Galactorrhea
Authors
INTRODUCTION
Galactorrhea, the inappropriate production and secretion of milk, with its associated hyperprolactinemia, is an extremely common clinical entity. It is often accompanied by menstrual disturbance and infertility and may herald the presence of pituitary tumors, which can produce considerable morbidity and rarely mortality. The management of this condition has been greatly simplified with the advent of newer radiologic and radiochemical techniques. The natural history of pituitary tumors is much better understood today than 20 years ago, and management of these lesions is less conservative than in the past.
HISTORICAL MILESTONES
In 1855, Chiari and colleagues1 reported two cases of puerperal atrophy of the uterus with amenorrhea and persistent lactation. This syndrome was redescribed by Frommel in 1882.2 A syndrome characterized by estrogen insufficiency, galactorrhea, and decreased urinary estrogens was described in 1952 and again in 1953.3,4 In 1954, Forbes and associates described a syndrome characterized by galactorrhea, amenorrhea, and low levels of urinary follicle-stimulating hormone (FSH).5 This observation first suggested the association between galactorrhea and the presence of pituitary tumors. The incidence of tumors in this group of patients was about 25%. This observation confirmed Costello's evaluation of 1,000 unselected pituitary glands obtained at autopsy.6 He found a remarkable 25% incidence of pituitary adenomas, with the frequency being 60% for men and 40% for women.
Despite these clinical observations, three events would have to occur before we gained contemporary understanding of prolactin physiology. First, Greenwood and Hunter7 described the technique for iodination of protein molecules. Then Berson and Yalow developed the techniques of radioimmunoassay for insulin that allowed endocrinologists to develop immunoassays to measure serum prolactin.8 Finally, the availability of computed tomography (CT) and magnetic resonance imaging (MRI) allowed radiologists to evaluate the pituitary gland and detect lesions as small as 2 mm.
PHYSIOLOGY OF PROLACTIN SECRETION
Historically, prolactin was shown to be unique from other pituitary tropic hormones by the fact that synthesis and secretion of the molecule occurred when the gland was placed in vitro, after transection of the pituitary stalk, or transferred to adjacent body sites, such as the kidney capsule or pneumoderma pouch.9 It was strongly suggested that prolactin secretion was inhibited by the central axis, and it was suspected that the central nervous system produced a small peptide (prolactin)-inhibiting factor (PIF) that was responsible for this inhibition. Numerous investigators attempted to isolate the mythical PIF without success. Currently, the principal inhibitory agent appears to be the neurotransmitter dopamine.10 This chemical inhibits prolactin secretion probably both in vivo and in vitro.11 The catecholamine is known to be synthesized in the median eminence and secreted into the hypothalamic portal vessels. The compound appears to account for 70% of central nervous system inhibitory activity. This conclusion was reached after dopamine concentrations were measured in hypothalamic portal blood. Subsequently, similar dopamine concentrations were infused into animals that had been pretreated with an inhibitor of dopamine synthesis, α-methylparatyrosine. Seventy percent of prolactin secretion could be blocked in these animals.12 The results of such studies have been confirmed in monkeys but not in humans.
Further evidence that dopamine is a principal regulator of prolactin secretion comes from suckling experiments performed in monkeys and rats. In the rat, a small decrease in hypothalamic dopamine secretion can be observed during simulated suckling. In addition, a small decrease in exogenous dopamine infusion in primates leads to a large increase in prolactin secretion.
Dopamine appears to act on lactotropes by both cyclic adenosine monophosphate (AMP)- and calcium-dependent mechanisms. High-affinity dopamine receptors have been isolated on lactotrope membranes, and adenyl cyclase inhibition occurs after establishment of the dopamine receptor complex.13 Furthermore, it has been demonstrated that dopamine inhibits prolactin synthesis at transcription, and the effect is amplified by cyclic AMP.
Other agents are involved in the control of prolactin secretion. The neurotransmitter γ-Aminobutyric acid (GABA) has been shown to be secreted into portal blood, and receptors for GABA have been demonstrated on the pituitary lactotrope membranes. Dopamine produces far greater inhibition than GABA, and it has been suggested that these two agents may serve different inhibitory functions within the lactotrope. For example, dopamine induces the storage of newly synthesized prolactin, which may be rapidly released after withdrawal of the catecholamine. Such a response is not seen with GABA.14
Numerous investigations have been performed to establish the presence and structure of a prolactin-releasing factor. It was shown that thyrotropin-releasing hormone (TRH) could stimulate both the synthesis and release of prolactin in vitro and in vivo.15 This releasing factor concomitantly stimulates the secretion of thyroid-stimulating hormone (TSH). Despite the apparent obligatory interaction, prolactin secretion may occur independently to that of TSH. For example, prolactin secretion is increased after suckling, which is not accompanied by an increase in TSH secretion.16 Therefore, TRH might function as a physiologic prolactin-releasing factor, and under certain circumstances it probably has a minor role in the control of prolactin.
Other compounds have been shown to stimulate prolactin secretion, including vasoactive intestinal peptide (VIP) and angiotensin II.17, 18 VIP was purified from porcine duodenum and is a 28-amino-acid polypeptide. This compound is found throughout the nervous system and has been measured in hypothalamic portal blood. Its release is mediated by prostaglandins. Studies have demonstrated that VIP is produced in the pituitary gland and has been localized to the lactotrope. It may function as an intrahypophyseal regulator of prolactin secretion. Angiotensin II is an octapeptide that has been identified throughout the brain. Its injection brings about a rapid release of prolactin of far greater magnitude than that produced by TRH. Angiotensin II receptors have been localized on lactotrope membranes. The prolactin-stimulating response to angiotensin II is inhibited by the antagonist saralasin.
Several other neurotransmitters appear to be involved in the control of prolactin secretion, including serotonin, endogenous opioid compounds, and histamine.19 The administration of serotonin precursors causes a significant increase in prolactin levels. This is blocked by the serotonin antagonist cyproheptadine. Opioids have been shown to affect dopamine turnover and release in the central axis, and histamine, a hypothalamic neurotransmitter, has been shown to stimulate prolactin secretion by binding to H1 receptors. Furthermore, neurotensin, a tridecapeptide, and substance P, a unidecapeptide, have been shown to stimulate prolactin secretion.20 If these neurotransmitters have a physiologic role in prolactin control, it is unclear at this juncture.
In addition to hypothalamic control, prolactin secretion appears to be regulated by both autocrine and paracrine mechanisms. Bergland and Page21 demonstrated retrograde flow in the hypothalamic portal system, which supports the concept that prolactin may be regulated by its own secretion through a short feedback mechanism. In the rat, the ventricular injection of prolactin results in increased dopamine turnover in the median eminence.22 A similar rate of turnover has been demonstrated during lactation and pregnancy. Intrahypophyseal mechanisms have also been demonstrated. VIP is synthesized by pituitary cells in culture and has been shown to stimulate prolactin secretion. Antibodies to VIP added to culture inhibit prolactin secretion. In addition, gonadotropes have paracrine regulatory influence on prolactin secretion by acting on the lactotrope. A synthetic gonadotropin-releasing hormone (GnRH) has been shown to release prolactin in vitro using rat superfusion and human pituitary cells in culture.23, 24 Incubation of GnRH with lactotropes separated from large gonadotropes failed to increase prolactin secretion, whereas coaggregation of these two cell types restored the stimulatory effects of GnRH on prolactin. Coincubation of a potent GnRH antagonist with GnRH inhibited the secretion of prolactin, whereas coincubation of the antagonist with synthetic TRH failed to alter the release pattern. Other studies have shown that incubation of the α-chain of luteinizing hormone (LH) with fetal rat lactotropes stimulates differentiation of these cell types. Further incubation of β-LH or FSH with human pituitary cells in vitro failed to stimulate prolactin secretion consistently; however, coincubation of antiserum to β-LH and FSH inhibited the GnRH-mediated release of prolactin.25 In addition, GnRH-associated peptide, a peptide component of the precursor to GnRH, has been reported to inhibit prolactin secretion in the rat. Therefore, all these studies would suggest that prolactin regulation is much more complex than a simple inhibition by a PIF.
