The Evaluation and Treatment of Women with Pelvic Pain
Authors
INTRODUCTION
Pelvic pain, in its many forms, is the presenting chief complaint in about one third of gynecology outpatients, making the diagnosis and treatment of the causative factors central to effective gynecologic practice. In many painful conditions of acute onset, medical and/or surgical treatment affords prompt relief, fulfilling the expectations of patient and physician alike. When the pain becomes chronic despite the best efforts of the clinician, frustration often mounts for all involved. The tried and true methods of diagnosis and treatment of acute pain often falter when applied without revision to chronically painful conditions.
The intent of this chapter is to provide a frame of reference for the gynecologic clinician that will maximize his or her own effectiveness in treating chronically painful gynecologic conditions and will provide guidelines for fruitful collaboration with other medical and mental health personnel.
Following a brief review of basic mechanisms of pain perception and modulation, I will compare acute and chronic pain problems from pathophysiologic and psychologic perspectives. A discussion of “chain reaction” pain syndromes, in which psychologic and physiologic factors may combine in a repeating cycle of pain augmentation, will be followed by a discussion of the diagnosis and treatment of nonacute pelvic pain problems.
THE EXPERIENCE OF PAIN
Merskey defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.”1 The traditional specificity theory of pain supposes that the intensity of pain is proportional to the amount of tissue damage present. However, psychologic data that document the influence of cultural background, personality style, attention, and the meaning of the pain to the individual,2 and neurologic data that report on new pains that emerge after the resection of nerve supply to painful areas,3 render this theory inadequate. In its place, more recent research has focused on the multidimensional nature of pain, with increasing attention directed to the psychologic influences on the experiencing and reporting of pain.
In the acute pain situation, such as that associated with clear-cut pelvic inflammatory disease, ectopic pregnancy, and similar disorders, an understanding of the psychologic and cultural factors in pain reporting may be of interest, but clinical judgment is more often swayed by laboratory data and physical findings. Hence, in dealing with a woman infected with Neisseria gonorrhoeae, an appreciation of the patient's personality will help the physician-patient relationship and is important in promoting patient compliance, but it will not often be a major factor in deciding which antibiotic to prescribe.
Once a painful condition of almost any type persists beyond a few months, a more complex model of pain perception is needed to guide our diagnostic efforts and to understand the effects of surgical, medical, and psychologic treatments. The gate theory of pain perception, although itself imperfect, provides such a model.4
GATE THEORY
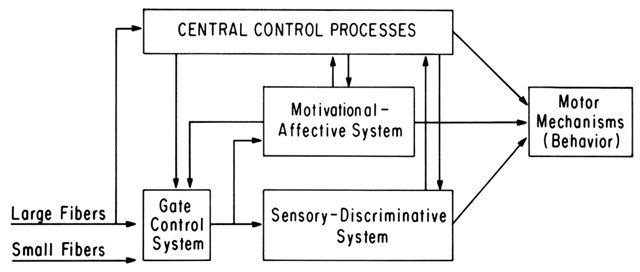
Nociceptive stimuli are thought to be transmitted to the neuraxis by small-diameter nerves, the unmyelinated A delta and C fibers. In the pelvis, fibers from the cervix, uterus, and upper vagina traverse the uterine and cervical plexuses, the pelvic plexus, the hypogastric plexus, and the superior hypogastric plexus and enter the lumbar and lower thoracic sympathetic chain. Near the spinal cord, they join the spinal nerves, entering the cord by way of the dorsal roots of T10 through L1, and synapse with neurons in the tract of Lissauer.5 At this level, signals may be modulated by impulses from longer somatic afferent nerves (Fig. 1), as well as descending signals from higher brain centers. The modulated signals may then be transmitted to the anterolateral horn to contribute to somatomotor and sympathetic nocifensive reflexes and may enter ascending afferent systems to the brain.
The small A delta and C fibers, in this conceptualization, are far more potent than the simple “pain fibers” of the specificity model. They are believed to facilitate transmission or “open the gate” at the spinal cord level, and thereby promote prolonged neuronal activity and spread of pain to other body areas. At the same time, the small fibers are also influenced by modulating signals caused by activity in the whole nervous system. The flexibility of this model of pain perception allows it to simultaneously incorporate physiologic, psychologic, and clinical data.6,7
This flexibility is important in the understanding of both acute and chronic pain. The ability to diminish the perception of acute pain through apparently centrally mediated mechanisms is well documented: the man who comfortably lies on a bed of nails and the wounded soldier who perceives pain only when he has reached safety are two dramatic examples. Chronic persistence of a painful condition, and for our specific purposes, chronic pelvic pain, allows the “motivational-affective” dimension (see Fig. 1) to play a more important role. The brain stem reticular formation and the limbic system, receiving fibers from the spinoreticular and paleospinothalamic components of the anterolateral somatosensory pathway, and having projections over wide cortical areas, are thought to be important in the regulation of this dimension of the experience of pain.7 This aspect of pain is an important part of the “suffering” experienced by pain patients. The person who has had a frontal lobotomy retains the sensory component of pain but no longer experiences aversion to the pain and has less drive for pain relief. Again, although accurate diagnostic and thoughtful handling of the acute pain victim may be aided by understanding these mechanisms, the effective management of the chronic pain sufferer depends more heavily on this understanding on the part of both the patient and the physician.
For a more complete discussion of the diagnosis and treatment of acute pain problems, the interested reader is referred elsewhere in these volumes to chapters dealing with acute gynecologic conditions and to other reviews.8 The remainder of this chapter will focus on chronic pain problems.
