Urinary Tract Infections in Obstetrics and Gynecology
Authors
INTRODUCTION
Urinary tract infections (UTIs) are frequent reasons for primary care physician office visits and account for more than 3.6 million office visits annually.1 When looked at another way, it is estimated that 11.3 million United States women had a presumed UTI for which they took a prescription medication.2 UTIs cost an estimated US$140.00 per case3 and account for $1.6 billion spent annually for outpatient treatment.3 A healthy female with a UTI will, on average, have 6 days of symptoms and 2½ days of restricted activities. New insight into the pathogenesis and genetics of these infections has been elucidated. Rational strategies have been devised for cost-effective treatment of lower UTIs and to change the usual inpatient treatment of UTIs. Fortunately, most UTIs are easily eradicated and for uncomplicated UTIs, there does not appear to be long-term medical consequences. Strategies for managing recurrent infections are highly effective and are presented in this chapter. Also, the management of UTIs in pregnancy and catheter-associated infections are discussed in detail as these problems can present more serious medical sequelae.
Recognizing that UTIs represent a broad spectrum of disease, five groups can be distinguished by pathogens, course, and management.4 This chapter addresses acute uncomplicated UTI, recurrent cystitis, acute uncomplicated pyelonephritis, complicated UTIs (including those in pregnancy), and asymptomatic bacteriuria.
EPIDEMIOLOGY
UTIs are considered to be the most common bacterial infection.5 The prevalence of UTIs in children is 1–5%. The prevalence increases greatly in young sexually active women, up to 20% of whom have acute cystitis each year.6 This prevalence is increased by a previous UTI, and in some populations nearly one-half of women with cystitis had one or more recurrences within 1 year of follow-up.7 Fifty to sixty percent of women report having had a UTI sometime during their life.6
Epidemiologic studies point to five risk factors for the development of acute uncomplicated UTI in young women. Sexual intercourse is a strong risk factor for UTI. It probably introduces bacteria into the introitus, and further from the introitus through the urethra into the bladder, and is thus a risk factor.8 Delayed postcoital voiding is a possible second factor, because bacteria are allowed to remain and proliferate in the bladder. Theoretically, local immune defense mechanisms can overcome a small concentration of recently introduced bacteria, but infection can ensue when bacteria are allowed to grow to large concentrations by delayed voiding. However, data are lacking to prove urination after intercourse is an efficacious strategy to prevent UTI.9 A third factor is a recent UTI, because approximately 27% of women with an initial episode of cystitis have recurrent infection. Some UTIs tend to cluster in time in the same individual from a persistent focus, but 90% of recurrent UTIs appear to be a newly reintroduced infection with new bacteria, occurring months apart.10 Diaphragm and spermicide use, which constitute the fourth risk factor, increase the risk of UTIs by killing H2O2-producing Lactobacillus while having no effect on Escherichia coli. This allows the persistence and overgrowth of E. coli in the vagina. Diaphragm use may also increase the risk by mechanically compressing the urethra. The last risk factor is use of selected antimicrobial agents, which also appears to alter the vaginal microflora and facilitate the survival of uropathogenic E. coli and other urinary tract pathogens in the vagina.
Elderly men and women (particularly the latter) also have problems with UTIs. A positive urine culture was present in 21% of women 65–70 years of age, 23–50% of women older than 80 years of age, 17–33% of older women living at home, and 23–27% of older women residing in a nursing home.11 The prevalence of positive urine cultures was 33% in hospitalized women and 34–50% among those in chronic care hospitals.12, 13 Some of the high prevalence of UTIs in postmenopausal women can be attributed to risk factors such as women with diabetes requiring treatment, poor health, vaginal itching, vaginal dryness, and urge incontinence.14 Recurrent UTIs in postmenopausal women are associated with incontinence, presence of a cystocele, and elevated postvoiding residual urine.15
Asymptomatic bacteriuria (ASB) is usually diagnosed when routine screening urine cultures are performed on patients without symptoms of a UTI. Only in specific conditions are screening and treatment now indicated for ASB. These are addressed later in this chapter. The overall prevalence of ASB is approximated to be 5%, although the prevalence increases with age.16 Other risk factors include parity, diabetes in women, a history of UTI, and lower education.
CAUSATIVE PATHOGENS
Two microorganisms account for the majority of cases of uncomplicated UTI and pyelonephritis. Approximately 80% of acute uncomplicated UTIs are caused by E. coli. Uropathogenic E. coli clones cause the majority of uncomplicated infections and consist of only a few serogroups. Staphylococcus saprophyticus accounts for 5–15% of uncomplicated UTIs, and most infections with S. saprophyticus occur in young women in the spring and summer.10Enterococcus and a variety of other primarily Gram-negative aerobes account for the other 5–10% of UTIs: Proteus mirabilis, Klebsiella species, and Pseudomonas species.4 More than 95% of UTIs are caused by a single bacterial species.
Complicated UTIs are defined as those occurring in a functionally, metabolically, or anatomically abnormal urinary tract or caused by bacteria resistant to antibiotics. Basically, complicated UTIs occur in anyone who is not young, healthy, and non-gravid. In complicated UTIs, E. coli is recovered in only approximately one-third of cases (34.5%); Streptococcal faecalis is recovered in 16%, Staphylococcus epidermidis in 13%, and Proteus mirabilis in 13%. Other bacteria involved in complicated UTIs include Pseudomonas sp. (5%), Staphylococcus aureus (4%), Enterobacter sp. (2.5%), Serratia sp. (1.5%), Streptococcus sp. (1.5%), Acinetobacter sp. (1.5%), Citrobacter sp. (1.0%), Providencia sp. (0.25%), Morganella morganii (0.25%), and Candida sp. (0.25%).17
Most elderly patients are categorized into the complicated UTI group. However, if age older than 65 years is the only risk factor, the bacterial pathogens differ only slightly from those in young women. E. coli predominates (approximately 70%), S. saprophyticus is rarely present, and P. mirabilis constitutes 10% of cases, whereas other Gram-negative bacilli (Pseudomonas, Klebsiella, Citrobacter, Enterobacter, and Serratia species) account for approximately 20% of cases.18
Among women older than 65 years with complicated UTIs, the pathogens change more markedly. E. coli is a less frequent pathogen (approximately 40%), but the other Gram-negative bacilli listed previously are more frequent (40%), and P. mirabilis occurs in about 10%.18 Symptomatic UTIs in patients older than age 65 years are associated with more than one organism in 16% of cases.18 Women in nursing homes with symptomatic UTIs also have more antibiotic-resistant bacteria, and up to 30% have infections with mixed bacteria.
Anaerobic bacteria, lactobacilli, streptococci (not including enterococci), and S. epidermidis are general urinary contaminants that are common in the introitus and are introduced into clean-catch urine specimens from the introitus but do not represent infectious agents in the urine.
Patients with acute uncomplicated pyelonephritis may be mildly ill with symptoms of acute uncomplicated UTI accompanied by mild flank pain, or they may be severely ill with fever, marked flank pain, and septicemia. E. coli causes more than 80% of cases of acute uncomplicated pyelonephritis in young women. For that reason, mildly ill patients with minimal flank pain can be treated with oral therapy as outpatients with careful follow-up.
PATHOGENESIS
UTIs usually arise from fecal bacteria that colonize the anterior urethra and/or vaginal introitus. These bacteria gain entry to the urinary tract by ascending into the urethra and bladder. Hematogenous seeding from bacteremia (usually S. aureus) or lymphatic spread is a rare cause of UTIs. Unresolved infectious foci in the kidney or previous infection from renal calculi may seed the bladder.
Community-acquired UTIs usually result from a retrograde ascent of bacteria in the external urethral meatus and/or vaginal introitus to the bladder. The proximity of the urethra to the vagina and rectum allows fecal flora (with coliforms such as uropathogenic E. coli) to colonize the periurethral area of women. Women with recurrent UTIs have colonization of the vaginal and urethral areas with the uropathogen before the onset of infection.16 The relatively short urethra of women in comparison with men and the mechanical effect of sexual intercourse facilitates movement of bacteria into the bladder and explains the 50-times greater UTI rate in women than in men and the connection between UTIs and sexual activity.
A critical step in colonization and subsequent infection of the lower urinary tract is the ability of bacteria to adhere to vaginal epithelial and urethral and bladder urothelial cells. Pathogenic E. coli possess pili or fimbriae, which are long, filamentous protein surface appendages. Pili have adhesion antigens and promote adherence to receptors on epithelial cells.19 Because pili are not always essential for the adherence of bacteria, it remains unclear how epithelial cells vary in their susceptibility to attachment of bacteria.
Several studies have investigated bacterial adherence to uroepithelial cells. Strains of E. coli from women with pyelonephritis adhered to epithelial cells in much greater number than did strains of E. coli from those with asymptomatic bacteriuria or strains from feces.19 Larger numbers of bacteria adhere to urothelium or buccal cells of women with recurrent UTIs than in women without UTIs.20, 21 These later data indicate a genetic susceptibility to UTIs determined by adherence of bacteria to uroepithelial cells. A genetic predisposition to UTIs is also suggested by findings of an increased risk of UTI and increased epithelial binding of E. coli in women who are nonsecretors of blood group antigens than in those who do secrete these antigens.22, 23 Two glycolipids isolated from the nonsecretor epithelial cells had binding sites for bacteria that appear to explain the increased risk of infection in nonsecretors.
