The Pathophysiology, Diagnosis, and Management of Rectoceles
Authors
INTRODUCTION
Since the early 19th century, surgeons have performed posterior colporrhaphy to manage complete tears of the perineum. In the early 1800s, the supports of the genital organs were largely a mystery, and there was little distinction between prolapses of the rectum, bladder, and uterus. As anatomic concepts developed, surgeons ascertained that the main support of the uterus was the vagina, which was supported by the insertion of the levator ani muscles into the perineum. This concept was the basis for the incorporation of plication of the levator ani muscles into posterior colpoperineorrhaphy, with the surgical goals of constriction of the vaginal tube, creation of a perineal shelf, and partial closure of the genital hiatus. Until more recently, little attention was given to the functional derangements that commonly are associated with rectoceles.
A rectocele is an outpocketing of the anterior rectal and posterior vaginal wall into the lumen of the vagina.1 Some rectoceles may be asymptomatic, whereas others may cause symptoms of incomplete bowel emptying, vaginal mass, pain, and pressure. The incidence of rectoceles is 20–80% in the general population and is thought to be increasing.2 A rectocele is fundamentally a defect of the rectovaginal septum, not of the rectum. The size of the defect does not correlate with the amount of functional derangement. This chapter reviews the anatomy, pathophysiology, diagnosis, and management of rectoceles.
ANATOMY
In 1839, Denonvilliers first described a layer of fascia found in men, which he named the rectovesical septum. Nichols and Milley later documented this septum to exist in surgical dissections and fresh female autopsies.3 This layer of connective tissue is fused to the undersurface of the posterior vaginal wall.
The rectovaginal fascia extends downward from the posterior aspect of the cervix and the cardinal-uterosacral ligaments to its attachment on the upper margin of the perineal body and laterally to the fascia over the levator ani muscles. Richardson3 stated that the rectovaginal septum and uterosacral ligaments provide suspensory support of the perineal body from the sacrum. Posterior to the rectovaginal septum lies the rectovaginal space, which provides a plane for dissection. In between the rectovaginal septum and the rectum, pararectal fascia exists. Inside this fibromuscular layer are blood vessels, nerves, and lymph nodes, which supply the rectum. The pararectal fascia, originating from the pelvic sidewalls, divides into fibrous anterior and posterior sheaths, which encompass the rectum. These layers provide additional support to the anterior rectal wall.4
The distal rectum is supported by the perineal body and its lateral fibrous attachments, through the perineal membrane, to the ischiopubic rami.5 In this orientation, the distal rectum abuts the perineal body. The supporting fibers become taut and resist displacement when force is applied caudally. Interruption of these fibers allows downward prolapse of the distal rectum.
Further support is provided by the levator ani, which is composed of paired iliococcygeus, puborectalis, and pubococcygeus muscles. These muscles function to maintain a constant basal tone and a closed urogenital hiatus. This constant basal tone prevents the urogenital hiatus from widening and the eventual descent of the pelvic viscera. When the levator muscles are damaged or relaxed, the vaginal canal opens, increasing tension in the midvagina.5 Loss of connection between the halves of the perineal membrane allows the levator muscles to move apart, again placing downward stress on the mid and distal rectum. These muscles also provide a contraction reflex to increased intra-abdominal pressures, preventing incontinence and prolapse. The anterior sacral nerve roots S2–4, which innervate these muscles, cross the pelvic floor and are stretched and compressed during labor, increasing the chance for injury.4, 6
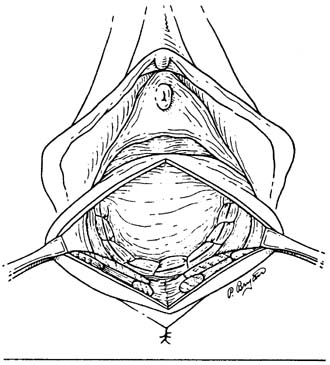
Anatomic studies7 have postulated that pelvic organ support and function should be thought of as a fluid process, constantly adjusting and changing. Level I support: The paracolpium is suspensory in nature attaching to the lateral pelvic walls with fibers attaching to the sacrum (i.e. ischial spine and sacrospinous ligament). Level II support: The vagina is attached laterally to the arcus tendineus fascia pelvis and superior fascia of the levator ani muscle. Level III support: The vagina is fused with the very strong support of the pubic rami, urogenital diaphragm and perineal body (see Figures 1 and 2).
Fig. 1. Pelvic floor support levels
Fig. 2. Normal pelvic floor fascia structures
CAUSE
Rectoceles were previously thought to be a condition affecting only multiparous women, resulting from obstetric damage or increased tissue laxity with aging and menopausal atrophy. More recently, rectoceles and enteroceles were noted to occur in approximately 40% of asymptomatic parous women.8 Shorvon and colleagues9 performed defecography on healthy, young, nulliparous, asymptomatic volunteers, noting 17 of 21 women had small or moderately sized rectoceles. Rectoceles may have a broader incidence than previously thought and may not be a result of parity.