GYNECOLOGIC CONDITIONS CAUSING CHRONIC PAIN
Familiarity with specificity theory, in which pain is thought to result from stimulation of “pain fibers” in damaged tissue, often leads the clinician to search for “enough” damage to “explain” the pain. As is apparent from the above discussion of gate theory, such a focused search may be misguided in the chronic pain patient. Similarly, the attempt to describe chronic pain as “either” physical “or” psychologic, or the attempt to “rule out” physical causes of pain before investigating psychologic aspects of the afflicted woman, is simplistic and unproductive. The psychologic assessment of the chronic pain patient will be discussed in greater detail later in the chapter. Nevertheless, the evaluation of the chronic pelvic pain patient should include investigation of chronic organic gynecologic conditions that may contribute to a pain problem. For the purpose of this brief review, the classification offered by Renaer and Guzinski of “episodic” and “continuous” chronic pain is useful9:
Episodic Chronic Pain
Dyspareunia
Mittelschmerz (midcycle pain)
Primary dysmenorrhea
Secondary dysmenorrhea
Adenomyosis
Endometriosis
Continuous Chronic Pain
Adhesions
Anatomic distortion
Chronic infection with tubal dilation
Residual ovary syndrome
Ovarian remnant syndrome
Pelvic tumors
Loss of pelvic support
Uterine retroversion
Allen-Masters “universal joint” syndrome
Genital prolapse
Endometriosis
Pelvic congestion syndrome
Chronic pain without obvious pathology
Episodic Chronic Pain
DYSPAREUNIA.
Physical problems such as a poorly healed and painful episiotomy incision, infected urethral diverticulum, and Bartholin's gland cyst or abscess are causes of introital dyspareunia that every practicing gynecologist will see at some point. On occasion, partial obstruction of the Bartholin's gland duct may result in gland enlargement that appears only during sexual arousal, when increased gland secretions cannot drain quickly enough.10 Perhaps more frequently, however, the cause of discomfort can be found by looking for one of the chain reaction syndromes described below or by tactful inquiry into the patient's sexual adjustment in general.
Vaginismus occurs with much greater frequency than is generally appreciated. Severe vaginismus will not escape the notice of even the most hurried clinician. The woman with poor voluntary introital muscle control during the pelvic examination may not be able to voluntarily relax during coitus. More subtle versions, which may be present only during coitus and not on the examining table, may be diagnosed only on careful history taking. Lamont reports that in 22% of his series, vaginismus was situational (i.e., present only during coitus but not during pelvic examination).11
Although vaginismus has traditionally been thought indicative of a “defense reaction against cohabitation (coitus)”,12 it has more recently been effectively treated as a conditioned response to painful vaginal events and/or fears of pain during vaginal entry. Teaching the afflicted woman how to regain voluntary control of vaginal muscles by using vaginal relaxation exercises is the cornerstone of modern treatment.10,13 Surgical revision of the introitus is rarely indicated and will only reinforce the conditioned muscle spasm if used in the inappropriately selected patient. Psychologic assistance beyond relaxation exercises is often appropriate and needs to be tailored to the individual case.
Deep dyspareunia is more often attributable to organic pathology such as endometriosis or chronic pelvic inflammatory disease. In some cases, it may be part of another “chain reaction” syndrome discussed below.
The interested reader is referred to recent reviews for a more complete discussion of this area.13,14
MITTELSCHMERZ.
Regularly occurring unilateral adnexal pain of brief duration (20–30 minutes) is usually attributed to release of a mature ovum from a follicle. If ovum release is accompanied by significant bleeding, the pain may last up to several days, but rarely does it become a significant ongoing problem for any individual patient. Suppression of ovarian function with oral contraceptives and/or midcycle prophylactic use of nonsteroidal anti-inflammatory agents will most often control the problem.
PRIMARY DYSMENORRHEA.
Defined as cramping midline lower abdominal or low back pain associated with menstruation and occurring in the absence of known organic pathology, primary dysmenorrhea was long thought to be psychologic or psycho-physiologic in nature. Recent investigations have described a probable role for prostaglandin F2α, produced in the endometrium, in mediating or causing painful uterine contractions during menstruation.15 Treatment with prostaglandin synthetase inhibitors such as mefenamic acid, naproxen, ibuprofen, acetylsalicylic acid, or combination oral contraceptive pills has been highly effective. In cases refractory to these medications, about 50% of women will derive long-term benefit from laparoscopic uterosacral nerve ablation.16
Although primary dysmenorrhea often responds well to such treatment, the clinician should remain alert for physical evidence of endometriosis, because the dysmenorrhea associated with this condition may also be relieved by prostaglandin synthetase inhibitors in some cases.
SECONDARY DYSMENORRHEA.
Endometriosis is perhaps the most common cause of dysmenorrhea that begins after years of relatively comfortable menstruation. In this situation, pain may be associated with menses at first, but it often increases and is present constantly. A careful chronologic history may therefore yield diagnostic clues. Rectal pain during defecation, rare in other gynecologic problems, is common when cul-de-sac involvement with endometriosis is present. Secondary dysmenorrhea due to endometriosis is often (75% of cases) relieved by meticulous surgical resection, via either laparoscopy or laparotomy; the utility of presacral neurectomy for additional pain relief has mixed support in controlled series.17,18
Adenomyosis, or the presence of endometrial glands deep within the uterine wall (more than two high-powered microscopic fields beneath the surface of the myometrium), is also a common cause of secondary dysmenorrhea, especially in women in their 30s or older. A boggy, softened, and mildly enlarged uterus is present on pelvic examination, especially in the luteal phase. Vaginal ultrasound may prove useful in the preoperative diagnosis of adenomyosis. Treatment with progestagens in the luteal phase or combined estrogen and progesterone may be palliative, but hysterectomy is often ultimately required.