Once attachment to the urothelium occurs, bacterial virulence factors other than adhesion come into play. Uropathogenic E. coli have virulence factors not present in nonuropathogenic clones. Most uropathogenic E. coli produce hemolysin (which may be important in cell damage and bacterial growth), may produce siderophores like aerobactin, produce increased K1 capsular antigen that protects bacteria from phagocytosis, and release endotoxins. E. coli may alter its behavior and invade macrophages, becoming an opportunistic intracellular organism.24 It is even possible that these hidden E. coli are able to cause recurrent infection and evade antibiotic therapy. Even without these mechanisms, an abundant reservoir of bacteria is present to colonize the periurethral area and cause recurrent ascending UTIs. In healthy women, pyelonephritis is almost always caused by bacteria with virulence factors.
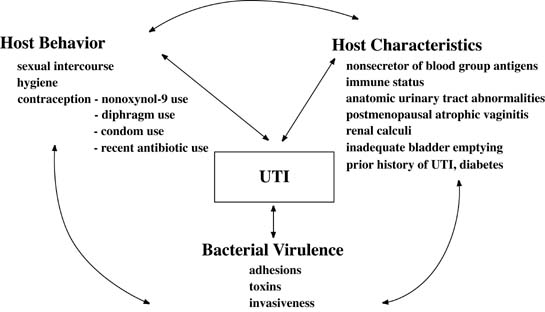
Several host defense mechanisms protect against infection. The flushing and diluting effects of urine and voiding help clear the bladder of bacteria. Bacteria are inhibited in urine, which is acidic and has a high urea level and high osmolality. The renal cortex is resistant to infection, whereas the renal medulla is very susceptible, because high urine osmolality inhibits leukocyte migration and phagocytosis. A summary of potential interactions between behavior, host characteristics, and bacterial virulence factors are shown in Figure 1. A small inoculum of bacteria into the bladder usually does not cause infection, but a large inoculation of bacteria, particularly fimbriated virulent uropathic strains in nonsecretor women, leads to UTI. Additionally, use of nonoxynol-9, male condoms, and particularly nonoxynol-9 coated condoms,25 poor personal hygiene, and frequency of sexual intercourse contribute to the increased susceptibility to UTIs.
|
Two more recently identified risk factors are age at first UTI and UTI history in the mother.26 Postmenopausal women not receiving hormone replacement therapy can develop vaginal pH and microflora changes that lead to recurrent UTIs (see Treatment). Lastly, women of any age are at increased risk for UTI after antibiotic use.4
CATEGORIES OF URINARY TRACT INFECTIONS AND CLINICAL MANIFESTATIONS IN ADULT WOMEN
A broad spectrum of anatomic levels and clinical manifestations of UTI that can be categorized separately (Table 1) are useful in choosing appropriate antibiotic therapy. Tables 2 and 3 present a guide for differentiating acute pyelonephritis, acute uncomplicated UTI, urethritis, and vulvovaginitis.
Acute uncomplicated UTI refers to a bacterial infection of the bladder that causes the abrupt onset of severe symptoms in a non-pregnant woman with a normal urinary tract and no underlying medical complications. Typical symptoms include internal dysuria, urgency, frequency, and suprapubic pain. Hematuria can occur. The spectrum of pathogens is quite narrow and predictable in acute uncomplicated UTI.
Table 1. Categories of urinary tract infection
| Acute uncomplicated cystitis |
| Recurrent cystitis |
| Acute uncomplicated pyelonephritis |
| Complicated urinary tract infection |
| Asymptomatic bacteriuria |
Table 2. Differential diagnosis of dysuria syndromes: history
| Dysuria | Onset | |||||||
| Internal | External | Acute | Subacute | Vaginal discharge or odor, pruritus, external lesions | New sexual partner, multiple sexual partners, with sexually transmitted disease | Frequency, irgency, hematuria, suprapubic pain, diaphragm dse | Fever, chills, sweats, nausea, vomiting | |
| Acute uncomplicated pyelonephritis | ± | − | + | − | − | − | ± | + |
| Acute uncomplicated cystitis | + | − | + | − | − | − | + | − |
| Urethritis caused by sexually transmitted disease | ||||||||
| Herpes simplex virus | + | ± | − | + | + | + | ± | − |
| Nesseria gonorrhoeae | + | − | + | − | + | + | ± | − |
| Chlamydia trachomatis | + | − | − | + | + | + | ± | − |
| Vulvovaginitis (bacterial vaginosis, trichomoniasis, candidiasis, genital herpes simplex) | − | + | − | + | + | − | − | − |
| Noninflammatory dysuria (trauma, irritant, allergy) | ± | ± | ± | ± | − | − | − | − |
(Adapted from Johnson JR, Stamm WE: Diagnosis and treatment of acute urinary tract infections. Infect Dis Clin North Am 1:773, 1987)
Table 3. Differential diagnosis of dysuria syndromes: physical examination
| Vaginal or cervical discharge, vulvar lesions | Suprapubic tenderness | Flank tenderness, fever | |
| Acute uncomplicated pyelonephritis | − | ± | + |
| Acute uncomplicated cystitis | − | ± | − |
| Urethritis caused by sexually transmitted disease | |||
| Herpes simplex virus | + | − | − |
| Nesseria gonorrhoeae | + | − | − |
| Chlamydia trachomatis | + | − | − |
| Vulvovaginitis (bacterial vaginosis, trichomoniasis, candidiasis, genital herpes simplex) | + | − | − |
| Noninflammatory dysuria (trauma, irritant, allergy) | − | − | − |
(Adapted from Johnson JR, Stamm WE: Diagnosis and treatment of acute urinary tract infections. Infect Dis Clin North Am 1:773, 1987)
Recurrent cystitis refers to repeated infections with different organisms or strains (usually of E. coli) with resolution of infection between clinical episodes. Recurrent cystitis must be distinguished from persistent cystitis. Persistent cystitis is an infection that never completely resolves, and cultures remain positive for the same organism and strain.
The definition of complicated UTI has expanded over time. Originally, it referred to infections complicated by urologic disorders such as urinary calculi or abnormal anatomy of the urinary tract. These disorders remain in this category; however, the definition has evolved to include any underlying condition that promotes UTI; leads to persistence, recurrence, or treatment failure; or leads to infection with unusual or multiple antibiotic-resistant organisms. Functional abnormalities include detrusor hyperreflexia in patients with cerebrovascular accidents, hypotonic bladder that impedes complete bladder emptying in patients with multiple sclerosis, spinal cord injury, diabetes, or outlet obstruction that leads to elevated postvoid residual urine volumes or necessitates prolonged catheterization. Pelvic floor relaxation with a large cystocele can lead to poor emptying. Indwelling catheters and intermittent self-catheterization also lead to UTIs.
Other patient groups that are at increased risk for complicated UTI include infants, pregnant patients, all elderly patients (because pathogens differ), and patients with diabetes mellitus or AIDS/HIV.27 Females with diabetes are at increased risk for not only UTIs but also more severe complications of pyelonephritis, emphysematous cystitis, and emphysematous pyelonephritis. Recent urologic instrumentation or surgery complicates a UTI as well. Recognizing host conditions that complicate UTIs is important because antibiotic resistance occurs more often, response to treatment is less rapid, and additional infectious complications occur more readily. The clinical symptoms are often more gradual in onset and more nonspecific than those in acute uncomplicated UTI.
Acute pyelonephritis is a clinical term used to describe the syndrome of acute illness with fever, chills, and low-back or flank pain and tenderness combined with bacteriuria. Lower urinary tract symptoms present in acute uncomplicated UTI also often occur. Other systemic symptoms include abdominal pain, nausea, vomiting, and malaise. The clinical syndrome of upper UTI probably represents an acute infectious inflammatory process evolving in the renal pelvis, parenchyma, or both. Renal parenchymal involvement is implicated when white blood cell casts are present in the urine.
Asymptomatic bacteriuria (ASB) refers to colonization of the urine with bacteria of significant quantity to be consistent with infection, but without producing symptoms of an acute UTI or pyelonephritis.
DIAGNOSIS
In healthy, non-pregnant adult females, UTI symptoms alone (dysuria, frequency, lower abdominal tenderness or urgency) are sufficiently reliable to confirm the diagnosis of UTI over 90% of the time. However, it is important to illicit the symptom of vaginal discharge, as vaginitis, urethritis and sexually transmitted diseases must be excluded. In certain circumstances, rapid urine tests can be used (described later in this section).
For complicated UTIs, a midstream urine culture remains the standard diagnostic tool. Suprapubic aspiration avoids potential contamination of the midstream urine collection, but it is usually reserved for research studies. Urethral catheterization also reduces contamination, but it causes patient discomfort, requires staff time, and thus is costly. The midstream, clean-catch urine collection technique remains the most used method of specimen collection. Careful instruction of specimen collection lessens contamination from introital bacteria. Women should be instructed to wash with an aseptic solution, start to void, and to catch midstream urine for culture.
