Tuberculosis of the Female Genital Tract
Authors
INTRODUCTION
Genital tuberculosis (TB) in females is by no means uncommon, particularly in communities where pulmonary or other forms of extragenital TB are common. TB can affect any organ in the body, can exist without any clinical manifestation, and can recur.
TB was recognized as a clinical entity as far back as 1000 BC. However, it was not until 1744 that Morgagni,1 following a postmortem examination of a 20-year-old woman who died of TB and whose uterus and fallopian tubes were found to be filled with caseous material, described the first case of genital TB. The word tuberculosis was first used in 1834, although Koch did not discover the tubercle bacilli until 1882.
TB is considered the most important communicable disease in the world. Since the beginning of the 20th century, the incidence of TB in general and genital TB more particularly has been steadily declining in developed countries. However, TB remains a major health problem in many developing countries, and in these areas, genital TB is responsible for a significant proportion of females presenting with infertility.2 TB affects almost 50% of the population in Third World countries. An estimated 30 million persons have active TB, and 7–10 million people die each year of TB.3, 4
A review of the literature reveals that the highest incidence of TB is still in India, followed by Scandinavia and Scotland. It is estimated that almost one half of the population of India has TB and that one person dies every minute from TB. The true incidence of genital TB is not known given that, owing to its subtle presentation, many cases remain undiagnosed. It has been estimated that approximately 5% of females presenting to subfertility clinics worldwide have genital TB.5 However, estimates of incidence vary tremendously based on country of origin, from less than 1% in the United States to 19% in India. In the United States, genital TB is rarely encountered, but the frequency varies considerably with geographic location and type of patients seen. The disease is more prevalent among immigrants, patients in inner-city living conditions, and personnel and long-term residents in certain chronic care institutions, such as nursing homes, mental institutions, and prisons.6, 7, 8 Reports from Scotland and Scandinavia suggest a higher incidence of genital TB.9
According to the Centers for Disease Control and Prevention, a 5% annual decrease in the incidence of TB has occurred since the 1970s. However, the increase since 1985 may be associated with an increase in acquired immunodeficiency syndrome (AIDS) and associated tuberculosis.10 In New York City, for example, TB cases increased from 1630 to 2223 (36% increase) from 1984 to 1986; during the same period, reported cases for the entire nation increased from 22,255 to 22,768 cases (2% increase).
Tripathy and Tripathy stated that genital TB is mostly a secondary manifestation of primary TB, the most common primary site being the lungs.11 They reported that the genital tract is vulnerable to this disease after puberty, and most cases occur during the childbearing period.
Female genital TB is typically understood as a disease of young women, with 80% to 90% of cases diagnosed in patients 20–40 years old, often during workup for infertility.5 More recent reports from Sweden and Scotland suggest a trend toward presentation in women in their 40s to 50s.12, 13
INCIDENCE
The actual incidence of genital TB cannot be determined accurately in any population because it is estimated that at least 11% of patients are asymptomatic and the disease is discovered incidentally.8 Incidence varies greatly according to socioeconomic and public health conditions; it usually parallels the incidence of pulmonary and abdominal TB.
The incidence of genital tract TB is 0.69% in Australia,5 0.07% in the United States,10 less than 1% in Finland,14 4.2% in Saudi Arabia,15 5.6% in Scotland,16 and 19% in India.17
Estimates of the frequency have been made on the basis of postmortem examination, operative specimens, and endometrial biopsy samples taken from patients with infertility. Autopsy studies by different authors reveal that 4–12% of women who have died of pulmonary TB also had evidence of genital TB.18 In a review published in 1976, Schaefer estimated that 5–10% of infertile females worldwide have genital TB,5 although this varies from less than 1% in the United States to nearly 13–19% in India.19 Falk and associates, in a review of newly diagnosed cases in 47 Swedish hospitals from 1968 to 1977, found an incidence of 2 cases per 10,000 gynecologic admissions.12
Francis stated that the incidence of genital TB varies not only with the prevalence of extragenital TB in the community but also with the physician’s interest in searching for the disease.20
Sivanesaratnam and coworkers reported that out of a total 39,204 gynecologic admissions during the study period, only 12 patients had genital TB, giving an incidence of 0.31 per 1000 gynecologic admissions during the 17-year period from March 1968 to February 1985 at the University Hospital, Kuala Lumpur, which acts as a major referral center in Malaysia.21 They stated that active immunization of all the newborns in Malaysia perhaps resulted in the decreased incidence of TB from 10 per 1000 in 1960 to 5.7 per 1000 in 1970, thus explaining the low incidence of pelvic TB of 0.31 per 1000 gynecologic admissions.22 Others report an incidence of 0.2–0.5 per 1000.12, 13
In a series conducted in India, 800 women with pelvic inflammatory disease were assessed; 6% of 48 cases were attributed to TB.23 Tripathy and Tripathy studied patients with a history of infertility, menstrual irregularity, and lower abdominal pain from July 1971 to February 1983.11 In 165 patients, endometrial TB was diagnosed histopathologically, with approximately 13 new cases each year.
Khilnani and colleagues reported that TB is one of the most common diseases in India.24 In cases of infertility, the incidence of genital TB is 17.4%. In women who died of pulmonary TB, postmortem studies revealed genital TB in 8%. Careful histologic examination by serial section of fallopian tubes resulted in an increased diagnosis of tubal TB from 7.7% to 20% in autopsy series.
In surgically removed adnexa, the frequency of genital TB, as reported in the examination of operative specimens, varies from 2% to 20%. The care with which excised fallopian tubes are examined histologically in different laboratories is an important and variable factor in determining the incidence of tuberculous salpingitis or endometritis.
Examination by Nogales-Ortiz and coworkers of more than 1400 pathologic specimen from patients with female genital TB removed at operation revealed that this disease had gradually decreased in their laboratory from 5.5% in the years 1950 to 1966 to about 0.27% in 1977.25
In Ibadan, Ojo and associates found that 3.5% of patients presenting with infertility had genital TB.26 Ojo and Unuigbe reported that the incidence of genital TB was 1 in 300 admissions.27 de Vynck and colleagues stated that the incidence of genital TB was 7.98% (36 of 451) in the group of women they studied between June 1986 and December 1987.28
Gini and Ikerionwu reported that the incidence of genital TB in Enugu, Nigeria, was 0.2%.29 They studied 4700 specimens of premenstrual endometrial curettings from infertile women attending the university hospital, Enugu. They found only 10 cases of tuberculous endometritis. They suggested that more deliberate and meticulous efforts should be made to search for genital TB in infertile women in developing countries. They also suggested that the lower incidence of TB found in their series might be the result of the inadequate search for disease in the genital tract. The incidence of pulmonary TB is high in Nigeria, and the low incidence of genital TB may very well be due to an inadequate search for the disease.30, 31
Marana and associates reported that the incidence of genital TB is decreasing in industrialized countries.32 Of their 101 patients with infertility associated with a tubal problem, only 2 had proven TB by both endometrial biopsy and culture, and 34 had laparoscopic findings suggestive of TB; Mycobacterium tuberculosis isolates were found only in the urine.
In 1960, the World Health Organization Expert Committee on Tuberculosis stated that TB is generally considered to be the most important specific communicable disease in the world as a whole, and its control should receive priority and emphasis. In 1900, TB was the second major cause of death in the United States. Today, TB is still one of the world’s biggest health problems. The incidence of TB is declining in America, but it still represents a massive economic burden to individuals and society, especially in the developing world. Muir and Belsey state that genital TB remains a major health problem in developing countries.2
PATHOGENESIS
Genital TB is almost always secondary to TB elsewhere in the body—usually pulmonary and sometimes renal, gastrointestinal, bone, or joint; occasionally it is part of a generalized miliary disease process. If the bacilli are not eradicated, there is a lifelong risk of reactivation, especially in conjunction with diseases or drugs that cause attenuation of T-cell response (e.g. Hodgkin’s lymphoma, AIDS, steroids, stress, or malnutrition). The mode of spread is usually hematogenous or lymphatic and occasionally occurs by way of direct contiguity with an intraabdominal or peritoneal focus.5, 33 The focus in the lung often heals, and the lesion may lie dormant in the genital tract for years, only to reactivate at a later time.
Hematogenous Spread
After tubercle bacilli invade the lung , in most cases the bacilli are disseminated by way of the bloodstream within a matter of hours and deposited in various organs of the body. This bacillemia may persist for 6 weeks or longer, if the disease is not recognized and treated promptly with antituberculous drugs. No organ or tissue of the human body is immune from the attack of the tubercle bacillus, although there are marked differences in the frequency with which different organs are infected. These differences are due to the degree to which the various organs are directly exposed to bacilli, to mechanical factors that influence the extent to which bacilli brought by way of the bloodstream will lodge in each organ, and in part to the ability of the different tissues to support the bacilli that lodge in them.
Tubercle bacilli also may reach the bloodstream and thus the genital tract from extrapulmonary and chronic pulmonary lesions. The fallopian tube forms a most favorable nidus for tubercle bacilli, with the earliest lesion found in the mucosa. The tendency of the tubercle bacillus to affect bilateral organs results in both tubes being involved in the tuberculous process. There is almost uniform initial pelvic involvement of the tubes, with subsequent dissemination to other genital organs and the peritoneum. Tuberculous peritonitis is commonly seen with genital tract involvement and may also be associated with rupture of a caseous abdominal lymph node or, less frequently, with spread from an intestinal focus.34
Lymphatic Spread
A less common mode of infection, lymphatic spread, occurs when the primary lesion is in the abdominal cavity. In some countries in which people drink raw milk (unpasteurized), infection which spreads by way of the alimentary tract and caused by the bovine tubercle bacillus is still reported. Gavaller and coworkers reported that in one area of Hungary, 33% of cases of female genital TB were due to bovine bacillus, which was spread to the fallopian tubes by way of the lymphatics.35
Direct Spread From a Neighboring Viscus
Direct extension to the genital tract organs from tuberculous abdominal viscera, such as the bladder, rectum, appendix, and intestines, has been described. Some researchers believe that this spread is along the peritoneal surface. However, peritoneal involvement can also be the result of spillage of infected material from the fallopian tubes; thus, the primary process is not always clear. It also may occur when adhesions bind the bladder or intestine to the fallopian tubes and perforation of a tuberculous ulcer results in direct spread to the genital organs.
Once the genital tract is colonized, granulomata containing viable tubercle bacilli form within various pelvic organs. After the development of tubercular hypersensitivity, these generally become clinically silent, and intervals of 1–10 years or even longer may pass before infection in this location is reactivated or becomes clinically manifest, if symptoms occur at all. Most foci are of no further significance clinically. Often, there is little or no remaining evidence of infection at the primary site once genital tract disease is established.36, 37 There is some evidence that when primary infection occurs close to the time of menarche, there is an increased likelihood of genital tract involvement.38
Most pathologists state that primary infection of the female genital organs does not occur. It is known that tuberculous foci may exist in the body and remain undetected for a long time. These lesions may precede the genital lesions and heal without leaving traces demonstrable on clinical examination. The criteria necessary for a diagnosis of primary genital TB are that (1) the genital lesions should be the first tuberculous infection in the body and (2) regional lymph nodes should demonstrate the same stage of tuberculous development as do the genital organs. Auerbach stated that no such case had ever been described in the literature he reviewed.39
There are reports of primary cervical and vulvar disease in which sexual partners have been thought to be the source of infection. This type of disease may also occur in a woman who has TB of another organ and who excretes tubercle bacilli in her stool, urine, or sputum. When these excretions come into contact with the external genitalia, TB of the vulva or vagina may result, particularly if the skin is abraded or broken. Sutherland found that of 128 women with proven TB, 5 (3.9%) of their husbands had active genitourinary TB; however, 3 of the 5 wives of these men also had evidence of TB outside the genital tract.40 Lattimer and colleagues reported the presence of M. tuberculosis in the semen of men with genitourinary TB in 1954.41
The lesions in the cervix and vagina are rare and usually present as isolated, chronic, ulcerative lesions.42 The infectious agent in TB is usually M. tuberculosis; occasionally Mycobacterium bovis may cause human disease, including genital tract infection, especially in underdeveloped countries lacking facilities for pasteurization of milk and an effective TB control program for cattle. The mycobacteria are obligate aerobes with a replicating cycle on the order of 17 –24 hours and are characterized by their acid-fast staining.
PATHOLOGY
When the tubercle bacilli infect a susceptible host, the initial reaction is a polymorphonuclear inflammatory exudate. Within 48 hours, this is replaced by mononuclear cells, which become the prime sites for intracellular tubercle replication. As cellular immunity develops, destruction of tubercle bacilli takes place and caseation necrosis occurs. Later reactivation of a focus of infection results in proliferative granulomatous lesion, classically with central caseation necrosis surrounded by concentric layers of epithelial and giant cells, with peripheral lymphocytes, monocytes, and fibroblasts.37
TUBERCULOSIS OF THE PELVIS
Pelvic TB may exist as tuberculous adenitis, of either the mesenteric or the pelvic lymph nodes, without involvement of the genital tract. Generalized miliary peritoneal TB, in which grayish white tubercles stud the abdomen, may involve the serosal surface of both abdominal and pelvic organs without penetrating to the mucosa. Such superficial lesions do not usually impair the reproductive function of the pelvic organs. It should be emphasized that pelvic TB is not the same disease as genital TB.