Treatment of endometriosis has traditionally involved hormonal regimens preceded or followed by conservative surgical resection of localized disease when preservation of fertility is desired. Hysterectomy with bilateral salpingo-oophorectomy has been the treatment of choice for relief of pain in patients whose families are complete or who have discontinued efforts to achieve pregnancy. Danocrine therapy and total ovarian suppression with gonadotropin-releasing hormone (GnRH) agonists have largely replaced other hormonal schemes (continuous progesterone, combination oral contraceptives, or “pseudo-pregnancy” with continuous oral contraceptives) by demonstrating more rapid and complete resolution of endometrial implants, as well as pain relief. Their present role is limited to enhancing fertility, although GnRH agonists may ultimately prove to be effective for long-term pain relief when combined with estrogen plus progesterone add-back therapy.
Fibroids are another common cause of secondary dysmenorrhea. Only submucous lesions are thought to be associated with cyclically recurring pain, whereas degeneration of a larger fibroid may result in acutely painful ischemic necrosis. Myomectomy, when surgically feasible, is the treatment of choice for the symptomatic fibroid in the patient who wishes to retain childbearing capacity, whereas hysterectomy is recommended for women for whom fertility is no longer a concern.
Asherman's syndrome, or intrauterine synechiae perhaps caused by a vigorous curettage of the uterus, and acquired stenosis of the cervix following radiation or conization are less common causes of episodic chronic pelvic pain. Both are treated with cervical dilation, and the synechiae may be removed by curettage or hysteroscopically controlled lysis.19
Continuous Chronic Pain
Anatomic changes and residua of acute disease processes may become components of a chronic pain syndrome. The scarring that often follows one or more bouts of acute pelvic inflammatory disease, chronic endometriosis, or a series of pelvic laparotomies is a prime example. In addition, loss of support for pelvic structures may sufficiently alter pelvic sensation to contribute to a pain problem.
ADHESIONS.
The question of whether or not adhesions cause pain has been the subject of continuous discussion in the surgical literature. The apparent absence of pain in some patients with massive adhesions in contrast to claims of intense pain by patients with only minimal adhesive disease has led many clinicians to doubt that adhesions alone are “enough” pathology to “cause” pain. The subjective nature of pain, the intrinsic difficulty in establishing reliable measures of clinical pain, and the lack of behavioral and psychologic assessment in reports of pelvic pain in the gynecologic literature have contributed to the confusion. Add to this the lack of noninvasive techniques for accurately observing and/or quantifying postoperative adhesion formation, and one has a difficult problem indeed. The magnitude of the problem is great: as many as 20% of patients with acute pelvic inflammatory disease will have chronic pain, much of which is felt to be associated with adhesions.20
Nonsurgical therapy for adhesions has generally been disappointing. Optimal bowel regulation with vegetable bulk laxatives, with appropriate dietary changes to minimize bowel irritability, may diminish pain caused by limitation of bowel motility by pelvic adhesions. Hot baths, local heat, and rectal diathermy are all time-honored palliative measures but seldom completely satisfy the patient's wish for relief.
The advent in the 1970s of microsurgical techniques for repair of damaged fallopian tubes sparked renewed interest in the surgical treatment of pelvic adhesive disease. The growing belief that laparoscopic adhesiolysis may reduce adhesion burden more effectively than laparotomy has further stimulated the search for agents that prevent adhesion re-formation while allowing normal healing to proceed. Interceed (oxidized cellulose), Gore-tex, and heparinized Ringer's lactate all have their proponents and may indeed occupy different therapeutic niches. The recent development of miniature laparoscopy for outpatient clinic use raises the intriguing possibility of economical repeated second-look laparoscopy for adhesiolysis or for the reapplication of adhesion-preventing agents.
ANATOMIC DISTORTION.
A fallopian tube dilated with serous or purulent fluid can be chronically painful, as can an abscessed ovary, presumably as a result of stretching of involved tissue and inflammatory response. In contrast, enlargement caused by either a benign or a malignant neoplastic growth is often painless in the absence of acute hemorrhage within or from the mass or in the absence of torsion of the tube.
On occasion, an ovary or ovaries that remain following hysterectomy may become continuously or cyclically painful or cause deep dyspareunia if they are adhered to the vaginal apex, if they are encased in an area of adhesions with insufficient room to accommodate normal cyclic changes in ovarian size, or if they undergo the “cystic degeneration” typical of the “residual ovary syndrome.”21,22 Hormonal suppression with oral contraceptives or the passage of time will often resolve the problem, but ovaries that are persistently enlarged and painful 1 year or more after hysterectomy may sometimes warrant surgical excision. Laparoscopic removal of the residual ovary is often possible but should be left for the laparoscopist substantially experienced in retroperitoneal dissection around the ureter.
DECREASED PELVIC SUPPORT.
The severely retroverted uterus may be quite tender to palpation and may be associated with pain referred to the low back or sacral area. The presence of adenomyosis or benign uterine enlargement due to fibroids may contribute to both deep dyspareunia and continuous chronic discomfort in the area, usually worse premenstrually.
