Uterine and Placental Blood Flow
Authors
INTRODUCTION
It should be obvious to any student of maternal-fetal medicine that a multiplicity of factors contribute to that intrauterine environment which permits normal growth and development of the fetus and also provides a safety factor for survival under stressful conditions. Aberrations of this environment are often referred to by such terms as uteroplacental insufficiency and placental dysfunction, which are no more than vague approximations of a less than optimal fetal environment. If, however, one considers those elements that must have a significant impact on the fetal environment, placental blood flow (PBF) is a critical factor. This is so not only because maternal blood carries necessary foodstuffs to the fetus and removes waste products throughout pregnancy but also because the quantity of oxygen delivered to the placenta is limited directly by the rate of PBF. Thus, since oxygen deprivation is such a critical determinant of fetal well-being, knowledge of that most important factor controlling oxygen delivery, PBF, is crucial irrespective of other compensatory responses.
In the half century since Barcroft and associates first quantitated uterine blood flow (UBF) in the pregnant rabbit, a significant body of knowledge has developed.1 Although we know the most about UBF and PBF in lower animals, primarily the ovine species, correlative data in subhuman primates and a paucity of observations in women indicate that despite obvious species variations in placentation, general principles of uterine vascular control are quite comparable.
DEVELOPMENT OF THE PLACENTAL CIRCULATION AND HOMEOSTASIS
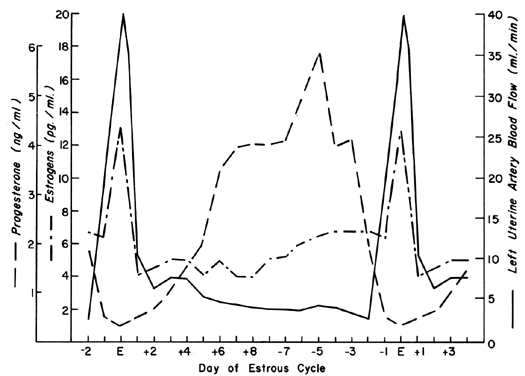
The uterine circulation is exquisitely sensitive to estrogen stimulation, responding with a degree of vasodilatation unparalleled by any other organ of the body to any other stimulus.2 During the ovarian cycle, repetitive patterns of UBF occur reflecting the effect of estrogen secretion and the modulating effects of progesterone. These cyclic patterns are demonstrated most dramatically in animals with short preovulatory cycle phases, such as the cow, sow, and ewe, and are illustrated in Figure 1 with peripheral levels of estrogen and progesterone.3, 4, 5 Following conception in these species, UBF patterns are similar to those in nonpregnant animals until interdigitation of maternal and fetal tissues occurs 17 to 28 days later; then, there is a definitive and progressive increase in UBF that continues until term pregnancy. Since erosion of endometrium by fetal trophoblast occurs much earlier after conception in the primate, it is reasonable to presume that UBF increases earlier in these species, although such responses have never been observed. The overall patterns of total UBF, UBF per unit weight of uterus and its contents, fraction of UBF supplying the placenta, and fetal weight throughout ovine pregnancy are illustrated in Figure 2. The period between 17 and 70 days of gestation is associated with the most rapid changes in UBF and PBF and corresponds with the time of definitive placentation in this species. By mechanisms incompletely understood, blood vessels supplying the placenta progressively dilate during this time, simultaneously causing an absolute increase in total UBF and shunting of this blood from nonplacental to placental tissues.* With subsequent placental maturation, the fraction of UBF supplying the placenta and the absolute amount of PBF increase, but at rates more similar to that of fetal growth; thus, during the last half of pregnancy, UBF per unit weight of uterus and its contents is essentially constant. During this same time, the amount of oxygen extracted from each milliliter of blood is constant and therefore the amount of oxygen delivered per given weight of gravid uterus is also constant.6 Correlative studies in women indicate similar homeostatic patterns from 10 to 40 weeks of gestation and similar oxygen consumption (approximately 10 ml/kg).7 Since women deliver the same amount of oxygen at lower UBF rates than the ewe (~150 versus ~270 ml/kg), it follows that the human placenta is more efficient in this regard. Thus, for fetuses of similar weight, less absolute UBF is required and the pregnancy load on the heart is diminished.
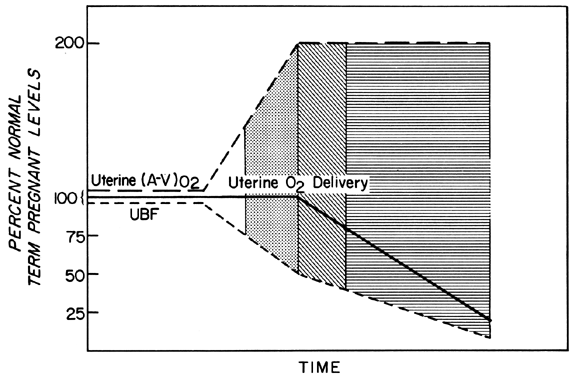
Changes in PBF are accomplished by an ever-increasing vasodilatation of the blood vessels supplying the placenta throughout the course of gestation. However, at any given moment during the second and third trimesters, essentially no further acute vasodilatation of these vessels can occur. Therefore, to maintain constant oxygen delivery during episodes of reduced PBF, increased oxygen extraction must occur (Fig. 3). Based on reported data, it would appear that the limit of increased extraction is twofold. Thus, PBF has to be reduced more than 50% before oxygen delivery is diminished. These considerations assume that the rate of homeostatic PBF was optimal prior to the flow reduction. We know, however, that homeostatic levels may be suboptimal either sub-acutely, as in toxemia, or chronically, as in nephropathies. In such cases, the safety factor, that is, the percent that PBF can be reduced before oxygen delivery is compromised, is lowered. This must always be considered when one evaluates the effect of an acute stimulus on the fetus.
