Materials for Reconstructive Gynecologic Surgery
Authors
INTRODUCTION
Reconstructive gynecologic surgeons face daily decisions regarding surgical materials. There is no currently available ideal reconstructive surgical material. Such a material would be biocompatible, appropriately strong and durable, cost-efficient, and easy to use. This chapter provides a broad overview of the properties and clinical performance of currently available reconstructive materials in gynecologic surgery. The properties of individual sutures are not reviewed here.
ENDOGENOUS TISSUES FOR RECONSTRUCTIVE SURGERY
As illustrated in Table 1,1, 2, 3, 4, 5, 6, 7, 8, 9, 10 all honest clinical series reporting repair of pelvic organ prolapse show (1) some outright failures and (2) deterioration of initially good outcomes over time. Such clinical observations have encouraged surgeons to enhance their outcomes by supplementing their repairs with exogenous materials.
Table 1. Objective success rates of pelvic organ prolapse repair procedures using endogenous tissues
Author, Year | Procedure | No. Patients | Follow-Up | Outcome (Objective) |
| Silva, 20061 | Uterosacral ligament suspension | 72 | Mean 5 years | 85% no recurrence of prolapse |
| Amundsen, 20032 | Uterosacral ligament suspension | 33 | 6-43 months | 82% no prolapse |
Tulikangas, 20013 | Enterocele repair | 54 | 6–29 months | 61% no prolapse |
Weber, 20014 | Anterior colporrhaphy | 33 | Median 23 months | 30% no prolapse |
Shull, 20005 | Uterosacral ligament suspension | 298 | Mean 1 year | 87% no support defects |
Comiter, 19996 | Transvaginal culdosuspension | 100 | 6–35 months | 96% no recurrence of prolapse |
Porter, 19997 | Rectocele repair | 89 | >6 months | 82% no prolapse |
Cundiff, 19988 | Rectocele repair | 69 | 12 months | 88% no prolapse |
Paraiso, 19969 | Sacrospinous ligament suspension | 243 | Mean 74 months | 58% no support defects |
Shull, 199210 | Sacrospinous ligament suspension | 81 | 2–5 years | 65% no prolapse |
The logical reconstructive materials to consider are the patient’s own tissues. For many years, gynecologic surgeons have relied on a variety of endogenous materials, including uterosacral ligaments, pelvic fascia, skin, muscle, and locally available connective tissue. These tissues may be used in their current location (e.g., uterosacral ligament suspension of vaginal apex) or moved to a new location for an alternative use (rectus fascia for suburethral sling). These materials are biocompatible and easy to use. The scientific evidence for their long-term durability varies. There are no randomized clinical trials comparing endogenous sources with other materials for reconstructive gynecologic surgery.
The uterosacral ligaments are probably the most used endogenous material for vaginal suspension. Every hysterectomy and many posthysterectomy vaginal support techniques rely on these native tissues. Several authors have studied the histologic components of this tissue, which is found to be composed of elastic, collagen, and smooth muscle fibers with scattered blood vessels.11 The term uterosacral ligament is a misnomer because these are not “ligaments” in any traditional sense given their composition of irregular connective tissue and abundant smooth muscle with its attendant autonomic nerve supply. Observant surgeons recognize how fleeting their form is when they are cut at the time of surgery. Their ligamentous appearance is secondary only to the anatomic tension of their environment. Surgeons selectively acknowledge the innervation of the uterosacral ligaments. Most commonly, these structures are transected and sewn without thought to the neural consequences. Certain surgical procedures use the opposite approach. During procedures to ablate midline pelvic pain, the uterosacral ligaments are transected or destroyed without regard to long-term vaginal support.
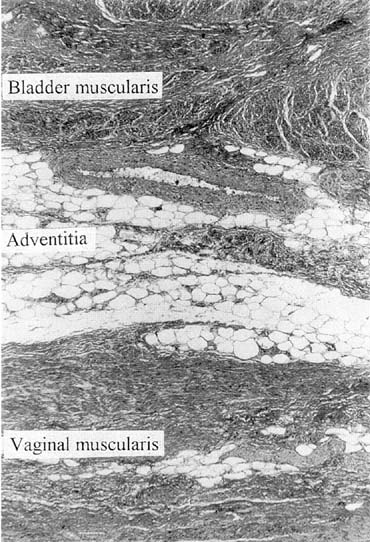
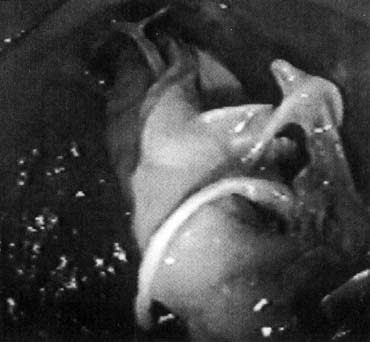
Vaginal fascia has been discussed in gynecologic operating rooms for more than 100 years, typically during anterior colporrhaphy. The histologic components of this material have been studied by Weber and Walters,12 who examined full-thickness sections of the bladder and vagina from autopsy specimens. Histologic examination confirmed that there is no vaginal “fascia”; rather the tissue between the vaginal mucosa and the bladder muscularis consists only of vaginal lamina propria and vaginal muscularis (Fig. 1). This layer is plicated during traditional anterior colporrhaphy. The histologic components of this tissue may help explain the relatively low anatomic success rates of anterior colporrhaphy. A layer that contains bladder adventitia and muscularis does not have the optimal biomechanical properties for long-term, durable anterior vaginal wall support. At the very least, this material is different from traditional fascia (rectus or fascia lata) and should not be considered to have similar biomechanical properties.
