The Role of Growth Factors in Ovarian Function and Development
Authors
INTRODUCTION
Ovarian folliculogenesis is a dynamic process marked by exponential expansion and differentiation of the granulosa cells, maturation of the oocyte, and neovascularization (Figure 1 to follow).
Although the central roles of gonadotropins and of gonadal steroids in this explosive agenda are well accepted, the variable fate of follicles within the same ovary suggests the existence of additional intraovarian modulatory systems.1 Stated differently, it is presumed that gonadotropin action is 'fine tuned' in situ, thereby accounting for observed differences in the rate and extent of development of ovarian follicles. Alterations in gonadotropin secretion cannot adequately explain the initiation and arrest of meiosis within the oocyte, the acquisition of follicular dominance, or the failure of follicular development, which leads to atresia. It is likely that the earlier stages of follicular growth, generally considered to be gonadotropin independent, may be controlled by intraovarian signaling.
The concept of local gonadal regulators originated when the embryology of the ovary was the subject of intense scrutiny. Gonadal differentiation was proposed by Witschi to result from the interaction of two morphogenic substances called cortexone and medullarine, the first of which was thought of as the stimulator of ovarian development and the latter as the promoter of testicular growth.2 Although multiple other contributors must undoubtedly be acknowledged, the notion of intraovarian regulators was promoted with special vigor by the late Cornelia Post Channing, whose pioneering experiments ushered in contemporary molecular endocrinology as it applies to ovarian physiology.3
Among potential novel intraovarian regulators, growth factors, cytokines, and neuropeptides have been the subject of increasingly intense investigation. Most of these agents are not expected to act in the traditional endocrine fashion because of their local intraovarian generation (as opposed to circulatory-derived influences emanating from distant endocrine glands). Speculation favors the notion that a host of putative intraovarian regulators may engage in subtle in situ modulation and coordination of growth and function of the varied follicular cell types: oocytes, granulosa, theca, and vascular epithelium (Figure 2 to follow).
In this capacity, a given putative intraovarian regulator may modulate the replication or cytodifferentiation of a developing ovarian cell, acting in its own right or as an amplifier-attenuator of gonadotropin action. Such putative intraovarian regulators may also be concerned with intercompartmental communication, allowing tighter linking of different cellular populations. For example, a growing body of evidence suggests that granulosa cell-derived modulators may regulate the adjacent theca-interstitial cell compartment in the interest of coordinated follicular development. In doing so, the granulosa cell may exert some control over its own destiny, in that it may regulate the very inflow of androgenic substrate from the neighboring theca. Together, gonadotropins, steroids, and locally derived peptidergic principles form a triad, which modulates the growth and differentiation of ovarian follicles (Fig. 3). According to contemporary views, potential intraovarian communication is mostly paracrine or autocrine in nature. Paracrine communication involves local diffusion of regulators from producer cells to distinct target cells within the same organ. This is a heteroregulatory phenomenon that could allow for intercompartmental communication, providing a tighter linkage of different cellular populations. In the ovary, the ability of increasing numbers of granulosa cells to produce estrogen depends on the concomitant ability of the thecal layer to provide the proper amounts of androgenic substrate. The granulosa cell, in the interest of efficient coupling, may elaborate substances (e.g. insulin-like growth factor-I [IGF-I] inhibin, activin) that could alter the function of the neighboring theca.
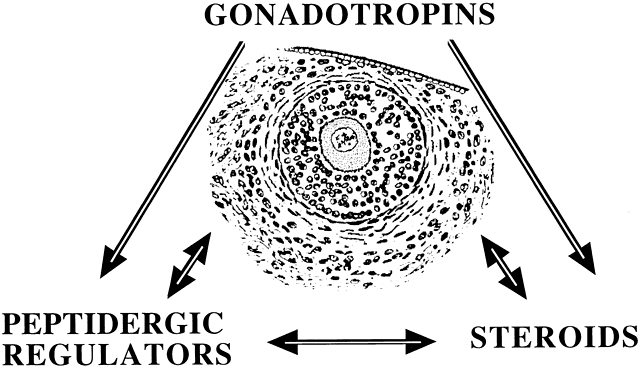 Fig. 3 Modulators of ovarian follicular growth and development: the regulatory triad
Fig. 3 Modulators of ovarian follicular growth and development: the regulatory triad
The other type of cellular communication, autocrine regulation, involves the action of a regulator on surface receptors at its cell of origin. This is a self-regulatory phenomenon wherein a single cell type modulates its own activity. In the ovary, granulosa cells elaborate substances such as IGF-I and activin that can alter granulosa cell function. Whereas steroids may be exerting intracrine (i.e. regulation within the cell of origin) effects, there is no evidence for juxtacrine (i.e. contact-dependent regulation between immediately adjacent cells) effects in the ovary.
To qualify as a bona fide intraovarian regulator, the putative agent needs to meet the minimal criteria of local production, local reception, and local action. Some evidence of indispensability to in vivo ovarian function needs to be provided. For the most part, few of the putative intraovarian regulators under study (Table 1) have satisfactorily met all of the previously described criteria (i.e. IGF-I, activin). Accordingly, the information provided later can be viewed as a prelude to what the future holds. Undoubtedly, additional information will become available with respect to the putative intraovarian regulators under consideration. It is equally certain that novel candidates will be added to this preliminary list, requiring modification of current views.
Table 1. Established and putative intraovarian regulators
Insulin-Like Growth Factor System
IGF-I
IGF-II
IGF binding proteins
Inhibin/Activin Systems
Inhibin
Activin
Follistatin
Interleukin-1 System
Interleukin-1
Interleukin-1 receptor antagonist
IL-1 binding protein (IL-1 receptor type II)
Other Growth Factors
EGF/TGFα
TGFβ1, TGFβ2
NGF
aFGF, bFGF
VEGF
TNFα
Other Peptidergic Factors
Ovarian renin angiotensin system
VIP
Oxytocin
Endothelin
The following sections describe a select group of putative intraovarian regulators reflecting different modes of action. The principle action of each regulator is briefly listed in Table 2.
Table 2. Principal actions of intraovarian regulators
Insulin-like growth factor-I
Follicle-stimulating hormone (FSH) amplification
Follicular growth
Follicular selection
Transforming growth factor-α
Follicular maturation
Oocyte maturation
Cellular differentiation
Potentiation of gonadotropin action
Regulation of apoptosis
Transforming growth factor-β1
Follicular rupture inhibition
Follicular differentiation
Basic fibroblast growth factor
Apoptosis inhibition
Regulation of folliculogenesis
Activin
Oocyte maturation
Follicular differentiation
Early embryogenesis
Regulation of steroidogenesis
Interleukin-1 (see also Fig. 4)
Ovulation induction
Glycolysis
Glucose transport
Tumor necrosis factor-α
Inhibits steroidogenesis
FSH antagonist
Induces apoptosis/luteolysis
Ovulation inhibition
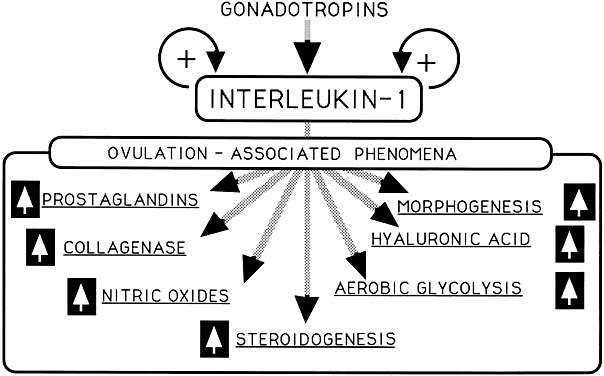 Fig. 4 Intraovarian interleukin-1 as a mediator of gonadotropin action
Fig. 4 Intraovarian interleukin-1 as a mediator of gonadotropin action
INSULIN-LIKE GROWTH FACTOR-1
A 70-amino acid polypeptide, IGF-I plays a variety of metabolic and endocrine roles, not the least of which is the promotion of linear skeletal growth. In keeping with its ubiquitous distribution, IGF-I is also known to serve a variety of autocrine or paracrine tissue-specific functions to suit the needs dictated by the tissues in question. In this respect, the ovary is but one example of many exemplifying the general concept of intraorgan regulation.4
A large body of information now strongly supports the view that the ovary is a site of IGF-I production, reception, and action (Fig. 5). Whereas the rat granulosa cell appears to be the only cellular site of IGF-I gene expression,5, 6 the granulosa7, 8 and the theca-interstitial cells9, 10 possess specific receptors for this peptidergic ligand. The mouse intraovarian IGF-I system is generally comparable to that of the rat, although they differ in several aspects.11, 12 These observations suggest that IGF-I may engage in intercompartmental communication in the interest of coordinated follicular development. IGF-I hormonal action appears subject to further modulation through the local elaboration of low-molecular-weight binding proteins (IGFBPs), the role and regulation of which are receiving increasing attention. Whereas the main IGFBP is IGFBP-3, being up-regulated by GH, other IGFBPs bear important roles in reproductive endocrinology. For instance, IGFBP-1 being down-regulated by insulin, has an important role in the pathophysiology of PCOS, by increasing the free, biological active IGF-I, augmenting the androgen generation in the theca layer.13 The discovery of IGFBP-4 mRNA in early-stage atretic follicles raises the intriguing possibility that depletion of IGF action may be necessary for the onset of the atretic process.14 Although multiple ovarian actions have been ascribed to IGF-I, its main role appears to be the amplification of gonadotropin action in theca-interstitial and granulosa cells. All markers of follicle-stimulating hormone (FSH) induction (e.g. production of progesterone inhibin, luteinizing hormone binding) are enhanced by IGF-I. Optimal gonadotropin hormonal action is contingent on the prior availability of granulosa cell-derived IGF-I and the consequent amplification of the gonadotropic signal. Given a hypothetical IGF vacuum created by excess exogenous IGF binding proteins, intrinsic FSH hormonal action proves to be relatively modest (Fig. 4). In contrast, given IGF-replete circumstances, FSH hormonal action in toto may be composed of a modest intrinsic component complemented by a substantial synergistic component.15 IGF-I has an obligatory role in granulosa cell replication in all species tested.16 Further consideration must be given to the possibility that there are two distinct types of granulosa cell related to their proximity to the oocyte.16 In addition, there appears to be an association between increased bioavailability of IGF-I in follicular fluid and selection of the dominant follicle.17, 18
 Fig. 5 The intraovarian insulin-like growth factor-I system
Fig. 5 The intraovarian insulin-like growth factor-I system
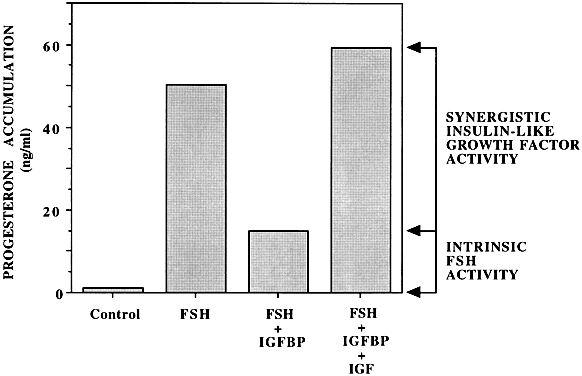 Fig. 6 Enhancing effect of insulin-like growth factor-I on follicle-stimulating hormone-stimulated progesterone accumulation
Fig. 6 Enhancing effect of insulin-like growth factor-I on follicle-stimulating hormone-stimulated progesterone accumulation
At the clinical level, ovarian IGF-I may have a bearing on the puberty-promoting effect of growth hormone. An association appears to exist between isolated growth hormone deficiency and delayed puberty in rodents and human subjects, a process reversed by systemic hormone replacement therapy. Given that ovarian IGF-I and its receptor may be growth hormone dependent, it is tempting to speculate that the ability of growth hormone to accelerate pubertal maturation in part may be caused by the promotion of ovarian IGF-I production and reception with the consequent local potentiation of gonadotropin action.
