Common Anorectal Problems
Authors
INTRODUCTION
Women frequently describe symptoms of anorectal disease to their gynecologist. These symptoms may coexist with pregnancy or pelvic floor disorders, or may occur independently. The most common symptoms are pain and bleeding with defecation, but may also include itching, drainage, and fullness from a mass (e.g., external hemorrhoid). These are most often attributed to benign disorders (e.g., hemorrhoids, fissures, abscesses), and a careful history and physical examination will help identify the source. Diet and lifestyle changes can ameliorate symptoms from fissures and hemorrhoids, but the patient with persistent symptoms or a more complicated entity may require evaluation and possibly surgical intervention by a specialist.
ANATOMY
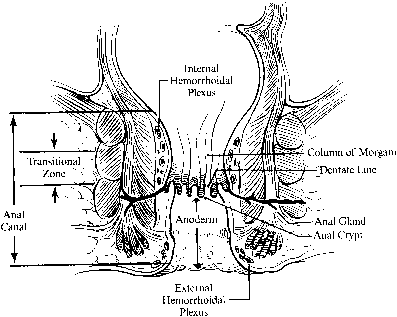
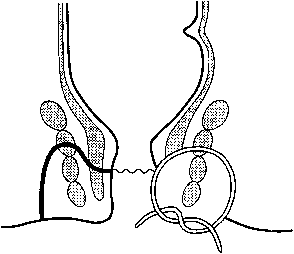
The anal canal is approximately 4 cm long and embryologically originates from proctoderm fused with the rectum (derived from the hindgut). Its caudal-most margin is the anal verge, marked by corrugated skin. The lower 2 cm of the anal canal is lined by the anoderm, a thin stratified squamous epithelium that lacks hair follicles, sweat or sebaceous glands. Approximately 2 cm above the anal verge is the dentate line, where anal valves and crypts house primitive anal ductal glands. Longitudinal folds of mucosa just above the dentate line form the columns of Morgagni. A transitional zone of cuboidal cells approximately 1 cm above the dentate line marks the vergence with the columnar mucosa of the rectum (Fig. 1).
|
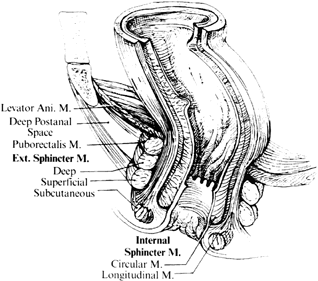
The anal sphincter is composed of two muscles. The internal sphincter is involuntary and is a continuation of the circular smooth muscle of the rectum. The external sphincter is a continuation of the striated muscle of the puborectalis muscle. The anorectal ring is a muscular structure at the junction of the anal canal and the rectum. It includes the puborectalis sling and upper portions of the internal and external sphincter (Fig. 2). Division of the anorectal ring results in incontinence. Blood is supplied to the anorectum via the superior hemorrhoidal artery (branch of inferior mesenteric artery), middle hemorrhoidals (branch of hypogastrics), and inferior hemorrhoidals (branch of pudendal artery). The anoderm and perianal skin are the only areas with somatic innervation. The upper anal canal and rectum are insensate.
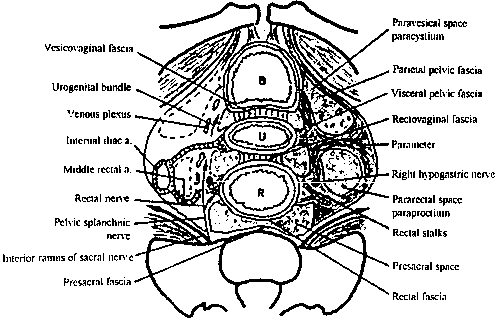
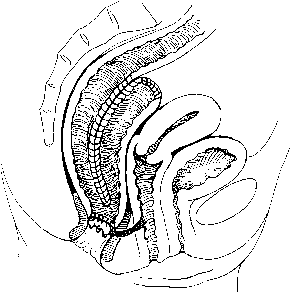
The fascial compartments surrounding the rectum are complex but clinically important. The rectal fascia propria surrounds the rectal wall and envelops the neurovascular supply and lymph nodes to the rectum. In women, the anterior rectovaginal fascia attaches to the vagina, forming the pouch of Douglas at the level of the superior vaginal recess. Relaxation of this fascia contributes to rectoceles and posterior support defects. Posteriorly, Waldeyer's fascia covers the presacral space. Laterally, the visceral pelvic fascia surrounds the mesorectum, is fixed superiorly to the internal iliac artery and hypogastric plexus, and forms the lateral rectal stalks (Fig. 3).