The most common causes of rectoceles are obstetric events. Traumatic obstetric events, which usually occur when the presenting part descends quickly in the second stage of labor, can predispose to rectocele formation. The forces of labor may separate, tear, or distend the pelvic floor, altering the functional and anatomic position of the muscles, nerves, and connective tissues. The rectal fascia may separate from the perineal body, causing a transverse defect and low rectocele. Low rectoceles are an isolated defect in the suprasphincteric portion of the rectovaginal fascia. They usually are caused by obstetric trauma that disrupts the attachments of the levator ani fascia and bulbocavernosus muscles. An eversion of the introitus is noted on physical examination. This eversion aggravates constipation and results in inefficient bowel movements and the need for stronger Valsalva maneuver.10, 11, 12, 13, 14 If mid or high rectoceles form, they may alter the vaginal axis.1, 4, 10, 11 Laxity of the levator ani secondary to either the levator detaching from the perineal body along the vaginal axis or the separation of the halves of the perineal body allows the pelvic organs to slide downward following the new altered axis. Individuals with an android pelvis are at increased risk because labor forces are directed toward the posterior vaginal wall and perineum, leaving the anterior vaginal wall relatively protected.
Midvaginal rectoceles most likely are caused by obstetric trauma not involving the levator ani. The rectovaginal fascia is damaged by the stretching and laceration of the tissue, which results in thinning of the fascia, leading to subsequent adhesion formation. This adhesion of the rectovaginal septum, vagina, and rectal capsule inhibits independent function. Symptoms may include incomplete bowel emptying, rectal pressure, and pain after bowel movements. These symptoms also may occur with a high rectocele.14
High rectoceles often occur from pathologic overstretching of the posterior vaginal wall. The cardinal ligaments fuse with the vagina and cervix, causing the cervix to fuse with the anterior vaginal wall. The rectovaginal septum is absent from the posterior vaginal wall, causing loss of the anterior rectal wall support. High rectoceles also may coexist with congenital deepness of the pouch of Douglas.14
Rectoceles may result secondarily from pathologic stretching of the pudendal nerves during descent of the fetal head, causing atrophy and denervation of the pelvic floor muscles. Sultan and Stanton15, 16 reported that most damage to pelvic support occurs with the first vaginal delivery. Electromyography studies showed an 80% occurrence rate of denervation of perineal muscles after vaginal delivery.6, 17, 18 Denervation most likely recovers after the postpartum period; however, it has been shown that injury may be cumulative with increasing parity.6 Increased labor duration and weight of the fetus directly influence perineal damage and denervation of the pelvic floor. This neuropathy can lead to weakening of pelvic floor muscles and development of a rectocele. Shortening of the second stage of labor by episiotomy or forceps may decrease the risk of denervation and subsequent pelvic floor damage.10
Defecation disorders may cause a subgroup of rectoceles. These disorders may lead to the weakening of the rectovaginal septum by continuous straining against an obstruction. One disorder, the perineal descent syndrome, is diagnosed clinically when the individual strains and the perineal plane descends past the ischial tuberosities.17 This disorder may be confused with a rectocele. Other conditions, such as paradoxical sphincter reaction (anismus), cause the individual unconsciously to contract the voluntary striated muscles when attempting to defecate. This constant straining with bowel movements has been shown to cause or worsen a pre-existing rectocele and increasingly weakens the rectovaginal septum by denervation injury.19 Anismus eventually leads to the accumulation of stool in the rectum, which may complicate pelvic outlet obstruction and cause a progressive cycle, worsening the rectocele.20
Congenital absence of the perineum may mimic a rectocele. This pseudorectocele has its posterior vaginal wall exposed because of the lack of inferior support. This condition may be corrected by surgical reconstruction of the perineum. Congenital absence allows for deepening of the cul-de-sac and weakening of the rectovaginal septum, leading to the development of a high rectocele and enterocele.8, 14
Some studies have observed differences in connective tissue strengths between races, which may contribute to rectoceles. Black women have been noted to have a decreased frequency of laceration after normal spontaneous deliveries and a subsequent decrease of uterine prolapse; this may be related to constitutional factors, such as pelvis type, connective tissue, and the ability to undergo fibrosis.14 Hispanic, Filipino, and Chinese women may have an increased risk for laxity of tissue.21
CLINICAL PRESENTATION
Clinical patient complaints of bowel dysfunction vary from being asymptomatic to severe. A common complaint is constipation, which may occur in 75% of women with rectoceles.2 Patients may complain of incomplete rectal emptying, a sense of rectal pressure, or a vaginal bulge.1, 22, 23 Vaginal digitation or perineal support is sometimes necessary to facilitate defecation.22 Many nonspecific symptoms, such as rectal pain, bleeding, fecal or gas incontinence, low back pain that worsens throughout the day but is relieved by lying down, and dyspareunia, may occur and many other defecatory disorders.8 As previously mentioned, most rectoceles are totally asymptomatic.
PHYSICAL EXAMINATION
Physical examination usually begins in the dorsal supine position for the gynecologist and the left lateral decubitus position for the colorectal surgeon. Delemarre and colleagues24 found that examination in the supine position leads to the underscoring of the rectocele, whereas the lateral decubitus position more closely matched the findings of defecography. After the external genitalia are inspected, the introitus and vaginal vault are inspected. The strength, integrity, and signs of descent (prolapse) of the perineum are tested. The patient is asked to strain down or cough forcefully. This maneuver allows the pelvic organs to descend to determine the extent of the prolapse. Digital support of the perineum opens the genital hiatus, allowing visualization of the anatomy. Posterior vaginal wall prolapse caused by a rectocele or enterocele is assessed by using a rectal finger to evaluate for anterior displacement of the rectovaginal septum and perineal body. A rectocele can be differentiated from an enterocele by noting bowel in the rectovaginal space. On standing, the rectovaginal examination can reveal small bowel herniating into this space when an enterocele is present. Of women with rectoceles, 80% are asymptomatic and can be diagnosed only on physical examination.9,20 Additional diagnostic tests may assist the physician in the evaluation of posterior vaginal wall defects and defecatory dysfunction.