|
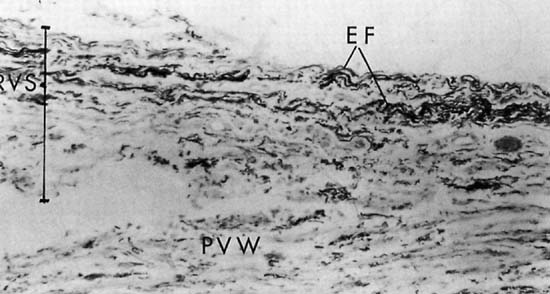
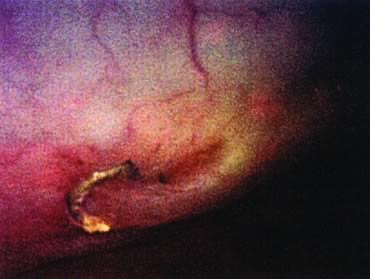
Repair of the posterior vaginal wall is a commonly performed gynecologic procedure. The most common material for this repair is the native rectovaginal fascia. There are no large case series that report the sufficiency of this tissue for its intended purpose. Experts commonly discuss the untoward side effects of posterior vaginal surgery without focus on anatomic recurrence of poor support. The exact histologic nature of the rectovaginal fascia has been the subject of some debate. One study found that the rectovaginal fascia consisted mainly of collagenous fibers13 and did not contain muscle cells (Fig. 2). In contrast, Milley and Nichols14 mentioned the presence of smooth muscle cells within the fascia. It may be that those muscle fibers originate from the external longitudinal muscle layer of the rectum.15 All authors agree that nerves of the hypogastric plexus run in the ventrolateral junction of the fascia with rectum. Surgical techniques that rely on this layer for posterior wall support conceivably could disable the delicate neuromusclar function of the rectovaginal axis. Techniques that document good efficacy with less dissection or plication7, 8, 16 may be preferable to traditional full-length colporrhaphy techniques.17, 18
The vaginal wall is also known to be a pliable, readily available surgical tissue. This tissue has been used for patch slings and vaginal repairs. It is unsuitable for these purposes because it does not have the biomechanical properties to resist stretching.19 This fact is easily appreciated by anyone who has witnessed vaginal birth during which the vaginal skin widely dilates to accommodate the emerging fetus. Similarly, skin throughout the body has phenomenal properties of stretch, which makes it unsuitable for long-term support.
|
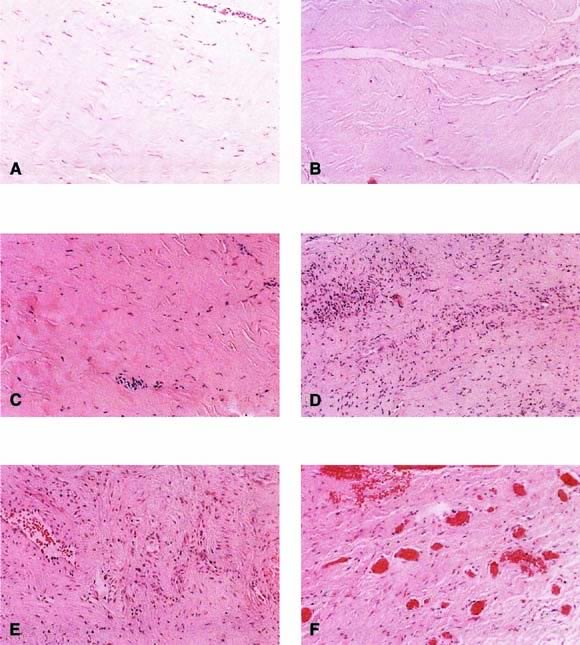
Autologous rectus fascia seems to be an ideal material for fascial reinforcement. This tissue is used commonly for suburethral sling and occasionally is used for sacrocolpopexy. Rectus fascia is biocompatible, strong, and appropriately pliable. Also it is readily available, although the amount harvested is directly related to the risk of incisional hernia. Despite its widespread use in suburethral slings and sacrocolpopexy, the rate of failure of this material is not well known. The surgeon would want this material to remodel to gain strength and appropriate vascularity. FitzGerald and coworkers20 reported on the histologic appearance of rectus fascia used for suburethral slings and found that after implantation there was fibroblast proliferation, neovascularization, and remodeling of the fascia graft. Some linear orientation of connective tissue and fibroblasts occurred, probably along the lines of force on the graft (Fig. 3). A randomized trial comparing materials in this area is needed.
|
Another source of endogenous fascia is fascia lata, typically harvested through a lateral incision in the thigh. This material is strong, durable, and readily available. One report of long-term problems after fascia lata harvest suggests caution, especially with increasing age. Walters and associates21 found that among 55 patients 2 years after fascia lata harvest, 25 (46%) had subjective complaints, including discomfort, weakness, lateral thigh bulge, or unacceptable incisional cosmesis. The histologic fate of fascia lata after implantation has not been reported.