EXAMINATION
Examination of the perineum can be performed in several patient positions (e.g., prone jackknife or left lateral decubitus with the knees bent toward chest), but is easily performed while the patient is in the lithotomy position for a pelvic exam. Inspection should include the entire perineum, noting asymmetry, masses, or skin changes. Condyloma, thrombosed or prolapsed hemorrhoids, anal skin tags, or fissures can be identified from external inspection. Induration or drainage suggests a fistula or abscess.
Palpation is used to evaluate tone of the anal sphincter, masses, tenderness, and induration. The tract of a fistula may be palpable and pus may be expressed from its external opening. In general, hemorrhoids are palpable only when thrombosed. The cervix is easily palpable, and tampons should not be mistaken for rectal masses. A bidigital examination to evaluate the rectovaginal septum should be performed, especially for patients with known or suspected cancer. The coccyx and ischial tuberosities can be identified and used as reference points to describe the location of a palpable lesion.
Anoscopy is the best method by which to inspect the anal canal for lesions near the dentate line. Fistula openings and internal hemorrhoids can be identified. If no identifiable lesions are found to explain rectal bleeding or pain, then rigid proctosigmoidoscopy or colonoscopy may be warranted. Patients 50 years of age or older should undergo screening for colorectal cancer, even if a benign anorectal problem has been identified.
HEMORRHOIDS
Symptomatic hemorrhoids are a common problem, believed to affect close to 25% of the American population.1 Most commonly occurring in the fourth to sixth decades, hemorrhoids may affect patients of all ages. They may be exacerbated by the hormonal changes of pregnancy, and may or may not resolve postpartum.
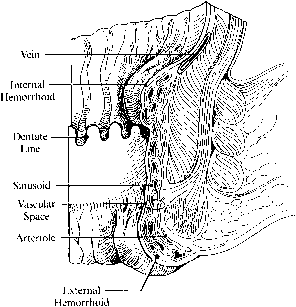
Hemorrhoids are vascular cushions that become congested with increased anal, rectal, and abdominal pressures. They are ubiquitous in the general population and the degree to which they cause symptoms depends on the integrity of their supporting connective tissue framework. Hemorrhoids are classified as internal (above the dentate line and covered by anal mucosa) or external (below the dentate line and covered with anoderm or perianal skin) (Fig 4).
On physical examination, they are typically located in the right anterior, right posterior, or left lateral position. Internal hemorrhoids usually present with bleeding associated with defecation and a sense of fullness from prolapsed tissue, which may require manual reduction. Pain is present only if an external hemorrhoid is thrombosed. Acute hemorrhoidal prolapse can be exquisitely tender, producing irreducible edematous tissue. Pressure necrosis, ulceration, and secondary infection can occur. Differential diagnosis includes painful bleeding from an anal fissure, full-thickness prolapse or procidentia, anal skin tags, and thrombosed external hemorrhoids.
Internal hemorrhoids are classified into four categories. First degree hemorrhoids project into the canal and cause bleeding, but do not prolapse. Second degree hemorrhoids prolapse, but spontaneously reduce back into the canal. Third degree hemorrhoids must be reduced manually, and fourth degree hemorrhoids are irreducible. Treatment for hemorrhoids ranges from dietary and lifestyle changes to surgical excision, and depends on the severity of symptoms and response to conservative management. For mild to moderate symptoms, a high-fiber diet and bulk dietary supplements can help alleviate pressure and bleeding. Patients should be encouraged to drink water and defecate without delay at the urge sensation.
Several procedures have been developed to fibrose the hemorrhoid tissue onto the underlying internal sphincter, thus restoring support for these vascular cushions and thereby preventing prolapse. When dietary and lifestyle modifications fail to reduce symptoms, sclerotherapy, rubber band ligation, and coagulation techniques may avoid surgical hemorrhoidectomy in up to 80% of patients with first and second degree internal hemorrhoids.
Sclerotherapy is effective in 60–80% of first and second degree internal hemorrhoids. It can be performed easily in the office and is relatively painless. Sclerosing agents, such as quinine and urea or 5% phenol in vegetable oil, are injected at the base of the hemorrhoid above the dentate line. Rare complications include sloughing of the mucosa, reaction to the injectate, and secondary infections. Sclerotherapy is not as effective on large prolapsing hemorrhoids.
Rubber band ligation of first and second degree hemorrhoids is effective in 65–75% of patients.2 Although less effective with third degree hemorrhoids, it can still be considered if one is attempting to avoid surgery. Banding around the base of the hemorrhoid causes necrosis and sloughing, and subsequent ulceration and fibrosis of the mucosa fix the tissue to the underlying sphincter muscle, preventing sliding of the anal mucosa. Banding is a simple procedure that can be performed in the office setting, and is relatively painless if performed properly. Common complications (less than 5% of patients) include bleeding, secondary infection, and pain if the band is placed too close to the dentate line. Despite rare reports of sepsis, rubber band ligation is one of the most common modalities for treating hemorrhoids.
