questions on reporting procedures in their area.182
Partner Notification and Services
Notifying and providing services to all sexual partners of patients with syphilis remains an essential component of patient care both because it prevents reinfection of the index patient and because it limits continued spread of syphilis in the community. Partner notification describes the process by which providers or health department or other personnel inform or locate the sex partners of persons with STDs to provide them with clinical care access and preventive measures.208, 209 Partner notification must be confidential and must link to quality clinical services to be effective. For the women's health care provider, who usually does not provide care to male patients, it can be challenging to ensure that all sex partners are notified, tested, and treated, if appropriate. Therefore, for the provider who is unable to notify, test, and treat patients' partners, health department referrals and follow-up for the patient and her sex partners are essential to prevent reinfection and further transmission. It is important that providers encourage their patients to make partners aware of potential risk and seek diagnosis and treatment, both with and without the assistance of local health agencies.21 Even in those areas where a diagnosis of syphilis is made in the office or where laboratory notification of the health department is mandatory, providers may need to engage the health department directly so that appropriate clinical information and follow-up can be provided. If local health agencies are engaged, the patient should be informed by her provider that health department personnel will inquire about sex partners for the previous 3 (primary), 6 (secondary), or 12 months (early latent), depending on her stage of disease. Also, the patient should be counseled about the importance of this confidential process to her own health, the health of her partners, and the prevention of further spread of disease.
Alternative methods of case finding, such as cluster interviewing, are being advocated because of their higher yield compared with routine partner notification when anonymous sex partners are involved.208, 210 Cluster interviewing is an alternative method in which patients and their contacts are asked to give information on sex partners and other persons in their social network who may be at risk for syphilis.210 This method has been shown to be helpful in identifying patients who may be marginalized from the health care system and otherwise never identified.211, 212
In conclusion, syphilis remains as elusive and multifaceted as when it was first described several centuries ago. As reported cases of infectious syphilis in the United States slowly begin to trend upward, it is even more important that providers not become complacent. Continued attention to the importance of early detection, treatment, and reporting of symptoms is crucial both to reducing the severe consequences of syphilis in women and their unborn children and to eliminating this disease.
REFERENCES
Chapel TA: The variability of syphilitic chancres. Sex Transm Dis 5: 68, 1978 |
|
Drusin LM, Singer C, Valenti AJ et al: Infectious syphilis mimicking neoplastic disease. Arch Intern Med 137: 156, 1977 |
|
Wasserheit JN, Aral SO: The dynamic topology of sexually transmitted disease epidemics: Implications for prevention strategies. J Infect Dis 174 (Suppl 2): S201, 1996 |
|
Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2006. Atlanta, GA: U.S. Department of Health and Human Services, November 2007 |
|
Koplan J: National syphilis elimination launch. Syphilis elimination: History in the making [opening remarks] Nashville, Tennessee, October 7, 1999. Sex Transm Dis 27: 63, 2000 |
|
CDC. The National Plan to Eliminate Syphilis from the United States. Atlanta, GA: U.S. Department of Health and Human Services, May 2006. |
|
Williams LA, Klausner JD, Whittington WL et al: Elimination and reintroduction of primary and secondary syphilis. Am J Public Health 89: 1093, 1999 |
|
Satcher D from the CDC: Syphilis elimination: History in the making. Sex Transm Dis 27:66, 2000 |
|
Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2006 Supplement, Syphilis Surveillance Report. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, December 2007 |
|
St. Louis ME, Gwinn M, Nakashima A et al: Covariation of HIV infection among childbearing women with other sexually transmitted diseases in the United States. 11th Meeting of the International Society for STD Research, 1995 |
|
Koumans EH, Sternberg M, Gwinn M et al: Geographic variation of HIV infection in childbearing women with syphilis in the United States. AIDS 14: 279, 2000 |
|
Fleming DT, Wasserheit JN: From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Dis 75: 3, 1999 |
|
Wasserheit JN: Epidemiological synergy: Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis 19: 61, 1992 |
|
Hook EW III: Syphilis and HIV infection. J Infect Dis 160: 530, 1989 |
|
Darrow WW, Echenberg DF, Jaffe HW et al: Risk factors for human immunodeficiency virus (HIV) infection in homosexual men. Am J Public Health 77: 479, 1987 |
|
Ghys PD, Diallo MO, Ettiegne-Traor'e V et al: Genital ulcers associated with human immunodeficiency virus-related immunosuppression in female sex workers in Abidjan, Ivory Coast. J Infect Dis 172: 1371, 1995 |
|
Greenblatt RM, Lukehart SA, Plummer FA et al: Genital ulceration as a risk factor for human immunodeficiency virus infection. AIDS 2: 47, 1988 |
|
Simonsen JN, Cameron DW, Gakinya MN et al: Human immunodeficiency virus infection among men with sexually transmitted diseases: Experience from a center in Africa. N Engl J Med 319: 274, 1988 |
|
Stamm WE, Handsfield HH, Rompalo AM et al: The association between genital ulcer disease and acquisition of HIV. JAMA 260: 1429, 1988 |
|
Blocker ME, Levine WC, St Louis ME: HIV prevalence in patients with syphilis, United States. Sex Transm Dis 27: 53, 2000 |
|
Centers for Disease Control and Prevention: 2001 Guidelines for treatment of sexually transmitted diseases. MMWR Morbid Mortal Wkly Rep (in press) |
|
Chhabra RS, Brian LP, Castro M et al: Comparison of maternal sera, cord blood, and neonatal sera for detecting presumptive congenital syphilis: Relationship with maternal treatment. Pediatrics 9: 88, 1993 |
|
Klass PE, Brown ER, Pelton SI: The incidence of prenatal syphilis at the Boston City Hospital: A comparison across four decades. Pediatrics 94: 24, 1994 |
|
Zenker PN, Berman SM: Congenital syphilis: Trends and recommendations for evaluation and management. Pediatr Infect Dis J 10: 516, 1991 |
|
Cohen DA, Boyd D, Pradbhudas I et al: The effects of case definition in maternal screening and reporting criteria on rates of congenital syphilis. Am J Public Health 80: 316, 1990 |
|
Centers for Disease Control and Prevention: Congenital syphilis—United States, 1998. MMWR Morbid Mortal Wkly Rep 48:757, 1999 |
|
Radolf JD, Sanchez PJ, Schulz KF et al: Congenital syphilis. In Holmes KK et al (eds): Sexually Transmitted Diseases, p 1165. 3rd edn. New York, McGraw-Hill. 1999 |
|
Centers for Disease Control and Prevention: Policy guidelines for the prevention and control of congenital syphilis. MMWR Morbid Mortal Wkly Rep 37:S-1, 1988 |
|
Centers for Disease Control and Prevention: Congenital syphilis—New York City, 1986-1988. MMWR Morbid Mortal Wkly Rep 38:825, 1989 |
|
Thompson BL: Congenital syphilis in Maryland, 1989-1991: The effect of changing the case definition and opportunities for prevention. Sex Transm Dis 22: 364, 1996 |
|
Sanchez PJ: Congenital syphilis: The Dallas experience. Pediatr Res 29: 286A, 1991 |
|
Gust DA, W. Levine WC. St. Louis M. Mortality associated with congenital syphilis in the United States, 1992-1998. Pediatrics, 2002; 109(5):E79-9 |
|
Evans HE, Frenkel LD: Congenital syphilis. Clin Perinatol 21: 149, 1994 |
|
Webber MP, Lambert G, Bateman DA et al: Maternal risk factors for congenital syphilis: A case-control study. Am J Epidemiol 137: 415, 1993 |
|
Fiumara NJ, Fleming WL, Downing JG et al: The incidence of prenatal syphilis at the Boston City Hospital. N Engl J Med 247: 48, 1952 |
|
Kaufman RE, Jones OG, Blount JH et al: Questionnaire survey of reported early congenital syphilis: Problems in diagnosis, prevention, and treatment. Sex Transm Dis 4: 135, 1977 |
|
