Lasers in Gynecology
Authors
INTRODUCTION
The use of laser technology in gynecology has become widespread since the CO2 laser was initially used by Kaplan and colleagues in 1973 for treatment of cervical erosions.1 Since then, many advancements in laser technology have been made, and several other types of lasers are now available, including the neodymium: yttrium-aluminum-garnet (Nd:YAG), potassium-titanyl-phosphate (KTP), and argon. At the same time, the laser has become a popular instrument in laparoscopy, especially in the area of infertility. Although the laser became extremely popular in the late 1980s, during the early nineties we have also been under pressure to decrease health care costs. Thus, alternative, less expensive technology, such as unipolar instruments and loop electrical excision, has diminished the enthusiasm for and use of laser energy. Nevertheless, laser technology offers the operator unique properties that can be of aid to the practicing gynecologist.
HISTORY
Laser is an acronym that stands for light amplification by the stimulated emission of radiation, a concept that was developed by Einstein in 1917.2 The first laser developed by Theodore Maiman in 1960 used a ruby as the active medium, and in 1961 the CO2 laser was introduced.3, 4 The CO2 laser was used in gynecology for the first time in 1973 for treatment of cervical erosions, and later by Bellina for treatment of cervical intraepithelial neoplasia (CIN), as well as for microsurgery of the fallopian tube.5, 6 The use of KTP, argon, and Nd:YAG lasers became popular in the early 1980s.
LASER PHYSICS
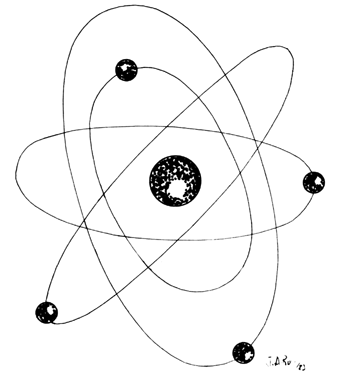
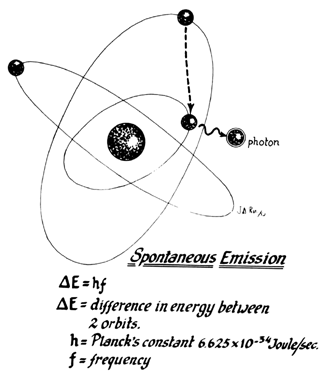
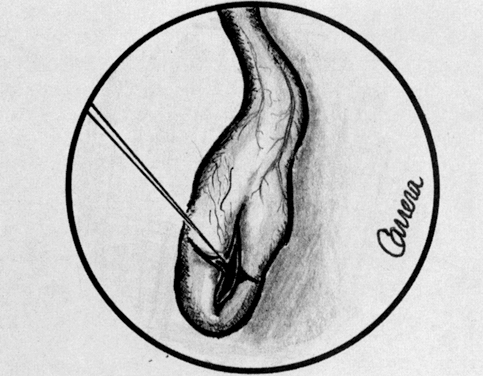
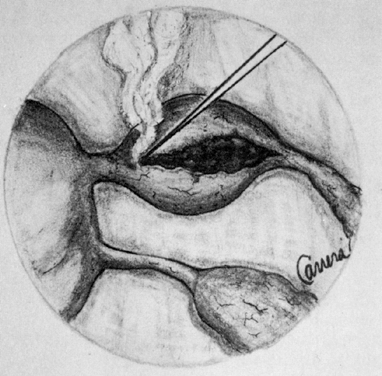
Lasers are named according to the medium that is activated. The common lasers in gynecology are CO2, argon, KTP, and Nd:YAG. Each medium produces light waves of specific wavelength giving it a characteristic color (monochromatic). A simple way to understand how light is emitted is to look at an atom with its surrounding electrons (Fig. 1). These electrons occupy discrete orbits that shift to higher orbits when they absorb energy (Fig. 2). Whenever the medium is activated, electrons are displaced to higher energy orbits. In the case of the CO2 laser, activation of the gas particles is done by using electrical wall current. The electrons that are displaced quickly return to their resting orbits, releasing a package of energy in the process referred to as a photon (Fig. 3). This process of light generation is known as spontaneous emission. An example of this process is a light bulb, which emits light waves of different frequencies in all directions out of phase.
|
|
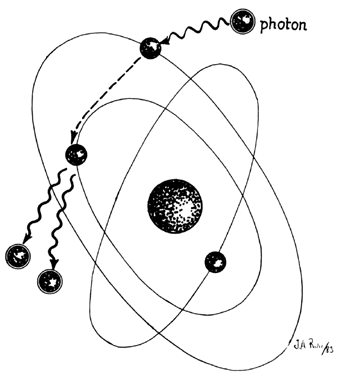
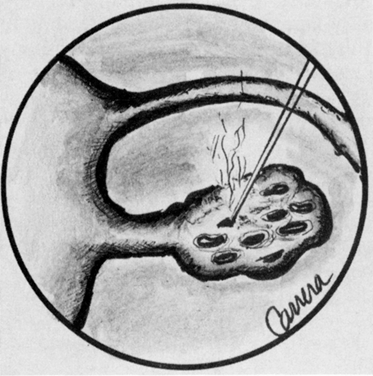
In the laser, however, these photons can further stimulate an already excited atom in its path to release an identical photon that is in phase (coherent), has the same wavelength and color (monochromatic), and travels in the same direction without divergence (collimated). This process is referred to as stimulated emission (Fig. 4).
|
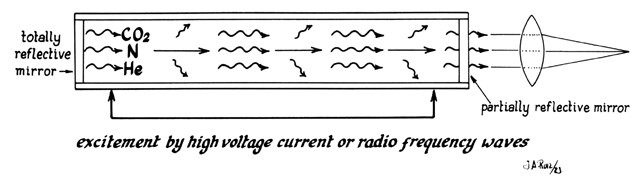
In the case of the CO2 laser, this process occurs within a tube located in the arm of the machine (Fig. 5). This tube is an optic resonator that has a totally reflective mirror and a partially reflective mirror at either end. Light generated is able to bounce back and forth from both mirrors, increasing the energy of the wave with each pass. Laser light is released through one of the mirrors that is partially transmissive and controlled by the foot pedal (Fig. 6).
|
|
Different colors are produced by different lasers. The argon laser produces a wavelength of 510 nm, making a blue-green light. The KTP produces a wavelength of 532 nm, making its light a green color. The CO2 laser, in contrast, produces a wavelength of 10,800 nm, which is in the nonvisible part of the electromagnetic spectrum; thus, a helium–neon laser is also used, which produces a red light to identify the location of the CO2 beam.
The laser beam comes out of the port as an unfocused beam. A lens system is used to focus the laser to a focal point. With the hand-held attachment, the focal point is usually 10 cm away from the focusing lens. With laparoscopy, the focal length is longer, taking into account the length of the laparoscope. Fine focusing can be done through a joystick or automatically with a coupler. Also, with the use of wave guides, one eliminates the problem of intermittent focusing of the beam that is associated with the joystick device (Fig. 7).
In contrast to the CO2 laser, in which the energy is transmitted through long tubes and reflected by mirrors, the argon, KTP, and Nd:YAG lasers are transmitted via a fiber (Fig. 8).
LASER–TISSUE INTERACTION
There are three basic parameters that determine the amount of energy being delivered to the tissue. The first is the wattage. For most gynecologic procedures using the CO2 laser, one rarely exceeds 20–30 W, which is used primarily for excision purposes. The second parameter is time. The longer the laser remains focused on one spot, the more energy is applied to that area. To limit tissue damage, especially in critical areas, one can simply move the beam back and forth, or select an intermittent, timed pulse mode, usually in fractions of a second. The third parameter that can be controlled is the spot size of the beam. As one gets closer to the target area, the spot size is made smaller, producing a more intense effect.
