Infection in Maternal-Fetal Medicine: An Overview
Authors
INTRODUCTION
The era in which puerperal sepsis was common has lapsed, leaving behind some historical footnotes which teach the obstetrician a need for vigilance in watching for infections in the obstetric population and which confer on him a sense of urgency when dealing with such infections. The modern physician entrusted with maternal and fetal care recognizes that the problem of infectious disease no longer revolves around the single entity of fulminating hemolytic streptococcal disease in the mother and newborn but that the etiologic agents of obstetric infections today span the microbiologic gamut. Hence, antimicrobial therapy, often erroneously conceived to be the final step in managing infections, is simply not useful for treating many of the currently encountered disease processes. Appropriate management involves much more than matching a disease-causing agent with a drug.
The challenge of dealing effectively with obstetric infections relates to several additional aspects of infectious disease and obstetric practice: 1) The majority of infectious agents encountered by the modern obstetrician are less virulent than those with which his predecessors had to deal, and the diseases which they cause may, therefore, be recognized less rapidly or may even go unnoticed; however, if these diseases are improperly managed, the ultimate oucome may be disastrous. 2) Antibiotics may be as ineffective for treating certain bacterial infections as they are for treating viral infections. First, microorganisms may display unusual or unexpected patterns of resistance to antimicrobial agents which might otherwise be the drugs of choice. Multiply resistant organisms compound this problem. Antibiotic therapy may also be limited by the pharmacodynamics of the drugs in the maternal-fetal system; while antibiotics do reach the fetus in utero, antibiotic treatment of the fetus is likely to fail and is not advisable. 3) Present medical practice includes a variety of procedures which may diminish the natural defenses of the host. For example, amniocentesis or intrauterine fetal monitoring may result in contamination of the amniotic fluid. Scalp abscess has been reported as a sequela of scalp electrode use. Although infrequent, serious pelvic or hip infections may occur in women who have had pudendal or paracervical local anesthesia during delivery. 4) Finally, the excessive susceptibility of the fetus to infection may be the most significant and distressing aspect of infectious diseases in obstetrics. A subclinical or inapparent disease process in the mother may cause severe damage to the fetus. This fetal damage may be manifested at birth or later.
PRINCIPLES OF INFECTION AND IMMUNITY
Contemporary experience with clinical infections indicates that virtually any organism may behave as a pathogen under appropriate circumstances. Therefore, rather than classifying organisms as pathogens or nonpathogens, it is better to place them along a continuum from lesser to greater virulence. Likewise, among individual human hosts, there is a continuum in the intrinsic ability of each host to resist infection. Perhaps the most perspicacious statement of the relationship between microbial virulence and host resistance to infection was suggested by Theobald Smith in 1934 1. Accurately reflecting the nature of the infectious process today despite modern changes in the ecology of infections, Smith's equation states:
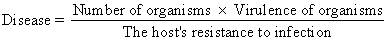
Implicit in this equation is the fact that the outcome of a host's encounter with an infectious agent, even an agent reputed to be a pathogen, will not necessarily be an infectious disease. However, disease may occur even with an organism of relatively low virulence if the host has diminished resistance or if the host is presented with an overwhelming number of organisms. In addition to its academic interest, this equation has practical significance. Specialists in maternal-fetal medicine are becoming more concerned with individualized care of obstetric patients, particularly high-risk patients. Therefore, the clinician should attempt to understand the role of the individual host. in infectious disease. This will not only help the physician to guide the care of the infected patient but also help him to recognize patients who are at special risk for developing sepsis.
IMPORTANT DISEASE ENTITLES IN OBSTETRICS
Areas of Physician Concern
There are no data to suggest that pregnancy precludes the occurrence of any specific infection. Therefore, the obstetrician in his role as primary care physician to the gravida must anticipate the occurrence and treatment of a variety of illnesses, many of which are irrelevant to the female genital tract or to the patient's pregnancy. However, there are a number of infectious diseases which are of special concern to the obstetrician because they are specific to the genitourinary system, because they occur more frequently or are more severe in the pregnant woman (such disease processes may be of no direct hazard to the fetus but represent a threat to the mother, especially during parturition), or because they threaten the well-being of the fetus, regardless of the effect of the infection in the mother.
Spectrum of Disease-Causing Agents
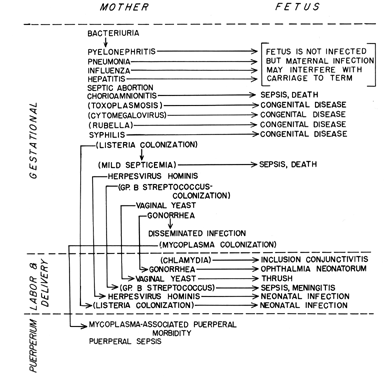
As an aid both to understanding the pathogenesis of an infectious disease and in developing a diagnosis, it is appropriate to group the diseases of interest according to which person of the maternal-fetal dyad is most severely affected and according to when during pregnancy the disease is likely to cause its greatest damage (Fig. 1).
As seen in Figure 1, the etiologic agents of the infectious processes of special concern to the obstetrician include viruses, bacteria, mycoplasmas, fungi, and protozoa. This diversity of causative agents is reflected in a diversity of mechanisms of transmission and pathogenesis. This diversity makes generalization difficult, with the result that an understanding of any particular disease entity will not be gained from this overview but will be found in one of the subsequent chapters.
Magnitude of the Problem
The following are three general questions that should be answered in assessing the magnitude of the problem of infectious diseases occurring in pregnancy. 1) What percentage of pregnant patients can be expected to acquire a specific disease? 2) In those women who get the disease, how frequently does the microorganism reach the fetus? 3) With what frequency does the microorganism seriously and permanently affect the fetus?
Such information is only partially available. Many problems exist in compiling the data, and the reader should be aware of these before placing undue reliance on such information. For certain infectious diseases, the significance during pregnancy has only recently been recognized, and few data have been made available. Although the national Center for Disease Control (CDC) collects and publishes disease incidence data, this source is of minimal value since many of the disease entities of interest to the obstetrician are not notifiable diseases and thus are not reported through the CDC. In addition, :notifiable diseases occurring coincidentally with pregnancy are not identified. An exception is rubella, for which a National Registry for Congenital Rubella Syndrome Surveillance was established in 1969 by the CDC. Congenital syphilis is also a notifiable disease and is reported through the CDC.
Perhaps the most useful information for the obstetrician comes from individual studies done for the purpose of developing disease incidence data. However, it must be remembered that such studies are limited to the time and population group from which they originated. Disease incidence is different for different socioeconomic groups, poverty being a positive correlate in some diseases. The time when disease incidence is determined is extremely important, as is illustrated by the changes in the incidence of the congenital rubella syndrome. In 1964, an epidemic year, Cooper 2 conservatively estimated that 30,000 infants were rubella casualties nationwide, whereas only 30 cases of congenital rubella syndrome were identified by the CDC in 1975. Thus, it is clear that disease incidence in the pregnant population is dynamic and differs with time, diagnostic methodology, disease control activities (eg, vaccination), and interest in reporting the disease.
Finally, disease incidence data are subject to variations in diagnostic criteria. Diagnosis of a viral infection is not often established by culture but is more frequently based on serology; however, it may be based on a symptom or combination of symptoms, serology, and/or culture. The criteria used for identifying a positive case affect the incidence and prevalence results, making it difficult to compare one study with another. In addition, particularly with vital diseases, one must be aware that symptoms may represent a reactivation of a previous disease and that positive serology may indicate a prior exposure.
Table 1 is presented as a generalization of the scope of infections in pregnancy and provides only a broad and admittedly imprecise overview.
