Pathology of Cervical Carcinoma
Authors
INTRODUCTION
In the past few decades, the epidemiology of cervical cancer has undergone some important changes. In the United States, it is currently the third most common gynecologic cancer following those of the uterine corpus and ovary, with 12,900 new cases and 4100 deaths estimated to have occurred in 2015.1 While the incidence of cervical cancer has been steadily decreasing in the United States, with an annual percentage change of –2.5% between 1992 and 2011,2 the survival rate for cervical cancer has not improved substantially since 1975.1 Worldwide, there were 527,600 new cases of cervical cancer and 265,700 cervical cancer-related deaths in 2012.3 Incidence and death rates of cervical cancer are particularly high in less developed countries, where it is the second most commonly diagnosed cancer and third leading cause of death in women based on 2012 data.3
Squamous cell carcinoma, which comprised more than 90% of primary cervical cancers before 1960, has decreased steadily in incidence, in large part owing to effective cytologic detection and subsequent eradication of its precursors. The incidence of cervical adenocarcinoma, in contrast, has increased significantly, with a reported 22.5% increase in age-adjusted incidence between 1973 and 1996.4
In addition to squamous and glandular neoplasms, neuroendocrine, mesenchymal, and metastatic tumors can also involve the cervix. New techniques in diagnosis have helped to modify histologic classifications. In particular, the use of immunohistochemistry has improved diagnostic accuracy. This chapter deals primarily with cervical malignancies and precursors of squamous and glandular carcinoma. The emphasis is on proper handling and reporting of surgical specimens, pathologic classification and criteria, and important pathologic parameters, all of which have an impact on the prognosis and management of the patient. Finally, recent updates in screening and prevention are discussed.
PROPER HANDLING AND PROCESSING OF CERVICAL SPECIMENS
The accuracy of histologic interpretation and diagnosis is strongly governed by the quality of tissue provided, as well as proper handling and processing of the specimen. In cervical biopsy specimens, many factors lead to unsatisfactory specimens. Distortion and crush artifact usually result from the use of dull or small instruments.5 Damage and denudation of the mucosa occur readily even with minor trauma to the cervix. Poor orientation with tangential sections not only precludes accurate interpretation of lesion grade or invasive extent, but also contributes to erroneous diagnoses. Additionally, use of the hemostatic agent Monsel’s solution can cause dark brown discoloration of the tissue, also limiting morphologic interpretation, although an effective destaining technique has been described.6 After the tissue is obtained, the specimen should be placed on a piece of paper towel with the mucosal surface upward and the base of the tissue downward. After the blood and mucus at the base of the tissue become adherent to the paper towel, the specimen and the paper towel are placed in buffered formalin and submitted to the laboratory accompanied by adequate demographic and clinical information.
The single most common cause of an inadequate biopsy specimen is the failure to provide abnormal tissue of sufficient amount and depth. Without the underlying stroma, an invasive neoplasm is likely to be interpreted as an in situ lesion. Verrucous squamous carcinoma and papillary, exophytic neoplasms are particularly prone to being underdiagnosed as benign proliferations when the specimen contains only the superficial layers of the neoplasm. Unless the specimen includes the base of the tumor and its underlying stroma, a correct diagnosis may not be made. In mixed tumors (e.g., adenosquamous carcinoma or carcinosarcoma), an inadequate biopsy specimen may not contain both squamous and glandular cellular elements, again resulting in misdiagnosis. Since the earliest squamous intraepithelial lesions occur at the squamocolumnar junction, biopsies from either endocervix or ectocervix only are inadequate for pathologic evaluation. Even in the setting of a negative colposcopic exam, random biopsy of the transformation zone has been demonstrated to be of use in detecting unseen high-grade lesions.7
For the treatment of high-grade squamous intraepithelial lesions (HSIL)/cervical intraepithelial neoplasia (CIN), cervical tissue removed by loop electrosurgical excision procedure (LEEP) has become widely accepted. The pathologist usually receives tissue from the anterior lip and the posterior lip, either separately or as a single circumferential portion of cervix. A third disc-shaped tissue fragment from the base of the excision or endocervical margin is sometimes received (“top hat”), and a post-LEEP endocervical curettage (ECC) is usually also received. Diagnostic problems in these specimens are most often caused by a lack of orientation and thermal damage. The specimen should be oriented by a suture or ink to indicate 12 o'clock. This allows sectioning radially around the endocervical canal. Without orientation, the specimen may be cut erroneously and the lesion may be missed entirely. Excessive maneuvers by the surgeon or pathologist lead to extensive denudation and loss of the cervical mucosa and potentially the lesion. Prolonged contact between the loop and the tissue results in broad zones of thermal damage, coagulative necrosis, and tissue distortion that preclude an accurate diagnosis of the lesion and the status of excision margins. In one study, 20% of ectocervical margins and 44% of endocervical margins removed by LEEP were unsatisfactory for evaluation.8 The use of the immunohistochemical stain p16 (see below) can help with assessment of excessively distorted and cauterized margins. With proper surgical technique, orientation of the specimen, marking of the margins by the surgeon, and appropriate use of immunohistochemistry, most LEEP specimens are reported to be adequate for interpretation. The status of excision margins correlates well with the subsequent recurrence of SIL/CIN.9
Cervical conization by cold knife is most often performed for extensive HSIL and for HSIL with suspected coexisting invasive carcinoma. If the cervical biopsy specimen or endocervical curettage specimen suggests the possibility of endocervical adenocarcinoma in situ, cervical conization is often used to confirm the diagnosis, excise the lesion, and exclude coexisting invasive adenocarcinoma.
Cervical conization specimens should be oriented by placing a suture at 12 o'clock, and should be submitted to the laboratory in the fresh state. After opening at 12 o'clock along the cervical canal, the specimen is pinned out on a cork board, immersed in fixative, and subsequently radially sectioned in a clockwise fashion. This method of sectioning provides perpendicular cuts through the mucosa and wall allowing for accurate determination of the disease process and its extent, depth, and relation to the surgical margins.5 An already fixed specimen may be too rigid for opening and pinning out.
In radical hysterectomy specimens, representative sections should include the most advanced area of tumor to determine the maximal stromal invasion. All surgical margins should be carefully identified and marked with ink. The parametrium needs special attention, as involvement of this area by the tumor has important clinical implications.
Pelvic lymph nodes should be properly labeled as to their anatomic sites. Benign Müllerian inclusions (endosalpingiosis) and decidual cells are known to occur in the peritoneum and in pelvic and paraaortic lymph nodes. Without knowledge of a pregnancy history, decidual cells in lymph nodes and pelvis may be misclassified as metastatic carcinoma cells. Serial sectioning of lymph nodes at 3 mm intervals may increase detection of small metastastic foci. Similarly, tissue reactions to prior surgery, radiation, and complications, such as intestinal perforation, can be difficult to separate from malignant tumor, especially on frozen sections. Thus, an accurate pathologic interpretation requires a close collaboration, communication, and understanding between the clinician and the pathologist.
In any cervical excision, the pathology report should include the following:
- Specimen/procedure type
- Tumor size
- Histologic type and grade
- Depth of stromal invasion
- Status of surgical margins
- For LEEP or cone specimens: endocervical, ectocervical, and deep margins
- For hysterectomy specimens: vaginal/ectocervical, deep/paracervical, and lateral/parametrial margins
- The presence or absence of lymph-vascular space invasion.
Involvement of endometrium should be noted, although its presence does not alter the FIGO stage. All pelvic and paraaortic lymph nodes received should be embedded and carefully studied, sometimes by multiple levels as indicated by institutional guidelines.
MORPHOGENESIS OF CERVICAL CANCER
The ectocervix is covered by mature squamous mucosa, whereas the endocervix is lined by mucus-secreting endocervical epithelium. The latter undergoes squamous metaplasia through both reserve cell hyperplasia (true squamous metaplasia) as well as squamous epithelialization (direct ingrowth of existing squamous epithelium). In addition, metaplastic glandular cells (ciliated tubal, tuboendometrioid, oxyphilic) and mesonephric remnants occur in the endocervix. Rare neuroectodermal cells (argyrophilic, neuroendocrine, melanocytic cells) also exist in the normal cervix. These epithelial and neuroectodermal cells are potential progenitors or components of cervical carcinoma. Cervical stromal cells may rarely become neoplastic, presenting as a pure mesodermal tumor or mixed with an epithelial neoplasm.
Most cervical squamous cell carcinomas likely originate from the metaplastic squamous epithelium located between the original and new squamocolumnar junctions, the transformation zone. Adenocarcinomas typically occur within the endocervical canal. Recent studies have demonstrated evidence that carcinogenic HPV-related squamous intraepithelial lesions and cervical cancers are linked to a small, discrete cell population of cells that localizes to the squamocolumnar (SC) junction of the cervix.10
Clinical and pathologic data support the concept that most invasive carcinomas develop from a preneoplastic intraepithelial lesion. Although many aspects of the cause of cervical cancer remain to be determined, human papillomavirus (HPV), particularly high-risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82),11 has been identified in squamous, glandular, and other less common types of cervical neoplasms. HPV infection is believed to play an important role at least as an initiator of cervical neoplasia (see below for additional discussion).
CLINICAL PRESENTATION AND NATURAL HISTORY OF CERVICAL CANCER
Carcinoma of the cervix may have a variety of clinical presentations. It may be discovered on routine Papanicolaou (Pap) smear in an asymptomatic woman; patients may present with irregular vaginal bleeding; or, in late stages, patients may present with symptoms of a mass lesion or metastatic disease. The cervix with early carcinoma has a poorly circumscribed granular or eroded appearance and bleeds easily on contact. At later stages, nodular, ulcerated lesions or an exophytic mass appear. Endophytic growth occurs in the cervical canal with direct infiltration into the wall causing diffuse enlargement and hardening of the cervix. The mucosal surface may be covered by normal epithelium, and the underlying malignant cells may escape detection by cytologic smear. Some cervical carcinomas are located in the cervical canal and grow endophytically without causing gross abnormality. When the cervix is diffusely enlarged, bulky, and larger than 6 cm in size, it is referred to as a barrel-shaped cervix. This gross appearance can be seen in any tumor type, although it is most commonly associated with adenocarcinoma.
Local extension of cervical carcinoma proceeds in a predictable manner to involve the endometrium superiorly and the upper vagina inferiorly. Parametrial involvement results from extension through the cervical stroma or lymph-vascular space invasion. From the parametrium the tumor may extend laterally to the pelvic sidewall, anteriorly to the bladder base, or posteriorly to the rectum. An unusual pattern of local spread is in the form of carcinoma in situ extending to the endometrium and/or fallopian tubes.
The International Federation of Gynecologists and Obstetricians (FIGO) updated their clinical staging system in 2009, taking into account the issues of surgical vs. clinical staging, early invasion amenable to more conservative treatment, and the utility of additional substages, among other considerations.12, 13 The current staging guidelines are outlined below (Table 1).
Table 1. Definition of FIGO clinical staging for cervical cancer
| Stage | Definition | ||
| I |
|
| Carcinoma strictly confined to the cervix (extension to the corpus should be disregarded) |
| IA |
| Invasive cancer identified only microscopically; invasion limited to stromal invasion with maximum depth of 5 mm and no wider than 7 mm (the depth of invasion should not be more than 5 mm taken from the base of the epithelium, either surface or glandular, from which it originates; vascular space involvement, either venous or lymphatic, should not alter the staging) | |
|
| IA1 | Measured invasion of stroma ≤3 mm in depth and ≤7 mm in width | |
|
| IA2 | Measured invasion of stroma >3 mm and <5 mm in depth and ≤7 mm in width | |
|
| IB |
| Clinical lesions confined to the cervix or preclinical lesions greater than stage IA |
|
| IB1 | Clinical lesions no greater than 4 cm in size | |
|
| IB2 | Clinical lesions greater than 4 cm in size | |
| II |
|
| The carcinoma extends beyond the cervix but has not extended to the pelvic wall or the lower third of the vagina |
|
| IIA |
| Involvement of up to the upper two thirds of the vagina, with no obvious parametrial involvement |
|
|
| IIA1 | Clinically visible lesion ≤4 cm |
|
|
| IIA2 | Clinically visible lesion >4 cm |
|
| IIB |
| Obvious parametrial involvement without involvement of pelvic sidewall. |
| III |
|
| The carcinoma has extended to the pelvic wall; on rectal examination, there is no cancer-free space between the tumor and the pelvic wall; the tumor involves the lower third of the vagina; all cases with a hydronephrosis or nonfunctioning kidney are included unless they are known to be due to other causes |
|
| IIIA |
| The carcinoma has spread to the lower third of the vagina but not to the pelvic wall |
|
| IIIB |
| The carcinoma has grown into the pelvic wall, or there is hydronephrosis/non-functioning kidney |
| IV |
|
| The carcinoma has extended beyond the true pelvis or has clinically involved the mucosa of the bladder or rectum |
|
| IVA |
| Spread to adjacent pelvic organs |
|
| IVB |
| Spread to distant organs |
Adapted from FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet 2014;125(2):97-8.14
SQUAMOUS INTRAEPITHELIAL LESIONS
Cervical intraepithelial neoplasia (CIN) is a relatively common problem, especially in women of reproductive age. Laboratory surveys from the mid-1990s from the College of American Pathologists suggest that more than 1 million women are diagnosed each year with low-grade cervical intraepithelial lesions and that approximately 500,000 are diagnosed with high-grade cervical cancer precursor lesions.15
Of the more than 150 HPV types known to exist, only about 15 types of HPV viruses are known to increase the risk of cervical cancer. High risk (carcinogenic) types of HPV include HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82. Of these, types 16, 18, 45, 31, 33, 52, 58, and 35 account for over 95% of cervical squamous cell carcinomas, with types 16 and 18 associated with the greatest risk of carcinoma.11 The most common low-risk HPV types are HPV 6 and 11, infection with which usually results in low-grade, non-preneoplastic lesions.
In recent years, an increased understanding of HPV biology has modified our understanding of cervical carcinogenesis and the significance of different grades of precursor lesions. Traditionally, cervical intraepithelial lesions have been classified using a three-tiered system (originally mild/moderate/severe dysplasia, subsequently CIN 1/2/3), corresponding to a presumed stepwise progression from low to intermediate to high-grade intraepithelial lesions, followed by invasive carcinoma. Additionally, some pathologists favor employing two additional tiers of HPV-related intraepithelial lesions: “HPV effect”/“koilocytic atypia”/“flat condyloma,” corresponding to viral cytopathic effect sans dysplasia, and “carcinoma in situ,” corresponding to full thickness dysplasia. However, it appears that the interaction between HPV and the cervical epithelium is actually dichotomous. Infection of the cervical epithelium may result in either transient productive infection with viral proliferation, or in persistent infection with progression to precancer. The latter is mediated primarily by two viral proteins, E6 and E7.
The early viral protein E6 binds to p53 protein in the host epithelial cell, resulting in its degradation and loss of its normal functions promoting growth arrest and apoptosis. The early viral protein E7 binds to pRb protein in the host epithelial cell, resulting in release of the DNA replication-promoting transcription factor E2F. In high-risk HPV types, the E6 and E7 proteins have greater affinity for host p53 and pRb, respectively. Disruption of the early viral protein E2 by viral integration into the host genome also plays a role in cell cycle disruption by causing increased expression of E6 and E7.16, 17
In order to better align diagnostic terminology with the current understanding of HPV biology, a multidisciplinary consensus effort was undertaken in 2012 to standardize diagnosis and terminology for HPV-related squamous lesions of all lower anogenital tract sites. This initiative was termed the Lower Anogenital Squamous Terminology (LAST) project, and was co-sponsored by the College of American Pathologists (CAP) and the American Society for Colposcopy and Cervical Pathology (ASCCP).18 One of the resulting consensus guidelines was switching to a two-tiered nomenclature for HPV-related lesions of the lower anogenital tract. Specifically, the authors recommended use of the terms “low-grade squamous intraepithelial lesion” (LSIL) and “high-grade squamous intraepithelial lesion” (HSIL). These terms had previously been used only for cytology interpretation under the Bethesda system. Histologic LSIL corresponds to CIN 1, including both lesions with loss of maturation in the lower third of the epithelium (mild dysplasia) and those with HPV cytopathic effect without dysplasia. Histologic HSIL corresponds to CIN 2 and CIN 3, and includes the spectrum from moderate dysplasia to carcinoma in situ.18
Justification for these new guidelines is multifold. Firstly, a dichotomous terminology better corresponds to the dichotomous biology of HPV infection. Secondly, switching to a two-tiered system improves diagnostic reproducibility.19, 20, 21, 22 In particular, CIN 2 has been demonstrated to have especially poor reproducibility, and no unique biology of CIN 2 vs. CIN 1 and 3 has been elucidated. The authors of the LAST project suggest that CIN 2 in fact consists of an admixture of CIN 1 and CIN 3 which cannot be accurately distinguished on morphology.18
In order to provide a smoother transition to the new system of nomenclature, as well as to allow for conservative management of young women with CIN 2 (which has a higher regression rate than lesions diagnosed as CIN 3), the LAST project guidelines recommend use of LSIL/HSIL terminology with parenthetical use of the older terminology (e.g. “High-grade squamous intraepithelial lesion (CIN 2)”). This practice will allow clinicians to continue to have the option for more conservative management of this lesion in younger patients.
The diagnosis of HSIL/CIN 2 in particular is also subject to certain caveats as per the LAST project guidelines, specifically the requirement for confirmation using p16 immunohistochemistry (discussed in more detail below).
Low-grade squamous intraepithelial lesion
Low-grade squamous intraepithelial lesion (LSIL) in the cervix is most commonly flat, but may rarely be exophytic (Fig. 1). Flat LSIL is characterized by dysplasia in the lower one third of the epithelium and/or HPV cytopathic effect. Condyloma acuminatum is an exophytic papillary lesion with viral cytopathic effect but no significant dysplasia. Although the LAST guidelines recommend classifying condyloma acuminatum as LSIL, with an optional parenthetical qualifier, an argument can be made that it is important to distinguish condyloma acuminatum from flat LSIL. Whereas up to 80% of flat LSIL is related to high-risk HPV,23 condyloma acuminatum is essentially always associated with the low-risk HPV types 6 and 11, with almost no risk for progression without co-infection with other HPV types.24, 25 Flat LSIL regresses in 57% of cases, persists in 32%, and progresses to HSIL or invasive carcinoma in 12%.26 It should be noted, however, that current management guidelines do not distinguish between these two lesions for the purposes of follow-up and treatment.
The typical cytopathic effect of HPV on squamous epithelial cells can be diagnosed by the presence of demarcated cytoplasmic clearing (“halos”), large, coarse and hyperchromatic nuclei, nuclear membrane irregularity (“raisinoid” nuclei), and bi- or multinucleation. Similar changes can be seen with reactive epithelial change in the setting of inflammation, as well as with degenerative change as is frequently encountered in detached epithelial fragments in cervical biopsy and endocervical curettage specimens. Careful assessment for typical cytologic features and for the presence of potentially confounding factors should therefore be undertaken.
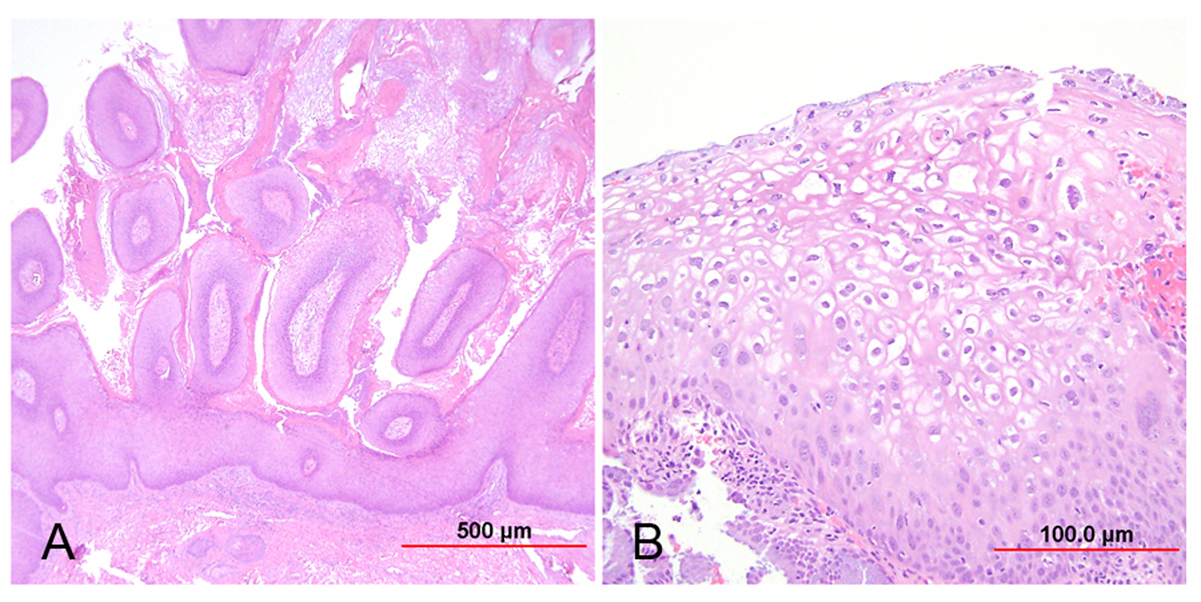 Fig. 1. Low-grade squamous intraepithelial lesion (LSIL). (A) Condyloma acuminatum shows papillomatosis, acanthosis, parakeratosis, and hyperkeratosis. Each papillary frond has a tiny blood vessel at its core. (B) Flat LSIL has koilocytotic atypia (bi/multinucleation, irregular nuclear contours, nuclear enlargement and hyperchromasia, and cytoplasmic clearing [halos]). Dysplasia may be present in up to the lower one third of the epithelium. (Hematoxylin-eosin stain, red bar: original magnification.)
Fig. 1. Low-grade squamous intraepithelial lesion (LSIL). (A) Condyloma acuminatum shows papillomatosis, acanthosis, parakeratosis, and hyperkeratosis. Each papillary frond has a tiny blood vessel at its core. (B) Flat LSIL has koilocytotic atypia (bi/multinucleation, irregular nuclear contours, nuclear enlargement and hyperchromasia, and cytoplasmic clearing [halos]). Dysplasia may be present in up to the lower one third of the epithelium. (Hematoxylin-eosin stain, red bar: original magnification.)
High-grade squamous intraepithelial lesion
High-grade squamous intraepithelial lesion (HSIL) is a precancerous lesion characterized by an abnormal parabasal-like cell proliferation with loss of polarity, overlapping nuclei, high nuclear-to-cytoplasmic ratio, increased mitoses, dyskeratosis, apoptosis, hyperchromasia, and significant nuclear atypia. Viral cytopathic effect may or may not be present. The immature and atypical epithelium extends to at least the middle third of the epithelium and may involve up to the full thickness of the epithelium.
HSIL encompasses both CIN 2 (dysplasia involving the lower two thirds of the epithelium) and CIN 3 (dysplasia extending to the upper third of the epithelium) (Fig. 2). Though both are considered preneoplastic lesions with a risk for progression, the rates of regression, persistence, and progression differ for these two lesion grades. Compared to CIN 3, CIN 2 regresses more frequently (43% vs. 32%), persists less frequently (35% vs. 56%), and progresses to invasive carcinoma less frequently (5% vs. 12%).26 Because CIN 2 is considered to be a somewhat ambiguous diagnostic entity without a biologic correlate, the LAST guidelines recommend use of ancillary testing to confirm the diagnosis of HSIL. Specifically, the authors of LAST recommend performing an immunohistochemical stain for p16 (see biomarker discussion below) to confirm the diagnosis of HSIL when the morphology is consistent with CIN 2, as well as to adjudicate CIN 1 vs. CIN 2 diagnostic uncertainty. Diffuse, strong, block positivity for p16 in at least the lower third of the epithelium supports the diagnosis of HSIL, while all other staining patterns (negative, focal, patchy) favor a diagnosis of LSIL or less (Fig. 3).18 It should be noted, however, that approximately half of LSILs will express diffuse block p16 positivity27, 28, 29 (correlating with high-risk HPV),30 and therefore it is important that the morphologic features also support a diagnosis of HSIL. If p16 immunostaining is not readily available, morphologic subclassification of HSIL (CIN 2 vs. 3) and characterization of the extent of the lesion may be helpful for patient management.
Fig. 2. High-grade squamous intraepithelial lesion (HSIL). (A) CIN 2: Dysplastic squamous cells in the basal two-thirds of the epithelium; the upper half of the epithelium shows koilocytic atypia; (B) CIN 3: Dysplastic squamous cells present throughout the full thickness of the epithelium; koilocytic atypia is present in the superficial layers. (Hematoxylin-eosin stain)
Fig. 3. p16 immunohistochemistry. (A) Diffuse strong p16 expression in area of atypical attenuated squamous epithelium, supporting diagnosis of HSIL. (Hematoxylin-eosin stain, red bar: original magnification) (B) Patchy p16 expression in area of atypical squamous epithelium, arguing against diagnosis of HSIL.
In contrast to LSIL, true in situ neoplasia is a monoclonal proliferations of cells that show evidence of genetic instability.31 The majority of such lesions are aneuploid and have loss of heterozygosity at nonrandom chromosomal loci that may be associated with neoplastic development.32 HSIL also has much less heterogeneity with respect to associated HPV types than do CIN 1 lesions.31
Current management guidelines from the ASCCP recommend excision of HSIL (CIN 2 or 3) in most women. However, in young women aged 21–24 years with CIN 2 specifically, observation is preferred.33
Biomarkers in the diagnosis of squamous intraepithelial lesions
Numerous biomarkers have been investigated for their potential utility in the diagnosis of cervical squamous intraepithelial lesions. These include p16ink4, Ki-67 (MIB-1), ProEx C, high-risk HPV, the late viral proteins L1 and L2, and telomerase. The discussion below focuses on p16 and Ki-67, the most widely used in clinical practice.
p16
The tumor suppressor gene CDKN2a encodes the protein p16ink4 (p16), a cyclin-dependent kinase inhibitor which promotes arrest of the cell cycle.34 Specifically, p16 inactivates cdk4-Cyclin D1 and cdk6-Cyclin D1 complexes, which normally phosphorylate pRb and cause it to release a transcription factor (E2F) which promotes transition of the cell into S phase. Due to the inverse relationship between p16 and pRb, binding of the viral protein E7 to the host cell pRb results in increased expression of p16.34 This inverse relationship allows p16 expression to be a convenient marker of high-risk HPV infection with cell cycle disruption.35 Immunohistochemical expression of p16 has been correlated with high-risk HPV and with increasing degree of dysplasia.23, 30, 36, 37, 38, 39, 40
Positivity for p16, defined as strong diffuse block positivity in at least the lower third of the epithelium, is present in the vast majority of HSILs (81.1–100% of CIN 2 and 100% of CIN 3) and a subset of LSILs (39–53%).28, 29, 35, 41 Therefore, while p16 is an excellent marker for the distinction of HSIL from benign mimics, it is not useful for LSIL vs. benign mimics. Additionally, given the expression of p16 in a significant percentage of LSILs, some caution is necessary in adhering to the new guidelines recommending use of p16 to adjudicate CIN 1 vs. CIN 2 diagnostic uncertainty, and some authors disagree with the use of p16 in this setting due to the risk of overdiagnosis and overtreatment of LSIL.
The use of p16 increases interobserver agreement and individual pathologist accuracy.29, 42, 43, 44, 45 It has also been demonstrated to be useful in identifying subtle foci of HSIL which were not initially identified on routine hematoxylin-and-eosin (H&E)-stained sections, and thus may be a useful screening tool in certain high risk scenarios (see below).29, 46
The potential prognostic significance of p16 has also been investigated, particularly for LSIL, in which it may predict a greater likelihood of progression and lesser likelihood of regression.47, 48, 49, 50, 51, 52 The data are, however, as yet insufficiently compelling to apply to clinical practice. Studies on CIN 2 and p16 as a predictor of progression are fewer, with somewhat inconsistent results, but there is some evidence that p16 may be a predictor of subsequent definitive HSIL.53, 54, 55, 56, 57, 58
The authors of the LAST project reviewed several biomarkers, and found sufficient evidence to make a recommendation only for p16. The guidelines recommend use of p16 in the following scenarios:18
- Distinguishing HSIL from benign mimics (e.g. atrophy, reactive change, tangential sectioning, immature metaplasia)
- Confirming the morphologic impression or consideration of CIN 2
- Adjudicating professional disagreement when HSIL is in the differential diagnosis
- Screening for subtle foci of HSIL in a biopsy specimen with CIN 1 or less, in the setting of a prior cytology diagnosis conferring a high risk for HSIL [specifically: HSIL, atypical squamous cells of uncertain significance (ASC-US)/HPV 16+, atypical squamous cells, cannot rule out HSIL (ASC-H), and atypical glandular cells, not otherwise specified (AGC NOS)].
The other frequently used immunohistochemical marker in the diagnosis of cervical intraepithelial lesions is Ki-67, a nuclear nonhistone protein which serves as a marker of cell proliferation.40 Typically, normal and metaplastic squamous epithelium expresses Ki-67 only in the parabasal cell layer, while it extends to the intermediate cell layers in LSIL, and is present in all layers in HSIL.59 However, increased expression of Ki-67 is not specific, as reactive and inflamed epithelium can also have an increased proliferation index.38 Additionally, some studies have found p16 staining to be more specific than Ki-67 for HSIL, without significant added utility of Ki-67 in conjunction with p16.27 While Ki-67 can have some utility in distinguishing HPV-related intraepithelial lesions from non-HPV-related lesions, it is less useful for distinguishing LSIL from HSIL.
High-risk HPV
Testing for high-risk HPV is currently performed on cervical cytology specimens as a primary screening test, as part of co-testing for women >30 years of age, and as a reflex test following an abnormal cytologic diagnosis.33, 60, 61 The use of genotyping assays is increasing, as it has been demonstrated that the presence of HPV 16 or 18 in the setting of ASCUS is associated with a significantly higher risk of HSIL than are other high-risk HPV types.62 On tissue sections, in situ hybridization (ISH) for different HPV types can be performed. While the mere presence of high-risk HPV would not be useful in grading lesions (although this can be used to confirm diagnosis of an HPV-related lesion vs. a non-HPV-related lesion), the presence of punctate rather than diffuse signal has been demonstrated to correlate with viral integration into the host cell genome.23, 63, 64 While this could theoretically be used to assist with grading lesions, the utility is limited by some variability in integration and signal type data, as well as issues with sensitivity compared with polymerase chain reaction (PCR) and p16.65, 66, 67, 68, 69
ProEx C
ProEx C targets topoisomerase II-alpha and minichromosome maintenance protein-2. The immunostain demonstrates an expression pattern that is similar to Ki-67,70, 71 and expression of ProEx C correlates with p16 and high-risk HPV positivity.40, 71, 72 Similar to p16, the utility of this marker seems to be in distinguishing HSIL from benign mimics, but limited for determining LSIL vs. HSIL, and the data are much less abundant than for p16.
L1 and L2
The late viral proteins L1 and L2 encode capsid proteins and are expressed only in terminally differentiated squamous cells.73 The expression of L1 is inversely correlated with lesion grade, and there is some evidence that absence of L1 may also predict progression or unsampled concurrent higher grade lesions.73, 74, 75, 76, 77 A similar inverse relationship with lesion grade has been described for L2, although the data are fewer.78 Currently, the data for both markers is insufficient for incorporation into routine practice.
SQUAMOUS CELL CARCINOMA AND ITS VARIANTS
Superficially invasive squamous cell carcinoma (SISCC)
This entity, previously called microinvasive carcinoma, was first described in 1847 as a squamous cell carcinoma of the cervix with ≤5 mm of invasion into the stroma.79 Since that time, various other terms and definitions have been employed, making “microinvasion” a somewhat confusing entity. For example, the Society of Gynecologic Oncologists defined microinvasive carcinoma of the cervix in 1973 as having a maximum depth of invasion of 3 mm, with the additional requirement of having no lymph-vascular space invasion.80 Also relevant to the definition of microinvasion is the evolution of the FIGO staging system for carcinoma of the cervix. The definition of FIGO stage IA carcinomas was refined in 1994, with stage IA1 defined as having a maximum depth of invasion of 3 mm a maximum horizontal extent of 7 mm, and stage IA2 defined as having a maximum depth of invasion between 3 and 5 mm and again a maximum horizontal extent of 7 mm.81 The 2009 revision of cervical cancer staging from FIGO retained these definitions.13
The importance of a consistent definition for microinvasive carcinoma in the cervix is its ability to identify patients who may be managed effectively with more conservative treatment. Specifically, it has been demonstrated that patients with microinvasive cervical carcinoma can be treated with LEEP, cold knife conization, or simple (rather than radical) hysterectomy, while still having a low risk of lymph node metastases and recurrence.80, 82, 83, 84, 85, 86, 87, 88, 89 Sources of controversy in the definition over the decades have related to the specific depth of invasion allowed, the need for a horizontal size cut-off, whether the absence of lymph-vascular space invasion should be required, and the significance of positive margins (for either invasive or intraepithelial lesions). It has been demonstrated that a depth of invasion of 5 mm or less confers a low risk of death or metastasis (1.2%), with some reporting that the specific depth less than 5 mm does not provide additional prognostic value.90, 91, 92 However, others have been able to demonstrate that a depth of invasion between 3 and 5 mm confers a higher risk of metastasis, recurrence, and death than a depth of invasion 3 mm or less.85, 87, 93, 94, 95, 96 These later data are reflected in the current FIGO staging system, with its subdivision of stage IA carcinomas as IA1 and IA2.
Given the historic controversy and inconsistency in the definition of microinvasion, the LAST project included in its guidelines updated and standardized definitions for this entity in each lower anogenital tract site. In order to avoid confusion with the various prior definitions of microinvasion, the authors proposed switching to the new terminology of “superficially invasive squamous cell carcinoma” (SISCC).18 In the cervix, SISCC was defined as a FIGO stage IA1 carcinoma, corresponding to a microscopic lesion measuring no more than 3 mm in depth and no more than 7 mm in horizontal extent (Fig. 4). Although the presence of lymph-vascular space invasion (which may increase the risk of lymph node metastasis and recurrence)85, 94, 95 as well as the presence of intraepithelial lesions at the margin (which increases the risk of recurrence and residual disease)97, 98, 99, 100, 101, 102, 103, 104, 105 should be reported, the absence of these features is not required to render a diagnosis of SISCC.18 While the invasive component must be completely excised to render a definitive diagnosis of SISCC, invasive carcinomas within the size cut-offs but extending to margins can be diagnosed as “at least superficially invasive squamous cell carcinoma” with a comment regarding positive margins.
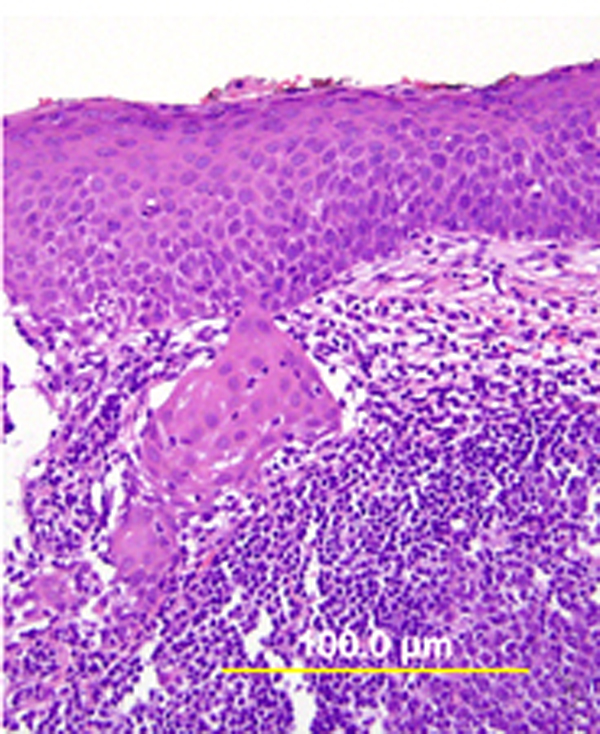 Fig. 4. Superficially invasive squamous cell carcinoma. HSIL with budding off of malignant cells downward into the underlying stroma and significant associated lymphocytic infiltrate. (Hematoxylin-eosin stain, yellow bars: original magnification.)
Fig. 4. Superficially invasive squamous cell carcinoma. HSIL with budding off of malignant cells downward into the underlying stroma and significant associated lymphocytic infiltrate. (Hematoxylin-eosin stain, yellow bars: original magnification.)
Invasive squamous cell carcinoma
Squamous cell carcinoma (SCC) is by far the most common tumor of the cervix. While the incidence of SCC has declined in the United States over the last few decades (approximately 40% between 1973 and 1996), it continues to account for 70–80% of cervical carcinomas in the United States,4 and the worldwide incidence of SCC is in fact increasing.106 SCC most commonly affects women in their mid-30s to mid-40s, but can affect women over a wide age range, from <20 to >80 years of age. The vast majority of these tumors (>99% worldwide) are related to infection with HPV.108
Microscopically, invasive SCC is characterized by infiltrating nests of neoplastic squamous epithelium in the stroma. These nests typically display an irregular, angulated shape, and may demonstrate increased cytoplasmic keratinization (so-called paradoxical maturation). Both of these features help distinguish true invasion from HSIL extending into endocervical glands (which is still considered an in situ process).