The combination of watts (power) and spot size determines the rate of tissue interaction. The higher the power density, the greater the laser's ability to vaporize and cut. This concept is expressed in watts/cm2 (unit/area) and referred to as intensity or power density. Power density is, therefore, inversely proportional to the area of the spot size and to the beam diameter. Doubling the beam diameter reduces the power density to one fourth. Conversely, by decreasing the diameter of the spot size, the power density is increased by 4 (Table 1).
Table 1. Power density in watts/cm2
Spot diameter (mm) | ||||
Power (watts) | 0.5 | 1 | 1.5 | 2 |
10 | 4,000 | 1,000 | 444 | 250 |
20 | 8,000 | 2,000 | 888 | 500 |
30 | 12,000 | 3,000 | 1,333 | 750 |
40 | 16,000 | 4,000 | 1,778 | 1,000 |
Of the various lasers available, the CO2 laser remains the most versatile and is relatively safe because of limited depth penetration. The CO2 beam is readily absorbed by tissue because of its high water content. The instantaneous boiling of intracellular water causes cells to explode, forming steam. Depending on the power density, the CO2 laser can be used effectively for vaporizing tissue, for excision, or for incision. Bleeding is reduced with the use of the CO2 laser because of its coagulating properties; it seals small vessels as it cuts.
When compared with other lasers, the depth of penetration and the lateral thermal damage of the CO2 laser are limited to less than 1 mm; thus, it can be used in areas of endometriosis on the pelvic side wall near the ureter. In contrast, the Nd:YAG laser has deeper penetration; thus, more caution is needed with its use (Table 2).
Table 2. Types of lasers used in gynecology
Type | Wavelength (nm) | Color | Fiber | Depth of penetration |
Argon | 488–512 | Blue-green | Yes | 0.5 mm |
KTP/532 | 532 | Green | Yes | 1–2 mm |
Nd:YAG | 1,064 | Infrared | Yes | 3–4 mm |
CO2 | 10,600 | Infrared | No | 0.1 mm |
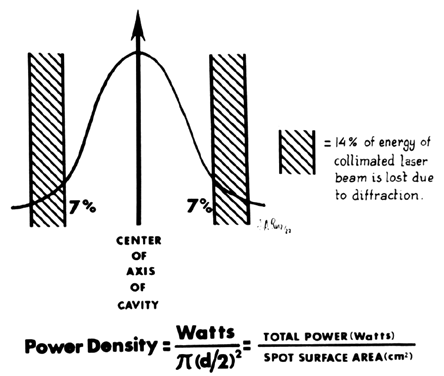
As a result of a laser beam impacting on tissue, a spot or crater is created. The diameter of this crater is a property of the divergence of the beam and the focal length of the lens through which the laser is focused. If the power of the laser is kept constant, the depth of the crater will vary inversely to the diameter of the spot. In other words, the smaller the spot, the deeper the crater. To obtain reproducible data, terminology relating to the level of tissue absorption must be reported. Intensity or power density refers to the transfer of laser energy to a given mass of tissue and is expressed in terms of watts per centimeter squared (Fig. 9). Because 86% of the laser power is absorbed by the spot, this figure should be multiplied by 0.86 to determine the power density.
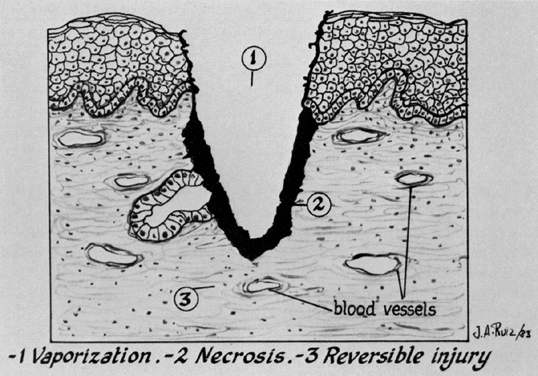
Three types of tissue injury may be identified following a laser wound. The zone of vaporization is characterized by an absence of tissue and a V-shaped defect, because energy is greater in the center of the beam than on the perimeter. Immediately below the zone of vaporization is a fixed zone of stromal necrosis measuring (in cervical tissue) approximately 50–100 μm in depth, regardless of the crater's extent. Within this zone, small vessels (<1 mm) are sealed. The third zone is one of reversible injury or potential repair. Laser wounds are clean, produce minimal tissue damage, and cannot be compared with cautery wounds, in which substantial devitalized debris remains behind. Therefore, laser and scalpel wounds are similar (Fig. 10). The CO2 laser may serve three principal functions: as a cutting or excisional instrument, as a vaporizing or ablating instrument, and as a defocused cauterizing instrument.
CO2 LASER
Of the various lasers available, the CO2 laser is the most versatile and is extremely safe because of its limited depth of penetration (0.1–0.5 mm) and lateral thermal damage (0.5 mm). This allows use of the CO2 laser in delicate areas where cautery would be unsafe, such as the bladder, lateral side wall near the ureter, and bowel serosa. Besides vaporization, the CO2 laser can be used for excision or incision by increasing the power density.
Disadvantages of the CO2 laser include focusing of the helium–neon beam as well as production of smoke referred to as “plume,” which needs frequent evacuation to allow adequate visualization of the target.
KTP–ARGON LASERS
The KTP and argon lasers have similar wavelengths, 532 nm and 514 nm, respectively, and are delivered via a fiberoptic fiber. They produce an intense green-blue light and can be transmitted through fibers of different diameter (400 μm and 600 μm), thus changing the spot size. The advantages of these lasers over the CO2 laser include: a selective absorption by hemoglobin, less plume production, and an easy delivery system that uses lower power settings in the range of 5–10 W. The main disadvantage is the need to wear special glasses that distort the view of the pelvis and make it difficult to visualize small implants of endometriosis. Keye and colleagues have reported pregnancy rates following argon laser treatment for mild, moderate, and severe endometriosis to be 38%, 30%, and 20%.7
Nd:YAG LASER
Nd:YAG lasers emit an invisible beam with a wavelength of 1064 nm and have to be guided similarly to the CO2 beam using a helium-neon spot. The Nd:YAG wave is readily absorbed by tissue with a deep penetration of 3–4 mm. The energy emitted by the Nd:YAG laser is poorly absorbed by fluids and thus makes it an excellent tool for hysteroscopic surgery. In addition, the wave can be transmitted through an operating hysteroscope. There are two modes of delivery of the Nd:YAG laser: the bare quartz fiber and the quartz fiber that has a sapphire contact tip. The bare fiber on contact with tissue creates an area of coagulation that can extend 3–5 mm into the tissue, as well as peripherally. By using a sapphire tip at the end of the fiber, the laser energy can be focused and converted into heat. This results in the ability to vaporize without the extensive tissue coagulation caused by the bare fiber. Sapphire tips need to be cooled with a coaxial flow of gas or liquid through the fiber and are contraindicated for hysteroscopic surgery, but they can be safely used for abdominal surgery. Recently, it has been made possible to modify bare fibers for use in a contact mode by molding the tip and creating sculptured tips of various types such as scalpel, tips, and balls. These fibers do not need to be cooled.
LASER SAFETY
Laser surgery in gynecology has been used for 20 years with a good safety record. However, as with any device used in surgery, a laser has the potential to cause serious injuries. Gynecologists requesting laser privileges should be certified for the specific type of laser used. Certification implies attendance of didactic instruction and practical use of the laser in the laboratory prior to its application in patients.