Taffel S: Prenatal care in the US, 1969-75. Set 21, no. 33. Data from the National Vital Statistics System, Washington, DC, US Department of Health, Education, and Welfare, publication no. PHS 78–1911, 1978 |
|
Centers for Disease Control and Prevention: Epidemic of congenital syphilis, Baltimore, 1996-1997. MMWR Morbid Mortal Wkly Rep 47:904, 1998 |
|
Smibert RM: Genus III: Treponema schaudinn 1905, 1728. In Kreig NR, Holt JG (eds): Bergey's Manual of Systematic Bacteriology, pp 49–57. Vol 1. Baltimore, Williams & Wilkins, 1984 |
|
Miao RM, Fieldsteel AH: Genetic relationship between Treponema pallidum and Treponema pertenue, two noncultivable human pathogens. J Bacteriol 141: 427, 1980 |
|
Noordhoek GT, Hermans PWN, Paul AN et al: Treponema pallidum subspecies pallidum (Nichols) and Treponema pallidum subspecies pertenue (CDC 2575) differ in at least one nucleotide: Comparison of two homologous antigens. Microb Pathog 6: 29, 1989 |
|
Centurion-Lara A, Castro C, Castillo R et al: The flanking region sequences of the 15-kDa lipoprotein gene differentiate pathogenic treponemes. J Infect Dis 177: 1036, 1998 |
|
Norris SJ, Larsen SA: Treponema and other host-associated spirochetes. In PR Murray, EJ Baron, MA Pfaller et al. (eds): Manual of Clinical Microbiology, pp 636–651. 6th edn. Washington, DC, ASM Press, 1995 |
|
Charon NW, Greenberg EP, Koopman MBH et al: Spirochete chemotaxis, motility, and the structure of the spirochetal periplasmic flagella. Res Microbiol 143: 597, 1992 |
|
Johnson RC, Ritzi DM, Livermore BP: Outer envelope of virulent Treponema pallidum. Infect Immun 8: 294, 1973 |
|
Radolf JD, Moomaw C, Slaughter CA et al: Penicillin-binding proteins and peptidoglycan of Treponema pallidum subspecies pallidum. Infect Immun 56: 1825, 1989 |
|
Hardy PH Jr, Levin J: Lack of endotoxin in Borrelia hispanica and Treponema pallidum. Proc Soc Exp Biol Med 174: 47, 1983 |
|
Cunningham TM, Walker EM, Miller JN et al: Selective release of the Treponema pallidum outer membrane and associated polypeptides with Triton X-114. J Bacteriol 170: 5789, 1988 |
|
Pavia CS, Folds JD, Baseman JB: Selective in vitro response of thymus-derived lymphocytes from Treponema pallidum-infected rabbits. Infect Immunol 18: 603, 1977 |
|
Baker-Sander SA, Sell S, Lukehart SA: Serum regulation of in vitro lymphocyte responses in early experimental syphilis. Infect Immunol 37: 568, 1982 |
|
Nichols HJ, Hough WH: Demonstration of Spirochaeta pallida in the cerebrospinal fluid. JAMA 60: 108, 1913 |
|
Norgard MV Miller JN: Cloning and expression of Treponema pallidum (Nichols) antigen in Escherichia coli. Infect Immun 42: 435, 1983 |
|
Walfield A, Hanff PH, Lovett MA: Expression of Treponema pallidum antigens in Escherichia coli. Science 216: 522, 1982 |
|
Gherardini FC, Hobbs MM, Stamm LV et al: Complementation of an Escherichia coli proC mutation by a gene cloned from Treponema pallidum. J Bacteriol 172: 2996, 1990 |
|
Stamm LV, Barnes NY: Nucleotide sequences of the proA and proB genes of Treponema pallidum, the syphilis agent. DNA Sequence 8: 63–70, 1997 |
|
Stamm LV, Young NR, Frye JG et al: Identification and sequences of the Treponema pallidum mglA and mglC genes. DNA Sequence 6: 293, 1996 |
|
Porcella SF, Popova TG, Hagman KE et al: A mgl -like operon in Treponema pallidum, the syphilis spirochete. Gene 177: 115, 1996 |
|
Fraser CM, Norris SJ, Weinstock GM et al: Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281: 375, 1998 |
|
Fieldsteel AH, Stout JG, Becker FA: Comparative behaviour of virulent strains of Treponema pallidum and Treponema pertenue in gradient cultures of various mammalian cells. Infect Immun 24: 337, 1979 |
|
Pillay A, Liu H, Chen CY et al: Molecular subtyping of Treponema pallidum subspecies pallidum. Sex Transm Dis 25: 408, 1998 |
|
Sutton MY, Liu, H, Steiner B, et al. Molecular subtyping of Treponema pallidum in a county with increasing syphilis morbidity using specimens from ulcers and blood. J Infect Dis 2001;183:1601-1606 |
|
Magnuson H, Thomas E, Olansky S: Inoculation syphilis in human volunteers. Medicine 35: 33, 1956 |
|
Magnuson HJ, Eagle H, Fleischman R: The minimal infectious inoculum of Spirochaeta pallida (Nichols strain) and a consideration of its rate of multiplication in vivo. Am J Syphilis Gonorrhea Vener Dis 32: 1, 1948 |
|