TABLE 1. Generalizations on the Risks Associated With Various Infections During Pregnancy
Infections | Mother | Fetus |
Bacterial |
|
|
Urinary tract | Approximately 6% of pregnant | Hazard to the fetus |
infections | women have bacteriuria; if | is not adequately |
| untreated, one third | established |
| will suffer renal invasion |
|
Pneumonia | 1/1,000 pregnancies are | Greatest proportion |
| complicated by pneumonia, | of fetal loss occurs |
| with the greatest incidence | in mild trimester; 26% |
| occurring in mid trimester; | fetal loss without |
| mortality rate is 12.5% without | antibiotics, 17% fetal |
| antibiotics, 3.5% with anti- | loss with antibiotics |
| biotics |
|
Septic abortion | Causes an estimated 5,000 |
|
| maternal deaths annually |
|
Chorioamnionitis | In patients who experience | In separate reports, 30% |
| rupture of membranes without | to 50% of neonatal deaths |
| labor for more than 24 | were associated with |
| hours, incidence approaches | clinically evident |
| 20% | amnionitis |
Group B β-hemolytic | Although present in the vaginal | Serious sepsis occurs in |
streptococci | flora of 5% to 15% of pregnant | 2 to 3/1,000 live |
| women, these organisms are | births |
| of little importance in |
|
| maternal infection |
|
Gonorrhea | Incidence in the pregnant | Ophthalmia occurs in |
| population is 2% to 5% | approximately 1% |
|
| of infants |
Syphilis |
| If mothers are untreated, |
|
| 70% to 100% of infants |
|
| are affected |
Tuberculosis | There is an estimated | Congenital tuberculosis |
| incidence of 1% in most | is extremely rare |
| obstetric populations | but usually fatal |
Viral |
|
|
Cytomegalovirus | Although less than 5% of women | Fifty percent of fetuses |
| become infected during | of mothers primarily |
| pregnancy,12% to 28% of | infected will shed |
| mothers harbor virus in | virus at birth, |
| the third trimester | and one fifth of |
|
| these are symptomatic |
Rubella | The attack rate is 8/10,000 | If mother is infected |
| pregnancies | during first trimester, |
|
| 70% of children will |
|
| show symptoms by age |
|
| 4 years; mortality |
|
| rate is greater than 30% |
Herpes simplex | Occurs in approximately | If herpes genitalis is |
(primarily herpes | 0.3% to 1% of pregnant | present at time of birth, |
genitalis) | patients; incidence is | approximately one third |
| dependent on socioeconomic | of infants will be |
| status | affected |
Hepatitis | Jaundice occurs in approxi- | Main fetal complication |
| mately 0.5 to 1/3,000 | is prematurity |
| pregnancies and is due to | resulting from maternal |
| viral infection approxi- | infection, most often |
| mately one half of the | seen in developing |
| time | countries |
Others |
|
|
Toxoplasmosis | Approximately 0.2% of | If the mother acquires |
| women acquire this | the disease, the inci- |
| disease during pregnancy | dence of fetal acquisi- |
|
| tion is 30% to 50% |
Candidiasis | Colonization occurs in | Incidence of fetal |
| approximately 15% to | infection approached |
| 30% of women, half of | 4% in one study |
| whom may have symptoms |
|
It may seem from Table 1 that the magnitude of any one of these problems is relatively small. However, in view of the many possible complications of infectious disease which many occur during pregnancy, the problem becomes more formidable. In a large collaborative study 3 completed in 1964, it was found that 5% of pregnancies were at some time complicated by some viral infection, not including the common cold. No similar overall estimate of the number of bacterial infections occurring during pregnancy is available, although it has been estimated that the neonatal mortality associated with bacterial infection acquired during the birth process is 30% despite modern diagnosis and therapy. If one considers the number of births nationally during a year, it is clear that the number of potential individual tragedies may be vast.
ORGANISMIC FACTORS OF DISEASE PROCESSES
Basic Steps in Pathogenesis
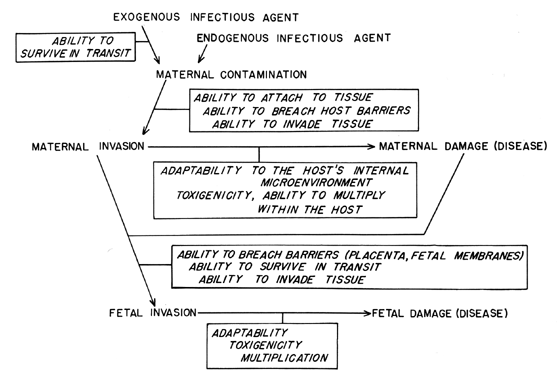
Figure 2 summarizes the infectious organism's role in the steps of the pathogenesis of obstetric infections. An infectious organism must possess certain attributes to consistently produce disease in susceptible hosts. First, the organism must be transmissible, ie, it must be able to survive in transit from one host to another. Secondly, it must gain entry into its host. A first step in this process may be the attachment of the infectious agent to some host tissue, with subsequent invasion. This is an essential step for viral diseases and is important in many bacterial infections as well. Organisms may possess certain invasive properties which allow them to gain access to the host. For example, many Gram-positive bacteria elaborate a hyaluronidase which depolymerizes a tissue's ground substance and is putatively a “spreading factor.” In some instances, a 'break in the normal integrity of the host's anatomic barriers provides access to the host's tissue or bloodstream. Some infections are of endogenous origin, or the offending organism may already be present on the host as indigenous flora. Such organisms gain access when host tissue is damaged or when normal defense mechanisms of the host are altered.
Having gained access to the host, the organism must adapt to the microenvironment within the host in order to survive and to multiply. Adaptation to the microenvironment may simply involve induction of metabolic pathways to utilize the available nutrients, or it may be more complex and involve processes which circumvent host antimicrobial properties, eg, production of an antiphagocytic capsule. Multiplication of the organism within the host may also be enhanced by production of a toxin which causes some specific damage to the host. Although not all infectious agents produce an identifiable toxin, the host reaction to their mere presence may actually damage the host. The clinical symptoms of infection result from the damage that microbial multiplication produces in the host.
For the obstetrician, extension of the disease process to the fetus is of great concern, and understanding how the fetus becomes infected is a separate study.
Transmission of Infectious Organisms to the Offspring
IN UTERO.
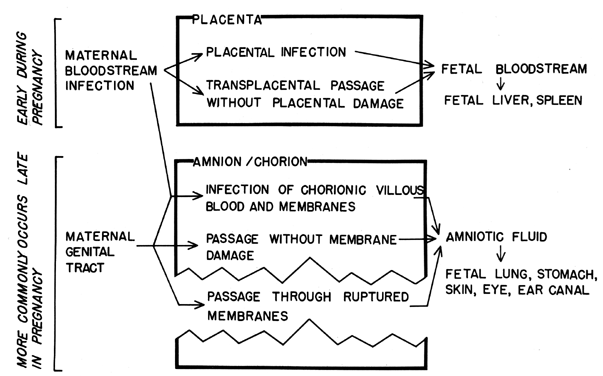
Intrauterine infection of the fetus may occur by several routes (Fig. 3). The most probable route to intrauterine infection during early pregnancy is transplacental passage, although transplacental infection may occur at any time during pregnancy. Table 2 lists a variety of organisms which may cross the placenta. There are no apparent features common to all of the organisms which would indicate reasons for their successful placental passage. The infectious agents most commonly involved are viruses. This may reflect the fact that viral invasion of the blood (and, thus, placental contact) is a more common feature in the natural history of viral infections than is bloodstream invasion in other types of infections. It has also been postulated that the placental barrier and the blood-brain barrier are analogous and that similar mechanisms of transit may be operational. This has not been substantiated.