*In man and subhuman primates, the spiral arterioles that will supply the intervillous space (approximately one per placental cotyledon) lose their capillary connections and dilate about tenfold as the result of replacement of the vessel walls by trophoblast. This process may extend to or below the myoendometrial junction, literally turning the spiral arterioles into spiral arteries. Blood flowing through these modified blood vessels into the intervillous space comprises PBF.
In the ovine species, multiple discrete areas of endometrium called caruncles are present in the nonpregnant state. During pregnancy, interdigitation of fetal and maternal tissues occurs in these caruncles, which enlarge to become individual placental cotyledons. Blood flowing to these areas collectively comprises PBF. Although the cotyledonary arterial blood vessels dilate significantly, they contain no trophoblastic elements, maternal-fetal transfer is by a capillary-capillary interface, and no intervillous space is present.
Dynamic Responses of the Uterine Circulation
The blood vessels of the nonpregnant uterus respond similarly to those of any other muscular organ with the exception of their unique reactivity to estrogenic stimulation, their response to local anesthetic agents, and, possibly, their response to prostaglandins (Table 1). During pregnancy, the blood vessels supplying the placenta progressively dilate, achieving a state in which minimal or no further acute dilatation can occur.24 Therefore, stimuli which evoke vasodilatation in the nonpregnant uterus are ineffective after definitive placentation has occurred. However, the placental vessels retain their ability to vasoconstrict as in the nonpregnant state. Since PBF approximates 80% to 90% of total UBF at term pregnancy, total uterine vascular responses will appear as those of the placental vessels. This point is often misinterpreted, and one must remember that the responses of the nonplacental vessels are the same whether pregnancy is present or not.
TABLE 1. Responses of the Nonpregnant Uterine Vasculature*
Vasoconstriction | Vasodilatation |
α-Adrenergic stimulation | β-Adrenergic stimulation |
(dopamine, epinephrine, | |
Acetylcholine and parasympathomimetic agents23 | |
Sympathomimetic agents11 | Adenosines17 |
(vasopressor drugs) | |
Hypoxemia(severe)12 | Cyanide19 |
Local anesthetic agents | Estrogenic substances |
(delayed but prolonged effect)2 | |
Nicotine | Glucosamine |
(α-adrenergic mediation)15 | (? osmotic effect)21 |
| Hypoxemia (mild)12 |
| Ischemia19 |
| Nitroglycerine19 |
| |
| Vasoactive intestinal polypeptide20 |
* The marked dilatation of the blood vessels supplying the placenta and the preponderant distribution of uterine blood flow to the placenta obliterate gravid responses; therefore the effects of vasodilator stimuli during pregnancy are controversial. When tested, nonplacental vascular responses are similar to nonpregnant responses.
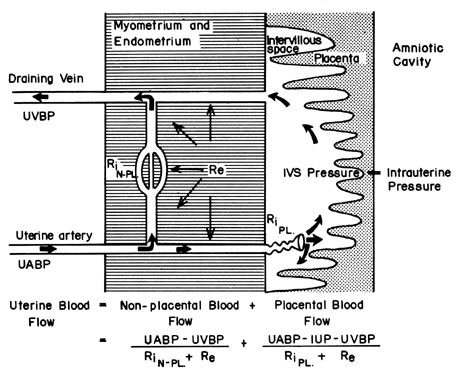
The hemochorial structure of the human placenta adds a unique factor to those that normally control blood flow in other vascular beds. That is, blood traverses the spiral arteries to enter the swamplike intervillous space, perfuses the fetal villi, and then returns to the general circulation by many collecting veins in the basal plate. Effectively, blood leaves normal vascular channels to circulate in a new extravascular space grafted onto the uterus for the duration of pregnancy. Since the intervillous space lies within the uterine cavity and since the placenta is a flexible structure, pressure generated by the contracting myometrium will be transmitted equally to the amniotic cavity and the intervillous space. Thus, a factor extraneous to usual vascular control, myometrial activity, can change intervillous space pressure and influence PBF by its effects on perfusion pressure. A schematic representation of blood flow to the non-placental tissues and to a single cotyledon is shown in Figure 4, along with the formulas pertinent to the control of each. It should be noted that these formulas are only applications of Ohm's law to these individual cardiovascular situations.
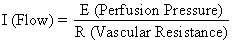
The perfusion pressure delivering blood to nonplacental tissues is the difference between uterine arterial and venous blood pressures. However, the perfusion pressure delivering blood to the intervillous space is the difference between uterine arterial blood pressure and the intervillous space pressure. The latter is best approximated by amniotic fluid pressure (IUP). In a muscular organ, resistance factors will include resistance from reactivity of vascular smooth muscle, or intrinsic resistance (Ri), and the squeeze imparted to blood vessels as they traverse the contracting myometrium, or extrinsic resistance (Re). It should be evident then that uterine contractions can affect PBF by two mechanisms: by increasing Re and by reducing placental perfusion pressure.