Turner TB, Hollander DH: Biology of the Treponematoses. Geneva, World Health Organization, 1957 |
|
Miller J: Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by gamma-irradiation. J Immunol 1206, 1973 |
|
Cameron CE, Castro C, Lukehart SA et al: Function and protective capacity of Treponema pallidum subsp. pallidum glycerophosphodiester phosphodiesterase. Infect Immun 66: 5763, 1998 |
|
Cameron CE, Lukehart SA, Castro C et al: Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J Infect Dis 181: 1401, 2000 |
|
Barbosa-Cesnik CT, Gerbase A, Heymann D: STD vaccines: An overview. Genitourin Med 73: 336, 1997 |
|
Stokes JH, Beerman H, Ingraham NR: Modern Clinical Syphilology. Philadelphia, W.B. Saunders, 1944 |
|
Schroeter AL, Turner RH, Lucas JB et al: Therapy for incubating syphilis: Effectiveness of gonorrhea treatment. JAMA 218: 711, 1971 |
|
Garnett GP, Aral SO, Hoyle DV et al: The natural history of syphilis: Implications for the transmission dynamics and control of infection. Sex Transm Dis 24: 185, 1997 |
|
Sanchez PJ, Wendel GD, Norgard MV: Congenital syphilis associated with negative results of maternal serologic tests at delivery. Am J Dis Child 145: 967, 1991 |
|
Dorfman DH, Glaser JH: Congenital syphilis presenting in infants after the newborn period. N Engl J Med, 323: 1299, 1990 |
|
Sparling PF: Natural history of syphilis. In Holmes KK, Sparling PF et al (eds): Sexually Transmitted Diseases, pp 473–478. 3rd edn. New York, McGraw-Hill, 1999 |
|
Schober PC, Gabriel G, White P et al: How infectious is syphilis? Br J Vener Dis 59: 217, 1983 |
|
Ingraham NR: The value of penicillin alone in the prevention and treatment of congenital syphilis. Acta Dermatol Venereol. 31 (Suppl 24): 60, 1951 |
|
Ricci JM, Fojaco RM, O'Sullivan MJ: Congenital syphilis: The University of Miami/Jackson Memorial Medical Center experience, 1986-1988. Obstet Gynecol 74: 687, 1989 |
|
McFarlin BL, Bottoms SF, Dock BS et al: Epidemic syphilis: Maternal factors associated with congenital infection. Am J Obstet Gynecol 170: 535, 1994 |
|
Van der Sluis JJ, Onvlee PC, Kothe FC et al: Transfusion syphilis: Survival of Treponema pallidum in donor blood. I. Report of an orientating study. Vox Sang 47: 197, 1984 |
|
Van der Sluis JJ, ten Kate FJ, Vuzevski VD et al: Transfusion syphilis: Survival of Treponema pallidum in donor blood. II. Dose dependence of experimentally determined survival times. Vox Sang 49: 390, 1985 |
|
Infectious disease testing for blood transfusions. In NIH Consensus statement 13, pp 13–14. Bethesda, MD, National Institutes of Health, 1995 |
|
Marfin AA, Liu H, Sutton MY, et al. Amplification of the DNA polymerase I gene of Treponema pallidum from whole blood of persons with syphilis. Diagnost Microbiol Inf Dis 2001;40:163-166 |
|
Stamm L: Biology of Treponema pallidum. In Holmes KK, Sparling PF et al (eds): Sexually Transmitted Diseases, pp 467–472. 3rd edn. New York, McGraw-Hill, 1999 |
|
Weiss RS, Joseph HL. Syphilis. 1st edn. New York: Thomas Nelson & Sons, 1951 |
|
Tramont EC. Spirochetes: Treponema pallidum (syphilis). In Mandell GL, Bennett JE, Dolin R (eds): Principles and Practice of Infectious Disease. 5th ed. Philadelphia: Churchill Livingstone, 2000 |
|
Public Health Service: Syphilis: A synopsis. 1st ed. Atlanta, Bureau of Disease Prevention and Environmental Control, National Communicable Disease Center, Venereal Disease Program, 1968 |
|
Fitzgerald TJ: Pathogenesis and immunology of Treponema pallidum. Ann Rev Microbiol 35: 29, 1981 |
|
Mahoney JF, Bryant KK: Time element in penetration of genital mucosa by Treponema pallidum. J Vener Dis Infect 15: 1, 1934 |
|
Raiziss GW, Severac M: Rapidity with which Spirochaeta pallida invades the bloodstream. Arch Dermatol Syphilol 35: 1101, 1937 |
|
Thomas DD, Navab M, Haake DA et al: Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc Natl Acad Sci USA 85: 3608, 1988 |
|
Lukehart SA, Holmes KK: Syphilis. In Isselbacher KJ et al (eds): Harrison's Principles of Internal Medicine, pp 726–737. 13th edn. New York, McGraw-Hill, 1994 |
|
Lukehart SA, Baker-Zander SA, Lloyd RM et al: Characterization of lymphocyte responsiveness in early experimental syphilis: II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol 127: 1361, 1980 |