TABLE 2. Organisms Which May Cause Intrauterine Infection and Most Common Routes to Infection
Hematogenously Acquired | Acquired by Ascending Infection |
Cytomegalovirus | Escherichia coli |
Rubella virus | Streptococcus faecalis |
Varicella-herpes zoster virus | Staphylococci |
Variola virus | β-Hemolytic streptococci |
Vaccinia virus | groups A and B |
Listeria monocytogenes | Anaerobic cocci |
Treponema pallidum | Bacteroides fragilis |
Toxoplasma gondii | Candida albicans |
Enteric bacteria | Herpes simplex virus |
Mumps virus | Clostridium perfringens |
Rubeola virus | Listeria monocytogenes |
Vibrio fetus | Proteus, klebsiella, and |
Coxsackievirus B | Pseudomonas |
Poliomyelitis virus |
|
|
Organisms which cross the placenta show a gradation in their production of placental pathology, which ranges from no apparent damage to production of severe placental lesions (Table 3). Whether the ability of an organism to cause placental damage aids its transplacental passage is not known.
TABLE 3. Gradation of Placental Damage With Transplacentally Acquired Infections
Effect on Placenta | Agent |
Severe | Vaccinia virus |
| Varicella virus |
| Variola virus |
| Treponema pallidum |
Moderate | Listeria monocytogenes |
Mild | Rubella virus |
| Toxoplasma gondii |
| Cytomegalovirus |
Minimal | Coxsackievirus B |
None | Poliomyelitis virus |
Organisms acquired by the transplacental route reach the fetal blood supply and are subsequently filtered by the fetal liver and spleen. Therefore, the finding of organisms in the fetal liver or spleen at autopsy can be used as evidence of this route of disease acquisition. More practically, a live-born infant infected hematogenously may show evidence of septicemia, meningitis, or infection of the heart, adrenal gland, liver, or spleen.
Infection may also result from fetal aspiration of amniotic fluid which has become contaminated following migration of organisms through the fetal membranes or following infection of decidual tissues and contiguous placental villi. Fetal infection following decidual infection is usually the result of cervical-vaginal bacteria and may result in spontaneous abortion or stillbirth or may cause “congenital pneumonia,” a condition in which lesions are found in the lungs of stillborn infants or those who die within approximately 24 hours after birth. However, whether this is a true lung infection or whether it results from fetal aspiration of amniotic fluid laden with white blood cells and debris is controversial.
The amniotic cavity is probably most commonly contaminated following rupture of the membranes and ascension of the cervical-vaginal bacteria into the amniotic cavity. The ascension may be hastened or encouraged by multiple and carelessly performed vaginal and/or rectal examinations. The risk of infection increases not only for the fetus but also for the mother as the time following rupture increases. Organisms associated with infections following membrane rupture are listed in Table 2.
Inhalation and swallowing are the most common ways by which the fetus contracts the infection; therefore, the effects are mainly in the lungs and gastrointestinal tract, eg, acute gastritis and pneumonia. The auditory canal, skin, and eyes are also exposed. Such exposure may be antecedent to meningitis, skin and ocular infection, or omphalitis.
DURING BIRTH.
During normal, spontaneous vaginal delivery, infants are subject to contact with a wide variety of organisms which may be present in the vagina. Some of these may colonize the infant, and most will pose no problem. However, some organisms, eg, group B β-hemolytic streptococci, Neisseria gonorrhoeae, Listeria monocytogenes, Candida albicans, Gram-negative rods, and herpes simplex virus (Herpesvirus hominis), may cause serious neonatal infections, including sepsis, which leads to death. Neonatal sepsis appears to be caused most frequently by aerobic Gram-negative rods, but anaerobic bacteria (usually Bacteroides) and group B β-hemolytic streptococci also cause infections with high mortality. Infants may also acquire Salmonella, Shigella, and enteropathogenic Escherichia coli from the bowel flora of asymptomatic maternal carriers.
Although herpes simplex virus may be acquired before birth, the most prevalent mode of acquisition is from passage through an infected birth canal or from an infected amniotic cavity after rupture of the membranes. Maternal herpes genitalis detected late in pregnancy is commonly an indication for cesarean section.
AFTER BIRTH.
After birth, the infant is subject to colonization and, possibly, infection by any of the microorganisms existing in his environment. Common routes of infection are contact with the mother or hospital personnel who are carriers of virulent organisms or contact with contaminated equipment. Streptococcal or staphylococcal infections usually result from the former, whereas infections caused by Gram-negative rods (especially those such as Pseudomonas, Serratia, and Flavobacterium, associated with humidifiers and contaminated bathing solutions) are usually associated with the latter. These infections may be spread from infant to infant throughout the nursery, thus becoming epidemic.
Neonates who require special care and procedures are at a greater :risk of developing an infection. For example, catheterization of the umbilical blood vessels or oxygen therapy may expose the infant to organisms which he would not otherwise contact, and infants who require these procedures are premature or debilitated and therefore are less likely to be able to combat infection.
Effects on the Mother
The physician is well acquainted with signs of infection, but he must be especially cautious in interpreting them in the pregnant woman. Associated with the pregnancy itself are many symptoms such as nausea, fatigue, vague myalgias, and the physiologic leukocytosis of pregnancy. A number of viral infections may cause similar symptoms and are likely to be ignored. Also, infections which may cause very severe problems for the fetus may cause minimal symptoms in the mother. For example, cytomegalovirus infection and toxoplasmosis usually evoke in the mother only symptoms resembling a common cold.
Because of her pregnancy, a woman may be more frequently or more severely affected by certain disease processes.
Poliomyelitis, oculogenital infections by Chlamydia trachomatis, herpes simplex virus type 2 infections, disseminated gonorrhea, vulvovaginal candidiasis, pyelonephritis, and septic shock apparently occur more frequently in the pregnant woman than in the nonpregnant woman.
Although herpes simplex virus type 2 infections occur more frequently during pregnancy, they appear to affect the pregnant woman no more severely than the nonpregnant woman.
Infection with N gonorrhoeae is more likely to become disseminated in the pregnant woman than in the nonpregnant woman. This dissemination is most commonly manifested as arthritis.
Vaginitis caused by yeasts, especially C albicans, occurs more frequently in pregnant women, and its incidence increases with gestational age. It does not usually present severe problems for the mother or the fetus, although the symptoms can be distressing. The disease often clears up spontaneously in the mother after delivery. Women may be asymptomatic or have a range of symptoms from mild to severe itching, discharge, pain, swelling, erythema, and ulceration. The most severe infections often occur in pregnant women who are also diabetics. This is notable, since gestational diabetes may occur in women who are not known to be diabetic.
Although bacteriuria is probably no more common in pregnant women than in nonpregnant women, the likelihood of its progression to pyelonephritis is greater in pregnant women. The probability of developing pyelonephritis may be related to the physiologic hydroureter of pregnancy.
Infectious diseases which may be more severe in pregnant women than in nonpregnant women include poliomyelitis, smallpox, condyloma acuminatum, pneumococcal pneumonia, and, possibly, influenza. The problems of poliomyelitis and smallpox have been minimized in industrialized society and are mainly of academic concern to obstetricians. Condyloma acuminatum is significant in that the growth may become large enough to interfere with vaginal delivery.
Reports of the effects of pneumonia on the pregnant woman and fetus are few since the advent of antibiotic therapy. Oxorn's study indicates that antibiotics have reduced maternal mortality and fetal mortality if the fetus is delivered after the 36th week of gestation 4. However, reports of pneumonia in pregnant women before the antibiotic era indicate that these women were more severely affected than nonpregnant women. Pneumonia which occurs later in pregnancy is more often rapidly fatal than pneumonia occurring early in pregnancy.