In clinical practice, three major characteristics of placental vascular control are important. These include the relationship between perfusion pressure and flow, the responses of the spiral arteries to vasoactive stimuli, and the effects of myometrial contractions. In addition, the unique effects of local anesthetic agents must be appreciated.
Pressure-Flow Relationship
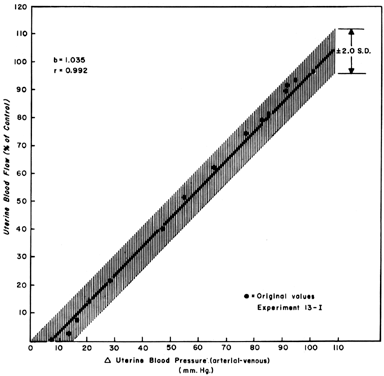
When one observes changes in UBF secondary to reductions in perfusion pressure during myome-trial quiescence, a straight-line relationship with a slope of one can be developed (Fig. 5). This reflects the widely dilated nature of the placental vasculature and indicates that PBF will decrease almost in identical proportion to the decrease in perfusion pressure. Since uterine venous pressure is quite constant under most circumstances, changes in systemic blood pressure (MBP) may be used to approximate PBF changes. That is, a 25% decrease in mean arterial pressure would be expected to cause a 25% decrease in PBF. Such measurements should be made in the lateral decubitus position, however, since pressure of the gravid uterus on the aorta alone has been shown to decrease blood pressure in the pelvic area below that observed in the brachial artery.
Responses to Vasoactive Stimuli
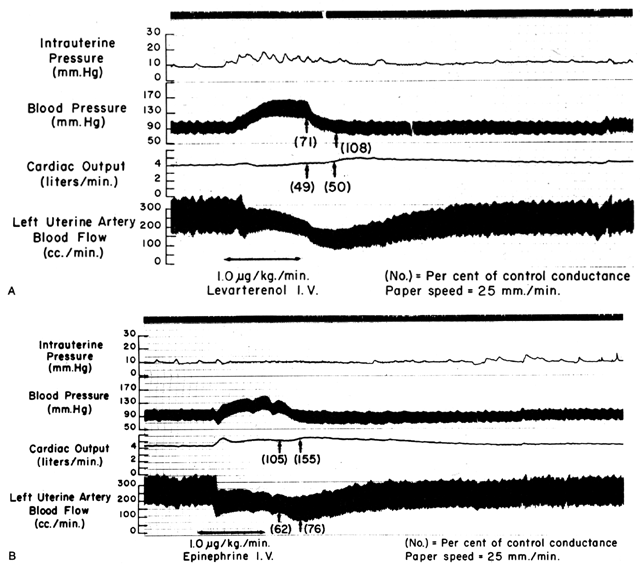
Since the spiral arteries approach maximum dilatation in the resting state, vasodilator agents or stimuli have little or no effect even though receptors for such agents are present. However, the smooth muscles of these vessels are exquisitely sensitive to vasoconstrictor agents or stimuli, more so than most other peripheral vascular beds (Fig. 6). This means that although MPB may increase in response to stimulation by a peripherally acting vasopressor drug such as phenylephrine, the proportionate increase in placental vascular resistance (Ri) is SO much greater that the net effect is a marked decrease in PBF. Such differences in vasoconstrictor sensitivity must be considered whenever a vasopressor drug is indicated. Use of a more centrally acting drug such as ephedrine, although causing a small amount of placental vasoconstriction, will result in a proportionately greater improvement in MBP with an absolute increase in PBF.
Myometrial Contractions
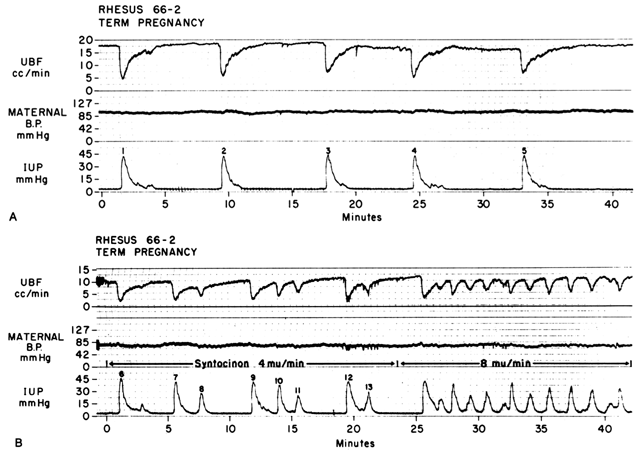
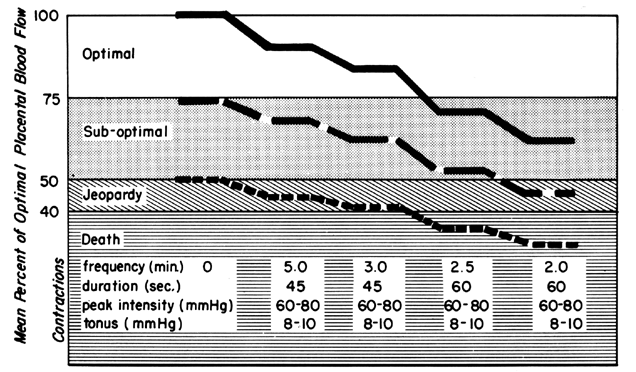
Acting by the two mechanisms discussed above, increasing Re and decreasing perfusion pressure, myometrial contractions decrease PBF in direct proportion to the intensity and duration of each contraction. The relationship is so precise that a tracing of intrauterine pressure is almost an exact inverse image of PBF (Fig. 7). Increasing the frequency of contractions decreases PBF during a given unit of time by decreasing the duration of myometrial diastole, that time when PBF is at homeostatic levels. In addition, should intercontraction tonus be elevated, as in a placental abruption, intercontraction PBF will be proportionately reduced. Radioangiographic studies in subhuman primates and women show that during the acme of myometrial contractions of average intensity, PBF ceases.25 It is evident then that labor is inherently stressful to the fetus, since the mean amount of PBF perfusing the fetal villi per given period of time progressively decreases as the frequency and intensity of uterine contractions increase.