TUBERCULOSIS OF THE FALLOPIAN TUBES
Various sources on the topic of genital TB appear to agree that the fallopian tubes are likely the initial source of infection, because both tubes are involved in nearly 100% of cases.5, 25, 43 The fallopian tubes constitute the initial focus of genital TB in the overwhelming majority of cases (Table 1), and TB has accounted for approximately 5% of all cases of salpingitis in many areas of the world.44 In more than 90% of patients with genital TB, the tubes are involved bilaterally. Although only one tube appears infected, there probably are microscopic lesions in the other. In the early stages, the tubes show little change, but as progression occurs, the diameter of the tube becomes larger. Usually, the ampullary region shows the earliest and most extensive changes, the fimbrial processes become greatly swollen, and the ostia remain open or closed (Fig. 1). The gross appearance varies and is nondiagnostic; the tubes may appear normal or only slightly edematous but are much more likely to present a picture consistent with chronic salpingitis of a nontubercular nature.
Table 1. Frequency of tuberculosis in genital organs
Organ | Frequency (%) |
Fallopian tubes | 90–100 |
Endometrium | 50–60 |
Ovaries | 20–30 |
Cervix | 5–15 |
Vulva and vagina | 1 |
(From Schaefer G: Female genital tuberculosis. Clin Obstet Gynecol 19:23, 1976)
|
The isthmus and the adjacent interstitial portion of the tube may remain free of TB. As the process continues, the tubes become softer, and caseation develops in the inner wall. At times, the peritoneal surfaces of the tubes will be studded with tubercles, and the cross sections may show them to be filled with caseous material.25 In 25–50% of cases of genital TB, the tubes remain patent with recognizable everted fimbriae, even if the remaining tube is enlarged and distended, the so-called tobacco-pouch appearance.37, 44
MICROSCOPIC APPEARANCE
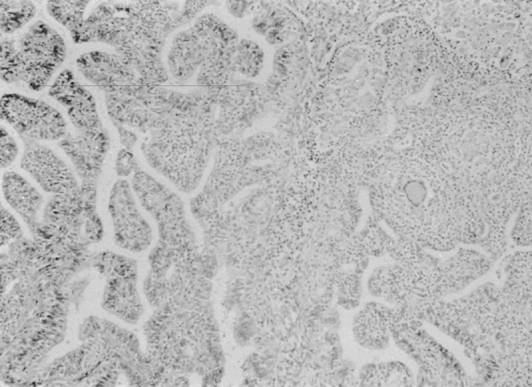
Microscopically, granulomata and a chronic inflammatory infiltrate may involve the full thickness of the tubal wall, and caseation necrosis is common in advanced states. Some tubercles have a caseous center, which, as they progress, involves the overlying mucous membrane or causes pressure atrophy. After liquefaction, the caseous foci pour their bacilli into the lumen and form an ulcer at the site. Caseation or a pyogenic membrane lines the ulcer; beyond the inner zone is an area of vascular granulation tissue containing epithelioid and giant cells. Adhesion of the individual foci may occur, resulting in large cystic spaces—pseudofollicular salpingitis. When healing occurs, the picture is further changed, and calcium deposits, hyalinization, and increased fibrous tissue may be seen. The mucosa frequently exhibits a hyperplastic, adenomatous pattern with a complex network of fused papillae that may be confused with adenocarcinoma (Fig. 2) and has been associated with ectopic pregnancies.37, 44 There is some suggestion that this pattern may actually predispose to the development of tubal adenocarcinoma, although the evidence is insufficient for statistical assessment.45 Tuberculous salpingitis may contain Schaumann bodies, which are conchoidal, laminated, calcified structures surrounded by foreign body giant cells (Fig. 3). In chronic tuberculous salpingitis, unless multiple sections are taken, the characteristic lesion may be missed.
TYPES OF TUBERCULOUS SALPINGITIS
Exudative
In the exudative type, the tube may be significantly enlarged. Although a large pyosalpinx may form, these tubes show few adhesions and usually are reasonably mobile if surgery is needed. Frequently, the organs contain a large amount of caseous material plus purulent exudate from secondary infection. This is a relatively acute phase of the process.
Productive-Adhesive
In the productive-adhesive form, which is found most frequently at laparoscopy or laparotomy, the tubes are studded with tubercles and are densely adherent to the surrounding organs. The tubercles are seen mostly near the attachment of the tube to the mesosalpinx. The tube wall is thickened and nodular, and the fimbriae and tube are slightly swollen. Eventually, when the process starts healing, it results in calcification and fibrosis.
MODE OF SPREAD FROM TUBES
After the initial involvement of the tubes, the tuberculous infection spreads to the uterus and ovaries by direct extension. Extension to the uterus is along the endometrium and rarely into the myometrium. Direct hematogenous spread to the uterus as part of a generalized hematogenous TB has rarely been reported.
The ovaries may be involved by direct spread from adjacent organs. In most cases, infection spreads from the tube, and the lesion is seen on the surface of the ovaries. Rarely, the infection extends from the peritoneum to the ovary. Hematogenous spread usually affects the center of the ovary, and the periphery appears normal.
The cervix is involved by spread from the endometrium or as part of the hematogenous infection. Tuberculous infection of the vagina and vulva may follow injury or abrasions to these structure in the presence of tubercle bacilli from the upper genital tract, intestinal tract, or lungs.
Dellepiane stated that the use of antituberculous drugs has tended to change the clinical picture of the disease, resulting in a decreasing incidence of acute forms and an increasing incidence of subacute and chronic forms.46 On the basis of 965 cases of genital TB in which the pathogenesis could be defined precisely by a series of clinical, laboratory, radiologic, and laparoscopic procedures, he described genital TB as primary in 0.2%, hematogenous in origin in 59.2%, and descending in 40.6%. The latter route is by way of the lymphatics from the lungs to the intestinal lymph nodes and the tubes.
TUBERCULOSIS OF THE ENDOMETRIUM
Grossly, the size and shape of the uterus may appear normal. The tuberculous process generally is localized to the endometrium, is most extensive in the fundus, and decreases toward the cervix. The myometrium is not usually involved. In premenopausal patients, much of the infected tissue is shed during the menstruation, only to have the endometrium reinfected from the tubes with each cycle.
In genital TB, there is a high incidence of involvement of the endometrium. Schaefer reported an incidence of 50–60%;5 Onuigbo, an incidence of 60%,47 and Nogales-Ortiz and coworkers an incidence of 79%,25 whereas Sutherland estimated 90% involvement of the endometrium in genital TB.16
In a large series of 1436 cases, Nogales-Ortiz and coworkers found 79% involvement using extensive endometrial sampling.25 Grossly, the endometrium appears unremarkable in most cases, probably because of the cyclic menstrual shedding. However, when extensive involvement of the endometrium occurs, there may be ulcerative, granular, or fungating lesions present, or the endometrial cavity may be obliterated with intrauterine adhesions. Sometimes, the macroscopic appearance may resemble carcinoma, and TB has been suggested microscopically.
In 2.5% of cases of tuberculous endometritis, Nogales-Ortiz and coworkers,25 Schenker and Margalioth,48 and Hasselgren and Bolin49 noted total destruction of the endometrium with resulting amenorrhea secondary to end-organ failure and predisposition to pyometra should the internal os becomes occluded.
MICROSCOPIC APPEARANCE
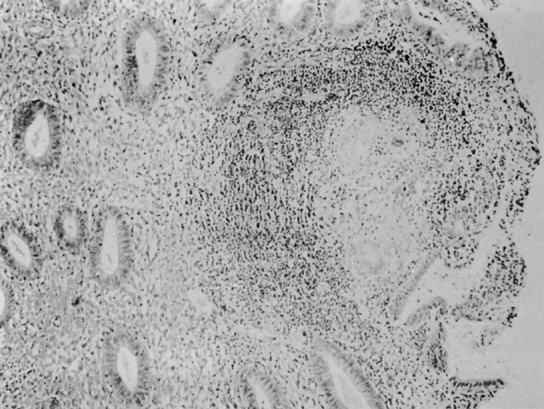
On histologic examination, the picture is one of infrequent, usually isolated, small tubercles scattered irregularly through the endometrium. In most cases, the lesions are extremely scanty, and careful search through all the sections of the endometrium removed at curettage may reveal only one or two foci of TB (Fig. 4).
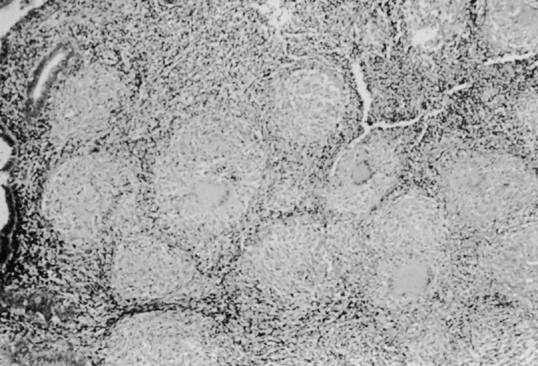
TB of the endometrium resembles TB in other tissues, but the advanced stages—caseation, fibrosis, and calcification—rarely are seen during the reproductive period of the regular cyclical shedding of the endometrium. The classic lesion in tuberculous endometritis is the noncaseating granuloma, composed of epithelial cells, Langhans giant cells, and lymphocytes. These granulomata are located throughout the endometrium but occur in greater density in the more superficial layers (Fig. 5). They occasionally perforate into gland lumina, causing an acute inflammatory reaction and giving the appearance of microabscesses. Endometrial glands adjacent to granulomata may not reveal a secretory response or may become compressed, resulting in a pseudoadenomatous appearance.25, 50, 51
|
Most often, the endometrial lesion consists of circumscribed lesions of endothelioid cells surrounded by a zone of lymphocytes and plasma cells. These inflammatory cells may be present in the stroma without focal lesions. However, the giant cells are not always indicative of tuberculous infection. Endometrial tuberculous lesions are frequently focal and immature because they tend to be shed monthly except in postmenopausal women or women with amenorrhea. Sutherland stated that the endometrium is reinfected on a regular basis from the tubes or from infections of the basalis by organisms in menstrual blood after sloughing of the superficial endometrium.52 The granulomatous lesions are usually best recognized on cycle days 24–26 or within 12 hours of the onset of menses.50
TUBERCULOSIS OF THE OVARY
There is disagreement in the literature regarding the frequency with which the ovaries are involved. Some studies estimate 20–30%,5 whereas a review of a large series of pathologic specimens found involvement in only 11% of cases.25 This is likely explained by varying definitions of involvement, because the latter source uses a stricter definition of true parenchymal granulomatous involvement.
Usually, the involvement is bilateral, although this cannot always be recognized with certainty at laparotomy. Two forms of ovarian TB are described: perioophoritis, in which the ovary may be surrounded by or encased in adhesions and studded with tubercles caused by direct extension from the tube; and oophoritis, in which infection starts in the stroma of the ovary, presumably from a hematogenous source that produces a caseating granuloma within the parenchyma.5, 18, 25
PERIOOPHORITIS
Extension of the tuberculous process from the tube involves the ovary in a tuboovarian mass, which is frequently adherent to omentum and intestines. This is the most common form of tuberculous involvement of the ovary, and the resulting lesion is that of perioophoritis, with the extension of the lesion from the periphery toward the center. The tough tunice albuginea is thought to protect the ovaries from tuberculous infection; hence, the less frequent involvement of the ovary compared with the tube.
OOPHORITIS
Oophoritis is a relatively rare condition and usually follows hematogenous spread. Typical tubercles or larger foci with caseous centers may be recognized on cross section in the hilum of the ovary. Thus, the ovary should be bisected before removal; if no caseous foci are present, the ovary may be retained, and the patient should be given antituberculous treatment postoperatively.
TUBERCULOSIS OF THE CERVIX
The cervix appears to be involved in 5–25% of cases, whereas involvement of the external genitalia occurs only rarely.53 The usual incidence of cervical involvement in genital TB is 5–15%. However, Nogales-Ortiz and Villar thought that cervical lesions were more common, especially in the endocervix, which was frequently overlooked.53
As with other parts of the female genital tract, there are no macroscopic changes in the cervix that are specific for TB. The cervix may appear normal or inflamed, and its condition may resemble invasive carcinoma, both grossly and with the colposcope.54, 55 The most common type is the ulcerative form, although papillomatous and miliary forms may also occur.
Nogales-Ortiz and coworkers stated that a velvety, polypoid appearance is seen frequently,25 whereas ulceration or destruction of surface epithelium is less common.