Influenza may be more severe in pregnant women. Often the data on influenza are mixed with data relating to pneumonia, and it is difficult to determine the impact of influenza itself. It appears, however, that it may lead to abortion or premature delivery of the fetus and/or death of the mother and that the effects are related to the virulence of the virus strain which causes the influenza. Influenza which mildly affects the general population seems to affect the pregnant woman in a similar manner.
The most common viral infections in pregnancy, ie, respiratory infections caused by adenovirus, coronavirus, and/or rhinovirus, appear to be no more severe in pregnant women than in nonpregnant women and appear not to affect the fetus.
It is difficult to know whether the pregnant patient has any particular propensity for the development of septic shock following a septic abortion, in the puerperium, or as a complication of chorioamnionitis. Some investigators feel that the shock syndrome may develop particularly rapidly in the pregnant woman. One explanation proposed is that endotoxic shock is similar to the generalized Shwartzman phenomenon. McKay 5 has demonstrated that in a pregnant host, in contrast to a nonpregnant host, a generalized Shwartzman reaction may be elicited without prior sensitization. In addition to the possibility of shock developing rapidly in pregnancy, the physiologic changes in clotting factors, which presumably prepare the host to deal with bleeding associated with delivery and placental separation, also predispose to development of disseminated intravascular coagulation. Therefore, the care of the patient with developing shock merits intensive and aggressive management.
Effects on the Fetus
A large number of microorganisms have been shown to be capable of crossing the placenta and infecting the fetus, with diverse results. Although the fetus may not be affected and the outcome of the pregnancy may be the delivery of a normal infant, the less fortunate results of fetal infection include abortion, stillbirth, prematurity, low birth weight, developmental abnormalities, congenital disease, and/or persistent neonatal infection.
LOW BIRTH WEIGHT.
Retardation of fetal growth, which is manifested by the birth of an infant considered small for gestational age, is most commonly associated, in terms of feud infection, with infection by rubella virus or cytomegalovirus. Associated with the small body size due to cytomegalovirus are unpredictability of organ size and a subnormal number of cells in most organs. The subnormal numbers of cells may be attributable to cell destruction by the virus or to vital interference with cell multiplication without cell destruction.
Maternal rubella infection has also been shown to result in small-for-date infants. Infants infected with rubella virus also have decreased numbers of cells in most organs. The mechanism for this appears to be related most directly to viral inhibition of cell multiplication by interference with mitotic activity. The virus also causes an obliterative angiopathy of small blood vessels and capillaries and, with persistent infection, progressive damage to the placental vasculature. This interference with blood flow and tissue oxygenation further handicaps fetal development.
Low birth weight has also been associated with maternal mumps, measles, and hepatitis. However, with mumps, the difference between infants of virus-infected mothers and the control group was not statistically significant, and the low birth weights in infants whose mothers had measles or hepatitis appeared to be related to prematurity rather than growth retardation.
PREMATURITY.
Prematurity, ie, birth of a live infant at less than 37 weeks' gestation, is a common result of several different maternal viral or bacterial infections and possibly some protozoan infections. Maternal viral infections with which premature delivery has been associated include cytomegalovirus, rubeola, variola, and herpes genitalis.
A variety of bacteria, including Treponema pallidum, Mycobacterium tuberculosis, N gonorrhoeae, L monocytogenes, Vibrio fetus, and Salmonella typhosa, have been incriminated as agents leading to premature births.
Although maternal pyelonephritis is associated with premature delivery, it has not been determined whether prematurity is associated with bacteriuria. A number of studies have been done, but not all were well controlled, and the results have been conflicting.
Studies have indicated that mycoplasmal infection may result in premature birth. Genital mycoplasmas have been found to be more common both in low birth weight infants and in the urine or cervixes of women who subsequently delivered babies with a lower mean birth weight.
DEVELOPMENTAL ANOMALIES.
Of all the organisms which may infect the fetus, only rubella virus and cytomegalovirus have been conclusively shown to cause developmental anomalies, ie, to act as teratogens in the fetus. These anomalies include CNS and cardiovascular abnormalities, deafness, and mental retardation. In addition, cataracts are associated with rubella, and chorioretinitis is associated with cytomogalovirus infections.
The possibility of the teratogenicity of certain coxsackieviruses has been advanced. Studies of the effects on the fetus of maternal infection with coxsackieviruses have shown that in comparison with infants born to noninfected mothers, there is 1) a significantly increased incidence of neonatal urogenital anomalies associated with maternal coxsackievirus B2 infections, 2) a significantly increased incidence of gastrointestinal anomalies associated with maternal coxsackievirus A9 infections, and 3) a significantly increased incidence of cardiovascular anomalies associated with maternal coxsackieviruses B3 and B4 infection 6. The projected risk of occurrence of congenital heart disease in an infant whose mother was infected with coxsackievirus B3 was determined to be somewhat greater than the occurrence of congenital heart disease in the general population. However, confirmation of this work is necessary. Unconfirmed reports have also attempted to show a relationship between congenital defects and maternal infectious mononucleosis and infections with paramyxoviruses (mumps), rubeola virus, varicella-herpes zoster virus, vaccinia virus, hepatitis viruses, herpes simplex virus, and influenza virus.
CONGENITAL DISEASE.
Most of the organisms which can cross the placenta to infect the fetus have been shown to cause congenital disease, ie, tissue damage and/or physiologic abnormalities caused directly by the infecting organism. Such congenital disease may affect any of the organ systems, but cardiovascular, CNS, and hepatic defects are often incompatible with life. Some of the effects of congenital infections, eg, deafness, microencephaly, mental retardation, and poor growth, may not become apparent until some time after birth.
In some of the diseases, the time of gestation at which the mother is infected is important in regard to the effects on the fetus. For example, adverse effects on the fetus from rubella are unlikely if the disease is contracted in the third trimester, and congenital syphilis may be prevented if the mother is treated before the 16th week of pregnancy.
PERSISTENT POSTNATAL INFECTION.
Rubella virus, cytomegalovirus, herpes simplex virus, varicella-herpes zoster virus, T pallidum, M tuberculosis, malaria organisms, and Toxoplasma gondii have been shown to survive in (and may be isolated from) infants for months or years after birth. The persistence of these organisms may result in disease, and the mechanisms by which some infants resist the consequences of infection and others do not are incompletely understood.
ABORTION AND STILLBIRTH.
Maternal infections caused by most organisms which can cross the placenta (including rubella, mumps, poliomyelitis, smallpox, rubeola, syphilis, malaria, toxoplasmosis, and infections caused by S typhosa, V fetus, L monocytogenes, cytomegalovirus, and herpes simplex virus) may result in abortion or stillbirth. Infections which persist without symptoms have been suggested as the cause of habitual abortion; these infections include listeriosis, toxoplasmosis, syphilis, and mycoplasmal infections.
Finally, infections in the mother which result in fever, anoxia, and/or toxin production, eg, 1obar pneumonia and typhoid fever, may result in stillbirth or spontaneous abortion.