Infrared photocoagulation transmits infrared radiation and coagulates the mucosa, with results similar to banding and sclerotherapy. If done properly, it also is relatively painless and complications are minimal. Laser photocoagulation offers no advantage over other treatment modalities as it is expensive and carries a greater risk of complications from unrecognized deep tissue destruction.3Electrocoagulation employs thermal injury to fibrose the hemorrhoidal tissue. Bipolar electrocautery employs similar instrumentation as the endoscopic devices and can be applied through an anoscope. Direct current electrotherapy requires more time and discomfort, but has shown better results in treating fourth degree hemorrhoids.4 A heater probe modified for use through an anoscope also shows good results with first and second degree hemorrhoids, but requires interval treatments.
Reducing increased anal sphincter tone, and thus preventing engorgement of the vascular cushions, is the theory behind anal dilation and sphincterotomy. Manual dilation traumatically disrupts fibrotic bands and sphincter muscle fibers that contribute to increased anal tone. The procedure must be done under anesthesia, and while having success rates comparable to other nonoperative treatments for patients with high intra-anal tone, it carries almost a 10% complication rate. Complications include mucosal tears, prolapse, and incontinence. Surgical sphincterotomy also reduces high canal pressures, but is usually performed in conjunction with other procedures and is generally not performed as the sole treatment modality for hemorrhoids. The internal sphincter is partially divided under direct visualization, thus reducing anal pressure in a more controlled and predictable fashion than anal dilation.
Surgical hemorrhoidectomy is indicated for grade 3 and 4 hemorrhoids and for patients with grade 1 and 2 hemorrhoids who have failed conservative management. One or all three hemorrhoidal complexes can be removed, generally in the operating room under local anesthesia. Hospitalization can be avoided for many patients. The swelling and discomfort of surgical hemorrhoidectomy requires a longer recovery period (minimum of 2 weeks) but, with a recurrence rate less than 3%, offers cure for most patients. Complications include bleeding, urinary retention, anal stenosis (less than 1%), and abscess or fistula (less than 0.01%).5 Surgical hemorrhoidectomy can be performed in combination with other modalities, including sphincterotomy, banding, and sclerotherapy and can be tailored to the individual patient. Several trials have compared laser excision of hemorrhoids with surgical excision; clear improvements in postoperative pain, analgesia, or recovery time have not been demonstrated with laser hemorrhoidectomy. Moreover, the added cost and delayed complications of bleeding, stenosis, intersphincteric abscess, and prolonged healing from laser excision leaves surgical excision the gold standard of hemorrhoid treatment.3
Newer surgical techniques include stapled hemorrhoidopexy or procedure for prolapsing hemorrhoids, which was first developed in the 1990s. Stapled hemorrhoidopexy is performed using a circular stapling device which is inserted into the anal canal. A purse string suture is then placed into the mucosa, approximately 2 cm above the superior aspect of the hemorrhoids. The opened stapler device is inserted into the purse string suture, which is then tightened. Next, the stapler device is closed and fired, thereby excising a ring of hemorrhoidal tissue. The results of this mucosectomy is thought to be twofold, both reducing the prolapsed anal mucosa and disrupting the vascular supply to the hemorrhoids.6 This procedure, when compared to the conventional excisional hemorrhoidectomy, is found to cause less postoperative pain and fewer complications from bleeding with comparable recurrence rates.7, 8
Doppler guided hemorrhoid artery ligation (DGHAL) was first described in 1995 by Morinaga and has since gained in popularity, especially in Europe. DGHAL is performed using a modified proctoscope which is combined with a Doppler probe. This is inserted into the anal canal and used to identify the hemorrhoidal arteries. Sutures are then placed into the areas of arterial signal through an opening in the proctoscope. This both ligates the arterial supply of the hemorrhoids and pexies the mucosa, reducing prolapse. Sohn et al. followed a cohort of 1415 patients for two years after being treated by DGHAL. Their experience found this procedure to be less painful than traditional banding secondary to the suturing occurring above the dentate line with fewer hospitalizations and less time off of work. Relatively few complications were encountered, but did include infection and hemorrhage, both with rates of less than 1%. Recurrence rates also were not significantly different than those found with excisional hemorrhoidectomy.9
Hemorrhoidectomy should not be performed in the presence of local perianal inflammation or dermatitis. Patients with ulcerative colitis should be treated only while in remission. Caution should be exercised for patients with Crohn's disease as well; if the patient has active anorectal Crohn's disease, then hemorrhoidectomy wounds may not heal. Hemorrhoidal flare-ups during pregnancy can be treated conservatively in most instances; however, grade 4 disease can be surgically treated during all three trimesters with acceptable results. Patients with AIDS may safely undergo hemorrhoidectomy, provided their immune status is stable.