The Allen-Masters syndrome is a variation on this theme and consists of rent(s) in the peritoneum covering the broad ligaments, excess peritoneal fluid, and retroversion of the uterus with excessive uterine mobility.23 Repair of the peritoneal defects and uterine suspension are reported to relieve pain, although most gynecologists will perform hysterectomy if fertility is no longer desired. Allen and Masters postulated that the severe uterine retroversion altered uterine perfusion so as to result in uncomfortable pelvic vasocongestion.
ENDOMETRIOSIS.
The dysmenorrhea associated with endometriosis may, with time, gradually encroach on the rest of the menstrual month, leaving the patient in continuous chronic pain. The pain may become quite diffuse and may become associated with bowel function because of endometriosis on the rectum, cul-de-sac of Douglas, and sigmoid colon.
The treatment of endometriosis in the woman wishing to preserve fertility is presented in more detail elsewhere in these volumes. Suffice it to say here that the woman afflicted with endometriosis often quickly learns the fallibility of all evaluation techniques short of laparoscopy. If she knows that pain may be her only warning of advancing disease, that the rate of progression is highly variable from person to person, that her fertility may be impaired, and that one or more surgeries may await her in the future, her vigilance may be increased. Discomfort is much more difficult to disregard when it carries such weighty meanings.
PELVIC CONGESTION SYNDROME.
The notion that either mechanically (by way of pelvic varicosity formation) or psychophysiologically mediated (anxiety-associated) chronic overdistension of pelvic veins may become uncomfortable has survived through decades of gynecologic literature. Duncan and Taylor described indirectly measured increases in vaginal mucosal blood flow during anxiety-producing interviews in 10 women they described as having “pelvic congestion syndrome.”24 Lack of orgasmic release was felt to be contributory to the problem. This study awaits replication in controlled fashion.
Beard and colleagues25 used intrauterine instillation of contrast material to document increased diameters of pelvic veins in women reporting symptoms similar to those described by Taylor24 (i.e., continuous heavy aching pelvic discomfort that worsens at the end of the day and premenstrually). Beard and colleagues26 have further characterized the psychiatric co-morbidity present in this group and have published controlled treatment trials.27 They observed that elimination of ovulatory cycles with continuous medroxyprogesterone diminished symptoms, but that lasting improvement more often occurred when the patient became involved in productive psychotherapy. These data taken together suggest the interaction of physical and emotional predispositions that may lead to either transitory psychophysiologic changes in pelvic vein volume and/or permanent changes in this vascular system. Furthermore, the nociceptive signals from the veins may be variably processed as they ascend toward conscious perception.
Treatment might reasonably begin with mild analgesics, then continue with ovulation suppression by the means most appropriate to the individual and the severity of her pain (e.g., oral contraceptives, continuous medroxyprogesterone), psychotherapy of an insight-oriented or behavioral nature, and finally extirpative surgery if all else fails and significant contraindications are not present.
MUSCULOSKELETAL COMPONENTS.
Musculature of the low back and pelvis may contribute to pain in the region of the pelvis, either as primary muscular syndromes or as sequelae to pain from other pelvic sources. Dysmenorrhea may be referred to the low midline of the back, particularly in the instance of uterine retroversion, and similar pain may accompany central pelvic endometriosis. Dense adhesion of the ovary to the pelvic side wall may produce referred pain to the ipsilateral low back.
Pelvic floor tension myalgia is perhaps the most common muscular problem producing pelvic pain.28 Intermittent or constant painful contraction of the levator plate may be present as a primary psychophysiologic problem but more commonly occurs in reaction to some other source of pain. The pain often presents as low pelvic aching, pressure, or “falling out” sensations that worsen with prolonged standing and are promptly relieved by reclining. The voluntarily tensed levators are tender during pelvic examination, reproducing at least some of the chief complaint. Modified Kegel exercises may help, using short contraction and long relaxation phases. Encouraging the patient to “go ahead and let everything fall out” produces muscle relaxation that reduces the discomfort.
Lumbar muscles may become tender in response to subtle changes in posture or motion, as described by King.29 Trigger points may be present in the low back, abdominal wall, and gluteal areas, and on occasion internally in the vaginal wall. Injection of local anesthetic into the trigger point for a series of two to four injections may produce long-term relief in up to 75% of patients.30 The prevalence of trigger points as an explanation for pelvic pain is debated but is noted in less than 5% of patients in my experience.
In many cases, musculoskeletal problems do not appear in pure form but rather are seen together with other physical and psychologic components of chronic pain. The diagnostic and therapeutic challenges multiply with this complexity.
When seeing women with chronic pelvic pain, clinicians often recognize that such patients may be clearly anxious, depressed, and emotionally constricted. It may be easy to conclude that significant “psychologic overlay” (an over-used and imprecise term) may exist. The implication is often made that the emotional distress may be etiologic, but a number of studies have found that pain patients without obvious organic findings may be psychologically indistinguishable from women with long-standing pain attributed to organic pathology.31,32 Simply being in pain for prolonged periods of time may induce psychologic symptoms in the appropriately predisposed individual, almost regardless of the magnitude of the “organic” contributions to the problem.33,34
Although careful diagnostic evaluation will often uncover at least some evidence of “organic” pathology, a distressingly large number of women experience disabling pelvic pain in the absence of palpable or laparoscopically visible tissue damage.
“Chain Reaction” Syndromes of Chronic Pelvic Pain
The classifications described so far look at chronic pain problems in “snapshot” fashion, as they present at any given time. A careful review of the chronology of a pain problem often reveals a series of interacting factors that reinforce each other and augment the pain in chain-reaction fashion. Several examples will suffice, although variations on the theme are numerous.
VAGINITIS-VAGINISMUS SYNDROME.