HOST FACTORS AND THE DISEASE PROCESS
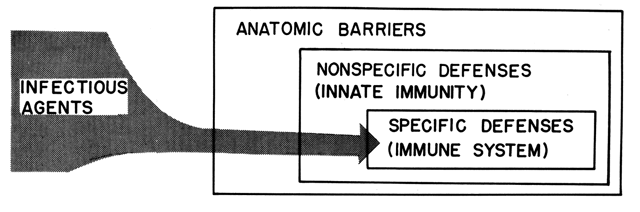
Host resistance, or host defense, refers to the sum of all host attributes mitigating the infectious properties of microorganisms. Therefore, host resistance to infection encompasses a variety of components (Fig. 4). Characteristically, an invading microorganism first encounters some barrier during its entry into the host; next, it comes in contact with nonspecific defenses; and finally, it activates the host's immune system.
|
The past 2 decades have been characterized by such an astounding proliferation of knowledge in the field of immunology that the significance of the nonspecific defenses is often in eclipse. The nonspecific defense system is important for two reasons: First, before active immunity becomes effective, the immune system requires both a sufficiently large antigenic exposure to become activated and adequate time for lymphoid proliferation to take place; thus, the nonspecific defenses protect the host from being overwhelmed by an infectious process prior to activation or specific immunity. Second, the nonspecific defense system is more frequently iatrogenically compromised in the pregnant patient than the specific defense system.
Nonspecific Defenses (Innate Immunity)
PHYSICAL BARRIERS.
The skin and mucous membranes provide the primary barriers to entry of microorganisms into the host. The adequacy of these barriers is enhanced by a variety of adjunctive antimicrobial factors. The skin, in addition to its physical barrier function, is hostile to microbial invasion because of its dryness and perhaps because of the presence of antibacterial lipids. The mucosal surfaces of the body also possess adjunctive antimicrobial properties that complement their barrier function. It is becoming increasingly apparent that tissues which have a characteristic microbial flora may be protected from microbial invasion by that resident flora. Alteration of the indigenous microflora may be a prerequisite for some infectious disease processes involving the upper respiratory tract, gut, or vaginal epithelium. Phagocytic cells may also be present in or on mucosal surfaces, and lysozyme and immunoglobul'm A are also usually present.
CELLULAR DEFENSES.
The cellular defenses involve phagocytic cells with various functions. When tissue injury occurs and microorganisms are implanted in the tissue, they may be phagocytized by local “wandering” macrophages. Eosinophils are abundant in skin, gut, vagina, and respiratory mucosa and may fulfill a similar protective role. Elimination of organisms from the tissue does not depend solely on resident phagocytes. Following injury, an inflammatory response occurs in which circulating polymorphonuclear leukocytes egress from the bloodstream and enter the site of injury. If the organisms persist in the tissue for more than approximately 24 hours, tissue macrophages infiltrate the lesion and become the predominant phagocytic cell type in the lesion. If the infectious process extends to the bloodstream or is primarily blood borne, phagocytic cells of the reticuloendothelial system (fixed macrophages) in the liver and spleen are responsible for removal of microbial contamination.
Three stages in the phagocytic process may be influenced by the host's physiology or exogenous factors. First, the chemotactic phase is characterized by the attraction of phagocytic cells, moving along a chemical gradient of chemotactic factors, to the infectious agent. Chemotactic factors include tissue components released when tissue is damaged, bacterial components, and breakdown products of complement. The chemotactic response is altered in uncontrolled diabetic states which can occur during pregnancy. The second stage of the phagocytic event is ingestion of the microorganisms. This step is aided by opsonic antibodies and basic peptides released from damaged tissues. The third major step in phagocytic defense is intracellular killing of the ingested microorganisms. A variety of intracellular factors are reported to be involved in intracellular inactivation of microorganisms within the phagocyte, but of particular importance is the increased oxidative metabolism of the phagocytic cell which accompanies the phagocytic event. The hormonal status of the host may influence this metabolic response to phagocytosis.
HUMORAL DEFENSES.
The phagocytic defenses described above take place in a milieu which contains soluble antimicrobial factors.
Transferrin, which is responsible for serum iron transport, also functions as an antimicrobial factor by binding iron and thus making the host's serum iron supplies unavailable for microbial growth. An analogous iron-binding protein, lactoferrin, is present in milk.
Lysozyme is an enzyme present in most body fluids and secretions. It depolymerizes the peptidoglycan of the cell walls of Gram-positive bacteria, but its activity is not strictly limited to Gram-positive bacteria. It may cooperate with other antibacterial factors, expanding its inhibitory role.
β-Lysin, a heat stable antimicrobial factor, is active primarily against a limited number of Gram-positive organisms. It is released from platelets when they rupture and is therefore found in greater abundance following trauma. The cationic or basic peptides are a diverse, poorly characterized group of peptides with similar antibacterial activity. They are generally of low molecular weight, are heat stable, and bind to anionic bacterial cells. They are reported to diminish bacterial respiration and to facilitate phagocytosis.
Viral infection and endotoxemia cause elaboration of interferon, which protects uninfected cells from vital infection. Interferon is species-specific but not virus-specific and appears to be a glycoprotein with a molecular weight of approximately 25,000 daltons. It is extremely potent in small quantities.
A zinc-polypeptide antimicrobial system has been identified in amniotic fluid, but its role in host defense is not clear 7.
Other nonspecific defense mechanisms probably exist but have not yet been adequately studied. The involvement of ascorbic acid in antibacterial defenses of the host has been proposed, and its cooperative effects with hydrogen peroxide or copper ion in low concentrations have been reported in in vitro studies. Additionally, it is now known that during infection the body dramatically redistributes its zinc and copper for reasons which are unknown. This will be an important area for future research, paticularly in view of the recognized association between infection and undernutrition.
Specific Defenses (Immune System)
CELLULAR IMMUNITY.
The immune response is classified as cellular or humoral, depending on the effector mechanism. Common to both humoral and cellular immune response is recognition of an antigenic stimulus, blastogenesis, and proliferation of a specific population of lymphocytes. For both cellular and humoral immunity, macrophages may trap, process, and present the antigen or some soluble message to the lymphocyte. In cellular immunity the lymphocyte population stimulated is the T-cell population. The effector cell may be the T lymphocyte itself. Although many of the activated T-cells' activities are directed against mammalian cells (ie, tumor or graft-cells), T-cells are important in defense against intracellular bacterial infection and viral infection. The activated T-cell may also produce an array of soluble factors such as interferon. Other soluble factors summon macrophages to the site of antigen stimulation and cause their activation. The activated macrophage is the principal effector in cell-mediated antimicrobial immunity.
HUMORAL IMMUNITY.
Antibody.
Humoral immunity results from the production of antibodies by plasma cells derived from B lymphocytes. Antibodies belong to one of five classes of immunoglobulins which possess different physical, chemical, and functional properties. Of the five classes, however, only three have any apparent role in antimicrobial immunity. IgG is quantitatively the most abundant of the immunoglobulins and is active in the serum and interstitial fluid and crosses the placenta. IgM is a larger molecule which will not pass into the extravascular fluid or cross the placenta and which, along with IgG, represents the main intravascular antibody defense. IgA is the dominant antibody in secretions of the body's internal and external surfaces.
The antibacterial effects of antibodies depend on their combination with the microbial agent. This binding to the infectious agent may inactivate the microorganism or may promote phagocytosis of the agent by leukocytes. The reaction of antigen with its antibody may also precipitate a reaction with complement, resulting ultimately in damage to the infectious agent's membrane and subsequent lysis of the microorganism.
Complement.
Complement is a group of nine separate proteins (C1 to C9) which act sequentially in cascade fashion, analogous to the clotting sequence, to produce a biologic effect. The classic pathway of complement activation is initiated by combination of antibody with its antigen. The subsequent orderly reaction of complement components C1q, C1r, C1s, and C2 to C9 results in damage to the cell membrane. Other biologic activities are generated during reaction of some of the components of the complement system. Anaphylatoxin activity is generated by two reaction steps and results in increased capillary permeability. Chemotaxis of polymorphonuclear leukocytes is also promoted by complement components. Complement bound to a microorganism may cause adherence of the organism to host phagocytes, facilitating the removal of the organism. Viruses and many bacteria are not lysed by effects depend on its associated opsonic and chemotactic activities.