ANAL FISSURE
Anal fissures are very common, and initially appear as an acute tear in the mucosal lining of the anal canal below the dentate line. Often precipitated by a hard bowel movement or diarrhea, patients complain of intense pain associated with defecation and bright red blood on the toilet paper. Symptoms resolve in a few hours. Patients often mistake their symptoms for hemorrhoids. The pain may be so severe that patients hold their stool as long as possible and refuse examination unless under anesthesia. On examination, anal fissures are most commonly located in the posterior midline, with approximately 10% located in the anterior midline. Any deviation from these anatomic locations should raise suspicion for another disease process (e.g., inflammatory disease, venereal infection), especially if the lesion is nontender or located above the dentate line. Hypertrophic anal papillae are associated with chronic anal fissures and are found internally at the dentate line; a sentinel tag or pile often marks the fissure externally at the anal verge. Fibers of the internal sphincter may be exposed at the base of the ulcer and are friable on examination. High anal sphincter tone fosters nonhealing, which is further exacerbated by relative ischemia of the posterior midline anoderm.
Treatment of anal fissures is aimed at relieving trauma and relaxing anal hypertonicity. The addition of bulking agents and dietary fiber should improve symptoms in 4–8 weeks. A local anesthetic ointment, such as 5% lidocaine, applied at the time of defecation helps relieve painful symptoms. Warm sitz baths relieve perineal pain, trigger the somatoanal reflex, and have been shown to relax hypertonic sphincter tone.[10] Application of glyceral trinitrate 0.2% ointment (nitric oxide donor) has been shown to increase anoderm blood flow and relax sphincter tone.[11] Healing of fissures has been noted in up to 60% of patients, with side-effects including headache and orthostatic hypotension. Initial optimism with glyceral trinitrate has waned, and some prospective randomized trials have shown no benefit in pain scores or rate of healing when compared with dietary fiber and bulking agents alone.[12] Botulinum toxin injected into the internal anal sphincter relaxes anal resting pressures by binding to the presynaptic cholinergic receptor. In patients with chronic anal ulcers not responding to conservative management, 86–100% responded to botulinum injection. No adverse effects or permanent sphincter damage was noted, and the effects of botulinum toxin reverse in 3 months.[13] It is reasonable to try conservative therapy with glyceral trinitrate or botulinum toxin; however, patients should be told that treatment may not be successful or the fissure may recur. Furthermore, some patients are so miserably affected by the rectal pain that several weeks of conservative therapy is not an option.
Manual dilation and relaxation of the anal sphincter under anesthesia can be performed; however, the extent of traumatic rupture of the internal sphincter muscles is unpredictable. Consequently, this treatment has not gained wide acceptance. Surgical lateral sphincterotomy remains the gold standard for release of high anal sphincter tone and treatment of chronic anal fissure. Performed under local anesthesia, the internal sphincter is divided from the dentate line to its distal most margin at either lateral position. This can be performed using either an open or closed technique (Fig. 5). To avoid fistula formation, the anoderm and subcutaneous external sphincter should not be violated. Fissurectomy can be performed at the same time. Success rates are high and recurrence rates are less than 5%.[5] Complications include incontinence of flatus and soiling (less than 15% of patients).[14] Fortunately, these complications are temporary for most patients who experience them.
ANORECTAL ABSCESS
Abscesses in the anorectal tissue spaces are believed to arise from infected anal glands in a crypt at the dentate line. They can also be associated with recent trauma, surgery, or a complication of fissures and hemorrhoids. Patients with Crohn's disease, diabetes, or hematologic or immune disorders are more likely to develop perirectal abscesses. A careful history of other constitutional complaints is important, as a perianal abscess may be the initial symptom of inflammatory bowel or another systemic disease. Patients usually present complaining of a constantly painful mass and often fever. Walking or sitting may exacerbate the tenderness. The pain is relieved promptly with drainage (either spontaneous or surgical). On examination, superficial abscesses will be indurated, erythematous, tender masses. Deeper abscesses may be palpated as a tender boggy mass on digital rectal exam; however, the exam may be deceivingly unremarkable. A high index of suspicion is paramount to avoid misdiagnosis. If the exam is unclear, MRI, endoluminal ultrasound, or CT scan may aide in diagnosis. Many abscesses are associated with a fistulous tract or go on to develop a fistula, and a thorough exam can help identify those patients who may need a fistulotomy. Hidradenitis, pilonidal sinus, Bartholin's gland abscess, and malignancy are in the differential diagnosis, as all of these entities may present with erythematous tender perianal masses associated with draining tracts.