Clear evidence for the central role of IGF-I in reproductive physiology has been gained from gene knockout technology. In the mouse, targeted null mutation of the Igf1 gene, encoding IGF-I, results in infertility secondary to failure to ovulate even after administration of gonadotropins.19 Given the IGF-I primary action of FSH amplification, further efforts have been made to elucidate its mechanism of action in that regard.20, 21, 22 IGF-I leads to increased estradiol synthesis and proliferation of granulosa cells, enhanced LH-induced androgen-synthesis in theca cells and increased inhibin-, activin- and follistatin secretion from granulosa cells.23
In human dominant follicles IGF-II receptor is expressed in both granulosa cells and theca cells, while IGF-II is expressed extensively in granulosa cells.24 In addition, IGF-II is expressed in the corpus luteum, where it coordinates vessel maintenance and angiogenesis.25 IGF-II increases in the presence of FSH, LH receptor synthesis in theca-interstitial cells and granulosa cells from antral follicles,26, 27 as well as proliferation and steroidogenesis in granulosa cells.26
GROWTH HORMONE
Growth hormone receptor (GH-R) is expressed in the rat ovary together with GH binding protein (GHBP) that regulates the bio-availability of GH.28, 29 GH and GH-R are detected in oocytes and granulosa cells in early developing human follicles.30 However, it is still not clear whether systemic GH binds to GH-R in the ovary, or the ovary itself produces GH.31 It is suggested, that in the ovary GH acts directly through ovarian GH-R, or indirectly via IGF-I and IGF-II.31 GH has a stimulatory effect on the formation of secondary follicles.32 GH-R and GHBP deficient mice have strongly reduced numbers of primary, secondary and antral follicles, but an elevated number of primordial follicles and a significant increased number of atretic follicles.33 In addition, GH is responsible for the development and maintenance of sensitivity to gonadotropin.34, 35 GH increases IGF-I secretion from theca cells and IGF-I stimulates proliferation of granulosa cells and steroidogenesis.36 On the other hand, it has been shown that GH also directly enhances estradiol production in cultured granulosa cells.37 GH promotes nuclear and cytoplasmic maturation and improves the developmental capacity of oocytes.38, 39
TRANSFORMING GROWTH FACTOR/EPIDERMAL GROWTH FACTOR
Purified on the basis of its ability to stimulate precocious eyelid opening and tooth eruption in newborn mice, epidermal growth factor (EGF) was initially found in male mouse submaxillary glands and later in human urine as urogastrone. Mature EGF comprises a single polypeptide chain of 53 amino acids displaying three internal disulfide bonds. Originally thought to have a limited range of tissue expression, in situ hybridization analysis of sections of whole newborn mice indicate that RNA complementary to cloned EGF probes may be present in a large variety of tissues.
Transforming growth factor-α (TGF-α), a structural analog of EGF, is a single-chain, 50-amino acid polypeptide capable of binding to an apparently common EGF/TGF receptor. EGF and TGF recognize the same cellular receptor, and they are apparently equipotent in most systems studied. EGF may be the adult form of the embryonic growth factor TGF. TGF is a member of a family of polypeptides best known for their ability to produce an acute, albeit reversible, phenotypic transformation of normal mammalian cells. TGF can be defined operationally by its ability to stimulate anchorage-independent growth in soft agar of cells, which are otherwise anchorage dependent.
At the level of the ovary, EGF exerts potent regulatory effects on granulosa cell proliferation and differentiation.40, 41, 42 These effects of EGF presumably are mediated by specific cell membrane receptors, the existence of which has been demonstrated on bovine, ovine, and murine granulosa cells.43 However, the identity of the endogenous ligand occupying the receptor in question under in vivo conditions remains uncertain. Norris et al., 201044 demonstrated that EGF receptor kinase contributes to LH-induced meiotic resumption of oocytes through the closure of gap junctions and through a decrease in follicle cGMP.
EGF-like growth factors
It has been shown, that the binding of LH to G-a-coupled LH receptors on the outer theca cells and mural granulosa cells leads to matrix metaloproteinase (MMP)-mediated release of EGF-like growth factors from the cell surface.45 Subsequently, the released EGF-like growth factors bind to EGF receptors located on cumulus cells. Such EGF-like growth factors are amphiregulin, betacellulin and epiregulin.46 It has been suggested, that amphiregulin is important for oocyte maturation and cumulus cell expansion.47
Androgens and progestins promote oocyte maturation through steroid receptors.48 LH-induced, MMP-mediated, release of EGF-like growth factors leads to activation of EGF receptors on cumulus granulosa cells and hence to phosphorylation of steroidogenic acute regulatory protein (StAR), resulting in subsequent up-regulation of steroidogenesis.49
TGF, like EGF, proved to be a potent inhibitor of gonadotropin-supported granulosa cell differentiation. TGF has been localized to the theca-interstitial cell compartment,50 thereby raising the possibility that theca-interstitial cell-derived TGF may exert paracrine effects at the level of the adjacent granulosa cell. Theca-interstitial cell-derived TGF may also engage in autocrine effects.51 It is tempting to speculate that TGF of theca-interstitial cell origin may orchestrate follicular activities at the granulosa and theca-interstitial cell level (Fig. 7). However, because TGF has also been shown to suppress gonadotropin-supported theca-interstitial cell differentiation,51 the possibility of an autocrine mode of action cannot be excluded. Further evidence supports the possibility that TGF may also be expressed by other compartments of the ovary (e.g. granulosa cells, oocytes). In humans, the expression pattern may be age and cycle dependent52 and may have a role in ovarian embryogenesis.53
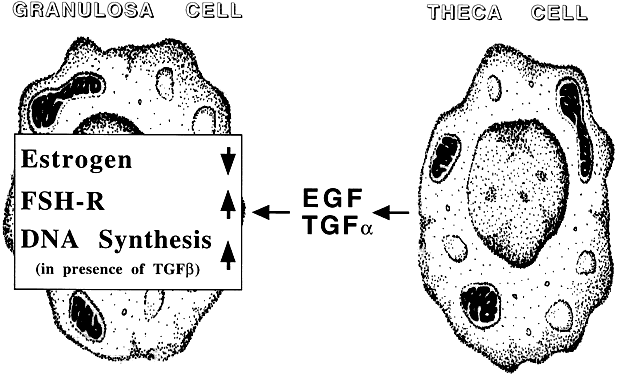 Fig. 7. The intraovarian epidermal growth factor/transforming growth factor-α system. EGF, epidermal growth factor; TGF, transforming growth factor; FSH, follicle stimulating hormone.
Fig. 7. The intraovarian epidermal growth factor/transforming growth factor-α system. EGF, epidermal growth factor; TGF, transforming growth factor; FSH, follicle stimulating hormone.
TGF is also involved in the process of follicular apoptosis, which is central in maintaining a balance between cell proliferation and demise. Treatment of cultured granulosa54 or theca-interstitial cells55 with TGF inhibits the spontaneous onset of apoptotic DNA cleavage.
TRANSFORMING GROWTH FACTOR BETA SUPERFAMILY MEMBERS
Transforming growth factor-β (TGF-β) superfamily is a group of about 35 proteins involved in pre- and postnatal physiological processes.56 Members of this superfamily are expressed by oocytes and ovarian somatic cells in key developmental stages.57, 58, 59, 60, 61 Throughout life ovarian follicles leave the resting pool to join the growing pool. The precise mechanism of follicular recruitment is not fully elucidated, however, members of the TGF-β family are involved in the process. Bone morphogenetic proteins (BMP) 7 and 4 promote primordial to primary follicle transition.62 Similarly, anti-Mullerian hormone (AMH), another member of the TGF-β superfamily, is involved in initiation of primordial follicle growth.63 Progression of primary follicles to early antral stage is enhanced by growth and differentiation factor-9 (GDF-9) and BMP-15 of oocyte origin, activins of granulosa origin, and BMP-4 and BMP-7 of thecal origin.61 Antral follicle growth and follicle selection mechanism involves the inhibin-activin system.64 Activin, TGF-β, and several BMPs exert paracrine actions on theca cells to attenuate LH-dependent androgen production in small to medium size antral follicles.65 Dominant follicle selection is influenced by changes in intrafollicular activins, GDF-9, AMH, and several BMPs. Activin plays a positive role in oocyte maturation, while inhibin upregulates LH-induced androgen secretion to sustain estradiol biosynthesis during the pre-ovulatory phase.
In human ovaries GDF-9 promotes the growth of early pre-antral follicles.66 GDF-9 inhibits FSH-induced steroidogenesis in pre-ovulatory follicles and promotes progesterone production in cumulus cells through enhancing the expression of an intrinsic prostaglandin-E2/EP2 receptor signaling pathway.67 Furthermore, GDF-9 promotes follicular survival during the transition to the antral stage by suppressing granulosa cell apoptosis and follicular atresia.68 During the latter stages of folliculogenesis cumulus cells require GDF-9 in order to support glycolysis and sterol biosynthesis prior to the LH-surge.69 It has been shown that GDF-9 protects granulosa cells from apoptosis through the activation of the PI3K/Akt pathway in pre-antral follicles 68. In addition, GDF-9 regulates granulosa cell mitosis through both Smad-dependent and independent pathways.70 Mutations in human GDF-9 contribute to ovarian insufficiency in women through defective GDF-9 production and/or activation.71 It has been shown that women with mutations leading to the activation of latent human GDF-9 reach menopause before the age of 35 years. These women have elevated FSH and LH levels and atrophic ovaries devoid of follicles. It is suggested that mutations in the latency-associated GDF-9 prodomain may contribute to premature ovarian failure through an increase in the number of growing follicles leading to a premature depletion of the ovarian reserve.71
Anti-Mullerian hormone
AMH is a member of the TGF-β superfamily.72 AMH is solely expressed in granulosa cells of small growing follicles. After the initiation of primordial follicle growth, AMH is expressed in granulosa cells and remains expressed until the small antral stage. Then, FSH leads to a decrease in AMH expression and is almost absent in granulosa cells during the FSH-dependent follicular growth.73 In contrast to this, AMH expression sustains in cumulus cells of pre-ovulatory follicles.74 AMH receptor II shows a similar expression pattern as AMH itself75 and is also expressed in theca cells.76
During early folliculogenesis AMH inhibits primordial to primary follicle transition and progression to the antral stage.77
In human granulosa cells AMH inhibits the effects of FSH.78 It has been shown, that AMH inhibits FSH-dependent aromatase synthesis and estradiol production in human granulosa cells.79 AMH probably keeps the follicle relatively insensitive to FSH until the follicle reaches a certain grade of maturity.80 In accordance to this, it has been shown that in AMH-knockout mice, more small antral follicles and also the larger, normally not FSH-responsive pre-antral follicles were recruited in the absence of AMH.81 It can be concluded that with decreasing AMH expression the FSH-sensitivity of the follicle increases and therefore the follicle can be recruited to enter the pool of follicles that may become dominant (Figure 8 to follow).
BASIC FIBROBLAST GROWTH FACTOR
Basic fibroblast growth factor (bFGF), a 146-amino acid polypeptide, is a mitogen for a wide variety of mesoderm-derived and neuroectoderm-derived cells. Its complete isolation and characterization has been accomplished from various organs; an amino terminally truncated form lacking the first 15 residues was identified in the ovarian corpus luteum.65 Although the physiologic relevance of bFGF to ovarian function remains under investigation,82 several lines of evidence suggest that bFGF may play a central role in supporting the growth and development of the granulosa-luteal cell. Basic FGF constitutes the main mitogenic factor isolated from crude extract and has previously been shown to stimulate the replicative lifespan of cultured granulosa cells of bovine, porcine, rabbit, guinea pig, and human origin.83, 84, 85 Because ovarian bFGF expression was not considered to be of granulosa cell origin,86 whereas FSH induces functional receptors for bFGF in the granulosa cells,87 it is tempting to speculate that locally produced bFGF88 may play autocrine or paracrine regulatory roles at or adjacent to its sites of synthesis. In so doing, it may participate in the differentiation and replication of the developing granulosa cell.89
Basic FGF is involved in early development of the human reproductive tract90 and partakes in suppression spontaneous onset of apoptosis.91 The latter may be associated with the ability of progesterone to maintain granulosa cell viability.92 In addition to granulosa cells, bFGF also inhibits apoptosis of the ovarian surface epithelial cells, acting in both sites on its own receptor.93 Basic FGF can be identified in a host of ovarian components, including granulosa cells, oocytes, follicular basement membrane, and surface epithelial cells.94, 95, 96
ACTIVIN
Activin is a 24-kD protein with structural homology to TGF-β1. It was discovered during the purification of inhibin and found to be a dimer of the β subunits of the heterodimeric inhibin molecule.97 Activin was concurrently discovered as capable of differentiating erythroleukemia cells98 and inducing mesoderm formation.99 Its presence in a variety of cell types suggests that it may regulate growth and differentiation in other tissues as well.97
Activins play a role in the local regulation of ovarian function. Acting through a set of receptors, postulated to be membrane-bound serine/threonine kinases,100, 101 activin alters the function of granulosa and theca-interstitial cells. For instance, activin treatment of cultured granulosa cells from immature follicles increases FSH-supported estradiol production, inhibin production, and FSH and luteinizing hormone binding. Activin may maintain the immature follicle during the period of declining FSH levels, which is induced by its partner inhibin. In contrast to its action on immature granulosa cells, activin decreases progesterone production by mature granulosa cells from preovulatory follicles.102 Based on these observations, Findlay and coworkers103 proposed an autocrine role for activin as a suppressor of spontaneous luteinization. Paracrine actions of activin are also a possibility because activin reduces luteinizing hormone-induced androstenedione production by cultured theca-interstitial cells.104, 105
Throughout follicle development the balance between activin and inhibin expression shifts. Activins are especially expressed in primary and antral follicles, while inhibins are expressed mainly in larger more developed follicles.106 Activin plays an essential role in primordial follicle assembly and in the establishment of the size of the primordial follicle pool. It has been suggested that during the time of follicle assembly the local activin concentrations determine the size of the ovarian follicle pool.107 As such it has been demonstrated that the administration of activin to neonatal mice at the time of germline cyst-breakdown led to an increase in the number of germ cells and pre-granulosa cells and also to an increase in the size of the primordial follicle pool.108 In accordance to this observation, Lei et al., 2010109 found that FSH treatment to a neonatal mouse ovarian culture model increased proliferation of pre-granulosa cells, expression of activin b and bB subunits and oocyte survival, and thus promoted overall primordial follicle formation. In addition, it is assumed that activin has the capacity to suppress early follicle growth.107 As such it was demonstrated that the presence of activin secreting secondary follicles inhibited the growth of pre-antral follicles in an in vitro co-culture model.110 Furthermore, activin increases LH induced androgen synthesis in small and medium sized antral follicles111 and promotes oocyte maturation and growth of pre-antral follicles.112 In this regard it has been shown that activin A alone, and in combination with FSH, promotes granulosa cell proliferation in granulosa cells of pre-antral follicles and oocyte growth.113
Activin stimulates the ERa promoter and estrogen receptor activity. On the other hand, estrogen suppresses activin b subunit and activin bB subunit. With the progression of folliculogenesis, activins may become inhibitory to steroidogenesis.114 In accordance to this, it has been demonstrated that in late tertiary follicles activin inhibits FSH-mediated aromatase activity.115 Activin has an anti-luteinization effect on granulosa cells. In this context, activin upregulates FSH-R and HSD11b2, downregulates LH-R, blocks the hCG-induced upregulation of StAR and downregulates ERa expression.116 In luteinized human granulosa cells activin suppresses progesterone production and the expression of CYP11a1, HSD3 b and CYP19.117 It is assumed that a reduction in activin signaling is essential for luteinization and the formation and maintenance of the corpus luteum. During luteolysis activin enhances MMP-2 expression, which can be reversed by follistatin.118 Activin receptors are expressed in the corpus luteum,119 while the level of follistatin decreases in regressing corpus luteum, thus resulting in increased bioavailability of activin.120
Follistatin, a glycoprotein with isoforms of 35–40 kD, was also discovered during the purification of inhibin.97 Its ability to bind activin121 provides a possible explanation for the observation that follistatin antagonizes the in vitro actions of activin. The presence of follistatin primarily in preovulatory follicles122 supports the idea that blocking activin is necessary for maturation and luteinization. In the developing follicles, FSH-induced granulosa cell proliferation and mitogenesis is facilitated by activin.123
The study of activin action is further complicated by the ability of its component subunits to combine with the α subunit of inhibin to form a molecule whose action in many experimental assays is diametrically opposed to that of activin. Although autocrine actions of inhibin have not been convincingly demonstrated, granulosa cell-derived inhibin can oppose the activin blockade of thecal androgen production.104 Activin, follistatin, and inhibin form a complex mix of intraovarian regulators (Fig. 9).