|
Abell E, Marks R, Jones EW: Secondary syphilis: A clinicopathologic review. Br J Dermatol 93: 53, 1975 |
|
Engelkens HJ, ten Kate FJ, Vuzevski VD et al: Primary and secondary syphilis: A histopathological study. Int J Sex Transm Dis Acquired Immune Defic Syndr 2: 280, 1991 |
|
Baughn RE, McNeely MC, Jorizzo JL et al: Characterization of the antigenic determinants host components in immune complexes from patients with secondary syphilis. J Immunol 136: 1406, 1986 |
|
Jorizzo JL, McNeely MC, Baughn RE: Role of circulating immune complexes in human secondary syphilis. J Infect Dis 153: 1014, 1986 |
|
Oppenheimer EH, Hardy JB: Congenital syphilis in the newborn infant: Clinical and pathological observations in recent cases. Johns Hopkins Med J 129: 63, 1971 |
|
Fraser JF: The pathology of congenital syphilis. Acta Dermatol Syph 38: 491, 1920 |
|
Riley BS, Oppenheimer-Marks N, Hansen EJ et al: Virulent Treponema pallidum activates human vascular endothelial cells. J Infect Dis 165: 484, 1992 |
|
Russell P, Altshuler G: Placental abnormalities of congenital syphilis. Am J Dis Child 128: 160, 1974 |
|
Malan AF, Woods DL, Van der Elst CW et al: Relative placental weight in congenital syphilis. Placenta 11: 3, 1990 |
|
McCord JR: Syphilis of the placenta: The histologic examination of 1,085 placentas of mothers with strongly positive Wasserman reactions. Am J Obstet Gynecol 28: 743, 1934 |
|
Fojaco RM, Hensley GT, Moskowitz L: Congenital syphilis and necrotizing funisitis. JAMA 261: 1788, 1989 |
|
Schwartz DA, Larsen SA, Beck-Sague C et al: Pathology of the umbilical cord in congenital syphilis: Analysis of 25 specimens using histochemistry and immunoflourescent antibody to Treponema pallidum. Hum Pathol 26: 784, 1995 |
|
Beck AC, Dailey WT: Syphilis in pregnancy. Public Am Assoc Adv Sci 6: 101, 1938 |
|
Bernirschke K: Syphilis: The placenta and the fetus. Am J Dis Child 128: 142, 1974 |
|
Nathan L, Bohman VR, Sanchez PJ: In utero infection with Treponema pallidum in early pregnancy. Prenat Diagn 17: 119, 1997 |
|
Harter C, Bernirschke K: Fetal syphilis in the first trimester. Am J Obstet Gynecol 124: 705, 1976 |
|
Silverstein AM: Congenital syphilis and the timing of immunogenesis in the human fetus. Nature 194: 196, 1962 |
|
Hook EW III, Marra CM: Acquired syphilis in adults. N Engl J Med 326: 1060, 1992 |
|
DiCarlo RP, Martin DH: The clinical diagnosis of genital ulcer disease in men. Clin Infect Dis 25: 292, 1997 |
|
Mindel A, Tovey SJ, Timmins DJ et al: Primary and secondary syphilis, 20 years' experience: II. Clinical features. Genitourin Med 65: 1, 1989 |
|
Chapel TA: The signs and symptoms of secondary syphilis. Sex Transm Dis 7: 161, 1980 |
|
Cunningham FG, MacDonald PC, Gant NF et al (eds): Williams Obstetrics, pp 1317–1321. 20th edn. Stamford, CT, Appleton & Lange, 1997 |
|
Mertz KJ, Trees D, Levine WC et al: Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities: The Genital Ulcer Disease Surveillance Group. J Infect Dis 178: 1795, 1998 |
|
Musher DM: Early syphilis. In Holmes KK, Sparling PF et al (eds): Sexually Transmitted Diseases, pp 479–485. 3rd edn. New York, McGraw-Hill, 1999 |
|
Campisi D, Whitcomb C: Liver disease in early syphilis. Arch Intern Med 139: 365, 1979 |
|
Hunte W, al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol 3:1351, 1993 |
|
Morrison A: On syphilis and the ear: An otologist's view. Genitourin Med 68: 420, 1992 |
|
Singh AE, Romanowski B: Syphilis: Review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev 12: 187, 1999 |
|
Gjestland T: The Oslo Study of untreated syphilis: An epidemiologic investigation of the natural course of syphilis infection based upon a re-study of the Boeck-Bruusgaard material. Acta Derm Venereol 35 (Suppl 34): 1, 1955 |
|
Roahn PD: Autopsy studies in syphilis. US Public Health Service, Venereal Disease Division. J Vener Dis Infect 21 (Suppl): 1, 1947 |
|
Olansky S: Untreated syphilis in the male negro: X. Twenty years of clinical observation of untreated syphilitic and presumably nonsyphilitic groups. J Chronic Dis 4: 177, 1956 |
|
Rockwell DH: The Tuskgee study of untreated syphilis. Arch Intern Med 114: 792, 1964 |
|
Merritt HH: The early clinical and laboratory manifestations of syphilis of the central nervous system. N Engl J Med 223: 446, 1940 |