When using the laser, an appropriate warning sign, such as “Laser in Use,” should be displayed on all doors of the operating room. Protective safety glasses appropriate for the laser in use should always be worn by surgeons and operating room personnel. When the laser is not being fired, it should always be on stand-by mode. Surgical drapes near the operating field should be fire retardant and kept wet if possible. Adequate suction should be available to collect all plume produced by laser use, because intact viral DNA and papilloma-virus have been detected in the plume.8
When using various types of laser wavelengths, it is important to understand their specific tissue interaction to avoid undesired trauma. It is much easier to cause damage to a vessel or ureter when using deep penetrating energy such as that produced by the Nd:YAG laser than when using the CO2 laser energy, if one is treating superficial endometriosis.
In addition, fibers used for transmission of laser energy are delicate and can break. If one is unaware of a broken fiber, laser energy will be delivered at the point of breakage, potentially injuring the patient and/or staff.
EXTRAPERITONEAL LASER SURGERY
Cervix–Cervical Intraepithelial Neoplasia
Although treatment of cervical intraepithelial neoplasia (CIN) is now more commonly performed by the large-loop excisional technique, the laser was the ideal method of treatment of the 1980s and still offers another option. Treatment of CIN can be performed by vaporization or by excisional conization using the laser as a substitute for the scalpel.9 The carbon dioxide (CO2) laser is the laser of choice for this. The major advantages of the CO2 laser for the treatment of CIN include:
- High degree of clinical efficacy
- Bloodless field
- Microscopic precision
- Sparing of normal tissue
- Rapid healing with minimal scar formation
- Small number of complications
- Outpatient methodology
The main disadvantages of the CO2 laser include the absence of a histologic sample when vaporization is performed and the expense of the laser machine.
VAPORIZATION
When using the laser coupled to the colposcope, one should first define the extent of the lesion. One should keep in mind that endocervical glands may lie deep in the stroma to a depth of 6–7 mm10; therefore, treatment should be carried out to a minimum of 9–10 mm with a peripheral margin of 3 mm. This procedure is performed with 30–40 W of power with a 2-mm-diameter spot and takes about 5–10 minutes to complete under local anesthesia. Vaporization is performed to a minimal depth of 1 cm and ends at the level of the endocervical canal. The cervical defect should resemble a funnel, as if one performed a small cone biopsy.
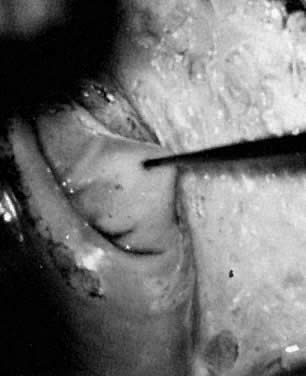
The operative site is circumferentially outlined with a 3- to 5-mm margin around the lesion (Fig. 11). The cervix is then divided into four quadrants. Power is increased to 30–40 W. Beginning in the lower quadrants and using a circular pattern, vaporization is carried down to a depth of 1 cm. The endocervical canal is usually spared. Measurements are made at frequent intervals, relating the depth to the surrounding ectocervical surface. When the lower half of the cervix has been vaporized, a similar procedure is followed for the anterior surface (Fig. 11, Fig. 12, and Fig. 13).
|
|
LASER EXCISIONAL CONIZATION
Laser excisional conization is performed when:
- The CIN extends into the endocervical canal
- The pathology suggests stromal extension
- Vaporization fails
- A disparity exists between the Papanicolaou smear and the biopsy, and the vaginal colposcopic examination is negative
- The colposcopic examination is unsatisfactory (i.e., the squamocolumnar junction extends into the endocervical canal)
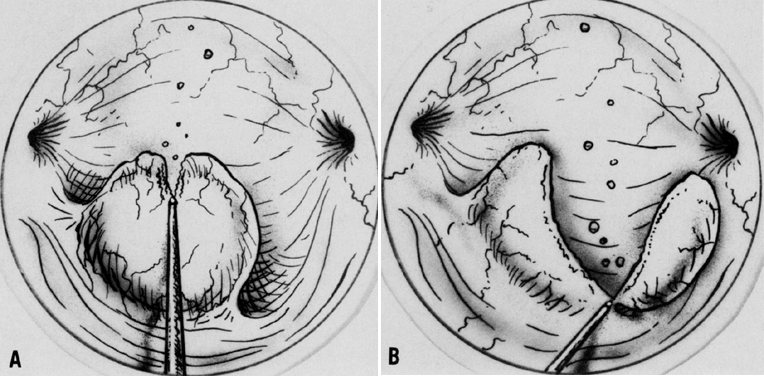
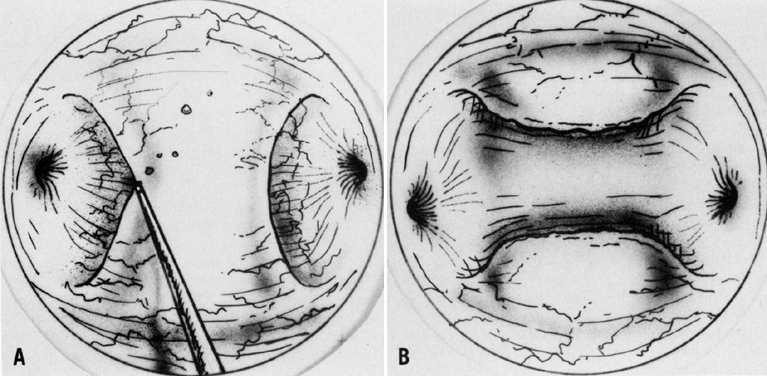
A colposcopic examination is first performed and the peripheral limits of the cone are mapped out with a series of shallow craters. Traction sutures are placed in the cervix at the 3 o'clock and 9 o'clock positions. A 1:30 diluted solution of vasopressin is next injected submucosally into the cervix by means of a 1-mL tuberculin syringe and a small-gauge needle. The injections are performed to cause deeper vascular channels to go into spasm; the constricted vessels are sealed by the traversing laser beam. Next, the tracing dots are connected by a shallow scoring incision. The power meter is advanced to 20 W, the spot size is reduced to approximately 0.5 mm in diameter, and a deep incision is cut into the cervical tissue (Fig. 14). A long-handled skin hook applies sharp traction and opposing countertraction to allow the laser beam to cut rapidly to a depth of 10–15 mm; this deep incision is extended around the cervix as the hooks are simultaneously moved in concert with the cutting beam (Fig. 15). Rather than a cone, a cylindrical-shaped mass of tissue will be incised (Fig. 16). Again, using the hooks, the specimen is sharply angulated and the endocervical margin is cut with a sharp scalpel blade. Minimal bleeding is encountered. When the cylinder of cervical tissue has been removed, small bleeding points are coagulated by reducing or enlarging the spot. The remaining endocervical canal is sounded and curetted. At the termination of the procedure, the defect is tightly packed with petrolatum gauze (Fig. 17). This procedure can be performed under local or general anesthesia. Patients should not insert anything vaginally for at least 2–3 weeks and should be warned that light spotting is common, occurring in 10% of patients. Postoperative discomfort is usually minimal, and discharge is common. Follow-up examinations are performed at 8 weeks, 3 months, and 6 months thereafter.
|
|
An important step in this technique, which reduces the possibility of cervical stenosis, is the creation of the endocervical button (Fig. 18). This is accomplished by vaporizing the stroma around the endocervical stump to a depth of 2–3 mm.
COMBINED EXCISIONAL AND VAPORIZATION CONIZATION
When CIN extends far out on the portio of the cervix, as well as into the endocervical canal, a combination laser procedure may be indicated. Multiple sampling biopsies are obtained from the ectocervical sites to ensure that intraepithelial disease is present. A small excisional cylinder of cervical tissue is removed to a depth of 1.5–2 cm and sent to a pathology laboratory for evaluation. The ectocervical extension is vaporized to a depth of 5–7 mm.