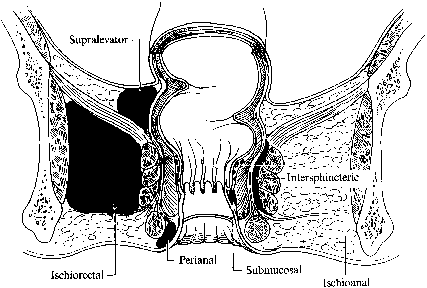
It is thought that perianal abscesses spread through the internal anal sphincter into various potential anorectal spaces. Most commonly, abscesses lie superficially beneath the perianal skin and can be easily drained through a small incision. Deeper abscesses can develop in the ischiorectal fossa, intersphincteric or supralevator spaces (Fig. 6). Multiple compartments can be infected, and if the deep anal space is involved, horseshoe extensions can develop into either ischiorectal space. In rare cases, life-threatening Fournier's gangrene can develop; this is a polymicrobial necrotizing myofasciitis capable of rapidly spreading throughout the tissue planes.
Treatment of abscess is always prompt surgical drainage, even of early abscesses that have not developed obvious fluctuance. Antibiotics should be used in adjunct, and not as sole therapy. Superficial abscesses can be drained under local anesthesia. Simple incision and drainage so that the cavity is adequately drained and explored, followed by packed dressing and sitz baths to allow secondary healing, is most commonly performed. A smaller incision with insertion of a catheter drain can also be used. It is important that all loculations and cavities be drained, and that the superficial tissue be kept open until the deeper tissues have healed to prevent a reaccumulation of loculated pus. Deeper abscesses may require counter incisions and placement of a Seton or Penrose drain to keep the tract open. Identified fistulous tracts should be treated with fistulotomy or Seton (discussed below); this is done in the operating room where a more thorough examination can be performed. If the causative crypt or source is identified, excision or definitive treatment should be attempted. After acute treatment of the abscess, the patient should have endoscopy performed if inflammatory bowel disease or malignancy is suspected. Expect approximately 25–50% of abscesses to resolve after initial treatment, with the remaining developing recurrence or fistulas in 1–3 months.15
ANAL FISTULAS
Anal fistulas are fibrinous tracts of granulation tissue connecting a primary opening within the anal canal to a secondary opening in the perianal skin. The primary opening is most commonly found within the dentate line and usually arises from an infected crypt or abscess. Other causes of fistulas include trauma or surgery, Crohn's disease, fissures, carcinoma, radiation therapy, or chlamydial infections. As the inflamed or infected area drains and granulates, the tract forms and remains patent or persistently draining.
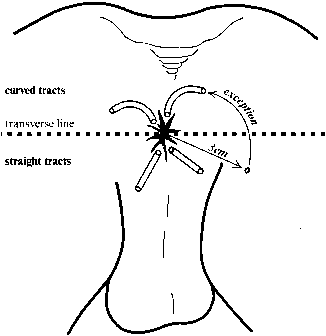
The secondary openings may be single or multiple, and are often the initial sign of an underlying problem. Patients may complain of drainage of pus, blood, stool, and/or mucus. Intermittent pain may be present if the tract becomes occluded and recurring abscesses form. On exam, the external opening is frequently a raised pink papule and may have a palpable cord running subcutaneously toward the anus. Drainage can usually be expressed. The location of the internal opening relative to the secondary opening can be predicted using Goodsall's rule (Fig. 7). Anterior fistulas drain in a radial pattern toward the anus, whereas posterior fistulas drain through a curvilinear tract that enters the anal canal at the posterior midline.
|
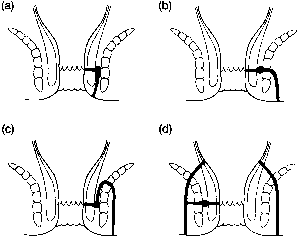
The internal opening usually can be identified during anoscopy. Probing of the internal opening should confirm its passage to the secondary opening, but reverse probing should be performed cautiously to prevent inadvertently creating a false passage. Once the tract is identified, its relationship to the sphincter will determine treatment (Fig. 8). Most commonly, an intersphincteric fistula is found. Transsphincteric fistulas are sequelae of ischiorectal abscesses. Suprasphincteric fistulas originate at the dentate line but course in a cephalad direction above the sphincter mechanism before turning caudally to exit on the perianal or gluteal skin. Extrasphincteric fistulas usually arise from the rectum above the anorectal ring and may be associated with foreign bodies, Crohn's disease, or malignancy. If the tract pathway is not easily determined, proctosigmoidoscopy may help. Barium enema, fistulograms using radiographic contrast material, endoscopic ultrasound, MRI, or CT may also be useful. Colonic, small bowel, and urethral fistulas can occur in an identical fashion, underscoring the importance of identifying an internal opening in the anal canal. Differential diagnosis also includes hidradenitis, pilonidal disease, and infected sebaceous cysts or Bartholin's glands. These entities will not have internal openings in the anal canal.