|
Merritt HH, Adams RD, Solomon HC: Neurosyphilis. New York, Oxford University Press, 1946 |
|
Hahn RD, Clark EG: Asymptomatic neurosyphilis: A review of the literature. Am J Syphilis Gonorrhea Vener Dis 30: 305, 1946 |
|
Swartz MN, Healy BP, Musher DM: Late syphilis. In Holmes KK, Sparling PF et al (eds): Sexually Transmitted Diseases, pp 487–509. 3rd edn. New York, McGraw-Hill, 1999 |
|
Moore JE: Asymptomatic neurosyphilis: VI. The prognosis of early and late neurosyphilis. JAMA 95: 1637, 1930 |
|
Simon RP: Neurosyphilis. Arch Neurol 42: 606, 1985 |
|
Loewenfeld IE: The Argyll Robertson pupil 1869-1969: A critical survey of the literature. Surv Ophthalmol 14: 199, 1969 |
|
Heggtveit HA: Syphilitic aortitis: A clinicopathologic autopsy study of 100 cases, 1950 to 1960. Circulation 29: 349, 1964 |
|
Jackman JD Jr, Radolf JD: Cardiovascular syphilis. Am J Med 87: 425, 1989 |
|
Sparling PF: Diagnosis and treatment of syphilis. N Engl J Med 284: 642, 1971 |
|
Howles JK: Synopsis of Clinical Syphilis. St. Louis, CV Mosby, 1943 |
|
Kampmeier RH: Saccular aneurysm of the thoracic aorta: A clinical study of 633 cases. Ann Intern Med 12: 624, 1938 |
|
Kampmeier RH: The late manifestations of syphilis: Skeletal, visceral and cardiovascular. Med Clin North Am 48: 667, 1964 |
|
Chung G, Kantor GR, Whipple S: Tertiary syphilis of the face. J Am Acad Dermatol 24: 832, 1991 |
|
Clark EG, Danbolt N: The Oslo Study of the natural history of untreated syphilis. J Chronic Dis 2: 311, 1955 |
|
Chawla V, Gupta K, Raghu MB: Congenital syphilis: A clinical profile. J Trop Pediatr 31: 204, 1985 |
|
Wile U, Mundt LK: Congenital syphilis: A statistical study with special regard to sex incidence. Am J Syphilis Gonorrhea Vener Dis 26: 70, 1942 |
|
Nabarro D: Congenital Syphilis. London, E Arnold, 1954 |
|
Rasool MN, Govender S: The skeletal manifestations of congenital syphilis: A review of 197 cases. J Bone Joint Surg Br 71: 752, 1989 |
|
Pendergrass EP, Bromer RS: Congenital bone syphilis: Preliminary report. Roentgenologic study with note on the histology and pathology of the condition. Am J Roent Ther 22: 1, 1929 |
|
Digre KB: Late-onset congenital syphilis: A retrospective look at University of Iowa Hospital admissions. J Clin Neuro Opthalmol 11: 1, 1991 |
|
Fiumara NJ, Lessell S: Manifestations of late congenital syphilis: An analysis of 271 patients. Arch Dermatol 102: 78, 1970 |
|
Berry MC, Dajani AS: Resurgence of congenital syphilis. Infect Dis Clin North Am 6: 19, 1992 |
|
Robinson RCV: Congenital syphilis. Arch Dermatol 99: 599, 1969 |
|
Hutchinson J: Clinical lecture on heredito-syphilitic struma and on the teeth as a means of diagnosis. Br Med J 1: 515, 1861 |
|
Radolf JD, Sanchez PJ, Schulz KF et al: Congenital syphilis. In Holmes KK, Sparling PF et al (eds): Sexually Transmitted Diseases, pp 1165–1189. 3rd edn. New York, McGraw-Hill, 1999 |
|
Reginato AJ: Syphilitic arthritis and osteitis. Rheum Dis Clin North Am 19: 379, 1993 |
|
Hutchinson CM, Hook EW III, Shepherd M et al: Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection. Ann Intern Med 121: 94, 1994 |
|
Schofer HM, Imhof M, Thoma-Greber E, et al: Active syphilis in HIV infection: A multicentre retrospective study. Genitourin Med 72: 176, 1996 |
|
Marra CM, Handsfield HH, Kuller L, et al: Alterations in the course of experimental syphilis associated with concurrent simian immunodeficiency virus infection. J Infect Dis 165: 1020, 1992 |
|
Marra CM: Syphilis and human immunodeficiency virus infection. Semin Neurol 12: 43, 1992 |
|
Larsen SA, Steiner BM, Rudolph AH: Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev 8: 1, 1995 |
|
Orle KA, Gates CA, Martin DH et al: Simultaneous PCR detection of Haemophilus ducreyi, Treponema pallidum, and herpes simplex virus types 1 and 2 from genital ulcers. J Clin Microbiol 34: 49, 1996 |
|
Chernesky MA: Laboratory services for sexually transmitted diseases: Overview and recent developments. In Holmes KK, Sparling PF et al (eds): Sexually Transmitted Diseases, pp 1281–1294. 3rd edn. New York, McGraw-Hill, 1999 |
|