Local Anesthetic Agents
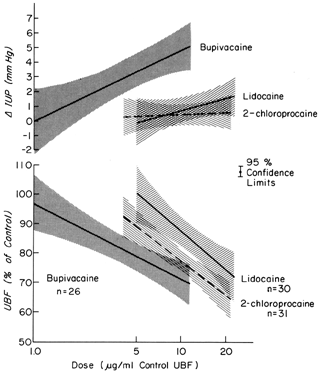
These drugs may exert effects on a vascular bed directly, as after inadvertent intravascular injection, and indirectly, as a result of paralysis of autonomic nerves that maintain normal vascular tonus. In most organs, intravascular injections have no significant effects on vascular resistance. However, the uterine and placental vasculatures respond to such stimuli with significant vasoconstriction. In addition, the myometrium is variably stimulated by such drugs (Fig. 8). Alone or together, these responses diminish PBF. Following paracervical block anesthesia administered during labor, a delayed fetal bradycardia may occur. The best hypothesis to explain the fetal response is that local anesthetic agents are injected close to the uterine arteries and that because of their excellent penetrance, they cross the arterial walls to cause their uterine effects decreasing PBF and causing fetal hypoxia.
CLINICAL APPLICATIONS
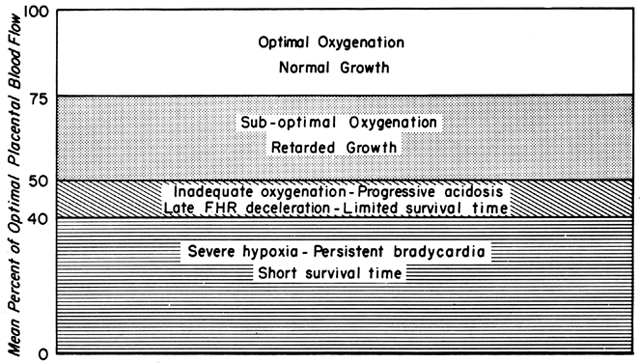
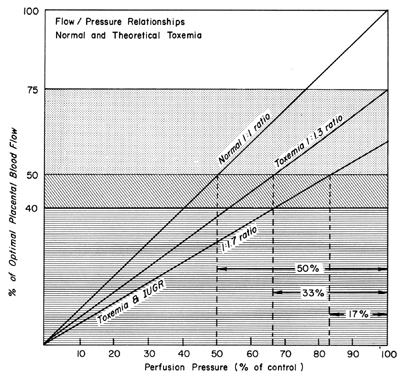
We have seen that all physiologic, pathologic, and iatrogenic stimuli usually decrease PBF and that we have no practical means of increasing PBF above homeostatic levels. Therefore, to optimize the fetal environment, the clinical goal must be to prevent, minimize, or reverse these adverse effects. In addition, the effects of any dynamic stimulus must always be interpreted on the background of the adequacy of the homeostatic levels of PBF prior to the stimulus. A theoretical depiction of this background based primarily on oxygen delivery is shown in Figure 9 and will serve to illustrate the interplay of homeostatic and dynamic factors.
Labor
The effects of labor on PBF at three different levels of prelabor homeostasis are illustrated in Figure 10. This shows mean PBF at four levels of uterine activity and assumes cessation of intervillous space flow for 30 seconds of a 45-second contraction and 45 seconds of a 60-second contraction. Whereas a fetus beginning labor at optimal maternal PBF levels is never jeopardized with respect to oxygen delivery even during a tumultuous labor, that fetus whose mother had suboptimal prelabor PBF could withstand average labor patterns but would have been depressed at birth or possibly stillborn had tumultuous labor occurred. It should be obvious in the third case that without any prelabor safety factor, even the mildest of uterine contractions would be sufficient to cause fetal compromise. This latter category probably describes the circumstances when a positive contraction stress test is evoked.