There are additional, more experimental native tissues being considered for surgical use. Bioengineering and cell culture techniques have used novel techniques to harvest material from the patient, expand and enhance the tissue, and replace it in a clinically useful manner. Such tissue techniques include culture of muscle cells,22 ear chondroblasts,23 and entire bladder wall.24 Although these are not currently ready for routine clinical use, this area of investigation is expanding rapidly.
DONOR BIOMATERIALS (ALLOGRAFTS AND XENOGRAFTS)
Clinical failures have tempted surgeons to consider adjunctive or alternative materials. The source of biologic materials is typically cadavers or animals. Although the use of cadaver tissue sources per se is not new, harvesting and processing fascia for gynecologic reconstruction is a more recent development. These human cadaver materials have an increased likelihood of biocompatibility, although there is some concern about immunogenicity and disease transmission.
Cadaver fascia is supplied commercially, typically in association with a tissue bank. Commercial processing of the native fascia varies significantly, and the steps involved are often confidential. The goals of processing include sterilization (bacterial and viral) and removal of immunogenic cellular components. Clinical case series are inconclusive regarding the use of cadaver fascia for gynecologic reconstruction. This material saves time and morbidity of harvesting. FitzGerald and associates25 reported concerns, however, about excess failure rates compared with historical rectus fascia controls in an early case series. With continued follow-up, a failure rate of 81% was reported for donor fascia sacrocolpopexies and of 52% for donor fascia suburethral slings.26 A volley of case series has reported successes and failures, suggesting that specific steps in the processing and preparation of the tissue may be an important factor. One case series mentioned fascia allograft erosion as a relatively frequent complication of suburethral sling and sacrocolpopexy procedures using this material.27Table 2 is a summary of case series and highlights the preparation methods and method of reporting success rates.26, 28, 29, 30, 31, 32, 33, 34, 35, 36 In favorable reports, there is a paucity of objective outcome data.
Table 2. Surgical outcomes of sling procedures using cadaver donor fascia
Author, Year | N, Tissue Processing | How Success Determined | Outcome |
O’Reilly, 200228 | 121, Fresh frozen/freeze-dried | Subjective | 78% success |
FitzGerald, 200226 | 27, Freeze-dried, irradiated | Subjective/objective | 48% success |
Carbone, 200129 | 154, Freeze-dried, irradiated | Subjective | 62% success |
Huang, 200130 | 18, Solvent-dehydrated, irradiated | Subjective | 72% success |
Soergel, 200131 | 12, Freeze-dried, irradiated | Objective (urodynamics) | 33% cured |
Amundsen, 200032 | 104, Freeze-dried | Subjective | 87% success |
Elliott, 200033 | 26, Solvent-dehydrated, irradiated | Subjective | 96% cured/improved |
Brown, 200034 | 121, Processing not stated | Subjective | 74% cured |
Wright, 199835 | 59, Freeze-dried | Subjective | 98% success |
Handa,199636 | 14, Fresh frozen/freeze-dried | Objective (urodynamics) | 79% cure |
The use of human dura mater allografts is limited to historical interest because of concerns about transmission of slow-virus neurologic diseases. These concerns have limited the use of this material, although there has never been a report of viral disease transmission from fascia transplantation in gynecologic surgery.
Several suppliers now market processed human cadaver skin as a material for reconstructive surgery. Cadaver skin may be processed to preserve the acellular dermal matrix with the intent of enhancing repopulation by endogenous host cells. These materials are approved by the Food and Drug Administration for dermal replacement but have not been tested or proved for reconstructive gynecologic surgery. Cadaver skin seems to be a viable technology and is likely to be a worthwhile source of tissue, although its exact role has yet to be established. Pessimists expect this line of investigation to produce tissue with biomechanical properties similar to skin, which would not be sufficient for prolapse repair. Marketing materials promise the tissue is “quickly revascularized and repopulated with cell populations of the patient’s own tissues.” Optimists believe that cellular ingrowth would result in enhanced strength suitable for reconstructive efforts. Case series are being reported before testing of efficacy in randomized clinical trials (Table 3).37, 38, 39, 40, 41, 42 Nonhuman sources for dermal tissue also have been investigated. Processed porcine skin has been implanted without the benefit of randomized clinical trials. Case reports and patient series offer only anecdotal outcome data (see Table 3).37, 38, 39, 40, 41, 42 Given the porcine source, religious prohibitions limit the use of this material in some patients.