One or more bouts of vaginal infection, with associated pain and/or repeated dyspareunia, may be sufficient to bring about involuntary vaginal introital and levator muscle contraction on attempted intromission as a conditioned response. The contraction may be situational (i.e., present during coital attempts but not present during pelvic examination), thus leaving the clinician baffled. The contraction may be a major factor in dyspareunia without being severe enough to prevent intromission. In its more subtle forms, the pain may disappear after intercourse continues for a while. In its severe forms, the pain may gradually progress to become virtually constant and spread to encompass the entire lower abdomen. Although this has not been well studied, some women in this group may then resemble the “pelvic congestion syndrome” group, because their sexual responses are impaired as a result of the pain.
VAGINISMUS-URETHRAL SYNDROME.
Every practicing urologist has a significantly large group of female patients who complain of intermittent or constant frequency, urgency, and dysuria in the absence of documented bacteriuria. Results of intravenous pyelography are almost always normal, and cystoscopic findings are limited to the demonstration of inflammatory changes in the trigone of the bladder and proximal urethra at most. Treatment with chronic antibiotics, antispasmodics, and analgesics will cure the majority of cases. However, many cases may be perpetuated by concomitant vaginismus.35 Coitally induced urethral trauma may be augmented by absent vaginal lubrication and by uncontrollable introital contraction. Treatment of the vaginismus may be a vital adjunct to traditional urologic therapies in these instances.
CERVICITIS—“BUMP” DYSPAREUNIA.
In similar fashion, cervicitis of either an intermittent or a chronic nature may cause pain on deep vaginal penetration when the cervix is struck. Over time, the anticipation of pain during intercourse, and the fact of it, may impede sexual response to the point that the uterine elevation normally occurring during the excitement and plateau phases of sexual response will be interrupted. The cervix may then lie more directly in the path of the entering penis, leaving the cervix vulnerable to painful stimulation and trauma.
Appropriate antibiotic and/or cryosurgical treatment of the cervicitis will often diminish the cervical inflammation. If the patient remains apprehensive and her sexual responses do not return, cervical stimulation during coitus may remain uncomfortable despite the absence of infection. Cervical trauma may be a factor in restarting the inflammation as well.
In the case of the patient who notes continuing deep dyspareunia after cervicitis has healed, the clinician must take a significantly detailed history to detect changes in sexual response. They may have resulted from the pain at first and may in turn have become part of the etiology of persistent or recurring cervical inflammation over a period of time. In addition to medical therapy for the cervicitis, the clinician should counsel the couple to avoid painful deep coital penetration until a higher level of sexual arousal is present. With patience, this approach can interrupt this form of the “chain reaction” problem.
This problem usually presents as chronic intermittent pain, but it can be a component of a chronic constant pain if the inflammation is severe enough and/or if the patient starts to assume some of the psychologic and behavioral characteristics of the chronic pain patient described below.
PELVIC CONGESTION—DEEP DYSPAREUNIA.
Deep dyspareunia is a frequent complaint of the woman with the pelvic congestion syndrome described above.36 By a sequence of events similar to that described for cervicitis above, anorgasmia and pain may become part of both the cause and the effect of the pelvic congestion problem. In addition to such commonly used symptomatic measures as mild analgesics, local heat, and rest in a position such that the pelvis is elevated, successful treatment may need to include counseling or therapy focused on sexual issues.
The above four “syndromes” are cited to provide common examples of how repeated episodes of acute illness (e.g., vaginitis, cystitis), associated pain, and sexual dysfunction may all contribute to a repetitive pattern that presents to the clinician as a pain problem. Assessment and treatment of such patterns must involve an approach to all accessible components.
ASSESSMENT AND TREATMENT OF CHRONIC PAIN PROBLEMS
In recognition of the complex nature of chronic pain problems and the interdisciplinary approach that seems needed to deal with many of them, “pain clinics” of varying descriptions have been started in many areas of the country over the past 15 years. Many of them employ behavioral approaches to treatment of chronic pain problems drawing from the work of Fordyce,37 Sternbach,38 and others.
The Evolution of Chronic Pelvic Pain Patients
Many patients certainly suffer through repeated episodes of pain or develop constant pain without having it significantly impair their work or their personal lives. Many others, however, begin to look and sound like chronic pain patients after 3 to 6 months of pain. Most research has focused on the pathology of the chronic pain patient, with little attention paid to how other chronic pain victims remain “well,” or at least outside the healthcare system.
The average age of the chronic pelvic pain sufferer is approximately 30 years (Table 1).11,24,39,40,41 On referral to a pain clinic, many will bring medical records describing multiple gynecologic and/or obstetric problems over the preceding 5 to 10 years or more and often many medical and/or surgical attempts to relieve the pain. For the typical victim, the pain problem may appear in retrospect to have started innocently enough with a commonplace problem such as dysfunctional uterine bleeding, a hemorrhagic corpus luteum cyst, spontaneous abortion, or one of the conditions described in the “chain reaction” syndromes above. Why, then, do some patients go on to develop chronic pain?
TABLE 1. Average Age of Patients in Studies of Chronic Pelvic Pain
Studies | No. Subjects | Mean Age |
Duncan and Taylor24 | 36 | 30.9 |
Castelnuovo-Tedesco and Kraut39 | 65 | 30 |
Beard and co-workers40 | 35 | 30 |
Lamont11 | 80 | 28 |
Renaer and co-workers41 | 15 | 34 |
Some of the more refractory may in fact be the “pain-prone patients” described by Engel, whose pain is an example of somatoform disorder or somatization or is part of depression, hypochondriasis, or schizophrenia.42 Many others, however, are less psychiatrically dramatic and seem to experience the original acute pain in a setting of unmet personal needs. They may “discover” (unconsciously) that these needs for dependence and care are more easily met when they are in the role of a sick person. With each recurrence of exacerbation of the pain, the patient becomes more firmly established in the role. Because with each episode there may be some hints of “organic” pathology in the physical examination and laboratory findings, the patient often becomes increasingly convinced that a physical problem is being overlooked, and the clinician hopes to find “enough” pathology to explain the pain.