Cultures that recover more than 105 bacteria per mL of voided urine have been considered evidence of UTI.28 Approximately one-fourth of women presenting with symptoms suggestive of acute uncomplicated UTI show no bacterial growth on urine culture.29 However, approximately one-third of women with acute uncomplicated UTI have midstream urine bacterial counts between 102 and 104 colony-forming units per mL. Acute pyelonephritis can also occur with lower bacterial counts in voided specimens. Thus, among acutely symptomatic women, recovery of 102 colony-forming units per mL or more of a single uropathogen can be used to establish diagnosis of a UTI.30, 31 Not all laboratories culture bacteria to this standard.
Because culture results are not available for 24–48 hours and cultures are not necessary for many acute, uncomplicated UTIs, the practitioner can use rapid diagnostic tests that are less sensitive and specific than culture to rapidly begin effective treatment. Microscopic urinalysis is the most accurate means of detecting pyuria, and the most valuable rapid test. The use of a hemocytometer to count leukocytes and detect pyuria in unspun voided urine is a highly sensitive indicator of UTI in women with acute uncomplicated UTI.32 Ten or more leukocytes per microliter are considered abnormal. Demonstration of pyuria in centrifuged urine is less accurate because of a lack of specificity from leukocytes in contaminating vaginal material. Pyuria is a less sensitive and less specific indicator in complicated UTIs. Microscopy can also detect hematuria, bacteria, leukocyte casts, and red blood cell casts. Hematuria, present in 40% to 60% of episodes of acute uncomplicated UTI, is a highly specific but not a sensitive indicator of UTI. Microscopic bacteriuria viewed on unspun, Gram-stained urine is specific for UTI caused by high (105 cfu/mL) bacterial counts but lacks sensitivity for infection with lower (102–104 cfu/mL) colony counts. White blood cell casts are diagnostic of upper urinary tract infection.
Urine dipstick tests are quick and readily available in most clinical settings. Unfortunately, leukocyte esterase activity is not as sensitive as microscopy or as specific as culture. Practitioners should be aware of the potential for false-positive dipstick tests for leukocyte esterase in patients already receiving antibiotics.33 The nitrite test depends on the ability of bacteria to reduce nitrates to nitrites, which are normally absent in urine. The nitrite test is specific but has only 50–75% of the sensitivity of urine culture. The nitrate test is fairly sensitive and specific for detecting large (>105 cfu) of Enterobacteriaceae. False positive dipsticks can occur if the patient has taken phenazopyridine, which turns the urine color.
Urine cultures should be performed when the diagnosis of UTI is uncertain or complicating factors are present. Initial urine cultures are indicated in patients with diabetes, symptoms lasting longer than 7 days, catheter-related infection, recent UTI, pregnancy, functional or anatomic abnormality of the urinary tract, immunosuppression, recent urinary tract instrumentation, and age older than 65 years. However, women with symptoms of acute uncomplicated UTI should receive one of the rapid tests to detect pyuria or bacteriuria and started on treatment without a culture, because the bacteria that cause acute uncomplicated UTI and their antimicrobial susceptibilities are so predictable. In women with symptoms of acute uncomplicated UTI, pretreatment cultures did not predict the outcome and were considered unnecessary.
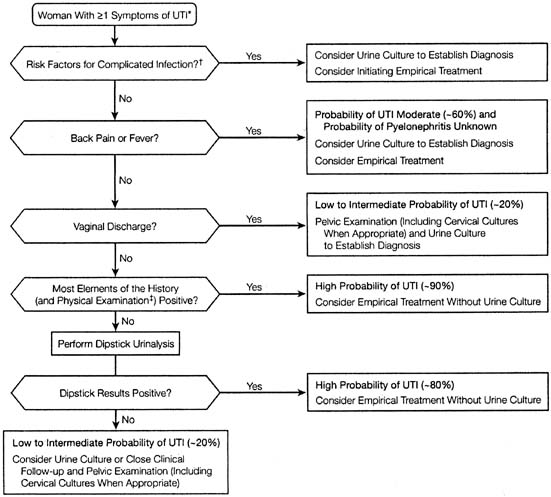
A detailed review article analyzing the diagnostic accuracy of the clinical assessment for acute uncomplicated UTI found that specific symptoms and, particularly, combinations of symptoms increase the probability of UTI diagnosis substantially. In fact, symptoms of dysuria and frequency without vaginal discharge or irritation raised the probability of UTI to more than 90%. An algorithm was proposed (Fig. 2).34
Radiologic evaluation with intravenous pyelography (IVP) and cystoscopy are not required in most women with a UTI. These tests should be reserved for women with a history of childhood UTIs, relapsing UTIs, history suggestive of calculi or obstruction, or infection with painless hematuria.
TREATMENT
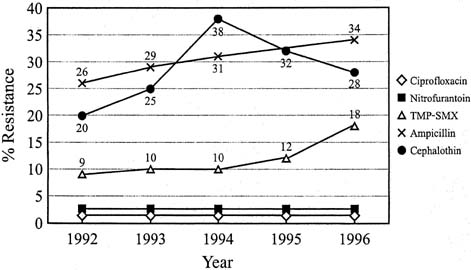
Antibiotic treatments are chosen after consideration of success rate in relation to duration of treatment, toxicity, effects on vaginal and bowel flora, antibiotic resistance, and cost. The recommended duration of treatment for acute uncomplicated UTI has varied from single-dose therapy to the traditional 7- to 10-day course. Single-dose therapy can be used but results in lower cure rates (approximately 60%) and more recurrences than do 3-day regimens.35, 36 Three-day antibiotic regimens have emerged as the preferred management because they are often as efficacious as 7- to 10-day courses for acute uncomplicated UTI, produce fewer side effects, and thus are associated with better patient compliance.37 In 1999, the Infectious Diseases Society of America (IDSA) published evidence-based treatment guidelines.37 A 3-day course of trimethoprim-sulfamethoxazole is more effective and less expensive than 3-day courses of oral nitrofurantoin, amoxicillin, or cefadroxil in treatment of acute uncomplicated UTI.38 Trimethoprim alone can be used for patients with sulfa allergies.39 Amoxicillin and nitrofurantoin for 3-day treatment were associated with a high rate of recurrent infections (67%) and treatment failure (61%). Trimethoprim-sulfamethoxazole is probably more effective because it eradicates E. coli from the rectum and perineum better than amoxicillin, nitrofurantoin, or cefadroxil.39 Unfortunately, resistance to trimethoprim-sulfamethoxazole now approaches 18% to 22% in some areas of the United States, making it less attractive as a first-line agent. (Fig. 3).40 A recent study of E. coli outpatient urinary isolates showed resistance to ampicillin at 37.7%, TMP-SMX 21.3%, nitrofurantoin 1.1%, ciprofloxacin 5.5% and levofloxacin 5.1%.41
Trimethoprim-sulfamethoxazole-resistant E. coli strains showed resistance to nitrofurantoin and fluoroquinolones at 1.9% and 9.5%, respectively,42 and makes them or fosfomycin potentially better first-line agents in certain regions (Table 4). Nitrofurantoin therapy is recommended for 5-day therapy. Fluoroquinolone therapy for 7 days or longer should be reserved for patients with complicated UTIs. Fluoroquinolones are also useful for cases of recurrent infections, treatment failures, patients with allergies to the other antibiotics, and documented antibiotic-resistant organisms.
Table 4. Antimicrobial agents for urinary tract infection
| Agent | Dosing schedule | Uncomplicated UTI dose (mg) | Special points |
| TMP-SMX* | q 12 h | 160/800 × 3 d | Increasing resistance |
| Not effective for Enteroccoccus or Pseudomonas | |||
| Sulfa allergy | |||
| Trimethoprim | q 12 h | 100 mg × 3 d | Not contraindicated with sulfa allergy |
| Megaloblastic anemia caused by folate deficiency. Can cause rash. | |||
| Nitrofurantoin | q 12 h macrocrystal/monohydrate | 100 mg × 5 d | Not useful in pyelonephritis |
| Little resistance | |||
| Not active against Pseudomonas or Proteus | |||
| Major side-effects are GI or CNS | |||
| Fluoroquinolones | |||
| Norfloxacin | q 12 h | 400 mg × 3 d | |
| Ofloxacin | q 12 h | 200 mg × 3 d | |
| Levofloxacin+ | one daily | 250 mg × 3 d | |
Ciprofloxacin+ | q 12 h | 100–250 mg × 3 d | Ciprofloxacin XR 500 mg of 24 hrs x 3d |
| Lomefloxacin | once daily | 400 mg × 3 d | |
| Gantifloxacin | once daily | 200 mg × 3 d | |
| Fosfomycin | once | 3 g × one dose | Less effective than 3-day regimens of TMP-SMX or fluoroquinolones |
| Cefpodoxime | q12 h | 100 mg x 3–7 days | Limited data |
+ Drugs of choice for treating UTI |
*Trimethoprim–sulfamethoxazole
DIf there is known antimicrobial resistance ≥ 20% the IDSA recommends 3-day fluoroquinolone treatment
Acute uncomplicated UTI symptoms usually resolve with antibiotics within 1–3 days, although only study reported 6 days of symptoms. Phenazopyridine (200 mg orally three times a day for 2 days) works as a urinary analgesic and may give symptom relief quickly. Patients should be warned it discolors the urine (orange). If the woman has urinary incontinence, it can permanently stain undergarments and clothes. Phenazopyridine is not for long-term use. Increasing oral fluids has been suggested for flushing out bacteria, but has not been studied and may dilute urine and affect antibiotic concentration.