Table 3. Success rates of procedures using processed dermis or small intestinal submucosa*
Author, Year | N, Tissue, Procedure | Follow-Up | How Outcome Assessed | Outcome |
Dambros, 200137 | 30, Porcine small intestinal submucosa, sling | 1–13 months | Subjectively | 93% cured |
Arunkalaivanan, 200138 | 46, Porcine dermis, sling | >6 months | Subjectively/objectively | 83% cured |
Deprest, 200139 | 30, Porcine dermis, sacrocolpopexy | 3–10 months | Objectively | 100% success |
Myers, 200140 | 6, Human cadaver dermis, cystocele repair | 6–24 months | Objectively | 100% cure |
Edwards, 200041 | 20, Porcine dermis, sling | 3 months | Subjectively | 100% cure |
Kohli, 200042 | 40, Human cadaver dermis, rectocele repair | Mean 8 months | Objectively | 97% cured |
* Derived from selected abstracts.
Similarly, intestinal submucosa has been obtained from canine and porcine sources. Nonhuman experiments showed that the tissue “becomes completely incorporated at 4 weeks.”43 Although this finding superficially suggests biocompatability, it also may represent efficient biodegradation. It is likely that successful tissue grafts undergo some degradation, but it seems essential that remodeling and reconstruction occur before complete tissue loss. As with other materials, clinical efficacy has not been well documented apart from published abstracts (see Table 3).37, 38, 39, 40, 41, 42
SYNTHETIC GRAFT MATERIALS
Native tissues have tremendous advantages and should be used whenever they are likely to accomplish the surgical reconstruction goals. As all surgeons are aware, however, the strength of native tissues can be insufficient. It is tempting to substitute stronger, more durable materials to enhance outcomes. One high-quality randomized trial compared inguinal hernia repair with synthetic mesh with careful repair of endogenous fascia using permanent sutures.44 There were high recurrence rates in both groups but an excess recurrence rate when sutures only were used without additional mesh (sutures 43% versus mesh 24%). This study also compared these materials for repeat hernia repair. The difference was more striking with a 58% recurrence for sutures only versus 20% with mesh. Even healthy endogenous materials may have insufficient biomechanical properties to compensate for abnormal physiology.
Reconstruction has long depended on synthetic materials, and a large variety of synthetic materials are available for use by gynecologic surgeons. The ideal surgical mesh would be chemically inert, biologically inactive, strong, flexible, convenient, and cheap—this ideal mesh currently does not exist. Available meshes differ as follows:
- Composition of component fibers: Mesh fibers are either monofilament or multifilament and composed of either absorbable or permanent materials.
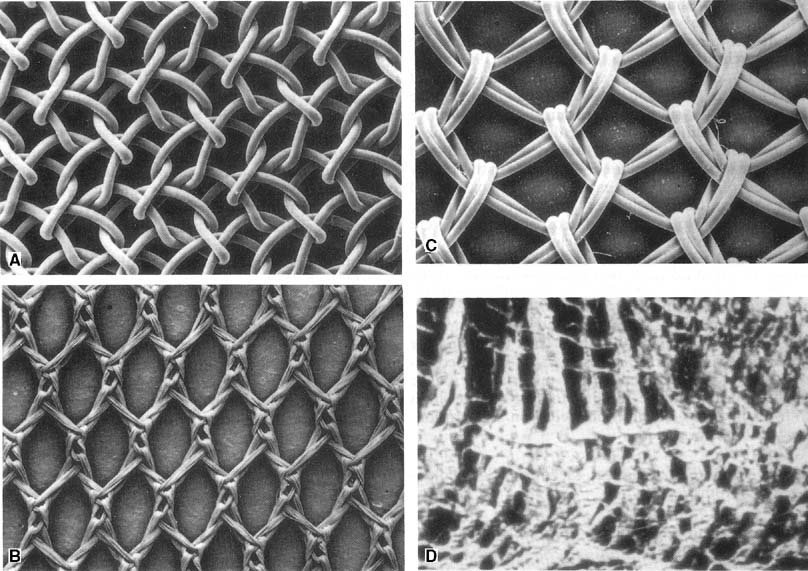
- Their pore size (Fig. 4): Theoretically, multifilament mesh interfiber pores are large enough to allow bacteria to enter but too small to allow macrophages to enter to combat infection.45
- Their stiffness: The flexibility of a mesh is related to the pore size and fiber composition. Marlex is stronger and stiffer than Mersilene.46 Expert opinion holds that stiffer meshes are more prone to erosion through the vagina.47
|
A systematic review of studies published over the past 60 years aimed to look specifically at the use of graft in transvaginal pelvic organ prolapse repair. It concluded that the data in the current literature are insufficient to allow for a complete assessment of anatomic or symptomatic efficacy of graft use in transvaginal prolapse repair.48 In addition, no particular material has proved superior to any other. Table 4 shows outcomes of transvaginal repair with graft versus native tissue. There is no study (analogous to the hernia study) that shows which patient group benefits from the use of synthetic mesh. In October 2008, the FDA put out a public health notification entitled "Serious Complications Associated with Transvaginal Placement of Surgical Mesh in Repair of Pelvic Organ Prolapse and Stress Urinary Incontinence." In it they state that physicians should obtain specialized training for each mesh placement technique and be aware of its risks. They also state that physicians should be vigilant for potential adverse events from the mesh, especially erosion and infection.49 Surgeons must develop their own philosophy about synthetic materials based on their own clinical outcomes (efficacy and complications), costs, and preferred route of surgery.