The diagnosis can be made with certainty only by histologic or bacteriologic examination. If bacteriologic examination of endocervical mucus was performed for tuberculous infection, as is done with infertility problems, any cases of tuberculous cervicitis could possibly be discovered.56 The cytopathologic examination of the cervix may reveal multinucleated giant cells, histiocytes, and epithelioid cells arranged in clusters, simulating the appearance of the granulomata that are characteristic of the Papanicolaou (Pap) smear in cervical TB. There may be associated epithelial atypia from which dyskaryotic cells are shed.54, 57
Histopathologic examination reveals granulomatous inflammation and sometimes marked inflammatory atypia along with frequent hyperplastic mucosal changes. Caseation may be seen. Endocervical involvement is common and usually results in an increased secretion of mucin.25 Samal and coworkers reported cervical involvement in 43.1% of the cases they studied.58
TUBERCULOSIS OF THE VULVA AND VAGINA
TB of the vulva and vagina is the rarest form of genital TB, occurring in less than 2% of cases.5, 25 In most cases, the lesions appear to be secondary to disease higher up in the genital tract but, rarely, the disease may be acquired from the male partner with an infected epididymis or seminal vesicles. In the vulva, it begins as a nodule on the labia or in the vestibular region, which breaks down and forms an irregular ragged ulcer, sometimes with sinuses discharging caseous material and pus. TB of Bartholin’s gland is rare. Rarely, a vulvar lesion presents as a hypertrophic, irregular warty growth sometimes resembling elephantiasis. A tuberculous lesion in the vagina may simulate carcinoma in its gross appearance.44, 59
The microscopic appearance is similar to TB occurring throughout the genital tract, with granulomatous inflammation tending to cause central caseation and an associated chronic inflammatory infiltrate.
TUBERCULOUS PERITONITIS
Tuberculous peritonitis is seen in combination with female genital tract TB approximately 45% of the time and is thought to be responsible for the often extensive adhesions seen in patients with pelvic TB.18 Two types of tuberculous peritonitis have been described: the plastic variety and the serous variety.
The plastic variety is less common and is characterized by tender abdominal masses and an abdomen “doughy” to palpation. The serous variety is seen more commonly and is characterized by ascitis, signs of peritoneal inflammation, fever, abdominal pain, weight loss, and anorexia. Most cases of the serous variety are insidious. Patients may be asymptomatic or may present acutely with chills, fever, ascitis, and sometimes, rebound tenderness. In the plastic variety, one may observe symptoms suggestive of partial intestinal obstruction.
With advanced diseases, all pelvic organs are densely matted together, often with tubercles studding peritoneal surface, foci of caseation, and calcified plaques, which represent attempts at healing.18, 34 The peritoneal fluid is exudative in character and generally contains 500–2000 cells, with a predominance of lymphocytes.
With peritonitis, an associated pleural effusion is not uncommon, although most patients have no parenchymal abnormalities on chest radiograph.
CLINICAL FEATURES
The clinical diagnosis of genital TB requires a high index of suspicion. About 20% of patients with genital TB give a history of TB in their immediate family.5 As a rule, they were exposed to an adult with TB during childhood. Approximately 50% of patients might have had tuberculous pleurisy, peritonitis, erythema nodosum, or renal, osseous, or pulmonary TB. A history of primary infertility in a woman in whom examination reveals no apparent cause and who gives a family history or personal history of TB should arouse suspicion of genital TB.
A history of poor general health persisting over months or years and associated with weight loss, undue fatigue, low-grade fever, or vague lower abdominal discomfort is often elicited in patients with genital TB.
According to most series, patients with genital TB will give a history of prior diagnosis or treatment of extragenital TB approximately 30–50% of the time.5, 9, 12
Age
Female genital TB is typically understood as a disease of young women, with 80% to 90% of cases diagnosed in patients 20–40 years old, often during workup for subfertility.5 Although in many developing countries, genital TB is more common among younger women, in developed countries most patients are older than 40 years.12, 13 Tripathy and Tripathy11 reported that the mean age of patients who had genital TB was 30.6 years; Ojo and Unuigbe27 reported that 80.9% of their patients were in the 20- to 39-year-old age group. Of the 1436 cases of the disease reported by Nogales-Ortiz and coworkers, 66% of patients were between 25 and 35 years of age; only 11% were postmenopausal.25 Gini and Ikerionwu reported that the age of their patients with tuberculous endometritis ranged from 23 to 30 years, with a mean age of 26.8 years.29
In 1982, Sutherland observed that the age incidence of genital TB had changed and that the proportion of patients older than 49 years was higher than in the past.40 Reports from Sweden and Scotland also suggest a trend toward presentation in women in their 40s to 50s.12, 13
Samal and coworkers reported that 65.8% of women were between 21 and 30 years of age, the youngest being 12 years and the oldest 65 years old, and 64.2% were nulliparous.58
SYMPTOMS
Systemic symptoms (Table 2) tend to be relatively mild, if present, and may include weight loss, fatigue, and a tendency toward a persistent mild evening elevation of temperature. Approximately 11% of patients are asymptomatic.5, 12, 36, 37
Table 2. Symptoms related to genital tuberculosis
Systemic
Weight loss
Fatigue
Low-grade fever
Infertility
Primary
Secondary
Menstrual disturbances
Amenorrhea
Menorrhagia
Metrorrhagia
Oligomenorrhea
Abdominal swelling
Postcoital bleeding
Vaginal discharge
Dyspareunia
In women with genital TB, four major presenting complaints are described with varying frequencies: infertility, abnormal bleeding, pelvic pain, and amenorrhea.
Infertility
The most common initial symptom is infertility.14, 20, 60, 61 Approximately 85% of patients with genital tract TB have never been pregnant; in the remaining 15%, symptoms of genital TB develop in one third to one half within 1 year after their last pregnancy was completed. Infertility is the presenting complaint in 40–50% of patients in most large series studied.33, 52, 62, 63
Sivanesaratnam and coworkers reported that the most common complaint in the group they studied was infertility.21 Tripathy and Tripathy stated that 64.2% of their patients with genital TB complained of infertility, compared with 22% in the control group.11 Ojo and Unuigbe reported that 54.4% of patients with genital TB presented with the complaint of infertility.27 de Vynck and colleagues reported that infertility is the common presenting symptom in women with genital TB.28 Dhillon and coworkers also reported that infertility was the main complaint in genital TB.4
Lower Abdominal Pain
The second most frequent complaint is lower abdominal pain or pelvic pain, present in approximately 25% to 50% of patients.12, 20, 52, 62 Usually, the pain has been present for several months before the patient sees a gynecologist. The pain is not usually severe and may be accompanied by swelling of the abdomen, although episodes of acute lower abdominal pain owing to secondary infection by pyogenic organisms may occur. The number of women who have pain as a feature of pelvic TB is proportional to the number of women with abdominal findings on physical examination.37 When progression of genital TB takes place, the pelvic pain becomes more severe and is usually aggravated by coitus, exercise, and menses.
Menstrual Disorders
The third most common symptom is some variety of menstrual problems. Abnormal uterine bleeding in genital TB has been reported in 10% to 40% of patients. Patients report menorrhagia, menometrorrhagia, intermenstrual bleeding, oligomenorrhea, and postmenopausal bleeding.36, 37
Samal and coworkers studied 120 cases of genital TB proved by histopathology. Analysis of their menstrual history showed that the common menstrual disorder oligohypomenorrhea was found in 54% of cases, menorrhagia in 19.9% of cases, and postmenopausal bleeding in 1.6% of cases. History of amenorrhea was present in 14.3% cases. There were 8 cases of secondary amenorrhea (6.6%) and 1 case was of primary amenorrhea.58 They stated that the genital TB was a disease of varied symptomatology. A high degree of suspicion and efficient investigation are important for diagnosis and adequate management.
Among women of reproductive age with genital TB, menstrual irregularities define a common form of the disorder. The menstrual cycle may be normal and undisturbed in cases of genital TB. Secretory endometrium has been found in a large number of patients. Superficial tuberculous endometritis does not interfere with the secretory response of the endometrium to hormonal stimulation.58
Disturbances of menstruation in genital TB do not follow the same pattern in different countries. Ylinen64 noted no menstrual irregularities in 56% of patients in Finland; Kirchhoff65, 66 described normal menstrual cycles in 62% of his patients in Germany; and Sutherland and Garrey67 in Scotland found no menstrual problem in 46% of their patients. In contrast, Aldea and colleagues68 found normal menstruation in only 7.3% of their patients in Romania, and Malkani and Rajani69 reported amenorrhea in 18% to 53% of their patients, depending on the part of India in which the patient lived. Tripathy and Tripathy studied the group of 165 women with histologically proven endometrial TB and compared them with a control group of 50 women without TB.11 They reported that menstrual irregularities were very common (85%) in cases of endometrial TB, among which secondary amenorrhea was the most common disorder (43.6%).
There has been some speculation on the cause of amenorrhea. It is well-known that advanced, active pulmonary TB may produce amenorrhea, particularly if it is associated with fever and weight loss. However, active pulmonary TB is rarely found concomitantly with active genital TB. Complete destruction of the ovary by genital TB seldom occurs, so that ovarian failure is not the cause of amenorrhea. The most likely explanation is that given by Malkani70 and Nogales-Ortiz and Villar,53 who attributed amenorrhea to end-organ failure secondary to endometrial caseation.
General Malaise
A history of poor general health persisting over a period of months or years and associated with weight loss, undue fatigue, low-grade fever, or vague lower abdominal pain often is elicited in patients with genital TB. Some patients gave a history of recurrent pelvic inflammatory disease that has not responded to the usual antibiotic therapy.
Other symptoms seen less frequently with pelvic TB include vaginal discharge, abdominal swelling, pelvic relaxation, and symptoms associated with fistula formation.40, 62, 71 Uterovesical, tubointestinal, and tuboperitoneal fistulas have all been described.33, 49 TB is significant because it may affect any organ in the body, may exist without manifesting clinical signs and symptoms, and may recur after being apparently arrested.72 The presence of long-standing genital TB in women without any symptoms has been reported.18, 73 Genital TB can mimic ovarian cancer.74, 75, 76, 77 These patients commonly present with adnexal masses and ascitis. To further confuse the picture, serum CA-125 levels can be elevated as well in genital TB, and diagnosis is often made only after laparotomy.74, 75, 76, 77
PHYSICAL SIGNS
Most series suggest physical examination can be normal in up to 50% of cases of female genital TB.9, 12, 43 When abnormal findings are present, they usually consist of adnexal masses or signs of ascitis.
Physical examination is important in establishing a diagnosis of genital TB (Table 3). However, it should be emphasized that no abnormal findings may be apparent or, at best, there may be only vague ones. There is little correlation between presenting complaints and physical findings in genital TB. In all, 35–50% of patients have an entirely normal examination.36, 40 In the remainder, bimanual examination often reveals an adnexal mass or fixation of pelvic organs. Tuberculous tuboovarian masses are less tender than those due to pyogenic infection, although secondary infection and acute exacerbation may produce sharp pain and tenderness. Other pelvic lesions, such as fibromyomas, ovarian cysts, and adenomyosis, as well as cervical cancer, may coexist with genital TB. The presence of bilateral tuboovarian masses in a virgin who has a history of pulmonary or extrapulmonary TB should make the clinician highly suspicious of genital TB.
Table 3. Physical signs in genital tuberculosis
Normal
Abdominal mass
Pelvic mass
Adnexal mass
Abdominal tenderness
Pelvic/adnexal tenderness
Ascites
Excessive vaginal discharge
Ulcer in the vulva, vagina, and cervix
Enlarged uterus with pyometra
Fistula
Sutherland’s large series spinning 30 years showed a significant decrease in the incidence of palpable adnexal masses, from 52% of 704 patients in the years 1951–60 to 26.5% in the years 1971–80.62 Falk and associates studied a group of 187 patients and reported that approximately 25% of the patients in their series had a palpable adnexal mass on examination, but 11% of these were found to be benign ovarian tumors or malignant lesions in the adnexa.12 Adnexal masses vary in size and in consistency and may result from thickened, edematous tubes, pyosalpinges, a conglomeration of pelvis organs matted together by adhesions, or a tubo-ovarian abscess.
A temperature greater than 38°C was seen in approximately one third of the 158 cases of Brown and associates from 1920 to 1950.78 Superimposition of acute bacterial infection, gynecologic operative procedures, or trauma to pelvic organs has been known to cause a flare-up of latent pelvic TB.79
Abdominal examination may reveal a “doughy” sensation, which has been ascribed to tubercle formation on the intestines and peritoneum. Ascitis, either general or sacculated, may produce distention of the abdomen. Tuberculous ascitis with increased intraabdominal pressure has been blamed for an occasional primary presentation such as uterine prolapse in an otherwise asymptomatic patient.71 Irregular masses caused by the matting together of intestines, omentum, and pelvic organs may be palpated. In an adolescent female presenting with ascitis, pain, and low-grade fever, the cause is frequently TB.37
In menopausal women, genital TB may cause an enlarged uterus that is tense and tender on examination, the result of pyometra formation.79, 80 Physical examination may suggest ovarian malignancy. A fistulous tract between the genital tract and the bowel, bladder, or cutaneous area may be identified. These are usually caused by rupture of a tuberculous pyosalpinx into adjacent organs. Less common findings include lesions of the cervix and external genitalia.
DIAGNOSIS
The possibility of TB infection of the genital tract should always be considered, especially in a patient from an area where TB is endemic, a patient with a family history of other exposure to TB, or a patient with some proven extragenital manifestations of the disease.