Further, using new research techniques such as cell blotting, new dimensions have been uncovered with regard to pituitary cell populations.26, 27 For instance, cells have been identified that store adrenocorticotropic hormone (ACTH), along with FSH, LH, TSH, and prolactin, which implies their multipotential capacity.28 Populations of lactotropes have been identified that are resistant to dopamine stimulation, and likewise, using the immunoblot technique, subpopulations of rat lactotropes have been identified that are also unresponsive.29, 30, 31 Lactotropes have been identified that secrete small amounts of prolactin but are highly responsive to TRH stimulation.32 Further, steroids can modulate the transdifferentiation of prolactin and growth hormone-secreting cells in bovine pituitary cell cultures.33 Prolactin expresses great heterogeneity, and various isotypes have been found to vary throughout the menstrual cycle, in pregnancy, and in hyperprolactinemic women with normal ovulatory function.34, 35
Growth hormone and prolactin evolved from a common ancestral gene. They share biologic and immunologic properties, immunoassay sequences, and homologies in nucleic acid sequence. Preprolactin consists of 914 base pairs in mammals, and the prolactin receptor consists of 830 amino acids in avians. The prolactin receptor exists in two forms and is a member of the growth hormone cytokine receptor superfamily. DNA cloning experiments have demonstrated a short 300-amino-acid and a long 600-amino-acid form. There is no clear-cut second messenger for prolactin that may account for its biologic diversity.36, 37, 38
The gene for prolactin has been isolated, cloned, and sequenced. It is located on the short arm of chromosome 6. Identifications of BG-2 polymorphisms have been made, but no mutations have been detected in patients with hyperprolactinemia. Interactions have been demonstrated between transcription regulatory regions of prolactin chromatin, with estrogen appearing to be the principal enhancer of the interaction.39, 40
Two other types of compounds are associated with prolactin secretion: galanin and granins.41, 42, 43 Galanin, which is widely distributed, is a regulatory peptide that modifies the secretion of prolactin and growth hormone. It is composed of 29 amino acids, and its secretion is regulated by estrogen. The exact role of galanin is unknown, but it probably represents a growth factor because immunoneutralization inhibits the mitogenic effect of estrogen. The granins are a family of tyrosine, sulfate, and secretory protein composed of two members, chromogranin-B and secretogranin-2. They have been coidentified in GH4C1 cell lines that secrete both prolactin and growth hormone. Although granins are subject to extensive post-translational modification, their function remains unknown.
GALACTORRHEA AND ITS ASSOCIATION WITH MENSTRUAL DYSFUNCTION
Historically, patients with hyperprolactinemia were believed to present with galactorrhea. In recent years, it has been demonstrated that hyperprolactinemia may not only be associated with galactorrhea but also with numerous ovulatory disorders, including amenorrhea, anovulation, oligo-ovulation, luteal phase defect, luteinized unruptured follicle syndrome, and perhaps unexplained infertility.44 In addition, patients may present with a polyendocrinopathy and associated increases in either growth hormone or ACTH. How hyperprolactinemia causes menstrual dysfunction is somewhat unclear. In rats, it has been shown that injection of prolactin increases central dopamine turnover. Dopamine exerts an inhibitory influence over GnRH secretion. A slowing of the GnRH pulse frequency at the level of the arcuate nucleus might result in dysfunctional gonadotropin secretion ranging from mild dysfolliculogenesis to amenorrhea. Restoration of the euprolactinemic state seems to result in the resumption of normal reproductive function.
It is also possible that hyperprolactinemia may have a direct effect on the ovary.45 Suggested mechanisms include atresia of a developing dominant follicle, interruption of ovulation and normal corpus luteum development, and premature involution of the corpus luteum. Most data come from the rat model, in which ovarian interstitial cells have been shown to contain a single class of specific high-affinity prolactin receptors. In this system, prolactin acts as a potent inhibitor of LH-mediated androgen synthesis. Disruption of androgen substrate production inhibits estrogen production by aromatization. Further high-affinity prolactin receptors have been demonstrated on granulosa cell membranes. Coincubation of follicles with high levels of prolactin (100 ng/mL) reduces aromatase activity in vitro by antagonizing the effect of FSH.46 Furthermore, prolactin has been detected in the intracellular environment of rat oocytes.
Further observations have been made in human oocytes. In the early follicular phase of the cycle, a fourfold to sixfold increase in prolactin was found in the fluid of developing follicles that had a high concentration of prolactin. These follicles were noted to have low concentrations of estradiol. In patients with hyperprolactinemia of about 100 ng/mL, atretic follicles are found almost 100% of the time. Native prolactin both stimulates and inhibits corpus luteum function. It appears to be involved in the induction of LH receptors that stimulate progesterone synthesis. In vitro, however, very high levels of prolactin have been found to inhibit both progesterone and estradiol synthesis. Therefore, it seems possible that hyperprolactinemia may produce alterations in normal reproductive function at the level of the central axis and ovary.
Several clinical papers have evaluated the effect of hyperprolactinemia on the results of in vitro fertilization.47, 48, 49, 50 Most of these findings suggest that hyperprolactinemia does not affect granulosa cell luteinization, the total number of oocytes, the number of mature oocytes, fertilization, cleavage, or pregnancy rate. Hyperprolactinemia has been demonstrated in up to 57% of patients undergoing in vitro fertilization. The use of bromocriptine to inhibit anesthesia-induced hyperprolactinemia has been touted as having a positive influence on embryonic development.
All these lines of evidence would seem to speak against a primary role of prolactin as a direct disrupter of follicle growth. However, the vast majority of women undergoing assisted reproductive technology had been subjected to pituitary downregulation with GnRH analogues, followed by treatment with gonadotropins that directly affect ovarian folliculogenesis. It appears that the main cause of anovulation in patients with hyperprolactinemia is an impairment in gonadotropin pulsatility and a derangement in the estrogen positive feedback on LH secretion. Subcutaneous pulsatile GnRH therapy combined with human chorionic gonadotropin (HCG) has been shown to compensate for these defects and to result in folliculogenesis and induction of ovulation.51, 52, 53, 54, 55
CAUSES OF GALACTORRHEA
Hyperprolactinemia is associated with numerous types of pathology (Table 1). The four most common causes of hyperprolactinemia are central dopamine metabolism disturbance (functional hyperprolactinemia), prolactinomas, hypothyroidism, and drug ingestion.56 Patients with hypothyroidism deserve special comment. These patients present with compensated primary hypothyroidism and may demonstrate a normal thyroxine level with markedly elevated TSH levels. They often have pituitary enlargement mimicking an adenoma and may have visual impairment.57 It is necessary to evaluate thyroid function in all patients before considering neurosurgical exploration.