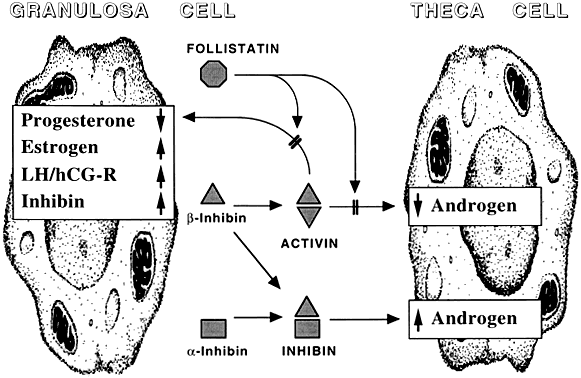 Fig. 9 The intraovarian activin/inhibin system. LH,luteinizing hormone; hCG, human chorionic gonadotropin
Fig. 9 The intraovarian activin/inhibin system. LH,luteinizing hormone; hCG, human chorionic gonadotropin
INHIBIN
Inhibin exists in two isoforms, inhibin A and inhibin B. Inhibin A consists of one a- and one bA subunit, while inhibin B comprises one a- and one bB subunit.107 It has been shown that both inhibin isoforms are expressed in granulosa cells of growing follicles.124 Inhibin A is especially produced by the dominant follicle after the preovulatory gonadotropin surge, and by the corpus luteum, whereas inhibin B is mainly secreted from the small antral follicles.125, 126 In the ovary inhibin antagonizes the action of activin through its competitive binding to activin receptors.127 Inhibin enhances in theca cells the LH-induced androgen synthesis.128 In neonatal mice the level of inhibin B drops drastically during the interval of follicle formation. This in turn lowers the inhibin to activin ratio and hence leads to sustained activin signaling in the ovary during the period of follicle formation.107 Vitale et al., 2002129 suggest that inhibin may play an important role in follicular selection, as inhibin secreted by the dominant follicle leads to apoptosis in subordinate follicles.
The corpus luteum is a major source of inhibin A during the human menstrual cycle.130 It has been demonstrated that antibodies to the a subunit of inhibin led to a decrease in hCG-induced progesterone secretion by luteal cells (Figure 10 to follow).131
INTERLEUKIN-1
Interleukin-1 (IL-1), a polypeptide cytokine previously referred to as lymphocyte-activating factor, is predominantly produced and secreted by activated macrophages. It possesses a wide range of biologic functions and plays a role as an immune mediator.132 At the level of the ovary, IL-1 suppresses the functional and morphologic luteinization of cultured murine and porcine granulosa cells.133, 134 Exerted at physiologic concentrations (10-9 M), IL-1 action could not be attributed to altered cell viability. Rather, the antigonadotropic activity of IL-1 appeared to involve sites of action proximal and distal to cAMP generation. Subsequent work by Kasson and Gorospe shed additional light on the ovarian relevance of interleukins.135 IL-1α and IL-1β augmented the FSH-stimulated accumulation of 20-dihydroprogesterone. In all cases, less IL-1β than IL-1α was required to produce a comparable effect. Other studies in the rat ovary indicate that the rat ovarian theca-interstitial cell is a site of IL-1β gene expression, the preovulatory acquisition of which is gonadotropin dependent.136 However, immediately after follicle rupture, granulosa cells stain positive for IL-1β in immunohistochemical studies in the mouse ovary.137 The possibility of a shift in IL-1β origin, receptor, and action to the granulosa cell compartment just before ovulation cannot be excluded.
Although the relevance of IL-1 to ovarian physiology remains a matter of study, it is tempting to speculate that IL-1 could be involved in mediation of gonadotropin action and in the luteinization process (Fig. 4). Such speculation appears particularly intriguing in light of the apparent progesterone dependence of IL-1 gene expression.138 In contrast, higher concentrations of progesterone significantly inhibit IL-1 activity.139 Although much remains to be learned on the intraovarian cellular origin of IL-1, resident interstitial ovarian macrophages could be sites of hormonally regulated IL-1 gene expression given the reported gonadotropin dependence of their testicular counterparts.140
Significant amounts of IL-1-like activity have been detected in follicular fluid.141 The ovarian reception of IL-1 involves the type I IL-1 receptor, whose transcripts have been identified in cultured human granulosa and theca cells.142 Interleukin and its receptors are maximally expressed in granulosa cells and theca cells in preovulatory follicles after the gonadotropin action.143 It was shown that IL-1 signaling occurs exclusively through the type I receptor,144 whereas the type II receptor inhibits IL-1 activity by acting as a 'decoy' target for IL-1.145 A growing body of evidence supports the role of IL-1 as an intermediary in the ovulatory process (Fig. 4). IL-1 is a potent stimulator of the ovarian phospholipase A2 system,146, 147, 148 and of prostaglandin endoperoxide synthase-1 and -2,149 both in the interest of upregulating prostaglandin biosynthesis. IL-1 is also involved in ovarian carbohydrate economy.150, 151, Ovarian IL-1 signaling occurs through the type I receptor, the expression of which is stimulated with ovulation.152In vivo models, using perfused ovaries or direct intrabursal injection, also support IL-1's central role in the process of ovulation.153, 154, 155
IL-1 can induce NO production in the ovary.156 In humans IL-1β can increase NO production by follicular cells after a 24 hour incubation period.157 Increasing NO production by IL-1β can inhibit apoptosis in rat ovarian follicles.158 In addition, it has been shown that IL-1beta inhibits estradiol production indirect via the stimulation of NO production.159 Furthermore, IL-1 inhibits gonadotropin stimulated secretion by granulosa cells. This may be due to a decrease in CYP19 activity.160 Altogether, it is suggested that IL-1 is involved in the intra-ovarian regulation of steroid biosynthesis.161
INTERLEUKIN-6
Granulosa cells synthesize IL-6162 and the IL-6 receptor is expressed in granulosa cells as well.163 It has been shown that IL-6 reduces the expression of CYP19163 as well as LH-R expression in granulosa cells.164 It is suggested that IL-6 mediates some of its effects in the ovary through the activation of the ERK1/2, JAK/STAT and p38MAPK pathways.165 As such IL-6 is a potent regulator of cumulus cell function and oocyte-cumulus cell expansion. In the corpus luteum, IL-6 inhibits hCG induced progesterone secretion from luteal cells and hence IL-6 is suggested to act as an autocrine and paracrine regulator in the corpus luteum at the time of luteolysis.166
TUMOR NECROSIS FACTOR-α
TNFα, a 157-amino acid polypeptide, was originally named for its oncolytic activity as displayed in the serum of bacillus Calmette-Guérin-immunized, endotoxin-challenged mice.167, 168 TNF proved capable of inducing tumor necrosis in vivo and of exerting non-species-specific cytolytic or cytostatic effects on a broad range of transformed cell lines in vitro. Although TNF was initially thought to be tumor selective, it has become clear that certain nontumor cells possess TNF receptors and that TNF may be a regulatory monokine with pleiotropic noncytotoxic activities in addition to its antitumor properties. TNF engages in the differentiation of a variety of cell types.
At the level of the ovary, TNF was found capable of attenuating the differentiation of cultured granulosa cells from immature rats.169 In other studies, TNF was found to effect complex dose-dependent alterations in the elaboration of progesterone and androstenedione, but not estrogen, by explanted preovulatory follicles of murine origin. Although the ovary contains TNF mRNA,170 its in vivo origins must be determined. In principle, two general possibilities are worthy of consideration. TNF may be locally derived from (activated) resident ovarian macrophages,171 as shown for regressing (but not young) corpora lutea. Although basal TNF activity was undetected in corpora lutea of pregnancy and pseudopregnancy, TNF activity was markedly stimulated in the presence of lipopolysaccharide.172 However, the detection of TNF activity in some luteal tissue on day 5, and the scarcity of macrophages at this stage raise the possibility that cells other than macrophages may also produce TNF in the corpus luteum. TNF may be of granulosa cell origin, as suggested by immunohistochemical studies wherein antral or atretic granulosa cells have been implicated as a possible site of TNF gene expression. Given such strong association between TNF elaboration and follicular and luteal decline, it is tempting to speculate that TNF may play a role in the still enigmatic processes of atresia or luteolysis. In this capacity, TNF of intraovarian origin may exert its effects at or adjacent to its site of synthesis, interacting with specific granulosa-luteal cell surface receptors to modulate gonadotropin hormonal action. TNF-induced luteolysis173 by apoptosis has been well documented.174 TNF induced apoptosis depends on the ceramide signaling pathway as its second messenger.175, 176
It has been shown that null mutations in TNF type I receptor (TNF-RI) impaired ovarian cycling in aged females and increased pre-pubertal ovarian responsiveness to gonadotropins, while null mutations in TNF-RII did not cause any effect regarding fertility in mice.143
TNFα is an inductor of apoptosis in granulosa cells and also of follicular atresia.177 TNFα mediates apoptosis through its receptors and the downstream mediators TRAIL, TRADD and TRAF2.
It has been shown that TNF stimulates progesterone synthesis in differentiated ovaries, while in undifferentiated ovarian cells TNF inhibits steroidogenesis.178
When pregnancy is established, the corpus luteum must produce progesterone to maintain the pregnancy. While TNFα and its receptors have been documented in the gravid uterus, placenta and embryo179 high affinity binding sites for TNFα were demonstrated in the corpus luteum.180 It is possible that locally produced TNFα plays an important role as an autocrine and/or paracrine mediator in the corpus luteum during pregnancy. Luteal TNFα may contribute to maintaining the pregnancy by stimulating the production of PGF2α and PGE2 by the corpus luteum of pregnancy,181 indirectly resulting in an increase in progesterone output.
Undoubtedly, future studies of the regulation of the TNF receptor and the elucidation of the in vivo source of its ligand will shed new light on the relevance of this system to the process of follicular development or demise.