Infertility for which no obvious cause can be found, chronic pelvic inflammatory disease refractory to standard antibiotic therapy, or adnexal disease with ascitis in virgin females should alert the clinician to look for TB of the genital tract.
In most series, a history of previous diagnosis or treatment for extragenital TB is present in 25% to 50% of patients.5, 10, 13, 20, 40, 62 In Sutherland’s large series of 638 patients, 80% had a history of TB elsewhere in the body or had evidence of such a lesion on chest or abdominal radiograph or in urine culture.52 A negative chest radiograph does not rule out the diagnosis because most pulmonary lesions are arrested by the time the genital tract becomes involved in the disease process.
Routine laboratory studies are of little value; most patients have a normal white blood cell count with differential, although there is a tendency to lymphocytosis. Anemia is sometimes seen, whereas microscopic examination of urine may show hematuria or abacteriuric pyuria if there is concomitant urinary tract involvement.38
Laboratory Investigations
The diagnosis of TB is based on the identification of M. tuberculosis or others of the M. tuberculosis complex—M. bovis, M. africanum, and M. microti—in culture. Isolation of mycobacteria from clinical specimens in pure cultures represents a challenge because of the prolonged period of cultivation required for most of them. Cultivation of M. tuberculosis requires special culture media. The most popular are Löwenstein-Jensen or other egg-based solid media, Middlebrook 7H10/7H11 agar, Middlebrook 7H9 broth, and Middlebrook 7H12 broth that is manufactured as radiolabeled BACTEC (Becton-Dickinson Diagnostic Instrument Systems, Sparks, MD) medium.81
The egg-based media are the most traditional ones for the isolation of M. tuberculosis, although it takes 3–8 weeks to achieve visible growth. Growth on agar-based media can be detected within 3 weeks for most M. tuberculosis isolates. The BACTEC 7H12 broth can be used for rapid detection of growth, often within 1 week for most isolates. Specimens are usually collected in sterile containers with liquid in the case of tissue (7H9 broth or saline) and transported to the laboratory in dry ice.
Investigations to confirm the diagnosis of genital TB are listed in Table 4.
Table 4. Investigations to confirm genital tuberculosis
Complete blood count
Chest radiographs
Tuberculin test
Menstrual blood for culture
Endometrial curettage
Histologic examination
Culture for Mycobacterium tuberculosis
Peritoneal fluid for culture
Peritoneal biopsy for culture and histology
Hysterosalpingography
Ultrasonography
Cervical cytology
Endoscopy
Laparoscopy
Hysteroscopy
Cystoscopy
Other serologic tests
Chest Radiograph
Given that genital TB is believed to be secondary to primary pulmonary infection in most cases, it seems intuitive that a chest radiograph might be helpful in evaluating these patients. Series that looked specifically at this issue found chest radiograph abnormalities in only 10–50% of cases.13, 14, 15, 43, 82 Most of the abnormalities found were suggestive of prior pulmonary TB, whereas active pulmonary TB in association with genital TB was rare.13, 15, 82 Signs of earlier pleurisy are of particular interest because pleural effusion in young people may be caused by TB.
Tuberculin Test
The tuberculin skin test has been the traditional method of demonstrating infection with M. tuberculosis.7 Although the currently available tuberculin skin tests are substantially less than 100% sensitive and specific for the detection of infection with M. tuberculosis, no better diagnostic methods have yet been devised. The test is based on the fact that infection with M. tuberculosis produces sensitivity to certain antigenic components of the organism that are contained in culture extracts called tuberculin. Two preparations of tuberculin are currently licensed for use in the United States: old tuberculin (OT) and purified protein derivative (PPD)
The reaction to intracutaneously injected tuberculin is the classic example of a delayed (cellular) hypersensitivity reaction. Characteristically, this reaction begins at 5– 6 hours, is maximal at 48–72 hours, and subsides over a period of days. Unfortunately, not all patients infected with M. tuberculosis have a reaction to the tuberculin skin test. The typical method of performing the tuberculin test is the intracutaneous, or Mantoux, method. Here 0.1 mL of PPD is injected intracutaneously into the dorsal or volar surface of the forearm. A discrete wheal should be produced. The test is read between 48 and 72 hours after the injection. The basis of reading is the presence or absence of induration. The diameter of the induration should be measured transversely to the long axis of the forearm and recorded in millimeters. The interpretation of the test should be influenced by the purpose for which the test was given and by the consequences of false classification.
A reaction of greater than or equal to 5 mm is classified as positive in patients with human immunodeficiency virus (HIV) infection or those with risk factors for HIV infection who have an unknown HIV status, in patients who have had recent close contact with infectious TB, and in those who have chest radiographs consistent with old healed TB. A reaction of greater than or equal to 10 mm is classified as positive in persons who do not meet the aforementioned criteria but who have other risk factors for TB. A reaction of greater than or equal to 15 mm is classified as positive in all other groups of patients.
Menstrual Blood Analysis
Definitive diagnosis of TB requires isolation of tubercle bacilli via culture, although diagnosis based on histologically characteristic granulomata is accepted by most authorities. Most experts recommend some form of endometrial sampling for histologic and microbiologic examination to make the diagnosis of genital TB. Because the endometrium is involved in the majority of cases and is readily accessible to sampling, it is often the first site at which attempts at definitive diagnosis are directed.
Bacteriologic examination of menstrual blood with acid-fast bacilli (AFB) smear and culture is recommended by some; however, sensitivity of these tests is quite low.5 Simon and coworkers reported that menstrual blood collected within 12 hours of the onset of menses and cultured was found to be positive for M. tuberculosis in 10% of cases.36 There are acid-fast organisms other than M. tuberculosis, and these may occasionally be mistaken for tubercle bacilli. de Vynck and colleagues advocate menstrual blood for culture to identify M. tuberculosis using Löwenstein-Jensen medium.28 They identified 36 positive cultures of 451 cultures tested, and subsequent laparoscopic examination revealed bilateral tubal block in 3 and peritubal adhesion in 17 (47.2%); in the remaining 16 (44.5%), the pelvis was considered normal. They stated that radiologic examination of the chest and histologic examination of the endometrium showed no evidence of infection.
ENDOMETRIAL CURETTAGE.
Endometrial biopsy is a frequent first diagnostic step, and although its sensitivity suffers from sampling errors, it can be very helpful if granulomata are found or, less commonly, if smears or cultures are positive for M. tuberculosis.12, 15
A definitive diagnosis of TB requires isolation of tubercle bacilli, although many authorities accept a diagnosis based on histologic examination, which confirms granulomata.20, 37 Although the newer culture techniques have increased the growth rate of the organism, it still takes 40 days or more for 75% of all positive cultures to show growth.6 An AFB stain is rapid and relatively simple and could be used initially to make the diagnosis while waiting for culture results to be available; however, it requires 10 organisms/mL for a positive test.
In investigating the possibility of genital tract TB, the most accessible tissue for study with a high frequency of involvement is the endometrium. The histologic examination of endometrial tissues removed by biopsy or curettage, especially from the cornual area, affords a rapid method of diagnosing genital TB in at least 50% of cases. AFB stain and culture and, occasionally, guinea pig inoculation, yield better microbiologic assessment. The optimal time for sampling is at the end of the menstrual cycle or within 12 hours after the onset of menstrual flow to allow the endometrial granulomata maximal time to develop. One negative biopsy or one negative curettage does not exclude the diagnosis of genital TB. Czernobilsky stated that serial sections needed to be studied because the lesions are frequently patchy.50 Falk and associates stated that a positive endometrial culture for TB was found only in 25% of cases of tuberculous endometritis.12 Dilation and curettage may increase the yield on endometrial specimens merely by increasing the amount of tissue available for histologic evaluation and cultures.
The demonstration of tuberculous endometritis may be assumed to indicate tuberculous salpingitis in practically 100% of cases. Negative endometrial evaluation may be the result of sampling error, or the disease may be in other genital organs without an associated tuberculous endometritis. Sometimes, the disease is diagnosed or suspected only at exploratory laparotomy with subsequent positive culture for tubercle bacilli or histologic confirmation. AFB culture or stain of peritoneal fluid shows variable results, but the sensitivity can be increased by peritoneal biopsy for culture, stain, and histologic evaluation. Laparoscopy with directed biopsies of suspicious areas may be helpful if less invasive methods fail to provide the needed diagnostic information.
Tissue has been obtained with percutaneous needle techniques, peritoneoscopy, culdoscopy, and laparoscopy, but all carry a significant risk of bowel injury secondary to a closed approach in the presence of dense adhesive disease. A small open biopsy can obtain tissue more safely.34, 36, 52
RADIOGRAPHY
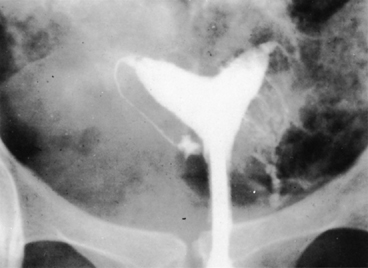
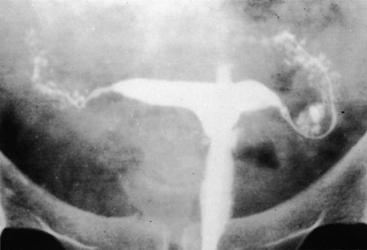
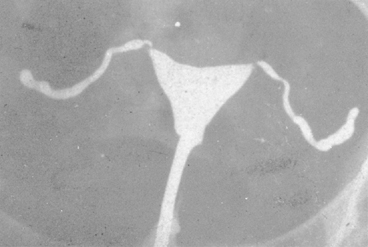
No characteristic radiographic features are pathognomonic for genital tract TB, although certain findings should raise suspicion of its possibility. Barter and associates reported that an abdominal film may show calcified pelvic and abdominal lymph nodes, a characteristic and recognized sequela of healed genital tract TBs.83 Hysterosalpingography may reveal certain abnormalities that suggest the possibility of pelvic TB. The uterine cavity is classically shriveled and deformed, with associated intrauterine adhesions and lymphatic extravasation (Fig. 6). The fallopian tubes often show ragged outlines with multiple strictures, giving a beaded appearance (Fig. 7); in some patients, the entire tube appears rigid and may exhibit small terminal sacculations of the ampullary end (Fig. 8). Fistulous tracts between the genital tract and other pelvic organs may be identified. Occlusion of the digital end of the fallopian tubes is common, although marked hydrosalpinx is usually uncommon (Fig. 9), Calcification of the organs may be visualized.84,85 If a water-soluble contrast medium is used and the usual precautions are observed, complications can be minimized. Hysterosalpingography is contraindicated in the presence of recent acute pelvic infection, and many reports described exacerbation of pelvic TB following the procedure.86
ULTRASONOGRAPHY
High-resolution abdominal and transvaginal ultrasonography may demonstrate loculated ascitis; bilateral, predominantly solid, adnexal masses containing scattered small calcification; thickened omentum; thickened peritoneum; and endometrial involvement, which might alert the clinician to suspect genital tract TB.87,88
CERVICAL CYTOLOGY
Khilnani and colleagues reported that when the cervical cytologic smear reveals the presence of clusters of epithelioid cells, it may be suggestive of a tuberculous lesion of the cervix.24 Angrish and Verma,57 Bhambani and coworkers,54 and Misch and associates89 also reported that the cytologic examination of the smear taken from the cervix may be useful in identifying cervical TB.
ENDOSCOPY
Merchant used endoscopy (laparoscopy, cystoscopy, hysteroscopy) in evaluating women for pelvic TB.90 Among 687 patients who underwent laparoscopy, pelvic TB was suspected in 101 (14.7%) from the appearance alone. Definitive evidence was found in 70 cases.
de Vynck and colleagues also used laparoscopy to diagnose and to confirm genital tract TB.28
RAPID MOLECULAR TECHNIQUES
Older microbiologic methods for diagnosis of M. tuberculosis that relied on cultures and conventional biochemical tests often took several weeks to yield definitive results. Given the importance of rapid diagnosis of TB for both an individual and at public health level, more rapid diagnostic techniques were needed. The development of broth-based culture media, available now as a component of automated, continuously monitored systems, has decreased average detection time for positive M. tuberculosis cultures to 9–14 days.91 The more recent arrival of molecular methods that can reliably identify M. tuberculosis has accelerated this process even further.
POLYMERASE CHAIN REACTION
The DNA probes primarily save time by providing a rapid alternative to the longer conventional identification process. Since 1987, DNA probes complementary to species-specific sequences of rRNA have been commercially available for the identification of M. tuberculosis complex, Mycobacterium avium complex, and others. These single-stranded DNA probes, labeled with acridinium ester, hybridize with complementary rRNA sequences of the target organisms. DNA-rRNA hybrids are captured by antibodies specific for them, and via signal amplification techniques, these enzyme-conjugated antibodies are then detected by a chemiluminescent substrate that emits a light measured by a luminometer.91
The concept of amplification of discrete fragments of bacterial DNA or RNA created a new opportunity for making the nucleic acid probe hybridization method highly sensitive and opened the possibility of detection and identification of mycobacteria directly in the patient’s specimen. Polymerase chain reaction (PCR) was first suggested for the detection of M. tuberculosis. The ability of the test to create a 1 billion-fold amplification from each copy of target DNA meant that fewer than 10 input molecules of target DNA could lead to a positive signal. Thus, diagnosis using the raw specimen could be completed in just a few days or perhaps a few hours after arrival of the specimen.