DIAGNOSTIC STUDIES
Defecography
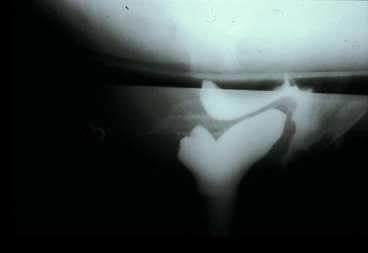
Defecography, first described by Burhenne in 1964,9 increasingly is being used for preoperative evaluation of pelvic organ prolapse. Defecography is believed useful by some because it provides objective outcomes and identifies anatomic abnormalities. The technique involves filling the rectum with a barium paste that is the consistency of stool and opacifying the vagina. Fluoroscopy is used at rest, on contracting the pelvic floor muscles, on straining down, and during defecation (Fig. 3).20 The fluoroscopic examination grades rectoceles during maximal distention as small (<2 cm), moderate (2–4 cm), and large (>4 cm).1, 11, 20 Kelvin and associates20 noted that barium was retained only in moderate-to-large sized rectoceles. The relevance of the rectocele size compared with symptoms has not been shown25; however, larger rectoceles are associated with fecal trapping.11
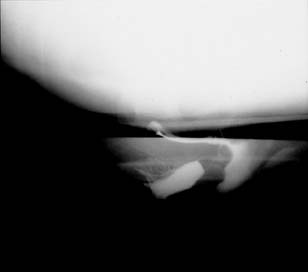
Defecography is a good diagnostic tool to help exclude other defecation disorders that may increase the risk of recurrence of symptoms despite anatomic repair. Radiographically, anismus or paradoxical sphincter response is observed as a decrease, or less than 5% increase, in the anal rectal angle during straining.18, 26 The anterior rectal wall enfolding 6–8 cm inside the rectum identifies internal intussusception.20 An enterocele may be suspected if the distance between the vagina and the rectum is 2 cm or greater and the concurrent herniation of small loops of bowel is seen within the rectovaginal space.20 Significant rectoceles tend to trap barium during defecography (Fig. 4). If barium entrapment is not present, some physicians1, 23 discourage surgical correction. Although defecography has been shown to be reproducible when clinical data are present,27 the significance of fecal trapping and usefulness of correlating data between fecal trapping and the need for repair are controversial and need to be studied further.
Scintigraphic defecography is another tool to evaluate the physiology of rectal emptying. It not only assesses the rate and completeness of emptying, but also assesses the anorectal angle and pelvic floor descent. Advantages include less radiation exposure and precise quantification. Disadvantages include its inability to evaluate the anatomic detail or view pelvic floor anatomy.18
Ultrasound
The use of endosonography of the anus has proved to be valuable in detecting incontinence disorders by imaging the anatomic integrity of the internal and external anal sphincter. Sandridge and Thorp28 stated anatomic defects are best detected with an endovaginal probe by measuring rectal length and diameter, puborectalis thickness and angle, thickness and integrity of the internal and external anal sphincters, and curvature of the anal canal.
Anal Mammometry
Anal mammometry measures rectal pressures by a transducer or balloon. Its measurement of rectal sensation evaluates first feeling, urge, and discomfort. This information is used to distinguish causes of constipation. When an individual is able to tolerate increased volumes without signs of increased discomfort or the urge to defecate, overflow incontinence may occur. Careful consideration must be made during this evaluation process because individuals with small volumes may have an irritable rectum causing incontinence or urgency. These disorders may mimic rectocele symptoms, such as incontinence or incomplete emptying. If misdiagnosis of a rectocele is made, rectocele repair may exacerbate these disorders by causing worsening of symptoms.18, 19, 29
Electromyography and Nerve Conduction Studies
Electromyography and nerve conduction studies also have been used to evaluate defecation disorders. Obstetric trauma denervates and causes atrophy of the pelvic floor muscles, which may lead to subsequent pelvic floor weakness. This denervation may be detected by electromyography studies, and pudendal terminal motor latency can be used as a method to detect the causes of pelvic floor weakness.