Activation of the complement system is not limited to the classic pathway involving all nine components. An alternate pathway which bypasses C1q, C1r, C1s, C2, and C4 has been described. Initiators, including bacterial endotoxin (lipopolysaccharide), zymosan, IgA, and subclass 4 of IgG, react with components of the properdin system to activate C3 activator and trigger the rest of the complement sequence (C3, C5 to C9). While the lytic activity of this system is minimal, antimicrobial effects are possible through generation of chemotactic and opsonic principles.
COOPERATIVE EFFECTS OF ANTIMICROBIAL SYSTEMS.
As indicated in the preceding paragraphs, the host defenses are many, and some are ostensibly redundant. However, systems which seem distinct may operate in concert, producing greater antimicrobial effects than are produced by a single system. For example, phagocytosis occurs slowly in the absence of opsonins; however, opsonizing entities are not derived primarily from phagocytic cells but from the immune system or from complement. Likewise, the lytic activity of complement is enhanced by lysozyme. Lysozyme also operates synergistically with IgA to produce a bactericidal effect on Gram-negative organisms which neither factor produces alone. Such synergistic effects emphasize the importance of the uncompromised host defense system, since cooperative effects may amplify the effect of a single deficiency.
Maternal Defenses
Because of the profound changes in maternal physiology resulting from pregnancy, it is appropriate to consider what effects these may have on her resistance to infectious challenge.
NONSPECIFIC DEFENSES.
Pneumonia and, to some extent, influenza appear to affect the pregnant woman more severely than the nonpregnant woman of the same age, suggesting a defect in some intrinsic defense mechanism in pregnancy. The reason for this is unknown, although diminished vital lung capacity and the progesterone-induced hyperventilation may aggravate respiratory infections.
Anatomic changes in the urinary tract occur during pregnancy and may predispose to infection. The hormones of pregnancy cause a relaxation of the ureter, which allows it to distend, resulting in the complement; in these infections, its antimicrobial physiologic hydroureter and hydronephrosis of pregnancy. Whether this is responsible for the susceptibility of women with bacteriuria to upper urinary tract involvement is not clear. Other reasons have been proposed. Bacteriuria has been associated with a urine-concentrating defect. It has been suggested that the normally high concentration of urea in the urine inhibits bacterial growth. It is possible that dilute urine may predispose to infection. Finally, a phagocytic defect may be involved in urinary tract infection in pregnant women.
The importance of barriers has been mentioned. In the pregnant woman the vaginal epithelium and cervix are of particular importance as they relate to ascending infection. Ascending intrauterine infection may be caused by vaginal microorganisms. However, it was noted in one study 8 that as pregnancy progressed, changes in the vaginal flora occurred which may be interpreted as being generally benign. The number of aerobic, Gram-positive rods increased during pregnancy; most other groups, with the exception of the yeasts, decreased in number. Bacterial interference by the normal flora may offer some protection against infection. However, as is well-known, the mechanisms responsible for colonization by benign bacterial flora may predispose to yeast vaginitis.
The cervical mucus plug is putatively the protective barrier between the contaminated vagina and the normally sterile uterus. As the cervix becomes more dilated, the mechanical barrier function of the cervical plug obviously diminishes. However, certain components of the cervical mucus may afford some protection against ascending infection. The presence of lysozyme and IgA in cervical mucus and the presence of an uncharacterized component that inhibits motility of Proteus have been documented.
The fetal membranes represent an additional barrier. The time-dependent increase in amniotic infection following premature rupture of membranes attests to their importance. However, as a barrier, the fetal membranes are not impermeable. Bacterial contamination of the amniotic fluid can occur with intact membranes, but the contamination appears to be minimal and probably is controlled by antibacterial factors in the amniotic fluid. The clinician should keep the function of this barrier in mind when performing amniocentesis and intrauterine fetal monitoring and when managing the patient with premature rupture of the membranes.
During parturition and thereafter, considerable alteration of these defenses occurs. The barrier function of the membranes is gone. The uterus contains blood and adherent clots which may provide a nidus for bacterial proliferation. Retained secundines likewise may be the site of bacterial growth. Vaginal or cervical laceration or episiotomy may provide an additional portal for entry of organisms into the host, as may sites of administration of paracervical or pudendal blocking agents. Further threatening the mother in the puerperium is the dramatic change in bacterial flora of the vagina within the first 3 days post partum 8. Group B streptococcal colonization nearly doubles, Baeteroides fragilis increases from 2.6% to 34.8%; E coli increases from 2.6% to 32%. Other groups of organisms are likewise increased.
In addition to altering the nonspecific host defense, pregnancy may alter the phagocytic defenses of the gravida. First, pregnancy causes a leukocytosis, primarily of neutrophilic leukocytes. Whether this affords any added protection to the host is not known.
Pregnancy is not known to affect the chemotactic activity of phagocytes, although it has been reported that hyperglycemia inhibits leukocyte chemotaxis. Thus, unrecognized gestational diabetes may represent a compromise of the host's defense.
The hormones of pregnancy, particularly estriol, appear to enhance the phagocytic activity of leukocytes and increase metabolic activities associated with intracellular killing. In one study, however, it was found that some pregnant women with bacteriuria failed to display leukocytic hyperbactericidal activity 9.
Soluble host defense factors may also undergo change during pregnancy. Transferrin concentration increases during pregnancy, and serum iron concentration declines. This should provide a more hostile environment for bacteria which gain access to the bloodstream. Other soluble nonspecific factors have not been adequately studied.
SPECIFIC DEFENSES.
The status of the immune system during pregnancy has been a topic of intense interest to immunologists because the fact that the fetus is not immunologically rejected implies that pregnancy alters the normal immune responsiveness of the host. The question has not been resolved satisfactorily. Some animal studies seem to support the concept of an immunosuppression during pregnancy, but less information is available on humans. It is known that the pregnant woman can rally an immune response, but whether this response is equivalent to that of the nonpregnant woman is not clear.
Cellular Immunity.
Because cellular immune responses are important in protection against vital infections, the increased morbidity and mortality reported for pregnant women with influenza, varicella, polio, herpesvirus, and coxsackievirus infections have been cited as evidence that cellular immunity is diminished in pregnant women. In addition, lymphocytopenia, thymic atrophy, decreased tuberculin skin test reactivity, and prolonged skin graft survival have also been associated with pregnancy and may reflect depressed cellular immunity.
Humoral Immunity.
It has been shown that IgG levels diminish during pregnancy; IgA and IgM levels do not change significantly. An increased rate of IgG catabolism and transplacental transport is believed to account for the decrease in IgG levels during gestation.
Alterations in the complement system have been noted. Serum C3 levels are diminished during the first trimester and rise throughout pregnancy, returning to normal by 6 weeks post partum. However, C3 is abundant in the serum, and a greater than 90% reduction in C3 is needed to effect a reduction in total complement activity. Total complement has been measured during pregnancy. Individual reports vary, and some of the earlier literature gave conflicting data. However, recent reports, while showing some difference in the control levels of complement, agree that total complement activity increases significantly during pregnancy.
Fetal Defenses
THE FETUS.
Two barriers protect the fetus from infectious assault, viz, the placenta and the fetal membranes. The placental barrier is not as impenetrable as was once thought. We have noted several organisms which commonly cross the placenta, but in addition to those organisms, there is evidence of the rare transmission of a larger group of organisms. Once an organism passes the placenta, it is immediately in contact with the blood-borne defenses of the fetus.
