Environmental Factors Causing Ovarian Failure in Humans
Authors
INTRODUCTION
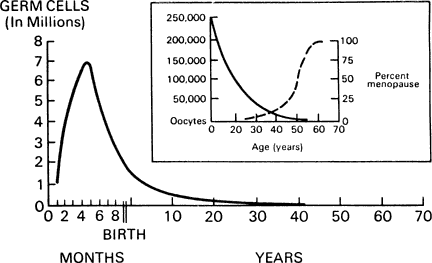
In contrast to gametogenesis in males, in females, oocytes are not continually replenished during reproductive life. After folliculogenesis in the latter half of gestation, the maximal number of oocytes is fixed. Ovarian failure occurs when these oocytes are depleted (Fig. 1). Exposure to an ovotoxin during or after embryogenesis may lead to irreversible ovarian dysgenesis or premature ovarian failure (POF).
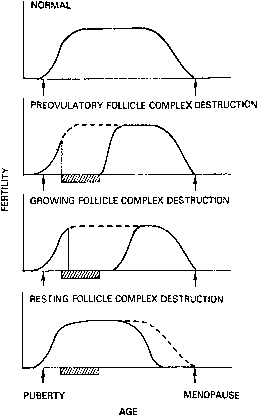
Response to a potential toxin is dependent on many factors, including the species and age of the subject and the size (or type) of exposed follicle. For example, three types of follicles are present in the ovary: resting(primordial) follicles, growing follicles, and preovulatory follicles. The different types of follicles have differing sensitivities to ovarian toxins, resulting in different patterns of ovarian failure (Fig. 2). A toxin that primarily destroyed preovulatory follicles would result in immediate loss of ovarian function, which might, however, be recovered. Destruction of growing follicles would result in somewhat delayed loss of function, which, again, might recover. Conversely, loss of all resting follicles would be evident only when the pool of preovulatory and growing follicles was exhausted. Ovarian function would then be permanently destroyed.1
Environmental agents have frequently been implicated as ovotoxins. However, for most, definitive evidence is difficult to obtain because so many variables affect response. In a few specific situations, however, ovarian failure is clearly the result of an environmental insult. The purpose of this chapter is to review agents that are well-documented causes of POF, as well as additional agents that have been implicated but not confirmed, as ovotoxins.
RADIATION
It is well-known that radiation can have a profound effect on both normal and tumor cells. However, effects do not depend solely on the physical characteristics of radiation, but also on various environmental conditions.
Type and quantity of radiation are obviously significant. For example, the distribution of x-rays will be relatively uniform in exposed embryos, whereas radioactive isotopes will not necessarily be uniformly distributed.2 Compared with an acute dose administered at the period of maximum sensitivity, fractionation of the dose reduces its effect.2,3,4 Similarly, lowering the dose rate increases the number of surviving oocytes, at least in the female mouse, suggesting that there may be repair processes in oocytes.2,5 Among important environmental and host factors are ambient temperature and oxygen levels, the lowering of which mitigates radiation effects.6 Concurrent drug or hormone regimen, genetic predisposition, age, reproductive history, pathologic condition, germ cell stage, and species2,3,7 also influence the effects of radiation on individuals, even when type, dose, and delivery rate are fixed. For example, primordial oocytes in juvenile mice are exquisitely radiosensitive in contrast to oocytes of macaques and human females.8 One explanation for the difference in species sensitivity is difference in chromosome organization within oocytes; dense chromosomes are more resistant to radiation than is diffuse chromatin.9 Another hypothesis is that it is the plasma membrane rather than the deoxyribonucleic acid (DNA) that is the target of ionization, at least in mouse oocytes.8 Presumably, the human oocyte membrane is less sensitive.
Because so many variables exist, generalizations are hazardous. Nonetheless, the effects of high-dose and low-dose ionizing radiation, radionuclides, and nonionizing radiation are discussed and conclusions attempted.