Regional Anesthesia
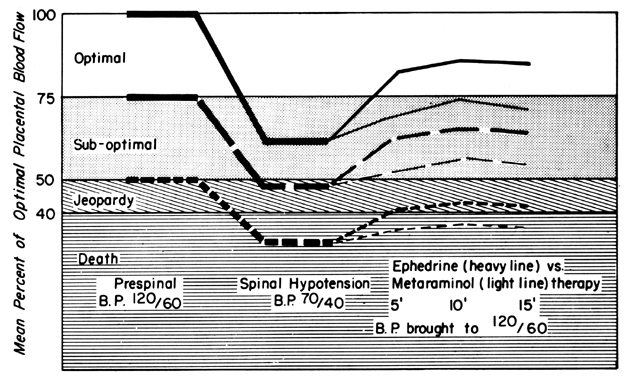
Epidural or spinal anesthesia may cause maternal hypotension by paralysis of sympathetic nerves, peripheral vasodilatation, peripheral pooling, and reduced cardiac return of blood. Since the placental vasculature is normally widely dilated, sympathetic nerve paralysis would have no effect on these vessels. We have then a clinical example of the pressure-flow experiment, and an × percent decrease in MBP would cause an × percent reduction in PBF. This response is illustrated in Figure 11, along with two methods of treating the hypotension: vasopressor drugs and volume replacement. While each restores normotension, their effects on PBF are radically different. Predictably from the preceding discussion, the peripherally acting agent phenylephrine caused no sustained increase in UBF since the degree of induced placental vasoconstriction far exceeded the rise in MBP. Treatment of the underlying pathophysiology, a discrepancy between the capacity and the volume of the vascular system, with volume replacement simultaneously restored MBP and UBF to normal. Sometimes, however, volume replacement is ineffective and a vasopressor agent must be used. Figure 12 illustrates the more favorable PBF effects that can be obtained by the use of centrally acting agents and the critical difference this choice may make depending on the amount of pre-existing reserve PBF.
Hemorrhage
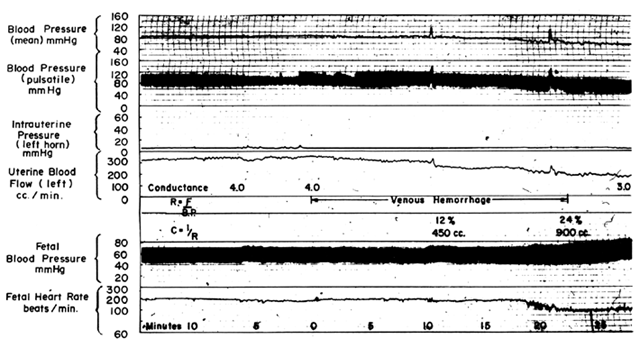
Following a bleed of 10% to 15% of circulating blood volume, MBP usually is maintained within normal limits primarily by peripheral vasoconstriction. The placental vasculature will participate in this generalized vasoconstriction with a reduction in PBF. With further blood loss, the limits of compensation are exceeded and hypotension occurs, adding an additional factor to reduce PBF. If mean blood pressure is decreased 25%, then PBF will be decreased 25% by the pressure-flow mechanism alone plus an additional decrease from the increased intrinsic vascular resistance. These predictable responses are confirmed experimentally in Figure 13. As with hypotension induced by a regional anesthetic block, therapy directed at the underlying problem, volume loss, will improve MBP and PBF simultaneously, and vasopressor agents should be used only as a last resort.
Inhibition of Labor
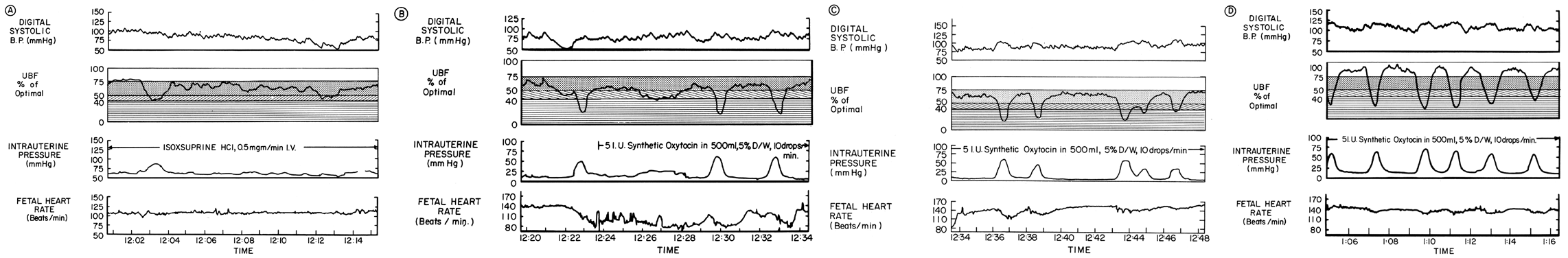
The β-adrenergic drugs used to inhibit labor act by their relaxing effect on myometrial contractility. The central and peripheral effects of these agents cause varying degrees of maternal tachycardia, peripheral vasodilatation, and hypotension. Since the placental vessels are widely dilated normally, no further vasodilatation can occur and we have another clinical example of the pressure-flow experiment, PBF varying in direct proportion to changes in MBP. An example of the interplay of the myometrial and systemic effects of isoxsuprine on PBF in a patient in active labor is shown in Figure 14. During isoxsuprine therapy, uterine contractions almost ceased and systemic blood pressure progressively decreased, causing a similar baseline reduction in PBF (Fig. 14A). Prudently, isoxsuprine was stopped when MBP was halved although the fetal heart rate was still normal. While MBP and PBF were still at low levels, occurrence of a strong uterine contraction further decreased PBF, causing fetal bradycardia (Fig. 14B). The wrong decision for proper management was made at this point due to a failure to recognize that hypotension alone was not stressing the fetus. Rather it was the additional PBF decreases associated with uterine contractions. Proper management would have been to allow normal uterine contractions to recur gradually, anticipating that by that time, MBP and PBF would be back to homeostatic levels. As it occurred, oxytocic stimulation initially caused a sustained contraction that although of low intensity was sufficient to keep PBF at jeopardy levels despite the progressive increase in MBP. As a more normal myome-trial contractile pattern evolved, the cessation of PBF during contractions continued to evoke deceleration patterns (Fig. 14C) until MBP and PBF rose to normal intercontraction levels (Fig. 14D). Fortunately in this patient, homeostatic PBF was within the normal range, so while transient fetal hypoxemic episodes occurred, the safety factor was adequate to permit normal fetal survival.