Various subtypes of cervical SCC have been described. In 1958 Wentz and Reagan109 divided cervical SCC into three cell types: large cell keratinizing, large cell nonkeratinizing, and small cell. With the advent of electron microscopy and immunohistochemistry, it became apparent that what had been termed small cell SCC really represents a heterogeneous group of tumors, including basaloid SCC and small cell neuroendocrine carcinoma. The current WHO subclassification of SCC is as follows:
- Keratinizing
- Non-keratinizing
- Basaloid
- Warty
- Papillary
- Verrucous
- Squamotransitional
- Lymphoepithelioma-like
These subtypes are discussed in greater detail below.
The value of separating SCC by subtypes was evaluated using data from the Gynecologic Oncology Group (GOG). Among women with stage I SCC treated surgically, the subtype was not predictive of pelvic nodal metastasis or outcome.110, 111 The percentages of patients who were progression-free at 5 years were 84% for large cell keratinizing SCC and 74% for large cell nonkeratinizing SCC, a difference which was not statistically significant. The differences among different grades were also not statistically significant, with the following percentages of patients who were progression-free at 5 years: 75% for grade 1, 82% for grade 2, and 78% for grade 3.110 However, the consistency and reproducibility among pathologists in separating keratinizing and nonkeratinizing tumors based on cervical biopsy specimens may have caused some problems in interpretation. In a GOG study of women with stage IIB–IVA SCC treated by radiation therapy, when the histologic criteria were modified to include all tumors with individual cell keratinization in the large cell keratinizing category, this group had a significantly higher recurrence/death rate than the large cell nonkeratinizing group (65.8% vs 53.5%, p = 0.0074).112
Keratinizing SCC
Keratinizing SCC is characterized by well-differentiated squamous cells infiltrating as nests, cords, and sheets of cells (Fig. 5). The cells have abundant cytoplasm, large pleomorphic nuclei, and inconspicuous nucleoli. Keratin pearls and intercellular bridges are evident. Mitotic figures are noted occasionally but are not typically numerous. The presence of even one keratin pearl has been considered sufficient for diagnosis. Keratin pearls are circular whorls of squamous epithelium with central nests of acellular keratin. Keratohyaline granules and individual cell keratinization are also seen.
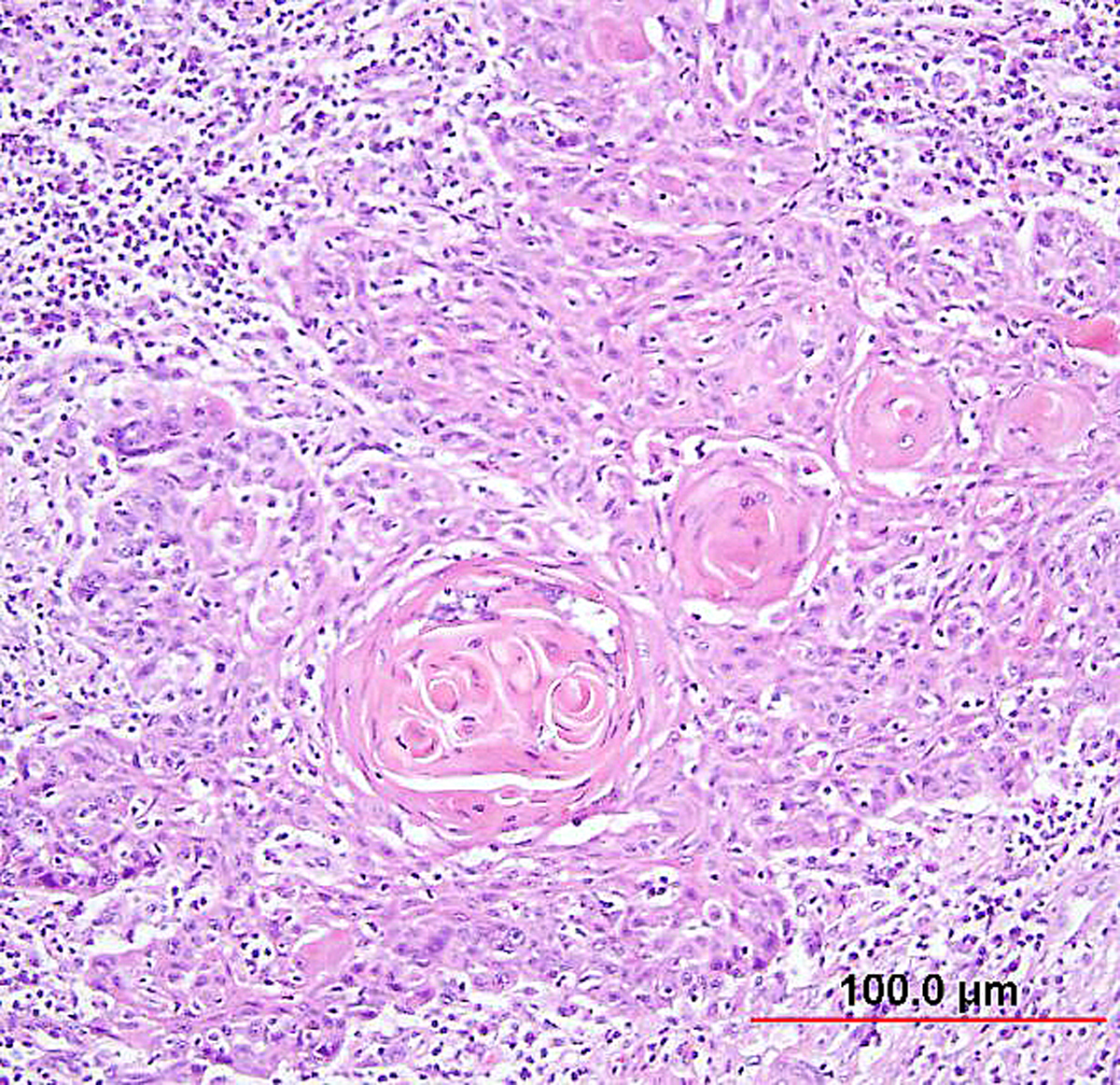 Fig. 5. Squamous cell carcinoma of the cervix, keratinizing type. Malignant squamous cells form irregular nests invading the stroma. In the center of the nest, laminated keratin pearls are present. Individual cells have abundant eosinophilic keratinized cytoplasm. (Hematoxylin-eosin stain, red bar: original magnification.)
Fig. 5. Squamous cell carcinoma of the cervix, keratinizing type. Malignant squamous cells form irregular nests invading the stroma. In the center of the nest, laminated keratin pearls are present. Individual cells have abundant eosinophilic keratinized cytoplasm. (Hematoxylin-eosin stain, red bar: original magnification.)
Non-keratinizing SCC
Non-keratinizing SCC is characterized by large cells of similar size and shape with indistinct cell borders, infiltrating as nests and sheets (Fig. 6). The cytoplasm is moderate in amount and eosinophilic to amphophilic. Individual cell keratinization may be seen, but keratin pearl formation should be absent. Nucleoli are prominent and mitotic figures are common.
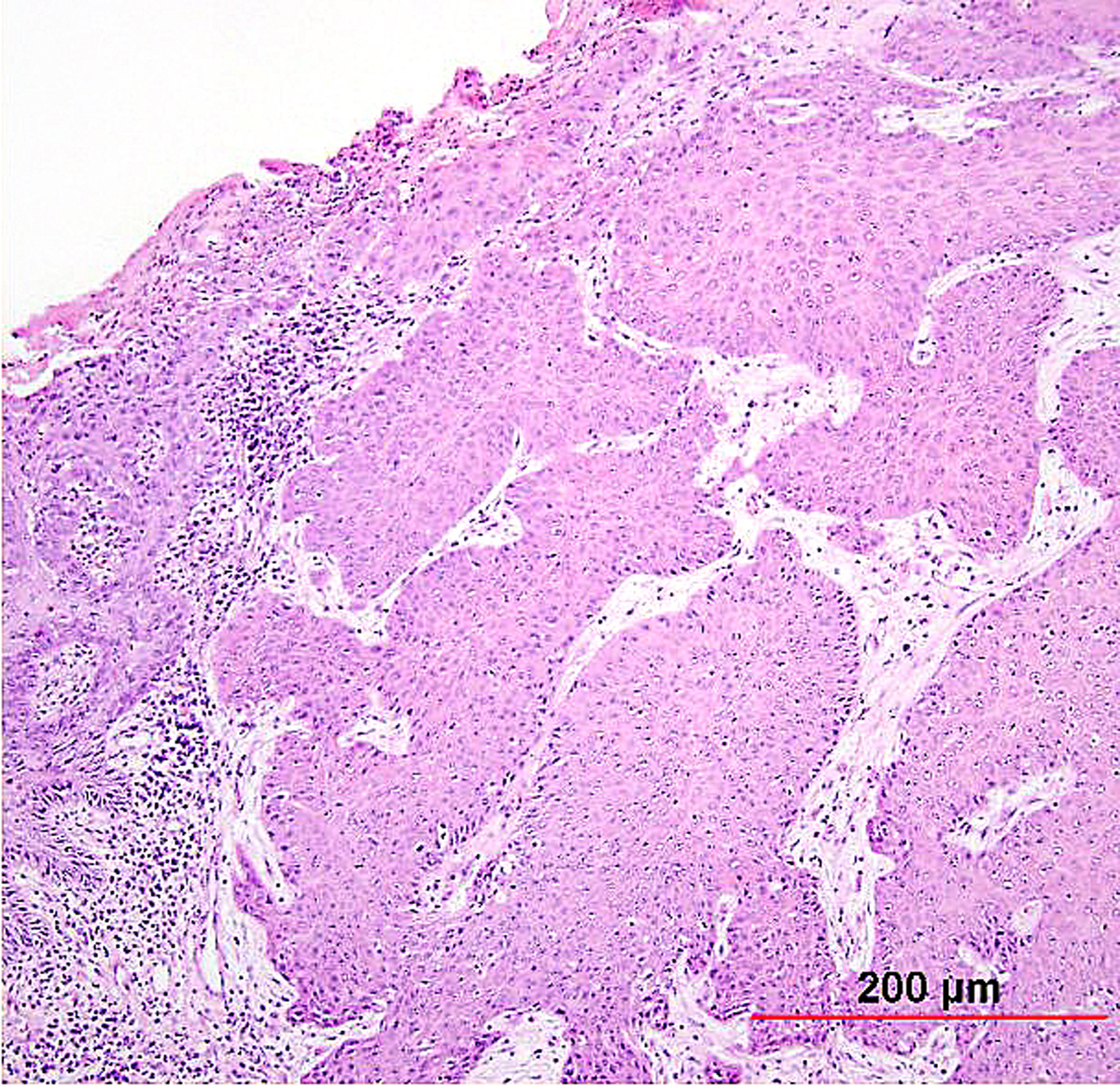 Fig. 6. Squamous cell carcinoma of the cervix, non-keratinizing type. Malignant squamous cells have abundant eosinophilic cytoplasm, distinct cell borders, and individual cell keratinization. The irregular, large nuclei contain multiple nucleoli. (Hematoxylin-eosin stain, red bar: original magnification.)
Fig. 6. Squamous cell carcinoma of the cervix, non-keratinizing type. Malignant squamous cells have abundant eosinophilic cytoplasm, distinct cell borders, and individual cell keratinization. The irregular, large nuclei contain multiple nucleoli. (Hematoxylin-eosin stain, red bar: original magnification.)
Basaloid SCC
Basaloid SCC (previously called small cell nonkeratinizing SCC) is an aggressive variant characterized by loosely cohesive nests and sheets of small to medium sized cells with hyperchromatic nuclei, scant cytoplasm, and small nucleoli. Keratinization is minimal or absent, and mitotic figures are abundant. The nuclear chromatin is finely to coarsely granular, and small nucleoli are often evident (Fig. 7). Crush artifact and nuclear smudging are not prominent. The nuclear-to-cytoplasmic ratio is lower than in small cell neuroendocrine carcinoma. The cell borders are also more distinct. Rare cytoplasmic keratinization may be present, allowing recognition of the squamous nature of the lesion. Necrosis is frequently observed.
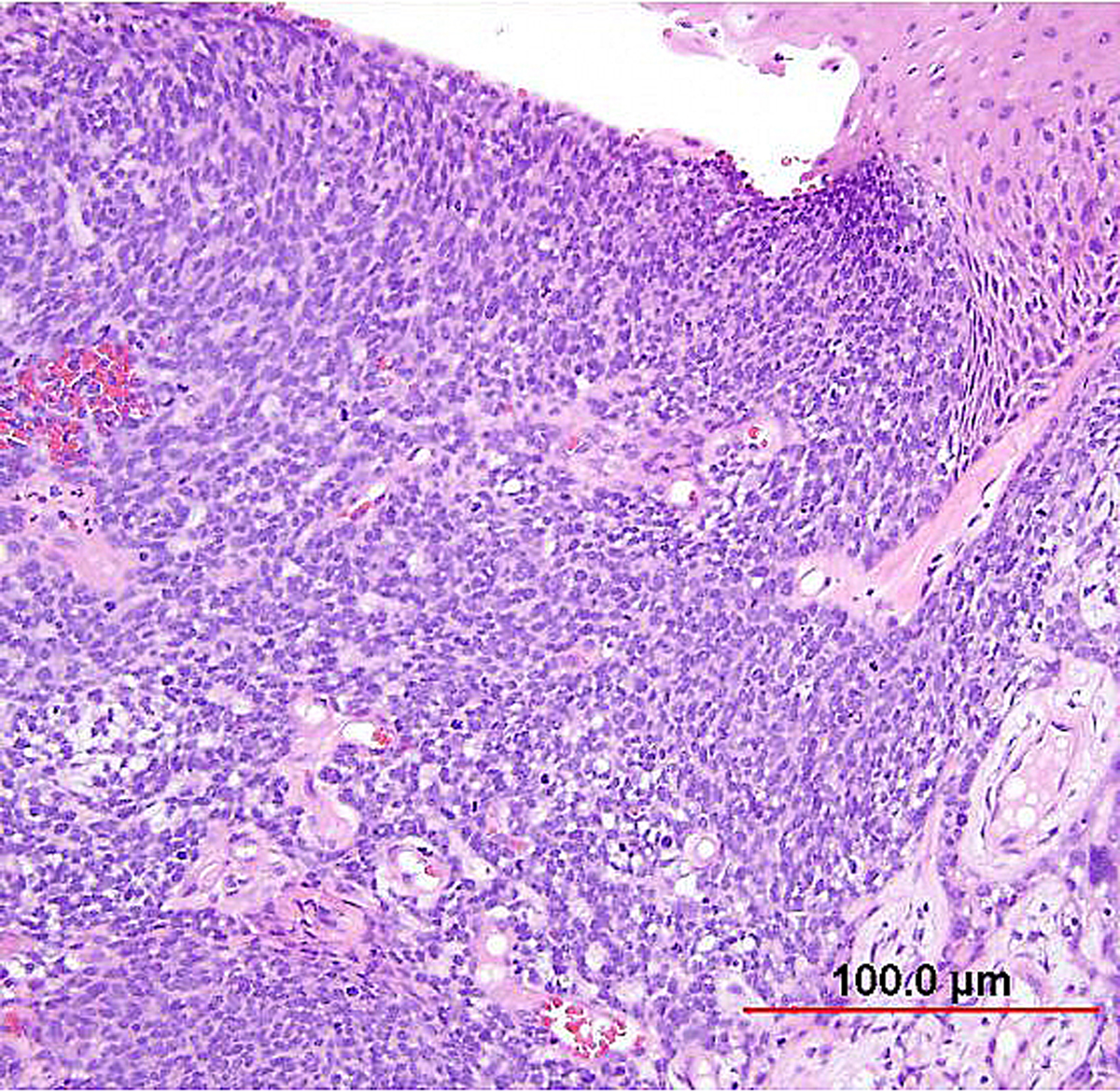 Fig. 7. Squamous cell carcinoma of the cervix, basaloid type. The malignant squamous cells have small round to oval nuclei, finely granular chromatin, and small nucleoli. Most of the tumor cells contain a small amount of eosinophilic cytoplasm. Mitotic figures are abundant. (Hematoxylin-eosin stain, red bar: original magnification.)
Fig. 7. Squamous cell carcinoma of the cervix, basaloid type. The malignant squamous cells have small round to oval nuclei, finely granular chromatin, and small nucleoli. Most of the tumor cells contain a small amount of eosinophilic cytoplasm. Mitotic figures are abundant. (Hematoxylin-eosin stain, red bar: original magnification.)
Warty SCC
Warty or condylomatous SCC demonstrates low-power architecture very similar to condyloma acuminatum. Cytologic features of koilocytosis are present. The deep edge of the tumor, however, displays features more consistent with conventional SCC, distinguishing these from verrucous carcinoma. This variant may be less aggressive than conventional well-differentiated SCC.113
Papillary SCC and squamotransitional carcinoma
Papillary SCC of the cervix is characterized by highly dysplastic squamous cells forming papillary fronds with thin to broad fibrovascular cores. Not surprisingly, the gross appearance of this lesion may be warty or fungating as in verrucous squamous carcinoma. Some papillary carcinomas of the cervix demonstrate features similar to transitional cell carcinoma of the urothelial tract, and terms such as papillary squamotransitional cell carcinoma and transitional cell carcinoma have been used.114, 115 Despite the morphologic resemblance to poorly differentiated transitional cell carcinoma of the urinary bladder, these tumors all display a similar immunohistochemical profile, with one study finding only two of 21 (9.5%) tumors expressing cytokeratin 20 (a marker supporting urothelial over squamous differentiation).114 Therefore, it is favored that these represent squamous cell carcinomas with a spectrum of morphologic appearances.
In a series of 32 women, the age of patients with papillary squamous cell carcinoma varied from 22 to 93 years (mean 50 years).114 The women presented with abnormal bleeding or abnormal cervical smears. The tumor size ranged from 0.7 to 6 cm (mean 3.0 cm). The usual diagnostic problem associated with this diagnosis is evident in the fact that only 20 of 32 (63%) specimens were considered to be adequate. The remaining 37% were too superficial to determine whether they were in situ or invasive carcinoma. Of those with suitable specimens, 90% (18 of 20) had stromal invasion.114 In another study, one tumor was found to be an in situ lesion, whereas the remaining eight cases ranged from stage I to stage IV invasive carcinoma.116 Since invasion is evident only in the stroma beneath the papillary surface components, deep biopsy specimens are necessary to distinguish in situ from invasive lesions. This is particularly important given that papillary SCC can behave aggressively with metastasis and recurrence.114, 116
Verrucous carcinoma
Verrucous carcinoma of the cervix, like that of other sites, represents a special variant of well-differentiated squamous carcinoma. Grossly, these tumors appear exophytic and warty, and may simulate a condyloma acuminatum. Histologically, the cells show orderly maturation and lack cytologic atypia. The tumor grows by expansion with smooth, pushing borders, as opposed to the infiltrating pattern of conventional SCC. To differentiate verrucous carcinoma from condyloma, pseudoepitheliomatous hyperplasia, or typical SCC, deep biopsy specimens are necessary. Some conventional SCCs have a verrucous appearance superficially but show severe nuclear atypia and foci of invasion by nests or single cells in the stroma. These tumors behave like conventional SCC and should be classified as such. Condyloma acuminatum has prominent koilocytosis and delicate fibrovascular cores, as opposed to the compressed cores and confluent epithelial growth pattern seen in verrucous carcinoma. Condylomata also lack the expansile, endophytic extension into the stroma seen in verrucous carcinomas.
Verrucous carcinomas can be deeply invasive and cases extending into the vagina and endometrium have been described. Local recurrence is common, but lymph node and distant metastases are rare.113, 117
Lymphoepithelioma-like carcinoma
Circumscribed carcinoma of the uterine cervix was described in 1977 by Hasumi and associates.118 In their study, the tumors were characterized by solid cords of cells with neither squamous nor glandular differentiation, surrounded by a dense lymphocytic infiltrate, which occasionally contained a considerable number of eosinophils and plasma cells. The tumor cells were fairly monomorphic, with large nuclei, one or more nucleoli, and clear to eosinophilic, granular cytoplasm. Many mitotic figures were seen. All 39 cases reported measured larger than 5 mm in depth, but only two (5%) had lymph node metastases at the time of surgery, compared with 18% of ordinary squamous carcinomas of comparable stage. Improved 5-year survival was also seen in these cases (97% vs. 79%, p <0.05).118
Studies of similar, if not identical, tumors use the term lymphoepithelioma-like carcinoma to indicate the histologic likeness to the lymphoepithelioma of the nasopharynx and the malignant lymphoepithelial lesions of the salivary glands.119, 120 In a study by Tseng and associates,121 15 such tumors were compared with conventional SCC by PCR. Epstein-Barr viral gene sequences were found in 11 of 15 tumors (73%), compared with four of 15 (27%) of the usual SCC (p = 0.001). Interestingly, HPV 16 and 18 types were detected in 20% (three of 15) of these tumors, compared with 80% (12 of 15) of the usual SCC (p = 0.001).121
Although the total number of cases in the literature is too small for complete understanding of these neoplasms, they appear to have a better prognosis than conventional SCC. After radical hysterectomy, all 15 patients in one study were alive and well.121
Spindle cell SCC
Although not part of the current WHO classification system, a spindled variant of SCC is also recognized. Spindle cell SCC is a rare variant of poorly differentiated SCC that may be confused with either melanoma or sarcoma.5, 122 This tumor is composed of cells with large, spindle shaped or oblong nuclei arranged in fascicles (Fig. 8). Keratin formation and the nesting pattern typical of epithelial tumors may be absent. Stromal changes such as heavy collagen deposition may give the appearance of a fibrosarcoma or osteosarcoma. When confronted with such a lesion, immunohistochemistry is often required to identify the epithelial nature. Positive immunohistochemical stains for epithelial markers (e.g. cytokeratin) favor the diagnosis of a spindle cell SCC over a mesenchymal tumor.
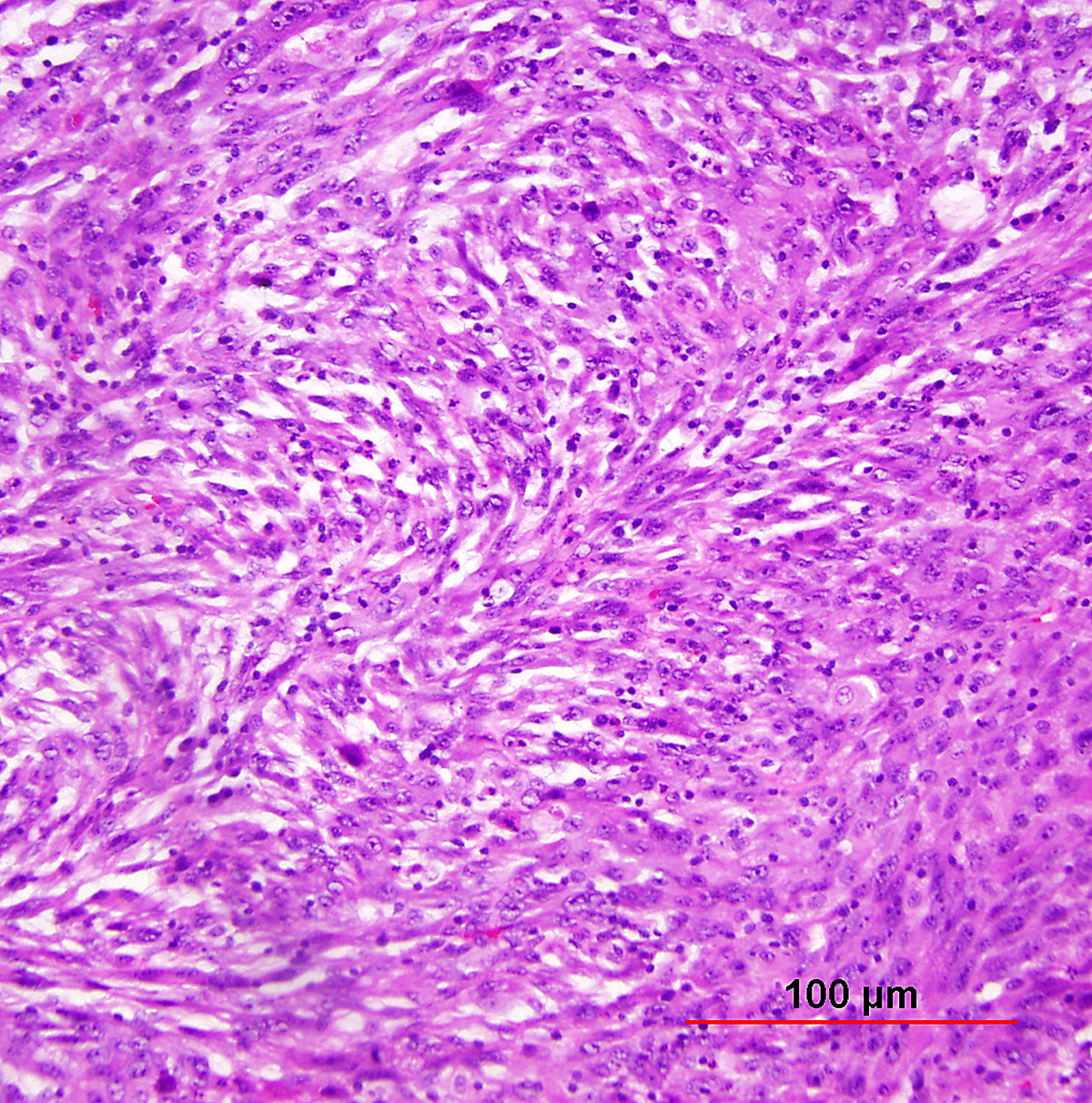 Fig. 8. Squamous cell carcinoma of the cervix, spindle cell type. Elongated tumor cells are arranged in bundles simulating spindle cell sarcoma. Immunohistochemical stain for cytokeratin was positive, confirming the diagnosis of carcinoma (not shown). (Hematoxylin-eosin stain, red bar: original magnification.)
Fig. 8. Squamous cell carcinoma of the cervix, spindle cell type. Elongated tumor cells are arranged in bundles simulating spindle cell sarcoma. Immunohistochemical stain for cytokeratin was positive, confirming the diagnosis of carcinoma (not shown). (Hematoxylin-eosin stain, red bar: original magnification.)
One may also rarely see abnormal spindle cells in the cervical stroma adjacent to typical SCC. This so called pseudosarcoma was reported by Watty and colleagues,123 who described individual atypical stromal cells with elongated, pleomorphic nuclei and frequent multinucleation. Rare abnormal mitotic figures were seen. The authors felt that this change represented a response to the nearby tumor and noted that similar lesions have been reported in squamous cell carcinomas of the head and neck region.
Histologic grade
The histologic grade reflects the degree of differentiation of the tumor cells. The most commonly used grading system for SCC is a modification of the original Broders' system, consisting of three grades based on the amount of keratin, the degree of nuclear atypia, and the mitotic activity. Well-differentiated (grade 1) tumors exhibit abundant intercellular bridges, cytoplasmic keratinization, and keratin pearls. The cells are relatively uniform with minimal nuclear pleomorphism. The mitotic rate is generally low. Moderately differentiated (grade 2) tumors show primarily individual cell keratinization, moderate nuclear pleomorphism, and more numerous mitotic figures than seen in grade 1 tumors. Poorly differentiated (grade 3) tumors show little evidence of squamous differentiation. The tumor cells are immature, with marked nuclear pleomorphism, scant cytoplasm, and numerous mitotic figures per high power field. Necrosis is also common.113, 124 Histologic grade may have an effect on response to therapy and prognosis (see section on Prognostic Factors later in this chapter). Currently, the grade of SCC is broadly used in daily practice and included in pathology reports.
GLANDULAR INTRAEPITHELIAL LESIONS
Endocervical glandular dysplasia
Endocervical adenocarcinoma in situ (AIS) is recognized as the precursor to HPV-related invasive endocervical adenocarcinoma. However, diagnosis of atypical lesions less severe than AIS is somewhat controversial and not standardized. Support for diagnosis of dysplastic lesions less than AIS stems from demonstration of dysplasia adjacent to AIS and invasive adenocarcinoma,125, 126, 127 the younger age of patients with dysplasia vs. AIS and invasive adenocarcinoma,127, 128 the presence of high-risk HPV in at least a subset of dysplastic lesions,125, 126, 129 and the presence of diffuse p16 expression in the majority of glandular dysplasias in some studies (Fig. 9).130 Other studies, however, have found differing results, include absence of coexisting AIS and dysplasia,131, 132 absence of HPV in many atypical glandular lesions,132, 133, 134 the existence of HPV-negative atypias with high Ki-67 proliferation indices,132 and absence of diffuse p16 positivity in glandular dysplasias.135, 136
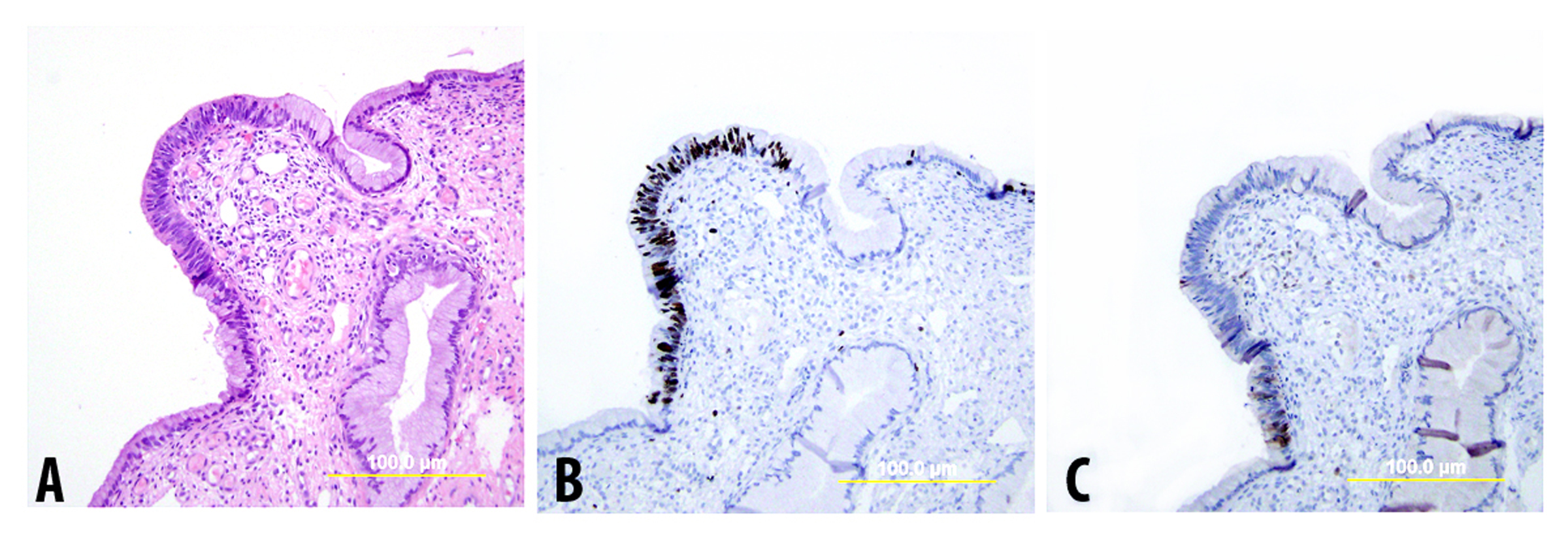 Fig. 9. Endocervical glandular dysplasia. (A) H&E-stained slide shows focal area of atypical glandular epithelium with partial stratification, moderate nuclear atypia, and one mitosis. (B),(C) Immunostains reveal an increased Ki-67 proliferation index (B) but only focal immunoreactivity for p16 (C). (Yellow bars: original magnification)
Fig. 9. Endocervical glandular dysplasia. (A) H&E-stained slide shows focal area of atypical glandular epithelium with partial stratification, moderate nuclear atypia, and one mitosis. (B),(C) Immunostains reveal an increased Ki-67 proliferation index (B) but only focal immunoreactivity for p16 (C). (Yellow bars: original magnification)
In 2003, Silverberg and colleagues137 proposed a three tier scoring system to differentiate among benign glandular lesions, endocervical glandular dysplasia (EGD), and AIS. This scheme gives scores from 0 to 3 to each lesion for: (1) nuclear atypia, (2) stratification, and (3) sum of mitoses/apoptoses per gland (counted in the two most active glands and then averaged per gland). These three scores are then added to result in the total score (Table 2). The study demonstrated a significant increase in agreement with use of the scoring system (52.2% agreement and kappa 0.565 without scoring system, vs. 77.6% agreement and kappa 0.705 with scoring system). It should be noted, however, that in the original study from Silverberg and colleagues, the best reproducibility was obtained by combining the benign and EGD categories into a single “benign/reactive” category (agreement 94%), allowing the accurate distinction between AIS and lesions less than AIS. The authors proposed that this practice be adopted given the poor understanding of EGD.137
Table 2. Silverberg scoring system for noninvasive endocervical glandular lesions137
| Score | Feature | ||
| Stratification | Nuclear atypia | Mitoses and apoptosis | |
| 0 | None | As normal | None |
| 1 | Mild (up 1/3 epithelial thickness) | Small or slightly enlarged, uniform, minimal hyperchromasia, little dispolarity, no nucleoli | <0.5 per gland |
| 2 | Moderate (up to 2/3 epithelial thickness) | Size up to 3X normal, moderate anisocytosis, moderate hyperchromasia, moderate dispolarity, occasional small nucleoli | 0.6-3.0 per gland |
| 3 | Up to luminal surface | Size >3X normal, marked anisocytosis, marked hyperchromasia, severe dispolarity, frequent prominent nucleoli | >3 per gland |
|
| |||
| Total Score | Interpretation | ||
| 0–3 | Benign | ||
| 4–5 | Endocervical glandular dysplasia (EGD) | ||
| 6–9 | Adenocarcinoma in situ (AIS) | ||
In the United Kingdom, a similarly tiered grading system is employed, termed “cervical glandular intraepithelial neoplasia (CGIN).” Two grades of CGIN are diagnosed: low-grade CGIN (LCGN), which generally corresponds to EGD, and high-grade CGIN (HCGIN), which generally corresponds to AIS.138
Given the lack of widespread consensus regarding the diagnosis of EGD, as well as its as yet questionable biologic significance, many authors recommend against its diagnosis.139, 140, 141 This is reflected also in the most recent WHO classification. Whereas the prior WHO classification included both glandular dysplasia and AIS under the classification of cervical glandular tumor precursors,142 the current WHO classification has eliminated the dysplasia category and includes only AIS as a precursor lesion.106
Adenocarcinoma in situ
The entity of endocervical adenocarcinoma in situ (AIS) has been widely accepted and recognized as the precursor of invasive adenocarcinoma. In addition to the usual HPV-related type of AIS, endometrioid,32 clear cell,143 and intestinal (see below) types have also been described. HSIL coexists with 35–71% of adenocarcinomas in situ.144, 145, 146
The majority of AIS lesions are detected initially in the cervical smears of asymptomatic women or incidentally in hysterectomy specimens removed for benign conditions. Some are found in cervical biopsy specimens, endocervical curettage specimens, or cone specimens removed for squamous neoplasia. Colposcopic findings are nonspecific, such as patchy acetowhite lesions in the cervical canal, fused and papillary columnar villi, and abnormal vessels.
Most AIS lesions begin in the region of the squamocolumnar junction and spread proximally. With few exceptions, both the endocervical mucosal surface and the underlying glands are involved. The affected surface may be flat, papillary, or villous in appearance. Whereas normal endocervical cells are arranged in a single layer and have basally located small nuclei with absent to rare nucleoli and mitotic figures, neoplastic endocervical cells demonstrate nuclear enlargement, hyperchromasia, and pseudostratification, with a typically cigar-shaped elongated appearance (Fig. 10). Nucleoli are multiple, and mitotic figures are easily identified within the apical mucin (imparting a “floating” appearance). Apoptotic figures are also frequent. The cytoplasm appears basophilic, clear, or vacuolated. In the deeper portion of the endocervical glands, a sharp transition between the normal and neoplastic cells is often apparent. The normal architecture and branching pattern of the glands is maintained, although some budding and intraglandular proliferation and cribriforming can be seen. The characteristic features distinguishing this lesion from invasive adenocarcinoma are the preservation of normal architecture, the smooth configuration of the glandular profiles, the absence of neoplastic glands deeper than the uninvolved glands, and normal fibromuscular stroma without desmoplasia.