High-Dose External Ionizing Radiation
High doses to the ovaries of external ionizing radiation cause permanent amenorrhea. Indeed, x-rays were used for this purpose 50 years ago. In 1939, Jacox10 reported that 500 rads to the ovaries was sufficient to cause permanent castration in most patients, although an occasional patient required significantly more. Peck and colleagues11 later clarified one of the reasons for this variation: observing that older patients were more sensitive than younger ones. Nearly 90% of patients older than 40 years of age required only 375 to 499 radiographs to ensure permanent castration, whereas younger patients usually required 625 to 749 radiographs.
Although large doses are sufficient to cause permanent loss of ovarian function, there appears to be a threshold below which fertility is not significantly affected. Blot and Sawada12 studied 2345 Japanese women in Hiroshima and Nagasaki 18 years after the atomic bomb explosions. They observed no significant difference in fertility among groups exposed to no radiation, 0 to 9 rads, 10 to 99 rads, and 100 rads or more. Seigel's findings were similar.13 Tabuchi14 also studied women exposed to the atomic bombs and claimed an increase in fertility in young women for 1 year after exposure, with no evidence of subsequent decreased fertility. No change in the age at menarche among exposed girls and no change in the age at menopause in exposed women were noted. Exposure doses and number of women studied were not stated in the latter study nor, apparently, were other variables controlled.
Exposure to high levels of external ionising radiation currently is essentially limited to women undergoing treatment for malignant diseases (e.g., Hodgkin's disease). Before the development of oophoropexy, women treated with pelvic irradiation for Hodgkin's disease (approximately 4000 rads) invariably had ovarian failure develop. To prevent castration, Ray and coworkers15 devised the technique of oophoropexy. At staging laparotomy, both ovaries and their attached vascular pedicles are moved to the middle of the true pelvis and sutured to either the anterior or the posterior surface of the uterus; metallic clips mark ovarian placement. A lead shield is placed over the central portion of the true pelvis before irradiation. Ray's group treated 22 patients (13 to 23 years of age) with oophoropexy and pelvic irradiation (mean follow-up interval, 10 months).15 In 13 (59%) of the 22, normal ovarian function was maintained, although in four of these 13, menses temporarily ceased. The remaining nine patients (41%) manifested permanent amenorrhea. The investigators were unable to relate differential maintenance of ovarian function to patient's age, ovarian dose, dose/fraction, or total treatment time.
Radiation-induced amenorrhea will sometimes be followed by spontaneous resumption of menses. This may be because of the destruction of preovulatory follicle complexes but not of resting follicle complexes. The resting follicles would, subsequently, still be available for recruitment to the growing and preovulatory follicle populations. Of four patients receiving 150 rads to the ovaries following oophoropexy, Thomas and colleagues found three initially showed menstrual disturbances that later spontaneously resolved.16,17 Of 12 patients who received 500 to 3500 rads to the ovaries, all initially became amenorrheic. However, three of the 12 (exposure, 600 to 700 rads; ages 24, 25, 32) resumed menses after 6 to 24 months of amenorrhea. Finally, Horning and associates18 observed that 13 of 19 patients (13 to 28 years of age) treated with oophoropexy and total lymphoid irradiation were temporarily amenorrheic. However, at follow-up (median interval after treatment, 87 months), 18 of 19 patients were menstruating.
Not surprisingly, several authors have found that the age of the patient at treatment is inversely related to the likelihood of continuation or resumption of regular menses.18,19 That is, younger women are more likely to retain ovarian function after treatment than are older women. Younger women have larger pools of oocytes before therapy than do older women. Logically, after therapy, younger women are more likely to have some functional oocytes persisting. Children seem especially resistant to ovarian failure caused by abdominal irradiation.19 However, at high doses (2000 to 3000 rads), even children will suffer ovarian failure.20,21
Because so few data are available, it is difficult to generalize about the response of the human fetal ovary to x-rays. As is true of other potential teratogens, the effects of radiation on the fetus are influenced by the specific type of agent, the dose, the time in gestation when the fetus is exposed, and the genetic susceptibility of mother and fetus. Fetal mice exposed to 120 to 200 rads on day 15 (total gestation, 18 to 20 days) show decreased fertility during their adult life.14 Similarly, rats exposed to 100 rads on day 15.5 in utero (total gestation, 21 to 22 days) show markedly decreased oocyte number.2 However, significant species' difference in fetal susceptibility may exist.2,7 Human fetal germ cells appear more resistant in vitro than do rat or monkey germ cells.22 For example, Tabuchi14 did observe delayed menarche in women exposed to 500 rads or more in utero at Hiroshima and Nagasaki at 1 to 3 months of fetal age. Among Japanese women exposed in utero to either 10 to 50 rads or 50 to 459 rads, Burrow and coworkers23 found no significant difference in age at menarche. Similarly, in Japanese women exposed prenatally, Blot and associates24 showed no difference in the number of childless marriages, the number of births, or the interval between marriages and first births.