Young women with acute uncomplicated pyelonephritis and mild symptoms can be treated as outpatients with oral antibiotics.43 A 7- to 14-day course of oral antibiotics with trimethoprim-sulfamethoxazole or a fluoroquinolone is appropriate if nausea or vomiting are not present and adequate compliance and follow-up take place. In a double-blind randomized study of 254 premenopausal women, clinical improvement or resolution of symptoms occurred in 96% of women after 7 days of ciprofloxacin and in 83% after 14 days of trimethoprim-sulfamethoxazole.44 The greater efficacy of both bacteriologic and clinical cure rates with ciprofloxacin were caused by trimethoprim-sulfamethoxazole resistance in infecting organisms.
Most patients with pyelonephritis require hospital admission. Hospital admission is necessary for an uncomplicated episode of acute pyelonephritis with nausea, vomiting, or suspected sepsis. Patients with complicated infection related to a catheter, urologic surgery, or renal tract abnormality also require hospitalization. The IDSA guidelines also addressed acute pyelonephritis in women.37 Parenteral therapy or oral therapy with fluoroquinolones is the first-line agent for moderate to severe pyelonephritis and can be initiated until culture results are available. The traditional approach to therapy with ampicillin or an extended-spectrum cephalosporin plus an aminoglycoside is an alternative, but the increased resistance of E. coli to ampicillin makes this a second-line therapy. If the infecting organism is known to be Gram-positive, the IDSA recommends ampicillin/sulbactam with or without an aminoglyside.37 The clinical illness with pyelonephritis usually abates within 48 to 72 hours of the onset of therapy. Oral therapy can be started once the patient is afebrile and able to take oral fluids. A total of 14 days of antibiotic therapy is indicated for pyelonephritis to prevent recurrence.45 Persistent symptoms after 72 hours of antibiotic therapy usually require further investigation with ultrasonography or computed tomography to investigate for urinary tract obstruction or abnormalities or to detect renal abscess. Complicated cases of pyelonephritis may require longer courses of therapy and urologic consultation.
Treatment of asymptomatic bacteriuria offers no benefit for most healthy, adult, nonpregnant women. Some studies have suggested increased mortality rates in elderly patients with bacteriuria, particularly those who are hospitalized,46 but these patients often had multiple confounding variables, including catheterization.47 Generally, treatment of asymptomatic bacteriuria is not necessary even in elderly women.48 ASB frequently resolves without treatment and has no long-term medical consequences. ASB is three-times as common in diabetic patients as in women without diabetes mellitus. A recent double-blind placebo-controlled study showed treatment of ASB in diabetic women did not reduce the complications including the number of symptomatic UTIs or hospitalizations.49
However, screening of and treatment for asymptomatic bacteriuria should be performed in women before urologic surgery, in pregnant women, and in patients with neutropenia or renal transplant. Clinical trials in patients with diabetes mellitus, spinal cord injury, indwelling urethral catheters and elderly nursing home residents all showed no benefit with treatment of asymptomatic bacteriuria.50 The 2008 U.S. Preventive Services Task Force reaffirmed their recommendation that pregnant women continue to need screening for asymptomatic bacteriuria, but not other adults (including diabetics).51
Many women have infrequent episodes of acute uncomplicated UTI, and approximately 3% experience recurrences often enough to consider prophylactic treatment. Recurrent UTIs occur by at least two different mechanisms. The most common is a new infection or re-infection from the bowel and vaginal reservoir. These patients often have a cluster of infections over a short time period and then have infection-free intervals of 6–12 months.52 A less common mechanism of relapse occurs from persistent infection with the same bacteria and strain, usually at close intervals. Relapsing infections also may be associated with a complicating factor such as urinary tract abnormalities or calculi. There is no evidence that recurrent UTIs pose risks for future renal disease or hypertension without other urologic abnormalities.
Sometimes factors associated with recurrent UTIs can be avoided or altered. Sexual intercourse is an independent risk factor for UTI. Voiding immediately after intercourse may reduce the incidence if intercourse is temporally related to infections although this technique is unproven.53 Other studies have not shown any particular voiding habit lessens or alters the risk of UTIs. Changing from a diaphragm and spermicide or condoms to a different form of contraception that does not include nonoxynol-9 may be effective in preventing infection. Three antibiotic prophylactic treatment regimens are effective to reduce recurrent infections. First, if reinfection correlates with sexual intercourse, a single-dose antibiotic postcoital strategy can be used.54 Second, self-start intermittent therapy with either single-dose or 3-day therapy is a reasonable option for women with relatively infrequent infections (two to three times per year). Trimethoprim-sulfamethoxazole is an inexpensive choice of antibiotic for either of these strategies. The third strategy consists of continuous low-dose prophylaxis with trimethoprim, trimethoprim-sulfamethoxazole, cephalexin, nitrofurantoin, or norfloxacin and is highly effective in suppressing infections for women with frequent recurrences.55, 56 Therapy is usually continued for 6 months, but infections often recur after suppressive therapy is discontinued. All are used in lower dosages than for treatment.
Estrogen vaginal cream is highly effective for reducing recurrences in postmenopausal women with recurrent infections.57, 58 Topical estrogen therapy lowers vaginal pH, restores atrophic mucosa, and increases lactobacilli while reducing vaginal colonization with Enterobacteriaceae from 67% to 31%.39 UTIs were reduced from 5.9 to .5 episodes per patient-year, probably as a result of alterations in vaginal flora.58 The Estring vaginal ring, used in a randomized, open, parallel study of postmenopausal women, prolonged the time to UTI recurrence and decreased the number of recurrences per year compared with controls.59
Cranberries and cranberry juice appear to reduce the incidence of UTIs because of proanthocyanidins inhibiting adherence of p-fimbriated E. coli to the urothelium.60, 61, 62, 63, 64 In elderly women, 300 mL daily of cranberry juice taken orally reduced the frequency of bacteriuria with pyuria in a randomized, double-blind, placebo-controlled trial.65 Data in younger women suggest possible efficacy of cranberry juice in preventing UTIs.66, 67 While the use of cranberries is interesting, there are no definite studies for treating UTIs with cranberries and this is not recommended. Cranberry use for preventing recurrent UTIs is more promising but definitie studies on the type of cranberry (juice versus pill) are still not available.
There are some preliminary data on nonantimicrobial agents for preventing UTIs. An anti-E. coli vaccine has been tried in women with recurrent UTIs.68 Vaginal probiotics containing lactobacillus have been tested to restore normal vaginal flora to reduce recurrent UTIs.69
URINARY TRACT INFECTIONS IN PREGNANCY
UTIs are the most common medical complication of pregnancy. The prevalence of asymptomatic bacteriuria in pregnancy is 4–7%.70, 71, 72 Acute cystitis occurs in 1.3%73 and acute pyelonephritis in 1% of pregnant women.74 The risk of asymptomatic bacteriuria increases with increasing parity, lower socioeconomic status, increased age, sexual activity, sickle cell trait or disease, diabetes, and previous UTI. The etiologic agents of UTIs in pregnancy are the same as those of acute uncomplicated UTI in nonpregnant women. E. coli causes 80–90% of UTIs in pregnancy, and P. mirabilis, K. pneumoniae, S. saprophyticus, and enterococci are the usual isolates from the remainder of patients with uncomplicated infections. In pregnancy, group B β-hemolytic streptococci are also potential urinary tract pathogens.75
During pregnancy, the female urinary tract undergoes profound physiologic changes that facilitate the development of acute pyelonephritis. As early as 7 weeks' gestation, a physiologic hydroureter develops from the hormonal effects of progesterone, which causes ureteral atony, increased ureteral volume, and urinary stasis. Mechanical obstruction from the enlarging uterus also delays ureteral emptying function. Physiologic bladder changes, including decreased smooth muscle tone, increased capacity, and incomplete emptying, predispose pregnant women to vesicoureteral reflux and ascending pyelonephritis. The urinary tract returns to normal size and function by 6 weeks' postpartum.
An astoundingly high rate of 30–40% of untreated pregnant women with bacteriuria have pyelonephritis76 in contrast to less than 1% of those without bacteriuria in early pregnancy.77 Asymptomatic bacteriuria is the most significant factor in the development of pyelonephritis during pregnancy.76 Treatment of asymptomatic bacteriuria reduces the risk of pyelonephritis to that of a nonbacteriuric population. Early pregnancy screening and treatment of asymptomatic bacteriuria prevents 70–80% of cases of pyelonephritis.