Hypertensive States
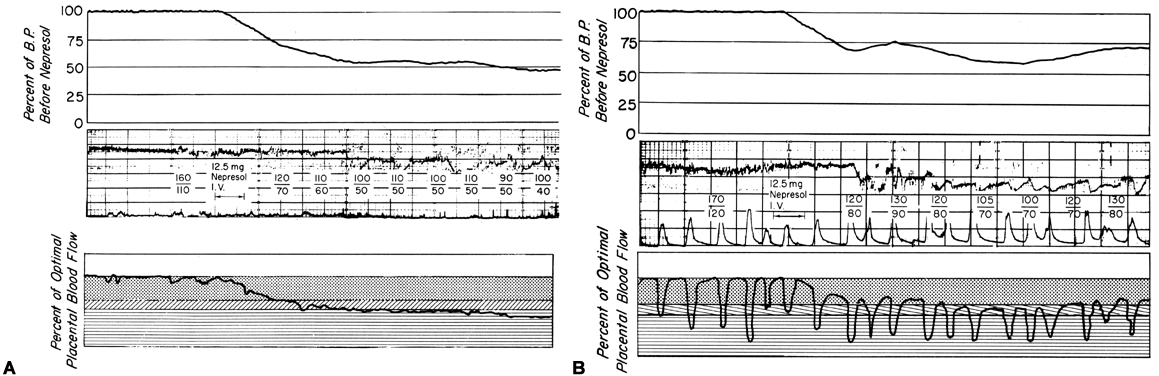
There is considerable evidence that toxemia of pregnancy and chronic cardiovascular-renal diseases are associated with suboptimal levels of homeostatic PBF.26 Presumably, mechanisms responsible for the normal dilatation of spiral arteries supplying the placenta are compromised or inhibited during the definitive placentation process and its subsequent maturation. This could result in spiral arteries with a capacity for further acute dilation or in vessels with walls immobilized by an atherotic process so that further dilation could not occur. In both instances, one would expect that the slope of the pressure-flow curve would be lowered, decreasing progressively as the severity of disease increases. Therefore, an × percent reduction in perfusion pressure would cause a less than × percent decrease in PBF (Fig. 15). Theoretically, an autoregulatory curve is possible that would further minimize PBF decreases during reductions in MBP, but most morphologic studies augur against such vascular reactivity. Irrespective of these considerations, hydralazine, a nonspecific vascular smooth muscle relaxant, is frequently used to control life-threatening maternal hypertension. Since we cannot measure homeostatic PBF and since the effect of hydralazine on the toxemic placental vasculature is unknown at present, prudent clinical management dictates that we must assume the least favorable effects. Those would be a decrease in PBF as MBP decreases according to the pressure-flow relationship and no vasodilatation of the spiral arteries. The end point of therapy should be a tolerable MBP of 150/90 and normal fetal heart rate patterns. This implies constant fetal monitoring during therapy and a gradual MBP reduction to prevent undesirable blood pressure and therefore PBF levels. Failure to observe these tenets may cause fetal compromise as illustrated in Figure 16. In both patients, intrauterine growth retardation was present, presupposing significant reductions in homeostatic PBF. In both patients, maternal hypertension was overtreated sufficiently to lower PBF to levels that evoked severe fetal distress and necessitated cesarean section. It should be noted in the first illustration that the reduction in MBP alone compromised the fetus. In the second illustration, MBP was reduced less but the added presence of uterine contractions reduced PBF to fetal jeopardy levels.
Unusual Problems
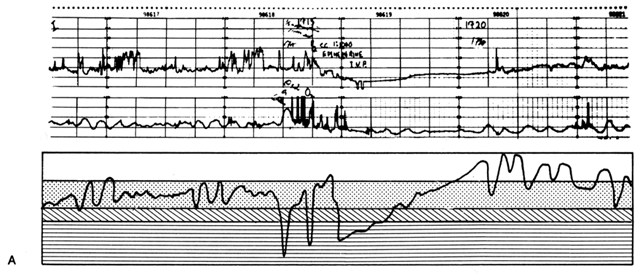
The physician must cope constantly with human errors. Figure 17 illustrates one such problem occurring in a normal laboring woman who accidentally received an injection of methylergonovine maleate (Methergine) prescribed for a post-partum patient. Ergotrates cause a sustained increase in myometrial tonus plus or minus superimposed rhythmic contractile patterns. Following the Methergine injection, intercontraction tonus progressively rose with an obligatory inverse PBF response, and fetal bradycardia ensued. Recalling the relaxing effect of epinephrine on the myometrium from β-adrenergic stimulation, the physician prescribed such therapy. Uterine hypercontractility was reduced, but the placental vasculature responded with vasoconstriction. The net effect of therapy was a decrease in PBF and persistence of fetal bradycardia. To properly manipulate PBF, the physician must assess the pathophysiology of each given circumstance and then apply the principles of placental vascular control to optimize fetal environment. As one reviews this record, the status of the fetus appeared to be improving and only close observation was indicated. Had the fetus evidenced a deteriorating condition, specific therapy in the form of a myometrial-relaxing general anesthetic agent such as halothane would have been indicated. The use of a β-adrenergic agonist such as ritodrine has been recommended in this situation but is theoretically a less optimal choice. Halothane should be a more predictable myometrial relaxant, and therapeutic levels would be achieved more rapidly without the development of confounding variables such as maternal hypotension.