Patients have reported three types of discomfort associated with laser vaporization of the cervix. The initial impact of the laser beam on the cervix is usually perceived as a pinch similar to that experienced with applying a tenaculum or taking a cervical punch biopsy. As vaporization progresses, many women complain of uncomfortable warmth as heat is transferred to peripheral cervical stromal tissue. The latter may be dissipated by stopping treatment for a moment. Invariably, heat build-up will lead to substantial discomfort if not relieved. Finally, as a large bulk of cervical tissue is removed, menstrual-like cramping is reported by virtually every woman. The cramping can be related to the liberation of prostaglandin-like substances. The cramping can be ameliorated by the administration of a prostaglandin synthetase inhibitor (e.g., Motrin) approximately 30 minutes prior to laser vaporization.
ANESTHESIA
In 95% of vaporizations, no anesthesia is used. In the remaining 5%, local infiltration, paracervical block, or general anesthesia may be used. All laser excisional conizations are performed with either general or paracervical block anesthesia.
RESULTS OF TREATMENT
The results of CO2 laser treatment of intraepithelial neoplasia are very acceptable and comparable to loop excision. Baggish, Dorsey, and Adelson reported a series of 954 laser excisional cones with 97% showing no evidence of persistent recurrent disease.10 Four cases of invasive cancer were identified, and 73 women had disease extending to the margin; 44 of the latter were followed with no further treatment and remained free of disease. There were 25 women in this series with persistent disease requiring repeat cone or hysterectomy.
COMPLICATIONS OF LASER CONE BIOPSY
Complication rates with laser cones are very low; cervical stenosis occurs in 1.3% of cases, cervical incompetence in 0.05%, and major bleeding in less than 1% of patients.11, 12
Vulva–Vulvar Intraepithelial Neoplasia (VIN)
The skin surface of the vulva may be afflicted with a variety of superficial diseases amenable to laser surgery, including vulvar intraepithelial neoplasia (VIN) and condylomata acuminata.
Vulvar intraepithelial neoplasia (VIN) is an intraepithelial neoplasia disorder that peaks in occurrence in women 35–54 years of age. Its coexistence with human papillomavirus infection 16 and 18 has been documented.13, 14 In women younger than 40 years of age, VIN appears to be less aggressive in growth than in those older than 50 years of age. There seems to be a more linear relationship between VIN and invasive disease in the over-50 group than in the younger group.15
Treatment of VIN in the past has included simple vulvectomy and skinning vulvectomy. These more aggressive and disfiguring procedures were replaced by wide local excision and laser ablative procedures of the affected vulvar area. Although the CO2 laser has been used widely for ablation of VIN, it does not allow tissue specimen for determination of possible existing invasive disease and identification of margins. Instead of performing laser ablation in treating VIN, the surgeon can obtain laser thin sections (Fig. 19). This method allows the intradermal removal of strips of vulvar skin affected by VIN and at the same time provides a tissue sample for the pathologist to inspect depth of penetration as well as the adequacy of surgical margins.16
Laser–thin-section treatment to the vulva requires anesthesia. General anesthesia is preferred for extensive disease, but local anesthesia can be used if the extent of tissue removal is limited to a small area. Thin section is performed by first using a 27-gauge needle for intradermal injection of either saline or local anesthetic plus 1:1000 diluted vasopressin in the involved area. This intradermal injection forms a barrier that absorbs heat and minimizes thermal energy. The spot size used is generally 0.5 mm with a superpulse power of 20–25 W. Once anesthesia has been applied, tracer spots are used to demarcate the boundaries of skin to be removed. Generally, the timing interval is set for pulses of 0.2 seconds and 10 W of power. Once these spots have been made, they are then connected with the higher power previously mentioned. A laser hook is placed at the margin of the incision, and a tissue plane is then developed. With traction placed upward, the thin section is completed by removing strips of epithelium to depths ranging from 1 to 2 mm. The laser-treated area heals in a manner similar to that in laser ablation, avoiding the use of suture or grafts. Using a beam with a larger diameter of 1–1.5 mm, superficial vaporization of tissue is carried out to a 3-mm peripheral margin from the incision edge. The char material can be swabbed off with 4% acetic acid. Laser ablation may be preferred to the thin-section method for small areas of VIN located in specific areas, such as the clitoral area, anus, or labia minora.
Postoperatively, the surgical sites are covered with bio-occlusive dressings to diminish pain. Covered wounds also appear to heal more rapidly than undressed wounds because they retain moisture. For more extensive laser wounds, patients are asked to take sitz baths using Instant Ocean four times a day (Fig. 20). These are more comfortable than saline baths and tend to debride the wounds, thereby enhancing healing. A 1:4 diluted solution of Betadine provided in plastic bottles is squirted on the perineum after urination or defecation. After bathing or irrigation, the areas are dried with an electric dryer on the air cycle. Alternatively, the skin may be thoroughly covered with Silvadene cream, reapplied several times per day. Patients are instructed to wear oversized cotton pants to provide adequate circulation of air to the vulva and to provide a barrier for the Silvadene cream. Healing is usually complete in 6 weeks. The patient should be seen at approximately 2-week intervals until healing is complete, and thereafter every 6 months.
Most patients experience little immediate pain. The explanation for this relative anesthesia relates to the nerve-sealing action of the laser, which results in a clublike nerve ending, compared with the shaving brush pattern seen after a knife cut. Delayed bleeding is rare after laser vaporization or thin section. Because full-thickness excision usually results in scarring, great care must be taken during any excision or vaporization treatment to avoid extending beyond reticular dermis. Deeper vaporization can be identified by the visualization of fine, white, subvulvar fat. Another concern is coaptation of the labia. This may be avoided by having patients manually separate their labia during sitz baths. With laser ablation for treatment of VIN, cure rates of approximately 80% can be expected.17, 18, 19 With thin section, these cure rates may exceed 90%. Careful long-term follow-up is imperative.
Because vulvar carcinoma in situ is a disease predominantly of younger women, conservative methods of treatment that preserve functional anatomy should be offered to every patient. Even with extensive lesions, laser therapy compares favorably with other methods of treatment. Paget's disease may be the exception; recurrent disease is quite common after laser therapy.
CONDYLOMATA ACUMINATA.
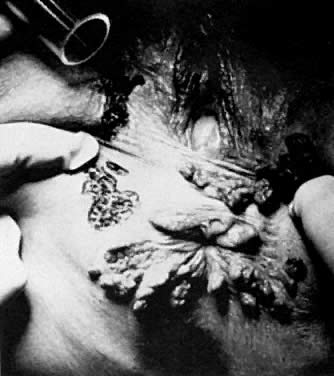
The most common indication for CO2 laser surgery in the lower genital tract is the treatment of condylomata acuminata.20 These lesions caused by the human papilloma virus (HPV) are most commonly found on the vulva and perianal skin. Although genital warts can cause irritation and some discomfort, they are not generally painful; most patients seek medical attention because of their unsightly growth. They spread principally by autoinoculation of the HPV virus and may undergo spontaneous regression. Pregnancy, oral contraceptive use, diabetes, immunosuppressive drug therapy, and immunodeficiency virus infections can accelerate growth of genital warts. These lesions may be polypoid, flat, papillomatous, fleshy, white, or pigmented in appearance. The indication for CO2 laser ablation of genital warts is the presence of gross disease, and not subclinical HPV infection, such as koilocytosis and papillosis.