To summarize the effects of high-dose external ionizing radiation, ovarian doses of 150 rads or less usually have no deleterious effects on young women; however, an occasional woman older than 40 years will be sterilized by this dose (Table 1). Four hundred rads will cause permanent amenorrhea in almost all women older than 40 years. A variable percentage of younger women will be rendered temporarily, or permanently, amenorrheic at doses of 250 to 800 rads. Acute or fractionated doses of greater than 800 rads will render virtually all women permanently sterile. Children show greater resistance to radiation castration than do adult women.
Table 1. Effect of Ionizing Radiation on Ovarian Function
Minimum Ovarian |
|
|
|
Dose | Dose Rate | Effect | Evidence |
150 | 150 in fractions over | No deleterious effect in most young | No permanent change in menstrual |
| 28 days | women; some risk of sterilization in | cycle in three patients aged 20–31 |
|
| women more than 40 years of age. | |
250–500 | 263–500 fractionated | Variable. Among women 15–40 years, | Of 14 patients 15–24 years old, 4 |
| over 12–41 days | about 30% are permanently sterilized; | showed permanent amenorrhea |
|
| some of the others may suffer | and 10 had normal menstrual cycles |
|
| temporary amenorrhea. | (some had temporary amenorrhea |
|
|
| 1 eventually became pregnant).15 |
| 400–700 in 1–4 fractions | Among women older than 40 years, almost | Artificial menopause was induced |
|
| 100% are permanently sterilized. | with 400 rads in women older than |
|
|
| 40 years.11 |
500–800 | 530–660 fractionated | Variable. About 60% of women | Of seven patients 13–32 years of age, |
| over 30–55 days | 15–40 years are permanently sterilized; | four showed permanent |
|
| of the others, some may suffer | amenorrhea and three had normal |
|
| temporary amenorrhea. | menstrual cycles.15 |
| 500–800 fractionated | Variable. About 70% of women | Of seven patients 19–36 years, five |
| over 28 days | 15–40 years are permanently sterilized; | showed permanent amenorrhea |
|
| others may suffer temporary | and two suffered temporary |
|
| amenorrhea. | amenorrhea; one of these |
|
|
| eventually became pregnant.16 |
>800 | 900–3500 fractionated | 100% permanently sterilized | Four patients 18–32 years showed |
| over 28 days |
| permanent amenorrhea.16 |
| 700–1500 | 100% permanently sterilized | Artificial menopause was induced in |
|
|
| women of all ages.4 |
(Adapted from Ash P: The influence of radiation on fertility in man. Br J Radiol 53:271, 1980.)
Low-Dose External Ionizing Radiation
To extrapolate the effects of high-dose irradiation to low-dose short-term or long-term (occupational) exposure would not necessarily be correct. In those instances in which oocyte killing has been measured directly, there did not appear to be a clear threshold dose under the observed conditions.3 However, Mondorf and Faber25 studied 180 women exposed to 1 to 5 rads during infancy. No differences were found between the control subjects and the exposed patients with respect to the number of children born and the age distribution of births. There was no evidence of a decrease in fertility later in life. In a second study, Meyer and colleagues26 investigated the effects of prenatal x-ray exposure due to pelvimetry on subsequent reproductive performance and found no decrease in the birth rate of women exposed as fetuses when compared with control subjects. Summarizing the effects of radiation in utero, Brent2 concluded that short-term doses of less than 25 rads will not cause sterility in human female fetuses. The above imply that even if no threshold exists, the number and proportion of oocytes killed by low-dose radiation are presumably small enough not to affect fertility. However, depletion of resting follicle complexes could accelerate ovarian failure and age at menopause.