Table 1. Causes of hyperprolactinemia
Idiopathic (functional)
Pituitary Disease
Prolactinomas
Nonsecreting pituitary adenomas
Acromegaly
Cushing’s disease
Lactotroph hyperplasia
Empty sella syndrome
Lymphocytic hypophysitis
Medications
Phenothiazines
SSRIs
Tricyclic antidepressants
Metoclopramide
Cimetidine
Methyldopa
Verapamil
Protease inhibitors
Oral contraceptive pills
Hypothyroidism
Physiologic
Pregnancy
Breast-feeding
Stress
Exercise
Sexual intercourse
Sleep
Hypothalamic Disease
Craniopharyngiomas
Meningiomas
Dysgerminomas
Other tumors
Sarcoidosis
Leukemia
Eosinophilic granuloma (Histiocytosis X)
Neuraxis irradiation
Peripheral Neural Stimulation
Breast/nipple stimulation
Nipple piercing
Breast augmentation & breast reduction surgery
Thoracotomy
Chest wall lesions
Herpes zoster
Spinal cord lesions
Medical illness
Renal failure
Cirrhosis
Adrenal insufficiency
Ectopic production
Renal cell carcinoma
Lung tumor
Gonadoblastoma
Ovarian teratoma
Other
Hysterectomy
Oophorectomy
Syphilis
Tuberculosis
Cavernous sinus thrombosis
Head trauma
Temporal arteritis
Burns
As mentioned previously, various drugs have been shown to elevate levels of serum prolactin (Table 2),58 most notably oral contraceptives and psychotropic agents. Antipsychotic tranquilizers (phenothiazines) are the most potent stimulators of prolactin secretion; class II phenothiazines seem to cause the greatest release of this hormone. In addition, tricyclic antidepressants can cause marked hyperprolactinemia; however, new-generation antidepressants such as fluoxetine hydrochloride (Prozac) do not seem to be associated with an elevation of milk-producing hormone. Other commonly used drugs for the control of high blood pressure, such as α-methyldopa (Aldomet), increase prolactin secretion, as does the commonly used antinuclear preparation cimetidine (Tagamet).
Table 2. Agents that alter prolactin secretion
Inhibitors
Dopamine
γ-Aminobutyric acid
Stimulators
Thyrotropin-releasing hormone
Vasoactive intestinal peptide
Angiotensin II
Opioids (exogenous)
Histamine
Neurotensin
Substrate P
Estrogen
Progesterone
In addition to the four most common causes of hyperprolactinemia, numerous types of trauma and pathology have been associated with increased prolactin production. These include head trauma, obstetric accident, intracranial tumor, encephalitis, meningitis, histiocytosis X, syphilis, tuberculosis, cavernous sinus thrombosis, temporal arteritis, chest trauma, chest surgery, breast augmentation, herpes zoster, oophorectomy, hysterectomy, and renal failure.59 A recent study has shown a 15% incidence of amenorrhea and/or galactorrhea and a 3% incidence of galactorrhea among 198 adult female patients in the post-burn period.60
PRESENTATION OF GALACTORRHEA
Galactorrhea may be intermittent or continuous, bilateral or unilateral, and free-flowing or expressible.61 By definition, fat droplets must be present on microscopic examination for a breast secretion to be considered milk and thus evidence of galactorrhea. Galactorrhea is frequently associated with hyperprolactinemia, and serum levels are evaluated by radioimmunoassay. Prolactin exists in several isoforms, including glycosylated prolactin, big prolactin, and big-big prolactin. Native prolactin (monomeric) appears to have the greatest biologic activity.
The institution and maintenance of lactation are species-dependent. For instance, in rabbits and sheep, prolactin alone is sufficient to cause milk production; in ruminants, milk secretion is restored by the addition of corticosteroids, thyroxine, growth hormone, and prolactin. In humans, prolactin appears to play a key role in the maintenance of lactation because bromocriptine administration blocks lactogenesis.62 The role of thyroid hormones is unclear: thyroidectomy inhibits lactation, and replacement therapy with thyroxine increases milk yield. It has been suggested that growth hormone and thyroxine synergize to alter milk yield and triiodothyronine acts directly on mouse mammary tissue in vitro to increase its sensitivity to prolactin.
Galactorrhea can often be confused with breast infections and malignancy.63 Patients presenting with a unilateral breast discharge that does not demonstrate fat globules by microscopy should be evaluated for other causes of pathology (e.g. streptococcal or Escherichia coli infection). If the discharge is greenish, Pseudomonas should be suspected. A galactocele or retention cyst can occur after the cessation of lactation and is the result of a duct obstruction. This can masquerade as mastitis and unless drained may serve as a future site for sepsis.
Anatomic lesions such as the intraductal papilloma may result in a bloody nipple discharge. If the patient presents with a small palpable mass associated with a bloody nipple discharge, there is a 75% chance an intraductal papilloma will be found. If no mass can be palpated, Paget's disease of the nipple or carcinoma should be considered. Intraductal papilloma is not premalignant and is best managed by excision of the duct by wedge resection.
Although galactorrhea is a common sign of hyperprolactinemia, adolescent and teenage patients often present with prolactin levels in the range of 2,000–3,000 ng/mL with no galactorrhea.64 Hyperprolactinemia has been shown to result in the inhibition of pubertal development and occasionally presents as primary amenorrhea after normal pubarche and adrenarche. Hyperprolactinemia is often associated with large macroadenomas that could be classified as invasive. These lesions are not spherical, grow along dural planes, and histologically closely resemble meningiomas.65 Children presenting with galactorrhea or menstrual dysfunction should be aggressively evaluated and treated to prevent both loss of reproductive capacity and visual impairment.
EVALUATION OF THE PATIENT WITH GALACTORRHEA
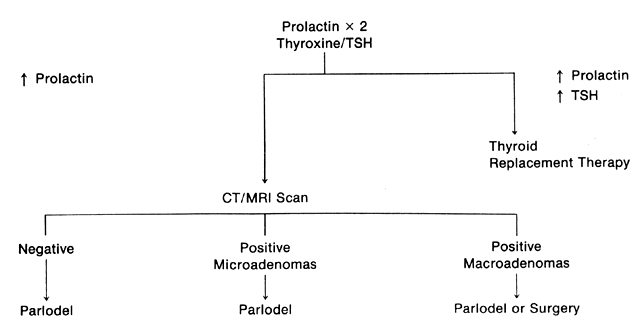
The patient who presents with galactorrhea should undergo an extensive history and physical examination to determine the cause of the problem (Fig. 1). A serum prolactin level should be measured because the absolute level is often predictive of the type of pathology. For instance, a pituitary tumor is found 25% of the time with a prolactin level of 50 ng/mL, 50% of the time with a level of 100 ng/mL, and almost 100% of the time with a level of 200 ng/mL.66 Drug intake rarely produces hyperprolactinemia above 60 ng/mL. When measuring serum prolactin levels, the physician should remember that this is a dynamic, stress-related hormone. Numerous physiologic events are associated with hyperprolactinemia, including prior breast or pelvic examination, food intake (particularly the amino acid arginine), and time of day, because prolactin has a sleep-entrained rhythm.67 In general, samples for serum prolactin levels should be drawn before examination, later in the morning to early in the afternoon, and at least an hour away from meals. If an elevated level is found on the initial sample, one or two repeat samples should be drawn, along with a sample for thyroxine and TSH determination to rule out compensated primary hypothyroidism. In addition, one could argue for the measurement of serum growth hormone and ACTH to rule out a polyendocrinopathy, although this is rarely discovered.
Considerable difference of opinion exists as to when radiographic surveillance should be instituted. Many reproductive endocrinologists believe that a CT or MRI scan should be performed in patients with prolactin levels of 100 ng/mL or greater.68, 69 Most reproductive endocrinologists would institute radiographic surveillance at levels of 50 ng/mL. However, we recommend that radiographic surveillance be undertaken if a persistent hyperprolactinemia is demonstrated at any level, because an adequate initial evaluation of hyperprolactinemia will give the patient a better understanding of the implications of her disease process and allow the clinician more insight into the appropriate long-term management of the problem.
Both CT and MRI scans are adequate for the diagnosis of both microadenomas and macroadenomas (Fig. 2 and Fig. 3). Both of these radiographic techniques can detect a pituitary tumor as small as 2 mm. There is considerable difference in the cost of a CT and MRI scan ($758 versus $1,490, respectively). MRI scanning is advantageous if longitudinal surveillance is necessary over a lifetime because no radiation exposure is incurred with this technique.
Visual field evaluation by Goldman bowl perimetry should be performed in the patient with a macroadenoma only. Visual field changes will not be demonstrated unless there is suprasellar extension of a lesion; in fact, these visual changes are found with such lesions only 60% of the time.70 The most subtle visual field finding is bilateral superior bitemporal hemianopsia. However, patients with extremely large lesions may demonstrate bitemporal hemianopsia and, in severe cases, blindness in one or both eyes.