Local anesthesia may be used for small isolated vulvar warts. However, with more extensive disease, general anesthesia is preferred. Warts should be vaporized no deeper than the level of the surrounding skin surface (Fig. 21, Fig. 22, and Fig. 23) using a power level of 40– 60 W with a beam diameter of 3 mm. After complete vaporization of the genital warts is accomplished, the 1–2 cm of skin surrounding the individual lesions is brushed lightly with 10 W of power and a 2-mm diameter spot size to destroy subclinical HPV involvement and diminish the recurrence of the disease. When the condyloma lesion has a well-defined pedicle, it is more efficient to excise rather than vaporize the entire lesion. Ablation of the remaining base is then accomplished to the level of the skin surface. The postoperative treatment regimen is identical to that described for VIN. Laser treatment of genital warts is effective; they are eliminated in over 90% of patients. For those patients who are immunologically compromised, chronic application of 5-fluorouracil cream has been recommended.21 The use of recombinant interferon after laser treatment by Reid and colleagues has also diminished their recurrence.22
|
|
Anal, genital, and urethral warts are vaporized more superficially with power settings of 10–15 W and a beam diameter of 1.5 mm.
VAGINA
Vaginal intraepithelial neoplasia (VAIN) is the least common of the group of lower genital tract premalignancies with an annual incidence of 0.2 per 100,000 women.23 It is found most often in women who have had CIN or VIN. Its location is most often in the upper third of the vagina, and lesions are usually multifocal. The lesion can be identified by colposcopy after swabbing suspected areas of the vagina with 4% acetic acid. Lesions in the vagina may be white or flat or have a raised condylomatous pattern. Although many treatment options for VAIN are available, including cryosurgery, electrocautery, radiation therapy, and surgical excision, the use of laser therapy is an excellent option. Townsend and colleagues, in 1982, reported a success rate of 90% in a series of 52 patients treated in this manner.24
Power density of approximately 500 W/cm2 with a beam diameter of 2 mm is recommended for laser vaporization of the vagina. Laser treatments should be performed with the aid of a laser hook to help increase vaginal exposure (Fig. 24). Vaporization should be carried to a depth of 1 mm or less (Fig. 25). Wide peripheral margins should be obtained to eliminate this multifocal disease. Disease in the upper third of the vagina should be treated by vaporizing the entire upper third of the vaginal canal. If there is spread of disease from the upper third to the middle third, then the upper two thirds of the vagina should be vaporized. If VAIN involves areas in all thirds of the vagina, then the entire vagina should be vaporized. This is best done in two planned sessions. The use of superpulse laser at settings varying from 100 to 300 pulses per second with a pulse interval of 0.1–0.3 milliseconds is recommended. This rapid pulse with rest intervals helps preserve underlying stromal tissue and shortens healing time. There is no scar formation and no reduction in vaginal capacity. This surgery is best done with a CO2 laser coupled to a colposcope under general regional anesthesia. Following laser vaporization, postoperative discomfort is minimal, and healing of the mucous membrane is restored within 4 weeks. Because postoperative bleeding is not usually a problem after laser vaporization, there is no need for routine use of solutions such as ferric subsulfate. A more recent study by Dorsey and Baggish involving 83 cases of VAIN treated by laser only resulted in an 80% cure rate with 40% of the cases requiring more than one laser treatment.25
CLINICAL APPLICATIONS OF LASERS IN INTRA-ABDOMINAL GYNECOLOGIC SURGERY
Carbon dioxide lasers were first used by Bellina and associates in 1974 for intra-abdominal applications.26 By adapting the laser to the operating microscope, reconstructive microsurgical procedures, including salpingolysis, salpingostomy, fimbrioplasty, and treatment of endometriosis, were possible. With improvement in technology and increased experience performing laparoscopic procedures, the use of the laser for laparotomy has been replaced by an endoscopic approach.
Laparoscopy Instrumentation and Lasers
The CO2 laser is the laser most commonly used in gynecologic surgery, for both extra- and intra-abdominal applications. It has the advantage of causing little peripheral thermal damage, usually less than 1 mm, and what is seen in penetration is the true end result. Thus, it can be used in critical areas adjacent to bowel, ureter, and bladder. CO2 lasers can be used to vaporize, excise, or coagulate tissue by modifying the power density.
The CO2 laser beam can be directed by a handpiece when used in laparotomy or, alternatively, through a first or second puncture when used in laparoscopy (Fig. 26).
The CO2 laser can be easily coupled to an operating laparoscope. The laser beam should be aligned so that it lies in the middle of the viewing lens, and it should be finely focused. A second or third puncture is necessary only to manipulate pelvic structures, to clear the field of plume production, or for irrigation purposes. It is also possible to use the CO2 laser for second punctures by using wave guides that attach directly to the CO2 laser and allow some flexibility in delivering the laser energy. Research in developing a truly flexible fiber to deliver CO2 laser energy is still needed. At present, fiber systems used in intra-abdominal laparoscopic surgery include primarily KTP, Nd:YAG, and argon lasers.
An advantage of the lasers using flexible delivery systems is that they allow tactile contact with the tissue. Because each of these lasers has a different wavelength and different absorption properties, it is important to recognize their tissue interaction effects before they are selected for surgery.
The KTP and argon lasers share very similar wavelengths (532 nm versus 458–515 nm) and thus produce similar tissue effects. The energy produced is selectively absorbed by tissue containing hemoglobin or which is heavily pigmented. By moving the fibers closer or farther away from the tissue, one can achieve cutting, vaporization, or coagulation effects on tissue. The main drawback of these lasers is the required use of goggles for eye protection, which distorts color discrimination.
Although the Nd:YAG laser was originally used in gynecology primarily for hysteroscopic application, it is now possible to use it solely intra-abdominally by the use of contact sapphire probes or specially made sculptured tips. These probes provide a tactile feel of the tissue, and by selecting a variety of probes, one can incise, excise, vaporize, or coagulate tissue.
All of these lasers have been shown to be effective in the treatment of various gynecologic conditions, including:
Endometriosis
Laser uterosacral nerve ablation
Adhesiolysis
Neosalpingostomy
Fimbrioplasty
Ectopic pregnancy
Uterine fibroids
Endometriosis
Areas of endometriosis can be vaporized or excised with the CO2 laser depending on the depth of penetration of the lesion. Superficial areas of endometriosis in the cul-de-sac, uterosacral areas, or peritoneum in general can be vaporized with wattages of 10–20 W with spot sizes of 1 mm. With lesions larger than 5 mm, it is recommended that the surgeon cut through a healthy margin of the peritoneum and totally excise the lesion. This scalpel effect can also be accomplished with flexible laser fibers, as well as with electrosurgery and scissors. In addition, the wavelengths of the argon and KTP lasers have an affinity for pigmented lesions such as endometriosis, and these lasers have been used extensively and effectively by Keye and Dixon to heat and vaporize these lesions.27 The CO2 laser, however, remains the most commonly used laser and is the method of choice for treating American Fertility Society (AFS) stage I to IV endometriosis. The main reason is its high margin of safety, which is due to its limited peripheral tissue injury and penetration. This makes it ideal for adhesion excision near ureters and bowel. Although the CO2 laser is ideal for surgical treatment of endometriosis, it does not offer an increased pregnancy rate over other options, including sharp dissection with scissors or electrosurgery.28 Reported pregnancy rates after laser treatment of endometriosis are 57%, 53%, and 61% for mild, moderate, and severe disease, respectively.29
Endometriomas can also be effectively treated with the CO2 laser.30 Initially, a small opening on the cyst wall is made to allow for aspiration of the chocolate contents and profuse irrigation with a 5-mm aspirator probe. The edge of the endometrioma and normal ovarian cortex are then grasped individually with atraumatic forceps. With the CO2 laser used intermittently at 10–20 W, and with traction and countertraction of the forceps holding the ovarian cortex and endometrium cyst wall, the cyst can be stripped off. Removal of the cyst wall may be in several portions. The base of the ovary after removal of the cyst wall can then be ablated by defocusing the beam and decreasing to 10 W of energy. This causes coagulation of small areas of oozing and destroys any small areas of cyst wall that may remain.