COLONY STIMULATING FACTOR
Ovarian follicles produce colont stimulating factor (CSF).182 It is suggested that CSF-1 acts locally in the ovary on gonadotropin receptors.143 As such it has been demonstrated that mutations in CSF-1 lead to dysfunctional LH secretion, impaired ovarian luteinization, decreased ovulation and reduced occurrence of antral follicles.143
OTHER GROWTH AND PEPTIDERGIC FACTORS
Other growth and peptidergic factors have potential physiologic relevance to folliculogenesis (Table 1). The ovary contains a complete renin-angiotensin system that may be involved with vascularization and with modulation of steroidogenesis.183 Vasoactive intestinal peptide is also produced locally in the ovary, and it can enhance estrogen production by granulosa cells of prepubertal rats.184
Nerve growth factor is another peptidergic factor whose mRNA has been detected in the ovary,185 but its modulatory role in the ovary is unknown. Similarly, endothelin, a potent vasoconstrictor, influences ovarian progesterone production.186
Relaxin
Relaxins are peptide hormones and bind to G-protein coupled receptors.187 Relaxin is a major product of the corpus luteum during pregnancy188 and is mainly produced by luteal granulosa cells.189 Relaxin is also expressed in theca interna cells of antral follicles before the LH-surge.190
In the ovary, relaxin binds to the G-protein coupled receptor, RXFP1,187 activating G-protein-mediated adenylyl cyclase that causes an intracellular elevation of cAMP.191 RXFP1 is expressed in granulosa cells and cumulus cells of antral follicles.192 It is assumed that relaxin is involved in oocyte maturation and influences granulosa/cumulus cell function in a paracrine fashion.193
Insulin-like peptide 3
Insulin-like peptide 3 (INSL3) is structurally related to relaxin.194 In the ovary, INSL3 is mainly produced in theca interna cells of antral follicles195 and also in the corpus luteum.196 INSL3 knock-out mice have reduced numbers of antral follicles, fewer corpora lutea and small litter sizes, pointing to that INSL3 plays an important role in promoting the number of growing antral follicles and ovulation.197 In addition, INSL3 exerts an anti-apoptotic and pro-survival effect on growing antral follicles.198 Glister et al., 2013199 demonstrated that INSL3 is involved in a paracrine/autocrine feedback system that regulates androgen production in theca cells.
Antral follicles are the major source of circulating INSL3 in non-pregnant female mammals200 suggesting that INSL3 reflects the growth of antral follicles.201 Accordingly, the number of growing antral follicles influences the level of circulating INSL3 and hence, circulating INSL3 levels are reduced in women with low ovarian reserve, while circulating INSL3 levels are significantly elevated in women with PCOS (Figure 11 to follow).201
GONADOTROPIN MODULATION AND MEDIATION
If there are any lessons to be learned at this time, it is that optimal gonadotropin hormonal action is highly contingent on the input of tissue-based regulatory principles. According to this view, gonadotropins may not be the omnipotent agents they were once thought to be. Rather, gonadotropins may best be viewed as 'team players' and as initiators of a cascade of events facilitated, attenuated, or mediated through interaction with putative intraovarian regulators. It is the special case of IGF-I that best illustrates the role of a tissue-based modulator in that optimal gonadotropin hormonal action is clearly highly dependent on the availability of IGF-I and the consequent amplification of gonadotropin hormonal action.15 In contrast, putative intraovarian regulators exemplified by TGF-α may attenuate gonadotropin hormonal differentiation in the interest of continued proliferative ability.
Another role for putative intraovarian regulators, exemplified by IL-1, is that of mediation of gonadotropin action. According to this view, IL-1 constitutes an extension of the gonadotropin signal, possibly one of several more distal effectors, the overall mission of which may well be the conveyance of the message (or portions thereof) imparted by the midcycle surge.
The development of the ovarian follicle is a continuum of growth and differentiation of at least three distinct cell types: thecal cells, granulosa cells, and oocytes. Much depends on the localization and timing of expression of the regulatory principles. Of equal importance is the ability of the target cell to receive and respond to the regulatory signal. Activin elicits a stimulatory and an inhibitory response, depending on the cell type being studied104 and the developmental stage of the follicle.102 This duality of action may be explained if the activin receptor isoforms101 prove to have a specific cell-type and developmental-stage distribution. The action of a given regulatory factor, such as EGF/TGF, can also be influenced by the presence or absence of other factors.202 The ability of IL-1203 and nerve growth factor204 to alter EGF/TGF binding in nonovarian cell types is an example of how one growth factor can impinge on the actions of another.
It is the net balance representing the integration of multiple transduction pathways (Fig. 12) and often opposing signals that determines final gonadotropin hormonal action. Moreover, a given intraovarian growth factor may play several roles, depending on its local concentration, availability of its receptors or binding proteins, the cell population with which it interacts, and the precise timing of that interaction. There is every reason to believe that future studies may reveal other modes of interaction between trophic ovarian principles and tissue-based regulatory elements. It is with a strong sense of excitement that future work in this evolving area is anticipated.
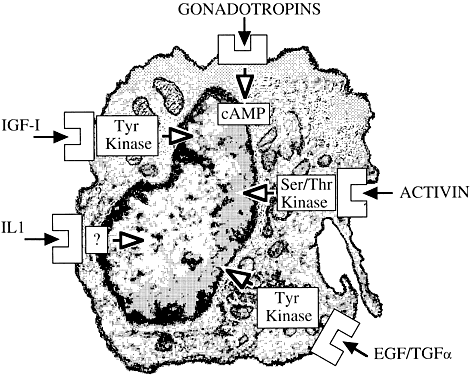 Fig. 12. Signal transduction pathways in the ovary
Fig. 12. Signal transduction pathways in the ovary
Pten AND mTORC
Survival, loss and activation of primordial follicles is mainly controlled by PI3K-signaling.205, 206 Oocyte specific deletion of Pten (phosphatase and tensin homolog deleted on chromosome ten), which is a negative regulator of PI3K or Pdk1207 results in a global activation of the entire primordial follicle pool208 leading to premature ovarian failure (POF) in Pten-deficient mice. Hence, a basal level of PI3K activation in oocytes is essential for the maintenance of the primordial follicle pool. In addition, AMH inhibits the PI3K induced activation of the primordial follicle pool and hence balances together with PI3K the ovarian reserve. Destruction of bigger, more mature follicles, for example through the administration of ovotoxic agents, diminishes AMH and thus, the loss of suppression leads through PI3K pathway activity to an accelerated activation of the primordial follicle pool, resulting in a “burn-out” effect of the ovarian reserve (Figure 13 to follow).209
Mammalian target of rapamycin complex 1 (mTORC1) in oocytes leads to premature activation of primordial follicles.210 Treatment of mice with oocyte specific deletion of Pten with rapamycin, an mTORC1- inhibitor, retains a significant amount of the ovarian reserve in mice with excessive activation of the primordial follicle pool. Hence, the suppression of mTORC1 signaling pathway is important in the maintenance of the dormant primordial follicle pool (Figure 14 to follow).210
HYPOXIA INDUCIBLE FACTOR-1 alpha
In the ovary it was shown that FSH leads to accumulation of antihypoxia inducible factor-1 alpha (anti-HIF-1α) protein in granulosa cells and rapamycin, an mTOR inhibitor and PI3 kinase inhibitor, inhibits the FSH-stimulated HIF-1 activity.211 In addition, PI3 kinase/AKT-mediated activation of mTOR and phosphorylation of FOXO1 are essential for the FSH-stimulated HIF-1 induced up-regulation of VEGF-A.212 In the corpus luteum the expression of HIF-1α is highest in luteal granulosa cells during luteal formation, but absent in the fully functional corpus luteum.213
VASCULAR ENDOTHELIAL GROWTH FACTOR-A
During folliculogenesis an increased number of follicular blood vessels are regulated by different factors, most notably by vascular endothelial growth factor-A (VEGF-A).214 VEGF-A is expressed in granulosa cells and theca cells of secondary follicles and is important for follicular maturation as the blocking of VEGF-A inhibits follicular growth.215, 216 After the LH-surge VEGF-A is up-regulated in luteal granulosa cells in the corpus luteum and remains expressed until the mid to late luteal phase.217 Inhibition of VEGF-A disrupts ovulation, blocks vascularization of the corpus luteum and suppresses progesterone secretion from the corpus luteum.218 On the other side of the spectrum, exaggerated levels of intraovarian VEGF play a cardinal pathophysiologic role due to increasing permeability of fluid through the ovarian blood vessels (therefore also called vascular permeability factor, VPF) the main modulator of the iatrogenic, and possibly serious, ovarian hyperstimulation syndrome (OHSS).
FIBROBLAST GROWTH FACTOR-2
Fibroblast growth factor-2 (FGF-2) is expressed especially during the follicular-luteal transition in the ovary, but is not expressed in granulosa cells and theca cells in the ovary until the antral stages.219 Inhibition of the FGF-receptor almost completely prevents the formation of the luteal endothelial networks.220 FGF-2 is expressed in oocytes of primordial and primary follicles221 and promotes the transition from the primordial to the primary follicular stages, pre-antral follicular growth and recruitment of theca cells.222 FGF-2 is expressed in theca interna cells and granulosa cells of antral follicles and modulates the action of VEGF-A.223 During the later stages of pre-ovulatory follicle development VEGF-A and FGF-2 expression increases.224 It has been shown that FGF-2 expression dramatically increases following the LH-surge and FGF-2 translocates from thecal endothelial cells to the nucleolus of granulosa cells.225 FGF-2 is critical for the formation of luteal endothelial networks.226 It is suggested that the increase in FGF-2 at the time during the follicular-luteal transition is important to stimulate the tissue remodeling after ovulation that accompanies angiogenesis.
ADIPOKINES
Adipokines are biologically important molecules that are secreted from adipose tissue. Adipokines modulate glucose and lipid metabolism as well as insulin sensitivity. Adipokines are molecules such as leptin, adiponectin, resistin, chemerin, and apelin.227
Leptin
Leptin is suggested to be an important signal in female reproduction, including control of ovarian function, beside its role in the regulation of body weight and energy expenditure.228 In the ovary, leptin receptor Ob-R is expressed in both granulosa cells and theca cells. It has been shown that leptin counteracts the synergistic effect of IGF-1 on FSH-stimulated estradiol and progesterone production in granulosa cells.229 In addition, leptin receptor Ob-R is also expressed in the oocyte.230 Leptin increases the rate of meiotic resumption in pre-ovulatory follicle enclosed oocytes, probably via indirect actions on theca cells.231
Adiponectin
Adiponectin activates peroxisome proliferation-activated receptors a (PPARa) and AMP-activated protein kinase. Adiponectin acts through two receptors, AdipoR1 and AdipoR2. Adiponectin and its receptors are expressed in the ovary.232, 233 Adiponectin decreases androgen and progesterone production indirect by insulin in theca cells, while in granulosa cells adiponectin increases progesterone and estradiol secretion in response to IGF-1.232
Resistin
Resistin activates 17-α-hydroxylase activity in theca cells in the presence of forskolin, suggesting a role of resistin in the regulation of androgen production in theca cells.234 Furthermore, in granulosa cells resistin modulates steroidogenesis and proliferation in response to IGF-1 and also in the basal state.235
Chemerin
Chemerin decreases IGF-1 induced progesterone and estradiol production through a decrease in the phosphorylation of IGF-1R b subunit and MAPK ERK1/2 signaling pathway.236
Apelin
Apelin is expressed in theca cells, while its receptor, AP J receptor, is expressed in both granulosa cells and theca cells.237 In granulosa cells, the increase in AP J receptor expression correlates with follicular atresia, while in theca cells apelin and AP J receptor are induced by LH.237
NEUROTROPHIC FACTORS
Neurotrophins and their receptors are expressed in the ovary238 and influence both ovarian somatic cells and oocytes.