To add to the complexity of the problem, the changes that happen in many “normal” individuals who suffer from chronic pain that started with a clear-cut organic problem, such as pelvic inflammatory disease, must be considered briefly. After a varying period of time, and repeated episodes, the non-narcotic and narcotic analgesics that were originally prescribed become less and less effective as physical tolerance develops. Sleep may then be impaired, leaving the patient chronically fatigued. Posture and gait may change subtly, allowing stiff low back and leg muscles to increase the discomfort. Constipation often ensues because of changes in diet and activity and as a side effect of analgesic medication. The longer it is impossible to work or carry out other routine functions, the more self-esteem is impaired. The patient then often becomes depressed, with early morning awakening, which further impairs her rest and lowers her pain tolerance. The usual variety of social supports gleaned from activities with friends and loved ones diminishes. Interaction with others becomes more constricted as pain and illness become pervasive themes of thought and conversation. Sexual relations become impossible because of pain, and sexual dysfunction is added to the list of complaints. Along with decreased capacity often comes decreased responsibility. When financial compensation is added to the list of “benefits” of being ill, the pattern becomes more firmly entrenched.
Once the patient begins to look and sound like the patient described above, the physician is often best advised to abandon the hope of finding a single cause for the pain, and thus a single effective course of treatment. He or she may also need to abandon the familiar course of trying multiple medications in series or adding one medication after another. When the feeling exists that no matter what medications are added the patient will come back with unremitting distress, it is time to stop, take stock of the situation, and re-evaluate the entire diagnosis and treatment of the patient. The following paragraphs describe such an assessment.
Evaluation Methods
HISTORY.
Engel gives very apt directions on taking a pain history. He writes that “an interview technic which permits the patient to speak for himself, his family, and his relationships as well as of his symptoms, which does not force a separation between what is regarded as organic and what is regarded as psychological or social, will be tremendously productive in clarifying the patient's illness.”42 A careful chronology of pain development and “spread” of the distress to other organ systems, matched up with a careful account of changes in relationships with peers and family, will give valuable insight into the meaning and function of the pain in the patient's life.
PHYSICAL EXAMINATION.
Thorough physical examination is assumed, but some aspects of the abdominal and pelvic examination in the pain patient are worthwhile. One can often overcome voluntary guarding and general apprehension by borrowing from the techniques of progressive muscle relaxation, that is, by asking the patient to first tense and then relax the muscle group of concern (e.g., the rectus abdominus muscles for the abdominal examination, or the vaginal introital muscles for the pelvic examination). Inability to voluntarily control introital muscles may be consistent with the diagnosis of vaginismus, if corroborated by history. In the calm and introitally relaxed patient, pain on palpation of the lateral margins of the introitus is very rare; if found, it is usually indicative of a patient who experiences almost any kind of pelvic palpation as painful. The exception to this rule is the woman afflicted with vulvar vestibulitis. In this case, exquisite tenderness is found in a precisely demarcated area of the vaginal vestibule when the area is systematically examined with a cotton-tipped applicator.
Careful palpation along the urethra and bladder base may reveal tenderness typical of trigonitis and urethritis. Gentle lateral and ventral traction on the cervix may produce pain due to pathology in the broad and uterosacral ligaments, respectively. Detailed description of the palpatory findings associated with the various gynecologic diseases will be found in the discussion of each disease entity and will not be reviewed here. In general, slower, more careful palpation of the introitus and vagina than is generally carried out may well be informative in the pelvic pain patient.
Many chronic pain victims have difficulty relaxing for the examination. I have found that they seem to fear that the examination will go on interminably, even if severe pain is experienced. I make a contract with the patient that when it starts to hurt, I will begin counting and will stop on the count of “three,” and give her a chance to recover. Placing limits on the duration of the discomfort seems to make it manageable and aids relaxation. With an experienced examiner, seldom is more than one or two cycles of counting necessary to evaluate each adnexal area.
PSYCHOLOGIC ASSESSMENT.
In addition to the standard gynecologic history and physical examination, in-depth evaluation of the emotional aspects of the person's life and relationships, including sexual history, should be done. This can be accomplished by a patient and experienced gynecologist but may also be done by a mental health professional. This should be done as part of the total patient evaluation and not after all “organic disease” has been “ruled out.” As Engel comments:
This is more economical in time and expense for both the physician and the patient than the currently traditional technique of “ruling out disease” and attempting to establish a diagnosis by exclusion. Such interminable diagnostic procedures may not be only a waste of time and money but may also render virtually impossible the establishment of correct diagnosis simply because the patient himself becomes increasingly oriented towards this type of approach and less spontaneous in revealing personal and psychological data.42
Psychometric testing may be used in the gynecologist's office as a general screening technique for depression or marital discord, or to measure the intensity of pain. The use of these measures conveys the message that the clinician values psychologic information, and it may serve to identify patients needing more extensive evaluation. More extensive psychologic testing is better left to the psychologist. Even so, it will help identify emotional needs, will describe individual strengths and weaknesses, and, when combined with a clinical interview, may suggest a psychiatric diagnosis such as anxiety, depression, or a personality disorder, but such evaluation rarely tells the gynecologist whether or not surgery should be performed. Such decisions, of course, must be based on the synthesis of all available clinical information.