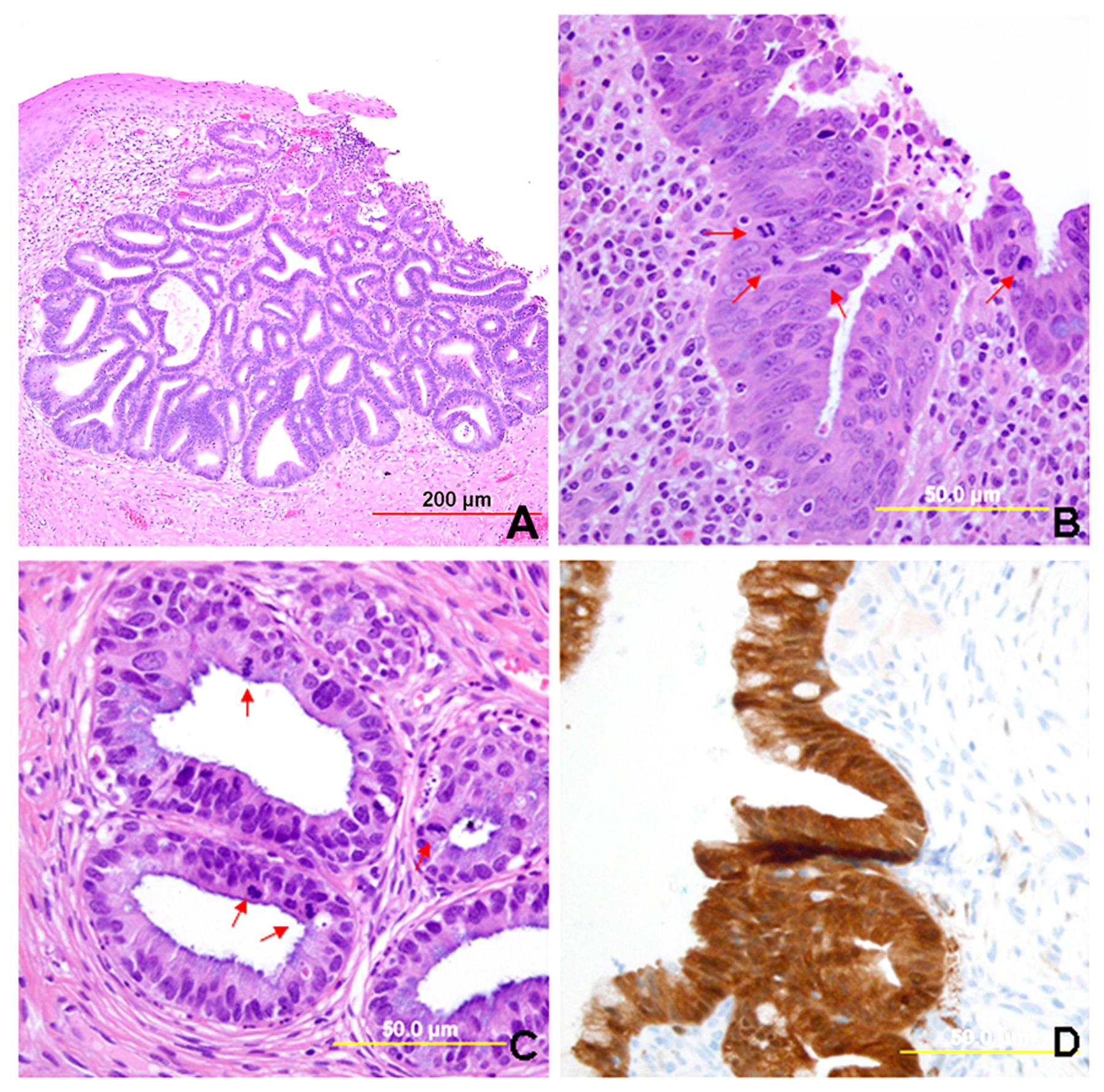 Fig. 10. Endocervical adenocarcinoma in situ. (A) The neoplastic endocervical glands retain the branching and budding pattern of normal endocervical glands. These glands have smooth borders and are surrounded by normal fibromuscular stroma without a desmoplastic reaction. At the base of the tumor, malignant cells replace normal endocervical cells. (B),(C) Higher magnification reveals tall columnar neoplastic cells with nuclear stratification (B), hyperchromasia, elongation, irregularity (C), and increased mitoses (red arrows, B,C). (Hematoxylin-eosin stain, red and yellow bars: original magnification). (D) The neoplastic glands are diffusely immunoreactive for p16.
Fig. 10. Endocervical adenocarcinoma in situ. (A) The neoplastic endocervical glands retain the branching and budding pattern of normal endocervical glands. These glands have smooth borders and are surrounded by normal fibromuscular stroma without a desmoplastic reaction. At the base of the tumor, malignant cells replace normal endocervical cells. (B),(C) Higher magnification reveals tall columnar neoplastic cells with nuclear stratification (B), hyperchromasia, elongation, irregularity (C), and increased mitoses (red arrows, B,C). (Hematoxylin-eosin stain, red and yellow bars: original magnification). (D) The neoplastic glands are diffusely immunoreactive for p16.
Although the diagnosis of AIS may be made on the basis of cytology and biopsy specimens, it is difficult to separate in situ carcinoma from well-differentiated invasive adenocarcinoma. As both lesions may occur concurrently, cervical conization is usually performed for a definitive diagnosis. Additionally, a newly proposed classification system for endocervical adenocarcinoma may make this distinction less crucial (see below).
Other important differential diagnoses to consider include tubal/tuboendometrioid metaplasia and reactive change. Like AIS, tuboendometrioid metaplasia demonstrates some nuclear enlargement and stratification. Mitotic activity and apoptotic bodies should, however, be rare, and the presence of cilia favors a diagnosis of tubal/tuboendometrioid metaplasia (although rare ciliated AIS does occur). Reactive endocervical cells are mostly seen in a background of inflammation, and demonstrate some nuclear enlargement, pleomorphism, and prominent nucleoli. In contrast to AIS, however, the nuclear chromatin demonstrates a more open appearance, and the nuclei sometimes appear smudged. Additionally, significant mitotic activity and pseudostratification are absent. Other entities which occasionally enter the differential diagnosis are microglandular hyperplasia (distinguished by its classic pattern of crowded glands with bland cuboidal lining cells, vacuoles, and neutrophils), Arias-Stella reaction (distinguished by its classic appearance of clear cytoplasm, enlarged nuclei, and prominent hobnailing, and aided by a known history of recent pregnancy), and endometriosis (distinguished by the usual presence of endometrial-type stroma).
Immunohistochemical stains can be of use in distinguishing benign mimics from AIS. Given that the vast majority of AIS is related to high-risk HPV, it is not surprising that diffuse strong p16 positivity is present in the majority of these lesions.130, 135, 147 An important caveat to the use of p16 in this differential diagnosis is that glands with tuboendometrioid metaplasia can also demonstrate p16 expression; however, careful assessment usually reveals a patchy (albeit sometimes extensive) staining pattern, compared with the diffuse pattern seen in AIS.130, 135, 147 The Ki-67 proliferation index is also elevated (usually >30%) compared to benign mimics (usually <10%).130, 147, 148 Additionally, estrogen and progesterone receptors are negative in AIS, whereas endometriosis and tubal/tuboendometrioid metaplasia are usually positive for ER and PR.136, 149
Estimates based on conization and hysterectomy specimens have found AIS extending as deep as 3–5 mm from the mucosal surface. The linear extent along the cervical canal varies from 0.5 to 25 mm with a mean of 12 mm.150 If measured from the external os, these lesions may reach up to 30 mm.151 This underscores the need for deep conization to encompass the entire lesion. Young women preferring to retain the uterus may be treated by conization alone and followed regularly by endocervical curettage.150 However, even if the surgical margins of the conization specimen appear uninvolved, residual tumor may exist. A possible explanation for this is multifocal disease, which is estimated to occur in 15% of cases.150
In additional to the usual type of AIS, an intestinal type is also occasionally encountered. This type is characterized by goblet cells, sometimes accompanied by Paneth cells and neuroendocrine cells.152 It is usually admixed with usual type AIS.152 Based on the rarity of intestinal metaplasia in benign endocervical glands, some authors recommend diagnosing any intestinal metaplasia in the cervix as AIS, even without significant cytologic atypia.153 The presence of intestinal metaplasia can also raise concern for a metastatic carcinoma from the gastrointestinal tract. However, the distinction from a lower gastrointestinal tract primary is aided by the typical CK7(+), CK20(-), and p16-diffuse immunoprofile which intestinal AIS appears to maintain.154 Of interest, however, CDX2, a marker of intestinal differentiation, has been demonstrated to be positive in these lesions.154
Adenosquamous intraepithelial lesions
The entity of adenosquamous carcinoma in situ described by Steiner and Friedell155 closely resembles squamous cell carcinoma in situ. Intermixed with the dysplastic squamous cells, however, are cells with vacuolated or basophilic cytoplasm. Mucicarmine and periodic acid-Schiff (PAS) stains reveal mucin production in these cells. Similar change sometimes occurs in the vicinity of adenosquamous carcinomas.
More recently, the entity of “stratified mucin-producing intraepithelial lesion (SMILE)” of the cervix has been described. Park et al.156 described 18 lesions demonstrating stratified atypical epithelium (similar to HSIL) and containing cytoplasmic mucin (similar to AIS), with distinctive cytoplasmic clearing or vacuolization (Fig. 11). While this lesion is likely akin to the previously described “adenosquamous carcinoma in situ,” the authors proposed an origin from reserve cells, an assertion supported by the location of these lesions near the transformation zone, the occasional undermining of normal columnar epithelium (like reserve cells), and the frequent co-existence of HSIL and AIS.156 Immunohistochemically, these lesions display a lack of definitive squamous differentiation; when encountered in isolation, they are likely best regarded as a stratified form of AIS or as a high-grade reserve cell dysplasia.153, 156 As with typical HSIL or AIS, SMILE demonstrates diffuse p16 positivity.157
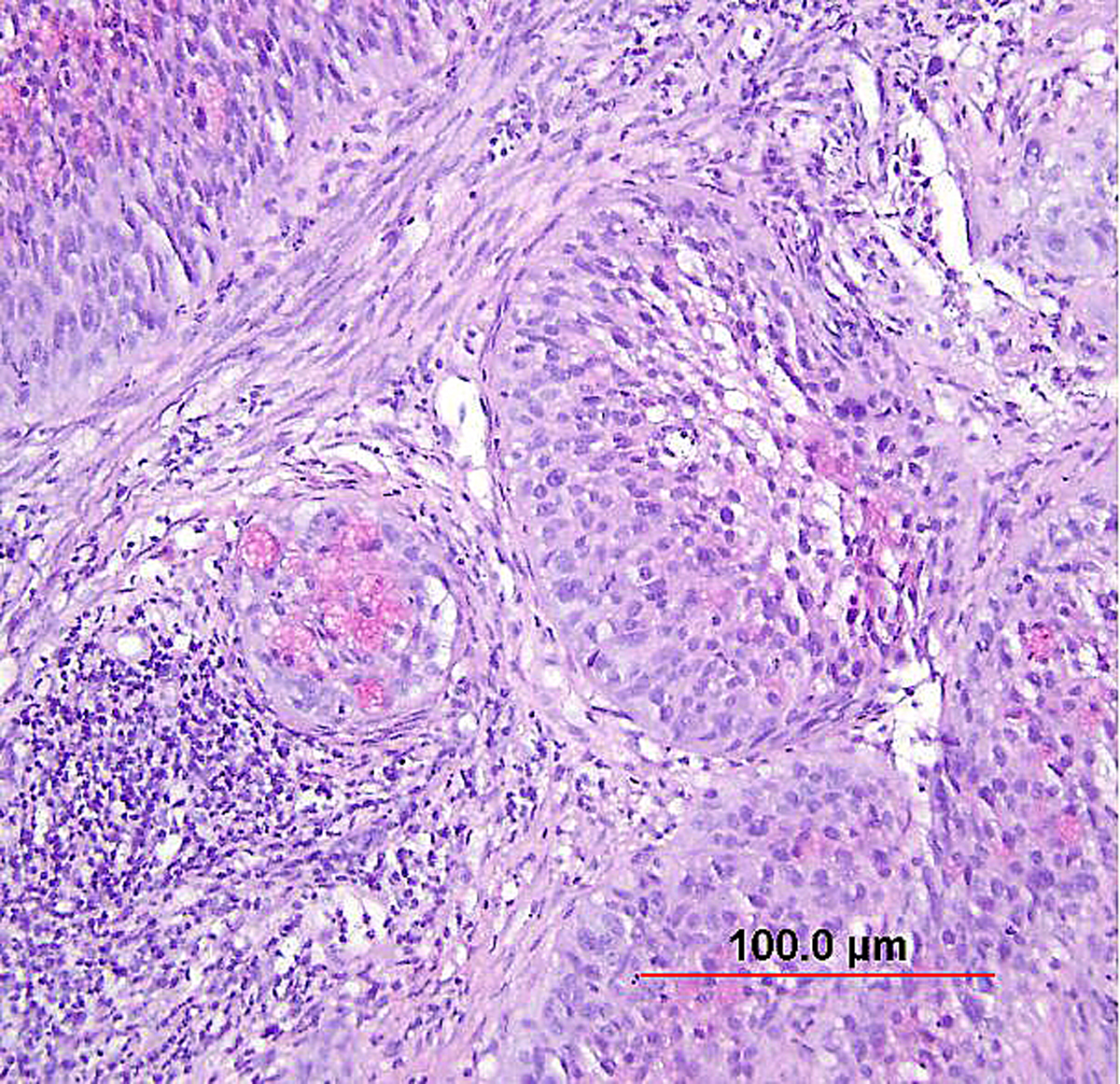 Fig. 11. Stratified mucin-producing intraepithelial lesion (SMILE)/adenosquamous carcinoma in situ. Nests of noninvasive, stratified, high-grade neoplasia with a mucin-producing component (pink intracytoplasmic mucin detected by counterstain of Mucicarmin). (Hematoxylin-eosin stain, red bar: original magnification.)
Fig. 11. Stratified mucin-producing intraepithelial lesion (SMILE)/adenosquamous carcinoma in situ. Nests of noninvasive, stratified, high-grade neoplasia with a mucin-producing component (pink intracytoplasmic mucin detected by counterstain of Mucicarmin). (Hematoxylin-eosin stain, red bar: original magnification.)
MALIGNANT GLANDULAR NEOPLASMS
Cervical carcinomas with glandular differentiation are of heterogeneous cell types, diverse growth patterns, and variable differentiation. The majority of glandular neoplasms are pure adenocarcinomas. The current WHO classification of glandular tumors of the uterine cervix106 is listed below. Adenosquamous carcinomas will be discussed separately.
· Endocervical adenocarcinoma, usual type
· Mucinous carcinoma, NOS
o Gastric type
o Intestinal type
o Signet-ring cell type
· Villoglandular carcinoma
· Endometrioid carcinoma
· Clear cell carcinoma
· Serous carcinoma
· Mesonephric carcinoma
· Adenocarcinoma admixed with neuroendocrine carcinoma
As previously stated, while the overall incidence of cervical carcinoma as well as the incidence of squamous cell carcinoma has decreased in recent decades in developed countries, the incidence of adenocarcinoma has been rising.4 The reasons for this are not yet understood.
The clinical presentation and gross appearance of cervical adenocarcinoma are basically similar to those of SCC. In a series of 55 women with IB adenocarcinoma by Greer and colleagues,158 44% of women had no symptoms and 58% (32 of 55) had no gross lesions. The diagnosis was suspected on the basis of abnormal cervical smears in 16 women (29%). Of these, 15 required conization for diagnosis.158
Early invasive adenocarcinoma
As with early invasive squamous lesions, the definitions of “microinvasion” for cervical adenocarcinoma vary. Several investigators have attempted to define the morphologic criteria for microinvasive adenocarcinoma. Teshima and associates159 defined early adenocarcinoma as less than 5 mm of stromal invasion as measured from the mucosal surface. All 30 patients in their study were treated by hysterectomy and one woman developed tumor recurrence. Among women with stage I and II cervical adenocarcinoma up to 5 mm in depth, Berek and colleagues160 found that two of 24 women (8%) had pelvic lymph node metastasis and the overall 5-year survival rate was 92%. Thus, adenocarcinomas as superficial as 5 mm have a small risk of pelvic nodal metastasis. In the United States, a FIGO stage IA1 tumor (≤3 mm in depth) is usually considered microinvasive, while some institutions in the United Kingdom include also FIGO stage IA2 (≤5 mm in depth) in the definition.153 Therefore, as with SCC, avoidance of the term “microinvasion” is preferable. Unlike with SISCC, however, no recent standardized guidelines have been established, and so reporting instead the FIGO stage without use of the term “microinvasion” is recommended by some authors.153 Depth of invasion should be measured from the basement membrane of the overlying abnormal epithelium to the point of deepest invasive tumor (Fig. 12).
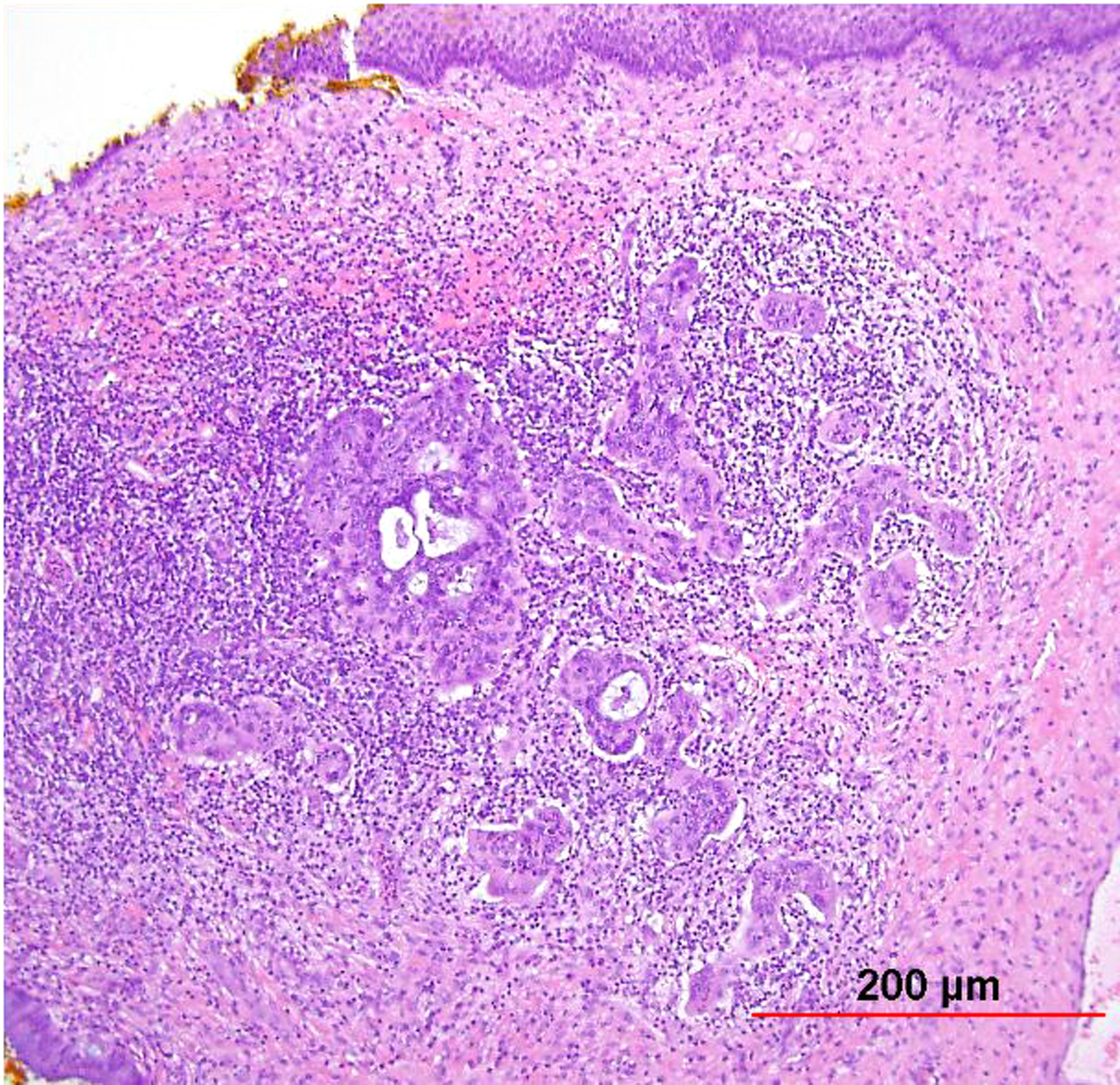 Fig. 12. Early invasive endocervical adenocarcinoma. This small focus of adenocarcinoma forms multiple irregular tongue-like protrusions from the periphery of endocervical glands. These protrusions are associated with fibrotic stroma and chronic inflammation. (Hematoxylin-eosin stain, red bar: original magnification.)
Fig. 12. Early invasive endocervical adenocarcinoma. This small focus of adenocarcinoma forms multiple irregular tongue-like protrusions from the periphery of endocervical glands. These protrusions are associated with fibrotic stroma and chronic inflammation. (Hematoxylin-eosin stain, red bar: original magnification.)
The distinction of early invasive adenocarcinoma and AIS can be challenging. In cases with clearly infiltrative glands, stromal desmoplasia, and/or destructive invasion, the diagnosis can be rendered without significant difficulty. However, frequently these features are absent, and the pathologist must rely on more subtle findings such as a haphazard low-power appearance of the glands, glandular confluence and complexity, excessively deep glands (beyond the deepest benign gland), and proximity to thick-walled vessels.161 A recently proposed pattern-based classification scheme for usual type endocervical adenocarcinoma may reduce the burden of making this distinction in difficult cases (see below).
Invasive endocervical adenocarcinoma, usual type
Endocervical adenocarcinoma of the usual type is the most common type of endocervical adenocarcinoma. Well-differentiated tumors demonstrate mostly glandular architecture, with cribriforming, confluence, and sometimes formation of papillae. The lining cells are columnar and often stratified, and have eosinophilic or finely vacuolated pale staining cytoplasm resembling normal endocervical cells. Mitotic activity and apoptotic bodies are abundant. Moderately differentiated tumors demonstrate a greater proportion of solid growth (11–50%), whereas poorly differentiated tumors demonstrate >50% solid growth (Fig. 13). Poorly differentiated tumors can also have anaplastic cells and cells with a signet ring cell appearance.
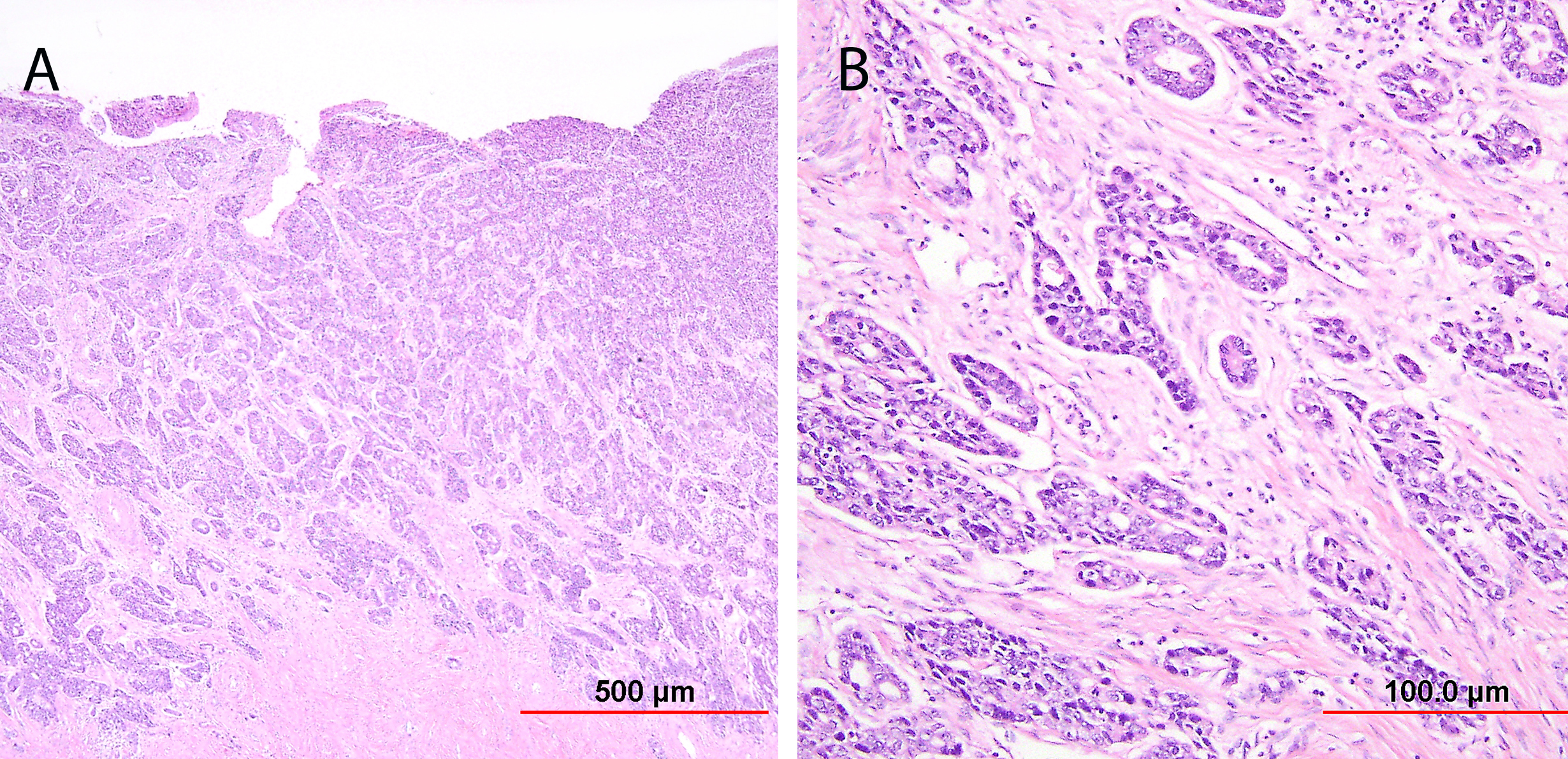 Fig. 13. Invasive endocervical adenocarcinoma, usual type. Moderately differentiated invasive adenocarcinoma consisting of small irregular glands and solid nests (A). Higher power view (B) shows vacuolated cytoplasm with mucinous features. Nuclear atypia is apparent. (Hematoxylin-eosin stain, red bars: original magnification.)
Fig. 13. Invasive endocervical adenocarcinoma, usual type. Moderately differentiated invasive adenocarcinoma consisting of small irregular glands and solid nests (A). Higher power view (B) shows vacuolated cytoplasm with mucinous features. Nuclear atypia is apparent. (Hematoxylin-eosin stain, red bars: original magnification.)
The traditional treatment for invasive endocervical adenocarcinoma was radical hysterectomy with pelvic lymph node dissection. However, the ability to avoid hysterectomy and therefore preserve fertility in women of child-bearing age, as well as to avoid the morbidity of lymphadenectomy, is clearly desirable. It has been demonstrated that FIGO stage IA1 carcinomas (≤3 mm depth, ≤ 7 mm horizontal extent) are amenable to more conservative treatment (e.g. cold knife cone or simple hysterectomy without lymph node dissection) with a very low risk of extracervical spread and recurrence.162 However, patients with stage IA1 carcinomas can still have lymph node metastases and die of disease.162, 163 Additionally, while more radical surgery is usually recommended for patients with stage IA2 carcinoma, the risk of lymph node metastasis and death has been demonstrated to be low in these patients as well.163 Compounding this issue is the difficulty and subjectivity involved in measuring depth, particularly in a well-differentiated carcinoma with associated AIS, in which the invasive and in situ component cannot always be reliably distinguished.
Given these issues, a new system of classification for usual type endocervical adenocarcinoma has recently been proposed based on a multi-institutional study.164 Termed the “Silva system,” this scheme relies on pattern, rather than tumor depth, to stratify patients into risk categories. Three patterns are defined (Table 3).
Table 3: The Silva system for pattern-based classification of endocervical adenocarcinoma of usual type164
| Pattern A | Well-demarcated glands with rounded contours No single cells or stromal desmoplasia Depth and relationship to large vessels not considered Complex intraglandular growth permitted No lymph-vascular invasion Well or moderate differentiation |
| Pattern B | Early destructive stromal invasion arising from Pattern A-like glands Lymph-vascular invasion may or may not be present |
| Pattern C | Diffuse destructive invasion |
In the initial study looking at data from 352 patients, the authors found that none of the patients with Pattern A adenocarcinomas had lymph node metastases, compared to 4.4% for Pattern B and 23.8% for Pattern C.164 Interestingly, although the average depth of invasion increased from Pattern A to C, there was significant overlap among the patterns, with 27.4% of Pattern A tumors having a depth of invasion >5 mm, and 10.6% of the Pattern C tumors having a depth of invasion ≤3 mm. Additionally, the average horizontal spread for Pattern A tumors was 8.6 mm (range 1.5–20 mm), compared to 16.3 mm (range 1.2–30 mm) for Pattern C. Therefore, a large number of the Pattern A tumors (81%) were beyond a FIGO stage IA1, and thus beyond the usual cut-off for conservative vs. radical treatment. Based on these data, it appears that the newly proposed pattern-based classification system may be superior to depth of invasion in predicting the risk for lymph node metastasis and identifying patients with tumors amenable to conservative treatment.164 Although reproducibility studies are as yet limited, one study using blinded review of 48 cases found that a consensus diagnosis with the new system was achieved in 50% of cases (kappa values between 0.24 and 0.84). Agreement was improved using two tiers (A vs. B/C), with a consensus diagnosis reached in 81.3% of cases (kappa values between 0.33 and 0.92).165 While additional studies are needed to validate this system, it appears very promising as a superior way to diagnose and treat patients with endocervical adenocarcinoma.
Mucinous carcinoma
Mucinous carcinomas of the cervix are generally classified according to the subtypes discussed in this section. However, mucinous carcinomas which do not demonstrate features of any of these specific subtypes are classified as “mucinous carcinoma, NOS.”106
Mucinous carcinoma, gastric type
This category includes the tumor known as “minimal deviation adenocarcinoma” or “adenoma malignum.” This is an extremely well-differentiated adenocarcinoma closely resembling normal endocervical glands,166, 167 which comprises approximately 1% of cervical adenocarcinomas.168 This variant deserves special recognition because of the inherent difficulty in its diagnosis, as well as its aggressive behavior, belied by its deceptively bland appearance. The tumor mostly retains the branching pattern of normal endocervical glands, has minimal nuclear atypia, and causes minimal stromal response (Fig. 14). Clues for the diagnosis of minimal deviation adenocarcinoma include angulated, cystic glands with a vaguely haphazard architecture at low power and the presence of glands deep in the cervical stroma. Encroachment of the blood vessels and nerve fibers adds further support for stromal invasion. Careful survey of individual lining cells reveals at least focal nuclear atypia with enlargement, hyperchromasia, and uneven chromatin. Nucleoli may be evident.
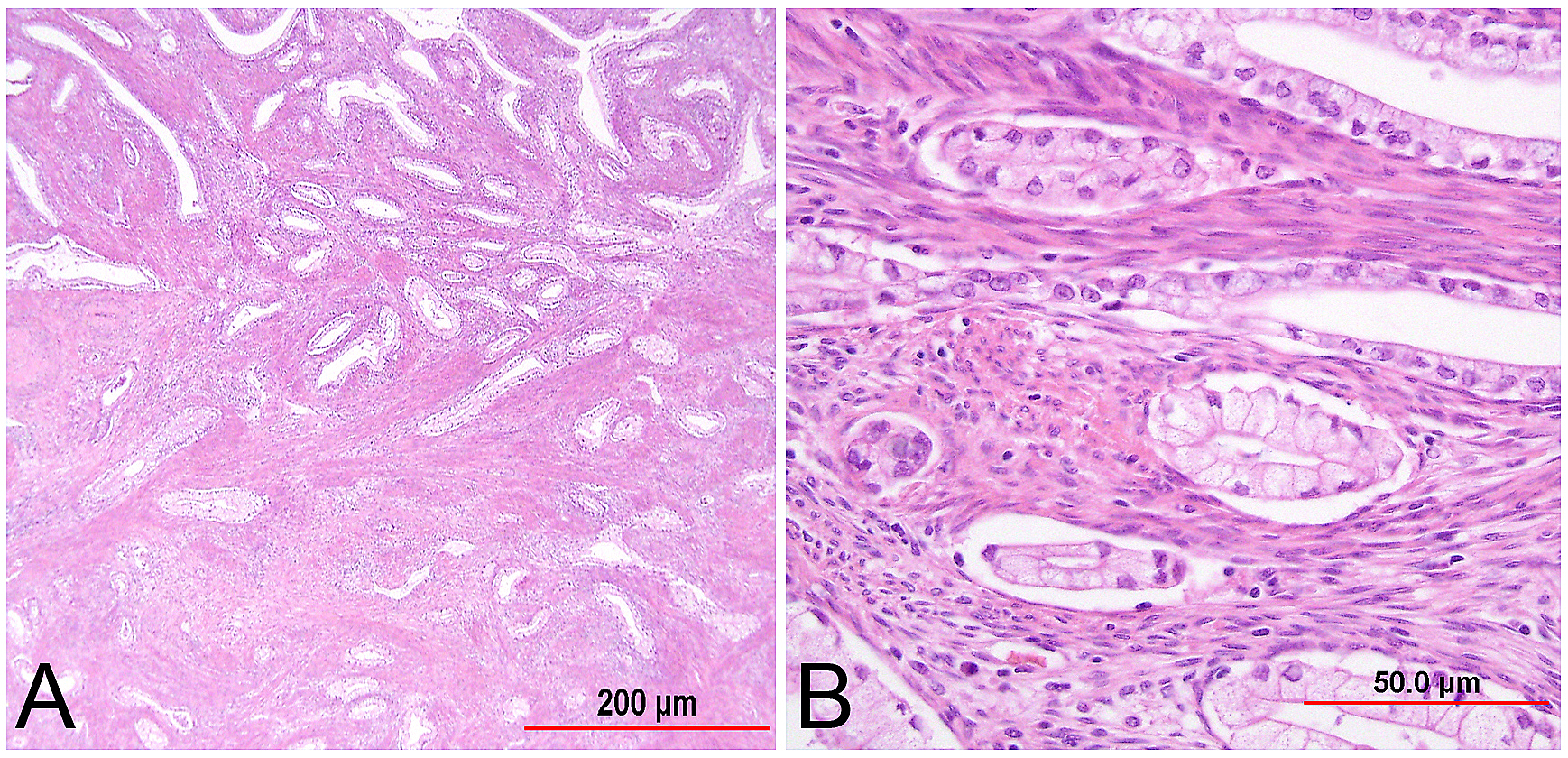 Fig. 14. Cervical adenocarcinoma, minimal deviation type (adenoma malignum). Haphazardly arranged, branching, budding glands infiltrate into deep cervical stroma (A). Neoplastic cells closely mimic normal endocervical cells, with tall columnar configuration, abundant mucinous cytoplasm, and small basally located nuclei (B). Focal nuclear irregularity and small nucleoli are however evident. (Hematoxylin-eosin stain, red bars: original magnification.)
Fig. 14. Cervical adenocarcinoma, minimal deviation type (adenoma malignum). Haphazardly arranged, branching, budding glands infiltrate into deep cervical stroma (A). Neoplastic cells closely mimic normal endocervical cells, with tall columnar configuration, abundant mucinous cytoplasm, and small basally located nuclei (B). Focal nuclear irregularity and small nucleoli are however evident. (Hematoxylin-eosin stain, red bars: original magnification.)
Although similar characteristics have also been observed in well-differentiated endometrioid, clear cell, and mesonephric carcinomas,168 most authors apply the terms adenoma malignum and minimal deviation adenocarcinoma only to the mucinous type.
Minimal deviation mucinous adenocarcinomas of the cervix are associated with Peutz-Jeghers syndrome, and 55% of sporadic cases in one study were demonstrated to have mutations in the Peutz-Jeghers syndrome-associated tumor suppressor gene STK11.169
Support for a gastric phenotype comes from studies demonstrating histochemical positivity for gastric mucin and immunohistochemical positivity for the gastric markers HIK1083 and MUC6.170, 171, 172 Additional immunohistochemical characteristics include negativity for ER and PR, at least focal positivity for CEA, positivity for CK7, and variable positivity for CK20, PAX8, and Her2/neu.170, 173, 174 Diffuse positivity for p53 has also been described in some tumors.174 Additionally, these tumors are not associated with high-risk HPV, and p16 is usually non-diffuse.173, 174
Tumors that have a gastric phenotype but which demonstrate non-well-differentiated areas may be classified as gastric-type adenocarcinoma.106
Gastric-type mucinous adenocarcinomas of the cervix, including the deceptively bland minimal deviation adenocarcinoma, are reported to be associated with aggressive behavior and a worse prognosis compared to usual type endocervical adenocarcinoma, with gastric-type adenocarcinomas having a higher risk for extracervical spread, a higher risk of recurrence, and a decreased 5-year disease-free survival (25% vs. 75%).173, 175 Delay in correct diagnosis due to difficulty in screening for these tumors may account for some of the advanced cases.
Equally important, benign conditions such as tunnel clusters, hyperplastic mesonephric ducts, deep Nabothian cysts, and endometriosis, should not be confused with minimal deviation adenocarcinoma. Careful assessment for the features discussed above as well as use of immunohistochemistry can aid in this differential diagnosis.