Glial cell line derived neurotrophic factor
Glial cell line derived neurotrophic factor (GDNF), a distant member of the TGF-beta superfamily and known for promoting the survival and differentiation of peripheral and central neurons239 is expressed in the ovary.240 GDNF is expressed in ovarian granulosa and stromal cells as well as in oocytes.241 GDNF stimulates oocyte nuclear and cytoplasmic maturation and cumulus cell expansion242 and increases DAZL expression.243 However, it has also been suggested that GDNF may contribute to the onset of ovarian tumorigenesis in aging mutant mice carrying a disruption in the FSH receptor gene.241
Brain derived neurotrophic factor
Brain derived neurotrophic factor (BDNF) is expressed in cumulus cells and in oocytes.244 BDNF promotes oocyte maturation.245 Furthermore, it has been shown that BDNF acts as a paracrine factor enhancing the extrusion of the first polar body.246
Nerve growth factor
Nerve growth factor (NGF) is expressed in human granulosa cells and oocytes.247 Injection of NGF into murine oocytes enhances the ability of oocytes to form parthenogenetic pronuclei in both oocytes within COCs and denuded oocytes.248
REFERENCES
Franchimont P, Channing CP (eds): Intragonadal Regulation of Reproduction. London: Academic Press, 1981 |
|
Witschi E: Migration of the germ cells of human embryos from the yolk sac to the primitive gonadal folds. Contrib Embryol 32: 67, 1948 |
|
Channing CP: Influences of the in vivo and in vitro hormonal environment upon luteinization of granulosa cells in tissue culture. Recent Prog Horm Res 26: 589, 1970 |
|
Adashi EY: Insulin-like growth factors as determinants of follicular fate. J Soc Gynecol Invest 2: 721, 1995 |
|
Hernandez ER, Roberts CT Jr, LeRoith D, Adashi EY: Rat ovarian insulin-like growth factor-I (IGF-I) gene expression is granulosa cell-selective: 5'-untranslated mRNA variant representation and hormonal regulation. Endocrinology 125: 572, 1989 |
|
Oliver JE, Aitman TJ, Powell JF et al: Insulin-like growth factor-I gene expression in the rat ovary is confined to the granulosa cells of developing follicles. Endocrinology 124: 2671, 1989 |
|
Adashi EY, Resnick CE. Rosenfeld RG: Insulin-like growth factor-I (IGF-I) and IGF-II hormonal action in cultured rat granulosa cells: Mediation via type I but not type II IGF receptors. Endocrinology 126: 216, 1990 |
|
Davoren JB, Kasson BO, Li CH, Hsueh AJW: Specific insulin-like growth factor (IGF) I- and II-binding sites on rat granulosa cells. Endocrinology 119: 2155, 1986 |
|
Cara JF, Fan J, Azzarello J, Rosenfeld RG: Insulin-like growth factor-I enhances luteinizing hormone binding to rat ovarian theca-interstitial cells. J Clin Invest 86: 560, 1990 |
|
Hernandez ER, Resnick CE, Svoboda ME et al: Somatomedin-C/insulin-like growth factor-1 (SM-C/IGF-I) as an enhancer of androgen biosynthesis by cultured ovarian cells. Endocrinology 122: 1603, 1988 |
|
Adashi EY, Resnick CE, Payne DW et al: The mouse intraovarian insulin-like growth factor I system: Departure from the rat paradigm. Endocrinology 138: 3887, 1997 |
|
Wandji SA, Wood TL, Crawford J et al: Expression of mouse ovarian insulin growth factor system components during follicular development and atresia. Endocrinology 139: 5205, 1998 |
|
Blumenfeld Z. PCO--from basic science to clinical practice, Eur J Obstet Gynecol Reprod Biol. 1994; 55:29-30 |
|
Erickson GF, Nakatani A, Ling N et al: Cyclic changes in insulin-like growth factor-binding protein-4 messenger ribonucleic acid in the rat ovary. Endocrinology 130: 625, 1992. |
|
Adashi EY, Resnick CE, Ricciarelli E et al: Local tissue modification of follicle stimulating hormone. In Genazzani AR, Petraglia F (eds): Hormones in Gynecolological Endocrinology, p 255. London: Parthenon Publishing Group, 1992 |
|
Khamsi F, Roberge S: Differential effects of insulin-like growth factor-I and gonadotropins on the proliferative activity of two subgroups of granulosa cells: cumulus oophorus andmural granulosa cells. Fertil Steril. 2001 May;75(5):997-1003. |
|
Fortune JE, Rivera GM, Evans AC et al: Differentiation of dominant versus subordinate follicles in cattle. Biol Reprod. 2001 Sep;65(3):648-54. |
|
Ginther OJ, Beg MA, Bergfelt DR et al: Follicle selection in monovular species. Biol Reprod. 2001 Sep;65(3):638-47. |
|
Baker J, Hardy MP, Zhou J et al: Effects of an IGFI null mutation on mouse reproduction. Mol Endocrinol 10: 903, 1996 |
|
LaVoie HA, Garmey JC, Veldhuis JD: Mechanisms of insulin-like growth factor I augmentation of follicle stimulating hormone-induced porcine steroidogenic acute regulatory protein gene promoter activity in granulosa cells. Endocrinology 140: 146, 1999 |
|
Li D, Kubo T, Kim H et al: Endogenous insulin-like growth factor-I is obligatory for stimulation of rat inhibin alpha-subunit expression by follicle-stimulating hormone. Biol Reprod 58: 219, 1998 |
|
deMoura MD, Choi D, Adashi EY, Payne DW: Insulin-like growth factor-I-mediated amplification of follicle-stimulating hormone-supported progesterone accumulation by cultured rat granulosa cells: enhancement of steroidogenic enzyme activity and expression. Biol Reprod 56: 946, 1997. |
|
Stewart RE, Spicer LJ, Hamilton TD, Keefer BE. Effects of insulin-like growth factor I and insulin on proliferation and on basal and luteinizing hormone-induced steroidogenesis of bovine thecal cells: involvement of glucose and receptors for insulin-like growth factor I and luteinizing hormone. J Anim Sci1995. 73:3719-31 |
|
El-Roeiy A, Chen X, Roberts VJ, LeRoith D, Roberts CT, Yen SS. Expression of insulin-like growth factor-I (IGF-I) and IGF-II and the IGF-I, IGF-II, and insulin receptor genes and localization of the gene products in the human ovary. J Clin Endocrinol Meta. 1993;77:1411-8. |
|
Amselgruber W, Sinowatz F, Schams D, Skottner A. Immuno-histochemical aspects of insulin-like growth factors I and II in the bovine corpus luteum. J Reprod Fertil;1994;101:445-51. |
|
Guidice LC. Insulin-like growth factors and ovarian follicular development. Endocr Rev.1992;13:641-69. |
|
Magoffin DA, Weitsman SR. Insulin-like growth factor-I regulation of luteinizing hormone (LH) receptor messenger ribonucleic acid expression and LH-stimulated signal transduction in rat ovarian theca-interstitial cell. Biol Reprod.1994;51:766-75. |
|
Zhao J, Tayerne MA, van de Weijden GC, Bevers MM, van den Hurk R. Immunohistochemical localization of growth hormone (GH), H receptor (GHR), insulin-like growth factor I (IGF-I) and type I IGF-I receptor, and gene expression of GH and GHR in rat pre-antral follicles. Zygote. 2002.10:85-94. |
|
Carlsson B, Nilsson A, Isaksson OGP, Billig H. Growth hormone-receptor messenger RNA in the rat ovary: regulation and localization. Mol Cell Endocrinol. 1993;95:59-66. |
|
Abir R, Garor R, Felz C, Nitke S, Krissi H, Frisch B. Growth hormone and its receptor in human ovaries from fetuses and adults. Fert Ster. 2008.90:1333-9. |
|
Lucy MC. Regulation of ovarian follicular growth by somatotropin and insulin-like growth factors in cattle. J Dairy Sci. 2000;83:1635-47. |
|
Liu XJ, Andoh K, Yokota H, Kobayashi J, Abe Y, Yamada K, et al. Effects of growth hormone, activin, and follistatin on the development of preantral follicle from immature female mice. Endocrinology. 1998;139:2342-7. |
|
Slot KA, Kastelijn J, Bachelot A, Kelly PA, Binart N, Teerds KJ. Reduced recruitment and survival of primordial and growing follicles in GH receptor-deficient mice. Reproduction. 2006;131:525-32. |
|
Eckery DC, Moeller CL, Nett TM, Sawyer HR. Recombinant bovine somatotropin (rbST, Sometribove) maintains the sensitivity of ovarian follicles to gonadotropins in hypophysectomized ewes. Biol Reprod. 1993;48(Suppl.) |
|
Eckery DC, Moeller CL, Nett TM, Sawyer HR. Localization and quantification of binding sites for follicle-stimulating hormone, luteinizing hormone, growth hormone, and insulin-like growth factor I in sheep ovarian follicles. Biol Reprod. 1997; 57:507-13. |
|
Kolodziejczyk J, Gertler A, Leibovich H, Rzasa J, Gregoraszczuk EL. Synergistic action of growth hormone and insulin-like growth factor I (IGF-I) on proliferation and estradiol secretion in porcine granulosa and theca cells cultured alone or in co-culture. Theriogenology. 2003;60:559-70. |
|
Karamouti M, Kollia P, Kallitsaris A, Vamvakopoulos N, Kollios G, Messinis IE. Growth hormone, insulin-like growth factor I, and leptin interaction in human cultured lutein granulosa cells steroidogenesis. Fert Ster. 2008;90:1444-50. |
|
Izadyar F, Hage WG, Colenbrander B, Bevers MM. The promotory effect of growth hormone on the developmental competence of in vitro matured bovine oocytes is due to improved cytoplasmic maturation. Mol Reprod Dev. 1998;49:444-53. |
|
Izadyar F, Van Tol HTA, Colenbrander B, Bevers MM. Stimulatory effect of growth hormone on in vitro maturation of bovine oocytes is exerted through cumulus cells and not mediated by IGF-I. Mol Reprod Dev. 1997;47:175-80. |
|
Vlodavsky I, Brown KD, Gospodarowicz D: A comparison of the binding of epidermal growth factor to cultured granulosa and luteal cells. J Biol Chem 253: 3744, 1978 |
|
Jones PBC, Welsh TH Jr, Hsueh AJW: Regulation of ovarian progestin production by epidermal growth factor in cultured rat granulosa cells. J Biol Chem 257: 11268, 1982 |
|
Knecht M, Catt KJ: Modulation of cAMP-mediated differentiation in ovarian granulosa cells by growth factor and platelet-derived growth factor. J Biol Chem 258: 2789, 1983 |
|
St. Arnaud R, Walker P, Kelly PA, Labrie F: Rat ovarian epidermal growth factor receptors: Characterization and hormonal regulation. Mol Cell Endocrinol 31: 43, 1983 |
|
Rachael P Norris, Marina Freudzon, Viacheslay O Nikolaev, Laurinda A Jaffe. Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction. 2010;140:655-662. |
|
Panigone S et al. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway Mol Endocrinol 2008;22(4):924–36. |
|
Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004 Jan 30;303(5658):682-4. |
|
Zamah AM, Hsieh M, Chen J, Vigne JL, Rosen MP, Cedars MI, Conti M. Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin. Hum Reprod. 2010 Oct;25(10):2569-78. doi: 10.1093/humrep/deq212. |
|
Ning G, Ouyang H, Wang S, Chen X, Xu B, Yang J, Zhang H, Zhang M, Xia G. 3',5'-cyclic adenosine monophosphate response element binding protein up-regulated cytochrome P450 lanosterol 14alpha-demethylase expression involved in follicle-stimulating hormone-induced mouse oocyte maturation. Mol Endocrinol. 2008 Jul;22(7):1682-94. doi: 10.1210/me.2007-0480. |
|
Andric N, Ascoli M. The luteinizing hormone receptor-activated extracellularly regulated kinase-1/2 cascade stimulates epiregulin release from granulosa cells. Endocrinology. 2008 Nov;149(11):5549-56. doi: 10.1210/en.2008-0618. |
|
Kudlow JE, Kobrin MS, Purchio AF et al: Ovarian transforming growth factor gene expression: immunohistochemical localization to the theca-interstitial cells. Endocrinology 121: 1577, 1987 |
|
Erickson GF, Case E: Epidermal growth factor antagonizes ovarian theca-interstitial cyto-differentiation. Mol Cell Endocrinol 31: 71, 1983 |
|
Reeka N, Berg FD, and Brucker C: Presence of transforming growth factor alpha and epidermal factor in human ovarian tissue and follicular fluid. Hum Reprod 13: 2199, 1998 |
|
Bennett RA, Osathanondh R, Yeh J: Immunohistochemical localization of transforming growth factor-alpha, epidermal growth factor (EGF), and EGF receptor in the human fetal ovary. J Clin Endocrinol Metab 81: 3073, 1996 |
|
Billig H, Cun SY, Eisenhauer K, Hsueh AJW: Gonadal cell apoptosis: Hormone-regulated cell demise. Hum Reprod Update 2: 103, 1996 |
|
Foghi A, Teerds KJ, van der Donk H, Dorrington J: Induction of apoptosis in rat thecal/interstitial cells by transforming growth factor alpha plus transforming growth factor beta in vitro. J Endocrinol 153: 169, 1997 |
|
Massague J, Wotton D: Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000 Apr 17;19(8):1745-54. |