The use of pituitary challenge testing was widely investigated in the late 1970s.71 Such techniques do not appear useful for routine clinical practice and should be reserved for research protocols evaluating the patient with unexplained infertility or intermittent hyperprolactinemia.
Measurement of Prolactin: Diagnostic Pitfalls
There is considerable variation among laboratories with conventional radioimmunoassays. Over recent years, chemiluminometric assays and two-site immunoradiometric assays have been widely used. At very high prolactin levels, the antibodies may become saturated thus preventing the formation of the prolactin-antibody sandwich and leading to a falsely low prolactin value. This is known as the ‘hook effect’. In order to exclude the ‘hook effect’, prolactin levels should be measured in both undiluted and 1:100 diluted serum. The clinician should consider excluding the ‘hook effect’ in the evaluation of patients with large pituitary macroadenomas who have normal or mildly elevated prolactin levels.72 Macroprolactin is a polymeric form of prolactin. Macroprolactin has reduced bioactivity compared to the monomeric form and it is detected by most prolactin assays. This results in falsely elevated measured prolactin level. Polyethylene glycol precipitation can be used to confirm macroprolactinemia. The alternative option, size exclusion chromatography, is time-consuming and is not suitable for routine clinical use.73
TREATMENT OF THE PATIENT WITH HYPERPROLACTINEMIA
Historically, patients with hyperprolactinemia were not treated unless they had large pituitary tumors. Generally, the patients underwent transsphenoidal hypophysectomy if visual impairment was noted or fertility was desired. The longitudinal studies by Weiss and associates74 at the University of Southern California showed that adenomas grow very slowly when untreated, and in fact only 10% of the lesions expand. In addition, 32% of the time a progressive decrease in prolactin level was demonstrated. Such observations might negate the need for treatment of patients with all but macroadenomas. However, four reports have appeared in the literature that associate hyperprolactinemia with profound bone demineralization.75, 76, 77, 78 Patients who are both hypoestrogenic and hyperprolactinemic demineralize bone more rapidly than patients who are hypoestrogenic. Klibanski and Greenspan79 have shown that treatment of these patients with bromocriptine and restoration of normal prolactin levels causes a reversal of the demineralization process. Therefore, until the issue of bone demineralization is clarified, one could argue that all patients with hyperprolactinemia should be maintained in an estrogenic state to prevent bone loss.
In view of the association of bone demineralization with hyperprolactinemia, it would seem prudent to treat patients with functional hyperprolactinemia (i.e. patients who do not have pituitary tumors demonstrated on radiographic surveillance) with pharmacologic agents that will render them estrogenic. This could be accomplished by administering cyclic overlapping estrogen-progestogen therapy, oral contraceptive agents, or dopamine agonists.
Concern had been expressed in the past about treating patients with functional hyperprolactinemia or prolactinomas with estrogens because it was believed that these compounds stimulated prolactin secretion and because of the clinical observation that pituitary tumors had been shown to grow during pregnancy.80 Review of the literature shows that the stimulation of prolactin secretion by both estrogens and progestogens occurs primarily in lower animal models and has not been demonstrated in humans. In addition, most microadenomas show little or no growth during pregnancy, and most patients with macroadenomas have an uncomplicated pregnancy, labor, and delivery. A poll of reproductive endocrinologists directly involved in prolactin research showed that most would treat patients with functional hyperprolactinemia or microadenomas with replacement estrogen or oral contraceptive agents.81
Further, oral contraceptive agent treatment of patients with microadenomas produced no long-term change in tumor size. In addition, although birth control pills have been shown to induce a small increase in prolactin secretion, this effect is not thought to be clinically significant.
Bromocriptine
The primary mode of therapy for the treatment of functional hyperprolactinemia involves the use of dopamine agonists. Bromocriptine (Parlodel) was the first compound approved for the treatment of hyperprolactinemia in the United States.82 This type of drug is an ergot derivative closely related to LSD. The ergots were originally isolated from a fungus (Claviceps purpurea) that grew on rye. Ingestion of such grain resulted in St. Anthony's fire and the cessation of lactation. Modification of the molecule has attenuated some of the undesirable ergot side effects, but many patients experience nausea and vomiting, nasal stuffiness, lethargy, and dysphoria when taking bromocriptine. A few patients are hypersensitive to ergots and have hypotensive episodes. When ergots are administered in extremely high doses for the treatment of Parkinson's disease, tachyarrhythmias (often fatal) have been reported, as has the induction of psychosis. The dose range for the treatment of hyperprolactinemia with bromocriptine is 5–10 mg/day. However, subtherapeutic doses have often been shown to suppress prolactin levels and shrink pituitary tumors.83 The medicine should be administered at bedtime to take advantage of the blockade of the sleep-associated surge in prolactin. It has also been suggested that patients who have side effects such as nausea with oral bromocriptine might benefit from vaginal delivery of this drug.84 Serum Parlodel levels have been shown to rise more slowly than those achieved with oral delivery, but serum levels are maintained for a longer duration.85 Also, bromocriptine has been shown to have no deleterious effect on sperm function when tested in vivo or in vitro.86, 87, 88
Patients with functional hyperprolactinemia are often treated for 12–14 weeks, followed by discontinuation of the medication. In general, spontaneous cures are rare, and patients require long-term therapy. After pregnancy, patients often have a lower basal prolactin level, and at times spontaneous cures occur.
Several new forms of bromocriptine have been introduced into the European market. Parlodel SRO, a long-acting oral bromocriptine, suppresses the prolactin level with a single oral dose for more than 24 hours.89 After 1 month's therapy, 63% of patients had normal prolactin levels and 43% had return of menstruation. Likewise, Parlodel LAR is a long-acting repeatable injectable form of bromocriptine.90 The polymer matrix has a total mass degradation of less than 3 months and is being used every 28 days for prolactin suppression. These drugs are not available on the current US market.
Cabergoline
Cabergoline (Dostinex) is a D2 selective agonist. It has a very long half-life and can be given orally once or twice weekly. After oral administration, prolactin-lowering effects are detectable at 3 hours which then increases gradually to reach a plateau between 48 and 120 hours. Cabergoline is usually started at a dose of 0.25–0.5 mg orally once or twice weekly. The dose is gradually increased monthly until prolactin levels normalize. Doses of less than 3 mg per week are usually sufficient to achieve this goal. Cabergoline has been shown to be more effective than bromocriptine in a multicenter, 24-week trial of 459 hyperprolactinemic women randomized to either cabergoline or bromocriptine.91 Cabergoline induced normal prolactin levels in 83% compared with 59% with bromocriptine and ovulation cycles or pregnancies were obtained in 72% versus 52%. In addition, side effects were less frequent, less severe, and of shorter duration in patients treated with cabergoline. These findings have also been supported by several retrospective studies. Cabergoline has also been shown to have a significant tumor-shrinking effect in patients with macroprolactinoma. In a long-term follow-up study, 12–24 months of cabergoline treatment led to a greater than 80% decrease in tumor size in 61% of cases, with complete disappearance of the tumor in 6 out of 23 (26%) patients. In addition, an improvement in visual field defects was obtained in 9 out of the 10 patients presenting with visual impairment prior to cabergoline treatment.92
The most common side-effects are nausea and vomiting (~35%), headache (~30%), and dizziness (~25%).93 Although a median decrease in blood pressure of 10 mm Hg can occur in about 50% of patients treated with cabergoline or bromocriptine, this degree of hypotension is generally asymptomatic.91 There have been reports of pleuropulmonary inflammatory-fibrotic syndrome in a few patients with Parkinson’s disease treated with high doses of cabergoline. Rare patients experiencing limiting nausea and vomiting may be treated with intravaginal cabergoline.
Pergolide
Pergolide (Permax) is an ergot dopamine agonist and had been used primarily for the treatment of Parkinson’s disease. Hyperprolactinemia can be controlled with single daily doses of 50–150 mg. There have been reports of valvular heart disease when pergolide was used at high doses in Parkinson’s disease.94 In March 2007, pergolide was voluntarily removed from the market in the United States.