Rein MF, Banks GW, Logan LC et al: Failure of the Treponema pallidum immobilization test to provide additional diagnostic information about contemporary problem sera. Sex Transm Dis 7: 101, 1980 |
|
Jurado RL, Campbell J, Martin PD: Prozone phenomenon in secondary syphilis: Has its time arrived? Arch Intern Med 153: 2496, 1993 |
|
Spangler AS, Jackson JH, Fiumara NJ et al: Syphilis with a negative blood test reaction. JAMA 189: 87, 1964 |
|
Wilkinson D, Sach ME: Accuracy of on-site screening for syphilis among women attending a rural mobile antenatal clinic. South Afr Med J 88: 783, 1998 |
|
Delport SD, van den Berg JH: On-site screening for syphilis at an antenatal clinic. SAMJ 88: 43, 1998 |
|
Lien TX, Tien NT, Chanpong GF et al: Evaluation of rapid diagnostic tests for the detection of human immunodeficiency virus types 1 and 2, hepatitis B surface antigen, and syphilis in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg 62: 301, 2000 |
|
Peeling R, Ye H: Diagnostic tools for preventing and managing maternal and congenital syphilis: an overview. Bull WHO 82(6): 439-446, 2004 |
|
Augenbraun MH, Rolfs R: Treatment of syphilis, 1998: Nonpregnant adults. Clin Infect Dis 28 (Suppl 1): S21, 1999 |
|
Burke JM, Schaberg DR: Neurosyphilis in the antibiotic era. Neurology 35: 1368, 1985 |
|
Davis LE, Sperry S: The CSF-FTA test and the significance of blood contamination. Ann Neurol 6: 68, 1979 |
|
Gourevitch MN, Selwyn PA, Davenny K et al: Effects of HIV infection on the serologic manifestations and response to treatment of syphilis in intravenous drug users. Ann Intern Med 118: 350, 1993 |
|
Hutchinson CM, Hook EW III, Shepherd M et al: Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection. Ann Intern Med 121: 94, 1994 |
|
Bordon J, Martinez-Vazquez C, de la Fuente-Aguado J et al: Response to standard syphilis treatment in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis 18: 729, 1999 |
|
Yinnon AM, Coury-Doniger P, Polito R et al: Serologic response to treatment of syphilis in patients with HIV. Arch Intern Med 156: 321, 1996 |
|
Johns DR, Tierney M, Felsenstein D: Alteration in the natural history of neurosyphilis by concurrent infection with the human immunodeficiency virus. N Engl J Med 316: 1569, 1987 |
|
Southwick KL, Guidry HM, Weldon MM et al: An epidemic of congenital syphilis in Jefferson County, Texas, 1994-1995: Inadequate prenatal syphilis testing after an outbreak in adults. Am J Public Health 89: 557, 1999 |
|
Blount JH, Holmes KK: Epidemiology of syphilis and the nonvenereal treponematoses. In Johnson RC (ed): The Biology of Parasitic Spirochetes, p 157. New York, Academic, 1976 |
|
Hogue MK, Poppe JA: Sexually Transmitted Diseases: A Policymaker's Guide and Summary of State Laws. Washington, DC, National Conference of State Legislatures, 1998 |
|
Centers for Disease Control and Prevention. 2006 Sexually Transmitted Diseases Treatment Guidelines. MMWR 55(RR11) 1-94, 2006 |
|
American Academy of Pediatrics and American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care, pp 65–92. 4th edn. Washington, DC, American Academy of Pediatrics and American College of Obstetricians and Gynecologists, 1997 |
|
Watts DH, Brunham RC: Sexually transmitted diseases, including HIV infection in pregnancy. In Holmes KK, Sparling PF et al (eds): Sexually Transmitted Diseases, pp 1089–1132. 3rd edn. New York, McGraw-Hill, 1999 |
|
Ingall D: Syphilis. In Remington JS, Klein JD (eds): Infectious Diseases of the Fetus and Newborn Infant. Philadelphia, WB Saunders, 1994 |
|
Stoll BJ: Congenital syphilis: Evaluation and management of neonates born to mothers. Pediatr Infect Dis J 13: 845, 1994 |
|
Ward JW, Greenspan JR: Public health surveillance for HIV/AIDS and other STDs: Guideposts for prevention and care. In Holmes KK, Sparling PF et al (eds): Sexually Transmitted Diseases, pp 1337–1352. 3rd ed. New York, McGraw-Hill, 1999 |
|
Centers for Disease Control and Prevention: Inadvertent use of Bicillin® C-R for treatment of syphilis: Maryland, 1998. MMWR Morbid Mortal Wkly Rep 48:777, 1999 |
|
Brown ST: Adverse reactions in syphilis therapy. J Am Vener Dis Assoc 3: 172, 1976 |
|
Farmer TW: The Jarisch-Herxheimer reaction in early syphilis. JAMA 138: 480, 1948 |
|
Klein VR, Cox SM, Mitchell MD et al: The Jarisch-Herxheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol 75: 375, 1990 |