|
Shimasaki S, Zachow RJ, Li D et al: A functional bone morphogenetic protein system in the ovary. Proc Natl Acad Sci U S A. 1999 Jun 22;96(13):7282-7. |
|
Drummond AE, Dyson M, Le MT et al: Ovarian follicle populations of the rat express TGF-beta signalling pathways. Mol Cell Endocrinol. 2003 Apr 28;202(1-2):53-7. |
|
Erickson GF, Shimasaki S: The spatiotemporal expression pattern of the bone morphogenetic protein family inrat ovary cell types during the estrous cycle. Reprod Biol Endocrinol. 2003 Feb 5;1:9 |
|
Bristol SK, Woodruff TK: Follicle-restricted compartmentalization of transforming growth factor betasuperfamily ligands in the feline ovary. Biol Reprod. 2004 Mar;70(3):846-59. Epub 2003 Dec 3. |
|
McNatty KP, Smith P, Moore LG et al: Oocyte-expressed genes affecting ovulation rate. Mol Cell Endocrinol. 2005 Apr 29;234(1-2):57-66. |
|
Nilsson EE, Skinner MK: Bone morphogenetic protein-4 acts as an ovarian follicle survival factor andpromotes primordial follicle development. Biol Reprod. 2003 Oct;69(4):1265-72. Epub 2003 Jun 11 |
|
Durlinger AL, Visser JA, Themmen AP: Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002 Nov;124(5):601-9. |
|
Glister C, Groome NP, Knight PG: Bovine follicle development is associated with divergent changes in activin-A,isoforms in follicular fluid. J Endocrinol. 2006 Feb;188(2):215-25. |
|
Glister C, Richards SL, Knight PG: Bone morphogenetic proteins (BMP) -4, -6, and -7 potently suppress basal andprimary culture: could ovarian hyperandrogenic dysfunction be caused by a defectin thecal BMP signaling? Endocrinology. 2005 Apr;146(4):1883-92. Epub 2004 Dec 29. |
|
Hreinsson JG, Scott JE, Rasmussen C, Swahn ML, Hsueh AJW, Hovatta O. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. Journal of Clinical Endocrinology and Metabolism 2002;87(1):316–321. |
|
Elvin JA, Yan C, Matzuk MM. Growth differentiation factor-9 stimulates progesterone synthesis in granulosa cells via a prostaglandin E2/EP2 receptor pathway. Proc Natl Acad Sci U S A 2000;97(18):10288–10293. |
|
Orisaka M, Orisaka S, Jiang JY, Craig J, Wang Y, Kotsuji F, Tsang BK. Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage. Mol Endocrinol 2006;20(10):2456–2468. |
|
Sugiura K, Pendola FL, Eppig JJ. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol 2005;279(1):20–30. |
|
Huang Q, Cheung AP, Zhang Y, Huang HF, Auersperg N, Leung PC. Effects of growth differentiation factor 9 on cell cycle regulators and ERK42/44 in human granulosa cell proliferation. Am J Physiol Endocrinol Metab 2009;296(6):E1344–1353. |
|
Simpson CM, Robertson DM, Al-Musawi SL, Heath DA, McNatty KP, Ritter LJ, Mottershead DG, Gilchrist RB, Harrison CA, Stanton PG. Aberrant GDF9 Expression and Activation Are Associated With Common Human Ovarian Disorders. J Clin Endocrinol Metab. 2014 Apr;99(4):E615-24. doi: 10.1210/jc.2013-3949. |
|
Visser JA.2003. AMH signaling: from receptor to target gene. Mol Cell Endocrinol. 211:65-73. |
|
Durlinger AL, Visser JA, Themmen AP.2002. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction;124:601-609. |
|
Grondahl ML, Nielsen ME, Dal Canto MB, Fadini R, Rasmussen IA, Westergaard LG, Kristensen SG,Yding Andersen C.2011. Anti-Mullerian hormone remains highly expressed in human cumulus cells during the final stages of folliculogenesis. Reprod Biomed;22:389-398. |
|
Baarends WM, Uilenbroek JT, Kramer P, Hoogenbrugge JW, vanLeeuwen EC, Hemmen AP, Grootegoed JA.1995. Anti-Mullerian hormone and anti-mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin induced follicle growth. Endocrinology;136:4951-4962. |
|
Ingraham HA, Hirokawa Y, Roberts LM, Mellon, SH, McGee E, Nachtigal MW, Visser JA.2000. Autocrine and paracrine Mullerian inhibiting substance homone signaling in reproduction. Recent Prog Horm Res;55:53-67. |
|
Knight PG, Glister C. TGF beta superfamily members and ovarian follicle development. Reproduction 2006;132:191-206. |
|
Pellatt L, Rice S, Dilaver N, Heshri A, Galea R, Brincat M, Brown K, Simpson ER, Mason HD.2011. Anti-Mullerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil Steril;96:1246-1251,e1241. |
|
Grossman MP, Nakajima ST, Fallat ME, Siow Y.2008. Mullerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril;89:1364-1370. |
|
Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, deJong FH, Uilenbroek JT, Grootegoed JA, Themmen AP.2001. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology;142:4891-4899. |
|
Visser JA, Durlinger AL, Peters IJ, vandenHeuvel ER, Rose UM, Kramer P, deJong FH, Themmen AP.2007. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Mullerian hormone null mice. Endocrinology;148:2301-2308. |
|
Gospodarowicz D: Fibroblast growth factor: Involvement in early embryonic development and ovarian function. Semin Reprod Endocrinol 7: 21, 1989 |
|
Gospodarowicz D III, Birdwell CR: Effects of fibroblast and epidermal growth factors on ovarian cell proliferation in vitro: I. Characterization of the response of granulosa cells to FGF and EGF. Endocrinology 100: 1108, 1977 |
|
Gospodarowicz D, Bialecki H: The effects of the epidermal and fibroblast growth factors on the replicative life-span of bovine granulosa cells in culture. Endocrinology 103: 854, 1978 |
|
Gospodarowicz D, Bialecki H: Fibroblast and epidermal growth factors are mitogenic agents for cultured granulosa cells of rodent, porcine, and human origin. Endocrinology 104: 757, 1979 |
|
Koos RD, Olson CE: Expression of basic fibroblast growth factor in the rat ovary: Detection of mRNA using reverse transcription-polymerase chain reaction amplification. Mol Endocrinol 3: 3041, 1989 |
|
Shikone T, Yamoto M, Nakano R: Follicle-stimulating hormone induces functional receptors for basic fibroblast factor in rat granulosa cells. Endocrinology 131: 1063, 1992 |
|
Shimasaki S, Emoto N, Koba A et al: Complementary DNA cloning and sequencing of rat ovarian basic fibroblast growth factor and tissue distribution study of its mRNA. Biochem Biophys Res Commun 157: 256, 1988 |
|
Adashi EY, Resnick CE, Croft CS et al: Basic fibroblast growth factor as a regulator of ovarian granulosa cell differentiation: A novel non-mitogenic role |
|
Yeh J, Osathanondh R: Expression of messenger ribonucleic acids encoding for basic fibroblast growth factor (FGF) and alternatively spliced FGF receptor in human fetal ovary and uterus. J Clin Endocrinol Metab 77: 1367, 1993 |
|
Tilly JL, Billig H, Kowalski KI, Hsueh AJ: Epidermal growth factor and basic fibroblast growth factor suppress the spontaneous onset of apoptosis in cultured rat ovarian granulosa cells and follicles by a tyrosine kinase-dependent mechanism. Mol Endocrinol 6: 1942, 1992 |
|
Peluso JJ, Pappalardo A: Progesterone maintains large rat granulosa cell viability indirectly by stimulating small granulosa cells to synthesize basic fibroblast growth factor. Biol Reprod 60: 290, 1999 |
|
Trolice MP, Pappalardo A, Peluso JJ: Basic fibroblast growth factor and N-cahedrin maintain rat granulosa cell and ovarian surface epithelial cell viability by stimulating the tyrosine phosphorylation of the fibroblast growth factor receptors. Endocrinology 138: 107, 1997 |
|
Di Blasio AM, Vigano P, Cremonesi L et al: Expression of the genes encoding basic fibroblast growth factor and its receptor in human granulosa cells. Mol Cell Endocrinol 96: R7, 1993 |
|
Yamamoto S, Konishi I, Nanbu K et al: Immunohistochemical localization of basic fibroblast growth factor (bFGF) during folliculogenesis in the human ovary. Gynecol Endocrinol 11: 223, 1997 |
|
van Wezel IL, Umapathysivam K, Tilley WD, Rodgers RJ: Immunohistochemical localization of basic fibroblast growth factor in bovine ovarian follicles. Mol Cell Endocrinol 115: 133, 1995 |
|
DePaolo LV. Bicsak TA, Erickson GF et al: Follistatin and activin—a potential intrinsic regulatory system within diverse tissues. Proc Soc Exp Biol Med 198: 500, 1991 |
|
Murata M, Eto Y, Shibai R et al: Erythroid differentiation factor is encoded by the same mRNA as that of the inhibin beta A chain. Proc Natl Acad Sci USA 85: 2434, 1988 |
|
Smith JC, Price BM, Van Nimmen K, Huylebroeck D: Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature 345: 729, 1990 |
|
Mathews LS, Vale WW: Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell 65: 973, 1991 |
|
Attisano L, Wrana JL, Cheifetz S, Massague J: Novel activin receptors: Distinct genes and alternative messenger RNA splicing generate a repertoire of serine threonine kinase receptors. Cell 68: 97, 1992 |
|
Miro F, Smyth CD, Hillier SG: Development-related effects of recombinant activin on steroid synthesis in rat granulosa cells. Endocrinology 129: 3388, 1991 |
|
Findlay JK, Sai X, Shukovski L: Role of inhibin-related peptides as intragonadal regulators. Reprod Fertil Dev 2: 205, 1990 |
|
Hsueh AJW, Dahl KD, Vaughan J et al: Heterodimers and homodimers of inhibin subunits have different paracrine action in the modulation of luteinizing hormone-stimulated androgen biosynthesis. Proc Natl Acad Sci USA 84: 5082, 1987 |
|
Hillier SO: Regulatory functions for inhibin and activin in human ovaries. J Endocrinol 131: 171, 1991 |
|
Hillier SG, 2009. Paracrine support of ovarian stimulation. Mol Hum Reprod; 15:843-850. |
|
Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009 Jan;27(1):14-23. doi: 10.1055/s-0028-1108006. |
|
Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol. 2006 Oct 1;298(1):132-48. |
|
Lei L, Jin S, Mayo KE, Woodruff TK, 2010. The interaction between the stimulatory effect of follicle-stimulating hormone and the inhibitory effect of estrogen on mouse primordial folliculogenesis. Biol Reprod;82:13-22. |
|
Mizunuma H, Liu X, Andoh K, et al. Activin from secondary follicles causes small preantral follicles to remain dormant at the resting stage. Endocrinology 1999;140(1):37–42. |
|
Xia Y, Scheyer AL. The biology of activin: recent advances in structure, regulation and function. J Endocrinol 2009;202:1-12. |
|
Thomas FH, Armstrong DG, Telfer EE. Activin promotes oocyte development in ovine preantral follicles in vitro. Reprod Biol Endocrinol 2003;1:76. |
|
Mc Laughlin M, Telfer EE.2010. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum free culture system. Reproduction;139:971-978. |
|
Kipp JL, Kilen SM, Woodruff TK, Mayo KE. 2007. Activin regulates estrogen receptor gene expression in the mouse ovary. J Biol Chem;282:36755-36765. |
|
Xiao S, Robertson DM, Findlay JK.1992. Effects of activin and follicle-stimulating hormone (FSH)-suppressing protein/follistatin on FSH receptors and differentiation of cultured rat granulose cells. Endocrinology;131:1009-1016. |
|
Myers M, vandenDriesche S, McNeilly AS, Duncan WC.2008. Activin A reduces luteinization of human luteinized granulosa cells and has opposing effects of human chorionic gonadotropin in vitro. J Endocrinol;199:201-212. |
|
Eramaa M, Hilden K, Tuuri T, Rityos O.1995. Regulation of inhibin/activin subunit messenger ribonucleic acids (mRNA) by activin A and expression of activin receptor mRNAs in cultured human granulosa-luteal cells. Endocrinology;136:4382-4389. |
|
Myers M, Gay E, Mc Neilly AS, Fraser HM, Duncan WC.2007. In vitro evidence suggests activin A may promote tissue remodeling associated with human luteolysis. Endocrinology;148:3730-3739. |
|
Kayani AR, Glister C, Kight PG.2009. Evidence for an inhibitory role of bone morphogenetic proteins in the follicular-luteal transition in cattle. Reproduction;137:67-78. |
|
Erickson GF, Shimasaki S.2003. The spatio temporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle. Reprod Biol Endocrinol;1:9. |
|
Nakamura T, Takio K, Eto Y et al: Activin-binding protein from rat ovary is follistatin. Science 247: 836, 1990 |
|
Nakatani A, Shimasaki S, Depaolo LV et al: Cyclic changes in follistatin messenger ribonucleic acid and its protein in the rat ovary during the estrous cycle. Endocrinology 129: 603, 1991 |
|
Miro F, Hillier SG: Modulation of granulosa cell deoxyribonucleic acid synthesis and differentiation by activin. Endocrinology 137: 464, 1996 |