Colonic Transit Studies
Colonic transit studies are used to assess distal bowel motility and may be indicated when infrequent stooling is reported by a patient with a rectocele. The patient ingests a capsule with 24 radiopaque markers, which are measured and counted in the right colon, left colon, sigmoid colon, and rectum. Serial abdominal X-rays are done. Five days following ingestion, if 80% of the markers are retained, a colonic motility disorder should be considered. Clinically slow transit time is defined as fewer than two bowel movements per week over several years. The motility in individuals with rectoceles is inconclusive, although some have normal transit times, whereas others have prolonged times.26 Individuals with unimproved symptoms after rectocele repair were found preoperatively to have longer transit times.22
MANAGEMENT
When the clinical diagnosis is made, potentially confirmed by ancillary studies, the decision to operate or to treat conservatively must be made. Most nonsurgical treatments consist of proper bowel training, following an active lifestyle, and eating an appropriate amount of dietary fiber.30, 31 These steps are most important when the main complaint is constipation. The only nonsurgical therapy available for prolapse symptoms is estrogen replacement therapy in postmenopausal patients and the use of a vaginal pessary. In our experience, pessaries have not been effective in women with isolated symptomatic rectoceles. Indications for surgery should include severe symptoms, the presence of an anatomic defect, or the need for other pelvic reconstructive surgery with an asymptomatic rectocele.11
Symptoms that predict good postoperative results include pelvic pressure and a vaginal bulge, vaginal digitalization or splinting (which occurs in 20–75% of symptomatic patients), and outlet obstruction constipation. Janssen and van Dijke29 noted that repair increased rectal sensitivity causing the urge to defecate earlier as a positive predictor of a good outcome. The colorectal literature noted that defecography showing a rectocele greater than or equal to 20 mm with symptoms is a good indicator for surgery; however, this finding has not been conclusive in all studies.2 Sullivan and coworkers32 reported that anoscopic evidence of the rolling-down of the anterior rectal wall must be present before surgical correction should be undertaken;2 however, this may not be valid for all female patients.29
Signs and symptoms that are predictive of a poor surgical outcome include a history of potent laxative use, incidence of preoperative pain, and large-volume rectoceles in women who previously had undergone hysterectomy.22, 23, 29, 33 A few studies in the colorectal literature have noted that hysterectomy disrupts parasympathetic nerves, causing decreased rectal sensation and increased rectal compliance, which may not be improved after anatomic correction.29, 33 The persistence or development of dyspareunia after rectocele repair has been variable and depends on the surgical technique performed. Levator plication and excessive narrowing of the introitus may lead to increased dyspareunia, whereas defect-specific repair has been associated with disappearance or improvement of dyspareunia.34
SURGICAL REPAIR
Three different surgical techniques currently are being used to repair symptomatic rectoceles: levator plication, site-specific repair, and transanal and transabdominal repairs. These all can be done with or without mesh/graft augmentation. Gynecologic surgeons traditionally have advocated a transvaginal repair involving a levatorplasty. Because of the development of dyspareunia in some patients and the relatively poor functional outcome of these repairs, some gynecologic surgeons have advocated a defect-specific rectocele repair. Colorectal surgeons continue to repair rectoceles via a transanal approach.
Defect-Specific Rectocele Repair
According to Richardson,2, 3 rectoceles are caused by a variety of specific breaks in the rectovaginal fascia (Fig. 5). Richardson described the most common break as being a transverse separation above the attachment to the perineal body, resulting in a low rectocele. Another common fascial break results from an obstetric tear or episiotomy that was repaired improperly. This midline vertical defect may involve the lower vagina and extend to the vaginal apex. Less common separations involving a lateral separation down one of the sides of the fascia also were found to exist. Richardson also stated that a U-shaped or L-shaped tear in the fascia might occur. Since Richardson’s observations, there has been an increased movement toward site-specific rectocele repair among gynecologists. Richardson recommended doing the repair with a finger in the rectum so that defects can be identified easily and fascia can be approximated appropriately with interrupted sutures.
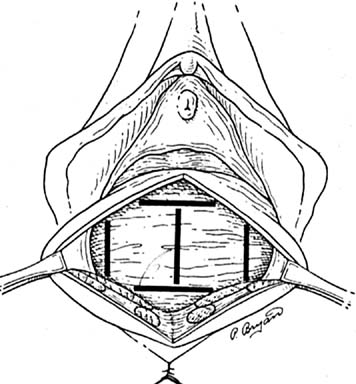
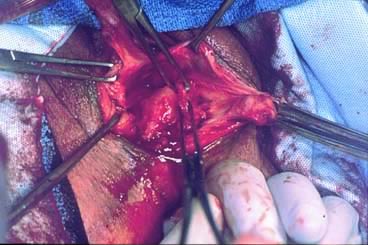
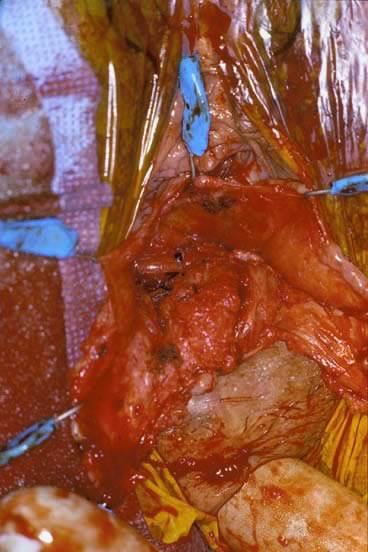
Before starting any rectocele repair, the surgeon should approximate the introitus by using Allis clamps bilaterally to help determine how much perineal and vaginal tissue needs to be excised to correct any gaping of the introitus. The repaired opening should accommodate three fingerbreadths, taking into account that the levator ani and perineal muscles are relaxed from general anesthesia and may constrict further postoperatively and with postmenopausal atrophy. The next step is to place Allis clamps on the posterior perineum; a diamond-shaped perineal incision is made, and the overlying skin is removed. The length and width of the perineal incision depend on the epithelium needed for restoration of the perineal body. Metzenbaum scissors are used to develop a plane in the rectovaginal space. An effort is made to leave as much fascia on the rectum as possible. Sharp dissection usually is required over the perineal body because of previous scarring from episiotomies. The surgeon performs blunt and sharp dissection between the mucosa and fascia to the apex of the vagina. This dissection is continued laterally to the tendinous arch of the levator ani and extends inferiorly to the perineal body. When hemostasis is ensured, irrigation may be performed to attain a clean operative field to allow inspection for defects. The rectovaginal fascia is inspected for defects by the surgeon inserting a finger of the nondominant hand into the rectum (Figs 6 and 7).