Pyelonephritis is a potentially serious threat for both the mother and the fetus. Pyelonephritis during pregnancy can lead to multiorgan dysfunction with hemodynamic (shock), hematologic (anemia, thrombocytopenia), renal (decreased creatinine clearance), and pulmonary (adult respiratory distress syndrome) effects. Approximately 1% of pregnant women with acute pyelonephritis have respiratory insufficiency from alveolar capillary leak. Most cases result in tachycardia, increased respiratory rate, and dyspnea.78 Prompt chest x-ray and arterial blood gas analyses are indicated. Although most patients respond to increased oxygen, occasionally full-blown adult respiratory distress requiring intubation and intense ventilatory support will ensue.78
Acute pyelonephritis in the late second and third trimesters is associated with a 20–50% risk of preterm delivery. Thus, acute pyelonephritis in the mother can lead to severe neonatal consequences from early preterm delivery. Potential mechanisms involved in preterm labor include maternal bacteremia and endotoxemia, which result in systemic levels of inflammatory mediators, including cytokines, which in turn lead to myometrial activity.
Whether asymptomatic bacteriuria alone leads to premature labor or other adverse perinatal outcomes in women without symptomatic pyelonephritis have remained a controversial subject. Kass initially reported a two-fold to three-fold increase in the neonatal death rate and prematurity rate of pregnant patients with asymptomatic bacteriuria;76 these findings were supported by others.79 However, not all studies have found an association between bacteriuria and poor perinatal outcome.80, 81 Using meta-analysis, evidence combined from multiple studies suggests that asymptomatic bacteriuria is associated with both preterm delivery and low birth weight.82Table 5 summarizes studies that associate bacteriuria with low birth weight, and Table 6 summarizes studies that associate bacteriuria with preterm delivery. In the meta-analysis, nonbacteriuric pregnant patients had a 35% reduction in the risk of low birth weight (relative risk [RR] = 0.65) and a 50% reduction in the risk of preterm delivery (RR = 0.50) compared with patients with untreated asymptomatic bacteriuria.82
Table 5. Meta-analysis of cohort studies of low-birth-weight (LBW) patients with or without bacteriuria (n = 17)
| Author | Untreated bacteriuria LBW/total | Nonbacteriurics LBW/total | RR | 95% CI |
| Kass | 26/95 | 88/1000 | 0.3 | 0.2–0.5 |
| Layton | 10/59 | 10/112 | 0.5 | 0.2–1.3 |
| Kincaid-Smith and Bullen | 12/56 | 25/500 | 0.2 | 0.1–10.5 |
| Stuart et al. | 20/88 | 83/729 | 0.5 | 0.3–0.8 |
| Little | 13/141 | 360/4735 | 0.8 | 0.5–1.4 |
| Wilson et al. | 14/137 | 592/6079 | 0.95 | 0.6–1.6 |
| Dixon & Brant | 4/71 | 68/1309 | 0.9 | 0.3–2.5 |
| Savage et al. | 21/98 | 67/496 | 0.6 | 0.4–1.0 |
| Patrick | 7/75 | 37/500 | 0.8 | 0.4–1.8 |
| Robertson et al. | 16/204 | 120/1980 | 0.8 | 0.5–1.3 |
| Wren | 14/90 | 140/3009 | 0.3 | 0.2–0.5 |
| Elder et al. | 15/122 | 20/107 | 1.5 | 0.8–3.0 |
| Brumfitt | 21/178 | 32/477 | 0.6 | 0.3–0.98 |
| Bryant et al. | 2/32 | 4/44 | 1.5 | 0.3–7.9 |
| Sleigh et al. | 7/100 | 7/100 | 1.0 | 0.35–2.8 |
| Norden and Kilpatrick | 17/114 | 14/109 | 0.9 | 0.4–1.7 |
| Whalley | 26/176 | 21/176 | 0.8 | 0.5–1.4 |
Typical RR = 0.65; 95% CI, 0.57–0.75.
RR, relative risk; CI, confidence interval.
(Adapted from Romero R, Oyarzun E, Mazor M, et al. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol 1989;73:576.)
Table 6. Meta-analysis of cohort studies of preterm delivery (PTD) patients with or without bacteriuria (n = 17)
| Author | Untreated bacteriuria PTD/total | Nonbacteriurics PTD/total | RR | 95% CI |
| Layton | 4/63 | 9/113 | 1.3 | 0.4–4.1 |
| Patrick | 7/75 | 21/500 | 0.5 | 0.2–1.1 |
| Robertson et al. | 13/204 | 62/1980 | 0.5 | 0.3–0.9 |
| Wren | 15/90 | 204/3009 | 0.5 | 0.2–0.7 |
Typical RR = 0.5; 95% CI 0.365–0.698.
RR, relative risk; CI, confidence interval.
(Adapted from Romero R, Oyarzun E, Mazor M, et al. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol 1989;73:576.)
In Table 7, meta-analysis of low birth weight in bacteriuric patients administered placebo versus antibiotic therapy in randomized clinical trials showed a highly significant reduction of low-birth-weight infants with antibiotic treatment of bacteriuria (RR = 0.56).82 Women with chronic renal disease have been shown to have low-birth-weight infants.83 However, few bacteriuric pregnant patients, even those with pyelonephritis, have chronic renal disease. The link between bacteriuria and prematurity remains largely unexplained. A greater frequency of amniotic fluid infection has been noted in women with bacteriuria compared with women without infection.84, 85 It is possible that cervicovaginal uropathic microorganisms can colonize and infect both the urinary and amniotic fluid compartments in the same patient, causing both UTI and premature labor and delivery. In support of this theory, one report of antibiotic treatment of women with group B streptococcal bacteriuria reduced the incidence of preterm delivery.86
Table 7. Meta-analysis of randomized clinical trials of low-birth-weight (LBW) patients with bacteriuria
| Author | Placebo LBW/total | Antibiotic LBW/total | RR | 95% CI |
| Kass | 26/95 | 6/84 | 0.3 | 0.1–0.6 |
| LeBlanc and McGanity | 6/27 | 7/101 | 0.3 | 0.1–0.9 |
| Kincaid-Smith and Bullen55 | 12/56 | 9/61 | 0.7 | 0.3–1.6 |
| Little | 13/141 | 10/124 | 0.9 | 0.4–2.0 |
| Savage et al. | 21/98 | 7/93 | 0.4 | 0.2–0.8 |
| Wren | 14/190 | 4/83 | 0.3 | 0.1–0.9 |
| Elder et al. | 16/122 | 17/107 | 1.2 | 0.6–2.4 |
| Brumfitt | 21/178 | 18/235 | 0.7 | 0.4–1.2 |
Typical RR = 0.56; 95% CI 0.43–0.73.
RR, relative risk; CI, confidence interval.
(Adapted from Romero R, Oyarzun E, Mazor M, et al. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol 1989;73:576.)
Women should be screened for bacteriuria at their first prenatal visit. A midstream clean-catch voided urine specimen for culture remains the standard for initial urine testing. Measures to detect leukocyte activity, either by urine dipstick test or by urinalysis, are not sufficiently sensitive to identify asymptomatic bacteriuria.87 Urine Gram stain is effective in detecting asymptomatic UTIs as a rapid same-day test.87 However, urine culture is still needed to guide therapy.
The treatment goals of UTI in pregnancy are to eradicate the infection with the shortest possible course of antibiotics and to maintain sterile urine for the remainder of pregnancy. Sulfonamides, nitrofurantoin, ampicillin, cephalexin, and nalidixic acid are all considered safe for use in pregnancy. Sulfonamides should not be used at term or during labor because of the theoretical risk of kernicterus in the infant. The sulfonamide can displace bilirubin from plasma albumin binding sites and lead to hyperbilirubinemia, but this risk is low with the presently available short-acting sulfonamides. Trimethoprim, a dihydrofolate reductase inhibitor, has been shown to have toxic effects in experimental animals, although no teratogenicity has been reported in pregnant humans. Tetracycline is contraindicated in pregnancy because of potential maternal liver damage and permanent discoloration of the deciduous teeth of the child. Fluoroquinolones are to be avoided because cartilage abnormalities have been reported in animal studies. Fosfomycin has not been shown to have teratogenic effects in animal studies, but few well-controlled studies have been performed in pregnant humans. Fosfomycin is rated category B during pregnancy.
A 3–7-day course of antibiotic therapy is recommended to treat asymptomatic bacteriuria or uncomplicated acute UTI in pregnancy.88 Single-dose therapy for asymptomatic bacteriuria in pregnancy is less effective than in a comparable nonpregnant group, with failure rates of 30%.89 Three-day courses of antibiotic therapy are effective if only one episode of infection has occurred and there are no complicating factors or evidence of renal involvement. It is unclear whether shorter courses of therapy eradicate the vaginal reservoir of uropathogenic bacteria in pregnancy. While 33% of bacterial strains causing acute uncomplicated UTI and pyelonephritis show resistance to amoxicillin, it is still useful in pregnancy when the infecting organism is know to be susceptible, and for Gram-positive organisms like group B streptococci.