Quinagolide
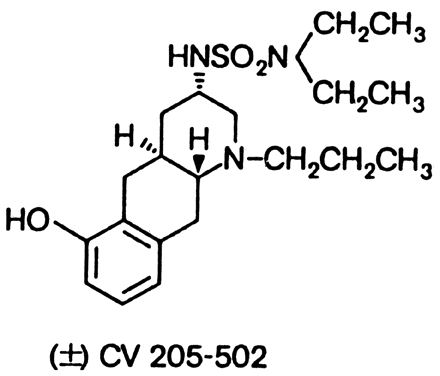
Quinagolide (CV 205-502) is a non-ergot dopamine agonist (Fig. 4). It is given once a day and has similar tolerance and efficacy compared to bromocriptine and pergolide. Although there are limited data on its use in pregnancy, quinagolide does not appear to be safe during pregnancy. It has been associated with fetal malfomations such as spina bifida, Trisomy 13, Down's syndrome, talipes, cleft lip, Zellweger syndrome, and arrhinencephaly.95 Quinagolide is not approved for use in the United States.
The patient with radiographic evidence of microadenoma may be treated in an identical manner to the patient with functional hyperprolactinemia. In general, bromocriptine is the treatment of choice on an indefinite basis to maintain bone stability. Considering the slow growth of these tumors, follow-up CT or MRI scanning is not recommended unless the patient were to break through bromocriptine suppression and develop symptoms of an expanding central nervous system lesion. Historically, transsphenoidal hypophysectomy was used to treat patients with microadenomas of the pituitary. However, studies have shown a 50% failure rate in terms of normalization of prolactin levels in patients undergoing such surgery. It is now recommended that surgery not be used as the primary mode of therapy in patients with microadenomas of the pituitary gland. The patient who fails to show suppression of prolactin levels or shrinkage of tumor with bromocriptine therapy possibly houses a non-endocrine-secreting tumor or one that produces another trophic hormone.96, 97
Patients with macroadenomas of the pituitary gland require lifelong therapy. The vast majority of these patients will promptly respond to bromocriptine therapy by lowering prolactin secretion and tumor reduction. These patients should be followed at intervals with either CT or MRI scanning until the tumor becomes nondetectable or stable. Subsequently, if continuous therapy is maintained and the patient has no sign of galactorrhea, headache, or menstrual dysfunction, she can be followed with visual field examinations yearly and serum prolactin evaluation every 6 months. Surgery should be reserved for patients refractory to bromocriptine therapy. About 70% of the patients undergoing transsphenoidal hypophysectomy with macroadenomas are not cured with surgery in terms of resolution of the pituitary tumor or restoration of the euprolactinemic state.98 Radiation treatment, either with cobalt or with Bragg peak proton therapy, can be used for refractory macroadenomas. Kjellberg and Kliman99 cured 678 patients with all types of adenomas (0.7% recurrence rate) using this type of therapy; however, some of these patients were rendered hypopituitary (10–20%) and must receive replacement therapy with thyroid, corticoid, and often posterior pituitary hormones.
Several investigators have recommended that bromocriptine therapy be used in persons with unexplained infertility. Ben-David and Chrambach100 have isolated an isoform of prolactin that may interfere with ovulation. It was suggested that when patients were rendered euprolactinemic in terms of native prolactin that the isoB form often remained elevated, requiring higher does of bromocriptine to produce suppression. Once isoB-prolactin returned to normal, a high conception rate was reported. Harrison and co-workers101 isolated a group of “prolactin spikers” with high anxiety scales. These women become pregnant when treated with bromocriptine as compared with controls. DeVane and Guzick102 treated patients with euprolactinemic galactorrhea; they attained a significant pregnancy rate when compared with patients treated with vitamin B6 or clomiphene. On the other hand, rendering a patient hyperprolactinemic with metoclopramide 4–5 days before ovulation does not appear to disrupt the process, and in a double-blind randomized placebo control study, investigators could not show a significant difference between patients treated with bromocriptine versus placebo in terms of conception rates.103, 104 Therefore, it is recommended that patients with unexplained infertility not be treated with dopamine agonist therapy empirically.
Although of historical importance, the choice of contraception in patients with hyperprolactinemia does not differ from that of the population at large, with the exception of patients with macroadenomas. These persons should not be treated with oral contraceptive agents, although prospective studies have not clearly shown an association between the use of birth control pills and tumor growth. Until this issue is resolved, it is probably best to have these patients treated by reproductive endocrinologists under protocol.
Another issue of historical importance is whether patients with hyperprolactinemia and prolactinoma should breastfeed. We recommend that patients with functional hyperprolactinemia or microadenomas be allowed to breastfeed because they are not at risk for exacerbating their condition. Patients with macroadenomas may also breastfeed, although they should be made aware that this process may stimulate growth of their tumor. In general, breastfeeding should be restricted to women who showed no sign of tumor on bromocriptine therapy before pregnancy.
The patient who presents with persistently elevated prolactin levels who is treated with bromocriptine can expect a pregnancy rate in excess of 80% within three or four cycles. There is no increased incidence of multiple births or malformations in patients treated with bromocriptine, although the drug should be discontinued as soon as a positive pregnancy test is obtained. Some reproductive endocrinologists treat patients with macroadenomas with bromocriptine throughout pregnancy. Long-term follow-up studies carried out in Japan, France, and Switzerland have shown no increased defects in motor or sensory development in children exposed to dopamine agonist therapy.105, 106
One issue that cannot be answered at this time is the natural history of pituitary tumors in the face of estrogen replacement therapy. It will take a generation for this question to be answered, using sophisticated radiographic techniques and serum prolactin monitoring to evaluate the growth pattern of microadenomas and macroadenomas in patients who do or do not receive estrogen replacement therapy.
LONG-TERM FOLLOW-UP OF HYPERPROLACTINEMIA
Patients with hyperprolactinemic syndromes require lifelong follow-up. The patient with no radiographic evidence of a pituitary tumor should be followed on an annual basis, and if she is taking medication to render her euprolactinemic, she should have a prolactin measurement performed at that time. If prolactin levels are within normal limits, the patient's menstrual function is undisturbed, and she has no evidence of galactorrhea, then radiographic surveillance is unnecessary.
The same mode of follow-up is recommended for a patient with a microadenoma. In general, these patients are on continuous bromocriptine therapy and should have a prolactin level measured every six months to one year. No visual field examination is needed, and it is recommended that radiographic surveillance not be reinstituted unless the patient has a precipitous rise in prolactin during therapy or develops symptoms of an expanding central nervous system lesion.
The patient with a macroadenoma requires lifelong suppressive therapy with bromocriptine or another second-generation dopamine agonist. She should be seen every six months, and prolactin levels should be measured. Once control of the tumor is achieved, the patient should undergo radiographic survey at widening intervals dependent on the length of follow-up (perhaps once every five years).
