The Mechanism of Ovulation
Authors
INTRODUCTION
The term ovulation refers to the release of a viable oocyte from the ovary. In the normal course of events, ovulation occurs once a month between the time of menarche and menopause. The release of a mature, fertilizable oocyte from the dominant follicle is the culmination of a wonderfully integrated and synchronized succession of hormonal actions and morphological changes involving principally the anterior hypothalamus, anterior pituitary and ovaries. The major players in this system are gonadotropin releasing-hormone (GnRH), FSH, LH, estrogen and progesterone but essential fine-tuning is provided by a large number of other factors including inhibin, activin and growth factors.
Oocyte release is preceded by estrogen-induced distinctive changes that prepare the reproductive tract for possible conception as if conception was being anticipated every month. For example, towards the time of ovulation the pH in the vagina becomes less acidic, the cervical mucus becomes more copious and less viscous and the cervical os becomes more patulous, all of which favor the progress of motile sperm towards the released oocyte. In addition estrogen serves as the ‘growth factor’ of the endometrium and prepares it for the transition of the endometrium from the proliferative to secretory stages by the post-ovulatory secretion of progesterone from the corpus luteum. The secretions of the corpus luteum, estrogen and progesterone prepare the uterus for the possible nidation of a fertilized egg. The corpus luteum is formed from the collapsed ovarian follicle, the ingrowth of capillaries and fibroblasts from the theca cell layer and luteinization of the granulosa cells. In the absence of pregnancy, ovulation defines the time of succeeding menstruation as the corpus luteum has a proscribed life span of 14 ± 2 days unless ‘rescued’ by human chorionic gonadotropin (hCG) from the trophoblast of the conceptus. An appreciation of the steps involved in the process of ovulation, necessitating the exact sequence of so many events, leaves one in awe of the ingenuity of the system and a little surprised that its breakdown, i.e. anovulation, does not occur much more frequently than is actually seen.
HYPOTHALAMIC-PITUITARY-OVARIAN AXIS
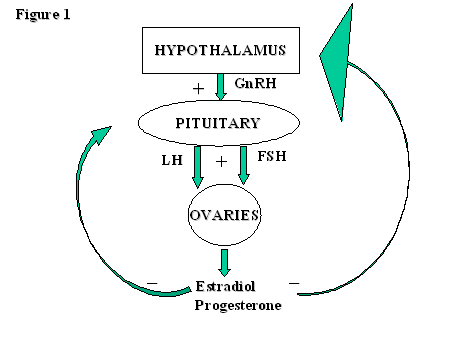
The normal functioning of this axis is dependent on the correct synchronization of the timing of release and the quantity of the hormones involved. These change dramatically throughout the cycle as a result of the various feedback mechanisms involved. First, we will consider the individual hormones involved, their target organs and actions, before piecing together the mosaic of the feedback mechanisms to complete the hormonal profile of the normal ovulatory cycle. Fig. 1 provides a very simple representation of the origin, target organ and feedback mechanisms of the principal hormones involved in this axis.
Fig. 1. A simple diagrammatic representation of the origins, target organs and feedback mechanisms of the principal hormones involved in the hypothalamic-pituitary-ovarian axis.
Gonadotropin releasing hormone (GnRH)
GnRH is a decapeptide which is synthesized and released by specific neuronal endings in the anterior and mediobasal hypothalamus. It is secreted into the portal vessels which run a very short course to the anterior pituitary. It is the compactness of the portal system which allows small quantities of GnRH to be concentrated enough to exert its action of gonadotropin release from the pituitary and explains why GnRH is undetectable in the peripheral circulation. The discharge of the gonadotropins, FSH and LH, induces the production of estradiol and progesterone from the ovary which, in turn, through a feedback mechanism, influence the pattern of release of GnRH from the hypothalamus.
GnRH is released in a pulsatile fashion and it is the frequency and amplitude of these pulses, in addition to the sensitivity of the pituitary gonadotrophs, that dictate the pattern of the release of the two gonadotropins. The GnRH pacemaker is principally influenced by the ovarian steroids but many other factors, including opiates, catecholamines, neuropeptide Y, etc., also play a role. If GnRH is released in a constant, non-pulsatile fashion, gonadotropin release is suppressed due to an apparent desensitization of the pituitary GnRH receptors. Pulsatile release of GnRH and fluctuations in the pattern of this pulsatility are thus integral features in the normal functioning of the ovulatory cycle.