|
Myles TD, Elam G, Park-Hwang E et al: The Jarisch-Herxheimer reaction and fetal monitoring changes in pregnant women treated for syphilis. Obstet Gynecol 92: 859, 1998 |
|
Schroeter AL, Lucas JB, Price EV et al: Treatment for early syphilis and reactivity of serologic tests. JAMA 221: 471, 1972 |
|
Schoch PE, Wolters EC: Penicillin concentrations in serum and CSF during high-dose intravenous treatment for neurosyphilis. Neurology 37: 1214, 1987 |
|
Alexander JM, Sheffield JS, Sanchez PJ et al: Efficacy of treatment for syphilis in pregnancy. Obstet Gynecol 93: 5, 1999 |
|
Riedner G, Rusizoka M, Todd J, et al. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N Engl J Med 2005;353:1236-44 |
|
Zhou P, Gu Z, Xu J, Wang X, Liao K. A study evaluating ceftriaxone as a treatment agent for primary and secondary syphilis in pregnancy. Sex Transm Dis 2005;32(8):495-498 |
|
Goeman J, Kivuvu M, Nzila N et al: Similar serologic responses to conventional therapy for syphilis among HIV-positive and HIV-negative women. Genitourin Med 71: 275, 1995 |
|
Telzak EE, Greenberg MS, Harrison J et al: Syphilis treatment response in HIV-infected individuals. AIDS 5: 591, 1991 |
|
Rolfs RT, Joesoef R, Hendershot EF et al: A randomized trial of enhanced therapy for early syphilis in patients with or without human immunodeficiency virus infection: The Syphilis and HIV Study Group. N Engl J Med 337: 307, 1997 |
|
Johnson RC, Bey RF, Wolgamot SJ: Comparison of the activities of ceftriaxone and penicillin G against experimentally induced syphilis in rabbits. Antimicrob Agents Chemother 21: 984, 1982 |
|
Korting HC, Walther D, Riethmuller U et al: Ceftriaxone given repeatedly cures manifest syphilis in the rabbit. Chemotherapy 33: 376, 1987 |
|
Augenbraun M, Workowski K: Ceftriaxone therapy for syphilis: Report from the emerging infections network. Clin Infect Dis 29: 1337, 1999 |
|
Hook EW III, Baker-Zander SA, Moskowitz BL et al: Ceftriaxone therapy for asymptomatic neurosyphilis: A case report and Western blot analysis of serum and cerebrospinal fluid IgG response to therapy. Sex Transm Dis 13: 185, 1986 |
|
Hook EW III, Roddy RE, Handsfield HH: Ceftriaxone therapy for incubating and early syphilis. J Infect Dis 158: 881, 1988 |
|
Moorthy TT, Lee C, Lim K et al: Ceftriaxone for treatment of primary syphilis in men: A preliminary study. Sex Transm Dis 14: 116, 1987 |
|
Dowell ME, Ross PG, Musher DM et al: Response of latent syphilis or neurosyphilis to ceftriaxone therapy in persons infected with human immunodeficiency virus. Am J Med 93: 481, 1992 |
|
Lukehart SA, Fohn MJ, Baker-Zander SA: Efficacy of azithromycin for therapy of active syphilis in the rabbit model. J Antimicrob Chemother 25 (Suppl A): S91, 1990 |
|
Verdon MS, Handsfield HH, Johnson RB: Pilot study of azithromycin for treatment of primary and secondary syphilis. Clin Infect Dis 19: 486, 1994 |
|
Hook EW III, Stephens J, Ennis DM: Azithromycin compared with penicillin G benzathine for treatment of incubating syphilis. Ann Intern Med 131: 434, 1999 |
|
Gunn RA, Rolfs RT, Greenspan JR, et al: The changing paradigm of sexually transmitted disease control in the era of managed health care JAMA. 279: 680, 1998 |
|
Azithromycin treatment failures in syphilis infections--San Francisco, California, 2002-2003. MMWR Morb Mortal Wkly Rep 2004;53:197-198 |
|
Lukehart SA, Godornes C, Molini BJ et al. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med 2004;351:154-158 |
|
Andrus JK, Fleming DW, Harger DR et al: Partner notification: Can it control epidemic syphilis? Ann Intern Med 112: 539, 1990 |
|
Peterman TA, Toomey K, Dicker LW et al: Partner notification for syphilis: A randomized controlled trial of three approaches. Sex Transm Dis 24: 511, 1997 |
|
Oxman G: A comparison of the case-finding effectiveness and average costs of screening and partner notification. Sex Transm Dis 23: 51, 1996 |
|
Centers for Disease Control and Prevention: Relationship of syphilis to drug use and prostitution—Connecticut. MMWR Morbid Mort Wkly Rep 37:755, 1988 |
|
Greenberg MS, Singh T, Htoo M et al: The association between congenital syphilis and cocaine/crack use in New York City. Am J Public Health 81:1316, |