SOClOLOGIC CONSIDERATIONS
With increased public awareness of physical fitness and the effects of environmental stimuli on well-being, the obstetrician must broaden his awareness to fetomaternal responses as well. Since the effects on PBF of only a few such stimuli have been studied, most comments in this area must be speculative. Two of these stimuli, cigarette smoking and exercise, are addressed in this chapter.
Cigarette smoking increases blood levels of nicotine and carbon monoxide. The latter decreases the oxygen-carrying capacity of maternal blood, limiting the amount of oxygen available for placental transfer. Nicotine, a stimulant of autonomic ganglia, apparently mediates its effects by the release of catecholamines, since maternal blood epinephrine and norepinephrine levels are increased and since direct infusions of nicotine into the uterine artery cause no change in UBF.15 When nicotine is administered intravenously to gravid sheep, low doses (125μg/min) cause no effect on UBF,27 while relatively high doses (1.0 to 1.5 mg/min) evoke significant UBF reductions.15 Presumably, at a given threshold level of nicotine, sufficient catecholamines are released to increase placental vascular resistance and decrease PBF. One must be cautious in applying such data from sheep to women since the effects of intravenous nicotine rather than smoking per se have been measured and since factors such as habituation and tolerance to chronic stimulation have not been evaluated. However, observations of pregnancy outcome in smoking mothers suggest a subtle, long-term, dose-related reduction in fetal growth. Therefore, it is reasonable to presume that smoking causes a qualitative long-term reduction in PBF and that smoking should be stopped or decreased during pregnancy, especially if high-risk conditions exist.
The effects of maternal exercise on UBF and the fetus have been observed only in the ovine species. In general, these studies indicate that mild to moderate exercise for up to 60 minutes is well tolerated by the fetus. When exercise was continued to the point of extreme fatigue or exhaustion, maternal respiratory alkalosis and decreased UBF occurred with reductions in fetal arterial oxygen tensions. In one study, fetal oxygen uptake was maintained during a 28% decrease in UBF by a 56% increase in oxygen extraction across the uterus.28 In another study on only four ewes, moderate to heavy daily exercise for 30 minutes during the last half of gestation resulted in lambs of low birth weight.29
Although the data are fragmentary, they support a fairly consistent response pattern. Cardiac output increases during exercise in response to the increasing metabolic demands of contracting muscles. The splanchnic and presumably the uterine circulations continue at only slightly reduced levels as long as this need can be met adequately. If peripheral demands exceed the cardiac capability, further splanchnic vasoconstriction and peripheral shunting will occur. During moderate to heavy exercise, the data suggest a degree of PBF reduction that is offset by increased oxygen extraction. During pregnancy, PBF will be affected by pre-pregnancy conditioning, the increased pregnancy load on the heart, and the degree of exercise. In addition, the effect on the fetus will be determined by the adequacy of homeostatic PBF. Recommendations regarding exercise during pregnancy must take all of these factors into consideration. It would be unwise for a nonjogger to begin such activity during pregnancy. Continuation of an established exercise program during early pregnancy with a gradual reduction in activity as the pregnancy load on the heart increases is logical. In the presence of conditions thought to reduce homeostatic PBF, perhaps all but mild exercise should be prohibited.
OVERVIEW
Uterine vascular adaptations to pregnancy require local blood vessels to dilate some tenfold to 20-fold to meet the requirements of the fetoplacental unit and the enlarging myometrial mass. While the spiral arterioles that perfuse the intervillous space probably undergo the greatest morphologic changes during this process, dilatation of supply arteries from the major uterine vessels down through the radial arteries is also critical to meet the downstream demand. For example, the middle uterine artery of a yearling ewe prior to conception is thin walled to the point of translucency and is no greater than 2 mm in diameter. At term pregnancy, the arterial walls are much thickened, with diameters between 8 and 10 mm. After the first pregnancy, the nonpregnant uterine artery retains its thickened wall with diameters of approximately 3 mm, and an experienced observer can easily tell from the appearance of the artery alone whether the ewe has been pregnant previously or not. Similar changes must occur in arteries within the uterus as well. There is increasing evidence that inadequate development of the uterine vasculatures to meet pregnancy requirements may be determined primarily during the definitive placentation process and that PBF so compromised may lead to the development of toxemia of pregnancy.30 The high frequency of toxemia during the initial pregnancy, that requiring the greatest uterine vascular adaptations, is consistent. If this theory is correct, then an improved understanding of the placentation process becomes critical to subsequent pregnancy performance and management. Our knowledge of the dynamic factors controlling PBF, although by no means complete, is adequate to guide rational management. However, we desperately need methods to evaluate precisely the adequacy of homeostatic PBF. Optimally these would be noninvasive techniques; therefore, application of ultrasonic methods seems to offer the most fruitful area for research. Development of such technology would permit increased understanding of PBF changes throughout pregnancy, knowledge that may delineate critical developmental times and an appreciation of normal as well as pathologic mechanisms.