Organisms which pass the fetal membranes are in contact with the amniotic fluid before reaching the fetus. Although there was once controversy as to whether amniotic fluid inhibited bacterial growth, it is now clear that at or near term, amniotic fluid from well-nourished, healthy women inhibits bacterial growth. Such inhibition is primarily due to a zinc-polypeptide inhibitor, the activity of which is abrogated by added phosphate ion. The phosphate-sensitive inhibitor is inactive when large numbers of bacteria are present, suggesting why the minimal contamination that may occur before membrane rupture may be contained but the massive contamination occurring with prolonged rupture without labor often leads to infection. The activity of this inhibitory factor is variable and frequently is absent before the 30th week of gestation; it is usually present at term and declines slightly in post-term pregnancies. No residual inhibition of Gram-negative organisms is ordinarily present after the addition of phosphate to amniotic fluid, although occasionally, inhibition may remain for Gram-positive bacteria. This may be due to any of several other known antibacterial substances in amniotic fluid, including transferrin, immunoglobulin, lysozyme, fatty acids, β-lysin, spermine, and steroids. Phagocytes may also enter the amniotic fluid, but their protective role is questionable. Phagocytosis is inefficient in a fluid environment; surface phagocytosis usually plays a more important role in protection of the host.
The innate defenses of the fetus have not been well defined. It is known that transferrin is present in cord blood at approximately haft the concentration of that in adult serum. Phagocytic cells develop early in pregnancy but may function suboptimally due to opsonic deficiency. Complement in fetal blood is present in approximately half the concentration of that in adult blood.
Immunocompetence is dependent on the production of stem cells and the maturation of these cells in the primary lymphoid organs. This occurs for T-cells and, presumably, B cells at approximately the eighth week of gestation. Therefore, by the time the fetus is mature, the necessary immunologic equipment for producing an immune response is present, but paradoxically, because the fetus is sequestered from antigenic challenge during pregnancy, the neonate faces a hostile environment immunologically unprepared. If infected in utero, the fetus responds immunologically, producing IgM and IgG. IgM production by fetal cells in culture has been detected by the 10th week of gestation, IgG is detected by the 12th week, and IgA appears at the 30th week. In the absence of infection, the fetus at birth has an IgG concentration that is greater than the maternal concentration, an IgM concentration that approximates 10% of the maternal levels and an IgA concentration that is less than 1% of the maternal level.
MATERNAL CONTRIBUTION TO FETAL DEFENSE.
The fetal contribution to humoral immunity is minimal. The fetal levels of IgG are primarily derived from the maternal serum. IgA and IgM do not cross the placenta, but IgG appears to be actively appropriated by the fetus against a concentration gradient. Passive diffusion provides the fetal circulation with minimal quantities of IgG as early as the 38th day of gestation. Levels remain low until the 17th to 22nd week. Thereafter, IgG levels in the fetal serum rise sharply, with the result that concentrations in maternal and fetal serum are approximately equal by the 25th week. Subsequently, fetal levels continue to rise gradually, and at term the fetal blood concentration of IgG is 5% to 10% greater than the maternal blood concentration. The antibodies thus transferred to the fetus reflect the maternal antigenic experience.
Neonatal Defenses
THE NEONATE.
The neonate at birth is challenged with a variety of potentially hazardous organisms, first in the birth canal and then from the environment, as organisms begin to establish themselves as normal inhabitants of the body. Little is known about the precise host defense deficiencies in the newborn. Certainly, the umbilical stump is a likely portal of entry for many organisms, as is the conjunctiva. At birth the concentrations of IgM, IgA, serum transferrin, and complement have been found to be diminished. The rapidity with which certain infectious processes overwhelm the newborn further emphasizes the fact that the neonate must be considered a compromised host.
Some information is available on how the infant begins to assume responsibility for his own immunologic defense. The passively transferred antibodies which are present in his serum at the time of birth may be somewhat protected from destruction by a diminished catabolic rate. The half-life of IgG in the neonate is approximately 25 to 30 days as compared with a 23-day half-life in adults. The rate of antibody synthesis, however, does not keep pace with this decreased catabolic rate, and IgG levels decrease. The decrement reported in one study 10 was 560 mg/dl, from 900 mg/dl at birth to 340 rag/ dl by the fourth week. Levels begin to rise by the third month but are still subnormal at 22 months of age. Significant quantities of IgA and IgM begin to appear by the fourth month of life.
MATERNAL CONTRIBUTION TO NEONATAL DEFENSE.
The neonate is denied passively transferred IgG after birth but has a ready source of IgA in his mother's milk or colostrum. In addition to the immunoglobulin acquired in the milk, protection is afforded by several other antimicrobial systems, including lactoferrin, a milk protein which inhibits bacterial growth by a mechanism similar to that of transferrin. Breast milk also contains complement components C3 and C4, interferon, and unsaturated fatty acids which are inhibitory to staphylococci. Cellular defensive elements, including T and B lymphocytes and macrophages, are also present in breast milk.
In addition to these antimicrobial factors, certain components, including high lactose and the bifidus factor, promote the orderly colonization of the neonatal gut with a benign microflora 11.
In support of the concept of the protective activity of the mother's milk are several reports which indicate that infectious disease problems of the neonate are more likely if human milk is withheld 12,13,14. The effect may be particularly significant in geographic areas where sanitation is poor and infectious challenge to the newborn is particularly great.
DIAGNOSIS OF OBSTETRIC INFECTIONS
The diagnosis of an obstetric infection is based on a careful history and thorough physical examination with utilization of appropriate diagnostic studies. Certain infectious diseases which are known to confer permanent immunity may be excluded if the patient has previously had the disease. However, the physician must consider the entire spectrum of diseases when confronted with a gravid patient with an infection. The symptoms of many infections of concern to the obstetrician may be vague, eg, malaise or upper respiratory complaints. Symptoms of a potentially serious infection may be minimized by the patient, or she may attribute them to the normal course of pregnancy. Fever and rash are less likely to be overlooked but may occur late in a disease process when fetal salvage is compromised. Clinical signs, symptoms, and history alone may not be sufficient to make a diagnosis; therefore, confirmatory or differential diagnosis in many cases can be made only by the use of appropriate laboratory studies. Many of the etiologic agents can be cultured from suitable specimens under proper conditions. Serologic tests which show a rise in antibody titer in paired serum samples are more practical to retrospectively confirm the diagnosis of certain infectious processes. Table 4 lists infectious diseases encountered in the pregnant woman which may have potentially serious sequelae in the fetus and the serologic tests which can be done or the material from which the etiologic agent may be cultured.
TABLE 4. Laboratory Methods for Detecting Infections Which May Be a Hazard to the Fetus
| Material From Which Etiologic | Serologic Tests Which |
Disease | Agent Can be Isolated | can Be Performed |
Cytomegalovirus infection | Urine, biopsy specimen | Indirect hemagglutination |
|
| Neutralizing antibody |
|
| Complement fixation |
|
| Fluorescent antibody |
Herpes simplex | Local lesion | Neutralizing antibody |
|
| Complement fixation |
Rubella | Pharynx | Hemagglutination inhibition |
|
| Neutralizing antibody |
|
| Complement fixation |
Syphilis | Material from lesion | Fluorescent treponemal |
| (dark-field examination) | antibody-absorption |
Toxoplasmosis | Infected tissue | Neutralizing antibody |
|
| Complement fixation |
|
| Sabin-Feldman dye test |
|
| Indirect fluorescent antibody |
|
| Hemagglutination |
Varicella-herpes zoster | Vesicular fluid | Complement fixation |
Gonorrhea | Endocervix, rectum, throat |
|
During pregnancy, certain disease entities may be diagnosed or suggested by cervical-smear cytology. Trichomoniasis or candidiasis may be evident, but more importantly, cervical cellular changes aid in diagnosis of genital Chlamydia or herpes simplex virus infection.