It has recently been suggested that idiopathic hyperprolactinemia may be an entirely different entity from the prolactinoma.107, 108, 109 Likewise, it may be that microadenomas and macroadenomas are entirely different entities. Macroadenomas frequently are detected in children and adolescents, are quite aggressive, and require continuous follow-up and intermittent treatment. On the other hand, the patient with idiopathic hyperprolactinemia rarely has a progression to prolactinoma and has a high frequency of spontaneous cure and pregnancy; prolactin levels are often shown to decline, particularly after pregnancy.110 Further, parous women have been shown to have diminished prolactin response to a challenge with metoclopramide, and there appears to be an age-related change in the ability of metoclopramide to induce prolactin release in nulliparous women, suggesting a gradual decrease in the dopaminergic tone in older women.111, 112, 113 Further, when one evaluates the dynamics of pulsatile prolactin released during the postpartum lactational period, it appears that this hyperprolactinemia is maintained by selectively altering the endogenous secretory rate in each prolactin release episode, with no change in the number of births or prolactin discharge, or the prolactin half-life. These findings are complicated by the fact that big prolactin has been detected in the serum of women with normal ovulatory function.114 Isoforms of human growth hormone have been found in the serum of normal ovulatory, normal prolactinemic women with galactorrhea, and there appears to be a change in molecular variance during in vitro transformation and release of prolactin by the pituitary glands of lactating rats.115, 116 All these factors may account for the wide variance in resumption of ovulation and menstruation after pregnancy and breastfeeding, and the variable contraceptive effect offered by lactation.117, 118, 119, 120 Finally, there appears to be a reduction in the risk of breast cancer among premenopausal women who have lactated, but there appears to be no reduction in the risk of breast cancer occurring in postmenopausal women with a history of lactation.121
REFERENCES
Chiari J, Braum C, Spaeth J: Report of two cases of puerperal atrophy of the uterus with amenorrhea and persistent lactation. In: Klinik der Geburtshilfe and Gynecology, p 371. Erlangen, Enke, 1855 |
|
Frommel R: Ueber puerperale atrophie des uterus. Gynakologue 7: 305, 1882 |
|
Argonz J, DelCastillo E: A syndrome characterized by estrogenic insufficiency, galactorrhea and decreased urinary gonadotropisms. J Clin Endocrinol Metabol 13: 79, 1953 |
|
Ahumada J, DelCastillo E: Amenorrhea/galactorrhea. Bol Soc Obstet Ginecol (Buenos Aires) 11: 64, 1954 |
|
Forbes A, Henneman P, Griswold G, Albright F: Syndrome characterized by galactorrhea, amenorrhea and low urinary FSH: Comparison with acromegaly and normal lactation. J Clin Endocrinol Metabol 14: 265, 1954 |
|
Costello RT: Subclinical adenoma of the pituitary gland. Am J Pathol 12: 205, 1936 |
|
Greenwood FC, Hunter WM: The preparation of 131 I-labeled human growth hormone of high specific radioactivity. Biochem J 89: 14, 1963 |
|
Guyda H, Hwang P, Friesen H: Immunologic evidence for monkey and human prolactin. J Clin Endocrinol Metabol 32: 267, 1971 |
|
Blackwell RE, Guillemin R: Hypothalamic control of adenohypophyseal secretions. Ann Rev Physiol 35: 357, 1973 |
|
MacLeod RM: Influence of norepinephrine and catecholamine-depleting agents on the synthesis and release of prolactin and growth hormone. Endocrinology 85: 916, 1969 |
|
Leblanc H, Lechelin G, Abu-Fadil S, Yen SSC: Effects of dopamine infusion in pituitary hormone secretion in humans. J Clin Endocrinol Metabol 43: 669, 1976 |
|
Gibbs OM, Neill JD: Dopamine levels in hypophyseal stalk blood in the rat are sufficient to inhibit prolactin secretion in vitro. Endocrinology 102: 1895, 1978 |
|
Schettini G, Cronin MJ, MacLeod RM: Adenosine 3´,5´-monophosphate (cAMP) and calcium-calmodulin interrelation in the control of prolactin secretion: Evidence for dopamine inhibition of cAMP accumulation and prolactin release after calcium mobilization. Endocrinology 112: 1801, 1983 |
|
Matsushita N, Kato Y, Shimatsu A et al: Effects of VIP, TRH, GABA and dopamine on prolactin release from superfused rat anterior pituitary cells. Life Sci 32: 1263, 1983 |
|
Vale W, Blackwell RE, Grant C, Guillemin R: TRF and thyroid hormones on prolactin secretion by rat pituitary cells in vitro. Endocrinology 93: 26, 1973 |
|
Jacobs LS, Snyder PF, Wilber JF et al: Increased serum prolactin after administration of synthetic thyrotropin-releasing hormone (TRH) in man. J Clin Endocrinol Metabol 33: 996, 1971 |
|
Neill JD, Luque J, Mulchahey J, Nagy G: Regulation of prolactin secretion. In: Blackwell RE, Chang RJ (eds): Prolactin-Related Disorders: Proceedings of a Symposium. Florham Park, NJ, Macmillan, 1987 |
|
Dufy-Barbe R, Rodriguez F, Arsaut J et al: Angiotensin II stimulates prolactin release in the rhesus monkey. Neuroendocrinology 35: 242, 1982 |
|
Knigge U, Dejguard A, Wollesen F et al: Histamine regulation of prolactin secretion through H1 -H2 -receptors. J Clin Endocrinol Metabol 55: 118, 1982 |
|
Enjalbert A, Arancibia S, Priam M et al: Neurotensin stimulation of prolactin secretion in vitro. Neuroendocrinology 34: 95, 1982 |
|
Bergland R, Page R: Can the pituitary secrete directly to the brain (affirmative anatomical evidence)? Endocrinology 102: 1325, 1978 |
|
Gudelsky GA, Porter JC: Release of dopamine from tuberoinfundibular neurons into pituitary stalk blood after prolactin or haloperidol administration. Endocrinology 105: 526, 1980 |
|
Denef C, Andries M: Evidence for paracrine interaction between gonadotrophs and lactotrophs in pituitary cell aggregates. Endocrinology 112: 813, 1983 |
|
Blackwell RE, Rogers-Neame NT, Bradley EL, Asch RH: Regulation of human prolactin secretion by gonadotropin-releasing hormone in vitro. Fertil Steril 46: 26, 1986 |
|
Blackwell RE, Garrison PN: Inhibition of prolactin secretion by antiserum to the &b.alpha;- and &b.beta;-subunits of gonadotropin. Am J Obstet Gynecol 156: 863, 1987 |
|
Kendall ME, Hymer WC: Cell blotting: A new approach to quantify hormone secretion from individual rat pituitary cells. Endocrinology 121: 2260, 1987 |
|
Tanaka T, Shiu RPC, Gout PW et al: A new sensitive and specific bioassay for lactogenic hormones: Measurement of prolactin and growth hormone in human serum. J Clin Endocrinol Metabol 51: 1058, 1980 |
|
Childs GV: Multipotential pituitary cells that contain adrenocorticotropin (ACTH) and other pituitary hormones. Trends Endocrinol Metabol 2: 112, 1991 |
|
Pellegrini I, Rasolonjanahary R, Gunz G et al: Resistance to bromocriptine in prolactinomas. J Clin Endocrinol Metabol 60: 500, 1989 |
|
Webb CB, Thominet JL, Barowsky H et al: Evidence for lactotroph dopamine resistance in idiopathic hyperprolactinemia. J Clin Endocrinol Metabol 56: 1089, 1983 |
|
Arita J, Kojima Y, Kimura F: Identification by the sequential cell immunoblot assay of a subpopulation of rat dopamine-unresponsive lactotrophs. Endocrinology 128: 1887, 1991 |