CONSULTATIONS.
Consultation with specialists in orthopedics, neurology, neurosurgery, and urology should be obtained in a logical sequence dictated by the findings in the individual case. The evaluation is most constructive, of course, if the consultant has an interest in pain problems and does not simply “rule out” pathology pertinent to the specialty. The orthopedist, together with the physical therapist, may contribute by defining changes in gait and posture that develop in reaction to the pain and add to total discomfort. The neurologist or neurosurgeon may discover subtle nerve root compression, or the occasional instance of meralgia paresthetica, which may present as lower quadrant pain.43
SUMMARIZING THE ASSESSMENT.
With all the consultations completed, a treatment plan may then be devised. At this point, the clinician should ask two separate questions:
- Is there pathology that requires medical or surgical treatment?
- Are there psychologic difficulties that warrant treatment?
This approach actively avoids the question of a cause-and-effect relationship between physical and psychologic problems, which is usually unanswerable in the early stages of working with a chronic pain patient, if it indeed can ever be completely answered. Avoiding the cause-and-effect question allows the clinician to suggest appropriate treatment for both areas. For example, the anxious and depressed post-hysterectomy patient with probable severe pelvic adhesions should perhaps be treated for any bowel irregularities that may increase the discomfort of adhesive disease, as well as receive appropriate medicinal and psychotherapeutic attention for her affective disorder. Telling the patient that she is in pain because she is depressed, or that she is depressed because she is in pain, has little practical value and short-circuits the treatment.
In a surgically oriented specialty such as gynecology, the question inevitably arises concerning the advisability of operative intervention. This is very often an extraordinarily difficult judgment to make, and even retrospective assessment is uncertain all too frequently. Diagnostic laparoscopy is very useful, but again this should not be undertaken to find “enough pathology to explain the pain,” but rather to guide future medical or surgical therapy for organic disease with more certainty. The pelvis that is “negative” to routine examination is found to have pathology of some type about 40% of the time.44
Patients and their families often think of pain in the too simplistic notion of pain stimulation and perception that used to hold sway in the medical profession, so they often look to surgery as the way to find the final answer. They often hope that organic pathology will be found that will lead to rapid surgical resolution and obviate the need for further uncomfortable psychologic evaluation. Insufficient preoperative consideration of the psychologic and behavioral dimensions of the illness only serves to set the physician up for experiencing feelings of futility and to set the patient up for having feelings of disappointment and rejection.
Treatment Plans
Having surrendered the idea that one single cause for the pain will be discovered by his evaluation, the thoughtful clinician is well prepared for the idea that treatment will need to be multifaceted. The list of medications suggested will not necessarily be novel, but the informed and caring approach with which the list is designed for the individual will increase the treatment's efficacy. Several examples will suffice: appropriate antimicrobials for bacterial, fungal, or trichomonal vaginitis, together with intermittent use of acidic vaginal gels and other vaginal hygiene measures to promote regrowth of normal flora; careful prescription of vegetable laxatives, high-fiber diets, fluids, and exercise for constipation; flexion exercises for low back muscle tension; relaxation training and/or biofeedback for recognizable muscle tension discomfort; and laparoscopic lysis of adhesions or treatment of endometriosis.
Extensive clinical research with low back pain sufferers has given rise to new treatment philosophies that may well prove applicable to the pelvic pain victim. Fordyce and Sternbach, among others, have developed behaviorally structured “pain wards,” which systematically reinforce “well behaviors” and avoid rewarding “pain behaviors” in any way.37,38 Pain medications are given in elixir form on a regular, around-the-clock basis, not on a pain-contingent basis, that is, not in response to pain complaints. As treatment progresses, the analgesic dose in the elixir is decreased without the patient being aware of the rate of reduction in dose. Concomitant individual psychotherapy, group therapy, family therapy, and treatment of sexual dysfunction are undertaken as part of the treatment plan, and continued medical/surgical evaluation and treatment are included. Recent changes in reimbursement for medical services have caused significant financial problems for many of these units.
Various components of this philosophy can be handled on an outpatient basis, especially with the help of appropriately informed and supportive family members, but it is more difficult.
SUMMARY
The patient with pain presents the doctor with a formidable challenge. The physician must appreciate the complex nature of pain itself and understand the effects of chronic pain on psychologic functioning, as well as the possible manifestation of psychopathology as a symptom of pain. Physicians must avoid making a cause-and-effect judgment about the relationship between psychologic states and pain, however. Such judgments are extraordinarily difficult to make and often detrimentally affect the therapeutic doctor-patient relationship. Finally, physician and patient alike must remain open to new understanding about the pain problem as clinical surveillance continues. The rapidly expanding field of pain research will no doubt provide us with more effective therapies in the future.