Table 4. Outcomes of graft versus native tissue for transvaginal prolapse repair
Author, Year | Graft (n) | Native Tissue (control) | Mean Follow-Up | Anatomic Failure (%) |
| Hiltunen, 200750 | Polypropylene (105) | 97 | 12 months | Polypropylene (7) Control (39) |
| Meschia, 200751 | Pelvichol (100) | 100 | 12 months | Pelvichol (7) Control (19) |
| Paraiso, 200652 | Fortagen (31) | 37 | 4–33 months | Fortagen (46) Control (14) |
Gandhi, 200553 | Tutoplast (76) | 78 | Median 13 months | Tutoplast (21) Control (30) |
| Sand, 200154 | Vicryl mesh (73) | 70 | 12 months | Vicryl mesh (9) Control (10) |
Weber, 200155 | Vicryl mesh (35) | 35 | Median 23 months | Vicryl mesh (58) Control (70) |
During vaginal surgery, most surgeons are reluctant to place mesh for supportive repair of vaginal walls, citing many anecdotal reports of erosion and rejection secondary to potential contamination by vaginal organisms. Placement of synthetic sling materials through the bacteria-laden vagina has seen several rises and falls in popularity. Young and colleagues56 reported 5-year success with Mersilene slings. A persistent but acceptable materials problem rate is reported. Newer sling developments attempt to shield the synthetic sling from the bacterial contamination of the vagina. Reliable long-term data regarding the fate of this material for this indication are not yet available for this technique. Synthetic meshes are used widely for sacrocolpopexy, despite a persistent rate of foreign body erosion and rejection that occasionally requires reoperation.
Absorbable meshes are poorly suited for long-term reconstruction and are not discussed further in this chapter. The permanent meshes currently available in the United States are briefly considered in alphabetical order by chemical composition (Table 5).
Table 5. Popular synthetic meshes used in reconstructive pelvic surgery
Fiber Composition | Trade Name (Manufacturer) | Type |
Expanded polytetrafluoroethylene | Gore-Tex (WL Gore, Flagstaff, AZ) | Multifilament, nonabsorbable |
Polyethylene terephthalate | Mersilene (Ethicon, Somerville, NJ) | Multifilament, nonabsorbable |
Polyglactin 910 | Vicryl (Ethicon, Somerville, NJ) | Multifilament, absorbable |
Polypropylene | Marlex (CR Bard, Branston, NJ) | Monofilament, nonabsorbable |
Prolene (Ethicon, Somerville, NJ) |
Mesh preparation chemical polymers are manufactured into filaments that create the individual fibers (monofilament) or yarns (multifilament). These fibers or yarns ultimately are woven in to mesh material that is unique to each brand-name mesh. Polypropylene is used to create Prolene, Marlex, and Surgipro meshes. Prolene is also a monofilament mesh with macropores. In its typical mesh formation, it is stiff, although there is additional flexibility in its preparation for use as a transvaginal tape. This highlights the difference between the chemical composition of the fiber itself and the gross properties when those fibers have additional processing and gross physical arrangement. Marlex is stiff, macroporous mesh with irregular pore sizes. It is probably poorly suited to placement on flexible body surfaces, such as the vagina.
Polyethylene terephthalate is the material that is woven to compose Mersilene mesh. Typically this mesh is prepared in a hexagonal weave with the use of multifiber filaments. It is a macroporous mesh with directionality that can be shown easily by pulling the mesh by its width versus its length. This gross characteristic is important in determining proper orientation to limit mesh stretch after reconstructive surgery.
Expanded polytetrafluoroethylene polymers are used to form a Gore-Tex mesh. Because there is no weaving involved, this mesh has a smaller pore size than either Mersilene or Prolene. This small pore size may contribute to the fact that fibrocollagenous infiltration of Gore-Tex mesh does not occur after implantation, and minimal inflammatory response to the mesh occurs.57, 58
There is a negative side to the use of synthetic meshes in the pelvis. Although mesh is believed to augment surgical success rates, complications related to rejection and erosions are significant. In the course of 40 years of synthetic use, erosions into the urinary tract, bowel (large and small), and vagina have been reported (Table 6).56, 59, 60, 61, 62, 63, 64 Symptoms of mesh erosion typically include a persistent vaginal discharge that may be blood-tinged at times. More commonly, mesh exposure is detected when the patient is asymptomatic. One case report details asymptomatic rectal erosion and ultimate graft passage without sequelae (Fig. 5). Early efforts at full extirpation are probably overzealous and should be avoided. Expert opinion suggests that in the absence of sinister symptoms, early mesh erosions can be managed conservatively with periodic observation. A few surgeons have reported techniques for management of mesh complications,61, 65 including transvaginal revision, transvaginal removal, laparotomy, and laparoscopy with removal.