Patients with endometriosis who suffer from significant dysmenorrhea are also candidates for laparoscopic uterosacral nerve ablation. This controversial procedure was originally described by Doyle in 1963 and refers to the denervation of sensory fibers in the lower uterine segment.31 The CO2 laser is used to vaporize a lesion 1 cm in diameter by 1 cm in depth at the junction of the uterosacral ligaments to the posterior cervix. A comparison between the CO2 laser and electrosurgery for treatment of dysmenorrhea revealed a 50% relief of pain from both procedures.32
Adhesiolysis
Pelvic adhesions are common sequelae to previous pelvic infections and pelvic surgery. Although adhesiolysis is commonly performed with scissors or electrosurgery, the laser is another option and represents an ideal instrument for lysis of vascular adhesions, because excision and coagulation can be performed simultaneously while limiting peripheral tissue trauma. The use of graspers to apply tension on the adhesions maximizes the efficiency of the laser (Fig. 27). The use of quartz rods, rods with backstops, and irrigation is recommended to avoid injury to adjacent normal tissue. The pregnancy rate outcome with the laser, however, does not significantly differ from that with other standard modalities used to treat adhesions.33
Salpingostomy
Correction of distal tubal obstruction by cuff salpingostomy was first reported in 1884 by Schroeder.34 Because of the dismal pregnancy rate, this procedure did not become popular until principles of microsurgery were emphasized. Despite improvements in surgical technique, pregnancy results remained low and dependent on the extent of tubal disease at the time of surgery. With mild disease there is a small (<15 mm) enlargement of the distal tube, fimbriae are inverted but recognized when patency is achieved, there is a paucity of peritubal adhesions, and a rugal pattern is evident by hysterosalpingogram (HSG). In moderate disease there is a distended tube (15–30 mm in size), fimbriae are not identified, and a rugal pattern on HSG is not evident. With severe disease, a large (>30 mm) hydrosalpinx is present, no fimbriae are present, and there are dense pelvic adhesions. The pregnancy rate is 30–40%, 10–30%, and 0–5% for mild, moderate, and severe disease, respectively.35, 36 In the 1980s, our technology improved with the use of operative laparoscopy and the use of lasers. Laser salpingostomy is routinely performed, with pregnancy rates similar to those with the laparotomy approach.
Treatment of distal tubal blockage begins with lysis of peritubal and ovarian adhesions until the fallopian tube is completely free and movable. The obstructed end of the tube can be assessed for diameter, presence or absence of fimbriae, and patency. With the CO2 laser at 30 W, two linear incisions in the form of a cross are made in the distal obstructed end of the tube (Fig. 28). It is recommended that the incision begin on the area where the tube finally closed, because this is the thinnest and most avascular area. Continuous dye injection helps to keep the distal tube distended while the incision is being performed. The edges can then be everted by defocusing the beam on the serosal edges and lowering the energy to 5–10 W. Continuous irrigation is applied during the procedure. The pregnancy rate with the laparoscopic laser technique is not superior to that with the standard microsurgery technique, and patients remain at risk for ectopic pregnancy.
In contrast to the low pregnancy rate with neosalpingostomy, fimbrioplasty offers much better results in the 30–60% range37 because of the limited mucosal damage in these patients. In performing fimbrioplasty, the CO2 laser is used in a continuous mode with 15–20 W of energy, with a backstop and irrigation used as needed. With a spot size of 0.5 mm, the adhesions are easily removed by vaporization.
Ectopic Pregnancy
A conservative procedure for ectopic pregnancy was first reported by Stromme in the English literature in 1953.38 This procedure did not become very popular until De Cherney and colleagues in the early 1980s published their success in treating unruptured ectopic pregnancies.39 Laparoscopic treatment of ectopic pregnancy was reported in the mid-1980s with a CO2 laser incision on the antimesenteric border of the tube.40
Paulson reported the treatment of 125 consecutive patients with ectopic pregnancy.41 Laparoscopy was successful in all but four patients who needed laparotomy. The CO2 laser offers another alternative to electrosurgery and scissors in the treatment of ectopic pregnancy by laparoscopy. A linear incision is made on the antimesenteric side of the tube over the dilated portion of the ectopic pregnancy using 30 W of energy with a finely focused beam (Fig. 29). A dilute concentration of vasopressin can be injected into the mesosalpinx below the ectopic tissue and on the serosa overlying the ectopic pregnancy to decrease intraoperative bleeding. With the use of hydrodissection, the ectopic tissue is then gently lifted from its tubal bed. Small amounts of vascular oozing can be controlled with the laser, or alternatively with bipolar forceps. The tubal lumen does not have to be closed because fistula formation has not proved to be a problem. Results of treatment of ectopic pregnancies with use of the laser do not differ from those found with the conventional use of scissors or electrocautery.
Uterine Fibroids
Uterine leiomyomas are extremely common benign smooth muscle tumors occurring in 25% of women older than 30 years of age.42 Although most women are asymptomatic, at least 25% of women have complaints of pain, pressure, abnormal bleeding, urinary frequency, constipation, and bloating. Recurrent abortion and infertility can also be linked to large intramural and submucous fibroids, and surgery is indicated only after a complete infertility evaluation has been performed and all other factors are excluded.
The laparoscopic approach is recommended only for pedunculated and subserosal fibroids. Intramural myomectomies result in poor laparoscopic closure with the potential for future uterine rupture during pregnancy.43
Laser energy can be delivered through a handpiece for laparotomies or via the laparoscope. Small fibroids can be vaporized directly with 20–30 W of energy or can be shelled out in their entirety, as during conventional surgery. Dilute vasopressin (1:30) is frequently used to decrease intraoperative bleeding. Although the laser is capable of coagulation, it is necessary to suture or coagulate larger vessels (greater than 0.5 mm) with a bovie. Large intramural defects are closed with3-0 Dexon sutures for the muscularis, followed by 4-0 Dexon for the serosa.
The advantages of the CO2 laser for removal of uterine fibroids over scissors and standard electrosurgery are improved hemostasis, decreased tissue trauma, and decreased severity of adhesion formation. Approximately 50–60% of patients ultimately achieve pregnancy with laser myomectomy.44
Ovarian Wedge Resection
Ovarian wedge resection by laparotomy, once a popular treatment option for patients resistant to clomiphene citrate, is now rarely performed because of the development of severe postoperative adhesions. However, with strict adherence to microsurgical technique and the use of fine electrocautery needle tips or lasers, it is possible to perform ovarian wedge resection with pregnancy rates of 40%, which may be due to less adhesion formation.45
Once the ovaries are exposed, they are draped with moist sponges. With 30 W of energy, a focused beam of 0.5 mm can be used as a scalpel to remove a wedge of the ovary. The use of hooks to retract the ovarian tissue is helpful because tension on the tissue that is being incised expedites the surgery. The ovarian defect is then closed with 3-0 Dexon interrupted sutures, and 6-0 nylon is used for the cortical layer.
To achieve the same endocrinologic effect of lower ovarian androgen levels and ovulation that occurs with laparotomy, laparoscopic electrocoagulation or laser coagulation of the ovary can be performed.46 In this technique, either each ovary is cauterized with a unipolar needle electrode, or laser energy is applied in multiple areas, causing small ovarian craters (Fig. 30). The CO2, KTP, argon, and Nd:YAG lasers have been used to treat polycystic ovaries with ovulation rates of 70% and pregnancy rates of 40%.45 The endocrinologic effect of this procedure is temporary and rarely exceeds 12 months.
Laser Hysteroscopy
The hysteroscope has been used for many years as a diagnostic instrument to evaluate the source of abnormal uterine bleeding. With refinement of light sources, the use of low-viscosity fluids, and newer operating hysteroscopes, it is now possible to use this technology as a therapeutic modality for patients with abnormal uterine bleeding. Various instruments can be used with the hysteroscope, including electrodes to cut and coagulate, operating graspers, scissors, as well as various laser fibers. The main laser delivery systems available for hysteroscopy are the Nd:YAG and KTP 532. Both of these lasers use a flexible fiber that can be passed easily through the operating sleeve of the hysteroscope. The fiber can be directed by use of an Albarrán's bridge that is attached to the operating hysteroscope. Hysteroscopic procedures that can be accomplished with the laser include removal of fibroids and polyps, transsection of uterine septa, lysis of adhesions, and endometrial ablation.