Treatment of fistulas must ensure that healing occurs from the base of the surgical wound to avoid fistula recurrence, while preserving normal sphincter function. Superficial fistulas may be treated by fistulotomy. The tract is exposed, débrided, and left open for the defect to heal secondarily. Marsupialization of the skin edges can be performed to prevent early closure. For higher fistulas that encompass the external sphincter, fistulotomy will cause incontinence; therefore, a staged procedure is performed. Most commonly a Seton drain is placed (Fig. 9). A rubber-band drain or heavy silk suture is placed through the fistula tract and secured, maintaining patency of the tract while promoting fibrosis. At intervals, the Seton is tightened, allowing the deeper portion to heal while maintaining drainage. Gradual fibrotic repair maintains the integrity of the external sphincter. Treatment of large complex fistulas, such as horseshoe abscesses in the supralevator anal space, may require a combination of staged fistulotomies, and Seton or Penrose drains; alternatively, a mucosal advancement flap may be used to close the internal opening.
Newer methods of fistula treatment include using either fibrin glue or a bioprosthetic plug to fill in the fistula tract. Both techniques require the fistula tracts to be first curetted and all local sepsis treated. Fibrin glue is employed after identifying both the external and internal openings, which are then injected with the glue. Bioprosthetic plugs are used in a similar manner. Once both defects have been clearly identifed, the plug is then passed from the internal opening towards the external, with the narrowest portion of the plug snugly fitting at the external opening. The excess material is then trimmed from the anal canal. Both ends of the plug are then further secured into place with suture. Although Johnson et al found recurrence rates to be reduced by utilizing the plug technique, due to the wide spectrum of fistula complexity, recurrence rates for both procedures vary widely, from 13 to 100%.16, 17 The best results have been noted in simple fistulas, which also are the easiest to treat surgically; therefore the role of fibrin glue is controversial.18
Recurrence of fistulas varies, but approaches 18%,19 and occurs within 2 years in 90% of patients with recurrence.20 Most commonly, recurrence is attributable to misidentification of the primary opening, but can also be attributed to failure to expose the entire fistula tract, and early closure of the superficial tissue. Patients with active inflammatory bowel disease will also have a high recurrence rate.
RECTOVAGINAL FISTULAS
Patients with rectovaginal fistulas may present with complaints of fecal incontinence or passage of flatus or stool from the vagina, as well as recurrent vaginitis and malodorous or purulent discharge. Most commonly associated with childbirth trauma, rectovaginal fistulas may also develop from Crohn's disease, carcinoma, and radiation therapy. Rarely, pelvic inflammatory disease, diverticulitis, or endometriosis may be implicated as the cause. The fistula openings are most commonly in the distal vagina and anterior dentate line (Fig. 10), but if not easily identified, colovaginal and cystovaginal fistulas must also be considered. Barium studies, fistulograms, MRI, or CT may be necessary to determine the tract course.
|
Repair of the fistula should take into consideration the cause of the fistula, and the presence of acute or active inflammation. Surgery should be delayed until local inflammation and sepsis have resolved.21 Obstetrical fistulas will close spontaneously in up to 50% of patients, lending further support to waiting after the injury has occurred. Superficial anovaginal fistulas may be treated with fistulotomy or reopening of the episiotomy. Layered closures through vaginal or transperineal approaches and endorectal advancement flaps may be necessary to reconstruct the rectovaginal wall and anal sphincter. The procedure chosen is determined by surgeon preference. Fecal diversion via a colostomy is rarely needed for first-time repairs; however, it should be considered if multiple previous attempts have failed. Crohn's disease should be in remission before any surgical intervention, and at times medical management alone can control these rectovaginal fistulas. Surgical treatment should be minimized in Crohn's patients. If the rectum is severely involved with Crohn's, proctectomy and permanent fecal diversion may be the only surgical option. Treatment of malignant fistulas depends on whether the cancer is resectable, and radiation and chemotherapy may be used in conjunction with surgery. Palliative or curative diverting colostomy or bowel resection with colo- or ileo anal pull-through procedures may be required for malignancy- or radiation-related rectovaginal fistulas. Fibrin glue has also been used for rectovaginal fistulas; however, the tract is usually too short to hold the fibrin glue in place and results have varied.
PERIANAL HIDRADENITIS SUPPURATIVA
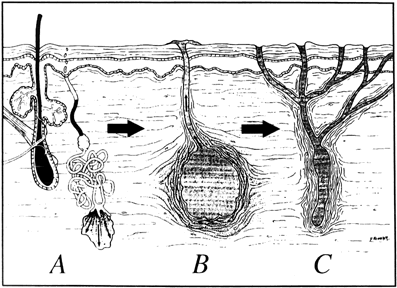
Hidradenitis suppurativa is chronic infection caused by occlusion of the apocrine glands by keratin comedo plugs. Secondary bacterial infection occurs and ruptures deep into the dermis (Fig. 11). Cellulitis, abscess, and chronic draining sinuses develop. There is a familial tendency and an association with cystic acne. Hidradenitis suppurativa usually does not develop until after the onset of puberty, and exacerbations are associated with excessive androgens, pregnancy, the late menstrual cycle, and oral contraceptives. Hidradenitis occurs most commonly in the axilla and inguinal region, and less commonly in the perianal region. The lesions are always distal to the dentate line, thereby distinguishing them from anal fistulas.