REFERENCES
Merskey H: Pain terms: A list with definitions and notes on usage, IASP Subcommittee on Taxonomy. Pain 6: 249, 1979 |
|
Melzack R: The Puzzle of Pain. New York, Basic/Harper Torchbooks, 1973 |
|
Noordenbos W: Pain. Amsterdam, Elsevier Press, 1959 |
|
Mendell LM, Wall PD: Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibers. Nature 206: 97, 1965 |
|
Bonica JJ: The Management of Pain. Philadelphia, Lea & Febiger, 1953 |
|
Melzack R, Wall PD: Pain mechanisms: A new theory. Science 150: 971, 1965 |
|
Melzack R, Dennis SG: Neurophysiological foundations of pain. In Sternbach RA (ed): The Psychology of Pain. New York, Raven Press, 1978 |
|
Chamberlain J: Gynaecological aspects of the acute abdomen. Ann R Coll Surg Engl 45: 174, 1969 |
|
Renaer M, Guzinski GM: Pain in gynecologic practice. Pain 5: 305, 1978 |
|
Sarrel PM, Steege JF, Maltzer M, Bolinsky D: Pain during sex response due to occlusion of the Bartholin gland duct. Obstet Gynecol 62: 261, 1983 |
|
Lamont JA: Vaginismus. Am J Obstet Gynecol 131: 632, 1978 |
|
Novak J: Nature and treatment of vaginismus. Urol Cutan Rev 52: 128, 1948 |
|
Fordney DS: Dyspareunia and vaginismus. Clin Obstet Gynecol 21: 205, 1978 |
|
Abarbanel AR: Diagnosis and treatment of coital discomfort. In LoPiccolo J, LoPiccolo L (eds): Handbook of Sex Therapy, pp 241–260. New York, Plenum Press, 1978 |
|
Hengl MR et al: A new perspective on dysmenorrhea: The role of prostaglandin inhibitors. J Reprod Med 25 (Suppl 4): 191, 1980 |
|
Licten EM, Bombard J: Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. J Reprod Med 32: 37, 1987 |
|
Candiani GB, Fedele L, Vercellini P et al: Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: A controlled study. Am J Obstet Gynecol 167: 100, 1992 |
|
Tjaden B, Schlaff WD, Kimball A, Rock JA: The efficacy of presacral neurectomy for the relief of midline dysmenorrhea. Obstet Gynecol 76: 89, 1990 |
|
March CM, Israel R: Intrauterine adhesions secondary to elective abortion, hysteroscopic diagnosis and management. Obstet Gynecol 48: 422, 1976 |
|
Westrom L: Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. Am J Obstet Gynecol 138: 880, 1980 |
|
Christ JE, Lotze EC: The residual ovary syndrome. Obstet Gynecol 46: 551, 1975 |
|
Grogan RH: Reappraisal of residual ovaries. Am J Obstet Gynecol 97: 124, 1967 |
|
Allen WM, Masters WH: Traumatic laceration of uterine support. Am J Obstet Gynecol 70: 500, 1955 |
|
Duncan CH, Taylor HC: A psychosomatic study of pelvic congestion. Am J Obstet Gynecol 64: 1, 1952 |
|
Beard RW, Highman JH, Pearce S et al: Diagnosis of pelvic varicosities in women with chronic pelvic pain. Lancet 2: 946, 1984 |
|
Beard W, Reginald PW, Wadsworth J: Clinical features of women with chronic lower abdominal pain and pelvic congestion. Br J Obstet Gynaecol 95: 153, 1988 |
|
Farquhar CM, Rogers V, Franks S et al: A randomized controlled trial of medroxyprogesterone acetate and psychotherapy for the treatment of pelvic congestion. Br J Obstet Gynaecol 96: 1153, 1989 |
|
Sinaki M, Merritt JL, Stillwell GK: Tension myalgia of the pelvic floor. Mayo Clin Proc 52: 717, 1977 |
|
King PM, Myers CA, Ling FW, Rosenthal RH: Musculoskeletal factors in chronic pelvic pain. J Psychosom Obstet Gynaecol 12 (Suppl): 87, 1991 |
|
Slocumb J: Neurological factors in chronic pelvic pain: Trigger points and the abdominal pelvic pain syndrome. Am J Obstet Gynecol 149: 536, 1984 |
|
Romano JM, Turner JA: Chronic pain and depression: Does the evidence support a relationship? Psychol Bull 97: 18, 1985 |
|
Blumer D, Heilbronn M: Chronic pain as a variant of depressive disease: The pain-prone disorder. J Nerv Ment Dis 170: 381, 1982 |
|
Lindsay P, Wycoff M: The depression-pain syndrome and its response to antidepressants. Psychosomatics 22: 511, 1981 |
|
Bradley JJ: Severe localized pain associated with the depressive syndrome. Br J Psychiatry 109: 741, 1963 |
|
Steege JF, Kaplan DL: The urethral syndrome: Sexual components. Sexuality Disabil 6 (2): 78, 1983 |
|
Gidro-Frank L, Gordon T, Taylor HC: Pelvic pain and female identity. Am J Obstet Gynecol 79: 1184, 1960 |
|
Fordyce WE: Behavioral Methods for Chronic Pain and Illness. St. Louis, CV Mosby, 1976 |
|
Sternbach RA: Pain Patients. New York, Academic Press, 1971 |
|
Castelnuovo-Tedesco P, Kraut BM: Psychosomatic aspects of chronic pelvic pain. Int J Psychol Med 1: 109, 1970 |
|
Beard RW, Belsey EM, Lieberman BA et al: Pelvic pain in women. Am J Obstet Gynecol 128: 566, 1977 |
|
Renaer M, Vertommen H, Nijs P et al: Psychological aspects of chronic pelvic pain in women. Am J Obstet Gynecol 134: 75, 1979 |
|
Engel GL: “Psychogenic” pain and the pain-prone patient. Am J Med 26: 899, 1959 |
|
Suber DA, Massey EW: Pelvic mass presenting as meralgia paresthetica. Obstet Gynecol 53: 257, 1979 |
|
Steptoe PC: Laparoscopy in Gynaecology. Edinburgh, Livingstone, 1967 |