As GnRH cannot be detected in human peripheral circulation, we have relied on the correlation with LH pulsatile release for our information on variations of pulsatility through the ovulatory cycle and in pathological conditions. Pulses of FSH are much more difficult to detect due to its longer half-life. In the follicular phase of a normal cycle, pulses of LH (reflecting GnRH) can be detected every 60–90 minutes.
Dramatic changes occur immediately preceding the pre-ovulatory LH surge. Hypothetically, the LH surge could be generated by an enormous discharge of GnRH or a temporary release from inhibition of pituitary LH discharge and a consequent increased pituitary sensitivity. Practically, both mechanisms are probably involved in creating the central event of the ovulatory cycle. Speculation is rife surrounding the existence of a proposed gonadotropin surge attenuating factor, produced by granulosa cells, which inhibits pituitary LH discharge. Although its structure is not yet known, a substance with this property has been isolated.
Following ovulation, under the influence of rising progesterone concentrations, the frequency of the LH pulses gradually decreases from one every 2–4 hours in the early luteal phase to every 8–12 hours towards the end of the cycle. The amplitude of LH pulses in the luteal phase is significantly greater than in the follicular phase. The fluctuations in the frequency and amplitude of GnRH pulsatile release are central in dictating the pattern of release of FSH and LH and, in turn, the triggering of the ovulatory process and ovarian steroid production.
This knowledge of the basic physiology of the pattern of release and action of GnRH has brought with it many clinical implications. Induction of ovulation for women who have hypothalamic hypogonadrophic hypogonadism is very successful when GnRH is administered in a pulsatile fashion with one pulse every 60–90 minutes. This is an ideal example of pure substitution therapy. The search for an agonist to boost GnRH action proved to have exactly the opposite eventual effect due to desensitization of GnRH receptors. These compounds are now very widely used before and during ovarian hyperstimulation for IVF to prevent premature LH surges. The use of GnRH antagonists is now also routine for use during controlled ovarian stimulation for IVF as they do not induce an initial, fleeting gonadotropin release as do the agonists, but an immediate decrease in their concentrations.
Follicle stimulating hormone (FSH)
The amount and timing of FSH release by the anterior pituitary changes throughout the ovulatory cycle. This mechanism is influenced by many factors. With the sudden demise of the corpus luteum which immediately precedes menstruation, the negative feedback effects of estradiol, progesterone and inhibin A on FSH secretion are suddenly lost so that FSH is secreted in relatively large quantities during menstruation itself. This rise in FSH concentrations stimulates the growth of antral follicles, granulosa cell proliferation and differentiation. It also encourages the action of the enzyme aromatase in the conversion of the basic androgens, androstendione and testosterone to estrogens. The sum total of these actions results in increasing estradiol and inhibin B concentrations, feedback mechanisms come into play and there is a consequent reduction of FSH concentrations. At mid-cycle, in tandem with the LH surge, there is a temporary increase in FSH secretion, more like a blip, whose significance is not clear. It may be a mere byproduct of the GnRH surge or may have a function in preparing a cohort of small antral follicles for the next cycle. With the formation of the corpus luteum and the outpouring of both estradiol and progesterone, the negative feedback mechanism comes into play and continues its suppression of FSH release until just before the next menstruation. The main undulations in FSH levels throughout the ovulatory cycle are very simply illustrated in Fig. 2.
Fig. 2. Hormonal, follicular and endometrial changes across the phases of the ovulatory cycle.
FSH is a hormone of many roles. It is a promotor of:
1. Granulosa cell proliferation and differentiation
2. Antral follicle development
3. Estrogen production
4. Induction of LH receptors on the dominant follicle
5. Inhibin synthesis
In addition to these functions, the decrease in FSH concentrations with rising estrogen concentrations is thought to play an important part in the selection of the dominant follicle. The declining secretion of FSH prevents multiple follicular development, as only the largest of the developing follicles stays above the FSH threshold, has the most FSH receptors, remains most sensitive to FSH and produces most estrogen. It is then less sensitive to the declining FSH concentrations and can continue to develop while others fade into atresia due to lack of enough FSH stimulation. The induction of LH receptors on the largest developing follicle(s) enables LH to take a part in the development of the dominant follicle in the late follicular phase and prepare it for the oncoming LH surge.