Nonsurgical management of perianal hidradenitis includes warm baths or whirlpool, steroids to reduce androgen production, and topical and systemic antibiotics. Common organisms cultured include Streptococcus and Chlamydia spp., but antibiosis should be tailored to cover the anaerobes and gram-negative organisms seen in perianal hidradenitis. Leuprolide used in a long-term daily dose suppresses ovarian and testosterone steroid production and has been successful in suppressing hidradenitis. Also, Accutane, more commonly used in cystic acne, has also shown therapeutic effects with hidradenitis.22
Incision and drainage in the acute setting will relieve symptoms temporarily, but will require further definitive treatment. A more extensive unroofing of the lesions with marsupialization to allow granulation may be adequate for smaller lesions. Limited local excisions have a 20% recurrence rate in the axilla and groin, but a higher success rate with perianal disease. If limited excision has failed, or disease is extensive, than wide en bloc excision should be performed. Healing by secondary intention followed by skin grafting has a high failure rate in the perianal region, which is why local excision is preferred for perianal hidradenitis.
PILONIDAL SINUS
Although pilonidal cysts have historically been thought to be a congenital lesion, the current theory held is that shed hair shafts embed into the skin and then become secondarily infected. Most commonly occurring in men between 16 and 20 years of age, patients present with complaints of pain, tenderness, and drainage from an abscess located in the gluteal fold just below the coccyx. Patients may present with an acute abscess, or a chronic draining pilonidal sinus.
In the acute setting, drainage can be performed with an incision lateral to the midline. For more definitive treatment, an elliptical excision of the sinus tracts should be performed with beveling or marsupialization of the skin edges to prevent early closure. Most surgeons will choose to have the wound granulate, but for sinuses that have been drained and infection controlled preoperatively, primary or Z-plasty closure can be performed.23
PERIANAL CONDYLOMA
Patients with genital condyloma may also have lesions in the perianal region. Patients may complain of pruritus, bleeding, or discharge, or they may be asymptomatic. The human papilloma virus strains are similar to genital condyloma, with subtypes 6 and 11 more commonly forming exophytic lesions, and subtypes 16 and 18 more likely associated with dysplasia and cancer. Condylomas are most commonly transmitted via sexual contact, although anal penetration does not need to occur for perianal condyloma. Transmission can also occur from mother to infant during birth. Differential diagnosis includes squamous cell carcinoma and condyloma lata of secondary syphilis; darkfield microscopy and biopsy are required to differentiate.
Treatment of perianal condyloma is similar to condyloma elsewhere. Podophyllin is a cytotoxin extract from the rhizome of the Podophyllum spp. It can be applied in weekly intervals to the perianal skin lesions, but should not be applied within the anal canal. Bichloracetic acid is also applied in weekly intervals, but it may be applied in the anal canal. 5-FU 5% cream has also been used for condyloma, but causes severe skin irritation, making it less well tolerated.
Several excisional treatment options exist, and each faces the same challenge: adequately removing the infected tissue while sparing minimal loss of unaffected tissue. Direct surgical excision by clipping the lesion at its base can be performed under local anesthesia.
Electrocoagulation and fulguration use heat coagulation to destroy the affected tissue. Care must be taken to avoid deep burns. Carbon dioxide laser ablation can be employed, but again, care to avoid deep burns must be taken. There is also added risk of transmission from aerosolized viral particles, and operating room personnel must wear special masks. Nitrogen oxide, liquid nitrogen or cryoprobes can be used for cryotherapy. All excisional treatments carry a high recurrence rate and multiple treatments are often required.
Several new immunotherapies have been developed. Abcarian developed an autologous vaccine from 5 g of condyloma tissue that was then injected intramuscularly in six weekly doses.24 Eighty-four percent of patients had complete remission, but preparation of the vaccine can be problematic. It is not widely practiced and it has not replaced surgical destruction or excision as the treatment of choice. Interferon-alpha has also been used as a systemic intramuscular injection, injected locally into the lesion, in combination with surgical excision. Results have varied with remission seen in up to 80% of treated groups.25
Infection with the human papilloma virus (HPV) has been implicated in the development of anal intraepithelial neoplasia (AIN) and invasive anal cancer. In this regard, there are parallels between anal and cervical malignancies. Malignant transformation is promoted by a state of immunodeficiency such as that seen with AIDS infection or with immunosuppression following organ transplantation. Just as Pap smears have reduced the mortality from cervical cancer, it is hoped that anal Pap smears for highrisk groups (HPV infection, HIV-positive patients, male homosexuals) will identify patients with high-grade dysplasia and AIN who are at risk for invasive cancer.