It has been proposed that the hyperplastic endocervical lesion termed “lobular endocervical glandular hyperplasia” (LEGH) may be a precursor to gastric-type adenocarcinomas, based on co-existence of the two entities, evidence of a gastric phenotype in LEGH, and shared chromosomal imbalances between LEGH and gastric-type adenocarcinomas.176, 177
Mucinous carcinoma, intestinal type
Intestinal type mucinous carcinomas of the cervix are defined in the current WHO classification as mucinous adenocarcinomas with areas of intestinal type differentiation.106
These tumors are composed of cells similar to those seen in colorectal adenocarcinomas, characterized by the presence of goblet cells. Glandular and papillary growth patterns can be seen (Fig. 15). Neuroendocrine cells and occasionally Paneth cells may be present. Intestinal differentiation may be found diffusely or only focally within a mucinous carcinoma. The main differential diagnosis is with metastatic colonic adenocarcinoma. Primary cervical intestinal type adenocarcinoma is generally immunoreactive with CK7 and negative or only focally positive for CK20.154 As with intestinal type AIS, positivity for CDX2, indicating intestinal differentiation, is also frequently present.154 The presence of high-risk HPV has been identified in many of these tumors,178 and diffuse positivity for p16 is often seen.154
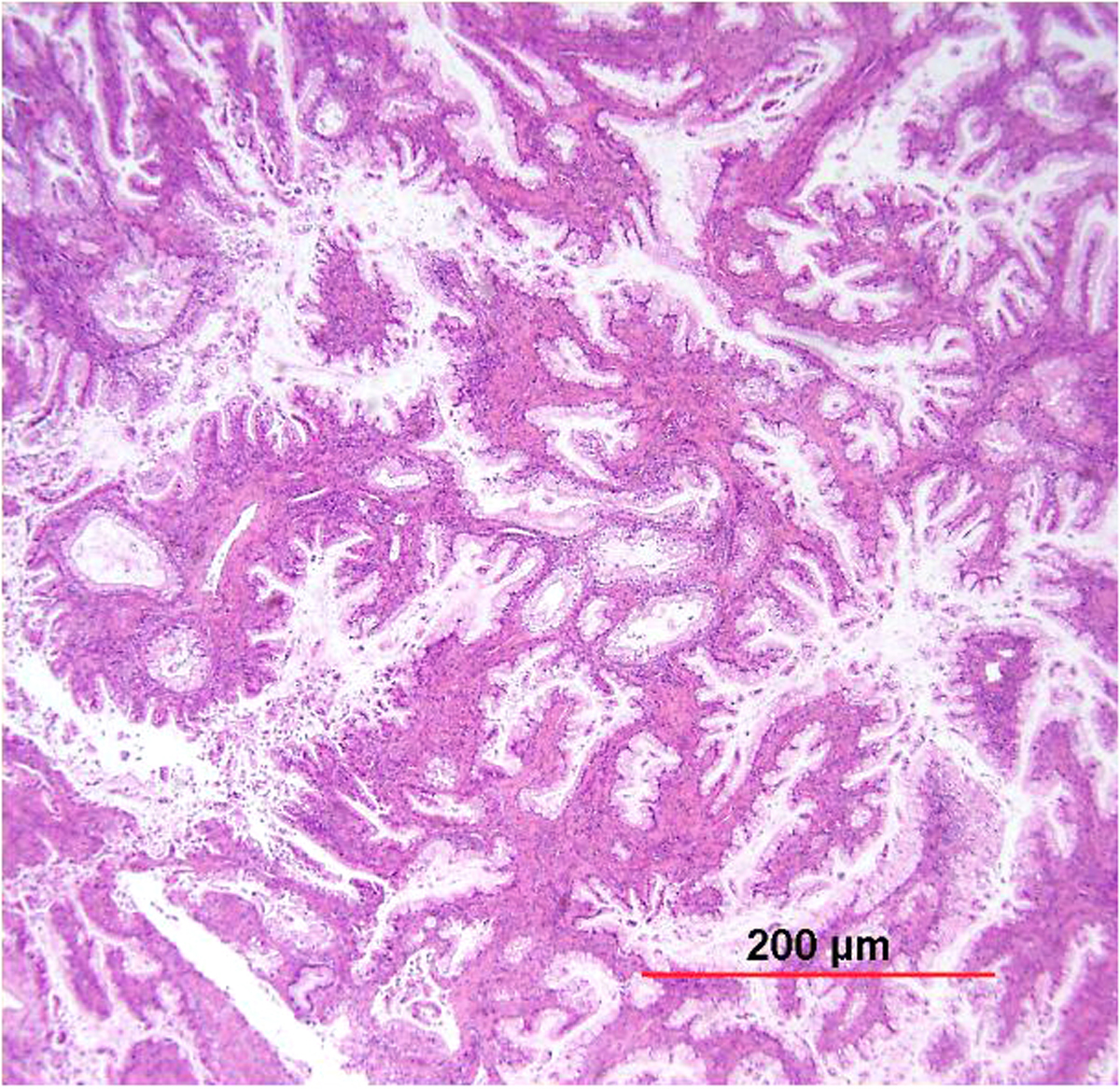 Fig. 15. Cervical adenocarcinoma of intestinal type. Intermediate power shows infiltrating adenocarcinoma with a glandular growth pattern. Tumor cells contain abundant intracytoplasmic mucin. Goblet cells can be appreciated. (Hematoxylin-eosin stain, red bar: original magnification.)
Fig. 15. Cervical adenocarcinoma of intestinal type. Intermediate power shows infiltrating adenocarcinoma with a glandular growth pattern. Tumor cells contain abundant intracytoplasmic mucin. Goblet cells can be appreciated. (Hematoxylin-eosin stain, red bar: original magnification.)
Mucinous carcinoma, signet-ring cell type
These rare carcinomas are defined as having focal or diffuse signet-ring cell differentiation.106 Signet-ring cell features may be seen in either usual-type endocervical adenocarcinomas which are associated with HPV, or with the HPV-independent gastric-type adenocarcinomas.106
Villoglandular carcinoma
Young and Scully179 reported a series of 13 villoglandular papillary adenocarcinomas of the uterine cervix. This tumor occurred mostly in young women (mean age, 33 years). The lesions were described clinically as polypoid, condylomatous, eroded, nodular, white, friable, or fungating. Microscopically, the superficial portion of the tumor consisted of complex papillae lined by well-differentiated endocervical cells (Fig. 16). In the deeper portion of the tumor, neoplastic cells formed branching tubular glands pushing into fibrous stroma. In six women, the tumor was confined to the superficial one third of cervical wall, whereas deep invasion occurred in two women. After hysterectomy, no tumor recurrence was noted in ten women observed for 2–14 years.179
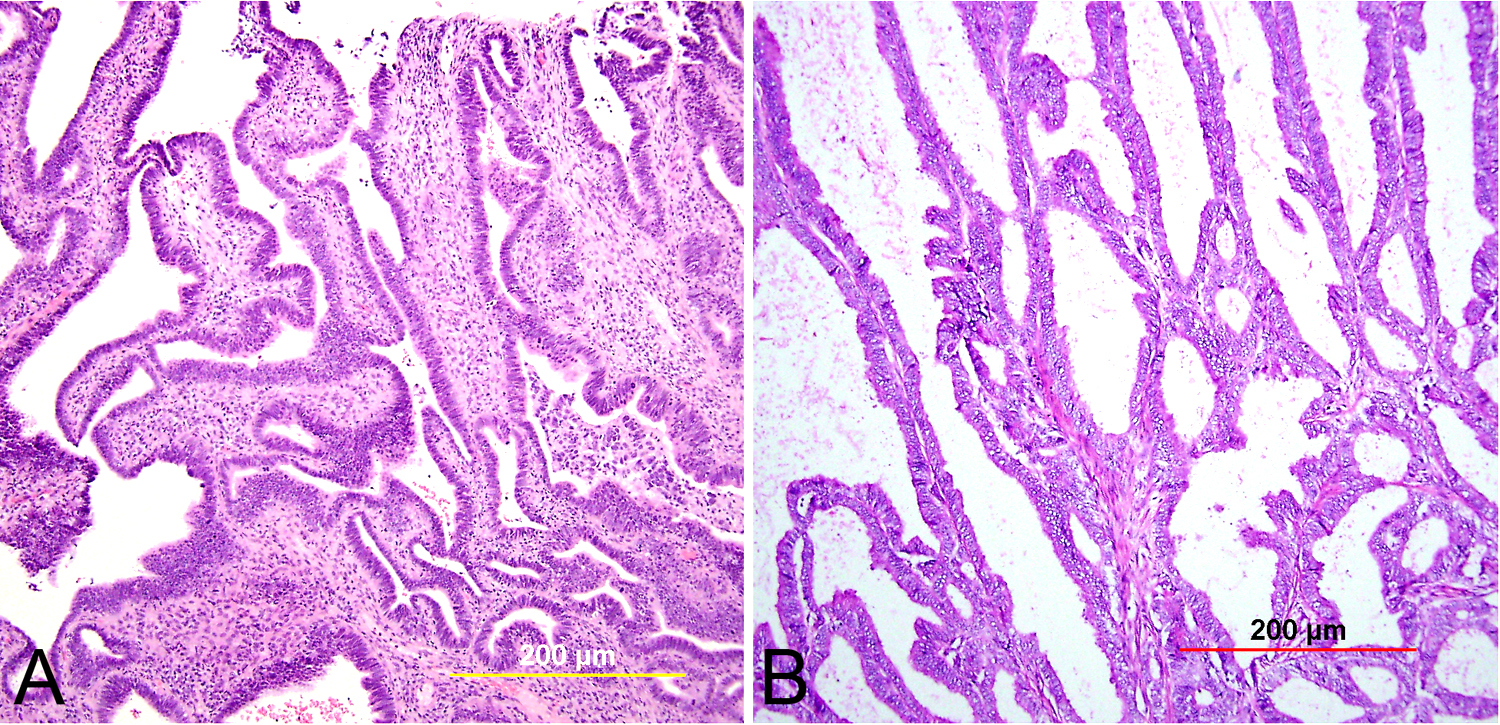 Fig. 16. Cervical adenocarcinoma, villoglandular type. On the surface are multiple papillary projections consisting of columnar cells with either thick (A) or thin (B) fibrovascular cores. The base of the tumor usually has smooth borders without an infiltrative pattern. (Hematoxylin-eosin stain, yellow and red bars: original magnification.)
Fig. 16. Cervical adenocarcinoma, villoglandular type. On the surface are multiple papillary projections consisting of columnar cells with either thick (A) or thin (B) fibrovascular cores. The base of the tumor usually has smooth borders without an infiltrative pattern. (Hematoxylin-eosin stain, yellow and red bars: original magnification.)
In a subsequent study of 24 cases by Jones and associates,180 the mean age was 37 years (range 27–54 years), and 50% of women were in their third decade of life. There was history of oral contraceptive use in 63% of women, in contrast to 28% among women having other types of cervical adenocarcinoma (p = 0.02). Grossly, the tumor had an exophytic polypoid appearance. Microscopically, the predominant cell type was endocervical in 50%, endometrioid in 33%, and intestinal in 17%. In all, 33% had coexisting squamous intraepithelial lesions. Five tumors (21%) were entirely in situ without stromal invasion; 75% (18 tumors) were superficially invasive, confined to the inner third of the cervical wall; and 4% (one tumor) invaded 75% of the cervical wall.180
Treatment modalities included local excision or cone biopsy in five women, simple hysterectomy in four women, and radical hysterectomy in 15 women.180 All tumors were confined to the cervix without pelvic lymph node metastasis. All women were alive and well 7–77 months (mean 36 months) later.180 Lymph node metastases, though reported, are rare.181 In view of their distinct clinical and pathologic features, these tumors are separated from other cervical adenocarcinomas. A conservative treatment approach is considered acceptable in many cases, especially in young women who want to retain fertility.180
It should be emphasized that the term villoglandular adenocarcinoma should be reserved only for those tumors which meet the stringent morphologic criteria. The degree of nuclear atypia should be no worse than moderate. The tumor borders should be smooth, and tumors made up of clear cells and serous cells are excluded. In cervical biopsy specimens and endocervical curettage specimens, there are often fragments of tumor that a have villous pattern. However, in the subsequently excised specimens, some of these tumors prove to have poorly differentiated elements or infiltrative borders. Thus, the diagnosis of well-differentiated villoglandular carcinoma should be made on completely excised specimens only. It has been recommended that tumors which do not meet these criteria or which have lymph-vascular space invasion or more than superficial invasion be treated more aggressively.181
Endometrioid carcinoma
This group of tumors has the appearance of FIGO grade 1 or grade 2 adenocarcinoma of the endometrium. The predominant growth pattern is glandular or less commonly papillary. The lining cells are tall, columnar, and have densely basophilic or eosinophilic cytoplasm (Fig. 17). Endometrioid carcinomas from the endometrium and cervix share a similar histology and immunoprofile, and therefore the diagnosis of cervical origin is justified only if the endometrium is normal after careful sampling for histologic examination. Sometimes, mature metaplastic squamous cells occur within the neoplastic glands.5, 182
Fig. 17. Endometrioid carcinoma of the cervix. This tumor has morphology similar to its uterine counterpart, with glandular and cribriform architecture, smooth luminal borders, and foci of squamous differentiation (A). High power demonstrates columnar cells with pseudostratified nuclei and mild to moderate atypia. (Hematoxylin-eosin stain, red bars: original magnification.)
Endometrioid carcinomas comprise 5% of endocervical adenocarcinomas.106 Some authors believe that these tumors have been overdiagnosed in the past due to the endometrioid appearance of some usual type endocervical adenocarcinomas.106, 138 Endometrioid carcinomas which represent endometrioid variants of usual type endocervical adenocarcinoma are related to HPV and will demonstrate diffuse p16 positivity. True endometrioid carcinomas, which can arise from cervical endometriosis, are HPV-independent.106
Clear cell carcinoma
Cervical clear cell adenocarcinoma affects women with or without exposure to diethylstilbestrol (DES) in utero. The age distribution has a bimodal peak, one around 20 years of age (mostly DES-exposed women) and the other in the fifth and sixth decades of life (mostly sporadic cases).183 In current practice, DES-associated clear cell carcinomas are rare.
The most common patient complaint is vaginal bleeding, and, on examination, a polypoid, exophytic, or fungating tumor is visible.184, 185 In the DES-exposed progeny, the cervical clear cell carcinomas are located mainly in the ectocervix and rarely in the endocervix.186
Under the microscope, the tumor cells have distinct clear, empty appearing cytoplasm and enlarged, hyperchromatic nuclei, which project into the apical cytoplasm, the so called hobnail appearance. The clear cytoplasm is attributed to the accumulation of abundant glycogen similar in appearance to that seen in secretory endometrial cells. Cells with eosinophilic cytoplasm can also be seen. The cells grow predominantly in tubulocystic, papillary, or solid patterns (Fig. 18). Significant nuclear atypia is appreciable at least focally. Intracytoplasmic hyaline globules and stromal hyalinization are common.
Fig. 18. Clear cell carcinoma of the cervix. This exophytic tumor is characterized by papillary and glandular growth patterns with hyalinized stroma. The papillae and glands are lined by intermediate to large tumor cells with clear or eosinophilic cytoplasm and high-grade nuclei. Scattered hobnail cells can be appreciated. (Hematoxylin-eosin stain, red bar: original magnification.)
There is evidence that a subset of these tumors is related to HPV.187 Survival appears to be similar to other types of cervical carcinoma.188
Serous carcinoma
A few cervical adenocarcinomas indistinguishable from serous carcinoma of the endometrium or adnexa have been reported.189, 190, 191 The tumor microscopically has a complex pattern of papillae with cellular budding and glands with slit-like spaces. Moderate to severe nuclear pleomorphism is usually seen (Fig. 19). Occasionally, psammoma bodies can be identified. An in situ component may be present. Nearly half of reported cases exhibited a second admixed pattern, most commonly low-grade villoglandular adenocarcinoma. Diagnosis of serous adenocarcinoma of the cervix can be made only when metastasis from the endometrium or adnexa is excluded.
Fig. 19. Cervical adenocarcinoma of serous type. Cervical serous carcinomas demonstrate the same high-grade cytologic features as their uterine counterparts, with marked nuclear atypia and pleomorphism as well as brisk mitotic activity. (Hematoxylin-eosin stain, red bar: original magnification)
When diagnosed at an advanced stage, these tumors behave aggressively, and poor prognostic factors include younger age, larger tumor size (>2 cm), deep invasion (>1 cm), lymph node metastasis, and elevated CA125. However, stage I tumors have a similar prognosis to other stage I cervical adenocarcinomas.191
Mesonephric carcinoma
The rare entity of mesonephric carcinoma arises from mesonephric remnants in the deep lateral cervical wall. Many of the mesonephric duct carcinomas reported earlier belong to the clear cell adenocarcinoma category by current classification. The tumors may be bulky or exophytic, and can invade deeply.106
Histologically, the tumors typically consist of tubules, glands, and microcysts containing eosinophilic hyaline material in the lumen (Fig. 20). This eosinophilic material is PAS-positive and mucicarmine-negative by histochemistry. The tubules are lined by cuboidal cells with variable cytologic atypia and mitotic activity. Other architectural patterns are also described, including ductal, retiform, solid, and sex cord-like structures. Rare cases with spindle cells resembling endometrial stromal sarcoma with osteoid and chondroid metaplasia have been described.192
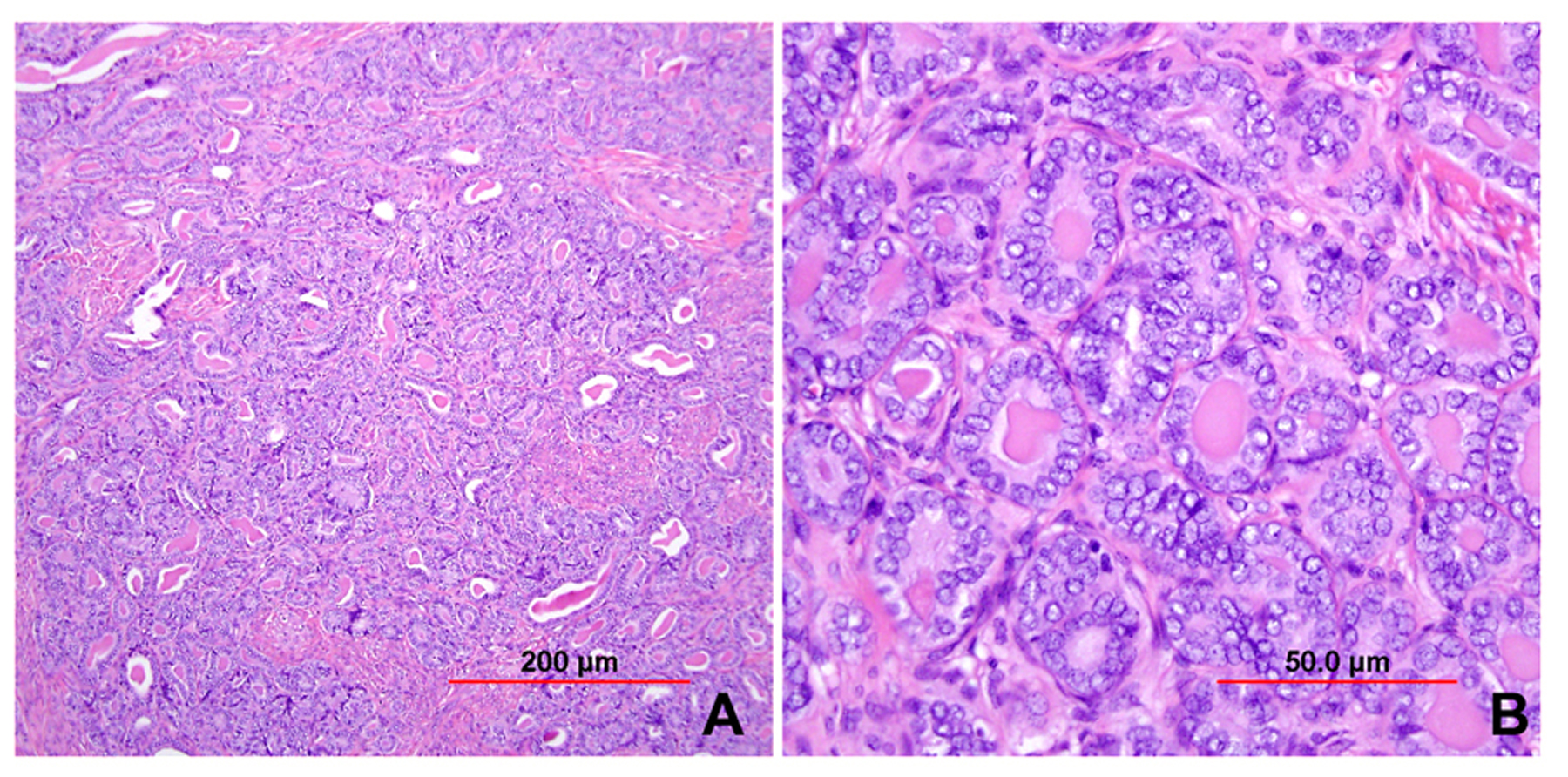 Fig. 20. Cervical adenocarcinoma of mesonephric type. Tumor consists of packed tubules and microcysts with infiltrating borders (A). A higher power view shows hyperchromatic and crowded nuclei with frequent mitoses (B). Intraluminal eosinophilic hyaline material is evident. (Hematoxylin-eosin stain, red bar: original magnification.)
Fig. 20. Cervical adenocarcinoma of mesonephric type. Tumor consists of packed tubules and microcysts with infiltrating borders (A). A higher power view shows hyperchromatic and crowded nuclei with frequent mitoses (B). Intraluminal eosinophilic hyaline material is evident. (Hematoxylin-eosin stain, red bar: original magnification.)
Immunohistochemistry is often necessary to confirm the diagnosis, given both the rarity of the tumor and its variable architectural patterns, which can mimic diverse tumor types. It has been demonstrated that mesonephric carcinomas are immunopositive for CD10, epithelial membrane antigen (EMA), vimentin, calretinin, PAX8, HMGA2, and CA125, with variable positivity for ER, CEA, inhibin, TTF1, and HNF1-beta.193 Interestingly, although these tumors are unrelated to high-risk HPV, diffuse p16 has been demonstrated in some cases.193
The prognosis of mesonephric carcinoma compared to other cervical adenocarcinomas has not been clearly elucidated; however, some cases with aggressive behavior, late recurrences, and distant metastases have been reported.194
It should be noted that benign hyperplasia of mesonephric ducts can be found deep in the cervical stroma, mimicking adenoma malignum and mesonephric adenocarcinoma.195 The presence of haphazard infiltrative growth, gland confluence, mitoses, and nuclear atypia assists in making the distinction between mesonephric hyperplasia and carcinoma.106
Adenocarcinoma admixed with neuroendocrine carcinoma
Mixed carcinomas consisting of adenocarcinoma and small cell or large cell neuroendocrine carcinoma have been described.196, 197, 198 Some evidence exists as to the monoclonality of the two components.196 The behavior is driven by the high-grade neuroendocrine component. Neuroendocrine carcinomas are discussed in more detail in a subsequent section.
Endometrial vs. endocervical carcinoma
Distinguishing endocervical from endometrial carcinomas can sometimes be difficult on biopsies or even in hysterectomies in which the tumor involves both sites. To compound the issue, endometrioid carcinomas of the endometrium can display focal to extensive mucinous differentiation, while endometrioid variants of endocervical adenocarcinoma exist (see above). Ancillary studies can be helpful in difficult cases. Low-grade endometrioid carcinomas of the endometrium are typically positive for ER, positive for vimentin, and negative for CEA; expression of p16 can be seen and is sometimes extensive, but usually with a patchy or mosaic pattern. Usual type endocervical adenocarcinomas, in contrast, are usually negative for ER, negative for vimentin, positive for CEA, and diffusely positive for p16. If the carcinoma in question demonstrates high-grade cytologic features (as with serous or undifferentiated types), the utility of p16 is limited, as high-grade tumors can express diffuse p16 via a non-HPV-related pathway. Useful ancillary studies in this case include p53 (often mutated in high-grade carcinomas) and high-risk HPV in situ hybridization. Additionally, immunohistochemical loss of one or more mismatch repair proteins, indicative of microsatellite instability, would favor an endometrial carcinoma.138, 153 It should be noted as well that some overlap in immunohistochemistry exists, especially for mucinous endometrioid carcinomas and endometrioid cervical carcinomas,199 and so the overall immunoprofile and morphologic setting must be considered. The presence of background preneoplastic lesions (atypical endometrial hyperplasia, endocervical AIS) should also be sought and considered.
Another diagnostic dilemma concerns the microglandular hyperplasia (MGH)-like variant of endometrioid carcinoma. This variant of endometrioid carcinoma demonstrates small closely packed glands, mucin production, and abundant luminal and stromal neutrophils, closely simulating MGH.200 Unlike typical MGH, however, these endometrioid carcinomas generally display more atypia and mitotic activity, and are more likely to present in postmenopausal patients.200 The presence of more typical endometrioid morphology in other areas of the tumor, as well as myometrial invasion, also assists in the distinction from a benign process, although these features may not be seen on the initial biopsy or curettage specimen. It should be noted also that MGH can be exuberant, and atypical forms have been described. Atypical MGH can display various unusual patterns, including solid, pseudoinfiltrative, myxoid, signet ring cell, and hobnail cell features.201 Although these lesions are benign, they can have more cytologic atypia (mild to moderate) than typical MGH, and can simulate both MGH-like endometrioid carcinomas as well as endocervical carcinomas. Helpful features in recognizing this entity include the presence of other more typical areas of MGH, continuity with normal cervical tissue, reserve cell hyperplasia, and intracellular vacuoles. In difficult cases, immunohistochemistry can be of use; atypical MGH demonstrates negative or patchy p16, positive ER and PR, and positive p63 in the accompanying reserve cells.
OTHER EPITHELIAL NEOPLASMS
Although squamous cell carcinoma and adenocarcinoma are the most common epithelial neoplasms to occur in the cervix, other less common tumors can also occur in this site. The following additional tumor types are included in the current WHO classification:106
- Adenosquamous carcinoma
- Glassy cell carcinoma
- Adenoid basal carcinoma
- Adenoid cystic carcinoma
- Undifferentiated carcinoma
- Neuroendocrine tumors
- Low-grade neuroendocrine tumor
- Carcinoid tumor
- Atypical carcinoid tumor
- High-grade neuroendocrine carcinoma
- Small cell neuroendocrine carcinoma
- Large cell neuroendocrine carcinoma
- Low-grade neuroendocrine tumor
Adenosquamous carcinoma
Adenosquamous carcinomas are defined by having both a squamous cell carcinoma and an adenocarcinoma component. In 1956, Glucksmann and Cherry202 subdivided adenosquamous carcinomas into mature (35%), signet-ring cell (44%), and glassy cell (21%) types. The current WHO classification includes only “adenosquamous carcinoma” and the poorly differentiated variant “glassy cell carcinoma.”
The squamous component must be malignant (benign squamous metaplasia does not qualify an adenocarcinoma as adenosquamous carcinoma) and usually demonstrates well-differentiated areas with keratinization.113 The adenocarcinomatous component should have recognizable glands (Fig. 21). Excluded from this diagnosis are SCC with occasional mucin-producing cells, as well as carcinomas with solid growth, mucin production, and no identifiable squamous differentiation (best diagnosed as poorly differentiated adenocarcinomas).106 Additionally, carcinomas with three cellular components (epidermoid, intermediate, and mucin-producing) are best classified as mucoepidermoid carcinomas; these, unlike adenosquamous carcinomas, demonstrate the t(11;19) translocation described in mucoepidermoid carcinoma of the salivary glands.203
Fig. 21. Adenosquamous carcinoma of the cervix. Well-differentiated squamous cell carcinoma with admixed areas of adenocarcinoma. (Hematoxylin-eosin stain, red bar: original magnification.)
Cervical adenosquamous carcinomas are associated with HPV types 18 and 16.204 Monoclonality between the squamous and glandular components has been demonstrated,205 and the tumors are thought to arise from pluripotent reserve cells.113 Co-existing in situ lesions can include HSIL, AIS, and SMILE (see above).
The prognosis of patients with adenosquamous carcinoma in comparison to SCC or adenocarcinoma is unclear, with conflicting results from studies reporting a worse prognosis for adenosquamous carcinoma vs. no difference in prognosis.206, 207, 208, 209
Glassy cell carcinoma
Glassy cell carcinoma is considered to be a poorly differentiated variant of adenosquamous carcinoma. These are rare tumors with rapid growth, which sometimes present with barrel-shaped cervix.106
Microscopically, large polygonal tumor cells are arranged in sheets and nests and have abundant eosinophilic to granular “ground-glass” cytoplasm, sharp cell borders, uniformly round to oval nuclei, and giant nucleoli (Fig. 22). Mitotic figures are abundant. Many eosinophils and plasma cells are present in the stroma. In the original description of this entity,202 tumor cells had PAS-positive material but no mucinous substance. However, in the study by Maier and Norris,210 seven of eight tumors studied by the mucicarmine stain were positive. Three of eight neoplasms contained glandular lumina, and three others had squamous differentiation. One tumor had both glandular and squamous foci. By electron microscopic study, the glassy appearance corresponded to abundant polyribosomes and rough endoplasmic reticulum. The tonofilaments were scant.211 Although no intracytoplasmic lumina were identified, there were intercellular spaces lined by microvilli to suggest glandular differentiation. Some tumor cells also contained mucinous material in the cytoplasm.211 These histochemical and ultrastructural findings support the poorly differentiated nature of these neoplasms and the presence of glandular differentiation in some of the tumor cells.211 Tumor cells are reactive for low and high molecular cytokeratin, MUC1, and MUC2, but are negative for estrogen or progesterone receptors.
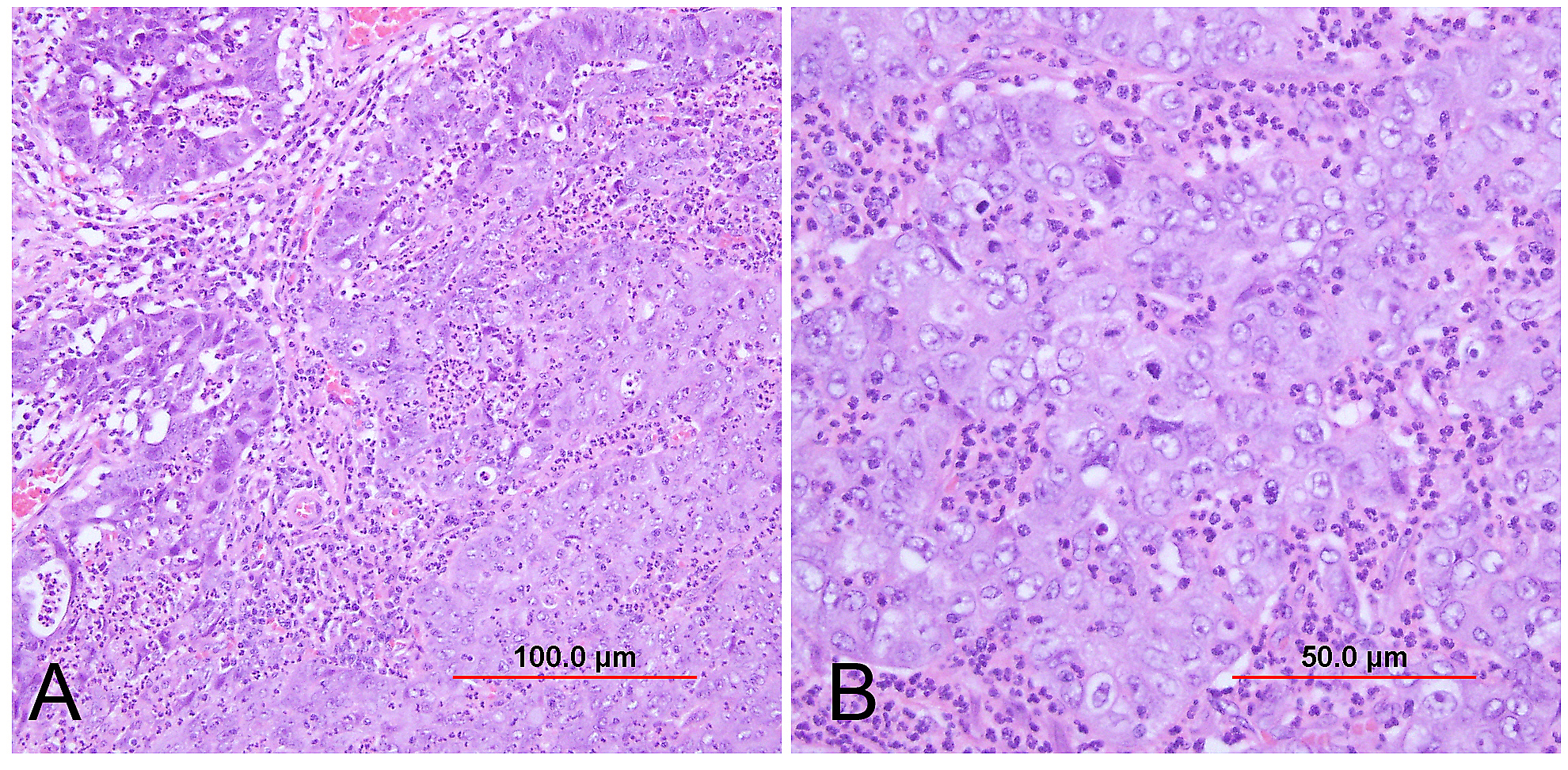 Fig. 22. Adenosquamous carcinoma of glassy cell type. (A) Tumor consists of sheets of large cells with abundant amphophilic "ground-glass" cytoplasm and well-defined cell borders. Areas of glandular architecture (upper left corner) can be appreciated. (B) Nuclei are large and relatively uniform with vesicular chromatin and prominent nucleoli. A significant inflammatory infiltrate is present. (Hematoxylin-eosin stain, red bars: original magnification.)
Fig. 22. Adenosquamous carcinoma of glassy cell type. (A) Tumor consists of sheets of large cells with abundant amphophilic "ground-glass" cytoplasm and well-defined cell borders. Areas of glandular architecture (upper left corner) can be appreciated. (B) Nuclei are large and relatively uniform with vesicular chromatin and prominent nucleoli. A significant inflammatory infiltrate is present. (Hematoxylin-eosin stain, red bars: original magnification.)
These tumors have been traditionally regarded as aggressive malignancies with a limited response to surgery and radiation,212 but some groups have reported greater treatment success with adjuvant or neoadjuvant chemotherapy.213, 214, 215, 216
Adenoid basal carcinoma
This rare cervical tumor resembles basal cell carcinoma of the skin in its histologic appearance. Most patients are postmenopausal and asymptomatic. The cervix is grossly normal and lesions are often detected following abnormal pap smears and incidentally in cervices removed for other reasons. Microscopically, basaloid cells with scanty cytoplasm and uniformly small, round nuclei are arranged in solid nests. These nests are grouped together in a lobular pattern. Cells in the outermost layer have a distinct palisaded nuclear arrangement. Centrally, glandular and squamous metaplasia, as well as cystic spaces containing necrotic debris, may occur (Fig. 23).
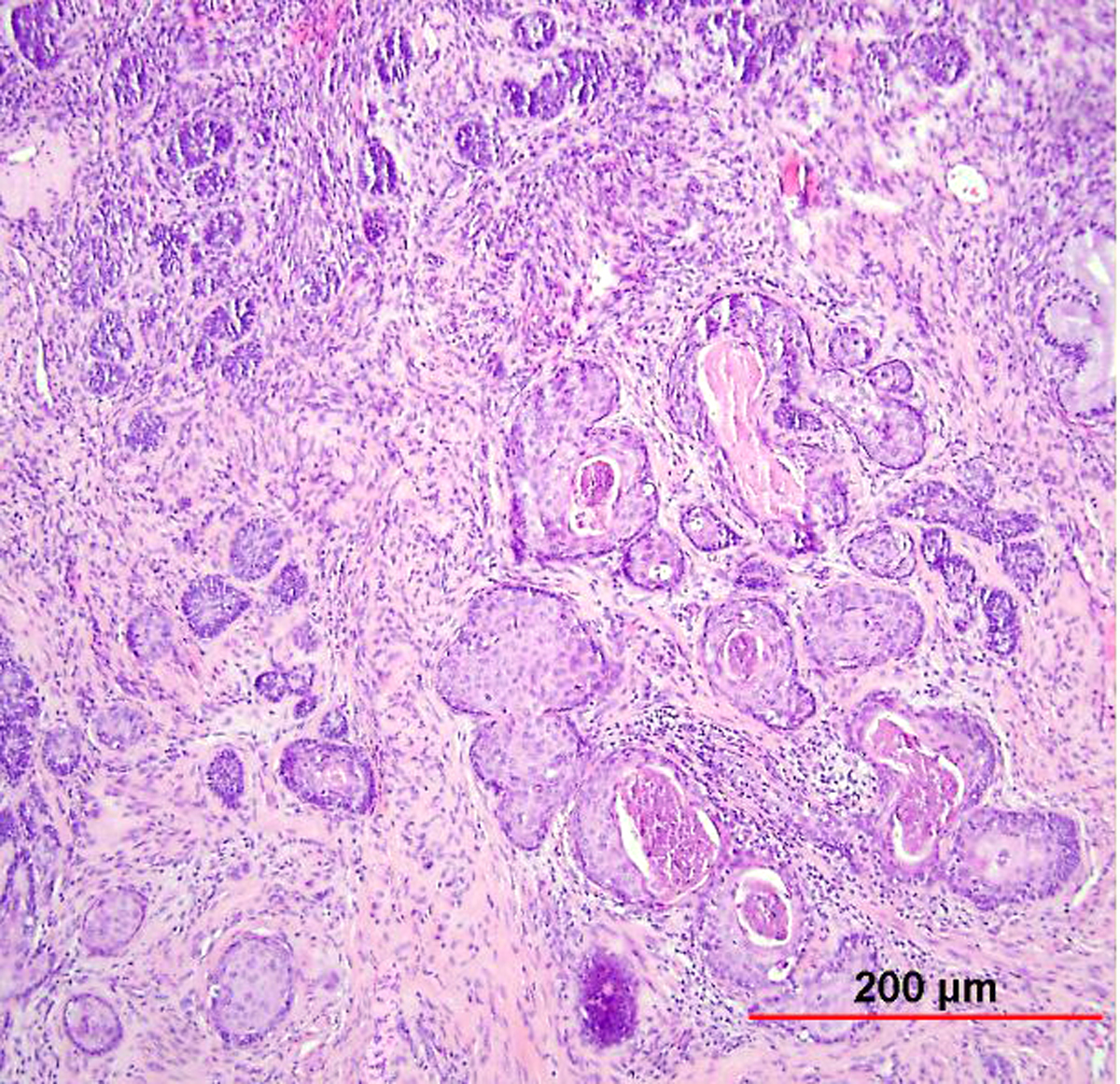 Fig. 23. Adenoid basal carcinoma of the cervix. This tumor consists of small basaloid nests (left) and large nests in a lobule with squamous metaplasia (right). The basaloid tumor cells are small with scant cytoplasm and a palisaded nuclear arrangement. (Hematoxylin-eosin stain, red bar: original magnification.)