|
Arita J, Kojima Y, Kimura F: Lactotrophs secreting small amounts of prolactin reveal great responsiveness to thyrotropin-releasing hormone: Analysis by the sequential cell immunoblot assay. Endocrinology 130: 3167, 1992 |
|
Kineman RD, Faught WJ, Frawley LS: Steroids can modulate transdifferentiation of prolactin and growth hormone cells in bovine pituitary cultures. Endocrinology 130: 3289, 1992 |
|
Sinha YN: Prolactin variants. Trends Endocrinol Metabol 3: 100, 1992 |
|
Larrea F, Escorza A, Valero A et al: Heterogeneity of serum prolactin throughout the menstrual cycle and pregnancy in hyperprolactinemic women with normal ovarian function. J Clin Endocrinol Metabol 68: 982, 1989 |
|
Cooke NE, Coit D, Shine J et al: Human prolactin: cDNA structural analysis and evolutionary comparisons. J Biol Chem 256: 4007, 1981 |
|
Chen X, Horseman ND: Cloning, expression, and mutational analysis of the pigeon prolactin receptor. Endocrinology 135: 269, 1994 |
|
Kelly PA, Djiane J, Edery M: Different forms of the prolactin receptor: Insights into the mechanism of prolactin action. Trends Endocrinol Metabol 3: 54, 1992 |
|
Cullen KE, Kladde MP, Seyfred MA: Interaction between transcription regulatory regions of prolactin chromatin. Science 261: 203, 1993 |
|
Moretuzzo RW, Layman LC, Tho SPT et al: Gonadotropin-releasing hormone-associated peptide gene sequences in women with hyperprolactinemia. Fertil Steril 58: 908, 1992 |
|
Hsu DW, El-Azouzi M, Black PM et al: Estrogen increases galanin immunoreactivity in hyperplastic prolactin-secreting cells in Fisher 344 rats. Endocrinology 126: 3159, 1990 |
|
Wynick D, Hammond PJ, Akinsanya KO, Bloom SR: Galanin regulates basal and oestrogen-stimulated lactotroph function. Nature 364: 529, 1993 |
|
Hinkle PM, Scammell JG, Shanshala ED: Prolactin and secretogranin-II, a marker for the regulated pathway, are secreted in parallel by pituitary GH4 C1 cells. Endocrinology 140: 3503, 1992 |
|
Pepperell RJ: Prolactin and reproduction. Fertil Steril 35: 267, 1981 |
|
Magoffin DA, Erickson GF: Prolactin inhibition of LH stimulated androgen synthesis in ovarian interstitial cells cultured in defined medium: Mechanism of action. Endocrinology 111: 2001, 1982 |
|
McNatty KP: Relationship between plasma prolactin and the endocrine microenvironment of the developing human antral follicle. Fertil Steril 32: 433, 1979 |
|
Kauppila A, Martikainen H, Puistola U et al: Hypoprolactinemia and ovarian function. Fertil Steril 49: 437, 1988 |
|
Lane TA, Chen TT: Heterologous down-modulation of luteinizing hormone receptors by prolactin: A flow cytometry study. Endocrinology 128: 1833, 1991 |
|
Adashi EY, Resnick CE: Prolactin as an inhibitor of granulosa cell luteinization: Implications for hyperprolactinemia-associated luteal phase dysfunction. Fertil Steril 48: 131, 1987 |
|
Martikainen H, Ronnberg L, Puistola U et al: Prolactin suppression by bromocriptine stimulates aromatization of testosterone to estradiol in women. Fertil Steril 52: 51, 1989 |
|
Matsuzaki T, Azuma K, Irabara M et al: Mechanism of anovulation in hyperprolactinemic amenorrhea determined by pulsatile gonadotropin-releasing hormone injection combined with human chorionic gonadotropin. Fertil Steril 62: 1143, 1994 |
|
Inaudi P, Reymond MJ, Rey F et al: Pulsatile secretion of gonadotropins and prolactin during the follicular and luteal phases of the menstrual cycle: Analysis of instantaneous secretion rate and secretory concomitance. Fertil Steril 58: 51, 1992 |
|
Huang K, Bonfiglio TA, Muechler EK: Transient hyperprolactinemia in infertile women with luteal phase deficiency. Obstet Gynecol 78: 651, 1991 |
|
Soules MR, Bremmer WJ, Steiner RA, Clifton DK: Prolactin secretion and corpus luteum function in women with luteal phase deficiency. J Clin Endocrinol Metabol 72: 986, 1991 |
|
Asukai K, Uemura T, Minaguchi H: Occult hyperprolactinemia in infertile women. Fertil Steril 60: 423, 1993 |
|
Blackwell RE: Diagnosis and treatment of hyperprolactinemic syndromes. Fertil Steril 43: 5, 1985 |
|
Atchison JA, Lee PA, Albright AL: Reversible suprasellar pituitary mass secondary to hypothyroidism. JAMA 262: 3175, 1989 |
|
Blackwell RE, Chang RJ, Cragun JR: Prolactin disorders in infertility. In Seibel MM (ed): Infertility: A Comprehensive Text. East Norwalk, CT, Appleton & Lange, 1990 |
|
Frantz A: Prolactin. N Engl J Med 298: 201, 1978 |
|
Goyal N, Gore MA, Shankar R. Galactorrhea and amenorrhea in burn patients. Burns, 2008 (in press) |
|
Blackwell RE, Younger JB: Assessment of pituitary function. In Wynn RM (ed): Obstetric and Gynecology Annual, Vol 10, p 349. New York, Appleton-Century-Crofts, 1981 |
|
Brun del Re R, del Pozo E, deGrandi P et al: Prolactin inhibition and suppression of puerperal lactation by a Bromergocriptine (CB154): A comparison with estrogen. Obstet Gynecol 41: 884, 1973 |
|
Oberman HA: Benign breast lesions confused with carcinoma. In McDuitt RW, Oberman HA, Ozzello K, Kaufman N (eds): International Academy of Pathology Monograph: The Breast. Baltimore, Williams & Wilkins, 1984 |
|
Blackwell RE, Younger JB: Long-term medical therapy and follow-up of pediatric-adolescent patients with prolactin-secreting macroadenomas. Fertil Steril 45: 713, 1986 |
|
Dollar JR, Blackwell RE: Diagnosis and management of prolactinomas. Cancer Metastasis Rev 5: 125, 1986 |
|
Blackwell RE, Boots LR, Goldenberg RL, Younger JB: Assessment of pituitary function in patients with serum prolactin levels greater than 100 ng/ml. Fertil Steril 39: 744, 1979 |
|
McAtee JW, Trenkle A: Effects of feeding, fasting, glucose or arginine on plasma prolactin levels in the bovine. Endocrinology 89: 730, 1971 |
|
Wiebe RH, Hammond CB, Handwerger S: Prolactin-secreting pituitary microadenoma: Detection and evaluation. Fertil Steril 29: 282, 1978 |
|
Keye WR, Chang RJ, Wilson CB, Jaffe RB: Prolactin-secreting pituitary adenomas: III. Frequency and diagnosis in amenorrhea-galactorrhea. JAMA 244: 1329, 1980 |
|
Halle AA, Drewry RD, Robertson JT: Ocular manifestations of pituitary adenomas. South Med J 76: 732, 1983 |
|
Lachelin GCL, Abu-Fadil S, Yen SSC: Functional delineation of hyperprolactinemia-amenorrhea. J Clin Endocrinol Metabol 44: 1163, 1977 |
|
St-Jean E, Blain F, Comtois R. High prolactin levels may be missed by immunoradiometric assay in patients with macroprolactinomas. Clinical Endocrinology 44; 305-309, 1996. |
|
Gibney J, Smith TP, McKenna TJ. The impact on clinical practice of routine screening for macroprolactin. J Clin Endocrinol Metab 90; 3927-3932, 2005. |
|
Weiss MH, Teal J, Gott P, Wsycoff R et al: Natural history of microprolactinomas: Six-year follow-up. Neurosurgery 12: 180, 1983 |
|
Schlechte JA, Sherman B, Martin R: Bone density in amenorrheic women with and without hyperprolactinemia. J Clin Endocrinol Metabol 56: 1120, 1983 |
|
Klibanski A, Need RM, Bettins IZ et al: Decreased bone density in hyperprolactinemic women. N Engl J Med 303: 1511, 1980 |
|
Cann CE, Martin MC, Genant HK et al: Decreased spinal mineral content in amenorrheic women. JAMA 251: 626, 1984 |
|
Koppelman MCS, Kurtz DW, Morrison KA et al: Vertebral body bone mineral content in hyperprolactinemic women. J Clin Endocrinol Metabol 59: 1050, 1994 |