Table 6. Mesh complication rates from selected surgical case series reporting complications resulting from use of synthetic mesh
Author, Year | Mesh Type | Surgical Procedure | Complications |
Bodelsson, 200259 | Prolene | Suburethral sling (TVT) | 2% mesh erosion |
Young, 200156 | Mersilene | Suburethral sling | 4% vaginal erosion |
Visco, 200160 | Mersilene, Gore-tex | Sacrocolpo(perineo)pexy | 5% mesh erosion |
Kohli, 199861 | Marlex, Mersilene | Sacrocopopexy | 12% mesh erosion |
Barbalias, 199762 | Gore-tex | Suburethral sling | 8% vaginal erosion |
Drutz, 199063 | Marlex | Suburethral sling | 6% vaginal erosion |
Morgan, 198564 | Marlex | Suburethral sling | 6% urethral erosions, 1% urethral transactions, 2% sinus tracts |
TVT, tension-free vaginal tape.
|
Graft complications can occur many years after placement. When a mature graft appears, expert opinion suggests that it is unlikely that trimming visible graft would result in a long-term solution. It is rarely necessary, however, to remove every remnant of graft from delicate areas, such as the presacral space, to resolve symptoms associated with erosion.
The patient may ask about her risk of prolapse recurrence when the graft is removed. The data are scant, although experts believe that the inflammatory reaction related to the graft rejection and erosion seems to take the place of the graft, and repeat reconstruction is rarely necessary.
Occasionally a patient forgets that she has had mesh placed during her surgery. When that mesh is detected with an imaging modality, exploratory surgery is often recommended. Typically there is an oncologic concern of malignancy in the pelvis, most commonly after sacrocolpopexy. The consultation of a reconstructive pelvic surgeon can save unnecessary laparotomy.
There are general principles guiding the use of synthetics in reconstructive gynecology. It is prudent to avoid synthetics when viable alternatives are available. If synthetics are deemed appropriate, it is wise to limit the amount of graft used and to ensure that the materials of the surgery are balanced (i.e., permanent materials for mesh and suture rather than a materials mismatch, such as permanent mesh with a rapidly absorbable suture).
Isolated reports of medical concerns with gynecologic mesh placement have appeared in the rheumatologic literature. One case report suggested a possible link between exacerbations of rheumatologic conditions and the use of synthetic mesh.66
METAL
Metal tacks, staples, and other fixation devices are available, but these are rarely needed given the strength and durability of less troublesome materials. Such metallic items fare poorly in the dynamic ecosystem of the pelvis, where the tissues require some mobility and flexibility. Case reports abound of metal present in unauthorized pelvic viscera, including the vagina, bladder, and bowel (Fig. 6),67 and use of bone anchors to anchor suburethral slings has been associated with pubic osteomyelitis.68
SUMMARY
Knowledge of available surgical materials in reconstructive gynecologic surgery is important for surgeons and patients. Wise choices facilitate and enhance reconstructive surgery. New materials or technologies that have not been scrutinized in appropriate clinical trials risks excess failure rates. Such trials should be relevant to the specific clinical situation and not generalize results from animal studies or nongynecologic human studies, such as orthopedics or dental surgery. Surgeons play an important role in requiring appropriate clinical trials before bringing new surgical materials into the operating room. Finally, it is important that patients be informed about materials that are to be used in their reconstruction.