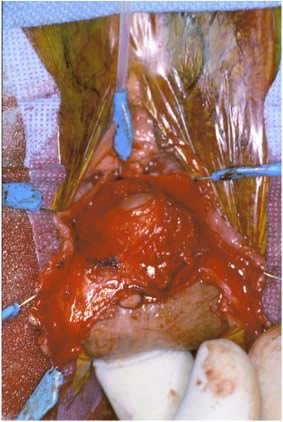
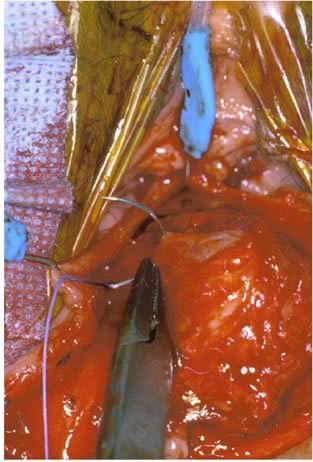
The rectal wall is brought forward, distinguishing the uncovered muscularis (fascial defect) from the muscularis covered by the smooth semitransparent rectovaginal septum. Frequently, based on the plane of dissection and the location of the defect, the rectovaginal fascia must be mobilized off the lateral vaginal epithelium. After the defect is identified, Allis clamps are used to grasp the connective tissue (perirectal or rectovaginal fascia) and pull it over the bare area to facilitate repair (Figs. 8 and 9). The rectal finger is used to determine if a defect has been corrected. Next, this area is sewn together with vertical mattress sutures, plicating the fascia over the rectal wall, which is repaired with No. 2-0 or 0 delayed absorbable suture (Fig. 10). The surgeon must examine after each suture to ensure that a smooth contour exists and that no vaginal narrowing occurs.3, 4, 8, 35, 36
|
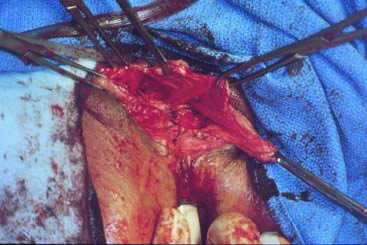
Completed repairs have complete resolution of the fascial defects (Figs. 11 and 12). The index finger of the surgeon is reinserted into the rectum, and the fascial defect cannot be shown (Fig. 11). Although rectocele repair is accomplished for identification of the fascial defect and reapproximation of the connective tissue, evaluation of the levator hiatus is a different issue. In women who have an enlarged levator hiatus, it may be appropriate to place another set of interrupted sutures horizontally to narrow the levator hiatus. This portion of the operation is not necessary in all patients and is independent of the rectocele repair.
If perineoplasty is necessary or desired, it is done at this time. The perineal body consists of the anal sphincter, the superficial and deep transverse perineus muscles, the bulbocavernosus muscles, and the junction of the rectovaginal fascia to the anal sphincter. Perineoplasty implies identification and reconstruction of these components. The first step is to remove any old scar tissue to the point that fresh viable tissue is revealed. If a grade 4 laceration is present, the rectal mucosa is completely mobilized from the vaginal wall and reapproximated with interrupted sutures. Then the external anal sphincter is reapproximated. A stepwise reapproximation of transverse perineal muscles is done, and the perineal body is sewn over the sphincter. Finally the bulbocavernosus muscle ends are attached to the perineal body. This musculofascial complex is covered by suturing the overlying vulvar skin with No. 3-0 suture in a running fashion. Vaginal packing is placed at the end of the procedure and is removed on postoperative day 1, and diet is advanced as tolerated.35, 36
Traditional Transvaginal Repair
The traditional rectocele approach has been described and illustrated by Nichols and Wheeless.37, 38 The opening of the vagina is performed as described in the previous section, via a midline incision or via removal of a triangular wedge of vaginal wall. Some surgeons place an initial row of 2-0 interrupted sutures approximating the rectovaginal fascia. The rectocele is depressed in the midline with the surgeon’s finger to reveal the margin of the puborectalis portion of the levator ani muscle. With the rectocele still depressed, a No. 1-0 absorbable suture is used to suture the margins of the levator ani muscles in an interrupted fashion. After all the sutures have been placed, they are tied. Posterior vaginal mucosa is trimmed appropriately and closed with either interrupted or continuous 3-0 absorbable sutures. The perineal body is repaired as previously described.