Based on the species in question and the number of mycobacterial organisms in the sample tested, these techniques have shown varying sensitivities of 47% to 100% for M. tuberculosis and 78% to 100% for M. avium complex.92 Specificity is thought to be high although, notably, specimens containing blood can lead to false-positive results owing to nonspecific chemiluminescence in this situation. This produces a potential problem given the bodily fluids cultured, such as bone marrow, blood, and blood-tinged sputum.91
Unfortunately, in reality, these expectations have not yet been fully realized. The insufficient sensitivities of the test have meant that these tests are limited to smear-positive sputum specimens only and they are not designated for specimens other than sputum. However, amplification techniques can be used for broth cultures at the earliest possible detection of growth. Under these conditions, diagnosis may be obtained within 7–10 days with a sensitivity and specificity of 100%.
NUCLEIC ACID AMPLIFICATION TECHNIQUES FOR TUBERCULOSIS
During the 1990s, several nucleic acid amplification (NAA) techniques evolved that dramatically altered the way in which we can detect and identify M. tuberculosis. Unlike the signal amplification technique, which relies on large numbers of organisms for detection and, thus, is useful only for cultured isolates, NAA techniques allow for detection of M. tuberculosis from samples containing relatively few M. tuberculosis bacilli. For this reason, NAA techniques can be utilized to identify M. tuberculosis directly from clinical specimens, avoiding the most time-consuming aspect of M. tuberculosis identification, the time required to culture the isolate.
In the mid-1990s, the United States Food and Drug Administration (FDA) approved two direct NAA tests for identification of M. tuberculosis complex in respiratory specimens that were smear-positive for AFB: the Gen-Probe M. tuberculosis Direct (MTD) test and the Roche Amplicor Mycobacterium Tuberculosis Test.93 The Amplicor test is a PCR-based test that amplifies M. tuberculosis DNA, whereas the Gen-Probe MTD test is a transcription-mediated amplification test that amplifies M. tuberculosis rRNA. The Gen-Probe MTD test takes only 3 hours to perform.
When used for AFB smear-positive respiratory samples, the Gen-Probe MTD test is quite reliable, with high sensitivity (83% to 100%), positive predictive value (94–100%), and negative predictive value (96–100%) reported in the literature.94, 95, 96 This high level of accuracy allows for early identification of AFB as either M. tuberculosis or nontuberculous mycobacteria (NTM), and treatment/perfection control decisions can be based on this information.
When used in AFB smear-negative respiratory samples, early studies suggested diminished reliability of this test with sensitivity of 50% and positive predictive value of 3–50%, although specificity remained high.93 More recent data with a new, enhanced MTD test (MTD2) suggest that even in AFB smear-negative respiratory samples, sensitivity and specificity approach 100%.96 Many studies suggest that the Gen-Probe MTD test is also useful in assessing extrapulmonary specimens with high sensitivity (84–100%), specificity (98–100%), and positive predictive value (90–100%).94, 95, 96, 97, 98
These new, rapid, highly sensitive molecular techniques do not solve all the problems detecting M. tuberculosis. Like any test, NAA assays are not perfect and should be used only in conjunction with traditional culture isolation methods to maximize sensitivity of the laboratory diagnosis of TB. Current NAA technology allows for the detection of M. tuberculosis in patient specimens within hours compared with the 14–28 days required for culture. The potential clinical benefits of this early detection, for both an individual and at public health level, are unimaginable.
SEROLOGIC DIAGNOSIS
Many research laboratories have shown that enzyme-linked immunosorbent assay (ELIZA) measurement of the immunoglobulin G (IgG) antibody to mycobacterial antigens can be used for the serologic diagnosis of tuberculosis. Limited data suggest that ELISA serologic diagnosis may be useful for diagnosis of extrapulmonary TB. Other serodiagnostic techniques such as radioimmunoassay (RIA), inhibition of monoclonal antibodies, and latex agglutination have had less extensive study but appear promising.7
DRUG SENSITIVITY TEST
Detection of drug resistance in a timely manner is one of the most important tasks in the proper management of TB patients. The decision about whether to perform drug sensitivity testing depends on the initial assessment of clinical and epidemiologic factors. Initial susceptibility testing should be done in persons known to be at high risk. These include patients with a history of antituberculous chemotherapy; patients in a geographic region in which there is a high prevalence of drug resistance, such as Asia, Africa, and Latin America; and contacts of known or suspected resistant cases.
DIFFERENTIAL DIAGNOSIS
Granulomatous lesion other than TB are sarcoid, Crohn’s disease, actinomycosis, leprosy, granuloma inguinale, lymphogranuloma venereum, syphilis, histoplasmosis, brucellosis, berylliosis, silicosis, tularemia, and foreign body reaction. Schistosomiasis and filariasis may directly damage the fallopian tubes and produce granulomata and may predispose to chronic infection.2,6,45,99,100,101
Conditions such as acute and chronic bacterial pelvic infection should also be considered when there is ascitis and peritonitis complicating genital TB. Other conditions that produce a similar clinical picture, such as hepatitis, cholecystitis, appendicitis, ovarian cancer, and renal and cardiac diseases, should be excluded.6,34
COMPLICATION OF GENITAL TUBERCULOSIS
Subfertility or Sterility
Despite effective therapeutic regimens for genital TB, sterility remains a major complication. Medical regimens have been successful at alleviating symptoms of menstrual disorders and pain, whereas follow-up endometrial sampling often reveals cure with an appropriate drug regimen. However, even in patients considered to be “cured,” extensive damage to the fallopian tubes and the endometrium is often irreversible, and chances of successful intrauterine pregnancy drop significantly. One extensive review of the literature published in 1976 described only 31 cases of successful pregnancy out of nearly 7000 cases of genital TB.5 Of 187 cases of genital TB in Sweden over 1968–77, no intrauterine pregnancies occurred after therapy.12 Somewhat more optimistic data came from a review of 710 cases of genital TB in Scotland, of which there had been 58 intrauterine pregnancies (35 healthy babies, 21 abortions, and 2 neonatal deaths) by the time of publication.82 Obviously, none of these series utilized the recent medical regimens considered to be standard of care for the treatment of extrapulmonary TB today.
Ectopic Pregnancy
The damage to the fallopian tubes can be extensive and irreparable if genital TB is not diagnosed and treated early in its course. After medical treatment, the risk of ectopic pregnancy in patients with pelvic TB is estimated to be 33–72%.43
Congenital Tuberculosis
A rare but potentially serious complication of female genital TB is congenital TB. Considering that genital TB causes sterility, cases of congenital TB involving transmission from maternal tuberculous endometrium to the fetus are rare, with only some 300 cases reported in the literature.102 Congenital TB can be an overwhelming systemic infection in the newborn and has considerable morbidity and mortality if untreated.102, 103.
MANAGEMENT
Once the diagnosis of genital tract TB is confirmed, it is important to rule out TB in other parts of the body. A chest radiograph and three early-morning sputum or gastric aspirate samples, or early morning urine samples for AFB stain and culture and intravenous urogram, are recommended.104 Daly and Monif reported that 10% of females with genital tract TB also show evidence of renal TB.37
To plan effective treatment, the gynecologist must consider the following: (1) Is active TB present elsewhere? (2) What is the extent of the genital tract lesion? (3) Will medical management cure genital TB? (4) When is surgical management needed? (5) Is pregnancy possible after treatment?
The presence of active extragenital foci of TB, as a rule, is rare when the genital lesion is discovered. The extent of genital lesion may be divided into minimal and advanced. Minimal genital TB is usually asymptomatic except for sterility. Examination of the pelvis may not reveal any abnormality. It is usually diagnosed from the bacteriologic or histologic examination of the endometrium or from the bacteriologic examination of the menstrual blood. In advanced genital TB, palpable tubo-ovarian masses are present. Histologic and bacteriologic examination of the endometrium or menstrual blood confirms the diagnosis of TB.
TREATMENT
In the past, irradiation, natural sun light, vitamins, sanatorium care and, for pelvic tuberculosis in particular, pelvic diathermy and injection of oxygen intraperitoneally, have all been tried. Before the advent of effective chemotherapy, surgery was the mainstay of treatment of genital tract TB, and postoperative complications such as bowel fistula (14%) and mortality from the primary disease (2.2%) were high.105
Unfortunately, there are sparse, prospective, controlled clinical studies regarding treatment of female genital TB, a gap that is found in the broader context of extrapulmonary TB. The small amount of data, however, suggest that extrapulmonary TB can be very effectively treated with some standard short-course regimen.106 Experts suggest that extrapulmonary TB may be even easier to treat than pulmonary TB owing to the decreased concentration of organisms in these lesions and the increased accessibility of many of the sites for antituberculous medication.
The advent of antituberculous drugs has revolutionized the management of this disease. If surgical intervention is needed, chemotherapy makes this approach safer, easier, and more effective. Three basic principles have emerged in the years following the advent of chemotherapy for TB:
- The regimen for treatment must contain multiple drugs to which the organism is susceptible.
- The drugs should be taken regularly.
- The drug therapy should continue for a sufficient period of time.
The treatment of extrapulmonary TB, including genital tract TB, is the same as the treatment of pulmonary TB (Tables 5 and 6). Thus, the current standards in the treatment of tuberculosis are:
- A 6-month regimen consisting of isoniazid (INH), rifampin (RIF) and pyrazinamide (PZA) for 2 months, followed by INH and RIF for 4 months, is the preferred treatment for patients with a fully susceptible organism who adhere to treatment. Ethambutol (EMB) or streptomycin (SM) should be included in the initial regimen until the results of drug sensitivity studies are available, unless there is little possibility of drug resistance. This four-drug, 6-month regimen is effective even when the infecting organism is resistant to INH. This recommendation applies to both HIV-infected patients and those who are noninfected with HIV. However, in the presence of HIV infection, the clinical course should be closely monitored, and treatment should be prolonged if the course is determined to be slow or suboptimal.
- A 9-month regimen of INH and RIF is acceptable in patients who cannot tolerate PZA. EMB or SM should be included until the drug susceptibility studies are available, unless there is little possibility for drug resistance. Consideration should be given to treating all patients with directly observed therapy (DOT)
- The major determinant of the outcome of treatment is patient adherence to the drug regimen. Virtually all treatment regimens may be given intermittently if directly observed, thus ensuring adherence.
Table 5. Drug regimens for genital tuberculosis
Initial therapy
INH + RIF + PZA daily for 2 months
Add EMB or SM where there is a high rate of resistance until drug susceptibility data are known
Continuation phase
INH + RIF daily for 4−10 months (total 6−12 months)
Add pyridoxine 25−50 mg daily to regimens that include INH
EMB, ethambutol; INH, isoniazid; PZA, pyrazinamide; RIF, rifampin; SM, streptomycin.
Table6. Dosage of antituberculous drugs
Dose (mg/kg)* | |||||
Regimen | INH | RIF | PZA | EMB | SM |
Daily | 5 (300) | 10 (600) | 15–30 (2000) | 15–25 | 15 (1000) |
2x/wk (DOT) | 15 (900) | 10 (600) | 50–70 (4000) | 50 | 25–30 (1500) |
3x/wk (DOT) | 15 (900) | 10 (600) | 50–70 (4000) | 25–30 | 25–30 (1500) |
DOT, directly observed therapy; EMB, ethambutol; INH, isoniazid; PZA, pyrazanimide; RIF, rifampin; SM, stretomycin.
*Maximum daily dose is in parentheses.
Dutt and Stead, based on their study of 478 patients with extrapulmonary TB including 65 patients with genitourinary TB from 1978 to 1987, concluded that treatment with short-course antituberculous chemotherapy—consisting of INH 300 mg orally 4 times a day and RIF 600 mg orally 4 times a day for 1 month, followed by INH 900 mg and RIF 600 mg twice a week for another 8 months—was successful in 95% of their cases.106
Arora and colleagues investigated the efficacy of short-course chemotherapy on the specific problem related to genital TB in a small prospective trial.107 Forty-one patients with proven genital TB were given SM/INH/RIF/PZA for 2 months, followed by INH/RIF for another 7 months. Thirty-two of these patients completed the full 9 months of therapy and, of these patients, 78% were considered cured, defined as both symptomatic and histologic microbiologic resolution.107
A similar outcome was achieved by Jindal and associates in a small prospective study of 14 female patients with genital TB who were treated with daily RIF 450 mg and INH 300 mg for 9 months. Follow-up endometrial biopsies were negative in all patients treated with this regimen. Three out of 14 patients (21%) conceived after starting therapy.108
Drugs in Current Use
ISONIAZID.