REFERENCES
Silva WA, Pauls RN, Segal JL et al: Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol. 2006 Aug;108(2):255-63. |
|
Amundsen CL, Flynn BJ, Webster GD: Anatomical correction of vaginal vault prolapse by uterosacral ligament fixationin women who also require a pubovaginal sling. J Urol. 2003 May;169(5):1770-4. |
|
Tulikangas PK, Piedmonte MR, Weber AM: Functional and anatomic follow-up of enterocele repairs. Obstet Gynecol 98:265, 2001 |
|
Weber AM, et al: Anterior colporrhaphy: A randomized trial of three surgical techniques. Am J Obstet Gynecol 185:1299, 2001 |
|
Shull BL, et al: A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol 183:1365, 2000 |
|
Comiter CV, Vasavada SP, Raz S: Transvaginal culdosuspension: Technique and results. Urology 54:819, 1999 |
|
Porter WE, et al: The anatomic and functional outcomes of defect-specific rectocele repairs. Am J Obstet Gynecol 181:1353, 1999 |
|
Cundiff GW, et al: An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol 179:1451, 1998 |
|
Paraiso MF, et al: Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol 175:1423, 1996 |
|
Shull BL, et al: Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol 166:1764, 1992 |
|
Mallipeddi P, et al: A study of the anatomy and histology of uterosacral ligaments. Int Urogynecol J Pelvic Floor Dysfunct 12(suppl 1):S20, 2001 |
|
Weber AM, Walters MD: Anterior vaginal prolapse: Review of anatomy and techniques of surgical repair. Obstet Gynecol 89:311, 1997 |
|
Ludwikowski B, Hayward IO, Fritsch H: Rectovaginal fascia: An important structure in pelvic visceral surgery? About its development, structure, and function J Pediatr Surg 37:634, 2002 |
|
Milley PS, Nichols DH: Correlative investigation of the human rectovaginal septum. Anat Rec 163:443, 1968 |
|
Silver PHS: The role of the peritoneum in the formation of the septum recto-vesicale. J Anat 90:538, 1956 |
|
Kenton K, Shott S, Brubaker L: Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol 181:1360, 1999 |
|
Arnold MW, Stewart WR, Aguilar PS: Rectocele repair: Four years’ experience. Dis Colon Rectum 33:684, 1990 |
|
Kahn MA, Stanton SL: Posterior colporrhaphy: Its effects on bowel and sexual function. Br J Obstet Gynaecol 104:82, 1997 |
|
Choe JM, et al: Autologous, cadaveric, and synthetic materials used in sling surgery: Comparative biomechanical analysis. Urology 58:482, 2001 |
|
FitzGerald MP, Mollenhauer J, Brubaker L: The fate of rectus fascia suburethral slings. Am J Obstet Gynecol 183:964, 2000 |
|
Walters AJ, et al: Harvesting autologous fascia lata for pelvic reconstructive surgery: Techniques and morbidity. Am J Obstet Gynecol 185:1354, 2001 |
|
Baskin LS, et al: Bladder smooth muscle cells in culture: I. Identification and characterization J Urol 149:190, 1993 |
|
Bent AE, et al: Treatment of intrinsic sphincter deficiency using autologous ear chondrocytes as a bulking agent. Neurourol Urodyn 20:157, 2001 |
|
Oberpenning F, et al: De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol 17:149, 1999 |
|
Fitzgerald MP, Mollenhauer J, Brubaker L: Failure of allograft suburethral slings. Br J Urol Int 84:785, 1999 |
|
FitzGerald MP, Fenner DE, Edwards SR: Longterm followup on use of freeze-dried, irradiated donor fascia for sacrocolpopexy and sling procedures. Int Urogyn J Pelvic Floor Dysfunct 15:238, 2004 |
|
Kammerer-Doak DN, Roger RG, Bellar B: Vaginal erosion of cadaveric fascia lata following abdominal sacrocolpopexy and suburethral sling urethropexy. Int Urogyn J Pelvic Floor Dysfunct 13:106, 2002 |
|
O’Reilly KJ, Govier FE: Intermediate term failure of pubovaginal slings using cadaveric fascia lata: A case series. J Urol 167:1356, 2002 |
|
Carbone JM, et al: Pubovaginal sling using cadaveric fascia and bone anchors: Disappointing early results. J Urol 165:1605, 2001 |
|
Huang YH, et al: High failure rate using allograft fascia lata in pubovaginal sling surgery for female stress urinary incontinence. Urology 58:943, 2001 |
|
Soergel TM, Shott S, Heit M: Poor surgical outcomes after fascia lata allograft slings. Int Urogyn J Pelvic Floor Dysfunct 12:247, 2001 |
|
Amundsen CL, et al: Outcome in 104 pubovaginal slings using freeze-dried allograft fascia lata from a single tissue bank. Urology 56(6 suppl 1):2, 2000 |
|
Elliott DS, Boone TB: Is fascia lata allograft material trustworthy for pubovaginal sling repair? Urology 56:772, 2000 |
|
Brown SL, Govier FE: Cadaveric versus autologous fascia lata for the pubovaginal sling: Surgical outcome and patient satisfaction. J Urol 164:1633, 2000 |
|
Wright EJ, et al: Pubovaginal sling using cadaveric allograft fascia for the treatment of intrinsic sphincter deficiency. J Urol 160:759, 1998 |