Transrectal Approach
The transrectal approach was described by Sarles and associates.13 In this method, all patients are treated preoperatively with oral laxatives and are given antibiotics: metronidazole, 500 mg twice a day for 2 days before surgery, and cefuroxime, 1.5 g intravenously 1 hour before surgery. The anus and vagina are cleansed with povidone-iodine. A Park retractor is inserted into the anal canal to expose the anterior rectal surface. An incision of the anterior rectal mucosa is made 1 cm above the dentate line. The submucosal plane is dissected sharply 8 to 10 cm from the anal verge. Bleeding is controlled by electrocoagulation. Dissection is formed anteriorly and laterally; the resulting bare areas are plicated using interrupted polyglycolic acid 2-0 suture placed 0.5 cm apart. The suture includes the rectal muscle and the rectovaginal septum. If the vaginal mucosa is perforated, the stitch is removed. The mucosal flap of at least 6 cm in length is excised. A second layer of 3-0 polyglycolic acid sutures closes the mucosal defect. Postoperative care includes delaying oral feedings for 48 hours after surgery and slowly advancing the diet over several days.
Transabdominal Repair
This approach is used in conjunction with an apical defect (enteroceles, vaginal vault) repair. Typically in an abdominal approach only a sacracalpopexy (high/mid rectoceles) or a combination abdominal/vaginal approach sacrocalpoperineopexy (low rectoceles) is performed. The rectocele defect is addressed by placing a synthetic mesh from the perineal body along the posterior vaginal wall superiorly to the periosteum of the sacrum.
Mesh/Graft Augmentation
Due to the high anatomic and functional success rates (>82%) of site-specific rectocele repairs,34, 39, 40 it is difficult to universally recommend posterior compartment graft augmentation. Literature review of posterior graft augmentation has been inconclusive with one study demonstrating significant increased anatomic failure in the graft group vs. posterior colporrhaphy.41 However, graft augmentation may be beneficial in patients who are risk for recurrence: poor tissue quality, prior surgical failure, impaired healing, older age (>60) or chronic increases in intrabdominal pressure (obstructive pulmonary disease, asthma or constipation).
Graft-augmented repairs are performed similarly to site-specific repairs. The hymenal ring is grabbed with Allis clamps and the ring is approximated to leave an introital opening of roughly 3 cm. Subsequently a self-retaining retractor is placed to provide counter traction for the dissection. Hydrodissection (normal saline and/or 0.5% lidocaine/epinephrine is used); this helps to dissect the underlying tissue away and to help with hemostasis (the typical total amount of fluid used is 75–200 ml).
A triangular incision is made over the perineal body and the skin is removed. A scalpel is used to make a small vertical incision. Most dissection is accomplished through tenting the vaginal mucosa and tunneling underneath it. Metzebaum scissors are then used for sharp dissection of the vaginal mucosa away from the rectovaginal fascia. In addition, some blunt dissection with a moist sponge can be used. The pararectal space is entered and the sacrospinous ligament and ischial spine are identified. Some dissection and removal of pararectal fat may be required to clearly identify the sacrospinous ligament.
It is at the surgeon's discretion to determine which anatomic support structure (sacrospinous ligament or iliococcygeus muscle) will be used for the apical support of the vagina/graft. It is also up to the surgeon to reduce the size of the prolapse by plicating the rectovaginal fascia in a site-specific repair with interrupted 2-0 absorbable/delayed absorbable suture (Fig. 14).
Fig. 14 Graft attached to the levator ani muscle laterally, pericervical ring and sacrospinous ligament superiorly.
Depending on the mesh graft technique and product that is chosen, the arms of the graft will either be attached directly to the sacrospinous ligament or iliococcygeus fascia or they will be retrieved by a cannula or trocar device.
Before cutting and attaching the graft, remember in vivo graft material may retract up to 30%. The graft is attached distally to the perineal body. Vaginal mucosa trimming is at the discretion of the surgeon. Tension from the self retraining retractor is released. The vaginal mucosa is reapproximated with absorbable suture. A standard perineorrhaphy may be required depending on the physical examination and the patient's wishes (Fig. 15).
Fig. 15 Graft in place between the vagina and rectum.
There are several commercially available mesh kits: PinnacleTM, ApogeeTM, AvaultaTM and ProliftTM. These kits provide a tension-free repair using precut polypropylene or biologic graft material suspended by polypropylene arms that are attached to the sacrospinous ligament or iliococcygeus fascia bilaterally. One problem with these kits (except PinnacleTM) is passing a trocar through the unfamiliar anatomy of the ischiorectal fossa. Although these kits tend to have a higher anatomic cure rate (94%),42 the surgeon must consider the benefits with the apparent increased risks of this technology. These complications include: mesh exposure (10%), prolapse recurrence, rectal injury (1%), vascular injury, rectovaginal fistulas, buttock pain (5%) and dyspareunia.