Fig. 23. Adenoid basal carcinoma of the cervix. This tumor consists of small basaloid nests (left) and large nests in a lobule with squamous metaplasia (right). The basaloid tumor cells are small with scant cytoplasm and a palisaded nuclear arrangement. (Hematoxylin-eosin stain, red bar: original magnification.)
In most reported cases, mitotic activity was absent to low, vascular lymphatic invasion was not seen, and behavior was reported to be essentially benign.217, 218 This led to the proposal that the term “adenoid basal epithelioma” be used for this type of tumor.218 The bulk of the cases reported in the literature would fall into this category, consisting of a low-grade tumor similar to basal cell carcinoma of the skin, with a generally benign behavior. It was also proposed that the entity termed “adenoid basal hyperplasia,” a similar lesion confined to superficial endocervical glands, represented early adenoid basal epithelioma.218 It has been proposed that the term “adenoid basal carcinoma” be reserved for infiltrative neoplasms with stromal desmoplasia and malignant cytologic features.113 One reported case of an adenoid basal carcinoma with metastasis to the lung demonstrated an appearance similar to morpheaform basal cell carcinoma of the skin.217 It should be noted, however, that in the current WHO classification, retention of the term “adenoid basal carcinoma” is recommended.106
Most adenoid basal carcinomas have coexisting HSIL, and some are associated with other invasive tumors, including invasive squamous cell carcinoma and adenoid cystic carcinoma.218, 219
The differential diagnosis includes adenoid cystic carcinoma, which presents with infiltrative cribriform glands associated with extracellular mucinous material or hyaline cylinders. Cytologic atypia is evident in the tumor cells. Also in the differential diagnosis is basaloid squamous cell carcinoma, in which the tumor cells are cytologically malignant and mitotically active, with a desmoplastic reaction associated with the infiltrative tumor nests.
The presence of high-risk HPV, along with diffuse p16 expression, has been demonstrated in these tumors.219, 220, 221 Mutation in TP53 has also been reported.220
Adenoid cystic carcinoma
Adenoid cystic carcinoma of the female lower genital tract occurs most commonly in the Bartholin gland. This is followed by the uterine cervix, affecting primarily postmenopausal women in their seventh decade of life, about 20 years later than squamous cell carcinoma. The histologic features are similar to those occurring in the salivary gland. It is suggested that multipotent reserve cells in the endocervical glands acquire myoepithelial differentiation, which is not normally seen in the cervix.222
Histologically, basaloid cells are typically arranged in cribriform glands with hyaline or mucinous material in the microcystic spaces (hyaline cylinders) (Fig. 24). Tubules and solid nests are less common, and tumors with predominantly solid growth patterns are associated with a worse prognosis.223 The individual cells have scanty cytoplasm and small, uniform, hyperchromatic nuclei. There is often palisading at the periphery of the cell nests. Mitotic figures are variable depending on the degree of differentiation.217 Necrosis is common, as is lymph-vascular space invasion.
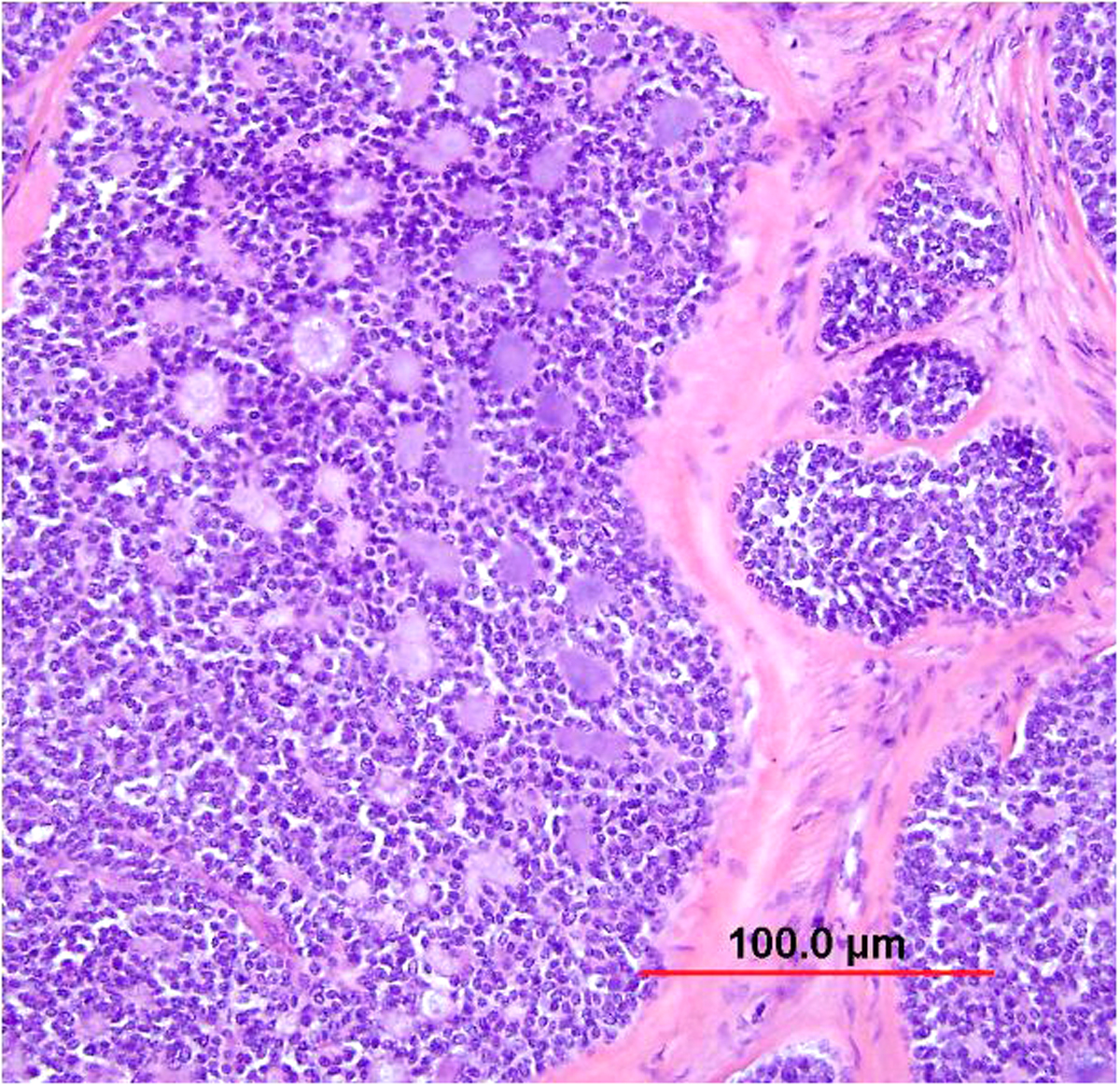 Fig. 24. Adenoid cystic carcinoma of the cervix. This infiltrating carcinoma displays large nests with solid and cribriform growth patterns. The tumor cells are basaloid with hyaline material in the lumen. (Hematoxylin-eosin stain, red bar: original magnification.)
Fig. 24. Adenoid cystic carcinoma of the cervix. This infiltrating carcinoma displays large nests with solid and cribriform growth patterns. The tumor cells are basaloid with hyaline material in the lumen. (Hematoxylin-eosin stain, red bar: original magnification.)
In about 50% of cases, squamous differentiation is apparent. In such cases, squamous cells replace the glandular lumina partially or completely. When squamous elements predominate, adenoid cystic carcinoma may not be recognized in a small biopsy specimen. Adenoid cystic carcinoma may also be associated with HSIL; because of this, some women are detected initially by abnormal cervical smears. Other types of adenocarcinoma, undifferentiated carcinoma, or sarcoma may sometimes coexist with adenoid cystic carcinoma.217, 224
In one study, 3–5 year survival rates for patients with stage I adenoid cystic carcinoma were 56% compared with 27% for stage II. None of the patients with stage III or IV disease survived.225Most of the treatment failures were caused by distant and/or local pelvic recurrence. Metastases occurred most frequently in the lung (44%) and less commonly in the bone, liver, and brain. With only 32% of patients free of disease at last follow-up, the overall prognosis of adenoid cystic carcinoma is worse than that of squamous cell carcinoma or pure adenocarcinoma of the cervix.225 In one review, bulky early stage tumors were found to be best treated with combined surgery and chemoradiation.226
Immunohistochemically, positivity for epithelial markers (e.g. AE1/AE3, CAM 5.2) and myoepithelial markers (e.g. p63, SMA, calponin) has been described.106, 217, 222 Recent studies have shown that CD117 is a useful biomarker for adenoid cystic carcinoma and can be used to differentiate it from adenoid basal carcinoma of the cervix.227 High-risk HPV has also been identified in these tumors.228 The MYB-NFIB gene fusion has been found in adenoid cystic carcinomas of various sites (although not specifically the cervix).229
Neuroendocrine tumors of the cervix
In the current WHO classification, neuroendocrine tumors of the cervix are divided into low-grade neuroendocrine tumors (NET) and high-grade neuroendocrine carcinomas. The low-grade tumors consist of carcinoid tumor and atypical carcinoid tumor, while the high-grade tumors include small cell and large cell neuroendocrine carcinomas (Fig. 25).106 Low-grade NET is very uncommon in the cervix. Typical carcinoid tumor, also known as low-grade NET, grade 1, demonstrates histologic features resembling the typical carcinoid seen in other parts of the body. Architectural patterns include organoid growth, spindling, nests, and trabeculae, and the cells are characterized by abundant cytoplasm and granular (“salt and pepper”) chromatin. Atypical carcinoid tumor, also known as low-grade NET, grade 2, is distinguished from typical carcinoid tumor based on its greater cytological atypia (mild to moderate), increased mitotic activity, and foci of necrosis. Unlike in other sites, the utility of specific Ki-67 proliferative indices or specific mitotic counts as thresholds for grading of NET has not been demonstrated in the cervix.106
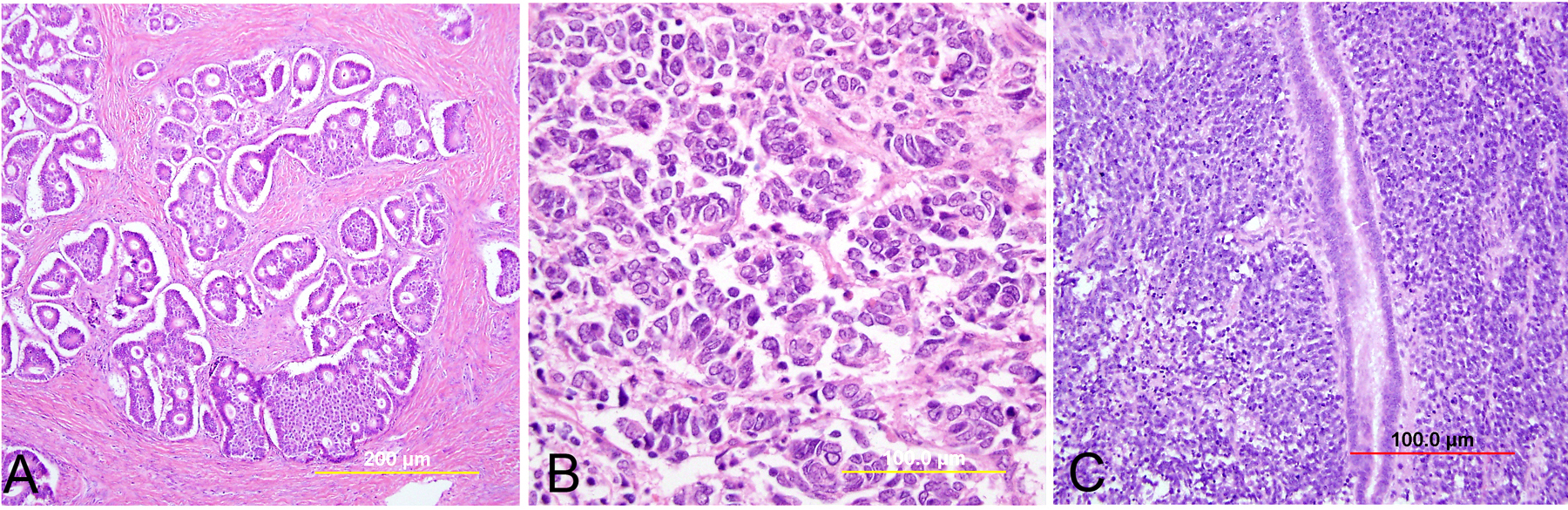 Fig. 25. Neuroendocrine tumors of the cervix. (A) Typical carcinoid tumor shows glandular and trabecular growth patterns with minimal cytologic atypia and rare mitosis. (B) Atypical carcinoid tumor has glandular and trabecular growth patterns with moderate cytologic atypia and increased mitoses. (C) Small cell neuroendocrine carcinoma has sheets and trabeculae of small ovoid to spindled tumor cells with scant cytoplasm and hyperchromatic nuclei. Mitoses and apoptoses are numerous. (Hematoxylin-eosin stain, yellow and red bars: original magnification.)
Fig. 25. Neuroendocrine tumors of the cervix. (A) Typical carcinoid tumor shows glandular and trabecular growth patterns with minimal cytologic atypia and rare mitosis. (B) Atypical carcinoid tumor has glandular and trabecular growth patterns with moderate cytologic atypia and increased mitoses. (C) Small cell neuroendocrine carcinoma has sheets and trabeculae of small ovoid to spindled tumor cells with scant cytoplasm and hyperchromatic nuclei. Mitoses and apoptoses are numerous. (Hematoxylin-eosin stain, yellow and red bars: original magnification.)
Small cell neuroendocrine carcinoma is the most common neuroendocrine tumor of the cervix. Small cell neuroendocrine carcinoma of the cervix is morphologically indistinguishable from small cell neuroendocrine carcinoma of the lung, and a large proportion also express TTF-1.230 Small cell neuroendocrine carcinoma (also known as neuroendocrine carcinoma, small cell type, grade 3) is characterized by sheets and cords of cells diffusely infiltrating a delicate fibrovascular stroma, simulating lymphoma (Fig. 26). The tumor cells have round to ovoid, small to intermediate sized nuclei. The nuclear chromatin ranges from coarsely granular (“salt and pepper”) to dark and sometimes smudged. Nucleoli are inconspicuous and mitotic figures are frequent. The cytoplasm is scant, resulting in nuclear molding and high nuclear-to-cytoplasmic ratios. Necrosis and crush artifact are common. Large cell carcinomas are characterized by more abundant cytoplasm and larger nuclei with prominent nucleoli. In addition to the diffuse infiltrative pattern, tumor cells are also arranged in trabeculae, ribbons, and rosettes. Occasionally, squamous cell carcinoma and/or adenocarcinoma coexist with neuroendocrine carcinoma.
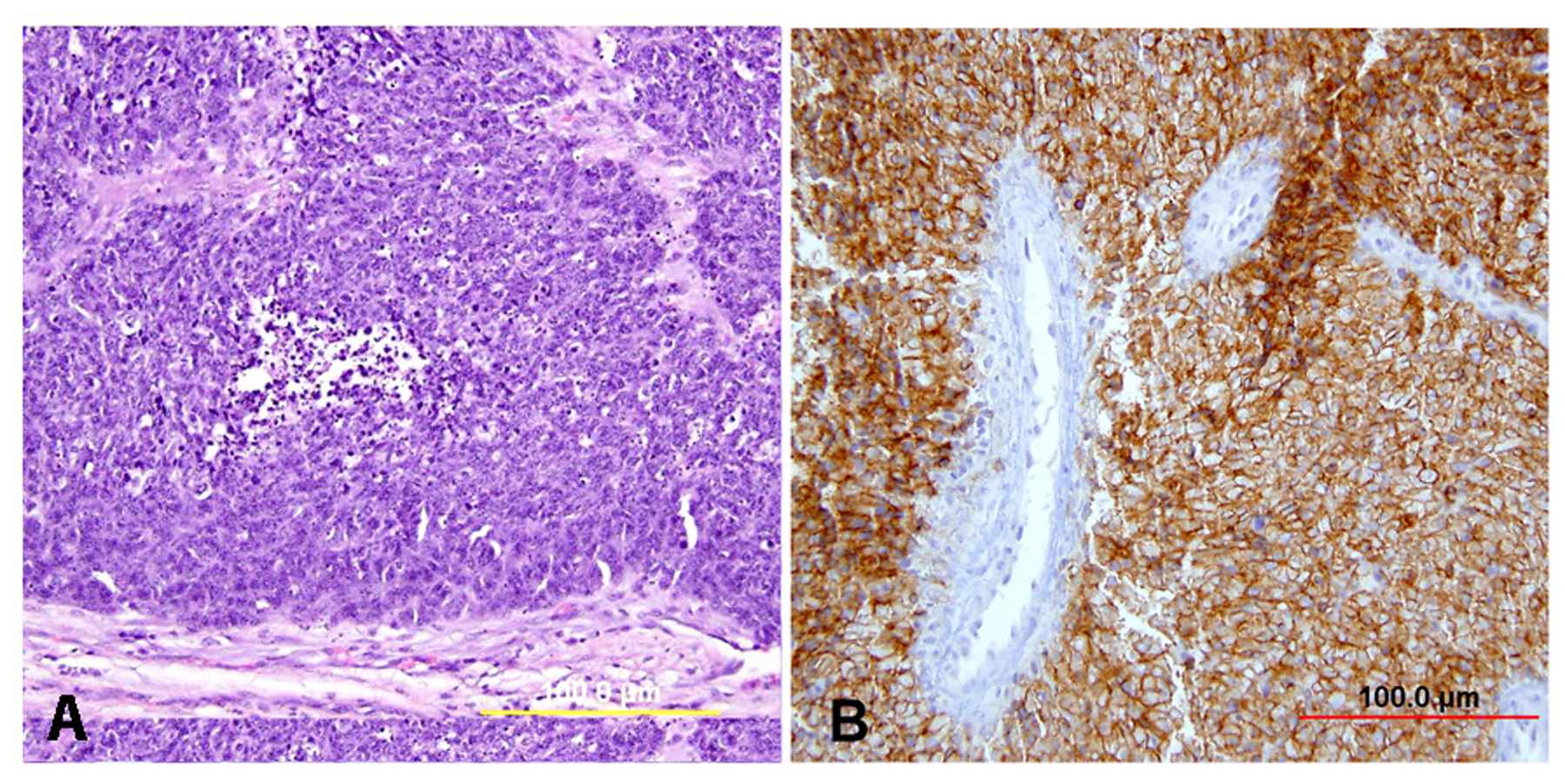 Fig. 26. Small cell neuroendocrine carcinoma. (A) Tumor demonstrates diffuse infiltrative pattern made up of sheets of malignant cells. The presence of crush artifact with smudged nuclei is characteristic. The small nuclei are hyperchromatic and have coarsely granular compact chromatin. The cytoplasm is scant, resulting in nuclear molding. Mitotic activity is high. (B) Tumor is diffusely immunoreactive for CD56. (Hematoxylin-eosin stain, yellow and red bars: original magnification.)
Fig. 26. Small cell neuroendocrine carcinoma. (A) Tumor demonstrates diffuse infiltrative pattern made up of sheets of malignant cells. The presence of crush artifact with smudged nuclei is characteristic. The small nuclei are hyperchromatic and have coarsely granular compact chromatin. The cytoplasm is scant, resulting in nuclear molding. Mitotic activity is high. (B) Tumor is diffusely immunoreactive for CD56. (Hematoxylin-eosin stain, yellow and red bars: original magnification.)
Small cell carcinomas are believed to derive from multipotent cells or argyrophilic cells in the basal cell layer of the endocervical mucosa.231 The diagnosis of neuroendocrine tumors in the cervix partly relies on immunoreactivity for neuroendocrine markers, which include synaptophysin, neuron-specific enolase (NSE), chromogranin, and CD56, although some high-grade neuroendocrine carcinomas fail to express these markers.230 There is a strong association with high-risk HPV, in particular HPV type 18.232, 233
The prognosis of this group of tumors is related to the extent of disease and the degree of differentiation. High-grade neuroendocrine carcinomas are highly aggressive with a propensity for local and distant spread.234, 235 One study of 31 neuroendocrine tumors of the cervix (including two atypical carcinoids, four large cell neuroendocrine carcinomas, and 25 small cell neuroendocrine carcinomas) demonstrated that the mean survival time for all patients was 32.3 months. The 2- and 5-year survival rates were 54.8% and 31.5% for all patients, respectively.236 When treated with radical hysterectomy and pelvic nodal dissection, one study of small cell carcinomas reported four of six (67%) patients to have pelvic nodal metastasis and five of eight (63%) patients to develop tumor recurrence.144 Sevin and colleagues237reported 12 women with small cell carcinoma, including one FIGO stage IA, ten stage IB, and one stage II. When compared with stage IB and II cervical squamous carcinoma and adenocarcinoma combined, small cell carcinoma had a higher frequency of lymph-vascular space invasion (82% vs. 62%), more frequent lymph node metastasis (45.5% vs. 18.9%), and a lower 5-year survival rate (36.4% vs. 71.6%). Only 42% (5 of 12) of patients were disease free at the time of report. In view of these findings, a combined therapy of surgery, radiotherapy, and cytotoxic chemotherapy is recommended.237
OTHER MALIGNANT TUMORS OF THE CERVIX
Some other rare malignancies of Müllerian origin can develop in the uterine cervix. These include carcinosarcoma (malignant mixed Müllerian tumor) (Fig. 27), leiomyosarcoma (Fig. 28), adenosarcoma (Fig. 29), and endometrial stromal sarcoma (Fig. 30). All these tumors show histology similar to their uterine and adnexal counterparts. Overall, carcinosarcoma and leiomyosarcoma behave more aggressively than adenosarcoma and stromal sarcoma. Recognition of these rare entities is valuable for early detection and appropriate clinical management.
Carcinosarcomas (malignant Müllerian mixed tumors, MMMT) of the uterine cervix are rare neoplasms. It seems that the epithelial differentiation of carcinosarcoma in the cervix is different from its counterpart in the uterus and ovaries. Grayson and colleagues238 reported eight cases of carcinosarcoma in the cervix. Patients' ages ranged from 32 to 93 years. Seven cases showed in situ squamous cell carcinoma. The invasive epithelial component was composed of combined adenoid basal carcinoma, basaloid SCC, and adenoid cystic carcinoma in two cases. Keratinizing SCC, large cell nonkeratinizing SCC, undifferentiated carcinoma, and basaloid SCC predominated in the remaining tumors, one of which had admixed adenoid cystic carcinoma. The sarcomatous component was homologous and spindled with admixed myxoid areas in three lesions. Polymerase chain reaction detected HPV DNA in all eight cases. In situ hybridization probes to HPV types 6, 11, 16, 18, 31, and 33 demonstrated integrated HPV 16 in three cases.238
Embryonal rhabdomyosarcoma, in particular the botryoid type (sarcoma botryoides), is an aggressive mesenchymal neoplasm which can occur in the gynecologic tract, most commonly in children. While the vagina is a more common site of occurrence for this tumor, cervical sarcoma botryoides has also been described, most often occurring in an older subset of patients (second decade) and also with a better prognosis than its vaginal counterpart.239, 240 Cervical rhabdomyosarcomas have also been reported in adult patients.241, 242 Sarcoma botryoides grossly presents as a “grape-like” mass (giving rise to the “botryoid” nomenclature), and histologically consists of a hypocellular and myxoid spindle cell neoplasm with scattered hypercellular tumor cell aggregates as well as a characteristic concentration of tumor cells just beneath the benign overlying epithelium (cambium layer) (Fig. 34). Rhabdomyosarcomas demonstrate skeletal muscle differentiation, and the presence of rhabdomyoblasts, present either as round cells with eosinophils globules and an eccentric nucleus, or as elongated cells with eosinophilic cytoplasm and sometimes characteristic cross-striations (“strap cells”), is a helpful diagnostic feature. Islands of benign cartilage may also been seen 40–45% of cases.240, 241 The tumors cells demonstrate immunohistochemical positivity for desmin and myogenin. In recent years, cervical sarcoma botryoides has been identified as one of the neoplasms associated with the DICER1-related inherited cancer syndrome.243
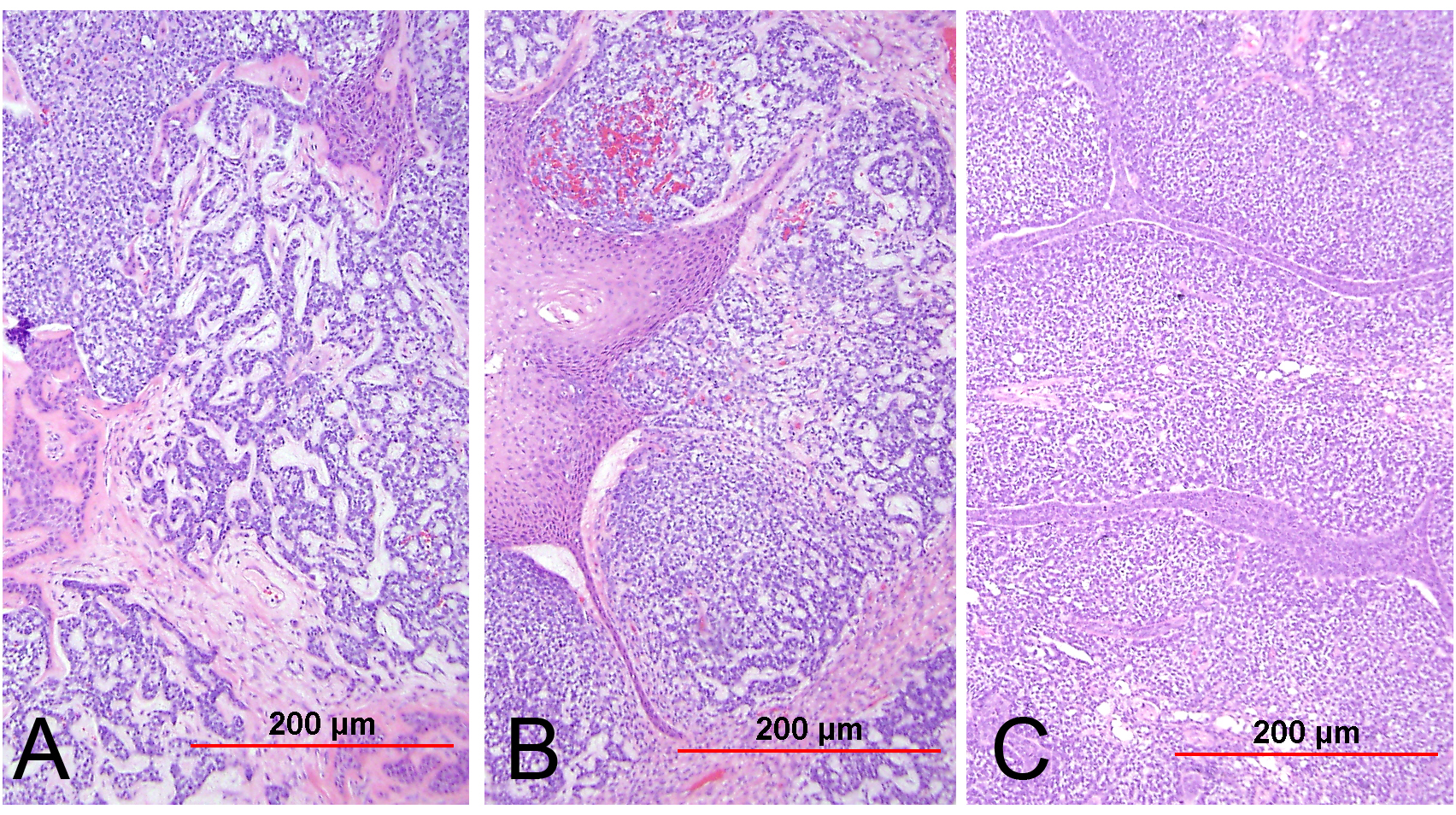 Fig. 27. Malignant mixed Müllerian tumor (carcinosarcoma) of the cervix. The tumor is biphasic. The epithelial component consists of squamous cell carcinoma, adenoid basal carcinoma, and adenoid cystic carcinoma (A–C). The stromal component is mainly undifferentiated sarcoma (C). (Hematoxylin-eosin stain, red bars: original magnification.)
Fig. 27. Malignant mixed Müllerian tumor (carcinosarcoma) of the cervix. The tumor is biphasic. The epithelial component consists of squamous cell carcinoma, adenoid basal carcinoma, and adenoid cystic carcinoma (A–C). The stromal component is mainly undifferentiated sarcoma (C). (Hematoxylin-eosin stain, red bars: original magnification.)
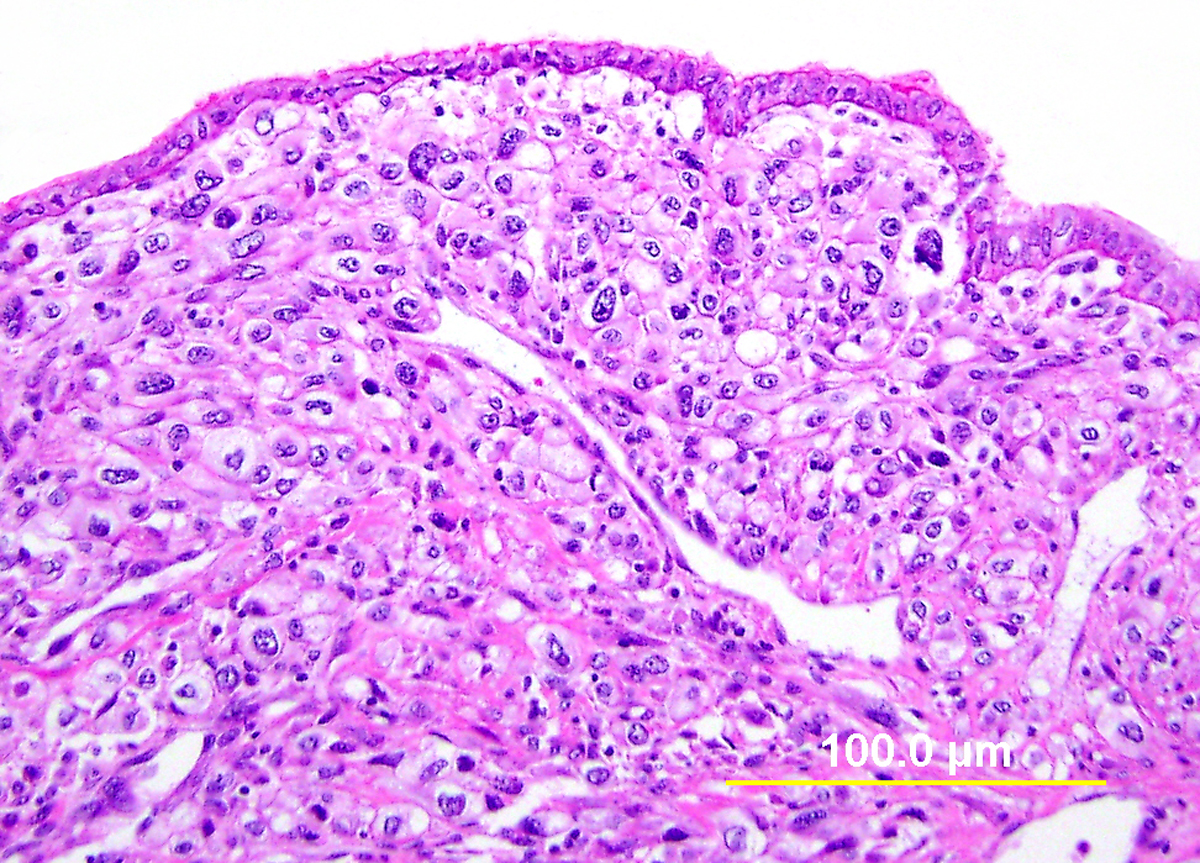 Fig. 28. Leiomyosarcoma of the cervix. This leiomyosarcoma from the cervix is composed of epithelioid tumor cells with atypical and pleomorphic nuclei. The overlying epithelium consists of normal endocervical cells. (Hematoxylin-eosin stain, yellow bar: original magnification.)
Fig. 28. Leiomyosarcoma of the cervix. This leiomyosarcoma from the cervix is composed of epithelioid tumor cells with atypical and pleomorphic nuclei. The overlying epithelium consists of normal endocervical cells. (Hematoxylin-eosin stain, yellow bar: original magnification.)
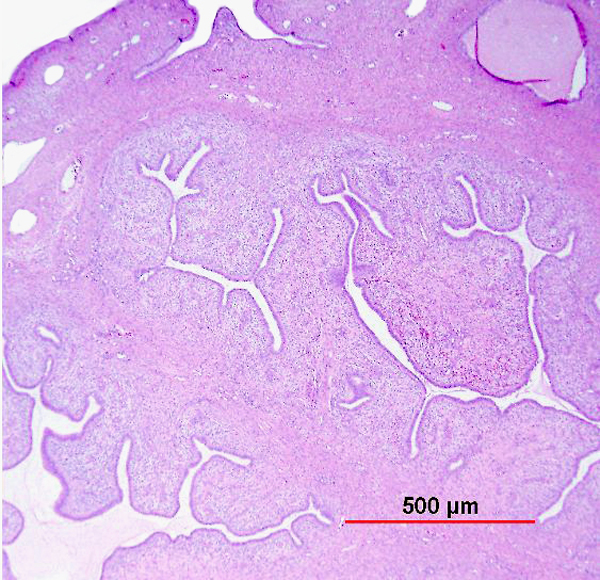 Fig. 29. Müllerian adenosarcoma of the cervix. The tumor consists of large and branching benign glandular structures surrounded by cellular atypical stromal cells (stromal cuffing). A focus of overlying normal endocervical epithelium can be seen in the upper left corner. (Hematoxylin-eosin stain, red bar: original magnification.)
Fig. 29. Müllerian adenosarcoma of the cervix. The tumor consists of large and branching benign glandular structures surrounded by cellular atypical stromal cells (stromal cuffing). A focus of overlying normal endocervical epithelium can be seen in the upper left corner. (Hematoxylin-eosin stain, red bar: original magnification.)
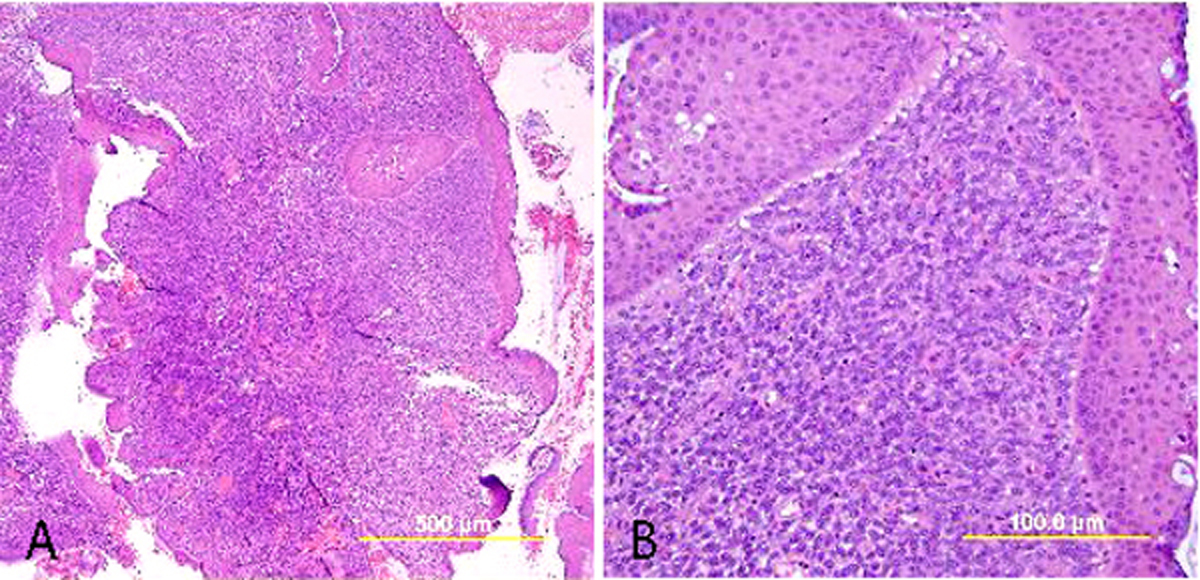 Fig. 30. Low-grade endometrial stromal sarcoma of the cervix. The tumor consists of small and uniform endometrial stromal cells infiltrating into endocervix (A) and ectocervix (B). (Hematoxylin-eosin stain, yellow bars: original magnification.)
Fig. 30. Low-grade endometrial stromal sarcoma of the cervix. The tumor consists of small and uniform endometrial stromal cells infiltrating into endocervix (A) and ectocervix (B). (Hematoxylin-eosin stain, yellow bars: original magnification.)
Other less common malignant neoplasms include aggressive angiomyxoma (Fig. 31), lymphoma (Fig. 32), malignant melanoma, choriocarcinoma (Fig. 33), yolk sac tumor, Wilms' tumor, and others. These are detailed elsewhere.5, 244
 Fig. 31. Aggressive angiomyxoma involving the cervix. The tumor is composed of mainly bland spindle and oval cells within a myxoid matrix. Thick and thin-walled vessels with hyaline changes are present. The tumor has an infiltrating growth pattern. (Hematoxylin-eosin stain, yellow bar: original magnification.)