This basic knowledge of the mode of action of FSH, particularly regarding the FSH threshold for follicular growth, has influenced a change in ovulation induction regimes. This has become particularly important in the development of a chronic low-dose regimen for the induction of mono-follicular ovulation and the avoidance of multiple pregnancies and ovarian hyperstimulation syndrome.
Luteinizing hormone (LH)
During the early and mid-follicular phase, the secretion of LH is relatively quiet with pulses every 60–90 minutes and a fairly constant low concentration of circulating LH. However, this is the calm before the storm. An enormous climax is reached with the onset of the LH surge in the late follicular phase, the central event of the ovulatory cycle (Fig. 2). Concentrations of LH rise to 10–20 times their resting level during the rest of the cycle. The duration of the surge is 36–48 hours.
The LH surge, without which ovulation does not occur, is brought about by a combination of circumstances. Principally, there is a dramatic switch from a negative to a positive feedback action of estradiol at both the pituitary and hypothalamic level, triggered when persistently increasing estradiol concentrations reach a critical point. LH secreting pituitary gonadotrophs clearly become highly sensitive to GnRH stimulation, probably by increasing their numbers of GnRH receptors, a GnRH surge occurs and a small rise in progesterone levels in the late follicular phase may also have a triggering role.
The pre-ovulatory LH surge has a number of key functions:
- Triggering of ovulation and follicular rupture about 36 hours after the surge.
- Disruption of the cumulus–oocyte complex.
- Induction of the resumption of oocyte meiotic maturation.
- Luteinization of granulosa cells.
Following the formation of the corpus luteum, increasing concentrations of progesterone slow down the frequency of the LH (GnRH) pulses to one every 3 then one every 4 hours. Concentrations of LH once again dip down to baseline levels. It is therefore, not clear what kind of influence LH levels have on the maintenance of the corpus luteum. This structure, which produces large quantities of hormones, seems to ‘have a mind of its own’ or a built-in program which terminates in a very constant manner after 14 days. The luteal phase is thus the constant part of the ovulatory cycle whereas the follicular phase is much more likely to be prone to changes in duration.
Two cells – two gonadotropins
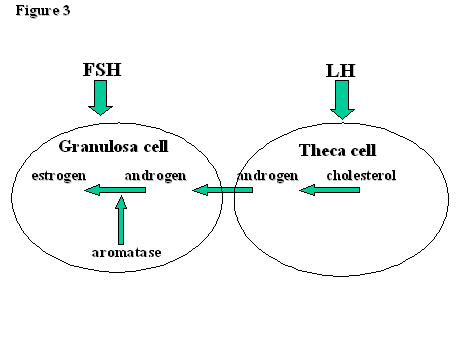
Outside the tumultuous events of the mid-cycle surge, the main function of LH is to encourage the production of androgens by theca cells. The androgens, androstendione and testosterone, are then ‘passed on’ to the granulosa cells. Here they meet aromatase (CYP19), whose function it is to convert them into estrogens, mainly estradiol but also estrone. Aromatase action, and therefore estrogen production, is controlled by FSH. Hence, the function of theca cells and granulosa cells are controlled by LH and FSH respectively (Fig. 3). There is some, however, as the LH receptors expressed by FSH on the granulosa cell membranes of developing follicles of >10mm diameter render LH capable of inducing estradiol production and follicular growth in the mid-late follicular phase.
Fig. 3. The two-cell, two gonadotropin hypothesis.
In clinical practice, hCG has been used as an excellent substitute for the LH surge in the triggering of ovulation as it binds to the LH receptor. It has a much longer half-life than LH. The current availability of pure, recombinant LH (and recombinant FSH) have enabled the further investigation of the physiology of the ovulatory cycle. High doses of recombinant LH are capable of triggering ovulation. The availability of these preparations as separate entities has prompted a large number of experiments to examine what is their exact function and necessity throughout the cycle.