|
Woodruff TK, D'Agostino J, Schwartz NB, Mayo KE. Dynamic changes in inhibin messenger RNAs in rat ovarian follicles during the reproductive cycle. Science 1988;239(4845):1296–1299 |
|
Bernard DJ, Chapman SC, Woodruff TK. Mechanisms of inhibin signal transduction. Recent Prog Horm Res 2001;56:417-50. |
|
Findlay JK, Drummond AE, Dyson M, Baillie AJ, Robertson DM, Ethier JF. Production and actions of inhibin and activin during folliculogenesis in the rat. Mol Cell Endocrinol 2001;180:139-44. |
|
Lewis KA, Gray PC, Blount AT, et al. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature 2000;404(6776):411–414 |
|
Nahum R, Thong KJ, Hillier SG. Metabolic regulation of androgen production by human thecal cells in vitro. Hum Reprod 1995;10(1):75–81. |
|
Vitale AM, Gonzalez OM, Parborell F, Irusta G, Campo S, Tesone M. Inhibin A increases apoptosis in early ovarian antral follicles of diethylstilbestrol-treated rats. Biol Reprod 2002;67:1989-95. |
|
Muttukrishna S, Fowler PA, Groome NP, Mitchell GG, Robertson WR, Knight PG.1994. Serum concentrations of dimeric inhibin during the spontaneous human menstrual cycle and after the treatment with exogenous gonadotrophin. Hum Reprod; 9:1634-1642. |
|
Webley GE, Marsden PL, Knight PG.1994. Differential control of immunoreactive aplpha inhibin and progesterone production by marmoset luteal cells in vitro: evidence for paracrine action of alpha-inhibin on basal gonadotropin- and progesterone production. Biol Reprod;50:1394-1402. |
|
Duff G: Immune diseases: Many roles for interleukin-1. Nature 313: 352, 1985 |
|
Fukuoka M, Mori T, Taii S, Yasuda K: Interleukin-1 inhibits luteinization of porcine granulosa cells in culture. Endocrinology 122: 367, 1987 |
|
Gottschall PE, Katsuura G, Hoffmann ST, Arimura A: Interleukin 1: An inhibitor of luteinizing hormone receptor formation in cultured rat granulosa cells. FASEB J 2: 2492, 1988 |
|
Kasson BG, Gorospe WC: Effects of interleukins 1, 2 and 3 on follicle-stimulating hormone-induced differentiation of rat granulosa cells. Mol Cell Endocrinol 62: 103, 1989 |
|
Hurwitz A. Ricciarelli E, Botero L et al: Endocrine- and autocrine-mediated regulation of rat ovarian (theca-interstitial) interleukin-1 gene expression: Gonadotropin-dependent preovulatory acquisition. Endocrinology 129: 3427, 1991 |
|
Simon C, Frances A, Pinquette C, Polan ML: Immunohistochemical localization of the interleukin-1 system in the mouse ovary during follicular growth, ovulation, and luteinization, Biol Reprod 50:449, 1994 |
|
Cannon JG, Dinarello CA: Increased plasma interleukin-1 activity in women after ovulation. Science 227: 1247, 1985 |
|
Pacifici R, Rifas L, McCracken R et al: Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci USA 86: 2398, 1989 |
|
Yee JB, Hutson JC: Testicular macrophages: Isolation, characterization and hormone responsiveness. Biol Reprod 29: 1319, 1983 |
|
Khan SA, Schmidt K, Hallin P et al: Human testis cytosol and ovarian follicular fluid contain high amounts of interleukin-l-like factor(s). Mol Cell Endocrinol 58: 221, 1988 |
|
Hurwitz A, Loukides I, Ricciarelli F et al: Human intraovarian interleukin-1 (IL-1) system highly compartmentalized and hormonally dependent regulation of the genes encoding IL-1, its receptor, and its receptor antagonist. J Clin Invest 89: 1746, 1992 |
|
Ingman WV, Jones RL. Cytokine knockouts in reproduction: the use of gene ablation to dissect roles of cytokines in reproductive biology. Hum Reprod Update, 2008; 14:179-92 |
|
Sims JE, Gayle MA, Slack JL et al: Interleukin-1 signaling occurs exclusively via the type I receptor. Proc Natl Acad Sci USA 90: 6155, 1993 |
|
Colotta F, Re F, Muzio M et al: Interleukin-1 type II receptor: A decoy target for IL-1 that is regulated by IL-4. Science 261: 472, 1993 |
|
Ben-Shlomo I, Kol S, Ando M et al: Ovarian expression, cellular localization and hormonal regulation of the rat secretory phospholipase A2: Increased expression by interleukin-1 and gonadotropins. Biol Reprod 57: 217, 1997 |
|
Kol S, Ruutiainen-Altman K, Ben-Shlomo I et al: The rat ovarian phospholipase A2 system: gene expression, cellular localization, activity characterization and interleukin-1 dependence. Endocrinology 138: 322, 1997 |
|
Kol S, Ben-Shlomo I, Ando M et al: Interleukin-1 beta stimulates ovarian phospholipase A2 (PLA2) expression and activity: Up-regulation of both secretory and cytosolic PLA2. Endocrinology 138: 314, 1997 |
|
Ando M, Kol S, Kokia E et al: Rat ovarian prostaglandin endoperoxide synthase-1 and -2: Periovulatory expression of granulosa cell-based interleukin-1-dependent enzymes. Endocrinology 139: 2501, 1998 |
|
Kol S, Ben-Shlomo I, Ruutiainen K et al: The midcycle increase in ovarian glucose uptake is associated with enhanced expression of glucose transporter 3: Possible role for interleukin-1, a putative intermediary in the ovulatory process. J Clin Invest 99: 2274, 1997 |
|
Ben-Shlomo I, Kol S, Roeder LM et al: Interleukin (IL)-1 beta increases glucose uptake and induces glycolysis in aerobically cultured rat ovarian cells: Evidence that IL-1 beta may mediate the gonadotropin-induced midcycle metabolic shift. Endocrinology 138: 2680, 1997 |
|
Scherzer WJ, Ruutiainen-Altman KS, Putowski LT et al: Detection and in vivo hormonal regulation of rat ovarian type I and type II interleukin-1 receptor mRNAs: Increased expression during the periovulatory period. J Soc Gynecol Invest 3: 131, 1996 |
|
Simon C, Tsafriri A, Chun SY et al: Interleukin-1 receptor antagonist suppresses human chorionic gonadotropininduced ovulation in the rat. Biol Reprod 51: 662, 1994 |
|
Takehara Y, Dharmarajan AM, Kaufman G, Wallach EE: Effect of interleukin-1 beta on ovulation in the in vitro perfused rabbit ovary. Endocrinology 134: 1788, 1994 |
|
Peterson CM, Hales HA, Hatasaka HH et al: Interleukin-1 beta (IL-1beta) modulates prostaglandin production and the natural IL-1 receptor antagonist inhibits ovulation in the optimally stimulated rat ovarian perfusion model. Endocrinology 133: 2301, 1993 |
|
Ahsan S, Lacey M, Whitehead SA: Interactions between interleukin-1 beta, nitric oxide and prostaglandin E2 in therat ovary: effects on steroidogenesis. Eur J Endocrinol. 1997 Sep;137(3):293-300. |
|
Tao M, Kodama H, Kagabu S et al: Possible contribution of follicular interleukin-1beta to nitric oxide generation Hum Reprod. 1997 Oct;12(10):2220-5. |
|
Chun SY, Eisenhauer KM, Kubo M et al: Interleukin-1 beta suppresses apoptosis in rat ovarian follicles by increasingnitric oxide production. Endocrinology. 1995 Jul;136(7):3120-7. |
|
Tobai H, Nishiya I: Nitric oxide mediates inhibitory efect of interleukin-1 beta on estrogen production in human granulosa- luteal cells. J Obstet Gynaecol Res 27, 53-59, 2001. |
|
Ghersevich S, Isomaa V, Vikho P: Cytokine regulation of the expression of estrogenic biosynthetic enzymes in cultured rat granulosa cells. Mol Cell Endocrinol 172, 21-30, 2001. |
|
Smolikova K, Mlynarcikova A, Scsukova S. Role of interleukins in the regulation of ovarian functions. Endocr Regul. 2012 Oct;46(4):237-53. |
|
Machelon V, Emilie D, Lefevre A, Nome F, Durand-Gasselin I, Testart J: Interleukin-6 biosynthesis in human pre-ovulatory follicles: some of its potential roles at ovulation. J Clin Endocrinol Metabol 79, 633-642, 1994. |
|
Tamura K, Kawaguchi T, Hara T, Sakamoto T, Tohei A, Miyajima A, Seishi T, Kogo H: Interleukin-6 decreases estrogen production and messenger ribonucleic acid expression encoding aromatase during in vitro cytodiferentiation of rat granulosa cell. Mol Cell Endocrinol 170, 103-111, 2000. |
|
Tamura, K, Kawaguchi, T, Kogo, H: Interleukin-6 inhibits the expression of of luteinizing hormone during the maturation of cultured rat granulosa cells. J Endocrinol 170, 121-127, 2001. |
|
Liu Z, de Matos DG, Fan Hy, Shimada M, Palmer S, Richards JS: Interleukin-6: An autocrine regulator of the mouse cumulus cell-oocyte complex expansion process. Endocrinol 150, 3360-3368, 2009. |
|
Sakumoto R, Komatsu T, Kasuya E, Saito T, Okuda K: Expression of mRNAs for interleukin-4, interleukin-6 and their receptors in porcine corpus luteum during estrous cycle. Dom Anim Endocrinol 31, 246-257, 2006. |
|
Unanue ER, Allen PM: The basis for the immunoregulatory role of macrophages and other accessory cells. Science 236: 551, 1987 |
|
Harrison LC, Campbell IL: Cytokines: An expanding network of immuno-inflammatory hormones. Mol Endocrinol 2: 1151, 1988 |
|
Roby KS, Terranova PF: Tumor necrosis factor alpha alters follicular steroidogenesis in vitro. Endocrinology 123: 2952, 1988 |
|
Sancho-Tello M, Perez-Roger I, Imakawa K et al: Expression of tumor necrosis factor-α in the rat ovary. Endocrinology 130: 1359, 1992 |
|
Zhao Y, Burbach JA, Roby KF et al: Macrophages are the major source of tumor necrosis factor alpha in the porcine corpus luteum. Biol Reprod 59: 1385, 1998 |
|
Bagavandoss P, Kunkel SL. Wiggins RC, Keyes PL: Tumor necrosis factor-α (TNF-α) production and localization of macrophages and T lymphocytes in the rabbit corpus luteum. Endocrinology 122: 1185, 1988 |
|
Wuttke W, Spiess S, Knoke I, et al: Synergistic effects of prostaglandin F2alpha and tumor necrosis factor to induce luteolysis in the pig. Biol Reprod 58: 1310, 1998 |
|
Quirk SM, Porter DA, Huber SC, Cowan RG: Potentiation of Fas-mediated apoptosis of murine granulosa cells by interferon-gamma, tumor necrosis factor-alpha, and cycloheximide. Endocrinology 139: 4860, 1998 |
|
Kaipia A, Chun SY, Eisenhauer K, Hsueh AJ: Tumor necrosis factor-alpha and its second messenger, ceramide, stimulate apoptosis in cultured ovarian follicles. Endocrinology 137: 4864, 1996 |
|
Santana P, Llanes L, Hernandez I et al: Ceramide mediates tumor necrosis factor effects on P450-aromatase activity in cultured granulosa cells. Endocrinology 136: 2345, 1995 |
|
Manabe N, Matsuda-Minehata F, Goto Y, Maeda A, Cheng Y, Nakagawa S, et al. Role Of cell death ligand and receptor system on regulation of follicular atresia in pig ovaries. Reprod Domest Anim 2008; 43 (Suppl. 2):268-72 |
|
Bornstein SR, Rutkowski H, Vrezas I. Cytokines and steroidogenesis. Mol Cell Endocrinol 2004; 215:135-41. |
|
Terranova PF, Hunter VJ, Roby KF et al: Tumor necrosis factor-alpha in the female reproductive tract. Proc Soc Exp Biol Med. 1995 Sep;209(4):325-42 |
|
Sakumoto R, Murakami S, Kishi H et al: Tumor necrosis factor-alpha and its receptor in the corpus luteum of pregnantcows. Mol Reprod Dev. 2000 Apr;55(4):406-11. |
|
Sakumoto R, Berisha B, Kawate N et al: Tumor necrosis factor-alpha and its receptor in bovine corpus luteum throughoutthe estrous cycle. Biol Reprod. 2000 Jan;62(1):192-9. |
|
Salamassi A, Mettler L, Jonat W, Bucks S, Koch K, Schmutzler AG. Circulating level of macrophage colony-stimulating factor can be predictive for human in vitro fertilization outcome. Fertil Steril.2010:116-23. |
|
Lightman A, Palumbo A, DeCherney AH, Naftolin F: The ovarian renin-angiotensin system. Semin Reprod Endocrinol 7: 79, 1989 |
|
Ojeda SR, Lara H, Ahmed CE: Potential relevance of vasoactive intestinal peptide to ovarian physiology. Semin Reprod Endocrinol 7: 52, 1989 |
|
Lara HE, Hill OF, Katz KH, Ojeda SR: The gene encoding nerve growth factor is expressed in the immature rat ovary: Effect of denervation and hormonal treatment. Endocrinology 126: 357, 1990 |
|
Iwai M, Hasegawa M, Taii S et al: Endothelins inhibit luteinization of cultured porcine granulosa cells. Endocrinology 129: 1909, 1991 |
|
Bathgate RA, Lin F, Hanson NF, Otvos L Jr, Guidolin A, Giannakis C, Bastiras S, Layfield SL, Ferraro T, Ma S, Zhao C, Gundlach AL, Samuel CS, Tregear GW, Wade JD Relaxin-3: improved synthesis strategy and demonstration of its high-affinity interaction with the relaxin receptor LGR7 both in vitro and in vivo. Biochemistry. 2006 Jan 24;45(3):1043-53 |
|
Sherwood OD.1994. Relaxin. In: Knobil E, Neill JD (Eds.), The Physiology of Reproduction, second ed. Raven Press,pp.861-1009. |
|