The author's investigation discussed in this chapter were supported by research grants No. HL-03941 and HD-11339 from the National Institutes of Health, United States Public Health Service.
REFERENCES
Barcroft J, Herkel W, Hill S: The rate of blood flow and gaseous metabolism of the uterus during pregnancy. J Physiol 77: 194, 1933 |
|
Rosenfeld CR et al: Effect of estradiol-17β on the magnitude and distribution of uterine blood flow in non-pregnant oophorectomized ewes. Pediatr Res 7: 139, 1973 |
|
Ford SP et al: Uterine blood flow of cows during the oestrous cycle and early pregnancy: Effect of the conceptus on the uterine blood supply. J Reprod Fertil 56: 53, 1979 |
|
Ford SP, Christenson RK: Blood flow to uteri of sows during the estrous cycle and early pregnancy: Local effect of the conceptus on the uterine blood supply. Biol Reprod 21: 617, 1979 |
|
Greiss FC, Jr, Anderson SG: Uterine blood flow during early ovine pregnancy. Am J Obstet Gynecol 106: 30, 1970 |
|
Huckabee WE, Metcalfe J, Prystowsky H et al: Blood flow and oxygen consumption of the pregnant uterus. Am J Physiol 200: 274, 1961 |
|
Assali NS, Rauramo L, Peltohen T: Measurement of uterine blood flow and uterine metabolism: VIII. Uterine and fetal blood flow and oxygen consumption in early human pregnancy. Am J Obstet Gynecol 79: 86, 1960 |
|
Greiss FC, Jr: Differential reactivity of the myoendometrial and placental vasculatures: Adrenergic responses. Am J Obstet Gynecol 112: 20, 1972 |
|
Ladher C, Brinkman CR, Weston P, Assail NS: Dynamics of uterine circulation in pregnant and non-pregnant sheep. Am J Physiol 218: 257, 1970 |
|
Fishburne JI et al: Vascular and uterine responses to dobutamine and dopamine in the gravid ewe. Am J Obstet Gynecol 137: 944, 1980 |
|
Greiss FC, Jr, Gobble FL, Jr Effect of sympathetic nerve stimulation on the uterine vascular bed. Am J Obstet Gynecol 97: 962, 1967 |
|
Greiss FC, Jr, Anderson SG, King LC: Uterine vascular bed: Effects of acute hypoxia. Am J Obstet Gynecol 113: 1057, 1972 |
|
Greiss FC, Jr et al: Effects of local anesthetic agents on the uterine vasculatures and myometrium. Am J Obstet Gynecol 124: 889, 1976 |
|
Fishburne JI, Jr et al: Responses of the gravid uterine vasculature to arterial levels of local anesthetic agents. Am J Obstet Gynecol 133: 753, 1979 |
|
Resnik R et al: Catecholamine mediated reduction in uterine blood flow following nicotine infusion in the pregnant ewe. Proc Soc Gynecol Invest 25: 93, 1978 |
|
Greiss FC, Jr: Unpublished data, 1971 |
|
Resnik R et al: The effect of various vasoactive compounds upon the uterine vascular bed. Am J Obstet Gynecol 125: 201, 1976 |
|
Still JG, Greiss FC, Jr: The effect of prostaglandins and other vasoactive substances on uterine blood flow and myometrial activity. Am J Obstet Gynecol 130: 1, 1978 |
|
Greiss FC, Jr: Differential reactivity of the myoendometrial and placental vasculatures: Vasodilatation. Am J Obstet Gynecol 111: 611, 1971 |
|
Alm P et al: Vasoactive intestinal polypeptide nerves in the human female genital tract. Am J Obstet Gynecol 136: 349, 1980 |
|
Greiss FC, Jr Still JG: A uterine vasoactive property of glucosamine. Gynecol Invest 8: 57, 1977 |
|
Resnik R, Brink GW: Uterine vascular response to prostacyclin in non-pregnant sheep. Am J Obstet Gynecol 137: 267, 1980 |
|
Greiss FC, Jr, Gobble FL, Jr, Anderson SG et al: Effect of acetylcholine on the uterine vascular bed. Am J Obstet Gynecol 99: 1073, 1967 |
|
Greiss FC, Jr et al: Uterine pressure-flow relationships during early gestation. Am J Obstet Gynecol 126: 799, 1976 |
|
Ramsey EM, Corner GW, Jr, Donner MW: Serial and cineradioangiographic visualization of maternal circulation in the primate (hemochorial) placenta. Am J Obstet Gynecol 86: 213, 1963 |
|
Landesman R, Knapp RC: Na24 uterine muscle clearance in late pregnancy. Am J Obstet Gynecol 80: 92, 1960 |
|
Kirschbaum TH et al: Some acute effects of smoking in sheep and their fetuses. Obstet Gynecol 35: 527, 1970 |
|
Clapp JF III: Acute exercise stress in the pregnant ewe. Proc Soc Gynecol Invest 26: 27, 1979 |
|
Longo LD et al: To what extent does maternal exercise affect fetal oxygenation and uterine blood flow? Proc Soc Gynecol Invest 25: 7, 1978 |
|
Robertson WB et al: Placental bed vessels. Am J Obstet Gynecol 117: 294, 1973 |