Estradiol
Estrogens are the basic female hormones and estradiol is the most important as far as the ovulatory cycle is concerned. The synthesis of estradiol by granulosa cells is a function of the action of FSH. FSH stimulates the enzyme aromatase (CYP19) to convert the substrate of basic androgens, androstendione and testosterone, to estradiol in granulosa cells. The production of this vital hormone thus requires the availability of the androgen substrate, whose production in theca cells is promoted by LH, and then the action of FSH.
The key functions of estradiol in the ovulatory cycle are:
- As a cog in a negative feedback mechanism suppressing the secretion of FSH and so aiding in the selection of the dominant follicle and preventing multifollicular development in the mid-late follicular phase.
- Triggering of the LH surge in mid-cycle by initiating a positive feedback mechanism when its concentrations rise to a critical level.
- As a ‘growth hormone’ for the development of the endometrium.
Estradiol concentrations are at their lowest during menstruation. The FSH induced follicular development brings about rapidly rising estradiol production in the mid-follicular phase. When estradiol levels attain a persistently high critical concentration in the late follicular phase, they induce the LH surge. Following ovulation, estradiol concentrations dip temporarily but are revived by corpus luteum activity. With the demise of the corpus luteum, estradiol concentrations sink rapidly to their lowest levels and invoke the FSH rise immediately preceding menstruation (Fig. 2).
A mistake of nature, hypogonadotrophic hypogonadism, in which both FSH and LH secretion are essentially missing, has provided a learning tool for the understanding of ovulatory physiology. The absence of FSH results in a lack of follicular development and estrogen production and the absence of LH in a lack of androgen substrate production. When treatment with pulsatile GnRH is administered, pure substitution therapy, everything falls into place and ovulation can be successfully induced. If pure FSH is used to induce ovulation by direct stimulation of the ovaries, the lack of LH and therefore lack of production of androgen substrate, allows the growth of follicles but not estradiol production. Even if ovulation can be triggered by hCG or recombinant LH when a large follicle is obtained, implantation cannot occur due to the lack of estrogen stimulation on the endometrium.
Progesterone
Progesterone is produced by luteinized granulosa cells. Large quantities are synthesized by the corpus luteum following ovulation. Progesterone concentrations rise to a peak 7–8 days following ovulation and fall rapidly with the failure of the corpus luteum (Fig. 2). The main function of progesterone from the corpus luteum is to fashion a secretory endometrium, capable of hosting the implantation of an embryo and to maintain this endometrium throughout the early weeks of pregnancy until trophoblastic/placental hormones take over this role. Under the influence of progesterone the endometrial glandular structures increase greatly in numbers and become more tortuous. Progesterone also plays a role in the expression of genes needed for implantation at the level of the endometrium.
Together with estradiol, progesterone suppresses pituitary gonadotropin release during the luteal phase. The increasing concentrations of progesterone following ovulation gradually reduce the frequency of the GnRH/LH pulses and increase their amplitude. During this phase, FSH is synthesized and stored ready for release when freed from the inhibition imposed by progesterone and estradiol when the corpus luteum fails. The initial rise of progesterone concentrations immediately preceding the LH surge may play a role in the triggering of this surge.
OVARIAN MORPHOLOGY
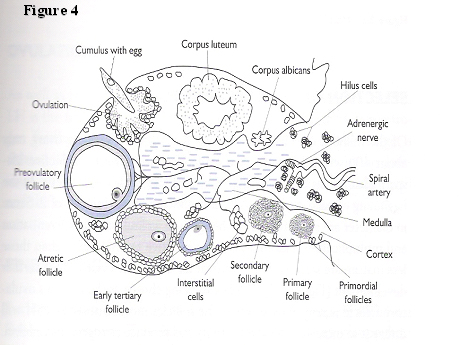
The ovary is, arguably, the most dynamically, constantly changing organ in the female body during the reproductive life span (Fig. 4). The inner, medullary or stromal section, is made up of connective tissue inundated by small capillaries and adrenergic nerves. The cortex, contains an enormous number of oocyte-containing follicles ranging from approximately 300,000 at menarche to 1500 at menopause. There is a constant state of flux in the various stages of development of the follicles from primordial (an oocyte with a single layer of granulosa cells around it), through primary and secondary stages with increasing numbers of layers of granulosa cells, antral stage containing follicular fluid, to a fully fledged, pre-ovulatory follicle. A corpus luteum can be seen in the luteal phase of the cycle and the picture is completed by the presence of corpora albicans (remnants of degenerate corpora lutea).