|
Handa VL, et al: Banked human fascia lata for the suburethral sling procedure: A preliminary report. Obstet Gynecol 88:1045, 1996 |
|
Dambros M, et al: Suprapubic pubovaginal sling using the porcine small intestine submucosa (SIS): A promising minimally invasive alternative for urinary stress incontinence. Int Urogyn J Pelvic Floor Dysfunct 12(suppl 3):S13, 2001 |
|
Arunkalaivanan AS, Barrington JW: Comparison of procine pubovaginal sling (Pelvicol) vs Tension free vaginal tape (TVT) in the surgical management of stress intoneincne. Int Urogyn J Pelvic Floor Dysfunct 12(suppl 3):S21, 2001 |
|
Deprest J, et al: Preliminary experience with laparoscopic sacrocolpoperineopexy using Pelvicol. Int Urogyn J Pelvic Floor Dysfunct 12:144, 2001 |
|
Myers DL, Arya LA: Alloderm graft for cystocele repair. Int Urogyn J Pelvic Floor Dysfunct 12(suppl 1):S60, 2001 |
|
Edwards GJ, Barrington JW: The use of pelvicol in a minimally invasive pubovaginal sling procedure. Int Urogyn J Pelvic Floor Dysfunct 11(suppl 1):S122, 2000 |
|
Kohli N, Miklos JR: Site-specific rectocele repair using cadaveric dermal graft. Int Urogyn J Pelvic Floor Dysfunct 11(suppl 1):S61, 2000 |
|
Stratisis graft. Surgisis product brochure, Cook Urological, Spencer, IN |
|
Luijendijk RW, et al: A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 343:392, 2000 |
|
Brun JL, et al: Physical and biological characteristics of the main biomaterials used in pelvic surgery. Biomed Mater Eng 2:203, 1992 |
|
Chu CC, Welch L: Characterization of morphologic and mechanical properties of surgical mesh fabrics. J Biomed Mater Res 19:903, 1985 |
|
Fenner DE: New surgical mesh. Clin Obstet Gynecol 43:650, 2000 |
|
Sung VW, Rogers RG, Schaffer JI et al: Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008 Nov;112(5):1131-42. |
|
www.fda.gov/cdrh/consumer/surgicalmesh-popsui.html |
|
Hiltunen R, Nieminen K, Takala T et al: Low-weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol. 2007 Aug;110(2 Pt 2):455-62. |
|
Meschia, M, Pifarotti P, Bernasconi F, Magatti F, Riva D, Kocjancic E. Porcine skin collagen implants to prevent anterior vaginal wall prolapse recurrence: a multicenter, randomized study. J Urol 2007;177:192-5. |
|
Paraiso MF, Barber MD, Muir TW et al: Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006 Dec;195(6):1762-71. |
|
Gandhi S, Goldberg RP, Kwon C et al: A prospective randomized trial using solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Am J Obstet Gynecol. 2005 May;192(5):1649-54. |
|
Sand PK, Koduri S, Lobel RW et al: Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol. 2001 Jun;184(7):1357-62; discussion 1362-4. |
|
Weber AM, Walters MD, Piedmonte MR et al: Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001 Dec;185(6):1299-304; discussion 1304-6. |
|
Young SB, Howard AE, Baker SP: Mersilene mesh sling: Short- and long-term clinical and urodynamic outcomes. Am J Obstet Gynecol 185:32, 2001 |
|
Pourdeyhimi B: Porosity of surgical mesh fabrics: New technology. J Biomed Mater Res 23(A1 suppl):145, 1989 |
|
Iglesia CB, Fenner DE, Brubaker L: The use of mesh in gynecologic surgery. Int Urogyn J Pelvic Floor Dysfunct 8:105, 1997 |
|
Bodelsson G, et al: Short term complications of the tension free vaginal tape operation for stress urinary incontinence in women. Br J Obstet Gynaecol 109:566, 2002 |
|
Visco AG, et al: Vaginal mesh erosion after abdominal sacral colpopexy. Am J Obstet Gynecol 184:297, 2001 |
|
Kohli N, et al: Mesh erosion after abdominal sacrocolpopexy. Obstet Gynecol 92:999, 1998 |
|
Barbalias GA, Liatsikos EN, Anthanasopoulos A: Gore-Tex sling urethral suspension in Type III female uriniary incontinence: Clinical results and urodynamic changes. Int Urogyn J Pelvic Floor Dysfunct 8:344, 1997 |
|
Drutz HP, et al: Clinical and urodynamic re-evaluation of combined abdominovaginal Marlex sling operations for recurrent stress urinary incontinence. Int Urogyn J Pelvic Floor Dysfunct 1:70, 1990 |
|
Morgan JE, Farrow GA, Stewart FE: The Marlex sling operation for the treatment of recurrent stress urinary incontinence: A 16-year review. Am J Obstet Gynecol 151:224, 1985 |
|
Clemens JQ, et al: Urinary tract erosions after synthetic pubovaginal slings: Diagnosis and management strategy. Urology 56:589, 2000 |
|
Bernard-Medina G, Gutierrez-Urena S, Orozco-Alcala J: Dermatomyositis exacerbated by abdominal Marlex mesh implantation: adjuvant effect? Clin Rheumatol 15:94, 1996 |
|
Kenton K, Oldham L, Brubaker L: Open Burch urethropexy has a low rate of peri-operative complications. Am J Obstet Gynecol 187:107, 2002 |
|
Glowacki CA, Wall LL: Bone anchors in urogynecology. Clin Obstet Gynecol 43:659, 2000 |