INH is the most widely used antituberculous agent.109 It is bactericidal, relatively nontoxic, easily administered, and inexpensive. Absorption from the gut is nearly complete, and it is highly active against M. tuberculosis. The usual dose is 3–5 mg/kg body weight. Hepatitis is the major toxic effect and is increased with increasing age to 65 years.110, 111 Alcohol consumption is also identified as a risk cofactor.112, 113 Peripheral neuropathy, most likely due to interference with pyridoxine metabolism, is associated with INH administration but is uncommon at a dose of 5 mg/kg. In patients at risk for peripheral neuropathy, concomitant pyridoxine administration is recommended, and it is also recommended in patients with seizure disorders and in pregnant women.
RIFAMPIN.
RIF is bactericidal to M. tuberculosis, relatively nontoxic, and easily administered. It is rapidly absorbed from the gut. It penetrates well into tissues and cells. The most common adverse effect of RIF is gastrointestinal upset. Other reactions include skin eruptions, hepatitis, and rarely, thrombocytopenia and cholestatic jaundice. RIF induces hepatic microsomal enzymes and may accelerate clearance of drugs metabolized by the liver.114 These include methadone, warfarin derivatives, glucocorticoids, estrogens, oral hypoglycemic agents, digoxin, antiarrhythmic agents, theophylline, anticonvulsants, ketoconazole, and cyclosporine. Because RIF interferes with estrogen metabolism, it can lower the effectiveness of oral contraceptives. In adults, intermittent administration of RIF in doses larger than 10 mg/kg may be associated with thrombocytopenia, influenza-like syndrome, hemolytic anemia, and acute renal failure. RIF is excreted in urine, tears, sweat, and other fluids, and it colors them orange. Patients should be advised of this discoloration and also of the possibility of permanent discoloration of soft contact lenses.
PYRAZINAMIDE.
PZA is bactericidal against M. tuberculosis in an acidic environment and is active against organisms in macrophages. Absorption from the gastrointestinal tract is nearly complete. PZA penetrates well into most tissues. The most important side-effect of this drug is hepatotoxicity. However, there does not appear to be an increase in hepatotoxicity when PZA is added to a regimen of INH and RIF in the initial 2 months of therapy. Hyperuricemia appears frequently, but acute gout is rare.34, 112 Skin rash and gastrointestinal irritation are also seen.
ETHAMBUTOL.
EMB in usual doses is generally considered to have a bacteriostatic effect on M. tuberculosis but may have a bactericidal effect when used in higher doses in intermittent therapy. The drug is easily administered and has a low frequency of adverse effects. It accumulates in patients with renal insufficiency. Retrobulbar neuritis is the most frequent and serious adverse effect of EMB. Symptoms include blurring of vision, central scotomata, and red-green colorblindness.112 The complication is dose-related and occurs in less than 1% of patients receiving a daily dose of 15 mg/kg, increases with a daily dose of 25 mg/kg, and is increased in patients with renal insufficiency.
STREPTOMYCIN.
SM is bactericidal in an alkaline environment and is given parenterally. Excretion of the drug is almost entirely renal. It has good tissue penetration. The most common serious adverse effect of SM is ototoxicity.115 This usually results in vertigo, but hearing loss may also occur. Nephrotoxicity may also occasionally occur. A total cumulative dose of more than 120 g should not be given unless other therapeutic options are not available.
OTHER DRUGS USED AGAINST MYCOBACTERIUM TUBERCULOSIS.
Other drugs that have been used against M. tuberculosis and are currently being used, especially in multidrug-resistant disease, are para-aminosalicylic acid (PAS), cycloserine, capreomycin, kanamycin, thiacetazone, amikacin, ciprofloxacin, and ofloxacin. Ciprofloxacin and ofloxacin are not licensed for the treatment of TB.
SUCCESS RATES AND FOLLOW-UPS.
Malkani and Rajani used weekly biopsies to follow a series of 30 patients receiving antituberculous drugs for the treatment of tuberculous endometriosis.69 From the 4th week, these patients showed an increasing number of histologically negative endometria, and after 12 weeks, the endometria in all 30 were negative by biopsy. According to Kardos, tuberculous endometritis may be considered “cured” as seen on histologic examination by using antimicrobial therapy of 3 months' duration, although he noted the recurrence of endometritis within 4–5 years if the fallopian tubes were not removed.116 Schaefer reviewed 387 cases of tuberculous endometritis treated for periods as long as 4 months.117 Twenty-two percent showed disease recurrence within 3 years. To determine the effect of antituberculous drug therapy on the fallopian tubes, which may be the cause of disease recurrence, and to arrive at the optimal duration of therapy, Schaefer treated a series of patients with genital TB for periods varying from 6 weeks to 3 years before removing the fallopian tubes.117 In patients with minimal genital TB, no evidence of active TB was found in the tubes after 10 months of therapy.
Schaefer subsequently advocated a regimen of treatment using INH 300 mg daily, and EMB 20 mg/kg or approximately 1200 mg/day.72 At 6 months and 12 months, endometrial curettings or biopsies are examined bacteriologically and microscopically. If, during the course of treatment, the endometrial curettings become positive or tubo-ovarian masses appear, RIF in a dose of 600 mg daily is added for 3 months, and the patient is advised to have surgery. Schaefer advocated treatment using INH and EMB for at least 2 years if there were no complications.117 Subsequently, his patients continued to receive INH for an indefinite period.
For advanced disease, Schaefer advocated INH and EMB for a period of 3–4 months, and if the tubo-ovarian mass still persisted, he advised surgery followed by antimicrobial therapy.117
In general, over 95% of patients treated for TB for the first time, using a combined drug regimen, undergo successful treatment if they complete the prescribed drug course. Most patients become noninfectious very rapidly, usually within 2 weeks.115 In patients who have had previous chemotherapy, who have had contact with a INH-resistant case, or who acquired their infection in an area with a high prevalence of resistant organisms, such as Asia, Latin America, or Africa, drug resistance should be suspected and drug sensitivity tests should be performed. Careful compliance is the most important factor in preventing the development of drug resistance.34 Corticosteroids have been proposed by some as an adjuvant therapy in the management of TB and may ameliorate certain aspects of the inflammatory response involved in the disease. Elsbach and Edsall stated that there was no evidence of an adverse effect on the course of the disease when chemotherapy was adequate.118
Patient response to treatment should be monitored by doing endometrial curettage 6 and 12 months after the initiation of treatment. Patients need to be followed closely for an indefinite period. Recurrence or dissemination to other organs is rare but occasionally occurs. Chest radiographs, urine cultures for M. tuberculosis, and uterine curettage should be repeated at the end of the treatment course, with a repeat curettage or endometrial biopsy every 6–12 months. Sutherland suggested that patients needed follow-up assessment for several years.40, 52
Surgical Treatment
The role of surgery in the treatment of genital TB in modern times is clearly as adjunctive therapy. Given the excellent results that can be obtained by treating extrapulmonary TB with antituberculous chemotherapy, surgery has become the back-up plan in most cases. Surgery is advocated only if there is no other choice of treatment.
Indications for surgical intervention in the management of pelvic TB include (1) persistent and recurrent disease despite adequate treatment; (2) persistent or recurrent pelvic masses after 6 months of adequate therapy; (3) persistent or recurrent symptoms such as pelvic pain and abnormal bleeding; (4) a persistent nonhealing fistula; (5) multidrug-resistant disease; and (6) concomitant genital tract neoplasia or other pathology.5, 9, 36, 37, 40, 119
When surgery is advocated, the patient should be given chemotherapy for at least 1–2 weeks preoperatively. Surgery should be performed at midcycle in premenopausal patients, and chemotherapy should be continued for 6–12 months postoperatively. Under antituberculous treatment, surgery is technically much less difficult, and morbidity and mortality are significantly reduced. Sutherland reported on 77 cases in which surgery was undertaken during antituberculous drug treatment; no patient developed a fistula, and there was no mortality.119 Late complications were infrequent.
When TB is first diagnosed postoperatively after histologic examination, antituberculous treatment is given immediately and continued for 6–12 months.
The operation of choice is total abdominal hysterectomy with bilateral salpingo-oopherectomy followed by hormone replacement therapy, especially in a premenopausal woman. If the patient is premenopausal and the ovaries look normal, they may be conserved.
GENITAL TUBERCULOSIS AND PREGNANCY
Ylinen stated that the prognosis for infertility seems nearly universal because of the difficulty of making an early diagnosis of genital TB.64 Those who report successful pregnancies attribute them to early treatment with antituberculous drugs and, in some cases, to the use of these drugs combined with steroid therapy. Halbrecht used antituberculous drugs and steroids for 4 months.120 Following this regimen, 3 patients of 42 became pregnant, 2 had intrauterine pregnancies, and 1 had an ectopic pregnancy. Of the 12 patients in this group who underwent tubal surgery, none conceived. It is estimated that if one or both fallopian tubes are patent at the start of treatment, the patient has a 50% chance of conceiving and delivering a child. Schaefer reviewed more than 7000 cases of genital TB from the literature.121 Among these, 155 women achieved full-term pregnancies, 67 had miscarriages, and 125 had ectopic pregnancies. Over 50% of the women who achieved full-term pregnancies were not adequately documented. In only 31 of 155 patients was there definite evidence of tuberculous infection, as assessed on the basis of histologic and bacteriologic evidence. Schaefer stated that although pregnancy may follow proved minimal TB in some instances, patients with advanced disease should be considered infertile.121
As previously noted, the most common presenting complaint for a woman with genital TB is infertility. A history of a previous pregnancy makes it more likely for the disease to have been acquired later in life.
Despite the advances in chemotherapeutic treatment, pregnancy after a diagnosis of genital tract TB is rare, and when it does occur, it is more likely to be an ectopic pregnancy or to result in spontaneous abortion.122 If an early diagnosis is made and adequate therapy is given immediately, a more favorable outcome may be expected.123 Sutherland reported the results of treatment of 206 women with genital TB with SM, PAS, and INH for 18–24 months.124 In 19 patients, surgery was needed because of failure of medical treatment; 45 pregnancies subsequently occurred in 26 patients—11 were ectopic and 11 resulted in miscarriage, but there were 23 live births in 14 women, for a posttreatment fertility rate of 6.7%. Tubal and endometrial damage usually prevents a normal conception and implantation, even though the fallopian tubes are patent. Tripathy and Tripathy reported that 2 of 165 women with genital TB had full-term normal deliveries and 3 had miscarriages after antituberculous treatment for 18 months.11 Ojo and Unuigbe reported that none of the 22 patients with proven genital TB conceived after antituberculous treatment.27 Sutherland, in a review of 710 cases, reported 84 pregnancies with only 35 healthy babies, 2 neonatal deaths, 21 miscarriages, and 26 ectopic pregnancies.82
Merchant treated 101 patients who were thought to have genital TB. Seventy had definitive evidence of tuberculosis.90 All 101 patients were treated. Eleven women (15%) had intrauterine pregnancy; 9 delivered normally, and 2 underwent medical termination of pregnancy. Three patients had tubal pregnancies (4%).
de Vynck and colleagues reported that of their 34 patients treated for TB, 13 (38.2%) became pregnant.28 Seven of the pregnancies were spontaneous, 5 patients became pregnant after gamete intrafallopian transfer (GIFT), and 1 patient achieved pregnancy by in vitro fertilization (IVF). Two abortions occurred, 1 in the spontaneous group, and the patient who underwent IVF treatment had a preterm delivery at 26 weeks’ gestation because of preeclampsia and multiple pregnancy. Two patients became pregnant before treatment started.
Tubal surgery has a poor prognosis. If it is to be attempted, the patient should be treated with a prolonged course of chemotherapy and the disease arrested for at least 18 months before surgery.33 Corticosteroid therapy has been tried without much success.120
Falk and associates published a retrospective study of 187 patients with a diagnosis of genital TB who were treated between 1968 and 1988.12 All their patients had chemotherapy, and 101 also had surgery. In 65.3%, the diagnosis was not established preoperatively and the patient underwent surgery for reasons other than known TB. At the end, only 139 case records contained adequate informatio: 105 patients (76%) became free of symptoms, and there were no intrauterine pregnancies, but there were 4 tubal pregnancies (3 of 4 occurred in the same patient).
Punnonen and coworkers stated that infertility was one of the main symptoms of female genital TB.14 They studied six patients with proven TB. All the patients had antituberculous therapy; four conceived, but only two had full-term deliveries.
Frydman and colleagues reported on 22 women with proven genital tract TB who presented with infertility and who underwent IVF and embryo transfer (ET) in 49 pregnancy attempts.125 Six patients conceived and had babies. The authors stated that IVF and ET probably offered a better chance of conception for those patients who had genital TB.