RESULTS
Few studies have addressed the long-term success of vaginal plastic procedures for treating rectoceles. Early recurrence of a rectocele is most likely the result of missed and unrepaired support defects. Rectoceles that occur late after repair are usually due to constitutional factors, such as weakening of the support tissue because of advancing age, chronic straining, postmenopausal atrophy, and other factors. Anatomic success rates ranged from 82%34,39 to 90%40 using the site-specific repair method. Site-specific studies also showed significant improvement in vaginal wall protrusion, difficult stool evacuation, dyspareunia, and quality of life. There have been variable effects on constipation and manual stool evacuation. Only a few studies have addressed objectively visceral and sexual function after rectocele repair.31,34
COMPLICATIONS
Postoperative complications include constipation, incontinence, incomplete rectal emptying, splinting, pain, bleeding, and sexual dysfunction (Table 1).18 Pain seems to be more persistent and severe after a transvaginal route.31, 43 Postoperative constipation develops or persists in 44–69% of patients.1, 12, 13, 43 Rectoceles may be associated with sexual dysfunction preoperatively and postoperatively. Levator plication leads to increased dyspareunia as a result of pressure atrophy of the included muscle and the resulting scarring.31, 38 Vaginal tightness and dyspareunia also have been reported in transanal rectocele repairs.43 Kahn and Stanton31 suggested that long-term sexual dysfunction may occur after vaginal posterior repair and may be increased with advancing age, postmenopausal atrophy, and other vaginal surgeries. There is a significant background prevalence of dyspareunia in the general population, however, which ranges from 17% to 34%.44 In 1961, Francis and Jeffcoate45 reported a 50% incidence of dyspareunia after vaginal surgery, usually from excessive narrowing of the introitus. Another study found dyspareunia in 9% of women, whereas 24% of patients stated there was improvement in their sexual life after surgery. The authors concluded this low rate of dyspareunia was due to early return to sexual intercourse, only 3 weeks after surgery.46 Kahn and Stanton31 also reported an increased rate of fecal incontinence (from 4% to 11%) after rectocele repair.
Table 1. Complications of rectocele repair
Bleeding | Difficulty with bowel emptying |
Constipation | Fecal incontinence |
Dyspareunia | Proctotomy |
Pelvic pain/pressure | Rectovaginal fistula |
If accidental proctotomy occurs during repair, the rectum should be repaired in layers. Oral feeding should be delayed until 48 hours after surgery and slowly increased from clear liquids, to soft diet, to low-residue diet over the next 48 hours. Laxatives should be avoided for 4–5 days to keep the terminal rectum and anus free of fecal material. If infection occurs, a rectovaginal fistula may develop.8
CONCLUSION
More than 11% of all women who reach age 80 undergo surgery for urinary incontinence or genital prolapse.47 As the society ages, the population suffering from rectoceles or defecation disorders will increase. The clinician will need to be well versed in the proper evaluation and the reparative techniques used to manage these defects. More research is needed to understand fully the correlation between anatomic defects and functional derangements that can occur secondary to posterior vaginal wall prolapse.
REFERENCES
Mellgren A, Anzen B, Nilsson BY, et al: Results of rectocele repair: A prospective study. Dis Colon Rectum 38:7, 1995 |
|
Mollen RMHG, van Larrhoven CJHM, Kuijpers JHC: Pathogenesis and management of rectoceles. Semin Colorectal Surg 7:192, 1996 |
|
Richardson AC: The rectovaginal septum revisited: Its relationship and its importance in rectocele repair. Clin Obstet Gynecol 36:976, 1993 |
|
Babiarz JW, Raz S: Pelvic floor relation. In Babiarz JW, Raz S (eds): Female Urology, 2nd edn, pp 445, 456. Philadelphia, WB Saunders, 1996 |
|
DeLancey JOL: Structural anatomy of the posterior pelvic floor compartment as it relates to rectocele. Am J Obstet Gynecol 180:815, 1999 |
|
Handa VL, Harris TA, Ostergard DR: Protecting the pelvic floor: Obstetric management to prevent incontinence and pelvic organ prolapse. Obstet Gynecol 88:470, 1996 |
|
DeLancey JO: Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992 Jun;166(6 Pt 1):1717-24; discussion 1724-8. |
|
Walters MD: Pelvic floor prolapse: Cystocele and rectocele. In Walters MD, Karram MM (eds): Clinical Urogynecology, pp 225, 236. St. Louis, Mosby-Year Book, 1993 |
|
Shorvon PJ, Mchugh S, Diamant NE, et al: Defecography in normal volunteers: Results and implications. Gut 30:1737, 1989 |
|
Nichols DH, Randall CL: Reduction of maternal injuries associated with childbirth. In Nichols DH, Randall CL (eds): Vaginal Surgery, 4th edn, pp 43, 57. Baltimore, Williams & Wilkins, 1996 |
|
Brubaker L: Rectocele. Curr Opin Obstet Gynecol 8:376, 1996 |
|
Khubchandani IT, Clancy JP III, Rosen L, et al: Endorectal repair of rectocele revisited. Br J Surg 84:89, 1997 |