Infections which occur in the mother after delivery are mainly bacterial and are often related to contamination of the pelvic area by the cervical-vaginal flora during delivery. Ideally, the diagnosis of endometritis rests on the isolation of the offending organisms from an endometrial culture. However, most often the clinical signs and symptoms are used to make the diagnosis since the results of an endocervical culture are of questionable value in the diagnosis of endometritis. Blood cultures should always be obtained in a febrile gravid patient; blood cultures are more often positive in pregnant patients with infection than in nonpregnant patients with similar diseases. When positive, blood cultures are extremely helpful in patient management.
Diagnosis of bacterial infections in the newborn can be aided by culture of material from the external auditory canal or of the gastric aspirate. Microscopic examination of the gastric aspirate for segmented neutrophils and Gram stain for bacteria may also be of some value. More than five segmented neutrophils per oil immersion field may be suggestive of neonatal infection.
INFECTION CONTROL
Screening
During the antepartum period, it is possible to screen the pregnant patient for a number of potentially hazardous diseases. The number of studies performed depends on economic considerations, the degree of risk involved, and the availability of laboratory procedures. Unfortunately, many of the diseases are more commonly found in patients in the lowest socioeconomic groups who are the least able to pay for a constellation of laboratory tests. The serologic test for syphilis must be obtained in all cases, and rubella titers should be done on any patient whose immune status is uncertain. A careful history and physical examination may identify patients who require further evaluation. For example, a histology or cultural study should be undertaken in patients with evidence of heretic lesions on the genitalia. Quantitative urine cultures to detect asymptomatic bacteriuria should be done in patients with bacteria seen on routine urinalysis or with urine giving a positive nitrate reduction test. The necessity of cultures for N gonorrhoeae may vary from practice to practice, but in our opinion, these cultures are sufficiently important to warrant their routine use. The routine endocervical culture for group B β-hemolytic streptococci has not been shown to be of value in patient management and is not recommended as standard procedure.
Surveillance
Surveillance of infectious morbidity is a necessary pan of any inpatient service. Although the recognition of infection in the individual patient is the responsibility of the attending physician, the prevalence of infectious morbidity on the maternity service and in the newborn nursery is the responsibility of both the attending physician and the hospital epidemiologist. Information regarding the prevalence of infections on the maternity service allows the physician to recognize and correct a problem early in its development.
The newborn must also be observed for signs of developing sepsis. Prolonged membrane rupture, prematurity, history of maternal infection (eg, syphilis) during pregnancy, obvious placental or membrane infection at the time of birth, meconium stained and/or foul-smelling amniotic fluid, and postpartum sepsis in the mother may identify the neonate at risk of infection. A variety of procedures are available to aid in determining the infected neonate. IgM levels or specific serologic studies of cord blood may show elevated levels, suggesting that an infant was infected in utero. The orogastric aspirate may also be examined microscopically or by culture. If a specific disease is suspected by virtue of symptoms or history of disease in the mother, surveillance activities may then be more definitively and intensively applied to a given infant.
Maternal Isolation
Because of various indications, different types of isolation areas should be available, including isolation on the maternity ward in which the patient can receive barrier nursing care, isolation by transfer to a different part of the hospital, or strict isolation in an infectious disease unit.
Indications for isolation cannot be dogmatically presented here, although certain minimum requirements must be met. Local situations vary with respect to both infectious diseases encountered and isolation facilities available. If a disease which is not on published lists of diseases recommended for isolation becomes a problem in a given hospital, medical officers should not hesitate to use isolation procedures to prevent spread of infection.
A reasonable protocol for maternal isolation includes isolation of active contagious vital infections such as hepatitis, rubella, and chickenpox. Special situations may be encountered, eg, if mother and neonate have rubella, they are isolated together and discharged as soon as is feasible. If the mother has herpes genitalis and the infant was delivered by cesarean section, the mother and infant are separated until the mother is no longer infectious.
For bacterial infections such as purulent wound discharges, particularly if staphylococci are present, the mother is transferred to another area and is isolated. If this cannot be accomplished, the patient should be strictly isolated on the maternity area. Patients with puerperal sepsis involving streptococci are isolated for 24 to 48 hours until effective chemotherapy has been instituted.
Neonatal Isolation
Because of the susceptibility of the infant, special precautions must be taken to minimize exposure to infectious agents. Many infections acquired during the neonatal period come from paramedical personnel or from cross contamination in the nursery due to breaks in handling techniques. Strategies to minimize this problem include minimal handling of infants and careful attention to handwashing between infant handling. Contact with the nursing staff may also be minimized if the nurses do not do anything for the newborn that can be done equally well by the mother.
Segregation of certain infants may also be necessary. This can be accomplished by rooming-in or by placement of infants in a nursery composed of multiple rooms in which one room is vacated and disinfected before more infants are admitted to that room.
Beyond these general guidelines for minimizing contact between infants, provision must be made for the strict isolation of certain babies. Whenever sepsis occurs in the newborn, whether the etiology is known or not, the infant should be isolated. Diarrhea presumed to be of infectious origin is an indication for isolation, as are staphylococcal and several viral infections in the newborn. Depending on the particular situation, the mother may be admitted to feed the isolated infant or may be isolated with the infant.
REFERENCES
Smith T: Parasitism and Disease. Princeton, N J: Princeton Univ Press, 1934 |
|
Cooper LZ: Rubella: A preventable cause of birth defects. In Bergsma D (ed): Birth Defects: Original Article Series: Symposium on Intrauterine Infections. New York: National Foundation, 1968, vol 4, p 23 |
|
Sever J, White LR: Intrauterine viral infections. Annu Rev Med 19: 471, 1968 |
|
Oxorn H: Changing aspects of pneumonia complicating pregnancy. Am J Obstet Gynecol 70: 1057, 1955 |
|
McKay DG, Merril S J, Weiner AE, Hertig AT, Ried DE: Pathologic anatomy of eclampsia, bilateral renal cortical necrosis, pituitary necrosis, and other acute fatal complications of pregnancy, and its possible relationship to the generalized Schwartzman phenomenon. Am J Obstet Gynecol 66: 507, 1953 |
|
Brown GC, Karunas RS: Relationship of congenital anomalies and maternal infection with selected enteroviruses. Am J Epidemiol 95: 207, 1972 |
|
Schlievert P, Johnson W, Galask RP: Isolation of a low-molecular-weight antibacterial system from human amniotic fluid. Infect Immun 14: 1156, 1976 |
|
Goplerud CP, Ohm M J, Galask RP: Aerobic and anaerobic flora of the cervix during pregnancy and the puerperium. Am J Obstet Gynecol 126: 858, 1976 |
|
Mitchell GW, Jr, McRipley RJ, Selvaraj RJ, Sbarra AJ: The role of the phagocyte in host-parasite interactions: IV. The phagocyte activity of leucocytes in pregnancy and its relationship to urinary tract infections. Am J Obstet Gynecol 96: 687, 1966 |
|
Orlandini IO, Sass-Kortsak A, Ebbs JH: Serum gamma globulin levels in normal infants. Pediatrics 16: 575, 1955 |
|
György P: The uniqueness of human milk: Biochemical aspects. Am J Clin Nutr 24: 970, 1971 |
|
Bullen CL, Willis AT: Resistance of the breastfed infant to gastroenteritis. Br Med J 2: 338, 1971 |
|
McCracken GH, Shinefield HR: Changes in the pattern of neonatal septicemia and meningitis. Am J Dis Child 112: 33, 1966 |
|
Head JR: Immunobiology of lactation. Semin Perinatol 1: 195, 1977 |