Fig. 4. Diagrammatic representation of ovarian morphology.
Although much of this changing picture of stages of follicular development is dependent on the stage of the (gonadotropin-dependent) ovulatory cycle, there is a constant, non-FSH dependent, progression in development of primordial to potentially ovulatory follicles being available at the start of the ovulatory cycle, a process that may take about 10 weeks. Progression from primordial, through primary, secondary to pre-antral follicles in the non-gonadotropin dependent part of follicle growth involves the apparent interplay of several factors. Among these, androgens encourage this progression whereas anti-Mullerian hormone (AMH) has an opposite function.
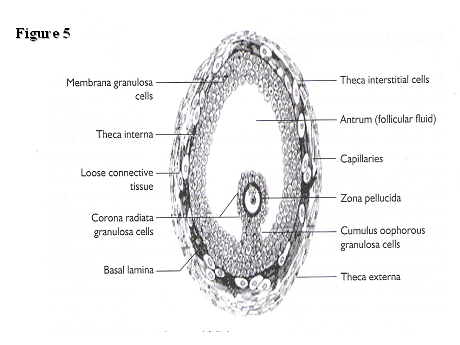
A diagrammatic representation of a pre-ovulatory follicle is illustrated in Fig. 5.
Fig. 5. Morphology of an antral follicle.
The oocyte itself grows during the preovulatory phase of follicular development. Oocyte development is suspended in the dictyate stage of the first meiotic division from embryonic life until just before ovulation. An oocyte remains in the germinal vesicle stage until after it has been stimulated by a preovulatory surge of LH upon which meiosis is resumed. Meiosis involves two divisions. In the first, the homologous chromosomes are separate entities, homologous pairs separate from each other. In the second division, each chromotid pair is joined by a centromere and each individual chromatid from the pair splits from its counterpart. The oocyte ovulates during metaphase II and reduction division is completed after the egg is penetrated by a spermatozoon.
SELECTION OF THE DOMINANT FOLLICLE
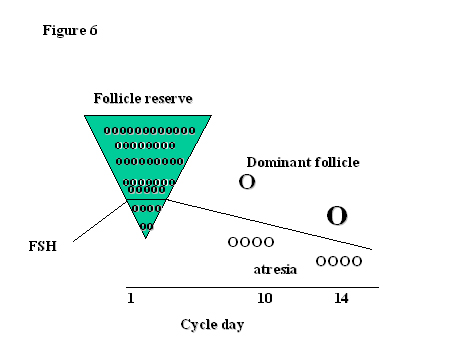
Of the millions of primordial follicles that started life in the ovary, only about 400 will actually achieve ovulation during the reproductive life span. That means that more than 99.9% of follicles become atretic. At the beginning of each cycle, a group of the most mature follicles of 2–5 mm diameter are recruited for further growth, granulosa cell differentiation and multiplication. The follicles more sensitive to FSH rather than those less mature are selected at the time of the FSH inter-cycle rise for further development (Fig. 6). The key to being chosen as the month’s ovulatory follicle is sensitivity to FSH. The follicles most sensitive to FSH will utilize it to increase aromatase activity and produce estrogens and inhibin. As FSH concentrations fall in response to rising estrogen and inhibin B levels and become less available, only the most sensitive follicle to FSH, that with the lowest threshold for a response to FSH, can survive, continue to thrive and produce the most estrogen and LH receptors. The rest, starved of the possibility of FSH stimulation, become atretic. The selection of the dominant follicle is an example of survival of the fittest for which a good start in life is extremely important!
Fig. 6. Selection of the dominant follicle in relation to FSH.
OVULATION
As well as playing a secondary role in follicular responsiveness to FSH, LH is the trigger for ovulation itself. In response to the switch in estrogen mediated feedback from negative to positive, the LH mid-cycle surge is created. This activates a whole cascade of inflammatory responses in the dominant follicle leading to the breakdown of the follicular boundary wall and the escape of the oocyte with its cumulus oophorus.