|
Sarles JC, Arnaud A, Selezneff I, Olivier S: Endo-rectal repair of rectocele. Int J Colorectal Dis 4:167, 1989 |
|
Nichols DH, Randall CL: Types of prolapse. In Nichols DH, Randall CL (eds): Vaginal Surgery, 4th edn, pp 101, 118. Baltimore, Williams & Wilkins, 1996 |
|
Sultan AH: Anal incontinence after childbirth. Curr Opin Obstet Gynecol 9:320, 1997 |
|
Sultan AH, Stanton SL: Preserving the pelvic floor and perineum during childbirth-elective caesarian? Br J Obstet Gynaecol 103:731, 1996 |
|
Benson JT: Vaginal approach to posterior vaginal defects: The perineal site. In Baden WF, Walker T (eds): Surgical Repair of Vaginal Defects, pp 219, 233. Philadelphia, JB Lippincott, 1992 |
|
Kahn MA, Stanton SL: Techniques of rectocele repair and their effects on bowel function. Int Urogynecol J 9:37, 1998 |
|
Johansson C, Nilsson BY, Holmstrom B, et al: Association between rectocele and paradoxical sphincter response. Dis Colon Rectum 35:503, 1992 |
|
Kelvin FM, Maglinte DDT, Benson JT: Evaluation proctography (defecography): An aid to the investigation of pelvic floor disorders. Obstet Gynecol 83:307, 1994 |
|
Green JR, Soohoo SL: Factors related with rectal injury in spontaneous delivery. Obstet Gynecol 73:732, 1989 |
|
Karlbom U, Graf W, Nilsson S, Pahlman L: Does surgical repair of a rectocele improve rectal emptying? Dis Colon Rectum 39:1296, 1996 |
|
Murthy VK, Orkin BA, Smith LE, Glassman LM: Excellent outcome using selective criteria for rectocele repair. Dis Colon Rectum 39:374, 1996 |
|
Delemarre JBVM, Kruyt RH, Dorrnbos J, et al: Anterior rectocele: Assessment with radiographic defecography, dynamic magnetic resonance imaging, and physical examination. Dis Colon Rectum 37:249, 1994 |
|
Hutchinson R, Mostafa AB, Kumar D: Rectoceles: Are they important? Int J Colorectal Dis 8:232, 1993 |
|
Pucciani F, Rottoli ML, Bologna A, et al: Anterior rectocele and anorectal dysfunction. Int J Colorectal Dis 11:1, 1996 |
|
Klauser AG, Ting KH, Mangel E, et al: Interobserver agreement in defecography. Dis Colon Rectum 37:1310, 1995 |
|
Sandridge DA, Thorp JM: Vaginal endosonography in the assessment of the anorectum. Obstet Gynecol 86:1007, 1995 |
|
Janssen LWM, van Dijke CF: Selection criteria for anterior rectal wall repair in symptomatic rectocele and anterior rectal wall prolapse. Dis Colon Rectum 37:1100, 1994 |
|
Infantino A, Masin A, Melega E, et al: Does surgery resolve outlet obstruction from rectocele? Int J Colorectal Dis 10:97, 1995 |
|
Kahn MA, Stanton SL: Posterior colporrhaphy: Its effects on bowel and sexual function. Br J Obstet Gynaecol 104:82, 1997 |
|
Sullivan ES, Leaverton GH, Hardwick CE: Transrectal perineal repair: An adjunct to improved function after anorectal surgery. Dis Colon Rectum 11:106, 1968 |
|
Smith AN, Varma JS, Binnie NR, Papachrysostomou M: Disordered colorectal motility in intractable constipation following hysterectomy. Br J Surg 77:1361, 1990 |
|
Cundiff GW, Weidner AC, Visco AG, et al: An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol 179:1451, 1998 |
|
Baden WF, Walker T: Evolution of the defect approach. In Baden WF, Walker T (eds): Surgical Repair of Vaginal Defects, pp 1, 7. Philadelphia, JB Lippincott, 1992 |
|
Baden WF, Walker T: Vaginal approach to posterior vaginal defects: The rectal site. In Baden WF, Walker T (eds): Surgical Repair of Vaginal Defects, pp 209, 218. Philadelphia, JB Lippincott, 1992 |
|
Nichols DH, Randall CL: Posterior colporrhaphy and perineorrhaphy. In Nichols DH, Randall CL (eds): Vaginal Surgery, 4th edn, pp 257, 289. Baltimore, Williams & Wilkins, 1996 |
|
Wheeless CR: Posterior repair. In Wheeless CR (ed): Atlas of Pelvic Surgery, 3rd edn, pp 46, 49. Baltimore, Williams & Wilkins, 1997 |
|
Porter WE, Steele A, Walsh P, et al: The anatomic and functional outcomes of defect-specific rectocele repairs. Am J Obstet Gynecol 181:1353, 1999 |
|
Kenton K, Shott S, Brubaker L: Outcome after rectovaginal fascia reattachment for rectocele repair. Am J Obstet Gynecol 181:1360, 1999 |
|
Paraiso MF, Barber MD, Muir TW et al: Rectocele repair: a randomized trial of three surgical techniques including graftaugmentation. Am J Obstet Gynecol 2006;195(6):1762-71 |
|
Toozs-Hobson P, Cardozo L: Retrospective multicentre study of the new minimally invasive mesh repair devicesfor pelvic organ prolapse. Br J Obstet Gynaecol 2008;115(7):919-20; author reply 920-921 |
|
Arnold MW, Stewart WRC, Aguilar PS: Rectocele repair: Four years’ experience. Dis Colon Rectum 33:984, 1990 |
|
Jamieson DJ, Steege JF: The prevalence of dysmenorrhea, dyspareunia, pelvic pain, and irritable bowel syndrome in primary care practices. Obstet Gynecol 87:55, 1996 |
|
Francis WJA, Jeffcoate TNA: Dyspareunia following vaginal operations. Br J Obstet Gynaecol 68:1, 1961 |
|
Hasses P, Skibsted L: Influence of operations for stress incontinence and/or genital descensus on sexual life. Acta Obstet Gynecol Scand 67:659, 1988 |
|
Olsen AL, Smith VJ, Bergstrom JO, et al: Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89:501, 1997 |