Stewart DR, Vandevoort CA.1997. Stimulation of human luteal endocrine function with granulose lutein cell culture. J Clin Endocrinol Metab;82:3078-3083. |
|
Blankenship T, Stewart DR, Benirschke K, King B, Lasley BL.1994. Immunocytochemical localization of non luteal ovarian relaxin. J Reprod Med;39:235-240. |
|
Nguyen BT, Dessauer CW.2005. Relaxin stimulates protein kinase C zeta translocation: requirement for cyclic adenosine 3,5-monophosphate production. Mol Endocrinol;19:1012-1023. |
|
Feugang JM, Greene JM, Willard ST, Ryan PL.2011. In vitro effects of relaxin on gene expression in porcine cumulus-oocyte complexes and developing embryos. Reprod Biol Endocrinol;9:15 |
|
Stewart DR, Vandevoort CA.1999. Relaxin secretion by human granulose cell culture is predictive of in vitro fertilization-embryo transfer success. Hum Reprod;14:338-344. |
|
Bullesbach EE, Rhodes R, Rembiesa B, Schwabe C.1999. The relaxin-like factor is a hormone. Endocrine;10:167-169. |
|
Hanna CB, Yao S, Patta MC, Jensen JT, Wu X.2010. Expression of insulin-like 3 (INSL3) and differential splicing of its receptor in the ovary of rhesus macaques. Reprod Biol Endocrinol;8:150. |
|
Bathgate R, Balvers M, Hunt N, Ivell R.1996. The relaxin like factor (RLF) is regulated in the bovine ovary of the cycle and pregnancy:sequence and mRNA analysis. Biol Reprod;55:1452-1457. |
|
Spanel-Borowski K, Schafer I, Zimmermann S, Engel W, Adham IM. 2001. Increase in final stages of follicular atresia and premature decay of corpora lutea in INSL3-deficient mice. Mol Reprod Dev;58:281-286. |
|
Amory JK, Page ST, Anawalt BD, Coviello AD, Matsumoto AM, Bremner WJ. 2007. Elevated end treatment of serum INSL3 is associated with failure to completely suppress spermatogenesis in men receiving male hormonal contraception. J Androl;28:584-554 |
|
Glister C, Satchell L, Bathgate RA, Wade JD, Dai Y, Ivell R, Anand-Ivell R Rodgers RJ, Knight PG. 2013. A functional link between Bone Morphogenetic Protein and Insulin-like Peptide 3 signaling in modulating ovarian androgen production. Proc Natl Acad Sci USA;110:E1426-E1435 |
|
Satchell L, Glister C, Bleach E, Bicknell AB, Dai Y, Anand-Ivell R, Ivell R, Knight PG. 2013. Ovarian expression of insulin-like peptide 3 (INSL3) and its receptor (RXFP2) during development of bovine antral follicles and corpora lutea and measurement of circulating INSL3 levels during synchronized estrous cycles. Endocrinology;154:1897-1906. |
|
Anand R, Dai Y, Ivell R. 2013. Neohormones as biomarkers of reproductive health. Fertil Steril;99:1153-1160. |
|
Bendell JJ, Dorrington JH: Epidermal growth factor influences growth and differentiation of rat granulosa cells. Endocrinology 127: 533, 1990 |
|
Bird TA, Saklatvala J: Down-modulation of epidermal growth factor receptor affinity in fibroblasts treated with interleukin 1 or tumor necrosis factor is associated with phosphorylation at a site other than threonine 654. J Biol Chem 265: 235, 1990 |
|
Brown AB, Carpenter G: Acute regulation of the epidermal growth factor receptor in response to nerve growth factor. J Neurochem 57: 1740, 1991 |
|
Adhikari D, Liu K. 2009. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev;30:438-464. |
|
Reddy P, Zheng W, Liu K.2010. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab;21:96-103. |
|
Reddy P, Adhikari D, Zheng W, Liang S, Hamalainen T, et al. 2009. PDK1 signaling in oocytes controls reproductive aging and life span by manipulating the survival of primordial follicles. Hum Mol Genet;18:2813-2824. |
|
Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, et al. 2008. Oocyte specific deletion of Pten causes premature activation of the primordial follicle pool. Science;319:611-613. |
|
Roness H, Gavish Z, Cohen Y, Meirow D. Ovarian follicle burnout: a universal phenomenon? Cell Cycle. 2013 Oct 15;12(20):3245-6. |
|
Adhikari D, Liu K. 2010. mTOR signaling in the control of activation of primordial follicles. Cell Cycle;9:1673-1674. |
|
Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, Hunzicker-Dunn M. 2004. Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidyl inositol 3-kinase AKT/Ras homolog enriched in brain (Rheb)/ mammalian target of rapamycin (mTOR) pathway is necessary for induction of selective protein markers of follicular differentiation. J Biol Chem;279(19):19431-19440. |
|
Alam H, Weck J, Maizels E, Park Y, Lee EJ, Ashcroft M, Hunzicker-Dunn M. 2009. Role of the phosphatidylinositol-3-kinase and extracellular regulated kinase pathways in the induction of hypoxia inducible factor (HIF)-1 activity and the HIF-1 target vascular and endothelial growth factor in ovarian granulosa cells in response to follicle stimulating hormone. Endocrinology;150(2);915-928. |
|
Vanden Drieschen S, Myers M, Gay E, Thong KJ, Duncan WC. 2008. HCG up-regulated hypoxia inducible factor-1 alpha in luteinized granulosa cells: implications for the hormonal regulation of vascular endothelial growth factor A in the human corpus luteum. Mol Hum Reprod;14(8):455-464. |
|
Fraser HM. 2006. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol;4:18.doi:10.1186/1477-7827-4-18. |
|
Fraser HM and Lunn SF. 2001. Regulation and manipulation of angiogenesis in the primate corpus luteum. Reproduction;121(3);355-362. |
|
Wulff C, Wiegand SJ, Saunders PT, Scobie GA, Fraser HM. 2001. Angiogenesis during follicular development in the primate and its inhibition by treatment with truncated Flt-1-Fc (vascular endothelial growth factor Trap (A40). Endocrinology;142(7):3244-3254. |
|
Jiang YF, Tsui KH, Wang PH, Lin CW, Wang JY, Hsu MC, Chen YC, Chiu CH. 2011. Hypoxia regulates cell proliferation and steroidogenesis through protein kinase A signaling in bovine corpus luteum. Anim Reprod Sci;129(3-4):152-161. |
|
Fraser HM, Mrris KD, Wiegand SJ, Wilson H. 2010. Inhibition of vascular endothelial growth factor during the postovulatory period prevents pregnancy in the marmoset. Contraception;82(6):572-578. |
|
Hayashi KG, Berisha B, Matsui M, Schams D, Miyamoto A. 2004. Expression of mRNA for the angiopoietin-Tie system in granulosa cells during follicular development in cows. Journal of Reproduction and Development;50:477-480. |
|
Woad KJ, Hunter MG, Mann GE, Laird M, Hammond AJ, Robinson RS. 2012. Fibroblast growth factor 2 is a key determinant of vascular sprouting during bovine luteal angiogenesis. Reproduction;143(1):35-43. |
|
Nilsson EE, Detzel C, Skinner MK. 2006. Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction;131:1007-1015. |
|
Matos MHT, van den Hurk R, Lima-Verde IB, Luque MCA, Santos KDB, Martins FS, Bao SN, Lucci CM, Figueiredo JR. 2007. Effects of fibroblast growth factor-2 on the in vitro culture of caprine preantral follicles. Cells, Tissues, Organs;186:112-120. |
|
Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang JH, Scheppke L, Stockmann C, Johnson RS, Angle N et al. 2008. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature;456:809-813. |
|
Berisha B, Schams D, Kosman M, Amselgruber W, Einspanier R. 2000. Expression and localization of vascular endothelial growth factor and basic fibroblast growtg factor during the final growth of bovine ovarian follicles. Journal of Endocrinology;167:371-382. |
|
Berisha B, Steffl M, Amselgruber W, Schams D. 2006. Changes in fibroblast growth factor 2 and its receptors in bovine follicles before and after GnRH application and after ovulation. Reproduction;131:319-329. |
|
Woad KJ, Hammond AJ, Hunter M, Mann GE, Hunter MG, Robinson RS. 2009. FGF2 is crucial for the development of bovine luteal endothelial networks in vitro. Reproduction;138:581-588. |
|
Campos DB, Palin MF, Bordignon V, Murphy BD. 2008. The “beneficial” adipokines in reproduction and fertility. Int J Obes (Lond);32:223-231. |
|
Brann DW, Wade MF, Dhandapani KM, Mahesh VB, Buchanan CD. 2002. Leptin and reproduction. Steroids;67:95-104. |
|
Agarwal SK, Vogel K, Weitsman SR, Magoffin DA. 1999. Leptin antagonizes the insulin-like growth factor-I augmentation of steroidogenesis in granulosa and theca cells of the human ovary. J Clin Endocrinol Metab;84:1072-1076. |
|
Craig J, Zhu H, Dyce PW, Petrik J, Li J. 2004. Leptin enhances oocyte nuclear and cytoplasmic maturation via the mitogen-activated protein kinase pathway. Endocrinology;145:5355-5363. |
|
Ryan NK, Woodhouse CM, Van der Hoek KH, Gilchrist RB, Armstrong DT, Norman RJ. 2002. Expression of leptin and its receptor in the murine ovary: possible role in the regulation of oocyte maturation. Biol Reprod;66:1548-1554. |
|
Maillard V, Uzbekova S, Guignot F, Perreau C, Rame C, Coyral-Castel S, Dupont J. 2010. Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development. Reprod Biol Endocrinol;8:23. |
|
Tabandeh MR, Hosseini A, Saeb M, Kafi M, Saeb S. 2010. Changes in the gene expression of adiponectin and adiponectin receptors (AdipoR1 and AdipoR2) in ovarian follicular cells of dairy cow at different stages of development. Theriogenology;73:659-669. |
|
Munir I, Yen HW, Baruth T, Tarkowski R, Azziz R, Magoffin DA, Jakimiuk AJ. 2005. Resistin stimulation of 17 alpha-hydroxylase activity in ovarian theca cells in vitro: relevance to polycystic ovary syndrome. J Clin Endocrinol Metab;90:4852-4857. |
|
Maillard V, Froment P, Rame C, Uzbekova S, Elis S, Dupont J. 2011. Expression and effect of resistin on bovine and rat granulosa cell steroidogenesis and proliferation. Reproduction;141:467-479. |
|
Reverchon M, Cornuau M, Rame C, Guerif F, Royere D, Dupont J. 2012. Chemerin inhibits IGF-1 induced progesterone and estradiol secretion in human granulosa cells. Hum Reprod;27:1790-1800. |
|
Shimizu T, Kosaka N, Murayama C, Tetsuka M, Miyamoto A. 2009. Apelin and APJ receptor expression in granulosa and theca cells during different stages of follicular development in the bovine ovary: Involvement of apoptosis and hormonal regulation. Anim Reprod Sci;116:28-37. |
|
Dissen GA, Garcia-Rudaz C & Ojeda SR 2009 Role of neurotrophic factors in early ovarian development. Seminars in Reproductive Medicine 27 24–31. |
|
Tomac A, Lindqvist E, Lin LF,Ogren SO, Young D, Hoffer BJ &Olson L 1995 Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 373 335–339. |
|
Golden JP, DeMaro JA, Osborne PA, Milbrandt J & Johnson EM Jr 1999 Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Experimental Neurology 158 504–528. |
|
Aravindakshan J, Chen XL & Sairam MR 2006 Age-dependent bimodal GDNF regulation during ovarian tumorigenesis in follitropin receptor mutant mice. Biochemical and Biophysical Research Communications 351 507–513. |
|
Linher K, Wu D & Li J 2007 Glial cell line-derived neurotrophic factor: an intraovarian factor that enhances oocyte developmental competence in vitro. Endocrinology 148 4292–4301. |
|
Liu J, Linher K & Li J 2009 Porcine DAZL messenger RNA: its expression and regulation during oocyte maturation. Molecular and Cellular Endocrinology 311 101–108. |
|
Martins da Silva SJ, Gardner JO, Taylor JE, Springbett A, De Sousa PA & Anderson RA 2005 Brain-derived neurotrophic factor promotes bovine oocyte cytoplasmic competence for embryo development. Reproduction 129 423–434. |
|
Seifer DB, Feng B, Shelden RM, Chen S & Dreyfus CF 2002 Brain-derived neurotrophic factor: a novel human ovarian follicular protein. Journal of Clinical Endocrinology and Metabolism 87 655–659. |
|
Kawamura K, Kawamura N,Mulders SM, Sollewijn Gelpke MD & Hsueh AJ 2005 Ovarian brain-derived neurotrophic factor (BDNF) promotes the development of oocytes into preimplantation embryos. PNAS 102 9206–9211. |
|
Abir R, Fisch B, Jin S, Barnnet M, Ben-Haroush A, Felz C, Kessler- Icekson G, Feldberg D, Nitke S & Ao A 2005 Presence of NGF and its receptors in ovaries from human fetuses and adults. Molecular Human Reproduction 11 229–236. |
|
Fedorushchenko AN, Koval T & Khamidov D 1999 The effect of in-situ nerve growth factor from different biological sources on the reinitiation of mouse oocyte meiotic maturation in culture and on parthenogenetic activation. Ontogenez 30 453–455. |