Fig. 31. Aggressive angiomyxoma involving the cervix. The tumor is composed of mainly bland spindle and oval cells within a myxoid matrix. Thick and thin-walled vessels with hyaline changes are present. The tumor has an infiltrating growth pattern. (Hematoxylin-eosin stain, yellow bar: original magnification.)
 Fig. 32. Diffuse large B-cell lymphoma of the cervix. This cervical biopsy is completely replaced by malignant lymphocytes with extensive tumor necrosis. Diagnosis was confirmed by immunostains (not shown). (Hematoxylin-eosin stain, yellow bar: original magnification.)
Fig. 32. Diffuse large B-cell lymphoma of the cervix. This cervical biopsy is completely replaced by malignant lymphocytes with extensive tumor necrosis. Diagnosis was confirmed by immunostains (not shown). (Hematoxylin-eosin stain, yellow bar: original magnification.)
 Fig. 33. Choriocarcinoma involving the cervix. The tumor consists of a sheet of cytotrophoblastic cells with areas of hemorrhage and scattered syncytiotrophoblastic cells. A normal endocervical gland is seen on the left side. (Hematoxylin-eosin stain, yellow bar: original magnification.)
Fig. 33. Choriocarcinoma involving the cervix. The tumor consists of a sheet of cytotrophoblastic cells with areas of hemorrhage and scattered syncytiotrophoblastic cells. A normal endocervical gland is seen on the left side. (Hematoxylin-eosin stain, yellow bar: original magnification.)
 Fig. 34. Botryoid embryonal rhabdomyosarcoma of the cervix. Low-power demonstrates a hypocellular neoplasm with a distinct cambium layer as well as hypercellular foci (A). Higher power of a hypercellular focus demonstrates spindled tumor cells with characteristic rhabdomyoblasts (arrows).
Fig. 34. Botryoid embryonal rhabdomyosarcoma of the cervix. Low-power demonstrates a hypocellular neoplasm with a distinct cambium layer as well as hypercellular foci (A). Higher power of a hypercellular focus demonstrates spindled tumor cells with characteristic rhabdomyoblasts (arrows).
Metastatic secondary tumors
Finally, secondary metastatic tumors to the uterine cervix occur most commonly from direct extension of an adjacent malignancy, such as those of the endometrium and vagina.5, 244 Less commonly, tumors of the breast (Fig. 35), ovary, colon, and stomach metastasize to the cervix by lymph-vascular spread.5, 244
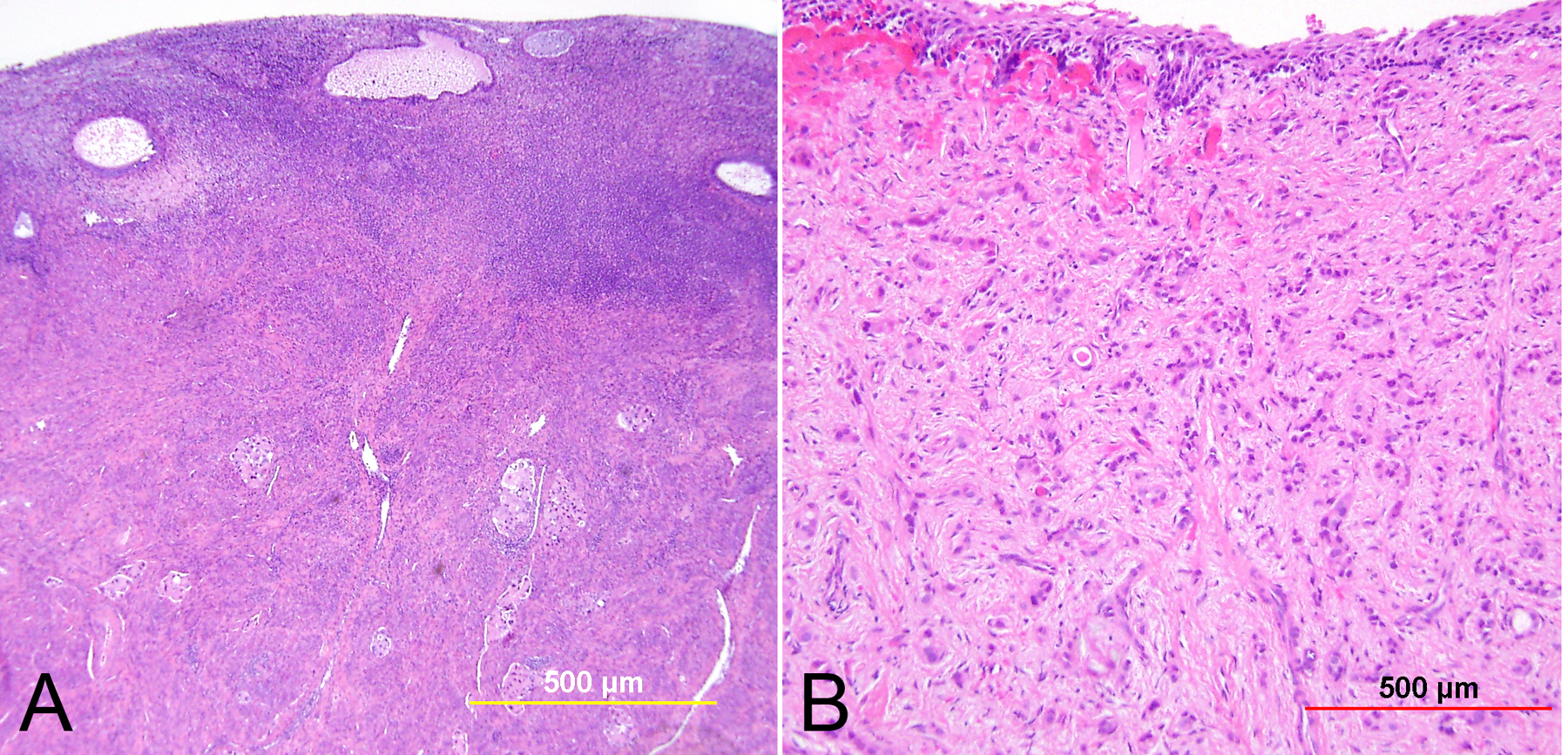 Fig. 35. Metastatic breast carcinoma in the cervix. (A) Metastatic ductal carcinoma in endocervix represented by multiple tumor nests in the stroma and lymph-vascular spaces. (B) Metastatic lobular carcinoma shows infiltrating cords and individual cells in the cervical stroma. (Hematoxylin-eosin stain, yellow and red bars: original magnification.)
Fig. 35. Metastatic breast carcinoma in the cervix. (A) Metastatic ductal carcinoma in endocervix represented by multiple tumor nests in the stroma and lymph-vascular spaces. (B) Metastatic lobular carcinoma shows infiltrating cords and individual cells in the cervical stroma. (Hematoxylin-eosin stain, yellow and red bars: original magnification.)
PROGNOSTIC FACTORS
Various pathologic features have been demonstrated to have prognostic significance in patients with cervical cancer. Some of these have been discussed earlier in this chapter. Selected pertinent literature is discussed below.
Several large series have applied statistical models to identify important prognostic parameters of cervical carcinoma among women treated by radical surgery and radiation therapy (Table 4). Sevin and associates245 evaluated women with stage I or II carcinoma who were treated by radical hysterectomy and pelvic lymphadenectomy. By univariate analysis, the disease free survival rates were closely related to the depth of stromal invasion, tumor size, presence or absence of lymph-vascular space invasion, pelvic lymph node status, tumor volume, and clinical stage.245 Kamura and colleagues246 examined a similar patient population and found that by univariate analysis, 5-year survival rates were correlated to the lymph node status, cell type (squamous cell carcinoma vs. adenocarcinoma), tumor dimension, depth of cervical wall invasion, lymph-vascular space invasion, and parametrial invasion.246 Fuller and colleagues247 studied 431 women with stage IB or IIA carcinoma treated by radical hysterectomy, including 85% squamous carcinomas, 9% adenocarcinomas, and 3% adenosquamous carcinomas. A decreased survival was strongly related to the presence of pelvic lymph node metastasis.247 Among node-negative cases, adenocarcinoma cell type, increasing tumor size, deeper stromal invasion, and high histologic grade were associated with decreased survival.247 Pelvic lymph node metastasis was found in none of 62 tumors involving the inner one third of the cervical wall, 12% of tumors invading up to the middle third, and 24% of tumors extending into the outer third.247
Table 4. 5-year survival rates in surgically treated cervical carcinomas
| Parameter | Kamura et al.246 | Sevin et al.245 | ||
| No. of cases | 211 (IB and II) | 370 (I and II) | ||
| Cell type | Squamous | 91.1% | Not significant |
|
|
| Adenocarcinoma | 70.1% | ||
| Tumor size | <2 cm | 97.6% | <1 cm | 93% |
|
| 2–4 cm | 85.6% | 1.1–2.0 cm | 76% |
|
| >4 cm | 80.1% | 2.1–3.0 cm | 64% |
| Stromal invasion | <3 mm | 95.7% |
|
|
|
| 3–5 mm | 91.7% | ||
|
| 5–10 mm | 83% | ||
|
| >10 mm | 81.8% | ||
|
| <1/3 | 94.8% | ||
|
| 2/3 | 88.1% | ||
|
| >2/3 | 79.9% | ||
| Lymph-vascular space invasion | Absent | 91.5% | Absent | 85% |
|
| Present | 82.4% | Present | 62% |
| Parametrial disease | Absent | 90.3% |
|
|
|
| Present | 77.3% | ||
| Lymph node metastasis | Negative | 90.8% | Negative | 77% |
|
| 1+ | 96.7% | 1–2+ | 55% |
|
| 2+ | 44.3% | >2+ | 39% |
|
| 3+ | 36% |
|
|
| Histologic grade | Not evaluated |
| Not significant |
|
Examining the question of depth from a different perspective, Kishi and colleagues248 measured the thickness of uninvolved cervical stroma from the deepest tumor to the external cervical wall in 287 stage IB, IIA, and IIB cervical squamous cell carcinomas. The pelvic nodal metastatic and 5-year cancer death rates were 7% and 8%, respectively, when the uninvolved stroma measured greater than 3 mm. Corresponding figures were 37% and 26% when the uninvolved stroma measured less than 3 mm. The authors felt that the uninvolved stroma acted as a barrier to cancer spread, and its width was therefore a more important measurement than the depth of tumor invasion.248
Parametrial spread carries important prognostic information. Its presence or absence should be noted in all pathology reports. Tumor extension to this highly vascular site occurs by contiguous spread, and less often from lymphatic invasion (Fig. 36). When present, parametrial invasion is associated with a higher incidence of vascular invasion, positive lymph nodes, recurrence, and death.111, 249
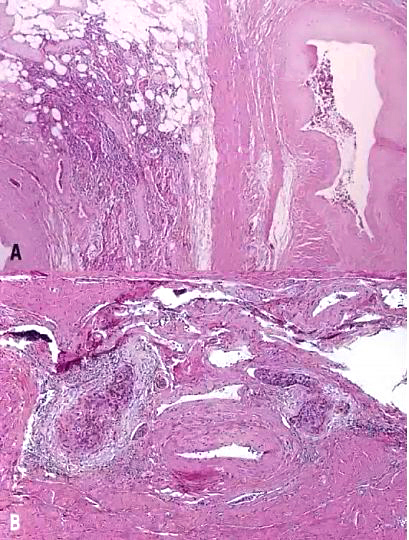 Fig. 36. (A) Tumor extension into the parametrial fibroadipose tissue (left), with adjacent deep cervical stroma containing smooth muscle and blood vessels (right). (B) Venous invasion in the parametrial tissue. The artery in the center of the field is not invaded by tumor. (Hematoxylin-eosin stain, original magnification A: 40X, B: 100X.)
Fig. 36. (A) Tumor extension into the parametrial fibroadipose tissue (left), with adjacent deep cervical stroma containing smooth muscle and blood vessels (right). (B) Venous invasion in the parametrial tissue. The artery in the center of the field is not invaded by tumor. (Hematoxylin-eosin stain, original magnification A: 40X, B: 100X.)
Assessing for the prognostically relevant factor of lymph-vascular space invasion can be challenging. Shrinkage artifact after formalin fixation often results in clear empty spaces at the periphery of tumor nests, simulating a vascular space. These artifactual spaces do not have well-defined endothelial cells (Fig. 37). In true vascular invasion, the tumor cells are partially adherent to the endothelial cells, which should be clearly identifiable. In addition, blood and sometimes fibrin thrombi are present in the vascular lumen. The frequency of lymph-vascular space invasion is closely related to the depth of stromal invasion.250 Vascular invasion is associated with higher rates of pelvic nodal metastasis and tumor recurrence, and with decreased survival in stage I and II squamous cell carcinoma and adenocarcinoma.111, 246, 247, 250, 251 This association between vascular invasion and disease recurrence has not been observed in later stages, however. In fact, several studies of stage II and III cervical squamous carcinomas have found that vascular invasion had no bearing on long-term survival.252
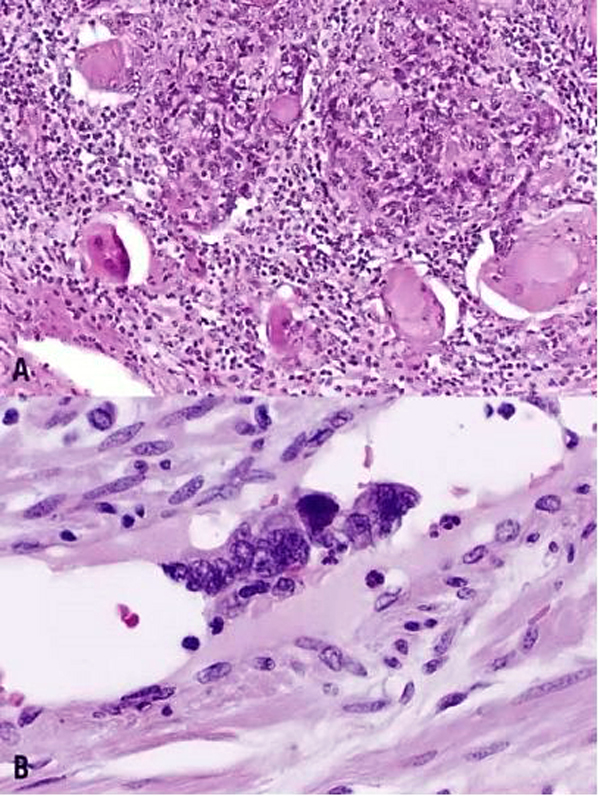 Fig. 37. Lymph-vascular space invasion. (A) Shrinkage artifact around the tumor nests simulates lymph-vascular space invasion. (B) True lymph-vascular spaces should demonstrate clearly identifiable endothelial cells. The malignant squamous cells are partially adherent to the vessel wall. (Hematoxylin-eosin stain, original magnification A: 200X, B: 400X.)
Fig. 37. Lymph-vascular space invasion. (A) Shrinkage artifact around the tumor nests simulates lymph-vascular space invasion. (B) True lymph-vascular spaces should demonstrate clearly identifiable endothelial cells. The malignant squamous cells are partially adherent to the vessel wall. (Hematoxylin-eosin stain, original magnification A: 200X, B: 400X.)
The frequency of pelvic lymph node metastasis is influenced by such parameters as FIGO stage, tumor size, depth of invasion, lymph-vascular space invasion, and histologic grade (Table 5).111, 247, 253, 254, 255 In some series, 15% of clinical stage I and 26–35% of stage II cervical carcinomas had positive lymph nodes at the time of diagnosis.247, 256, 257 The nodal groups most frequently involved are the paracervical, obturator, and external iliac lymph node chains (Fig. 38).258 The prognostic significance of lymph node involvement is dramatic. In one study of women with stage I or II cervical adenocarcinoma treated surgically, lymph node metastases in the pelvic or paraaortic region occurred in 15% of stage I and 40% of stage II tumors. In the presence of pelvic lymph node metastasis, the 5-year survival rate decreased from 92% to 10%.160 The likelihood of recurrence and death is increased in the presence of pelvic lymph node metastasis and is related to the number of positive nodes.254, 255 In a series of 97 patients with stage IB–IIB carcinoma, one third of patients with one positive lymph node and two thirds with three or more positive nodes had recurrence of tumor within 5 years.259 In another study of stage I and II carcinomas, the rate of survival was 59% with unilateral and 20% with bilateral lymph node metastases.255 From the iliac lymph nodes, tumor may spread to involve paraaortic nodes, and, from there, the scalene nodes. Tumor involvement of these sites is associated with disseminated disease in more than 75% of patients.260 Paraaortic lymph node involvement is present in 6% of stage IB carcinoma and 30% of stage II and III disease.261, 262 When all series are combined, the overall incidence of scalene lymph node metastasis from cervical carcinoma is 15%.263
Table 5. Frequency of lymph node metastasis in surgically treated cervical carcinomas
| Delgado et al.111 (SCC >3 mm in depth) | Fuller et al.247 (SCC, adenocarcinoma, & others) | |||
| No. of cases | 745 |
| 431 |
|
| FIGO stage | I | 15.5% | IB | 15% |
|
|
|
| IIA | 22% |
| Stromal invasion | 3–5 mm | 3.4% | <1/3 | 0% |
|
| 6–10 mm | 15.1% | 2/3 | 12% |
|
| 11–15 mm | 22.2% | >2/3 | 24% |
|
| 16–20 mm | 38.8% |
|
|
|
| 21 mm | 22.6% |
|
|
| Lymph-vascular space invasion | Absent | 8.2% | Absent | 14% |
|
| Present | 25.4% | Present (lymphatic) | 36% |
| Parametrial disease | Absent | 13.5% | Not evaluated |
|
|
| Present | 25% |
|
|
| Tumor size | Occult | 8.9% |
|
|
|
| Gross | 20.9% |
|
|
| Histologic grade | Grade 1 | 9.7% | Grade 1 | 9% |
|
| Grade 2 | 13.9% | Grade 2 | 16% |
|
| Grade 3 | 21.8% | Grade 3 | 25% |
| Cell type | LCK | 17.2% | Squamous carcinoma | 18% |
|
| LCNK | 17.2% | Adenocarcinoma | 18% |
|
| Small cell and others | 17.6% | Adenosquamous carcinoma | 17% |
LCK, large cell keratinizing; LCNK, large cell nonkeratinizing
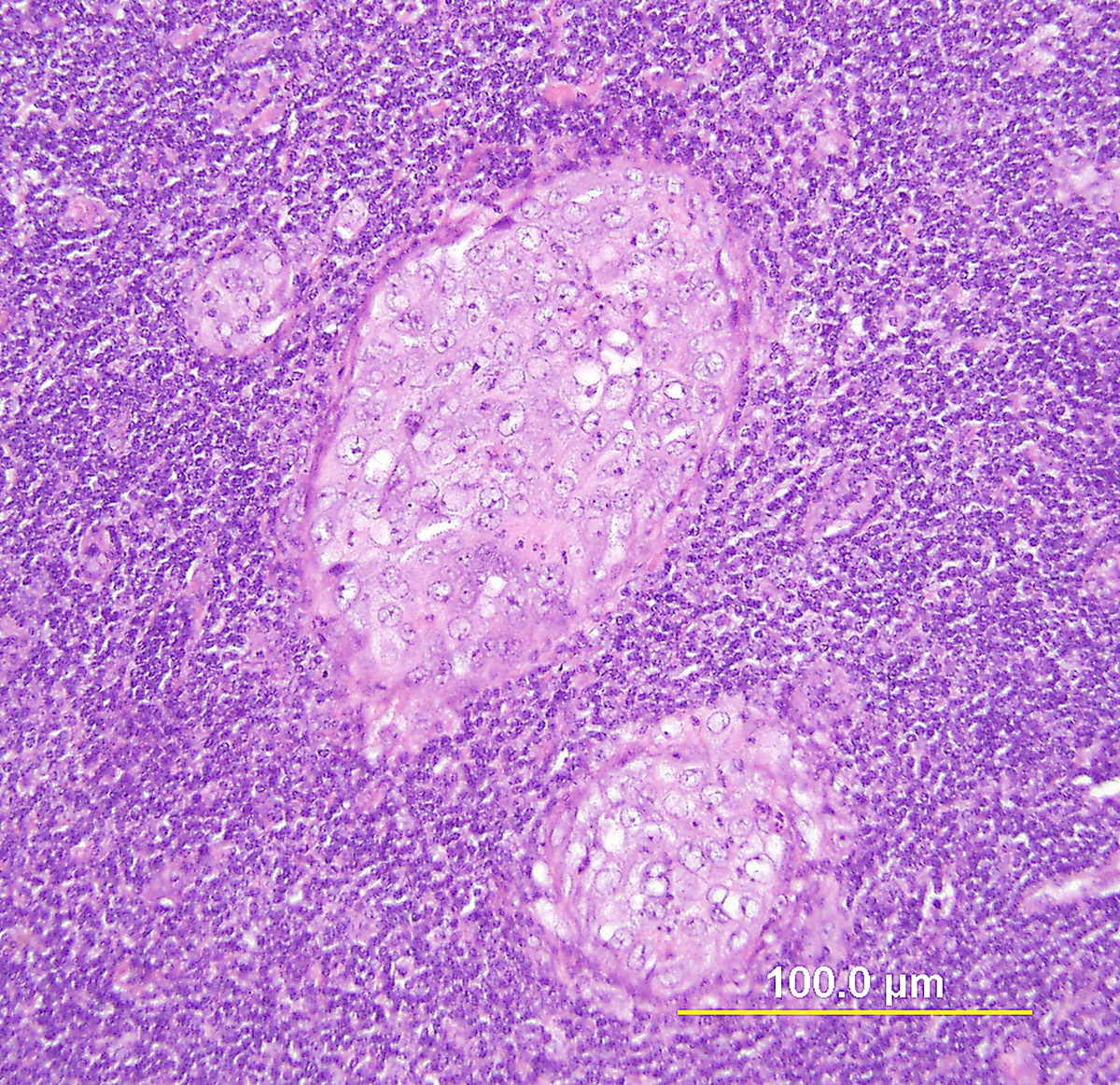 Fig. 38. Metastatic squamous cell carcinoma in an iliac lymph node. The image shows a high-power view of clusters of malignant squamous cells within an iliac lymph node. (Hematoxylin-eosin stain, yellow bar: original magnification.)
Fig. 38. Metastatic squamous cell carcinoma in an iliac lymph node. The image shows a high-power view of clusters of malignant squamous cells within an iliac lymph node. (Hematoxylin-eosin stain, yellow bar: original magnification.)
Similar prognostic findings have been found in studies of patients who underwent radiation therapy. Barillot and colleagues264 reviewed 1875 women with cervical carcinoma treated by radiation therapy alone. By univariate analysis of stage I/II cases, FIGO stage, tumor diameter, and lymph node status were significant prognostic parameters. The 5-year survival rates were 83.5% for stage IB, 81% for stage IIA, 71% for stage IIB, 65% for stage IIIA, and 59% for stage IIIB. For tumors smaller than 3 cm, the 5-year survival rate was 86%, vs. 76% for tumors measuring 3–5 cm, and 61.5% for tumors >5 cm. By multivariate analysis, FIGO stage and nodal involvement remained significant for all stages. For stage I/II tumors, tumors larger than 5 cm and adenocarcinoma were poor prognosticators.264
Overall, most authors have agreed that the depth of stromal invasion, tumor dimension, the presence or absence of lymph-vascular space invasion, pelvic lymph node status, and parametrial involvement are important prognosticators. The value of classification by cell type and histologic grade continues to be controversial. While some authors have noted worse survival for women with adenocarcinoma and adenosquamous carcinoma compared with squamous cell carcinoma,246, 265 others have found no difference in survival for these three tumor types when compared stage by stage.264, 266 However, some specific tumor types have been more consistently associated with a more aggressive course. For example, studies on glassy cell carcinoma have shown a high frequency of pelvic and extrapelvic spread, poor response to surgery and/or radiotherapy, and 5-year survival rates in the range of 31–33%.267 This poor outcome is thought in part to be related to radioresistance and understaging of the tumor.267
A variety of growth patterns and stromal reactions have been described in cervical squamous cell carcinoma, but none are prognostically useful. Similarly, the results of vascular density counts are conflicting in relation to radiosensitivity of the tumor and prognosis. However, the newly proposed pattern-based classification system for cervical adenocarcinomas (discussed earlier in this chapter) appears to have some promise in predicting tumor behavior and prognosis.164
Contradictory results have been reported for the prognostic significance of tumor grade. In surgically treated patients with stage I and II SCC, some studies have found histologic grade to influence prognosis and pelvic nodal metastasis.111 In a study of stage I SCC from the GOG, the histologic grade was correlated with the frequency of pelvic nodal metastasis, which occurred in 9.7%, 13.9%, and 21.8% of grade 1, grade 2, and grade 3 tumors, respectively.111 Comparable results were reported by Fuller and colleagues,247 who found that stage IB and IIA grade 3 tumors were larger and had a higher incidence of lymph node metastasis than lower-grade lesions.247 In their study, 25% of grade 3 tumors had pelvic lymph node metastases, compared with 9% and 16% of grade 1 and 2 tumors, respectively. The degree of differentiation in adenocarcinoma has also been demonstrated by some groups to be related to prognosis and pelvic nodal metastasis, with one study finding that in stage I and II tumors, pelvic nodal metastases were found in 5% of grade I, 11% of grade II, and 50% of grade III tumors.160 Another study, however, showed that among 445 patients with stage IIB through IVA squamous cell carcinoma treated by radiation therapy following GOG protocols, the histologic grade had no impact on prognosis.112 Similar findings were reported by others, including Sevin and associates.245, 252, 268 Of note, the newly proposed pattern-based classification system for adenocarcinoma (discussed above) also incorporates tumor grade, as pattern A tumors are defined as being well or moderately differentiated.164
Tumor recurrence
Tumor recurrences are divided into three categories: central (involving vaginal cuff, bladder, or rectum), pelvic sidewall, and distant or extrapelvic. Tumor recurrence is reported to be central in 14–28%, sidewall or sidewall and central in 37–59%, and distant in 35–42% of cases.269, 270, 271 In a large study, 40 of 303 (13%) women with stage IB/IIB cervical cancers developed recurrence following radical hysterectomy and pelvic lymphadenectomy.269 In a literature review, tumor recurrence occurred within 12 months of initial treatment in 45–58% of cases, and within 3 years in 70–90% of cases.269 Pelvic recurrence may be asymptomatic and detected only on physical examination, or may be suspected by nonspecific symptoms such as vaginal bleeding or discharge. Pelvic sidewall recurrence may produce pain in the lower abdomen, back, hip, or leg. Distant metastases may present with pain or a mass lesion.
Usual sites of metastasis in cervical carcinoma include periureteral, abdominal, hepatic, and paraaortic regions. Spread to scalene node occurs via the thoracic duct. Unusual sites of recurrence, such as cutaneous lymphatic dissemination, are occasionally reported.272
Treatment with cisplatin-based combination chemotherapy and more recently with angiogenesis inhibitors (in particular bevacizumab) has been found to increase overall and progression-free survival and may be useful in recurrent or advanced stage cervical carcinoma.273
BIOMARKERS IN CERVICAL CANCER PROGNOSIS
A variety of tumor antigens have been identified in cervical carcinoma using immunohistochemical techniques on tissue sections. Selected antigens with possible clinical utility are discussed here.
CEA is found in both squamous cell carcinoma and adenocarcinoma, as well as in normal cervical epithelium.274 In a series of 241 patients, 63% of large cell nonkeratinizing squamous carcinomas, 78% of keratinizing squamous carcinomas, 67% of small cell carcinomas, and 36% of adenocarcinomas were positive for CEA.275 The diagnostic utility of this antigen is limited, however, as it is found in benign, dysplastic, and malignant conditions. In addition, the presence of CEA in malignant tissue sections by itself has no prognostic significance.275, 276
In the study of Borras and associates,277 serum tumor antigens were determined by immunoradiometric assay in 96 women with invasive carcinoma. Elevated levels of serum CEA, CA 19-9, and CA125 were found in 33%, 32%, and 21.5% of cases, respectively. Increased CEA and CA 19-9 were related to clinical stage. Serum CA125 and CA 19.9 were higher in patients with adenocarcinoma than squamous cell carcinoma. When disease free, these antigens tended to decrease. All progressive cases had an elevated level of one of these antigens.277 Thus, as serum markers, these antigens are useful for monitoring tumor recurrence, especially for adenocarcinoma. Preoperative levels of CA125 have also been found to correlate with tumor size, depth, lymph-vascular space invasion, and lymph node metastasis.278
Squamous cell carcinoma antigen is one of 14 subfractions of the TA-4 tumor antigen, isolated in 1977 from cervical squamous cell carcinoma by Kato and Torigoe.279 Like CEA, SCC antigen can be demonstrated in both benign and malignant cervical epithelium, and increased serum levels occur almost exclusively in cancer patients.280 In a study of 96 cervical carcinoma patients who had increased serum levels of SCC antigen at the time of diagnosis, persistently high levels following completion of radiotherapy were significantly associated with biopsy-proven residual disease. In 60% of patients with persistently high SCC antigen levels, residual tumor was found on cervical biopsy.281 SCC antigen levels have also been found to be predictive of recurrence, with one study finding that 81% of patients with recurrent cervical squamous cell carcinoma had elevated SCC antigen levels.282 Additionally, preoperative serum SCC antigen levels have been found to be predictive of tumor size, depth, lymph-vascular space invasion, and lymph node metastasis.278
Cytokeratins may also be useful serum biomarkers in cervical cancer. Elevated pretreatment levels of serum fragments of cytokeratin 19 (CYFRA) have been correlated with higher stage, tumor size, lymph node metastasis, and depth of invasion.283, 284 CYFRA levels may also be a useful measure of treatment response to radiation.285One recent study reported CYFRA 21-1 levels to be an independent predictor of overall survival in squamous cell carcinomas and non-squamous cell carcinomas of the cervix.283
Mandai and associates286 used immunohistochemical stains to examine the expression of nm23-H1 and c-erb-2 proteins. The nm23-H1 gene was originally cloned from murine melanoma cell lines with low and high metastatic potential. It was identified later in humans. nm23-H1 expression is associated with a better prognosis and lower rates of lymph node metastasis in breast ductal carcinoma, hepatocellular carcinoma, and gastric carcinoma patients.286 c-erbB-2 (HER2/neu), a member of the epidermal growth factor receptor family, when overexpressed, is associated with poor prognosis in breast carcinoma. In the study of Mandai and associates, nm23-H1 was expressed in 46% of cervical adenocarcinomas and 36% of squamous cell carcinomas, and c-erb-2 was overexpressed in 49% of cervical adenocarcinomas and 38% of squamous cell carcinomas. A negative nm23-HI and an overexpressed c-erb-2 were associated with increased lymph node metastasis and poor prognosis in adenocarcinoma. These findings were not applicable to squamous cell carcinoma.286 Other groups have found that the rate of c-erbB-2 amplification and overexpression in cervical carcinomas is extremely low, arguing against its potential utility.287
A number of hypoxia and angiogenesis-related factors have also been studied as potential biomarkers in cervical cancer. These include HIF-1ɑ, VEGF, and CA9. One study of 67 patients who had received radiation therapy for cervical cancer found HIF-1ɑ immunohistochemical expression to be a predictor of only partial response to therapy, shorter progression-free survival, and shorter disease-specific survival.288 Another study also found HIF-1ɑ to be an independent predictor of overall survival.289 Immunohistochemical expression of VEGF is also reported to be an independent predictor of survival,290 and immunohistochemical expression of CA9 is associated with lymph node metastasis and a worse disease-free survival,291 as well as worse overall survival following radiation therapy.292
The apoptotic marker COX-2 has also been studied, immunohistochemical expression of which has been associated with lymph-vascular space invasion, poor treatment response, and decreased overall and progression-free survival.293, 294, 295 A greater association with adenocarcinoma vs. squamous cell carcinoma is also reported.293, 295
The role of oncogenes in cervical carcinoma has also been investigated. Mutations and overexpression of both the c-myc and ras oncogenes have been demonstrated in cervical carcinoma.296, 297, 298, 299 Some studies have correlated the degree of abnormal expression with prognosis. In one study, expression of the c-myc oncogene was increased in 25 of 72 stage I and II cervical carcinomas, and was associated with an eightfold or higher increase in the rate of early relapse in these patients.299
Estrogen and progesterone receptors have been detected in both benign and malignant cervical epithelia.300, 301 While the techniques used to detect these receptors vary among studies, most investigators have found no prognostic significance of the level of estrogen or progesterone receptors in cervical squamous cell carcinoma. In a study of 70 cases, Hunter and colleagues300 found no significant correlation between steroid receptors and a variety of parameters, including stage, menopausal status, cell type, histologic grade, or survival.
Flow cytometric analysis of cervical squamous cell carcinoma has been carried out by numerous investigators, and the results concerning the prognostic significance of DNA ploidy and S-phase rates in cervical carcinoma have been conflicting. Aneuploidy has been found in 28–44% of cervical carcinomas,302 with some authors finding this factor to be predictive of a worse overall survival303and correlated with a higher stage.304 Similarly, some groups have found high S-phase rates to be associated with early recurrence,305 and aggressive histopathologic features (infiltrative growth pattern, vascular invasion, sparse lymphoplasmacytic infiltrate).304 Others, however, have found that DNA ploidy and S-phase rates do not correlate with recurrence or survival.306, 307, 308
This review of biomarkers is far from comprehensive, and the reader is referred to several excellent recent reviews on the subject for further information.302, 309, 310
UPDATES IN CERVICAL CANCER SCREENING AND PREVENTION
Updated screening guidelines
The advent of screening programs for cervical cancer using cervical cytology (Papanicolaou smear) in the mid-1900s has significantly reduced the mortality of cervical cancer worldwide, particularly in developed countries.311 HPV testing has greater sensitivity than does cytology for the diagnosis of cervical intraepithelial lesions,312 and its addition to screening programs allows for consideration of longer screening intervals, and also provides the advantage of detecting more glandular lesions. In 2012, the American Society for Colposcopy and Cervical Pathology (ASCCP), along with the American Cancer Society (ACS) and American Society for Clinical Pathology (ASCP), updated their guidelines for cervical cancer screening.313 The highlights of the current guidelines are summarized below.
Screening for cervical cancer is recommended to begin at 21 years of age. This does not represent a change in the previous guidelines, and is based on the rarity of cervical carcinoma in patients younger than 21 and the potential for overtreatment and harm if these young patients are screened.313
For women ages 21–29 years, screening is recommended with cytology alone every 3 years. HPV testing is not recommended for screening in these patients. The lengthening of the screening interval, which was originally only 1 year, is based on the very small increase in cancers prevented by shorter screening intervals compared with the harm of overtreating patients whose HPV-associated lesions may yet regress. The prevalence of HPV infection is high in this age group, and as most of these infections will regress, screening with HPV testing is not recommended.313
For women ages 30–65 years, screening with both cytology and HPV testing (“cotesting”) every 5 years is recommended, but screening with cytology alone every 3 years is also considered acceptable. The justification for the longer interval for cotesting is based on the increased sensitivity for CIN 3 detection afforded by the addition of HPV testing, resulting in a lower subsequent risk of cervical carcinoma following negative cotesting vs. negative cytology alone.313
The 2012 recommendations did not endorse primary screening via HPV testing alone, due to concerns regarding the lower specificity of HPV testing vs. cytology, the lack of data on cytology as a follow-up test, and the possibility of unrecognized specimen inadequacy as a cause for false negative HPV tests.313 However, in 2014, the United States Food and Drug Administration (FDA) approved the Cobas HPV test manufactured by Roche Molecular Systems for primary cervical cancer screening.314 It is expected that new screening guidelines addressing this will be forthcoming in the next few years.
Also of note, the 2012 recommendations do not advocate any change in screening practices based on the introduction of the HPV vaccine (discussed below), due to as yet limited data as well as limited vaccine uptake in the United States.313
Updated management guidelines were also put forth by the ASCCP in 2012, and the reader is referred to the relevant publications for further details of both screening and management.33, 313
The cervical cancer screening recommendations in the 2014 Guide to Preventive Services, put forth by the United States Preventive Services Task Force (USPSTF), are very similar to the current ASCCP guidelines, including the initiation of screening at age 21 years, the use of cytology for screening every 3 years in women age 21–65 years, and the acceptability of cotesting every 5 years for women age 30–65 years.315 The ASCCP screening guidelines have also been endorsed by the American College of Obstetricians and Gynecologists (ACOG).316
HPV vaccine
In 2006, the US FDA approved the first vaccine against HPV, Gardasil, for use in women ages 9–26 years as a three-injection protocol. The original Gardasil, manufactured by Merck, is a quadrivalent vaccine covering HPV types 6, 11, 16, and 18.317 In 2009, the bivalent vaccine Cervarix, manufactured by GlaxoSmithKline and covering HPV types 16 and 18, was also approved for use in women age 10–25 years.318 More recently, in December of 2014, the FDA approved the new 9-valent Gardasil, covering HPV types 16, 18, 31, 33, 45, 52, 58, 6, and 11, with the potential to prevent 90% of cervical, vulvar, vaginal, and anal cancers.319 Currently, HPV vaccination is recommended for females ages 9–26 years, with several countries (including the United States, Canada, Austria, and Australia) also recommending vaccination for males to protect against genital warts, prevent HPV infection in their sexual partners, and reduce the incidence of anal cancer in men who have sex with men (MSM).320
Vaccination against HPV has been demonstrated to have excellent efficacy. One large, randomized, placebo-controlled study from Australia involving 5455 women found an efficacy of 100% for the quadrivalent vaccine in the prevention of genital warts, vulvar/vaginal intraepithelial lesions or cancer, and cervical intraepithelial lesions or cancer associated with the four covered HPV types.321 In the same study, an intention-to-treat analysis found that the vaccine reduced the rate of any cervical lesions (including those related to non-covered HPV types) by 20%.321 Australia’s HPV vaccination program has been very successful, with an impressive decrease in the prevalence of the covered HPV types in the 4 years following initiation of their national vaccination program (6.7% post-vaccine vs. 28.7% pre-vaccine).322 Vaccine uptake in the United States has been much lower than in many other developed nations, with 57.3% of adolescent females (13–17 years) and 34.6% of adolescent males receiving at least one dose in 2013, and only 37.6% of adolescent females and 13.9% of adolescent males receiving three doses in 2013.323 Even so, the vaccine has proven effective in vaccinated individuals, with a significant decrease in the prevalence of HPV 16/18-related HSIL in women who received at least one dose (53.6% in 2008 vs. 28.4% in 2012).324
The protective effect of the vaccine against the covered HPV types has been demonstrated for at least 8 years after complete vaccination,320 and there is some cross-protection against non-covered HPV types.325, 326, 327, 328 It has been demonstrated that in women with HPV infection by 1 or more of the covered HPV types at the time of vaccination, the vaccine is still 100% effective at preventing lesions caused by the remaining covered HPV types.329 Additionally, the vaccine also provides protection against reinfection or reactivation by covered HPV types in women with evidence of past infection by one or more of these HPV types.330 Despite some controversy, particularly from a number of political groups in the United States, the safety of the vaccine has been demonstrated in numerous large studies.331, 332
Currently, therapeutic vaccination against HPV-associated lesions is under investigation. These vaccines are targeted against E6 and E7 viral peptides, with promising results from phase I and II testing, and phase III testing planned for 2016.333, 334, 335, 336, 337
CONCLUSION
Optimal management of cervical cancer patients requires accurate assessment of the quality and the quantity of the neoplasm. Proper handling of the surgical specimens allows for comprehensive reporting of prognostically important pathologic findings. The use of immunohistochemistry and other techniques further enhances the validity of diagnosis and prognosis.