Endometrial Ablation
Hysterectomy is the most common major operation performed in women, with 591,000 of these procedures reported in 1990 by the Department of Health and Human Services.47 Although there are many indications for hysterectomy, dysfunctional uterine bleeding is the indication given in as many as half of these procedures when there is no organic cause.48 An alternative to hysterectomy to treat dysfunctional uterine bleeding, unresponsive to medical therapy, is endometrial ablation, which was first reported by Goldrath and associates in 1981.49
The purpose of an endometrial ablation is to destroy the entire endometrium and avoid future regeneration and menstrual bleeding. Patients are initially pretreated with a gonadotropin-releasing hormone (GnRH) agonist or danazol for a period of 4 weeks to decrease the endometrial thickness to that seen in the menopausal state, thus facilitating the penetration of the energy to the level of the myometrium.
After adequate visualization of the entire uterine cavity, the laser fiber can be inserted through the operating channel of the hysteroscope. The Nd:YAG fiber can be used as either a touch or nontouch technique. With the touch technique, the laser fiber is activated with 40–50 W of energy and dragged on the endometrial surface beginning on the fundus and traveling down toward the endocervix in successive strokes (Fig. 31). This is done in a systematic way so that the entire surface is eventually covered. With the nontouch or blanching technique, the laser fiber is placed a few millimeters away from the endometrial surface while the laser energy is activated. Of the two methods, the touch technique is preferred because penetration is deeper, extending 4–6 mm into the uterine wall. This depth is sufficient for destruction of the endometrium.50
Patients requesting endometrial ablation as a treatment for dysfunctional uterine bleeding should understand that amenorrhea is possible only in 50–65% of patients, whereas oligomenorrhea is observed in 25–30% and no response to treatment is observed in 10–15% of patients.
Endometrial ablation with the resectoscope using the roller ball technique is an alternative to using the laser technique.
Laser Excisional Hysteroscopic Procedures
In addition to endometrial ablation, the laser can be used via hysteroscopy for removal of submucous myomas and polyps, transsection of uterine septa, and lysis of intrauterine adhesions. By using the laser fiber as a scalpel with the touch technique, the tissue to be removed can be morcellated and later removed with polyp forceps or a curette (Fig. 32). The advantage of the laser over scissors is that simultaneous coagulation can be accomplished. Media used for distention do not have to be free of electrolytes because electrical energy is not being used.
Myomas larger than 4 cm can be pretreated with GnRH agonists to decrease the size of the tumor, allow better visualization of the cavity, and decrease presurgical uterine bleeding. An alternative instrument to scissors and laser for removal of myomas is the resectoscope, which is now the preferred method.
Although the laser could be used for removal of polyps, these are usually of very soft consistency and are easily removed with hysteroscopic scissors. The laser should be reserved for larger myomatous lesions, where use of the instrument is more efficient and cost-effective.
Septate Uterus
The uterine septum is one of several congenital uterine abnormalities that arise from incomplete resorption of the müllerian ducts, and it occurs in approximately 1–3.5% of women.51 The uterine septum is commonly associated with habitual miscarriage and is transmitted as a polygenic or multifactorial pattern of inheritance. Diagnosis of a septate uterus is made by both hysterosalpingogram and diagnostic laparoscopy. This will exclude the possibility of an arcuate or bicornuate uterus, which does not necessitate surgical intervention.
Resection of a uterine septum can be performed with various instruments, including scissors, resectoscope, and Nd:YAG laser. Operative hysteroscopy is performed in conjunction with laparoscopy. This allows a more uniform depth of incision throughout the septum and warns the operator when a perforation is imminent.
The Nd:YAG laser fiber is usually set at 40 W and used by the touch technique, as if one were using a scalpel. The laser tip must be oriented so that it incises the septum at the midline and does not deviate from this line of incision (Fig. 33). This will ensure a relatively bloodless field of incision and avoids injury to the myometrium. The laser incision is continued until there is uniformity in light transmission throughout the fundus as observed by laparoscopy, or until bleeding from the fundal myometrium is visualized. The advantage of the laser fiber technique over the scissor technique is primarily one of diminished bleeding. The procedure usually takes 20–30 minutes, with gratifying results such as a 70–80% delivery rate, and it is similar to the more classic Tomkin's and Jones' intra-abdominal approach to metroplasty. The main advantages of operative hysteroscopy compared with the abdominal approach include quickness of surgery, minimal blood loss, no abdominal or uterine scar, minimal morbidity, no reduction in intrauterine volume, and diminished costs. In addition, patients are allowed to deliver vaginally.
Intrauterine Adhesions
Lysis of intrauterine adhesions can be readily accomplished with the Nd:YAG laser. Unlike curettage, with hysteroscopy one can selectively cut the scar tissue, limiting trauma to surrounding normal tissue. An IUD or pediatric Foley catheter is left in the cavity for a few weeks while the patient takes exogenous estrogens and antibiotics.
Complications with Hysteroscopic Laser
Complications inherent to hysteroscopy include uterine perforation, bleeding, fluid overload, and, very rarely, infection. The use of a sapphire tip with the Nd:YAG laser is contraindicated because its use with CO2 gas for cooling has been implicated in several deaths due to gas emboli.
OTHER CLINICAL APPLICATIONS OF LASERS IN GYNECOLOGY
The use of lasers in cervical–vulvar neoplasia as well as in laparoscopic intraabdominal–pelvic conditions such as endometriosis and adhesions was extremely popular in the 1970–2000s. During this last decade we have seen a gradual and continued decrease in laser use for these conditions as other less expensive and equally successful alternatives have been utilized. Examples of these alternate methods include rollerball and radiofrequency for endometrial ablations. Small electrode catheter tips and loop electrodes are also used for lysis of adhesions, resection of endometriosis, and for cervical cone biopsies.
At the same time, however, other uses of laser technology have been made available primarily in the area of cosmetic gynecology and have been utilized by dermatologists, gynecologists, and general and plastic surgeons. These novel uses include hair removal,52 removal of wrinkles53 and removal of varicose veins.54
Other controversial areas regarding the use of lasers that have been promoted recently without rigid scientific validation includes vaginal rejuvenation, and design laser vaginoplasty. These reconstructive techniques are being promoted by a few physician entrepeneurs mostly as an office procedure without regulation or oversight, and are mostly handled in cash.