SUMMARY
Anorectal disorders such as abscesses, fistulas, fissures, and hemorrhoids are common. Women afflicted with these conditions will frequently seek treatment from their gynecologist or internist first, and a basic working knowledge of their management is essential. Distinguishing one condition from another is not always easy because symptoms and physical characteristics overlap. If doubt exists as to the correct diagnosis, referral to a specialist is indicated.
REFERENCES
Nelson RL et al: Prevalence of benign anorectal disease in randomly selected populations. Dis Colon Rectum 38: 341, 1995 |
|
MacRae HM, MacLeod RS: Comparison of hemorrhoidal treatment modalities. A meta-analysis. Dis Colon Rectum 38: 687, 1995 |
|
Endres JC, Steinhagen RM: Lasers in anorectal surgery. Surg Clin North Am 74: 1415, 1994 |
|
Randall GM, et al: Prospective randomized comparative study of bipolar versus direct current electrocoagulation for treatment of bleeding internal hemorrhoids. Gastrointest Endosc 40: 403, 1994 |
|
Mazier WP: Hemorrhoids, fissures and pruritus ani. Surg Clin North Am 74: 1277, 1994 |
|
Hard A. Chan CLH. Cohen CRG: The Surgical Management of Hemorrhoids - A Review. Dig Surg 22:26-33, 2005 |
|
Gravié JF, Lehur PA, Huten N, et al: Stapled hemorrhoidopexy versus Milligan-Morgan hemorrhoidectomy: a prospective, randomized, multicenter trial with 2-year postoperative follow up. Ann Surg 2005; 242: 29–35 |
|
Sutherland LM, Burchard AK, Matsuda J, et al: A systematic review of stapled hemorrhoidectomy. Arch Surg 2002; 137:1395-1406 |
|
Sohn N. Aronoff JS. Cohen FS. Weinstein MA: Transanal hemorrhoidal dearterialization is an alternative to operative hemorrhoidectomy. Am J Surg 182(5): 515-19, 2001 |
|
Jiang JK et al: Local thermal stimulation relaxes hypertonic anal sphincter: Evidence of somatoanal reflex. Dis Colon Rectum 42: 1152, 1999 |
|
Schouten WR et al: Relationship of anal pressure and anodermal bloodflow: The vascular pathogenesis of anal fissures. Dis Colon Rectum 37: 664, 1994 |
|
Altomare DF et al: Glyceryl trinitrate for chronic anal fissure: Healing or headache? Dis Colon Rectum 43: 174, 2000 |
|
Maria G et al: Botulinum toxin injections in internal anal sphincter for the treatment of chronic anal fissure: Long-term results after two different dosage regimens. Ann Surg 228: 664, 1998 |
|
Pernikoff BJ et al: Reappraisal of partial lateral internal sphincterotomy. Dis Colon Rectum 37: 1291, 1994 |
|
Lunniss PJ, Phillips RK: Surgical assessment of acute anorectal sepsis is a better predictor of fistula than microbiological analysis. Br J Surg 81: 368, 1994 |
|
Hammond TM, Grahn MF, Lunniss PJ: Fibrin glue in the management of anal fistulae. Colorectal Dis 6(5): 308-319, 2004 |
|
Johnson EK. Gaw JU. Armstrong DN: Efficacy of anal fistula plug vs. fibrin glue in closure of anorectal fistulas. Dis Colon Rectum 49(3):371-6, 2006 |
|
Abel ME et al: Autologous fibrin glue in the treatment of rectovaginal and complex fistulas. Dis Colon Rectum 36: 447, 1993 |
|
Vasilevsky CA: Fistula-in-ano and abscess. In Beck DE, Wexner SD (eds): Fundamentals of Anorectal Surgery. London, WB Saunders, 1998 |
|
Rosen L: Anorectal abscess-fistulae. Surg Clin North Am 74: 1293, 1994 |
|
Senatore PJ: Anovaginal fistulae. Surg Clin North Am 74: 1361, 1994 |
|
Rubin RJ, Chinn BT: Perianal hidradenitis suppurativa. Surg Clin North Am 74: 1317, 1994 |
|
Surrell JA: Pilonidal disease. Surg Clin North Am 74: 1309, 1994 |
|
Abcarian H, Shawn N: The effectiveness of immunotherapy in the treatment of anal condyloma acumination. J Surg Res 22: 231, 1977 |
|
Luchtefeld MA: Perianal condylomata acuminata. Surg Clin North Am 74: 1327, 1994 |