REFERENCES
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29. |
|
Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, et al. Annual Report to the Nation on the Status of Cancer, 1975-2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. Journal of the National Cancer Institute. 2015;107(6). |
|
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. |
|
Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States--a 24-year population-based study. Gynecol Oncol. 2000;78(2):97-105. |
|
Fu YS, Reagan JW: Pathology of the Uterine Cervix, Vagina, and Vulva. Philadelphia, WB Saunders, 1989. 397 p. p. |
|
Schlosshauer PW, W C, Chanderdatt D, Antonio L. Monsel's Artifact in Gynecologic Biopsies: A Simple Remedy. Journal of Histotechnology. 2005;28(3):161-2. |
|
Huh WK, Sideri M, Stoler M, Zhang G, Feldman R, Behrens CM. Relevance of random biopsy at the transformation zone when colposcopy is negative. Obstet Gynecol. 2014;124(4):670-8. |
|
Montz FJ, Holschneider CH, Thompson LD. Large-loop excision of the transformation zone: effect on the pathologic interpretation of resection margins. Obstet Gynecol. 1993;81(6):976-82. |
|
Felix JC, Muderspach LI, Duggan BD, Roman LD. The significance of positive margins in loop electrosurgical cone biopsies. Obstet Gynecol. 1994;84(6):996-1000. |
|
Herfs M, Yamamoto Y, Laury A, Wang X, Nucci MR, McLaughlin-Drubin ME, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A. 2012;109(26):10516-21. |
|
Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518-27. |
|
Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2009;105(2):107-8. |
|
Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2009;105(2):103-4. |
|
FIGO Committee on Gynecologic Oncology. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2014;125(2):97-8. |
|
Davey DD, Neal MH, Wilbur DC, Colgan TJ, Styer PE, Mody DR. Bethesda 2001 implementation and reporting rates: 2003 practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2004;128(11):1224-9. |
|
Burd EM. Human papillomavirus and cervical cancer. Clinical microbiology reviews. 2003;16(1):1- |
|
Doorbar J. The papillomavirus life cycle. Journal of Clinical Virology. 2005;32, Supplement(0):7-15. |
|
Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, Luff RD, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012;16(3):205-42. |
|
McCluggage WG, Bharucha H, Caughley LM, Date A, Hamilton PW, Thornton CM, et al. Interobserver variation in the reporting of cervical colposcopic biopsy specimens: comparison of grading systems. J Clin Pathol. 1996;49(10):833-5. |
|
Genest DR, Stein L, Cibas E, Sheets E, Zitz JC, Crum CP. A binary (Bethesda) system for classifying cervical cancer precursors: criteria, reproducibility, and viral correlates. Hum pathol. 1993;24(7):730-6. |
|
McCluggage WG, Walsh MY, Thornton CM, Hamilton PW, Date A, Caughley LM, et al. Inter- and intra-observer variation in the histopathological reporting of cervical squamous intraepithelial lesions using a modified Bethesda grading system. British journal of obstetrics and gynaecology. 1998;105(2):206-10. |
|
Ismail SM, Colclough AB, Dinnen JS, Eakins D, Evans DM, Gradwell E, et al. Observer variation in histopathological diagnosis and grading of cervical intraepithelial neoplasia. BMJ. 1989;298(6675):707-10. |
|
Kalof AN, Evans MF, Simmons-Arnold L, Beatty BG, Cooper K. p16INK4A immunoexpression and HPV in situ hybridization signal patterns: potential markers of high-grade cervical intraepithelial neoplasia. Am J Surg Pathol. 2005;29(5):674-9. |
|
Pirog EC, Chen YT, Isacson C. MIB-1 immunostaining is a beneficial adjunct test for accurate diagnosis of vulvar condyloma acuminatum. The American Journal of Surgical Pathology. 2000;24(10):1393-9. |
|
Maniar KP, Ronnett BM, Vang R, Yemelyanova A. Coexisting high-grade vulvar intraepithelial neoplasia (VIN) and condyloma acuminatum: independent lesions due to different HPV types occurring in immunocompromised patients. Am J Surg Pathol. 2013;37(1):53-60. |
|
Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12(2):186-92. |
|
Galgano MT, Castle PE, Atkins KA, Brix WK, Nassau SR, Stoler MH. Using biomarkers as objective standards in the diagnosis of cervical biopsies. Am J Surg Pathol. 2010;34(8):1077-87. |
|
Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. International journal of cancer Journal international du cancer. 2001;92(2):276-84. |
|
Klaes R, Benner A, Friedrich T, Ridder R, Herrington S, Jenkins D, et al. p16INK4a immunohistochemistry improves interobserver agreement in the diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol. 2002;26(11):1389-99. |
|
Benevolo M, Mottolese M, Marandino F, Vocaturo G, Sindico R, Piperno G, et al. Immunohistochemical expression of p16(INK4a) is predictive of HR-HPV infection in cervical low-grade lesions. Mod Pathol. 2006;19(3):384-91. |
|
Wright Jr TC. Pathogenesis and Diagnosis of Preinvasive Lesions of the Lower Genital Tract. In: Barakat RR, Markman M, Randall M, editors. Principles and Practice of Gynecologic Oncology. 5th ed. Baltimore: Lippincott Williams & Wilkins; 2009. p. 524. |
|
Nasu I, Meurer W, Fu YS. Endocervical glandular atypia and adenocarcinoma: a correlation of cytology and histology. Int J Gynecol Pathol. 1993;12(3):208-18. |
|
Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1-S27. |
|
Li J, Poi MJ, Tsai MD. Regulatory mechanisms of tumor suppressor P16(INK4A) and their relevance to cancer. Biochemistry. 2011;50(25):5566-82. |
|
Wang SS, Trunk M, Schiffman M, Herrero R, Sherman ME, Burk RD, et al. Validation of p16INK4a as a marker of oncogenic human papillomavirus infection in cervical biopsies from a population-based cohort in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13(8):1355-60. |
|
Keating JT, Cviko A, Riethdorf S, Riethdorf L, Quade BJ, Sun D, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25(7):884-91. |
|
Benevolo M, Terrenato I, Mottolese M, Marandino F, Muti P, Carosi M, et al. Comparative evaluation of nm23 and p16 expression as biomarkers of high-risk human papillomavirus infection and cervical intraepithelial neoplasia 2(+) lesions of the uterine cervix. Histopathology. 2010;57(4):580-6. |
|
Agoff SN, Lin P, Morihara J, Mao C, Kiviat NB, Koutsky LA. p16(INK4a) expression correlates with degree of cervical neoplasia: a comparison with Ki-67 expression and detection of high-risk HPV types. Mod Pathol. 2003;16(7):665-73. |
|
Murphy N, Ring M, Heffron CC, King B, Killalea AG, Hughes C, et al. p16INK4A, CDC6, and MCM5: predictive biomarkers in cervical preinvasive neoplasia and cervical cancer. J Clin Pathol. 2005;58(5):525-34. |
|
Conesa-Zamora P, Domenech-Peris A, Orantes-Casado FJ, Ortiz-Reina S, Sahuquillo-Frias L, Acosta-Ortega J, et al. Effect of human papillomavirus on cell cycle-related proteins p16, Ki-67, Cyclin D1, p53, and ProEx C in precursor lesions of cervical carcinoma: a tissue microarray study. Am J Clin Pathol. 2009;132(3):378-90. |
|
Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Immunohistochemical overexpression of p16 protein associated with intact retinoblastoma protein expression in cervical cancer and cervical intraepithelial neoplasia. Pathology international. 1998;48(8):580-5. |
|
Sayed K, Korourian S, Ellison DA, Kozlowski K, Talley L, Horn HV, et al. Diagnosing cervical biopsies in adolescents: the use of p16 immunohistochemistry to improve reliability and reproducibility. J Low Genit Tract Dis. 2007;11(3):141-6. |
|
Horn LC, Reichert A, Oster A, Arndal SF, Trunk MJ, Ridder R, et al. Immunostaining for p16INK4a used as a conjunctive tool improves interobserver agreement of the histologic diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol. 2008;32(4):502-12. |
|
Bergeron C, Ordi J, Schmidt D, Trunk MJ, Keller T, Ridder R. Conjunctive p16INK4a testing significantly increases accuracy in diagnosing high-grade cervical intraepithelial neoplasia. Am J Clin Pathol. 2010;133(3):395-406. |
|
Dijkstra MG, Heideman DA, de Roy SC, Rozendaal L, Berkhof J, van Krimpen K, et al. p16(INK4a) immunostaining as an alternative to histology review for reliable grading of cervical intraepithelial lesions. J Clin Pathol. 2010;63(11):972-7. |
|
Ordi J, Garcia S, del Pino M, Landolfi S, Alonso I, Quinto L, et al. p16 INK4a immunostaining identifies occult CIN lesions in HPV-positive women. Int J Gynecol Pathol. 2009;28(1):90-7. |
|
Negri G, Vittadello F, Romano F, Kasal A, Rivasi F, Girlando S, et al. p16INK4a expression and progression risk of low-grade intraepithelial neoplasia of the cervix uteri. Virchows Arch. 2004;445(6):616-20. |
|
Ozaki S, Zen Y, Inoue M. Biomarker expression in cervical intraepithelial neoplasia: potential progression predictive factors for low-grade lesions. Hum pathol. 2011;42(7):1007-12. |
|
del Pino M, Garcia S, Fuste V, Alonso I, Fuste P, Torne A, et al. Value of p16(INK4a) as a marker of progression/regression in cervical intraepithelial neoplasia grade 1. Am J Obstet Gynecol. 2009;201(5):488 e1-7. |
|
Hariri J, Oster A. The negative predictive value of p16INK4a to assess the outcome of cervical intraepithelial neoplasia 1 in the uterine cervix. Int J Gynecol Pathol. 2007;26(3):223-8. |
|
Liao GD, Sellors JW, Sun HK, Zhang X, Bao YP, Jeronimo J, et al. p16 immunohistochemical staining and predictive value for progression of cervical intraepithelial neoplasia grade 1: A prospective study in China. International journal of cancer Journal international du cancer. 2013. |
|
Cortecchia S, Galanti G, Sgadari C, Costa S, De Lillo M, Caprara L, et al. Follow-up study of patients with cervical intraepithelial neoplasia grade 1 overexpressing p16Ink4a. Int J Gynecol Cancer. 2013;23(9):1663-9. |
|
Genoves J, Alameda F, Mancebo G, Sole JM, Bellosillo B, Lloveras B, et al. Human papillomavirus detection and p16INK4a expression in cervical lesions: a comparative study. Hum pathol. 2014;45(4):826-33. |
|
Guedes AC, Brenna SM, Coelho SA, Martinez EZ, Syrjanen KJ, Zeferino LC. p16(INK4a) Expression does not predict the outcome of cervical intraepithelial neoplasia grade 2. Int J Gynecol Cancer. 2007;17(5):1099-103. |
|
Omori M, Hashi A, Nakazawa K, Yuminamochi T, Yamane T, Hirata S, et al. Estimation of prognoses for cervical intraepithelial neoplasia 2 by p16INK4a immunoexpression and high-risk HPV in situ hybridization signal types. Am J Clin Pathol. 2007;128(2):208-17. |
|
Nishio S, Fujii T, Nishio H, Kameyama K, Saito M, Iwata T, et al. p16(INK4a) immunohistochemistry is a promising biomarker to predict the outcome of low grade cervical intraepithelial neoplasia: comparison study with HPV genotyping. Journal of gynecologic oncology. 2013;24(3):215-21. |
|
Zhang G, Yang B, Abdul-Karim FW. p16 Immunohistochemistry is Useful in Confirming High-grade Squamous Intraepithelial Lesions (HSIL) in Women With Negative HPV Testing. Int J Gynecol Pathol. 2015;34(2):180-6. |
|
Maniar KP, Sanchez B, Paintal A, Gursel DB, Nayar R. Role of the Biomarker p16 in Downgrading -IN 2 Diagnoses and Predicting Higher-grade Lesions. Am J Surg Pathol. 2015;39(12):1708-18 |
|
al-Saleh W, Delvenne P, Greimers R, Fridman V, Doyen J, Boniver J. Assessment of Ki-67 antigen immunostaining in squamous intraepithelial lesions of the uterine cervix. Correlation with the histologic grade and human papillomavirus type. American Journal of Clinical Pathology. 1995;104(2):154-60. |
|
Media Release: FDA Advisory committee unanimously recommends Roche's HPV Test as primary screening tool for detection of women at high risk for cervical cancer. Basel, Switzerland: F. Hoffman-La Roche Ltd; 2014 [updated 3/13/2014; cited 2014 3/30/2014]; Available from: http://www.roche.com/media/media_releases/med-cor-2014-03-13.htm. |
|
Brief Summary of the Microbiology Devices Panel Meeting – March 12, 2014. Maryland: U.S. Food and Drug Administration; 2014 [cited 2014 3/30/2014]; Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/MicrobiologyDevicesPanel/UCM389192.pdf. |
|
Stoler MH, Wright TC, Jr., Sharma A, Apple R, Gutekunst K, Wright TL. High-risk human papillomavirus testing in women with ASC-US cytology: results from the ATHENA HPV study. Am J Clin Pathol. 2011;135(3):468-75. |
|
Cooper K, Herrington CS, Stickland JE, Evans MF, McGee JO. Episomal and integrated human papillomavirus in cervical neoplasia shown by non-isotopic in situ hybridisation. J Clin Pathol. 1991;44(12):990-6. |
|
Berumen J, Unger ER, Casas L, Figueroa P. Amplification of human papillomavirus types 16 and 18 in invasive cervical cancer. Hum pathol. 1995;26(6):676-81. |
|
Evans MF, Cooper K. Human papillomavirus integration: detection by in situ hybridization and potential clinical application. J Pathol. 2004;202(1):1-4. |
|
Guo M, Gong Y, Deavers M, Silva EG, Jan YJ, Cogdell DE, et al. Evaluation of a commercialized in situ hybridization assay for detecting human papillomavirus DNA in tissue specimens from patients with cervical intraepithelial neoplasia and cervical carcinoma. J Clin Microbiol. 2008;46(1):274-80. |
|
Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24(10):1295-305. |
|
Kong CS, Balzer BL, Troxell ML, Patterson BK, Longacre TA. p16INK4A immunohistochemistry is superior to HPV in situ hybridization for the detection of high-risk HPV in atypical squamous metaplasia. Am J Surg Pathol. 2007;31(1):33-43. |
|
Dabic MM, Hlupic L, Babic D, Jukic S, Seiwerth S. Comparison of polymerase chain reaction and catalyzed signal amplification in situ hybridization methods for human papillomavirus detection in paraffin-embedded cervical preneoplastic and neoplastic lesions. Archives of medical research. 2004;35(6):511-6. |
|
Badr RE, Walts AE, Chung F, Bose S. BD ProEx C: a sensitive and specific marker of HPV-associated squamous lesions of the cervix. Am J Surg Pathol. 2008;32(6):899-906. |
|
Conesa-Zamora P, Domenech-Peris A, Ortiz-Reina S, Orantes-Casado FJ, Acosta-Ortega J, Garcia-Solano J, et al. Immunohistochemical evaluation of ProEx C in human papillomavirus-induced lesions of the cervix. J Clin Pathol. 2009;62(2):159-62. |
|
Bala R, Pinsky BA, Beck AH, Kong CS, Welton ML, Longacre TA. p16 is superior to ProEx C in identifying high-grade squamous intraepithelial lesions (HSIL) of the anal canal. Am J Surg Pathol. 2013;37(5):659-68. |
|
Yemelyanova A, Gravitt PE, Ronnett BM, Rositch AF, Ogurtsova A, Seidman J, et al. Immunohistochemical detection of human papillomavirus capsid proteins L1 and L2 in squamous intraepithelial lesions: potential utility in diagnosis and management. Mod Pathol. 2013;26(2):268-74. |
|
Melsheimer P, Kaul S, Dobeck S, Bastert G. Immunocytochemical detection of HPV high-risk type L1 capsid proteins in LSIL and HSIL as compared with detection of HPV L1 DNA. Acta cytologica. 2003;47(2):124-8. |
|
Huang MZ, Li HB, Nie XM, Wu XY, Jiang XM. An analysis on the combination expression of HPV L1 capsid protein and p16INK4a in cervical lesions. Diagnostic cytopathology. 2010;38(8):573-8. |
|
Xiao W, Bian M, Ma L, Liu J, Chen Y, Yang B, et al. Immunochemical analysis of human papillomavirus L1 capsid protein in liquid-based cytology samples from cervical lesions. Acta cytologica. 2010;54(5):661-7. |
|
Yu L, Wang L, Zhong J, Chen S. Diagnostic value of p16INK4A, Ki-67, and human papillomavirus L1 capsid protein immunochemical staining on cell blocks from residual liquid-based gynecologic cytology specimens. Cancer cytopathology. 2010;118(1):47-55. |
|
Lin Z, Yemelyanova AV, Gambhira R, Jagu S, Meyers C, Kirnbauer R, et al. Expression pattern and subcellular localization of human papillomavirus minor capsid protein L2. Am J Pathol. 2009;174(1):136-43. |
|
Mestwerdt G. Die fruhdiagnose des Kollumkarzinoms. Zentralbl Gynàkol. 1847;69:98–202. |
|
Creasman WT, Parker RT. Microinvasive carcinoma of the cervix. Clinical obstetrics and gynecology. 1973;16(2):261-75. |
|
Creasman WT. New gynecologic cancer staging. Gynecol Oncol. 1995;58(2):157-8. |
|
Foushee JH, Greiss FC, Jr., Lock FR. Stage IA squamous cell carcinoma of the uterine cervix. Am J Obstet Gynecol. 1969;105(1):46-58. |
|
Andersen ES, Husth M, Joergensen A, Nielsen K. Laser conization for microinvasive carcinoma of the cervix. Short-term results. Int J Gynecol Cancer. 1993;3(3):183-5. |
|
Simon NL, Gore H, Shingleton HM, Soong SJ, Orr JW, Jr., Hatch KD. Study of superficially invasive carcinoma of the cervix. Obstet Gynecol. 1986;68(1):19-24. |
|
Raspagliesi F, Ditto A, Solima E, Quattrone P, Fontanelli R, Zanaboni F, et al. Microinvasive squamous cell cervical carcinoma. Critical reviews in oncology/hematology. 2003;48(3):251-61. |
|
Lee SW, Kim YM, Son WS, You HJ, Kim DY, Kim JH, et al. The efficacy of conservative management after conization in patients with stage IA1 microinvasive cervical carcinoma. Acta obstetricia et gynecologica Scandinavica. 2009;88(2):209-15. |
|
Creasman WT, Fetter BF, Clarke-Pearson DL, Kaufmann L, Parker RT. Management of stage IA carcinoma of the cervix. Am J Obstet Gynecol. 1985;153(2):164-72. |
|
Andersen ES, Nielsen K, Pedersen B. Combination laser conization as treatment of microinvasive carcinoma of the uterine cervix. Eur J Gynaecol Oncol. 1998;19(4):352-5. |
|
Orlandi C, Costa S, Terzano P, Martinelli GN, Comerci G, Guerra B, et al. Presurgical assessment and therapy of microinvasive carcinoma of the cervix. Gynecol Oncol. 1995;59(2):255-60. |
|
Roche WD, Norris HJ. Microinvasive carcinoma of the cervix. The significance of lymphatic invasion and confluent patterns of stromal growth. Cancer. 1975;36(1):180-6. |
|
Ostor AG. Studies on 200 cases of early squamous cell carcinoma of the cervix. Int J Gynecol Pathol. 1993;12(3):193-207. |
|
Ostor AG, Rome RM. Micro-invasive squamous cell carcinoma of the cervix: a clinico-pathologic study of 200 cases with long-term follow-up. Int J Gynecol Cancer. 1994;4(4):257-64. |
|
Seski JC, Abell MR, Morley GW. Microinvasive squamous carcinoma of the cervix: definition, histologic analysis, late results of treatment. Obstet Gynecol. 1977;50(4):410-4. |
|
Benedet JL, Anderson GH. Stage IA carcinoma of the cervix revisited. Obstet Gynecol. 1996;87(6):1052-9. |
|
Buckley SL, Tritz DM, Van Le L, Higgins R, Sevin BU, Ueland FR, et al. Lymph node metastases and prognosis in patients with stage IA2 cervical cancer. Gynecol Oncol. 1996;63(1):4-9. |
|
van Nagell JR, Jr., Greenwell N, Powell DF, Donaldson ES, Hanson MB, Gay EC. Microinvasive carcinoma of the cervix. Am J Obstet Gynecol. 1983;145(8):981-91. |
|
Sedlis A, Sall S, Tsukada Y, Park R, Mangan C, Shingleton H, et al. Microinvasive carcinoma of the uterine cervix: a clinical-pathologic study. Am J Obstet Gynecol. 1979;133(1):64-74. |
|
Jones WB, Mercer GO, Lewis JL, Jr., Rubin SC, Hoskins WJ. Early invasive carcinoma of the cervix. Gynecol Oncol. 1993;51(1):26-32. |
|
Gurgel MS, Bedone AJ, Andrade LA, Panetta K. Microinvasive carcinoma of the uterine cervix: histological findings on cone specimens related to residual neoplasia on hysterectomy. Gynecol Oncol. 1997;65(3):437-40. |
|
Marana HR, de Andrade JM, Matthes AC, Spina LA, Carrara HH, Bighetti S. Microinvasive carcinoma of the cervix. Analysis of prognostic factors. Eur J Gynaecol Oncol. 2001;22(1):64-6. |
|
Lin H, Chang HY, Huang CC, Changchien CC. Prediction of disease persistence after conization for microinvasive cervical carcinoma and cervical intraepithelial neoplasia grade 3. Int J Gynecol Cancer. 2004;14(2):311-6. |
|
Phongnarisorn C, Srisomboon J, Khunamornpong S, Siriaungkul S, Suprasert P, Charoenkwan K, et al. The risk of residual neoplasia in women with microinvasive squamous cervical carcinoma and positive cone margins. Int J Gynecol Cancer. 2006;16(2):655-9. |
|
Costa S, Marra E, Martinelli GN, Santini D, Casadio P, Formelli G, et al. Outcome of conservatively treated microinvasive squamous cell carcinoma of the uterine cervix during a 10-year follow-up. Int J Gynecol Cancer. 2009;19(1):33-8. |
|
Kim WY, Chang SJ, Chang KH, Yoo SC, Ryu HS. Conservative management of stage IA1 squamous cell carcinoma of the cervix with positive resection margins after conization. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2010;109(2):110-2. |
|
Leman MH, Jr., Benson WL, Kurman RJ, Park RC. Microinvasive carcinoma of the cervix. Obstet Gynecol. 1976;48(5):571-8. |
|
Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO Classification of Tumours of Female Reproductive Organs. 4th ed. Lyon, France: International Agency for Research on Cancer; 2014. |
|
SEER Cancer Statistics Factsheets: Cervix Uteri Cancer. Bethesda, MA: National Cancer Institute; [cited 2015 8/5/2015]; Available from: http://seer.cancer.gov/statfacts/html/cervix.html. |
|
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12-9. |
|
Wentz WB, Reagan JW. Survival in cervical cancer with respect to cell type. Cancer. 1959;12(2):384-8. |
|
Zaino RJ, Ward S, Delgado G, Bundy B, Gore H, Fetter G, et al. Histopathologic predictors of the behavior of surgically treated stage IB squamous cell carcinoma of the cervix. A Gynecologic Oncology Group study. Cancer. 1992;69(7):1750-8. |
|
Delgado G, Bundy BN, Fowler WC, Jr., Stehman FB, Sevin B, Creasman WT, et al. A prospective surgical pathological study of stage I squamous carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol Oncol. 1989;35(3):314-20. |
|
Stock RJ, Zaino R, Bundy BN, Askin FB, Woodward J, Fetter B, et al. Evaluation and comparison of histopathologic grading systems of epithelial carcinoma of the uterine cervix: Gynecologic Oncology Group studies. Int J Gynecol Pathol. 1994;13(2):99-108. |
|
Kurman R, Ronnett B, Ellenson L. Blaustein's Pathology of the Female Genital Tract. New York: Springer; 2010. |
|
Koenig C, Turnicky RP, Kankam CF, Tavassoli FA. Papillary squamotransitional cell carcinoma of the cervix: a report of 32 cases. Am J Surg Pathol. 1997;21(8):915-21. |
|
Albores-Saavedra J, Young RH. Transitional cell neoplasms (carcinomas and inverted papillomas) of the uterine cervix. A report of five cases. Am J Surg Pathol. 1995;19(10):1138-45. |
|
Randall ME, Andersen WA, Mills SE, Kim JA. Papillary squamous cell carcinoma of the uterine cervix: a clinicopathologic study of nine cases. Int J Gynecol Pathol. 1986;5(1):1-10. |
|
Tiltman AJ, Atad J. Verrucous carcinoma of the cervix with endometrial involvement. Int J Gynecol Pathol. 1982;1(2):221-6. |
|
Hasumi K, Sugano H, Sakamoto G, Masubuchi K, Kubo H. Circumscribed carcinoma of the uterine cervix, with marked lymphocytic infiltration. Cancer. 1977;39(6):2503-7. |
|
Mills SE, Austin MB, Randall ME. Lymphoepithelioma-like carcinoma of the uterine cervix. A distinctive, undifferentiated carcinoma with inflammatory stroma. Am J Surg Pathol. 1985;9(12):883-9. |
|
Halpin TF, Hunter RE, Cohen MB. Lymphoepithelioma of the uterine cervix. Gynecol Oncol. 1989;34(1):101-5. |
|
Tseng CJ, Pao CC, Tseng LH, Chang CT, Lai CH, Soong YK, et al. Lymphoepithelioma-like carcinoma of the uterine cervix: association with Epstein-Barr virus and human papillomavirus. Cancer. 1997;80(1):91-7. |
|
Steeper TA, Piscioli F, Rosai J. Squamous cell carcinoma with sarcoma-like stroma of the female genital tract. Clinicopathologic study of four cases. Cancer. 1983;52(5):890-8. |
|
Watty EI, Johnston LW, Bainborough AR. Polypoid carcinoma of the uterine cervix simulating "pseudosarcoma" and "carcinosarcoma" of esophagus and upper respiratory tract. Diagnostic gynecology and obstetrics. 1981;3(3):205-11. |
|
Fu YS KJ. Histopathology of preinvasive and invasive squamous neoplasia. In: Rubin SC HW, editor. Cervical Cancer and Preinvasive Neoplasia. Philadelphia: Lippincott-Raven Publishers; 1996. p. 77-92. |
|
Higgins GD, Phillips GE, Smith LA, Uzelin DM, Burrell CJ. High prevalence of human papillomavirus transcripts in all grades of cervical intraepithelial glandular neoplasia. Cancer. 1992;70(1):136-46. |
|
Leary J, Jaworski R, Houghton R. In-situ hybridization using biotinylated DNA probes to human papillomavirus in adenocarcinoma-in-situ and endocervical glandular dysplasia of the uterine cervix. Pathology. 1991;23(2):85-9. |
|
Kurian K, al-Nafussi A. Relation of cervical glandular intraepithelial neoplasia to microinvasive and invasive adenocarcinoma of the uterine cervix: a study of 121 cases. J Clin Pathol. 1999;52(2):112-7. |
|
Brown LJ, Wells M. Cervical glandular atypia associated with squamous intraepithelial neoplasia: a premalignant lesion? J Clin Pathol. 1986;39(1):22-8. |
|
Nielsen AL. Human papillomavirus type 16/18 in uterine cervical adenocarcinoma in situ and adenocarcinoma. A study by in situ hybridization with biotinylated DNA probes. Cancer. 1990;65(11):2588-93. |
|
Negri G, Bellisano G, Carico E, Faa G, Kasal A, Antoniazzi S, et al. Usefulness of p16ink4a, ProEX C, and Ki-67 for the diagnosis of glandular dysplasia and adenocarcinoma of the cervix uteri. Int J Gynecol Pathol. 2011;30(4):407-13. |
|
Goldstein NS, Ahmad E, Hussain M, Hankin RC, Perez-Reyes N. Endocervical glandular atypia: does a preneoplastic lesion of adenocarcinoma in situ exist? Am J Clin Pathol. 1998;110(2):200-9. |
|
Lee KR, Sun D, Crum CP. Endocervical intraepithelial glandular atypia (dysplasia): a histopathologic, human papillomavirus, and MIB-1 analysis of 25 cases. Hum pathol. 2000;31(6):656-64. |
|
Tase T, Okagaki T, Clark BA, Twiggs LB, Ostrow RS, Faras AJ. Human papillomavirus DNA in glandular dysplasia and microglandular hyperplasia: presumed precursors of adenocarcinoma of the uterine cervix. Obstet Gynecol. 1989;73(6):1005-8. |
|
Anciaux D, Lawrence WD, Gregoire L. Glandular lesions of the uterine cervix: prognostic implications of human papillomavirus status. Int J Gynecol Pathol. 1997;16(2):103-10. |
|
Riethdorf L, Riethdorf S, Lee KR, Cviko A, Loning T, Crum CP. Human papillomaviruses, expression of p16, and early endocervical glandular neoplasia. Hum pathol. 2002;33(9):899-904. |
|
Lu X, Shiozawa T, Nakayama K, Toki T, Nikaido T, Fujii S. Abnormal expression of sex steroid receptors and cell cycle-related molecules in adenocarcinoma in situ of the uterine cervix. Int J Gynecol Pathol. 1999;18(2):109-14. |
|
Ioffe OB, Sagae S, Moritani S, Dahmoush L, Chen TT, Silverberg SG. Proposal of a new scoring scheme for the diagnosis of noninvasive endocervical glandular lesions. Am J Surg Pathol. 2003;27(4):452-60. |
|
McCluggage WG. Endocervical glandular lesions: controversial aspects and ancillary techniques. J Clin Pathol. 2003;56(3):164-73. |
|
Loureiro J, Oliva E. The spectrum of cervical glandular neoplasia and issues in differential diagnosis. Arch Pathol Lab Med. 2014;138(4):453-83. |
|
Lee KR. Symposium part 4: Should pathologists diagnose endocervical preneoplastic lesions "less than" adenocarcinoma in situ?: Counterpoint. Int J Gynecol Pathol. 2003;22(1):22-4. |
|
Zaino RJ. Symposium part I: adenocarcinoma in situ, glandular dysplasia, and early invasive adenocarcinoma of the uterine cervix. Int J Gynecol Pathol. 2002;21(4):314-26. |
|
Tavassoli FA, Devilee P, editors. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon, France: IARC Press; 2003. |
|
Hasumi K, Ehrmann RL. Clear cell carcinoma of the uterine endocervix with an in situ component. Cancer. 1978;42(5):2435-8. |
|
Gloor E, Ruzicka J. Morphology of adenocarcinoma in situ of the uterine cervix: a study of 14 cases. Cancer. 1982;49(2):294-302. |
|
Christopherson WM, Nealon N, Gray LA, Sr. Noninvasive precursor lesions of adenocarcinoma and mixed adenosquamous carcinoma of the cervix uteri. Cancer. 1979;44(3):975-83. |
|
Wolf JK, Levenback C, Malpica A, Morris M, Burke T, Mitchell MF. Adenocarcinoma in situ of the cervix: significance of cone biopsy margins. Obstet Gynecol. 1996;88(1):82-6. |
|
Cameron RI, Maxwell P, Jenkins D, McCluggage WG. Immunohistochemical staining with MIB1, bcl2 and p16 assists in the distinction of cervical glandular intraepithelial neoplasia from tubo-endometrial metaplasia, endometriosis and microglandular hyperplasia. Histopathology. 2002;41(4):313-21. |
|
McCluggage WG, Maxwell P, McBride HA, Hamilton PW, Bharucha H. Monoclonal antibodies Ki-67 and MIB1 in the distinction of tuboendometrial metaplasia from endocervical adenocarcinoma and adenocarcinoma in situ in formalin-fixed material. Int J Gynecol Pathol. 1995;14(3):209-16. |
|
McCluggage WG. Immunohistochemistry as a diagnostic aid in cervical pathology. Pathology. 2007;39(1):97-111. |
|
Ostor AG, Pagano R, Davoren RA, Fortune DW, Chanen W, Rome R. Adenocarcinoma in situ of the cervix. Int J Gynecol Pathol. 1984;3(2):179-90. |
|
Bertrand M, Lickrish GM, Colgan TJ. The anatomic distribution of cervical adenocarcinoma in situ: implications for treatment. Am J Obstet Gynecol. 1987;157(1):21-5. |
|
Jaworski RC, Pacey NF, Greenberg ML, Osborn RA. The histologic diagnosis of adenocarcinoma in situ and related lesions of the cervix uteri. Adenocarcinoma in situ. Cancer. 1988;61(6):1171-81. |
|
McCluggage WG. New developments in endocervical glandular lesions. Histopathology. 2013;62(1):138-60. |
|
McCluggage WG, Shah R, Connolly LE, McBride HA. Intestinal-type cervical adenocarcinoma in situ and adenocarcinoma exhibit a partial enteric immunophenotype with consistent expression of CDX2. Int J Gynecol Pathol. 2008;27(1):92-100. |
|
Steiner G, Friedell GH. Adenosquamous Carcinoma in Situ of the Cervix. Cancer. 1965;18:807-10. |
|
Park JJ, Sun D, Quade BJ, Flynn C, Sheets EE, Yang A, et al. Stratified mucin-producing intraepithelial lesions of the cervix: adenosquamous or columnar cell neoplasia? Am J Surg Pathol. 2000;24(10):1414-9. |
|
Boyle DP, McCluggage WG. Stratified mucin-producing intraepithelial lesion (SMILE): report of a case series with associated pathological findings. Histopathology. 2015;66(5):658-63. |
|
Greer BE, Figge DC, Tamimi HK, Cain JM. Stage IB adenocarcinoma of the cervix treated by radical hysterectomy and pelvic lymph node dissection. Am J Obstet Gynecol. 1989;160(6):1509-13; discussion 13-4. |
|
Teshima S, Shimosato Y, Kishi K, Kasamatsu T, Ohmi K, Uei Y. Early stage adenocarcinoma of the uterine cervix. Histopathologic analysis with consideration of histogenesis. Cancer. 1985;56(1):167-72. |
|
Berek JS, Hacker NF, Fu YS, Sokale JR, Leuchter RC, Lagasse LD. Adenocarcinoma of the uterine cervix: histologic variables associated with lymph node metastasis and survival. Obstet Gynecol. 1985;65(1):46-52. |
|
Wheeler DT, Kurman RJ. The relationship of glands to thick-wall blood vessels as a marker of invasion in endocervical adenocarcinoma. Int J Gynecol Pathol. 2005;24(2):125-30. |
|
Reynolds EA, Tierney K, Keeney GL, Felix JC, Weaver AL, Roman LD, et al. Analysis of outcomes of microinvasive adenocarcinoma of the uterine cervix by treatment type. Obstet Gynecol. 2010;116(5):1150-7. |
|
Webb JC, Key CR, Qualls CR, Smith HO. Population-based study of microinvasive adenocarcinoma of the uterine cervix. Obstet Gynecol. 2001;97(5 Pt 1):701-6. |
|
Diaz De Vivar A, Roma AA, Park KJ, Alvarado-Cabrero I, Rasty G, Chanona-Vilchis JG, et al. Invasive endocervical adenocarcinoma: proposal for a new pattern-based classification system with significant clinical implications: a multi-institutional study. Int J Gynecol Pathol. 2013;32(6):592-601. |
|
Paquette C, Jeffus SK, Quick CM, Conaway MR, Stoler MH, Atkins KA. Interobserver variability in the application of a proposed histologic subclassification of endocervical adenocarcinoma. Am J Surg Pathol. 2015;39(1):93-100. |
|
McKelvey JL GR. Adenoma malignum of the cervix: A cancer of deceptively innocent histological pattern. Cancer. 1963;16:549. |
|
Silverberg SG, Hurt WG. Minimal deviation adenocarcinoma ("adenoma malignum") of the cervix: a reappraisal. Am J Obstet Gynecol. 1975;121(7):971-5. |
|
Kaminski PF, Norris HJ. Minimal deviation carcinoma (adenoma malignum) of the cervix. Int J Gynecol Pathol. 1983;2(2):141-52. |
|
Kuragaki C, Enomoto T, Ueno Y, Sun H, Fujita M, Nakashima R, et al. Mutations in the STK11 gene characterize minimal deviation adenocarcinoma of the uterine cervix. Lab Invest. 2003;83(1):35-45. |
|
Toki T, Shiozawa T, Hosaka N, Ishii K, Nikaido T, Fujii S. Minimal deviation adenocarcinoma of the uterine cervix has abnormal expression of sex steroid receptors, CA125, and gastric mucin. Int J Gynecol Pathol. 1997;16(2):111-6. |
|