The local events within the ovary itself that lead to follicular rupture are far from clearly understood. Although increased intrafollicular pressure would seem to be the obvious cause of follicular rupture, this has not proved to be the case. It is likely that many factors complement one another in the necessary mechanics involved in follicular rupture. These include proteolytic enzyme activity on the follicular wall, morphological changes in the stigma that favor follicular rupture, perifollicular ovarian smooth muscle contractions and vascular alterations in the perifollicular vessels. Some of these changes have been attributed to increased concentrations of prostaglandins in the ovarian follicles and some to a cascade of enzymatic steps resulting in collagenolysis. The confusion is compounded by the suggested participation of various cytokines, oxygen free-radicals, nitric oxide and angiotensin II. More study is clearly needed to elucidate the complicated mechanism of follicular rupture.
FINE TUNING
Such an intricate process as ovulation would not be complete without a fine-tuning system. This involves a large number of compounds, endocrine, autocrine and paracrine factors.
Inhibin
Inhibin is secreted by granulosa cells. Inhibin A and inhibin B are dimers which differ in their pattern of secretion. Inhibin A concentrations are low during most of the follicular phase but start to rise during its latest stages and peak in the mid-luteal phase. In contrast, inhibin B concentrations start rising early in the follicular phase, paralleling but later than the FSH rise. Inhibin B negatively influences FSH concentration and also reflects the size of the follicle cohort. Estrogens and inhibin B are both inhibitory factors for the secretion of FSH.
Activin and follistatin
Activin is a promotor of many actions of FSH in that it increases FSH secretion, promotes ovarian follicular development and inhibits androgen production. Follistatin is an activin-binding protein that neutralizes activin bioactivity.
Growth factors
Many growth factors form a network of interactions within the ovary and its compartments. The most well known are the insulin-like growth factors (IGFs) I and II which are very active and are counteracted by IGF binding proteins, six of which have been identified so far. Insulin, as well as binding to IGF receptors, has its own ovarian receptors and is known to promote androgen production. The transforming growth factor (TGF) family is also well represented in the ovary as is epithelial growth factor (EGF). All play a passive role in the regulation of gonadotropin activity within the follicles.
Anti-Mullerian hormone (AMH)
AMH, a dimeric glycoprotein and member of the transforming growth factor-beta family, is produced by ovarian follicular granulosa cells in late pre-antral and small antral follicles. It seems to have a role in the regulation of folliculogenesis at the two extremes of this process: (a) by restricting the progression of development of primordial follicles; and (b) by an inhibition of the sensitivity of antral follicles to FSH and inhibition of aromatase activity during an ovulatory cycle. Production of AMH is not seen in a maturing pre-ovulatory follicle, possibly inhibited by rising estradiol levels, leaving uninhibited FSH to carry the process further forward. These roles of AMH suggest not only that it plays an important function in the regulation of folliculogenesis, but also that its concentrations are able to reflect ovarian reserve or, more practically, the number of small antral follicles in a cohort available for ovarian stimulation. The age-related decline in ovarian reserve is mirrored by serum AMH concentrations which can also reliably predict the number of oocytes collected following ovarian stimulation or, at the least, enable a prediction of low, normal and high responders.
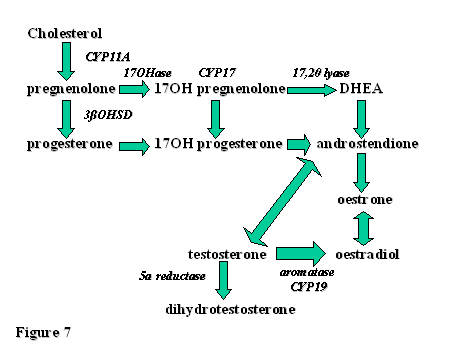
OVARIAN STEROIDOGENESIS
The pathways of ovarian steroid production are diagrammatically illustrated in Fig. 7. The enzymes involved in these complicated processes are also signified. A basic knowledge of these actions is necessary not only for the understanding of normal ovarian physiology but especially for pathophysiological conditions such as polycystic ovary syndrome (PCOS).
Fig. 7. Pathways of ovarian steroid production and some of the enzymes involved.
DHEA, dehydroepiandrosterone
ACKNOWLEDGMENT
This article has been largely adapted, with permission, from a chapter in the book by this same author, Roy Homburg Ovulation Induction and Controlled Ovarian Stimulation – A Practical Guide published by Taylor & Francis (now Informa), 2005. ISBN 1-84184-429-2. The second edition, published by Springer, is available from summer 2014.