Patients should have a follow-up urine culture 1 week after completing therapy and monthly urine cultures thereafter. From 16–23% of women will have recurrent asymptomatic bacteriuria during the same pregnancy.80 For persistent asymptomatic bacteriuria, nitrofurantoin 50–100 mg nightly is recommended for the remainder of pregnancy and 12 weeks postpartum for suppressive therapy. Ampicillin 250 mg twice per day would be a reasonable alternative. Antibiotic suppression is also recommended after two acute episodes of UTI in the same pregnancy.
Pregnant women with acute pyelonephritis should be hospitalized. Sepsis is reported to occur in 10% of pregnant women with pyelonephritis. Intravenous fluids, urine output monitoring, and parenteral antibiotics are generally recommended. Urine and blood cultures should be obtained in all pregnant women suspected of having pyelonephritis before initiating antibiotic therapy. Twenty to 30% of organisms that cause pyelonephritis are resistant to amoxicillin and first-generation cephalosporins in vitro; therefore, these antibiotics are no longer used alone for empirical treatment.
Parenteral β-lactams like ceftriaxone (not first generation cephalosporins), gentamicin (with or without ampicillin), aztreonam, or trimethoprim-sulfamethoxazole are recommended for initial therapy until culture and sensitivity results are available. Response to parenteral antibiotics is usually rapid, with 85% of pregnant women becoming afebrile within 48 hours and 97% becoming afebrile within 4 days of initiating treatment. Intravenous antibiotics should be continued until the patient is afebrile and can tolerate oral medications. A 10- to 14-day course of antibiotics should be completed. If the patient does not have improvement after 4 days of therapy, resistant organisms, obstructive uropathy, and perinephric abscess should be considered.
The incidence of recurrent acute pyelonephritis during the same gestation is 10–18%.90, 91 Without suppressive antimicrobial therapy, 60% of acute pyelonephritis patients had a recurrent episode in one study.90 Patients maintained on suppressive antibiotics had a 2.7% recurrence rate for the duration of the pregnancy.90
Antimicrobial agents used for suppression include nitrofurantoin 50–100 mg, sulfisoxazole 500 mg, or cephalexin 250–500 mg at bedtime daily. An acceptable alternative to suppressive therapy is evaluation every 2 weeks in the clinic, with urine cultures and prompt treatment of asymptomatic bacteriuria. Recurrent pyelonephritis occurred in 7% of the group on suppressive antibiotics and 8% of the group monitored with frequent urine cultures.92
Long-term follow-up of women with bacteriuria in pregnancy reveals that 25–35% have evidence of renal infection or urinary tract abnormalities including vesicoureteral reflux, IVP evidence of chronic pyelonephritis, or recurrent bacteriuria.93, 94
CATHETER-ASSOCIATED INFECTIONS
Nosocomial UTIs account for 40% of hospital-acquired infections and are the most common nosocomial infection.95 Approximately 80% of these infections are catheter-associated or occur after other types of urologic instrumentation. With sterile closed collecting systems, bacteriuria occurs in 10–25% of catheterized patients.96, 97, 98 In addition, 1–3% of patients with catheter-associated UTIs have bacteremia,99, 100 and Gram-negative bacteremia in hospitalized patients has been associated with a three-fold increase in mortality.101
The duration of catheterization is the most important risk factor for the development of bacteriuria.102, 103, 104 Host factors associated with an increased risk of infection related to catheters or instrumentation include female gender, older age, and increasing severity of underlying illness. Other independent risk factors linked to catheter-associated bacteriuria include lack of antibiotic use, diabetes mellitus, absence of use of a urinometer, microbial colonization of the drainage bag, indications for catheter other than surgery or output measurement, abnormal serum creatinine, and errors in catheter care.105
Aerobic Gram-negative rods account for most catheter-associated UTIs. The individual species vary from hospital to hospital and by location in the hospital (i.e., intensive care versus surgical ward). Overall, Enterobacteriaceae, pseudomonads, and enterococci account for most of the infections. Candidal infections may be isolated in the urinary tract, particularly when antibiotics are in use and in the intensive care unit setting.
Bacteria may take different routes to enter the catheterized urinary tract. The first route is direct introduction of periurethral microorganisms at the time of catheterization or instrumentation. With proper sterile catheterization technique, this should be infrequent, and based on time of onset, most catheter-associated infections arise later during the period of catheterization. A second route is the intraluminal route, in which microorganisms have entered the collecting bag or some junction in the collecting tube and ascend into the bladder.103, 106 With the adoption of sterile closed drainage systems, the incidence of infection via the intraluminal route has been markedly reduced. The third route appears to be responsible for the majority of catheter-associated infections and involves migration of periurethral organisms along the catheter-urethral interface into the bladder.107, 108 Organisms adhere to catheter surfaces and embed in a biofilm layer that consists of host factors such as fibrin, fibronectin, and a exopolysaccharide material. Approximately two-thirds of women with catheter-associated infections have had previous urethral and rectal colonization with their infecting strain,107 similar to uncatheterized women with UTI.106
Preventive guidelines have been published by the United States Centers for Disease Control and Prevention.109 Avoiding catheterization is often not possible but should be considered. Indwelling catheters are often used to manage incontinence in hospitalized women, but incontinence can often be managed without a catheter. The Agency for Health Care Policy and Research (AHCPR) Clinical Practice Guideline for Urinary Incontinence in Adults stresses the use of alternatives to indwelling catheterization.109 As mentioned previously, use of a closed sterile drainage system can significantly reduce the incidence of catheter-associated infections. Decreasing the duration of catheterization, using clean intermittent catheterization, inserting catheters aseptically, and maintaining gravity drainage of urine are all useful in preventing indwelling catheter infections. Efforts to reduce periurethral colonization of organisms with povidone–iodine application twice daily have not resulted in decreased rates of UTI,110 except in women with previous meatal colonization who were treated with polyantimicrobial ointment twice daily.111 Although antimicrobial-impregnated catheters have not been shown to reduce infection rates,112 silver-impregnated catheters were shown to reduce UTIs in some studies,113, 114 but did so only in limited subgroups in another study.115 Antibiotic irrigation of the bladder with polymyxin–neomycin has not been shown to reduce infection and in fact has resulted in infections with more resistant organisms.116 Systemic antibiotic agents have been shown to decrease the likelihood of bacteriuria with short-term catheterization.97, 110, 111, 116 However, with long-term catheterization, antimicrobial use promotes the emergence of resistant strains.117
When considering treatment of nosocomial UTIs, it is important to realize that approximately 50% of patients who have bacteriuria while catheterized are asymptomatic; no treatment is needed until the catheter is removed unless a urease-producing organism is present.118 Because urease-producing organisms such as Proteus species can lead to urinary tract calculi, they should be eradicated during catheterization. Treatment may be considered in certain high-risk asymptomatic patients as well (e.g., renal transplant recipient, immunocompromised patients, those at high risk for sepsis). Symptomatic nosocomial UTIs from catheterization can range from mild cystitis to acute pyelonephritis or septicemia.118 Symptomatic infections should be treated, and it is preferable to remove the catheter and begin appropriate antimicrobial therapy. If catheterization is still required, consider clean, intermittent catheterization or placing a new, sterile closed drainage system, because bacteria may be sequestered in a biofilm on the catheter surface. Antibiotic therapy is based on culture and sensitivity results. Seven to 10 days of therapy is generally adequate. Yeast isolation does not necessarily require treatment. Removal of the catheter results in the clearance of candiduria in approximately one third of patients. Repeated isolations or symptomatic infections can be treated with 2-day continuous amphotericin B bladder irrigation119 or oral fluconazole.
The National Surgical Infection Prevention Project has reported a retrospective review of 35,904 Medicare inpatients undergoing major surgery. A total of 86% of patients had perioperative indwelling catheters and 50% had catheters longer than 2 days. These patients were twice as likely to develop UTIs (9.4% versus 4.5%, p = 0.004).120 Among patients with UTIs, approximately 3.6% will develop bacteremia. Length of post-operative urinary catheterization is becoming a target for quality improvement initiatives.