|
Klibanski A, Greenspan SL: Increase in bone mass after treatment of hyperprolactinemic amenorrhea. N Engl J Med 315: 542, 1986 |
|
Blackwell RE, Chang RJ: Report of the national symposium on the clinical management of prolactin-related reproductive disorders. Fertil Steril 45: 607, 1986 |
|
Barbieri RL, Ryan KJ: Bromocriptine: Endocrine pharmacology and therapeutic applications. Fertil Steril 39: 72, 1983 |
|
Soto-Albors CE, Walters CA, Riddick DH, Daly DC: Titrating the dose of bromocriptine when treating hyperprolactinemic women. Fertil Steril 41: 58, 1984 |
|
Blackwell RE, Bradley EL Jr, Kline LB et al: Comparison of dopamine agonists in the treatment of hyperprolactinemic syndromes: A multicenter study. Fertil Steril 39: 744, 1983 |
|
Vermesh M, Fosum GT, Kletzky OA: Vaginal bromocriptine: Pharmacology and effect on serum prolactin in normal women. Obstet Gynecol 72: 693, 1988 |
|
Katz E, Weiss BE, Hassell A et al: Increased circulating levels of bromocriptine after vaginal compared with oral administration. Fertil Steril 55: 882, 1991 |
|
Chenette PE, Siegel MS, Vermesh M, Kletzky OA: Effect of bromocriptine on sperm function in vitro and in vivo. Obstet Gynecol 77: 935, 1991 |
|
Rojas FJ, Djannati E, Rojas IM: The effect of bromocriptine on the motility of human spermatozoa and its capacity to penetrate the cervical mucus. Fertil Steril 55: 48, 1991 |
|
Scommegna A, Ye SH, Prins GS: Bromocriptine reverses the inhibitory effect of macrophages on human sperm motility. Fertil Steril 61: 331, 1994 |
|
Weingrill CO, Mussio W, Moraes CRS et al: Long-acting oral bromocriptine (Parlodel SRO) in the treatment of hyperprolactinemia. Fertil Steril 57: 331, 1992 |
|
Ciccarelli E, Miola C, Avataneo T et al: Long-term treatment with a new repeatable injectable form of bromocriptine, Parlodel LAR, in patients with tumorous hyperprolactinemia. Fertil Steril 52: 930, 1989 |
|
Webster J, Piscitelli G, Polli A, et al. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. Cabergoline Comparative Study Group. N Engl J Med 331; 904-909, 1994. |
|
Colao A, Di Sarno A, Landi ML, et al. Long term and low-dose treatment with cabergoline induces macroprolactinoma shrinkage. J Clin Endocrinol Metab 82; 3574-3579, 1997. |
|
Rains CP, Bryson HM, Fitton A. Cabergoline. A review of its pharmacological properties and therapeutic potential in the treatment of hyperprolactinemia and inhibition of lactation. Drugs 49; 255-279, 1995. |
|
Schade R, Andersohn F, Suissa S, et al. Dopamine agonists and the risk of cardiac valve regurgitation. N Engl J Med 356; 29, 2007. |
|
Webster. A comparative review of the tolerability profiles of dopamine agonists in the treatment of hyperprolactinemia an dinhibition of lactation. Drug saf 14; 228-238, 1996. |
|
Domingue JN, Richmond IL, Wilson CB: Results of surgery on 114 patients with prolactin-secreting pituitary adenomas. Am J Obstet Gynecol 137: 102, 1980 |
|
Faria MA, Tindall GT: Transsphenoidal microsurgery for prolactin-secreting pituitary adenomas: Results in 100 women with the amenorrhea-galactorrhea syndrome. J Neurosurg 56: 33, 1982 |
|
Serri O, Rasio E, Beuregard H et al: Recurrence of hyperprolactinemia after selective transsphenoidal adenomectomy in women with prolactinoma. N Engl J Med 309: 280, 1982 |
|
Kjellberg R, Kliman B: Proton radiosurgery for functioning pituitary adenoma. In Tindall GE, Collins WF (eds): Clinical Management of Pituitary Disorders, p 315. New York, Raven Press, 1979 |
|
Ben-David M, Chrambach A: A method for isolation by gel electrofocusing of isohormones B and C of human prolactin from amniotic fluid. J Endocrinol 84: 125, 1980 |
|
Harrison FR, O'Moore A, Mosurski K et al: Intermittent hyperprolactinemia and the unexplained infertile couple: A placebo-controlled study of combined clomiphene citrate, bromocriptine therapy. Infertility 9: 1, 1986 |
|
DeVane G, Guzick D: Bromocriptine therapy in normoprolactinemic women with unexplained infertility and galactorrhea. Fertil Steril 46: 1026, 1986 |
|
McBain JC, Pepperell RJ: Use of bromocriptine in unexplained infertility. Clin Reprod Fertil 1: 145, 1982 |
|
Yikoek LO, Kauppila A: The effects on the ovulatory cycle of metoclopramide-induced increased prolactin levels, during follicular development. Fertil Steril 35: 588, 1981 |
|
Turkalj I, Braun P, Krupp P: Surveillance of bromocriptine in pregnancy. JAMA 247: 1589, 1982 |
|
Kurachi K, Aono T, Koike K et al: A follow-up survey of infants born to mothers treated with bromocriptine. Sanka to Fujinka 50: 126, 1983 |
|
Sluijmer AV, Lappohn RE: Clinical history and outcome of 59 patients with idiopathic hyperprolactinemia. Fertil Steril 58: 72, 1992 |
|
Schlechte J, Dolan K, Sherman B et al: The natural history of untreated hyperprolactinemia: A prospective analysis. J Clin Endocrinol Metabol 69: 412, 1989 |
|
Corenblum B, Taylor PJ: Idiopathic hyperprolactinemia may include a distinct entity with a natural history different from that of prolactin adenomas. Fertil Steril 49: 544, 1988 |
|
Musey VC, Collins DC, Musey PI et al: Long-term effect of a first pregnancy on the secretion of prolactin. N Engl J Med 316: 229, 1987 |
|
Parra A, Alarcon J, Gavino F et al: Age-related changes in the metoclopramide-induced prolactin release in nulliparous women. Fertil Steril 60: 34, 1993 |
|
de los Monteros AE, Cornejo J, Parra A: Differential prolactin response to oral metoclopramide in nulliparous versus parous women throughout the menstrual cycle. Fertil Steril 55: 885, 1991 |
|
Nunley WC, Urban RJ, Kitchin JD et al: Dynamics of pulsatile prolactin release during the postpartum lactational period. J Clin Endocrinol Metabol 71: 287, 1991 |
|
Fraser IS, Lun ZG, Zhou JP et al: Detailed assessment of big prolactin in women with hyperprolactinemia and normal ovarian function. J Clin Endocrinol Metabol 69: 585, 1989 |
|
Ochoa R, Mason M, Fonseca E et al: Distribution of growth hormone isoforms in sera from women with normal ovarian function, galactorrhea, and normoprolactinemia. Fertil Steril 60: 272, 1993 |
|
Mena F, Hummelt G, Aguayo D et al: Changes in molecular variants during in vitro transformation and release of prolactin by the pituitary gland of the lactating rat. Endocrinology 130: 3365, 1992 |
|
Lewis FR, Brown JR, Renfree MB, Short RV: The resumption of ovulation and menstruation in a well-nourished population of women breastfeeding for an extended period of time. Fertil Steril 55: 529, 1991 |
|
Diaz S, Cardenas H, Brandeis A et al: Early difference in the endocrine profile of long and short lactational amenorrhea. J Clin Endocrinol Metabol 72: 196, 1991 |
|
Short RV, Lewis PR, Renfree MB, Shaw G: Contraceptive effects of extended lactational amenorrhea: Beyond the Bellagio Consensus. Lancet 337: 715, 1991 |
|
Dewey KG, Lovelady CA, Nommsen-Rivers LA et al: A randomized study of the effects of aerobic exercise by lactating women on breast-milk volume and composition. N Engl J Med 330: 449, 1994 |
|
Newcomb PA, Storer BE, Longnecker MP et al: Lactation and a reduced risk of premenopausal breast cancer. N Engl J Med 330: 81, 1994. |