REFERENCES
Kaplan I, Goldman J, Ger R: The treatment of erosions of the uterine cervix by means of the CO2 laser. Obstet Gynecol 41: 795, 1973 |
|
Einstein A: Zur Quantentheorie der Strahlung. Physikalische Zeitschrift 18: 121, 1917 |
|
Maiman TH: Stimulated optical radiation in ruby. Nature 187: 493, 1960 |
|
Patel CKN: Continuous wavelength action on vibrational rotational transitions of CO2. Physical Review 136: 1187, 1964 |
|
Bellina JH: Gynecology and the laser. Contemp Obstet Gynecol 4: 24, 1974 |
|
Bellina JH: Microsurgery of the fallopian tube with the carbon dioxide laser: Analysis of 230 cases with a two year follow-up. Lasers Surg Med 3: 255, 1973 |
|
Keye WR Jr, Hansen LW, Astin M: Argon laser therapy of endometriosis: A review of 92 consecutive patients. Fertil Steril 47: 208, 1987 |
|
Garden JM, O'Banion MK, Shelnitz LS et al: Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA 259: 1199, 1988 |
|
Baggish MS: Management of cervical intraepithelial neoplasia by carbon dioxide laser. Obstet Gynecol 54: 565, 1979 |
|
Baggish MS, Dorsey JH, Adelson M: A ten year experience treating intraepithelial neoplasia with the CO2 laser. Am J Obstet Gynecol 161: 60, 1989 |
|
Indman PD, Arndt BC: Laser treatment of cervical intraepithelial neoplasia in an office setting. Am J Obstet Gynecol 152: 674, 1985 |
|
Larson G, Gullberg B, Grundsell H: A comparison of complications of laser and cold knife conization. Obstet Gynecol 62: 213, 1983 |
|
Buscema J, Woodruff D, Parmley TH et al: Carcinoma in situ of the vulva. Obstet Gynecol 55: 225, 1980 |
|
Wolcott HD, Gallup DG: Wide local excision in the treatment of vulvar carcinoma in situ: A reappraisal. Am J Obstet Gynecol 150: 695, 1984 |
|
Baggish MS: Carbon dioxide laser treatment of vulvar intraepithelial neoplasia. In Baggish MS (ed): Basic and Advanced Laser Surgery in Gynecology. Norwalk, CT, Appleton-Century-Crofts, 1985 |
|
Baggish MS, Sze EH, Adelson MD et al: Quantitative evaluation of the skin and accessory appendages in vulvar carcinoma-in-situ. Obstet Gynecol 74: 169, 1989 |
|
Baggish MS, Dorsey JH: CO2 laser for the treatment of vulvar carcinoma in situ. Obstet Gynecol 57: 371, 1981 |
|
Baggish MS, Dorsey JH: Carbon dioxide laser for combination excisional vaporization conization. Am J Obstet Gynecol 151: 23, 1985 |
|
Wright VC, Davies E: Laser surgery for vulvar intraepithelial neoplasia: Principles and results. Am J Obstet Gynecol 156: 374, 1987 |
|
Baggish MS: Carbon dioxide laser treatment for condylomata acuminata venereal infections. Obstet Gynecol 55: 711, 1980 |
|
Krebs HB: Prophylactic topical 5-fluorouracil for the treatment of human papilloma virus associated lesions of the vulva and vagina. Obstet Gynecol 68: 837, 1989 |
|
Reid R: Superficial laser vulvectomy III. Am J Obstet Gynecol 166: 815, 1992 |
|
Kramer DN, Cutler SJ: Incidence and histo-pathology of malignancies of the female genital organs in the US. Am J Obstet Gynecol 118: 443, 1976 |
|
Townsend DE, Levin RU, Crun CP, Richart RM: Treatment of vaginal carcinoma in situ with carbon dioxide laser. Am J Obstet Gynecol 143: 101, 1982 |
|
Dorsey JH, Baggish MS: Multifocal vaginal intraepithelial neoplasia with uterus in situ. In Sharp F, Jordan J (eds): Gynecological Laser Surgery. Proceedings of the Fifteenth Study Group of the Royal College of Obstetricians and Gynecologists. Ithaca, NY, Perinatology Press, 1986 |
|
Bellina JH, Fick AC, Jackson JD: Application of the CO2 laser to infertility surgery. Surg Clin North Am 64: 899, 1984 |
|
Keye WR, Dixon JA: Photocoagulation of endometriosis by the argon laser through the laparoscope. Obstet Gynecol 62: 383, 1983 |
|
Lotze EC, Grunert GM: The use of lasers in infertility surgery. Clin Obstet Gynecol 323: 79, 1989 |
|
Martin DC (ed): Laparoscopic Appearance of Endometriosis, 2nd edn, p 38. Memphis, Resurge Press, 1990 |
|
Sutton CJ, Jones KD. Laser laparoscopy for endometriosis and endometriotic cysts. Surg Endosc 16: 1513-17, 2002 |
|
Doyle JB, DesRosiers JJ: Paracervical uterine relief of pelvic pain. Clin Obstet Gynecol 6: 72, 1963 |
|
Lichten EM, Bombard J: Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. J Reprod Med 32: 37, 1987 |
|
Martin DC, Diamond MP: Operative laparoscopy: Comparison of lasers with other techniques. Curr Probl Obstet Gynecol Fertil 9: 563, 1986 |
|
Siegler AM: Tubal surgery for infertility. In Sciarra JJ (ed): Gynecology and Obstetrics, p 123. Hagerstown, MD, Harper & Row, 1975 |
|
Boer-Meisl ME, te Velde ER, Habbema JD et al: Predicting the pregnancy outcome in patients treated for hydrosalpinx: A prospective study. Fertil Steril 45: 23, 1986 |
|
Bateman BG, Nunley WC Jr, Kitchin JD III: Surgical management of distal tubal obstruction: Are we making progress? Fertil Steril 48: 523, 1987 |
|
Patton GW Jr: Pregnancy outcome following microsurgical fimbrioplasty. Fertil Steril 37 (2): 150, 1982 |
|
Stromme WB: Salpingostomy for tubal pregnancy. Obstet Gynecol 1: 472, 1953 |
|
De Cherney AH, Romero R, Naffolin F: Surgical management of unruptured ectopic pregnancy. Fertil Steril 33: 411, 1980 |
|
Johns DA, Hardie RP: Management of unruptured ectopic pregnancy with laparoscopic CO2 laser. Fertil Steril 46: 703, 1986 |
|
Paulson JD: The use of carbon dioxide laser laparoscopy in the treatment of tubal ectopic pregnancies. Am J Obstet Gynecol 167: 382, 1992 |
|
Buttram VC, Reiter RC: Uterine leiomyomata: Etiology, symptomatology, and management. Fertil Steril 36: 433, 1981 |
|
Starks GC: CO2 myomectomy in an infertile population. J Reprod Med 2: 184, 1988 |
|
Harris WJ: Uterine dehiscence following laparoscopic myomectomy. Obstet Gynecol 80:3(part 2), 1992 |
|
Gurgan T, Kisnisa H, Yarali H et al: Evaluation of adhesion formation after laparoscopic treatment of polycystic ovarian disease. Fertil Steril 56: 1176, 1991 |
|
Farquhar C, Lilford RJ, Marjoribanks J, Vandekerckhove P. Laparoscopic drilling be diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev, 2007 |
|
US Department of Health and Human Services: Health United States 1991, p 231. Hyattsville, MD, 1992 |
|
Goldfarb HA: A review of 35 endometrial ablations using Nd:YAG laser for recurrent menometrorrhagia. Obstet Gynecol 76: 833, 1990 |
|
Goldrath MH, Fuller TA, Segal S: Laser photovaporization of endometrium for the treatment of menorrhagia. Am J Obstet Gynecol 104: 14, 1981 |
|
Baggish MS: Hysteroscopic laser surgery in clinical practice of gynecology. In: Endoscopic Laser Surgery, Vol. 2, p 187. New York, Elsevier, 1990 |
|
Ashton D, Amin HK, Richart RM et al: The incidence of asymptomatic uterine anomalies in women undergoing transcervical tubal sterilization. Obstet Gynecol 72: 28, 1988 |
|
Clayton WJ, Lipton M, Elford J, Rustin M, Sherr L: A randomized controlled trial of laser treatment among hirsute women with polycystic ovary syndrome. Br J Dermatol 152(5):986-92, 2005 |
|
D. Kopera, J. Smolle, S. Kaddu, H. Kerl: Nonablative laser treatment of wrinkles: meeting the objective? Assessment by 25 dermatologists. Br J Dermatol 150(5): 936-9, 2004 |
|
Mundy L, Merlin TL,Fitridge RA,Hiller JE: Systematic review of endovenous laser treatment for varicose veins. Br J Surg 92(10): 1889-94, 2005 |