REFERENCES
Schappert SM. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1997. Vital and health statistics. Series 13. No. 143. Atlanta: National Center for Health Statistics, Nov 1999 (DHHS publication no. (PHS) 2000-1714.) |
|
Foxman B, Barlow R, D'Arcy H et al: Urinary tract infection: Self-reported incidence and associated costs. Ann Epidemiol 10:509-515, 2000 |
|
Johnson JR, Stamm WE: Urinary tract infections in women: Diagnosis and treatment. Ann Intern Med 111:906, 1989 |
|
Stamm WE, Hooton TM: Management of urinary tract infections in adults. N Engl J Med 329:1328, 1993 |
|
Nicolle LE: Epidemiology of urinary tract infections. Infect Med 2001;18:153-162, 2001 |
|
Foxman B: Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 113 Suppl 1A:5S, 2002 |
|
Ikaheimo R, Siitonen A, Heishanen T et al: Recurrence of urinary tract infection in a primary care setting: Analysis of a one year follow-up of 179 women. Clin Infect Dis 22:91, 1996 |
|
Nicolle LE, Harding GKM, Preiksaitis J et al: The association of urinary tract infections with sexual intercourse. J Infect Dis 146:579, 1982 |
|
Hooton TM, Scholes D, Hughes JP et al: A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med 335:468, 1996 |
|
Jordan PA, Irvani A, Richard GA et al: Urinary tract infection caused by Staphylococcus saprophyticus. J Infect Dis 142:510, 1980 |
|
Boscia JA, Abrutyn E, Kaye D: Asymptomatic bacteriuruia in elderly persons: Treat or do not treat? Ann Intern Med 106:764, 1987 |
|
Nicolle LE: Urinary tract infection in the institutionalized elderly. Infect Dis Clin Pract 1:68, 1992 |
|
Kaye D: Urinary tract infections in the elderly. Bull NY Acad Med 56:209, 1980 |
|
Brown JS, Vittinghoff E, Kahaya AM et al: Urinary tract infections in postmenopausal women: effect of hormone therapy and risk factors. Obstet Gynecol 98:1045-1052, 2001 |
|
Raz R, Gennesin Y, Wasser J et al: Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis 30:152-156, 2000 |
|
Stamey TA, Timothy MM, Millar M et al: Recurrent urinary infections in adult women: The role of introital Enterobacteriaceae. Calif Med 115:1, 1971 |
|
Naber KG: Use of quinolones in urinary tract infections and prostatitis. Rev Infect Dis 11:(S5):S1321, 1989 |
|
McCue JD: Urinary tract infections in the elderly. Pharmacotherapya 1993;13:51S, Armitage. Infect Dis Clin Pract 2:260, 1993 |
|
Svanborg-Eden C, Hanson LA, Jodal U et al: Variable adherence to normal human urinary tract epithelial cells of Escherichia coli strains associated with various forms of urinary tract infection. Lancet 2:490, 1976 |
|
Svanborg-Eden C, Jodal U: Attachment of E. coli to urinary sediment epithelial cells from urinary tract infection-prone and healthy children. Infect Immun 26:837, 1979 |
|
Schaeffer AJ, Jones JM, Duncan JK: Association of in vitro E. coli adherence to vaginal and buccal epithelial cells with susceptibility of women to recurrent urinary tract infections. N Engl J Med 304:1062, 1981 |
|
Kinane DF, Blackwell CC, Brettle RP et al: ABO blood group, secretor state, and susceptibility to recurrent tract infection in women. Br Med J 285:7, 1982 |
|
Lamberg H, Adergren B, Leffler H et al: Influence of blood group on the availability of receptors for attachment of uropathogenic E. coli. Infect Immun 51:919, 1986 |
|
Baorto DM, Gao Z, Malaviya R et al: Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature 389:636, 1997 |
|
Handley MA, Reingold AL, Shiboski S et al: Incidence of acute urinary tract infection in young women and use of male condoms with and without nonoxynol-9 spermicides. Epidemiology 13:431-436, 2002 |
|
Scholes D, Hooton TM, Roberts PL et al: Risk factors for recurrent urinary tract infection in young women. J Infect Dis 182:1177-1182, 2001 |
|
Schonwald S, Begovac J, Skerk V: Urinary tract infections in HIV disease. Int J Antimicrob Agents 11:309-311, 1999 |
|
Kass EH: Asymptomatic infections of the urinary tract. Trans Assoc Am Phys 69:57, 1956 |
|
Gallagher DJA, Montgomerie JZ, North JDK: Acute infections of the urinary tract and the urethral syndrome in general practice. Br Med J 1:622, 1965 |
|
Stamm WE, Counts GW, Running KR et al: Diagnosis of coliform infection in acutely dysuric women. N Engl J Med 307:463, 1982 |
|
Stamm WE: Quantitative urine cultures revisited (editorial). Eur J Clin Microbiol 3:279, 1984 |
|
Stamm WE: Measurement of pyuria and its relationship to bacteriuria. Am J Med 75:(S1B):53, 1983 |
|
Beer JH, Vogt A, Neftel K et al: False positive results for leucocytes in urine dipstick test with common antibiotics. Br Med J 313:25, 1996 |
|
Bent S, Nallamothu BK, Simel DL et al: Does this woman have an acute uncomplicated urinary tract infection? JAMA 287:2701-2710, 2002 |
|
Norrby SR: Short-term treatment of uncomplicated lower urinary tract infections in women. Rev Infect Dis 12:458, 1990 |
|
Hooton TM, Stamm WE: Management of acute uncomplicated urinary tract infection in adults. Med Clin North Am 75:339, 1991 |
|
Warren JW, Abrutyn E, Hebel JR et al: Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis 29:745, 1999 |
|
Hooton TM, Winter C, Tiu F et al: Randomized comparative trial and cost analysis of 3-day antimicrobial regimens for treatment of acute cystitis in women. JAMA 273:41, 1995 |
|
Nicolle LE: Urinary tract infection: traditional pharmacologic therapies. Am J Med 113:(SA):35S-44S, 2002 |
|
Gupta K, Sahm DF, Mayfield D et al: Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: a nationwide analysis. Clin Infect Dis 33:89-94, 2001 |
|
Zhanel GG, Hisanaga TL, Laing NM et al: Antibiotic resistance in Escherichia coli outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents 27(6):468-75, 2006 |
|
Karlowky JA, Thornsberry C, Jones ME, Sahm DF: Susceptibility of antimicrobial-resistant urinary Escherichia coli isolates to fluoroquinolones and nitrofurantoin. Clin Infect Dis 2003;36(2):183-7. Epub 2003 |
|
Stamm WE, McKevitt M, Counts GW: Acute renal infection in women: Treatment with trimethoprim-sulfamethoxazole or ampicillin for two or six weeks. A randomized trial Ann Intern Med 106:341, 1987 |
|
Talan DA, Stamm WE, Hooton TM et al: Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women: a randomized trial. JAMA 283:1583, 2000 |
|
Ronald AR: Optimal duration of treatment for kidney infection (editorial). Ann Intern Med 106:467, 1987 |
|
Nordenstam GR, Brandberg CA, Oden AS et al: Bacteriuria and mortality in an elderly population. N Engl J Med 314:18, 1986 |
|
Dontas AS, Kasviki-Charvati P, Papanayiotou PC: Bacteriuria and survival in old age. N Engl J Med 304:939, 1981 |
|
Abrutyn E, Mossey J, Berlin JA et al: Does asymptomatic bacteriuria predict mortality and does antimicrobial treatment reduce mortality in elderly ambulatory women? Ann Intern Med 120:827, 1994 |
|
Harding GKM, Zhanel GG, Nicolle LE, et al: Antimicrobial treatment in diabetes women with asymptomatic bacteriuria. N Engl J Med 347:1576-1583, 2002 |
|
Nicolle LE: Asymptomatic bacteriuria: review and discussion of the IDSA guidelines. Int J Antimicrob Agents 28S:S42-S48, 2006 |
|
Lin K, Fajardo K: Screening for asymptomatic bacteriuria in adults: evidence for the U.S. Preventive Services Task Force Reaffirmation Recommendation Statement. Ann Intern Med 149: W20-24, 2008 |
|
Kraft JK, Stamey TA: The natural history of symptomatic recurrent bacteriuria in women. Medicine 56:55, 1977 |
|
Beisel B, Hale W, Graves RS et al: Does postcoital voiding prevent urinary tract infections in young women. J Fam Pract 51:977, 2002 |
|
Vosti KL: Recurrent urinary tract infections: Prevention by prophylactic antibiotics after sexual intercourse. JAMA 231:934, 1975 |
|
Stamm WE, Counts GW, Wagner KF et al: Antimicrobial prophylaxis of recurrent urinary tract infection. Ann Intern Med 92:770, 1980 |
|
Stamey TA, Condy M, Mihara G: Prophylactic efficacy of nitrofurantoin macrocrystals and trimethoprim-sulfamethoxazole in urinary infections. N Engl J Med 296:14, 1977 |
|
Privette M, Cade R, Peterson J et al: Prevention of recurrent urinary tract infections in postmenopausal women. Nephron 50:24, 1988 |
|
Raz R, Stamm WE: A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med 329:753, 1993 |
|
Eriksen BC: A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol 180:1072, 1999 |
|