Soussis and colleagues described their attempts at IVF and ET in 13 patients with 21 treatment cycles in histologically documented genital tract TB. Six intrauterine pregnancies resulted (28.6% success rate).126 Gurgan and coworkers evaluated the outcomes of IVF and ET in patients with proven genital tract TB compared with patients without the disease. They concluded that patients with genital TB had higher basal follicle-stimulating hormone levels, required more exogenous gonadotropins, reached lower peak estradiol levels, and yielded fewer oocytes and embryos when compared with patients who had tubal factors but no tuberculous lesion. Furthermore, in women with genital TB, the clinical pregnancy rate per cycle was lower, and the spontaneous abortion rate was higher.127
In a recent publication, Namavar and associates studied retrospectively 3088 cases of TB from 1989 to 1999; 46 women were diagnosed to have genital TB.128 The diagnoses in 41 cases were based on the pathologic criteria of tissue specimens. The mean age of the patients at the time of diagnosis was 30.4 years; 17.07% presented with abdominal or pelvic pain; and 7 underwent surgery, 3 for abdominal mass, 4 for tuboovarian abscess. In 31 cases (75.6%), TB was diagnosed during investigations performed to evaluate the cause of their infertility; the most common procedure was endometrial curettage (25 cases). Female genital TB accounted for 1.32% of all tuberculous patients in this study. Of these, 75.6% were infertile. Tuberculous endometritis was detected in 72.01%, tubal involvement in 34.03%, ovarian lesion in 12.9%, and cervical lesion in 2.4% of the patients. This study confirms the strong relationship between genital TB and infertility. The possibility of this condition should be considered in the evaluation of every infertile patient in areas in which TB is endemic.
Despite the rather dismal prognosis concerning successful pregnancy outcome in a woman who has had genital TBs, the possibility of this disease should be considered in an infertile patient for whom no etiology for infertility can be discovered. If the diagnosis is confirmed, conservative treatment is recommended. Conception does occasionally occur, but the pregnancy must be monitored carefully for possibility of an ectopic pregnancy or miscarriage. For this group of women, IVF with ET is an option to achieve pregnancy. The success rate following such assisted reproductive technique is not as good that in women who have no genital tract TB.
PERINATAL OUTCOME OF PREGNANCY FOLLOWING TREATMENT FOR GENITAL TUBERCULOSIS
Figueroa-Damian and Arredondo-Garcia studied the impact of TB on perinatal outcome in a cohort of 25 women with TB treated at the National Institute of Perinatology in Mexico City from March 1990 to September 1995.129 They were compared with a cohort of normal pregnant women matched by age, gestational age, and socioeconomic status. Nine women started treatment either before or at the beginning of pregnancy; the remaining 16 started the treatment for TB in the second or third trimester of gestation. Thirteen women (52%) had pulmonary TB, 7 (28%) had renal lesions, and the remaining 5 had lesions elsewhere. Obstetric morbidity and neonatal mortality were significantly high in the group with TB who started the treatment late in pregnancy. Perinatal morbidity was similar in pregnant women who received treatment early in pregnancy compared with the control group.
The prevalence of TB, especially extrapulmonary TB, is increasing worldwide. However, we know little about the outcome of pregnancy. Jana and coworkers studied the course of pregnancy and labor and the perinatal outcome in these women and their infants.130 From 1983 to 1993, Jana and coworkers studied 33 pregnant women who had extrapulmonary TB: 12 had lymphadenitis; 9 had intestinal TB; and 7 had skeletal lesions, 2 with renal and 1 with endometrial lesion. Of the 33, 29 received antituberculous treatment during pregnancy. The outcome was compared with 132 healthy pregnant women without TB who were matched for age, parity, and socioeconomic status.
Tuberculous adenitis did not affect the course of pregnancy or labor or the perinatal outcome compared with the control group: 21 women with other extrapulmonary lesions had higher rates of antenatal hospitalization (24% versus 2%) and infants with low Apgar scores (less than or equal to 6) and low-birth-weight (less than 2500 g) infants (33% versus 11%). Extrapulmonary TB lesions other than lymph adenitis are associated with adverse outcome following pregnancy and childbirth.
Yip and colleagues stated that TB of the endometrium is usually associated with infertility. IVF and ET offer the only realistic treatment of infertility associated with genital TB.131 Yip and colleagues reported one case of spontaneous conception with a normal pregnancy outcome in a patient with M. tuberculosis of the endometrium. A 34-year-old woman who had 10 years of infertility was investigated. The patient underwent diagnostic laparoscopy, chromohydrotubation, and endometrial biopsy, which showed patency of both tubes and normal pelvic organs with no evidence of endometriosis or previous pelvic inflammatory disease. There was nothing in her history to suggest that she had the risk of genital TB. Histologic examination of the endometrium showed no granulomata; however, culture of the endometrium yielded M. tuberculosis.
When she was called back for antituberculous chemotherapy, she was found to be just pregnant following the biopsy. Antituberculous chemotherapy was commenced at 14 weeks’ gestation with RIF, INH, PZA, and pyridoxin. The pregnancy remained uneventful, and the patient delivered a normal female infant weighing 3380 g at term. This is an extremely rare, successful event, but the standard management still remains IVF and ET for this group of women.
Figueroa-Damian and Arredondo-Garcia stated that the incidence of TB has increased worldwide.132 It is considered that pregnant women will acquire this infection more frequently. M. tuberculosis infection during pregnancy may represent a risk for maternal and neonatal complications. These authors studied the perinatal events of 35 consecutive pregnancies complicated by TB from March 1990 to June 1998; 105 apparently healthy pregnant women were included as controls, matched in age, gestational age on arrival to the clinic, and socioeconomic status. Relative risk (RR) with 95% confidence interval (CI) was calculated, and a stratified analysis was also performed.
Seventeen (48.5%) tuberculous mothers had a pulmonary TB and 18 (51.5%) had an extrapulmonary TB. The neonatal morbidity rate in children born to women with TB was 23% against 3.8% of the children of the control group (p < 0.05). The average weight of newborn infants in the TB group was 2859 ± 78.5 g, whereas the average weight of the infants in the control group was 3099 ± 484 g (p = 0.03). The incidence of premature birth, perinatal death, and weight at birth less than 2500 g was higher in the TB group.
An active vaccination program with vigilant suspicion for TB, especially in endemic areas, will help our future generations.
ACKNOWLEDGMENT
I would like to express my gratitude to Dr. Nandita Thakkar for helping me with the references.
REFERENCES
Morgagni JB: De Sedibus et Causis meorboram. Epistola 38:34, 1744 |
|
Muir DG, Belsey MA: Pelvic inflammatory disease and its consequences in the developing world. Am J Obstet Gynecol 138:913, 1980 |
|
Ellner JJ: Tuberculosis. In Kelley A (ed): Textbook of Internal Medicine. p 1569, Philadelphia, JB Lippincott, 1989 |
|
Dhillon SS, Gosewehr JA, Julian TM, et al: Genital tuberculosis: Case report and literature review. WMJ 89:14, 1990 |
|
Schaefer G: Female genital tuberculosis. Clin Obstet Gynecol 19:223, 1976 |
|
Alvarez S, McCabe WR: Extrapulmonary tuberculosis revisited: A review of experience at Boston City and other hospitals. Medicine 63:25, 1984 |
|
American Thoracic Society: Diagnostic standards and classification of tuberculosis. Am Rev Respir Dis 142:725, 1990 |
|
Goldin AG, Baker WT: Tuberculosis of the female genital tract. J Ky Med Assoc 83:75, 1985 |
|
Varma TR: Genital tuberculosis and subsequent fertility. Int J Gynaecol Obstet 35:1, 1991 |
|
Klein TA, Richmond JA, Mishell DR: Pelvic tuberculosis. Obstet Gynecol 48:99, 1976 |
|
Tripathy SN, Tripathy SN: Endometrial tuberculosis. J Indian Med Assoc 85:136, 1987 |
|
Falk V, Ludviksson K, Agren G: Genital tuberculosis in women: Analysis of 187 newly diagnosed cases from 47 Swedish hospitals during the ten-year period 1968 to 1977. Am J Obstet Gynecol 138:933, 1980 |
|
Hutchins CJ: Tuberculosis of the female genital tract: A changing picture. Br J Obstet Gynaecol 84:534, 1977 |
|
Punnonen R, Kilholma P, Meurman L: Female genital tuberculosis and consequent infertility. Int J Fertil 28:235, 1983 |
|
Chattopadhyay SK, Sengupta BS, Edress YB, et al: The pattern of female genital tuberculosis in Riyadh, Saudi Arabia. Br J Obstet Gynaecol 93:363, 1986 |
|
Sutherland AM: Genital tuberculosis in women. Am J Obstet Gynecol 79:486, 1960 |
|
Bateman BG, Nunley WC, Kitchin JD, et al: Genital tuberculosis in reproductive age women. J Reprod Med 31:287, 1986 |
|
Schaefer G: Tuberculosis of the female genital tract. Clin Obstet Gynecol 13:965, 1970 |
|
Krishna UR, Sathe A V, Mehta H, et al: Tubal factors in sterility. J Obstet Gynecol India 29:663, 1979 |
|
Francis WJA: Female genital tuberculosis. J Obstet Gynaecol Br Commonw 71:418, 1964 |
|
Sivanesaratnam V, Lim BH, Sivanesan S, Menon A.: Pelvic tuberculosis: An uncommon gynaecological problem in Malaysia. J Trop Med Hyg 89:167, 1986 |
|
Prathap G: The National Tuberculosis Control Program. In The 1983 Annual Report of the National Tuberculosis Centre, p 25. Malaysia 1984 |
|
Shah HN, Patel S, Nagpol S: Pelvic inflammation: A study of 800 cases. J Obstet Gynecol India 28:429, 1978 |
|
Khilnani PH, Pandit AA, Krishna UR: Cytology as an aid in the diagnosis of genital tuberculosis. J Post Grad Med 34:100, 1988 |
|
Nogales-Ortiz F, Tarancion I, Nogales FF Jr: The pathology of female genital tuberculosis: A 31-year study of 1436 cases. Obstet Gynecol 53:422, 1979 |
|
Ojo OA, Onifade A, Bunnerman RHO: The pattern of female genital tuberculosis in Ibadan. Isr J Med Sci 61:365, 1968 |
|
Ojo VA, Unuigbe JA: Genital tuberculosis at the University of Benin Teaching Hospital: A nine year review. Cent Afr J Med 33:129, 1987 |
|
De Vynck WE, Kruger TF, Joubert JJ, et al: Genital tuberculosis associated with female infertility in the western Cape. S Afr Med J 77:630, 1990 |
|
Gini PC, Ikerionwu SE: Incidental tuberculous endometritis in premenstrual curetting from infertile women in Eastern Nigeria. Int J Gynecol Obstet 31:141, 1990 |
|
Dada BAA: Reported cases and deaths from notifiable diseases. Medical Statistics, 1971–1980. Federal Republic of Nigeria 1981 |
|
Lawson JB, Sultana HM: Pregnancy and pulmonary tuberculosis in West Africa. J Obstet Gynaecol Br Commonw 69:956, 1962 |
|
Marana R, Muzii L, Lucisano A, et al: Incidence of genital tuberculosis in infertile patients submitted to diagnostic laparoscopy: Recent experience in an Italian University hospital. Int J Fertil 36:104, 1991 |
|
Siegler AM, Kontopoulous V: Female genital tuberculosis and the role of hysterosalpingography. Semin Roentgenol 14:295, 1979 |
|
DesPrez RM, Goodwin RA: Mycobacterium tuberculosis. In Mandell GL, Douglas RG, Bennett JE (eds): Principles and Practices of Infectious Diseases. p 1383, 2nd ed.. New York, John Wiley & Sons, 1985 |
|
Gavaller J, Suranyi S, Berencsi G: Neue Gesichttpunkte in der Klinik der Genital tuberkulose. Zentralbl Gynakol 78:496, 1956 |
|
Simon HB, Weinstein AJ, Pasternak MS, et al: Genitourinary tuberculosis: Clinical features in a general hospital population. Am J Med 63:410, 1977 |
|