Utsugi K, Hirai Y, Takeshima N, Akiyama F, Sakurai S, Hasumi K. Utility of the monoclonal antibody HIK1083 in the diagnosis of adenoma malignum of the uterine cervix. Gynecol Oncol. 1999;75(3):345-8. |
|
Mikami Y, Kiyokawa T, Hata S, Fujiwara K, Moriya T, Sasano H, et al. Gastrointestinal immunophenotype in adenocarcinomas of the uterine cervix and related glandular lesions: a possible link between lobular endocervical glandular hyperplasia/pyloric gland metaplasia and 'adenoma malignum'. Mod Pathol. 2004;17(8):962-72. |
|
Karamurzin Y, Parkash V, Kiyokawa R, Soslow RA, Park KJ. Immunohistochemical profile of gastric type endocervical adenocarcinoma, including HER2 ⁄ neu status. Mod Pathol. 2012;25(Suppl. 2):1172A. |
|
Park KJ, Kiyokawa T, Soslow RA, Lamb CA, Oliva E, Zivanovic O, et al. Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol. 2011;35(5):633-46. |
|
Kojima A, Mikami Y, Sudo T, Yamaguchi S, Kusanagi Y, Ito M, et al. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol. 2007;31(5):664-72. |
|
Kawauchi S, Kusuda T, Liu XP, Suehiro Y, Kaku T, Mikami Y, et al. Is lobular endocervical glandular hyperplasia a cancerous precursor of minimal deviation adenocarcinoma?: a comparative molecular-genetic and immunohistochemical study. Am J Surg Pathol. 2008;32(12):1807-15. |
|
Nara M, Hashi A, Murata S, Kondo T, Yuminamochi T, Nakazawa K, et al. Lobular endocervical glandular hyperplasia as a presumed precursor of cervical adenocarcinoma independent of human papillomavirus infection. Gynecol Oncol. 2007;106(2):289-98. |
|
An HJ, Kim KR, Kim IS, Kim DW, Park MH, Park IA, et al. Prevalence of human papillomavirus DNA in various histological subtypes of cervical adenocarcinoma: a population-based study. Mod Pathol. 2005;18(4):528-34. |
|
Young RH, Scully RE. Villoglandular papillary adenocarcinoma of the uterine cervix. A clinicopathologic analysis of 13 cases. Cancer. 1989;63(9):1773-9. |
|
Jones MW, Silverberg SG, Kurman RJ. Well-differentiated villoglandular adenocarcinoma of the uterine cervix: a clinicopathological study of 24 cases. Int J Gynecol Pathol. 1993;12(1):1-7. |
|
Utsugi K, Shimizu Y, Akiyama F, Hasumi K. Villoglandular papillary adenocarcinoma of the uterine cervix with bulky lymph node metastases. Eur J Obstet Gynecol Reprod Biol. 2002;105(2):186-8. |
|
Fu YS, Reagan JW, Hsiu JG, Storaasli JP, Wentz WB. Adenocarcinoma and mixed carcinoma of the uterine cervix. I. A. clinicopathologic study. Cancer. 1982;49(12):2560-70. |
|
Dabbs DJ, Geisinger KR, Norris HT. Intermediate filaments in endometrial and endocervical carcinomas. The diagnostic utility of vimentin patterns. Am J Surg Pathol. 1986;10(8):568-76. |
|
Fawcett KJ, Dockerty MB, Hunt AB. Mesonephric carcinoma of the cervix uteri: a clinical and pathologic study. Am J Obstet Gynecol. 1966;95(8):1068-79. |
|
Kaminski PF, Maier RC. Clear cell adenocarcinoma of the cervix unrelated to diethylstilbestrol exposure. Obstet Gynecol. 1983;62(6):720-7. |
|
Herbst AL, Anderson D. Clear cell adenocarcinoma of the vagina and cervix secondary to intrauterine exposure to diethylstilbestrol. Seminars in surgical oncology. 1990;6(6):343-6. |
|
Pirog EC, Lloveras B, Molijn A, Tous S, Guimera N, Alejo M, et al. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod Pathol. 2014;27(12):1559-67. |
|
Reich O, Tamussino K, Lahousen M, Pickel H, Haas J, Winter R. Clear cell carcinoma of the uterine cervix: pathology and prognosis in surgically treated stage IB-IIB disease in women not exposed in utero to diethylstilbestrol. Gynecol Oncol. 2000;76(3):331-5. |
|
Young RH, Scully RE. Invasive adenocarcinoma and related tumors of the uterine cervix. Seminars in diagnostic pathology. 1990;7(3):205-27. |
|
Costa MJ, McIlnay KR, Trelford J. Cervical carcinoma with glandular differentiation: histological evaluation predicts disease recurrence in clinical stage I or II patients. Hum pathol. 1995;26(8):829-37. |
|
Zhou C, Gilks CB, Hayes M, Clement PB. Papillary serous carcinoma of the uterine cervix: a clinicopathologic study of 17 cases. Am J Surg Pathol. 1998;22(1):113-20. |
|
Clement PB, Young RH, Keh P, Ostor AG, Scully RE. Malignant mesonephric neoplasms of the uterine cervix. A report of eight cases, including four with a malignant spindle cell component. Am J Surg Pathol. 1995;19(10):1158-71. |
|
Kenny SL, McBride HA, Jamison J, McCluggage WG. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-beta. Am J Surg Pathol. 2012;36(6):799-807. |
|
Silver SA, Devouassoux-Shisheboran M, Mezzetti TP, Tavassoli FA. Mesonephric adenocarcinomas of the uterine cervix: a study of 11 cases with immunohistochemical findings. Am J Surg Pathol. 2001;25(3):379-87. |
|
Ferry JA, Scully RE. Mesonephric remnants, hyperplasia, and neoplasia in the uterine cervix. A study of 49 cases. Am J Surg Pathol. 1990;14(12):1100-11. |
|
Yasuoka H, Tsujimoto M, Ueda M, Kodama R, Iwahashi Y, Inagaki M, et al. Monoclonality of composite large-cell neuroendocrine carcinoma and invasive intestinal-type mucinous adenocarcinoma of the cervix: a case study. Int J Gynecol Pathol. 2013;32(4):416-20. |
|
Ramalingam P, Malpica A, Deavers MT. Mixed endocervical adenocarcinoma and high-grade neuroendocrine carcinoma of the cervix with ovarian metastasis of the former component: a report of 2 cases. Int J Gynecol Pathol. 2012;31(5):490-6. |
|
Rekhi B, Patil B, Deodhar KK, Maheshwari A, R AK, Gupta S, et al. Spectrum of neuroendocrine carcinomas of the uterine cervix, including histopathologic features, terminology, immunohistochemical profile, and clinical outcomes in a series of 50 cases from a single institution in India. Annals of diagnostic pathology. 2013;17(1):1-9. |
|
Kamoi S, AlJuboury MI, Akin MR, Silverberg SG. Immunohistochemical staining in the distinction between primary endometrial and endocervical adenocarcinomas: another viewpoint. Int J Gynecol Pathol. 2002;21(3):217-23. |
|
Young RH, Scully RE. Uterine carcinomas simulating microglandular hyperplasia. A report of six cases. Am J Surg Pathol. 1992;16(11):1092-7. |
|
Young RH, Scully RE. Atypical forms of microglandular hyperplasia of the cervix simulating carcinoma. A report of five cases and review of the literature. Am J Surg Pathol. 1989;13(1):50-6. |
|
Cherry CP, Glucksmann A. Incidence, histology, and response to radiation of mixed carcinomas (adenoacanthomas) of the uterine cervix. Cancer. 1956;9(5):971-9. |
|
Lennerz JK, Perry A, Mills JC, Huettner PC, Pfeifer JD. Mucoepidermoid carcinoma of the cervix: another tumor with the t(11;19)-associated CRTC1-MAML2 gene fusion. Am J Surg Pathol. 2009;33(6):835-43. |
|
Yoshida T, Sano T, Oyama T, Kanuma T, Fukuda T. Prevalence, viral load, and physical status of HPV 16 and 18 in cervical adenosquamous carcinoma. Virchows Arch. 2009;455(3):253-9. |
|
Ueda Y, Miyatake T, Okazawa M, Kimura T, Miyake T, Fujiwara K, et al. Clonality and HPV infection analysis of concurrent glandular and squamous lesions and adenosquamous carcinomas of the uterine cervix. Am J Clin Pathol. 2008;130(3):389-400. |
|
Farley JH, Hickey KW, Carlson JW, Rose GS, Kost ER, Harrison TA. Adenosquamous histology predicts a poor outcome for patients with advanced-stage, but not early-stage, cervical carcinoma. Cancer. 2003;97(9):2196-202. |
|
Shingleton HM, Bell MC, Fremgen A, Chmiel JS, Russell AH, Jones WB, et al. Is there really a difference in survival of women with squamous cell carcinoma, adenocarcinoma, and adenosquamous cell carcinoma of the cervix? Cancer. 1995;76(10 Suppl):1948-55. |
|
Vesterinen E, Forss M, Nieminen U. Increase of cervical adenocarcinoma: a report of 520 cases of cervical carcinoma including 112 tumors with glandular elements. Gynecol Oncol. 1989;33(1):49-53. |
|
Yazigi R, Sandstad J, Munoz AK, Choi DJ, Nguyen PD, Risser R. Adenosquamous carcinoma of the cervix: prognosis in stage IB. Obstet Gynecol. 1990;75(6):1012-5. |
|
Maier RC, Norris HJ. Glassy cell carcinoma of the cervix. Obstet Gynecol. 1982;60(2):219-24. |
|
Costa MJ, Kenny MB, Hewan-Lowe K, Judd R. Glassy cell features in adenosquamous carcinoma of the uterine cervix. Histologic, ultrastructural, immunohistochemical, and clinical findings. Am J Clin Pathol. 1991;96(4):520-8. |
|
Littman P, Clement PB, Henriksen B, Wang CC, Robboy SJ, Taft PD, et al. Glassy cell carcinoma of the cervix. Cancer. 1976;37(5):2238-46. |
|
Matsuura Y, Murakami N, Nagashio E, Toki N, Kashimura M. Glassy cell carcinoma of the uterine cervix: combination chemotherapy with paclitaxel and carboplatin in recurrent tumor. The journal of obstetrics and gynaecology research. 2001;27(3):129-32. |
|
Mikami M, Ezawa S, Sakaiya N, Komuro Y, Tei C, Fukuchi T, et al. Response of glassy-cell carcinoma of the cervix to cisplatin, epirubicin, and mitomycin C. Lancet. 2000;355(9210):1159-60. |
|
Nagai T, Okubo T, Sakaguchi R, Seki H, Takeda S. Glassy cell carcinoma of the uterine cervix responsive to neoadjuvant intraarterial chemotherapy. International journal of clinical oncology. 2008;13(6):541-4. |
|
Takekuma M, Hirashima Y, Takahashi N, Yamamichi G, Furukawa N, Yamada Y, et al. A case of glassy cell carcinoma of the uterine cervix that responded to neoadjuvant chemotherapy with paclitaxel and carboplatin. Anti-cancer drugs. 2006;17(6):715-8. |
|
Ferry JA, Scully RE. "Adenoid cystic" carcinoma and adenoid basal carcinoma of the uterine cervix. A study of 28 cases. Am J Surg Pathol. 1988;12(2):134-44. |
|
Brainard JA, Hart WR. Adenoid basal epitheliomas of the uterine cervix: a reevaluation of distinctive cervical basaloid lesions currently classified as adenoid basal carcinoma and adenoid basal hyperplasia. Am J Surg Pathol. 1998;22(8):965-75. |
|
Parwani AV, Smith Sehdev AE, Kurman RJ, Ronnett BM. Cervical adenoid basal tumors comprised of adenoid basal epithelioma associated with various types of invasive carcinoma: clinicopathologic features, human papillomavirus DNA detection, and P16 expression. Hum pathol. 2005;36(1):82-90. |
|
Jones MW, Kounelis S, Papadaki H, Bakker A, Swalsky PA, Finkelstein SD. The origin and molecular characterization of adenoid basal carcinoma of the uterine cervix. Int J Gynecol Pathol. 1997;16(4):301-6. |
|
Grayson W, Taylor LF, Cooper K. Adenoid basal carcinoma of the uterine cervix: detection of integrated human papillomavirus in a rare tumor of putative "reserve cell" origin. Int J Gynecol Pathol. 1997;16(4):307-12. |
|
Grayson W, Taylor LF, Cooper K. Adenoid cystic and adenoid basal carcinoma of the uterine cervix: comparative morphologic, mucin, and immunohistochemical profile of two rare neoplasms of putative 'reserve cell' origin. Am J Surg Pathol. 1999;23(4):448-58. |
|
Albores-Saavedra J, Manivel C, Mora A, Vuitch F, Milchgrub S, Gould E. The solid variant of adenoid cystic carcinoma of the cervix. Int J Gynecol Pathol. 1992;11(1):2-10. |
|
Lawrence JB, Mazur MT. Adenoid cystic carcinoma: a comparative pathologic study of tumors in salivary gland, breast, lung, and cervix. Hum pathol. 1982;13(10):916-24. |
|
Prempree T, Villasanta U, Tang CK. Management of adenoid cystic carcinoma of the uterine cervix (cylindroma): report of six cases and reappraisal of all cases reported in the medical literature. Cancer. 1980;46(7):1631-5. |
|
Dixit S, Singhal S, Vyas R, Murthy A, Baboo HA. Adenoid cystic carcinoma of the cervix. Journal of postgraduate medicine. 1993;39(4):211-5. |
|
Chen TD, Chuang HC, Lee LY. Adenoid basal carcinoma of the uterine cervix: clinicopathologic features of 12 cases with reference to CD117 expression. Int J Gynecol Pathol. 2012;31(1):25-32. |
|
Grayson W, Taylor L, Cooper K. Detection of integrated high risk human papillomavirus in adenoid cystic carcinoma of the uterine cervix. J Clin Pathol. 1996;49(10):805-9. |
|
Brill LB, 2nd, Kanner WA, Fehr A, Andren Y, Moskaluk CA, Loning T, et al. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol. 2011;24(9):1169-76. |
|
McCluggage WG, Kennedy K, Busam KJ. An immunohistochemical study of cervical neuroendocrine carcinomas: Neoplasms that are commonly TTF1 positive and which may express CK20 and P63. Am J Surg Pathol. 2010;34(4):525-32. |
|
Fetissof F, Serres G, Arbeille B, de Muret A, Sam-Giao M, Lansac J. Argyrophilic cells and ectocervical epithelium. Int J Gynecol Pathol. 1991;10(2):177-90. |
|
Stoler MH, Mills SE, Gersell DJ, Walker AN. Small-cell neuroendocrine carcinoma of the cervix. A human papillomavirus type 18-associated cancer. Am J Surg Pathol. 1991;15(1):28-32. |
|
Wistuba, II, Thomas B, Behrens C, Onuki N, Lindberg G, Albores-Saavedra J, et al. Molecular abnormalities associated with endocrine tumors of the uterine cervix. Gynecol Oncol. 1999;72(1):3-9. |
|
Pazdur R, Bonomi P, Slayton R, Gould VE, Miller A, Jao W, et al. Neuroendocrine carcinoma of the cervix: implications for staging and therapy. Gynecol Oncol. 1981;12(1):120-8. |
|
Stassart J, Crum CP, Yordan EL, Fenoglio CM, Richart RM. Argyrophilic carcinoma of the cervix: a report of a case with coexisting cervical intraepithelial neoplasia. Gynecol Oncol. 1982;13(2):247-51. |
|
Wang KL, Yang YC, Wang TY, Chen JR, Chen TC, Chen HS, et al. Neuroendocrine carcinoma of the uterine cervix: A clinicopathologic retrospective study of 31 cases with prognostic implications. J Chemother. 2006;18(2):209-16. |
|
Sevin BU, Method MW, Nadji M, Lu Y, Averette HA. Efficacy of radical hysterectomy as treatment for patients with small cell carcinoma of the cervix. Cancer. 1996;77(8):1489-93. |
|
Grayson W, Taylor LF, Cooper K. Carcinosarcoma of the uterine cervix: a report of eight cases with immunohistochemical analysis and evaluation of human papillomavirus status. Am J Surg Pathol. 2001;25(3):338-47. |
|
Atlante M, Dionisi B, Cioni M, Di Ruzza D, Sedati P, Mariani L. Sarcoma botryoides of the uterine cervix in a young woman: a case report. Eur J Gynaecol Oncol. 2000;21(5):504-6. |
|
Daya DA, Scully RE. Sarcoma botryoides of the uterine cervix in young women: a clinicopathological study of 13 cases. Gynecol Oncol. 1988;29(3):290-304. |
|
Li RF, Gupta M, McCluggage WG, Ronnett BM. Embryonal rhabdomyosarcoma (botryoid type) of the uterine corpus and cervix in adult women: report of a case series and review of the literature. Am J Surg Pathol. 2013;37(3):344-55. |
|
Ditto A, Martinelli F, Carcangiu M, Solima E, de Carrillo KJ, Sanfilippo R, et al. Embryonal rhabdomyosarcoma of the uterine cervix in adults: a case report and literature review. J Low Genit Tract Dis. 2013;17(4):e12-7. |
|
Foulkes WD, Bahubeshi A, Hamel N, Pasini B, Asioli S, Baynam G, et al. Extending the phenotypes associated with DICER1 mutations. Human mutation. 2011;32(12):1381-4. |
|
Clement PB. Miscellaneous primary tumors and metastatic tumors of the uterine cervix. Seminars in diagnostic pathology. 1990;7(3):228-48. |
|
Sevin BU, Lu Y, Bloch DA, Nadji M, Koechli OR, Averette HE. Surgically defined prognostic parameters in patients with early cervical carcinoma. A multivariate survival tree analysis. Cancer. 1996;78(7):1438-46. |
|
Kamura T, Tsukamoto N, Tsuruchi N, Saito T, Matsuyama T, Akazawa K, et al. Multivariate analysis of the histopathologic prognostic factors of cervical cancer in patients undergoing radical hysterectomy. Cancer. 1992;69(1):181-6. |
|
Fuller AF, Jr., Elliott N, Kosloff C, Hoskins WJ, Lewis JL, Jr. Determinants of increased risk for recurrence in patients undergoing radical hysterectomy for stage IB and IIA carcinoma of the cervix. Gynecol Oncol. 1989;33(1):34-9. |
|
Kishi Y, Hashimoto Y, Sakamoto Y, Inui S. Thickness of uninvolved fibromuscular stroma and extrauterine spread of carcinoma of the uterine cervix. Cancer. 1987;60(9):2331-6. |
|
Burghardt E, Pickel H. Local spread and lymph node involvement in cervical cancer. Obstet Gynecol. 1978;52(2):138-45. |
|
Boyce JG, Fruchter RG, Nicastri AD, DeRegt RH, Ambiavagar PC, Reinis M, et al. Vascular invasion in Stage I carcinoma of the cervix. Cancer. 1984;53(5):1175-80. |
|
White CD, Morley GW, Kumar NB. The prognostic significance of tumor emboli in lymphatic or vascular spaces of the cervical stroma in Stage IB squamous cell carcinoma of the cervix. Am J Obstet Gynecol. 1984;149(3):342-9. |
|
Crissman JD, Budhraja M, Aron BS, Cummings G. Histopathologic prognostic factors in stage II and III squamous cell carcinoma of the uterine cervix. An evaluation of 91 patients treated primarily with radiation therapy. Int J Gynecol Pathol. 1987;6(2):97-103. |
|
Rettenmaier MA, Casanova DM, Micha JP, Moran MF, Ramsanghani NS, Syed NA, et al. Radical hysterectomy and tailored postoperative radiation therapy in the management of bulky stage 1B cervical cancer. Cancer. 1989;63(11):2220-3. |
|
Giaroli A, Sananes C, Sardi JE, Maya AG, Bastardas ML, Snaidas L, et al. Lymph node metastases in carcinoma of the cervix uteri: response to neoadjuvant chemotherapy and its impact on survival. Gynecol Oncol. 1990;39(1):34-9. |
|
Pilleron JP, Durand JC, Hamelin JP. Prognostic value of node metastasis in cancer of the uterine cervix. Am J Obstet Gynecol. 1974;119(4):458-62. |
|
Matsuyama T, Inoue I, Tsukamoto N, Kashimura M, Kamura T, Saito T, et al. Stage Ib, IIa, and IIb cervix cancer, postsurgical staging, and prognosis. Cancer. 1984;54(12):3072-7. |
|
Timmer PR, Aalders JG, Bouma J. Radical surgery after preoperative intracavitary radiotherapy for Stage IB and IIA carcinoma of the uterine cervix. Gynecol Oncol. 1984;18(2):206-12. |
|
Lifshitz S, Buchsbaum H. The spread of cervical carcinoma. In: JJ S, editor. Gynecology and Obstetrics. Philadelphia: Harper and Row; 1980. |
|
Berman ML, Bergen S, Salazar H. Influence of histological features and treatment on the prognosis of patients with cervical cancer metastatic to pelvic lymph nodes. Gynecol Oncol. 1990;39(2):127-31. |
|
Brandt B, 3rd, Lifshitz S. Scalene node biopsy in advanced carcinoma of the cervix uteri. Cancer. 1981;47(7):1920-1. |
|
Lagasse LD, Creasman WT, Shingleton HM, Ford JH, Blessing JA. Results and complications of operative staging in cervical cancer: experience of the Gynecologic Oncology Group. Gynecol Oncol. 1980;9(1):90-8. |
|
Rubin SC, Brookland R, Mikuta JJ, Mangan C, Sutton G, Danoff B. Para-aortic nodal metastases in early cervical carcinoma: long-term survival following extended-field radiotherapy. Gynecol Oncol. 1984;18(2):213-7. |
|
Vasilev SA, Schlaerth JB. Scalene lymph node sampling in cervical carcinoma: a reappraisal. Gynecol Oncol. 1990;37(1):120-4. |
|
Barillot I, Horiot JC, Pigneux J, Schraub S, Pourquier H, Daly N, et al. Carcinoma of the intact uterine cervix treated with radiotherapy alone: a French cooperative study: update and multivariate analysis of prognostics factors. International journal of radiation oncology, biology, physics. 1997;38(5):969-78. |
|
Gallup DG, Harper RH, Stock RJ. Poor prognosis in patients with adenosquamous cell carcinoma of the cervix. Obstet Gynecol. 1985;65(3):416-22. |
|
Shingleton HM, Gore H, Bradley DH, Soong SJ. Adenocarcinoma of the cervix. I. Clinical evaluation and pathologic features. Am J Obstet Gynecol. 1981;139(7):799-814. |
|
Pak HY, Yokota SB, Paladugu RR, Agliozzo CM. Glassy cell carcinoma of the cervix. Cytologic and clinicopathologic analysis. Cancer. 1983;52(2):307-12. |
|
Reagan JW, Fu YS. Histologic types and prognosis of cancers of the uterine cervix. International journal of radiation oncology, biology, physics. 1979;5(7):1015-20. |
|
Krebs HB, Helmkamp BF, Sevin BU, Poliakoff SR, Nadji M, Averette HE. Recurrent cancer of the cervix following radical hysterectomy and pelvic node dissection. Obstet Gynecol. 1982;59(4):422-7. |
|
Larson DM, Copeland LJ, Stringer CA, Gershenson DM, Malone JM, Jr., Edwards CL. Recurrent cervical carcinoma after radical hysterectomy. Gynecol Oncol. 1988;30(3):381-7. |
|
Look KY, Rocereto TF. Relapse patterns in FIGO stage IB carcinoma of the cervix. Gynecol Oncol. 1990;38(1):114-20. |
|
Bachaud JM, Mazabrey D, Berrebi A, Maisongrosse V. Cutaneous metastatic lymphangitis from squamous cell carcinoma of the cervix. Dermatologica. 1990;180(3):163-5. |
|
Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol. 2015. |
|
Flint A, McCoy JP, Jr., Schade WJ, Hofheinz DA, Haines HG. Cervical carcinoma antigen: distribution in neoplastic lesions of the uterine cervix and comparison to other tumor markers. Gynecol Oncol. 1988;30(1):63-70. |
|
van Nagell JR, Jr., Donaldson ES, Gay EC, Hudson S, Sharkey RM, Primus FJ, et al. Carcinoembryonic antigen in carcinoma of the uterine cervix. 2. Tissue localization and correlation with plasma antigen concentration. Cancer. 1979;44(3):944-8. |
|
Lindgren J, Wahlstrom T, Seppala M. Tissue CEA in premalignant epithelial lesions and epidermoid carcinoma of the uterine cervix: prognostic significance. International journal of cancer Journal international du cancer. 1979;23(4):448-53. |
|
Borras G, Molina R, Xercavins J, Ballesta A, Iglesias J. Tumor antigens CA 19.9, CA 125, and CEA in carcinoma of the uterine cervix. Gynecol Oncol. 1995;57(2):205-11. |
|
Takeda M, Sakuragi N, Okamoto K, Todo Y, Minobe S, Nomura E, et al. Preoperative serum SCC, CA125, and CA19-9 levels and lymph node status in squamous cell carcinoma of the uterine cervix. Acta obstetricia et gynecologica Scandinavica. 2002;81(5):451-7. |
|
Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977;40(4):1621-8. |
|
Crombach G, Scharl A, Vierbuchen M, Wurz H, Bolte A. Detection of squamous cell carcinoma antigen in normal squamous epithelia and in squamous cell carcinomas of the uterine cervix. Cancer. 1989;63(7):1337-42. |
|
Ngan HY, Chan SY, Wong LC, Choy DT, Ma HK. Serum squamous cell carcinoma antigen in the monitoring of radiotherapy treatment response in carcinoma of the cervix. Gynecol Oncol. 1990;37(2):260-3. |
|
Bolli JA, Doering DL, Bosscher JR, Day TG, Jr., Rao CV, Owens K, et al. Squamous cell carcinoma antigen: clinical utility in squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 1994;55(2):169-73. |
|
Piao X, Kong TW, Chang SJ, Paek J, Chun M, Ryu HS. Pretreatment serum CYFRA 21-1 level correlates significantly with survival of cervical cancer patients: a multivariate analysis of 506 cases. Gynecol Oncol. 2015;138(1):89-93. |
|
Bonfrer JM, Gaarenstroom KN, Kenter GG, Korse CM, Hart AA, Gallee MP, et al. Prognostic significance of serum fragments of cytokeratin 19 measured by Cyfra 21-1 in cervical cancer. Gynecol Oncol. 1994;55(3 Pt 1):371-5. |
|
Suzuki Y, Nakano T, Ohno T, Abe A, Morita S, Tsujii H. Serum CYFRA 21-1 in cervical cancer patients treated with radiation therapy. Journal of cancer research and clinical oncology. 2000;126(6):332-6. |
|
Mandai M, Konishi I, Koshiyama M, Komatsu T, Yamamoto S, Nanbu K, et al. Altered expression of nm23-H1 and c-erbB-2 proteins have prognostic significance in adenocarcinoma but not in squamous cell carcinoma of the uterine cervix. Cancer. 1995;75(10):2523-9. |
|
Lesnikova I, Lidang M, Hamilton-Dutoit S, Koch J. HER2/neu (c-erbB-2) gene amplification and protein expression are rare in uterine cervical neoplasia: a tissue microarray study of 814 archival specimens. APMIS. 2009;117(10):737-45. |
|
Bachtiary B, Schindl M, Potter R, Dreier B, Knocke TH, Hainfellner JA, et al. Overexpression of hypoxia-inducible factor 1alpha indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin Cancer Res. 2003;9(6):2234-40. |
|
Burri P, Djonov V, Aebersold DM, Lindel K, Studer U, Altermatt HJ, et al. Significant correlation of hypoxia-inducible factor-1alpha with treatment outcome in cervical cancer treated with radical radiotherapy. International journal of radiation oncology, biology, physics. 2003;56(2):494-501. |
|
Loncaster JA, Cooper RA, Logue JP, Davidson SE, Hunter RD, West CM. Vascular endothelial growth factor (VEGF) expression is a prognostic factor for radiotherapy outcome in advanced carcinoma of the cervix. Br J Cancer. 2000;83(5):620-5. |
|
Lee S, Shin HJ, Han IO, Hong EK, Park SY, Roh JW, et al. Tumor carbonic anhydrase 9 expression is associated with the presence of lymph node metastases in uterine cervical cancer. Cancer science. 2007;98(3):329-33. |
|
Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, et al. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61(17):6394-9. |
|
Bandyopadhyay R, Chatterjee U, Mondal SK, Nag D, Sinha SK. A study on expression pattern of cyclooxygenase-2 in carcinoma of cervix. Indian journal of pathology & microbiology. 2011;54(4):695-9. |
|
Chen HH, Su WC, Chou CY, Guo HR, Ho SY, Que J, et al. Increased expression of nitric oxide synthase and cyclooxygenase-2 is associated with poor survival in cervical cancer treated with radiotherapy. International journal of radiation oncology, biology, physics. 2005;63(4):1093-100. |
|
Kim YB, Kim GE, Pyo HR, Cho NH, Keum KC, Lee CG, et al. Differential cyclooxygenase-2 expression in squamous cell carcinoma and adenocarcinoma of the uterine cervix. International journal of radiation oncology, biology, physics. 2004;60(3):822-9. |
|
Crook T, Greenfield I, Howard J, Stanley M. Alterations in growth properties of human papilloma virus type 16 immortalised human cervical keratinocyte cell line correlate with amplification and overexpression of c-myc oncogene. Oncogene. 1990;5(4):619-22. |
|
Sagae S, Kuzumaki N, Hisada T, Mugikura Y, Kudo R, Hashimoto M. ras oncogene expression and prognosis of invasive squamous cell carcinomas of the uterine cervix. Cancer. 1989;63(8):1577-82. |
|
Agnantis NJ, Spandidos DA, Mahera H, Parissi P, Kakkanas A, Pintzas A, et al. Immunohistochemical study of ras oncogene expression in endometrial and cervical human lesions. Eur J Gynaecol Oncol. 1988;9(5):360-5. |
|
Riou G, Barrois M, Le MG, George M, Le Doussal V, Haie C. C-myc proto-oncogene expression and prognosis in early carcinoma of the uterine cervix. Lancet. 1987;1(8536):761-3. |
|
Hunter RE, Longcope C, Keough P. Steroid hormone receptors in carcinoma of the cervix. Cancer. 1987;60(3):392-6. |
|
Henry RJ, Goodman JD, Godley M, Raju KS, Coffer AI, King RJ. Immunohistochemical study of cytoplasmic oestradiol receptor in normal, dysplastic and malignant cervical tissue. British journal of obstetrics and gynaecology. 1988;95(9):927-32. |
|
Gadducci A, Guerrieri ME, Greco C. Tissue biomarkers as prognostic variables of cervical cancer. Critical reviews in oncology/hematology. 2013;86(2):104-29. |
|
Susini T, Olivieri S, Molino C, Amunni G, Rapi S, Taddei G, et al. DNA ploidy is stronger than lymph node metastasis as prognostic factor in cervical carcinoma: 10-year results of a prospective study. Int J Gynecol Cancer. 2011;21(4):678-84. |
|
Strang P. Cytogenetic and cytometric analyses in squamous cell carcinoma of the uterine cervix. Int J Gynecol Pathol. 1989;8(1):54-63. |
|
Strang P, Eklund G, Stendahl U, Frankendal B. S-phase rate as a predictor of early recurrences in carcinoma of the uterine cervix. Anticancer Res. 1987;7(4B):807-10. |
|
Connor JP, Miller DS, Bauer KD, Murad TM, Rademaker AW, Lurain JR. Flow cytometric evaluation of early invasive cervical cancer. Obstet Gynecol. 1993;81(3):367-71. |
|
Kristensen GB, Kaern J, Abeler VM, Hagmar B, Trope CG, Pettersen EO. No prognostic impact of flow-cytometric measured DNA ploidy and S-phase fraction in cancer of the uterine cervix: a prospective study of 465 patients. Gynecol Oncol. 1995;57(1):79-85. |
|
Wolfson AH, Winter K, Crook W, Krishan A, Grigsby PW, Markoe AM, et al. Are increased tumor aneuploidy and heightened cell proliferation along with heterogeneity associated with patient outcome for carcinomas of the uterine cervix? A combined analysis of subjects treated in RTOG 9001 and a single-institution trial. International journal of radiation oncology, biology, physics. 2008;70(1):111-7. |
|
Dasari S, Wudayagiri R, Valluru L. Cervical cancer: Biomarkers for diagnosis and treatment. Clinica chimica acta; international journal of clinical chemistry. 2015;445:7-11. |
|
Noordhuis MG, Eijsink JJ, Roossink F, de Graeff P, Pras E, Schuuring E, et al. Prognostic cell biological markers in cervical cancer patients primarily treated with (chemo)radiation: a systematic review. International journal of radiation oncology, biology, physics. 2011;79(2):325-34. |
|
Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24 Suppl 3:S3/11-25. |
|
Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579-88. |
|
Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain JM, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16(3):175-204. |
|
U.S. Food and Drug Administration. FDA NEWS RELEASE: FDA approves first human papillomavirus test for primary cervical cancer screening. 2014 [updated 4/24/2014; cited 2015 9/8/2015]; Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm394773.htm. |
|
Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2012;156(12):880-91, W312. |
|
ACOG Practice Bulletin Number 131: Screening for cervical cancer. Obstet Gynecol. 2012;120(5):1222-38. |
|
U.S. Food and Drug Administration. FDA Licenses New Vaccine for Prevention of Cervical Cancer and Other Diseases in Females Caused by Human Papillomavirus. 2006 [updated 4/8/2013; cited 2015 9/8/2015]; Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108666.htm. |
|
U.S. Food and Drug Administration. FDA Approves New Vaccine for Prevention of Cervical Cancer. 2009 [updated 4/17/2013; cited 2015 9/8/2015]; Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm187048.htm. |
|
U.S. Food and Drug Administration. FDA approves Gardasil 9 for prevention of certain cancers caused by five additional types of HPV. 2014 [updated 12/11/2014; cited 2015 9/8/2015]; Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm426485.htm. |
|
Nicol AF, de Andrade CV, Russomano FB, Rodrigues LS, Oliveira NS, Provance DW, Jr., et al. HPV vaccines: their pathology-based discovery, benefits, and adverse effects. Annals of diagnostic pathology. 2015. |
|
Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928-43. |
|
Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Cummins E, Liu B, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206(11):1645-51. |
|
Elam-Evans LD, Yankey D, Jeyarajah J, Singleton JA, Curtis RC, MacNeil J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years--United States, 2013. MMWR Morbidity and mortality weekly report. 2014;63(29):625-33. |
|
Hariri S, Bennett NM, Niccolai LM, Schafer S, Park IU, Bloch KC, et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States - 2008-2012. Vaccine. 2015;33(13):1608-13. |
|
Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301-14. |
|
Wheeler CM, Castellsague X, Garland SM, Szarewski A, Paavonen J, Naud P, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet oncol. 2012;13(1):100-10. |
|
Wheeler CM, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16-26 years. J Infect Dis. 2009;199(7):936-44. |
|
Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis. 2009;199(7):926-35. |
|
FUTURE II Study Group. Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis. 2007;196(10):1438-46. |
|
Olsson SE, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Human vaccines. 2009;5(10):696-704. |
|
Arnheim-Dahlstrom L, Pasternak B, Svanstrom H, Sparen P, Hviid A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ. 2013;347:f5906. |
|
Macartney KK, Chiu C, Georgousakis M, Brotherton JM. Safety of human papillomavirus vaccines: a review. Drug safety. 2013;36(6):393-412. |
|
Kash N, Lee MA, Kollipara R, Downing C, Guidry J, Tyring SK. Safety and Efficacy Data on Vaccines and Immunization to Human Papillomavirus. Journal of clinical medicine. 2015;4(4):614-33. |
|
Welters MJ, Kenter GG, de Vos van Steenwijk PJ, Lowik MJ, Berends-van der Meer DM, Essahsah F, et al. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc Natl Acad Sci U S A. 2010;107(26):11895-9. |
|
Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Science translational medicine. 2012;4(155):155ra38. |
|
Bae SH, Park YJ, Park JB, Choi YS, Kim MS, Sin JI. Therapeutic synergy of human papillomavirus E7 subunit vaccines plus cisplatin in an animal tumor model: causal involvement of increased sensitivity of cisplatin-treated tumors to CTL-mediated killing in therapeutic synergy. Clin Cancer Res. 2007;13(1):341-9. |
|
Sin JI, Kim JM, Bae SH, Lee IH, Park JS, Ryoo HM. Adoptive transfer of human papillomavirus E7-specific CTL enhances tumor chemoresponse through the perforin/granzyme-mediated pathway. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17(5):906-13. |