Sobota AE: Inhibition of bacterial adherence by cranberry juice: Potential use for the treatment of urinary tract infections. J Urol 131:1013, 1984 |
|
Schmidt DR, Sobota AE: An examination of the antiadherence activity of cranberry juice on urinary and nonurinary bacterial isolates. Microbios 55:173, 1988 |
|
Zafriri D, Ofek I, Adar R et al: Inhibitory activity of cranberry juice on adherence of type 1 and P fimbriated E. coli to eukaryotic cells Antimicrob Agents Chemother 33:92, 1989 |
|
Jepson RG, Craig JC: Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev Jan 23(1):CD001321, 2008 |
|
Howell AB, Foxman B: Cranberry juice and adhesion of antibiotic-resistant uropathogens. JAMA 287:3082-3083, 2002 |
|
Avorn J, Monane M, Gurwitz JH et al: Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA 271:751, 1994 |
|
Kontiokari T, Laitinen J, Jarvi L et al: Dietary factors protecting women from urinary tract infection. Am J Clin Nutr 77:600-604, 2003 |
|
Strothers L: A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol 9:1558-1562, 2002 |
|
Zakri RH, Dasgupta R, Dasgupta P, Khan MS: Preventing recurrent urinary tract infections: role of vaccines. 2008;102(9):1055-6. Epub 2008 Aug 14 |
|
Reid G: The role of cranberry and probiotics in intestinal and urogenital tract health. Crit Rev Food Sci Nutr 42:293, 2002 |
|
Sweet RL: Bacteriuria and pyelonephritis during pregnancy. Semin Perinatol 1:25, 1977 |
|
Whalley PJ: Bacteriuria in pregnancy. Am J Obstet Gynecol 97:723, 1967 |
|
Norton CW, Kass EH: Bacteriuria of pregnancy: A critical appraisal. Annu Rev Med 19:431, 1968 |
|
Harris RE, Gilstrap LC: Cystitis during pregnancy: A distinct clinical entity. Obstet Gynecol 57:578, 1981 |
|
Gilstrap LC, Cunningham FG, Whalley PJ: Acute pyelonephritis in pregnancy: An anterospective study. Obstet Gynecol 57:409, 1981 |
|
Mead PJ, Harris RE: Incidence of group B beta-hemolytic streptococcus in antepartum urinary tract infections. Obstet Gynecol 51:412, 1978 |
|
Kass EH: The role of asymptomatic bacteriuria in the pathogenesis of pyelonephritis. In: Quinn EL, Kass EH, (eds): Biology of Pyelonephritis, pp 399-412. Boston, Little, Brown & Co, 1960 |
|
Kincaid-Smith P, Bullen M: Bacteriuria in pregnancy. Lancet 1:395, 1965 |
|
Cunningham FG, Lucas MS, Hankins GD: Pulmonary injury complicating antepartum pyelonephritis. Am J Obstet Gynecol 156:797, 1987 |
|
Kass EH: Phylonephritis and bacteriuria: A problem in preventative medicine. Ann Intern Med 56:46, 1962 |
|
Whalley PJ, Cunningham FG: Short term versus continuous antimicrobial therapy for asymptomatic bacteriuria in pregnancy. Obstet Gynecol 49:262, 1977 |
|
Whalley P: Bacteriuria of pregnancy. Am J Obstet Gynecol 97:723, 1967 |
|
Romero R, Oyarzun E, Mazor M et al: Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol 73:576, 1989 |
|
Harris RE, Thomas VL, Shelokov A: Asymptomatic bacteriuria in pregnancy: Antibody-coated bacteria renal function, and intrauterine growth retardation. Am J Obstet Gynecol 126:20, 1976 |
|
Naeye RL: Causes of the excessive rates of perinatal mortality and prematurity in pregnancies complicated by maternal urinary tract infection. N Engl J Med 300:819, 1979 |
|
Patrick MJ: Influence of maternal renal infection on the foetus and infant. Arch Dis Child 42:208, 1967 |
|
Thomasen AC, Morup L, Brogaard Hansen K: Antibiotic elimination of group-B streptococci in urine in prevention of preterm labour. Lancet 1:591, 1987 |
|
Bachman JW, Heise RH, Naessens JM et al: A study of various tests to detect asymptomatic urinary tract infections in an obstetric population. JAMA 270:1971, 1993 |
|
Vazquez JC, Villar J: Treatments for symptomatic urinary tract infections during pregnancy. Cochrane Database Syst Rev (3):CD002256,2003 |
|
Harris RE, Gilstrap LC, Pretty A: Single dose antimicrobial therapy for asymptomatic bacteriuria during pregnancy. Obstet Gynecol 59:546, 1982 |
|
Harris RE, Gilstrap LC: Prevention of recurrent pyelonephritis during pregnancy. Obstet Gynecol 44:637, 1974 |
|
Cunningham FG, Morris GB, Mickael A: Acute pyelonephritis of pregnancy: A clinical review. Obstet Gynecol 42:112, 1973 |
|
Lenke RR, Van Dorsten JP, Schifrin BS: Pyelonephritis in pregnancy: A prospective randomized trial to prevent recurrent disease evaluating suppressive therapy with nitrofurantoin and close surveillance. Am J Obstet Gynecol 146:953, 1983 |
|
Zinner SH, Kass EH: Long term (10–14 years) follow-up of bacteriuria of pregnancy. N Engl J Med 285:820, 1971 |
|
Whalley PJ, Martin FG, Peters PC: Significance of asymptomatic bacteriuria detected in pregnancy. JAMA 193:879, 1965 |
|
Haley R, Culver D, White J et al: The nationwide nosocomial infection rate: A new need for vital statistics. Am J Epidemiol 121:159, 1985 |
|
Finkelberg Z, Kunin CM: Clinical evaluation of closed urinary drainage systems. JAMA 207:1657, 1969 |
|
Kunin CM: Detection, Prevention and Management of Urinary Tract Infections, 4th edn. Philadelphia, Lea & Febiger, 1985 |
|
Thorton GF, Andriole VT: Bacteriuria during indwelling catheter drainage: II. Effect of a closed sterile drainage system JAMA 214:339, 1970 |
|
Schaeffer AJ: Catheter-associated bacteriuria. Urol Clin North Am 13:737, 1986 |
|
Kreiger JN, Kaiser DL, Wenzel RP: Urinary tract etiology of blood-stream infection in hospitalized patients. J Infect Dis 148:57, 1983 |
|
Platt R, Polk BF, Murdock B et al: Mortality associated with nosocomial urinary-tract infection. N Engl J Med 307:637, 1982 |
|
Garaibaldi RA, Burke JP, Dickman ML et al: Factors predisposing to bacteriuria during indwelling urethral catheterization. N Engl J Med 291:215, 1974 |
|
Hartstein AL, Garber SB, Ward TT et al: Nosocomial urinary tract infection: A prospective evaluation of 108 catheterized patients. Infect Control 2:380, 1981 |
|
Platt R, Polk BF, Murdock B et al: Risk factors for nosocomial urinary tract infection. Am J Epidemiol 124:977, 1986 |
|
Warren JW: Catheter-associated urinary tract infections. Infect Dis Clin North Am 1:823, 1987 |
|
Garibaldi RA, Burke JP, Britt MR et al: Meatal colonization and catheter-associated bacteriuria. N Engl J Med 303:316, 1980 |
|
Daifuku R, Stamm WE: Association of rectal and urethral colonization with urinary tract infection in patients with indwelling catheters. JAMA 252:2028, 1984 |
|
Wong ES, Hooton TM: Guidelines for prevention of catheter-associated urinary tract infections. Infect Control 2:125, 1982 |
|
Fantl JA, Newman DK, Colling J et al: Urinary Incontinence in Adults: Acute and Chronic Management. Clinical Practice Guideline, no. 2, 1996 Update, AHCPR Publication No. 96–0682. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research 1996 |
|
Burke JP, Garibaldi RA, Britt MR et al: Prevention of catheter-associated urinary tract infections: Efficacy of daily meatal care regimens. Am J Med 70:655, 1981 |
|
Burke JP, Jacobson JA, Garibaldi RA et al: Evaluation of daily meatal care with polyantibiotic ointment in prevention of catheter-associated bacteriuria. J Urol 129:331, 1983 |
|
Butler HK, Kunin CM: Evaluation of polymyxin catheter lubricant and impregnated catheters. J Urol 100:560, 1968 |
|
Lundeberg T: Prevention of catheter-associated urinary-tract infections by use of silver-impregnated catheters [letter]. Lancet 1:1031, 1986 |
|
Schaeffer AJ, Story KO, Johnson SM: Effect of silver oxide/trichloroisocyanuric acid antimicrobial drainage system on catheter-associated bacteriuria. J Urol 139:69, 1988 |
|
Johnson JR, Roberts PL, Olsen RJ et al: Prevention of catheter-associated urinary tract infection with a silver oxide-coated urinary catheter: Clinical and microbiologic correlates. J Infect Dis 162:1145, 1990 |
|
Warren JW, Platt R, Thomas RJ et al: Antibiotic irrigation and catheter-associated urinary tract infections. N Engl J Med 299:570, 1978 |
|
Warren JW, Anthony WC, Hoopes JM et al: Cephalexin or susceptible bacteriuria in afebrile, long-term catheterized patients. JAMA 248:454, 1982 |
|
Meares EM Jr: Current patterns in nosocomial urinary tract infections. Urology 37:9, 1991 |
|
Hsu CCS, Ukleja B: Clearance of candida colonizing the urinary bladder by two-day amphotericin B irrigation. Infection 18:280, 1990 |
|
Wald HL, Ma A, Bratzler DW, Kramer AM: Indwelling urinary catheter use in the postoperative period. Arch Surg 143 (6), 2008 |