Daly JW, Monif GRG: Mycobacteria. In Monif GRG (ed): Infectious Diseases in Obstetrics and Gynaecology. p 301, 2nd ed.. Philadelphia, Harper & Row, 1982 |
|
Burnie JC: The age of onset of genital tuberculosis in women. J Obstet Gynaecol Br Emp 63:96, 1956 |
|
Auerbach O: Tuberculosis of the female genital organs. Surg Gynecol Obstet 75:712, 1942 |
|
Sutherland AM: Postmenopausal tuberculosis of the female genital tract. Obstet Gynecol 59:549, 1982 |
|
Lattimer JK, Colmore HP, Sanger G, et al: Transmission of genital tuberculosis from husband and wife via the semen. Am Rev Tuberc 69:618, 1954 |
|
Miller JW, et al: Vulval tuberculosis. Tubercle 60:173, 1979 |
|
Saracoglu OF, et al: Pelvic Tuberculosis. Int J Gynaecol Obstet 37:115, 1992 |
|
Novak ER, Woodruff JD: Novak’s Gynecologic and Obstetric Pathology. p 328, 8th ed.. Philadelphia, WB Saunders, 1979: |
|
Vinall PS, Buxton N, Cowen PN: Primary carcinoma of the fallopian tube associated with tuberculosis salpingitis: A case report. Br J Obstet Gynaecol 86:984, 1979 |
|
Dellepiane G: The pathogenesis of tuberculosis of the female genital organs. In Rippmann ET, Wenner RS (eds): Latent Female Genital Tuberculosis. pp 16, 22 Basel, S Karger, 1966 |
|
Onuigbo WIB: Genital tuberculosis and reproductive function. J Reprod Med 21:249, 1979 |
|
Schenker JG, Margalioth EJ: Intrauterine adhesions: An updated appraisal. Fertil Steril 37:593, 1982 |
|
Hasselgren PO, Bolin T: Postmenopausal tuberculous pyometra. Acta Obstet Gynecol Scand 56:23, 1977 |
|
Czernobilsky B: Endometritis and infertility. Fertil Steril 30:119, 1978 |
|
Macintosh OC, Saxon RD: Endometritis due to Mycobacterium tuberculosis. Can Med Assoc J 133:667, 1985 |
|
Sutherland AM: Tuberculosis: Gynaecological tuberculosis. Br J Hosp Med 22:569, 1979 |
|
Nogales-Ortiz F, Villar E: Diagnosis and treatment of tuberculous cervicitis. Feburtshinfe Frauenheilkd 17:677, 1957 |
|
Bhambani S, Das DK, Singh V, et al: Cervical tuberculosis with carcinoma in situ: A cytodiagnosis (letter). Acta Cytol 29:87, 1985 |
|
Kobayashi-Kawata T, Haramik C: Tuberculous cervicitis. Acta Cytol 22:193, 1978 |
|
Hok TT, Leon LK, Tijiat NT, et al: The isolation of tubercle bacilli from endocervical mucus in infertile women. Am J Obstet Gynecol 99:397, 1967 |
|
Angrish K, Verma K: Cytologic detection of tuberculosis of uterine cervix. Acta Cytol 25:160, 1981 |
|
Samal S, Gupta U, Agarwal P: Menstrual disorders in genital tuberculosis. J Indian Med Assoc 98:2000 |
|
Battacharya P: Hypertrophic tuberculosis of the vulva. Obstet Gynecol 51:215, 1978 |
|
Govan ADT: Tuberculous endometritis. J Pathol Bacteriol 83:363, 1962 |
|
Russel PMG, Jackson MH, Midgley RL: A personal study of forty cases of pelvic tuberculosis. J Obstet Gynaecol Br Emp 58:712, 1951 |
|
Sutherland AM: The changing pattern of tuberculosis of the female genital tract: A thirty year survey. Arch Gynecol 234:95, 1983 |
|
Bazax-Malik G, Maheshwari B, Lal N: Tuberculous endometritis: A clinicopathological study of 1000 cases. Br J Obstet Gynaecol 90:84, 1983 |
|
Ylinen O: Genital tuberculosis in women: Clinical experience of 348 proved cases. Acta Obstet Gynecol Scand 40:1, 1961 |
|
Kirchoff H: Diagnosis of female genital tuberculosis from menstrual blood. Geburtshilfe Frauenheilkd 8:690, 1951 |
|
Kirchoff H: Die Genital tuberkulose der Frau (Pathogenese, Klinik, Diagnose and Therapie). Arch Gynaekol 186:278, 1955 |
|
Sutherland A, Garrey M: Female genital tuberculosis: A twenty-year clinical survey. Glasgow Med J 32:231, 1951 |
|
Aldea A, Filipescu I, Cristea A: Die Genital tuberkulose und die Menstruation. Z Geburtshilfe Gynaekol 153:150, 1969 |
|
Malkani PK, Rajani C: Endometrial tuberculosis. Indian J Med Sci 8:684, 1954 |
|
Malkani PK: Epidemiology of genital tuberculosis in India. In Rippman ET, Wenner R (eds): Latent Genital Tuberculosis. pp 27, 35 Basal, S Karger, 1966 |
|
Montz FJ, Dizerega GS: Genital tuberculosis in an elderly woman with primary symptoms of pelvic prolapse: Case report. Am J Obstet Gynecol 152:42, 1985 |
|
Schaefer G: Tuberculosis of the female genital tract, Vol 1. In Sciarra J (ed): Gynecology and Obstetrics. Hagerstown, MD, Harper & Row, 1981 |
|
Jeffcoate TNA: Principles of Gynaecology. 4th ed.. London, Butterworth, 1975 |
|
Sheth SS: Elevated CA 125 in advanced abdominal or pelvic tuberculosis. Int J Gynecol Obstet 52:167, 1996 |
|
Irwin WP, et al: Abdominal tuberculosis mimicking metastatic ovarian CA. Obstet Gynecol 92:709, 1998 |
|
Gurgen T. et al: Pelvic-peritoneal TB with elevated serum and peritoneal fluid CA-125 levels. Gynecol Obstet Invest 53:60, 1993 |
|
Sinha P: Pelvic tuberculosis: An uncommon gynaecological problem presenting as ovarian mass. Br J Obstet. Gynecol 107:139, 2000 |
|
Brown AB, Gilbert CRA, TeLinde RW: Pelvic tuberculosis. Obstet Gynecol 2:476, 1953 |
|
Schaefer G, Marcus RS, Kramer EE: Postmenopausal endometrial tuberculosis. Am J Obstet Gynecol 112:681, 1972 |
|
Lee J, Warner L, Khaleghian R: Sonographic features of tuberculous endometritis. J Clin Ultrasound 11:331, 1983 |
|
Heifets L: Mycobacteriology laboratory. Clin Chest Med 18:35, 1997 |
|
Sutherland AM: Surgical treatment of tuberculosis of the female genital tract. Br. J. Obstet Gynaecol 87:610, 1980 |
|
Barter JF, Addison WA, Rosenberg ER, et al: Calcific pelvic lymphadenopathy presenting as a postmenopausal adnexal mass: A case report. J Reprod Med 29:209, 1984 |
|
Siegler AM: Hysterosalpingography. Fertil Steril 40:139, 1983 |
|
Deutsch AL, Gosnik BB: Non-neoplastic gynecologic disorders. Semin Roentgenol 17:269, 1982 |
|
Winifred JAF: Female genital tuberculosis. J Obstet Gynaecol Br Commonw 71:418, 1977 |
|
Walzer A, Koenigsberg M: Ultrasonographic demonstration of pelvic tuberculosis. J Ultrasound Med 2:139, 1983 |
|
Yapar EG, Ekici E, Karasahin E, et al: Sonographic features of tuberculous peritonitis with female genital tract tuberculosis. Ultrasound Obstet Gynecol 6:121, 1995 |
|
Misch KA, Smithies A, Twomey D, et al: Tuberculosis of the cervix: Cytology as an aid to diagnosis. J Clin Pathol 29:3131, 1976 |
|
Merchant R: Endoscopy in the diagnosis of genital tuberculosis. J Reprod Med 34:468, 1989 |
|
Murray P, Baron E, Pfaller M: Manual of Clinical Microbiology, p 410. 1999 |
|
Badak FZ, et al: Use of nucleic acid probes for identification of M. tuberculosis directly from MD/Bact bottles J Clin Microbiol 37:1602, 1999 |
|
Catanzaro A, et al: ATS workshop: Rapid diagnostic tests for tuberculosis. Am J Respir Crit Care Med 155:1804, 1997 |
|
Gamboa F, et al: Comparative evaluation of initial and new versions of the Gen-Probe Amplified Mycobacterium Tuberculosis direct test for direct detection of M. tuberculosis in respiratory and nonrespiratory specimens J Clin Microbiol 33:2699, 1998 |
|
Vlaspolder F, et al: Diagnostic value of an Amplification Method (Gen-Probe) compared with that of culture for diagnosis of tuberculosis. J Clin Microbiol 33:2699, 1995 |
|
Chedore Pn, Jamieson FB: Routine use of the Gen-probe MTD2 Amplification Test for detection of M. tuberculosis in clinical specimens in a large public mycobacteriology laboratory Diagn Microbiol Infect Dis 35:185, 1999 |
|
Gamboa F, et al: Direct Detection of M. tuberculosis complex in nonrespiratory specimens by Gen-Probe Amplified Mycobacterium Tuberculosis Direct Test J Clin Microbiol 35:307, 1997 |
|
Ehlers S, Ignatius R, Regnath T, et al: Diagnosis of extrapulmonary tuberculosis by Gen-Probe amplified Mycobacterium tuberculosis direct test. J Clin Microbiol 34:2275, 1996 |
|
Skehan M, McKenna P: Sarcoidosis of the endometrium: A case presentation. Ir J Med Sci 154:114, 1985 |
|
Brooks JJ, Wheeler JE: Granulomatous salpingitis secondary to Crohn’s disease. Obstet Gynecol 49:31, 1977 |
|
Schaefer G: Tuberculosis of the genital organs. Am J Obstet Gynecol 91:714, 1965 |
|
Lee LH, et al: Congenital tuberculosis in a neonatal ICU. Case report, Epidemiological investigation and management of exposures Clin Infect Dis 27:474, 1998 |
|
Spark RP, et al: Perinatal tuberculosis and its public health impact: A case report. Tex Med 92:50, 1996 |
|
American Thoracic Society: Diagnostic standards and classification of tuberculosis and other mycobacterial disease. Am Rev Respir Dis 123:343, 1981 |
|
Jedberg H: A study on genital tuberculosis in women. Acta Obstet Gynecol Scand 31:170, 1950 |
|
Dutt AK, Stead WW: Treatment of extrapulmonary tuberculosis. Semin Respir Infect 4:225, 1989 |
|
Arora R, et al: Prospective analysis of short course chemotherapy in female genital tuberculosis. Int J Gynecol Obstet 38:311, 1992 |
|
Jindal UN, et al: Short course chemotherapy for endometrial tuberculosis in infertile women. Int J Gynecol Obstet 32:75, 1990 |
|
Anderson JR: Novak’s Textbook of Gynecology, 11th edn. Baltimore, Williams & Wilkins, 1988: 557, 569 |
|
Black M, Mitchell JR, Zimmerman HJ, et al: Isoniazid associated hepatitis in 114 patients. Gastroenterology 69:289, 1975 |
|
Byrd RB, Horn BR, Briggs GA, et al: Isoniazid chemoprophylaxis: Association with detection and incidence of liver toxicity. Arch Intern Med 137:1130, 1977 |
|
American Thoracic Society: Treatment of tuberculosis and tuberculosis infection in adults and children. Am Rev Respir Dis 134:355, 1986 |
|
Kopanoff DE, Snider DE Jr, Caras GJ: Isoniazid-related hepatitis: A US Public Health Service cooperative surveillance study. Am Rev Respir Dis 117:991, 1978 |
|
Skolnick JL, Stoler BS, Katz DB, et al: Rifampin, oral contraceptives, and pregnancy. JAMA 236:1382, 1976 |
|
American Thoracic Society: Control of tuberculosis. Am Rev Respir Dis 128:336, 1983 |
|
Kardos F: Surgical experiences in 200 cases of genital tuberculosis in women submitted to sanatorial treatment. In Rippman ET, Wenner RS (eds): Latent Female Genital Tuberculosis. pp 181, 188 Basel, S Karger, 1966 |
|
Schaefer G: Antimicrobial treatment of tuberculous salpingitis. Am J Obstet Gynecol 77:996, 1959 |
|
Elsbach P, Edsall JR: ACTH and cortisone as adjuncts in the treatment of advanced pulmonary tuberculosis. Ann Intern Med 46:332, 1957 |
|
Sutherland AM: Surgical treatment of tuberculosis of the female genital tract. Br J Obstet Gynaecol 87:610, 1980 |
|
Halbrecht I: Cortisone in the treatment of tubal occlusion caused by healed genital tuberculosis. Fertil Steril 13:371, 1962 |
|
Schaefer G: Full term pregnancy following genital tuberculosis. Obstet Gynecol Surv 19:81, 1964 |
|
Stallworthy J: Fertility and genital tuberculosis. Fertil Steril 14:284, 1963 |
|
O’Herlihy C: Early successful pregnancy following tuberculous endometritis. Acta Obstet Gynecol Scand 58:57, 1979 |
|
Sutherland AM: The treatment of tuberculosis of the female genital tract with streptomycin, PAS, and isoniazid. Tubercle 57:137, 1976 |
|
Frydman R, Eibscheitz I, Belsaisch-Allart JC, et al: In vitro fertilization in tuberculous infertility. J In Vitro Fert Embryo Transfer 2:184, 1985 |
|
Soussis I, Trew G, Matalliotakis I, et al: In vitro fertilization treatment in genital tuberculosis. J Assist Reprod Genet 15:378, 1998 |
|
Gurgan T, Urman B, Yarali H: Results of in vitro fertilization and embryo transfer in women with infertility due to genital tuberculosis. Fertil Steril 65:367, 1996 |
|
Namavar Jahromi B, Parsanezhad ME, Ghane Shiraz R: Female genital tuberculosis and infertility. Int J Gynecol Obstet 75:269, 2001 |
|
Figueroa-Damian R, Arredondo-Garcia JL: Pregnancy and tuberculosis, influence of treatment on perinatal outcome:. Am J Perinatol 15:303, 1998 |
|
Jana N, Vasishta K, Saha SC, et al: Obstetrical outcomes among women with extrapulmonary tuberculosis. N Engl J Med 26:645, 1999 |
|
Yip SK, Wong SP, Fung TY, et al: Unassisted conception with a normal pregnancy outcome in a woman with active Mycobacterium tuberculosis infection of the endometrium—A case report. J Reprod Med 44:974, 1999 |
|
Figueroa-Damian R, Arredondo-Garcia JL: Neonatal outcome of children born to women with tuberculosis. Arch Med Res 32:66, 2001 |