Radionuclides
Radionuclides are internal emitters, radioisotopes that are concentrated in specific tissues, potentially emitting radioactivity as long as they remain in the body. The effects of radioisotopes have been less well studied than the effects of externally administered radiation. Again, it is difficult to generalize about their effects, because such effects are dependent on the specific radionuclide, the dose and dose rate, tissue distribution, genetically controlled susceptibility, and the pathologic condition of the recipient. In the case of fetal exposure, the time during gestation in which the agent is administered and its ability to cross the placenta are also crucial.
Although not implicated in ovarian failure after exposure in adulthood, the effects on the fetus of several internal emitters (radioactive isotopes) should be considered. Although therapeutic doses of 131I (2 to 10 mCi) to the mother can ablate the fetal thyroid, the effect of 131I on the human fetal ovary is unknown. Oral administration of 5 mCi 131I delivers only 1 to 2 rads to the adult gonad. Presumably, then, the dose to the fetal gonad after maternal administration is of similar magnitude or less and thus unlikely to be significant. Certainly, small doses of 131I (5 to 50 μ Ci) administered to the mother for diagnostic purposes (thyroid scan) have not been reported to be harmful to the fetus.2
Tritium (3H) is a potential pollutant from nuclear energy production. Animal studies have shown that tritiated drinking water (HTO) and tritiated thymidine have a deleterious effect on the in utero development of the rat, mouse, and monkey oocyte.2,3 Cesium-137 (137Cs), 8.4 rads/day, produces sterility in female mice exposed during gestation and the early neonatal period.3 Human exposure in this range is unlikely, barring a major nuclear reactor accident.
Nonionizing Radiation
Nonionizing radiation is a form of electromagnetic radiation with a longer wavelength and lower energy than ionizing radiation. Such low-energy waves produce biologic effects by means of hyperthermia, excitation, and possibly other mechanisms. This energy could, in theory, be ovotoxic in humans. Prediction of the biologic effects of nonionizing radiation depends, again, on many factors. In particular, the wavelength, frequency, and intensity are important. In general, the higher the frequency, the lower the tissue-penetrating power and the less likely an effect occur.
Microwaves, shortwaves, and ultraviolet light are forms of nonionizing irradiation. Ultraviolet light does not penetrate tissue well; therefore, it cannot affect either adult or fetal gonadal tissue. Shortwaves are of lower frequency than microwaves and ultraviolet light and have greater tissue-penetrating power. For example, 27.5 MHz (cycles/second) shortwave radiation can heat mammalian tissues to a depth of 10 to 12 cm.2 In one set of experiments, pregnant rats exposed to 27 MHz shortwaves showed an increased rate of embryo resorption.2
In contrast to shortwaves, the frequency of most microwave ovens and diathermy machines is 2450 MHz. Such waves cannot penetrate human tissue and produce a significant thermal effect beyond 3 to 4 cm.2 It seems unlikely, then, that 2450-MHz microwaves actually reach the human fetus in any common clinical situation. Rubin and Erdman reported four women who were treated with microwave radiation (2450 MHz) for pelvic inflammatory disease and who, in addition, were pregnant or became pregnant during therapy.27 Three of the women delivered healthy infants. The fourth patient spontaneously aborted, but subsequently delivered a healthy baby during a later pregnancy, in which she also received microwave therapy.
In summary, there is no evidence that clinically relevant microwave exposure(2450-MHz microwave ovens, most diathermy machines) could cause ovarian failure in either fetuses or adults. Even a microwave oven that leaked would not cause significant ovarian exposure. The same is true of ultraviolet light. Short-wave exposure, however, may have deleterious effects.
CHEMOTHERAPY
Because the function of antineoplastic agents is to kill cells, not surprisingly, some of these agents are ovotoxic. Although the precise mechanism responsible for ovotoxicity is unknown, in vitro chemotherapy induces apoptotic changes in pregranulosa cells, leading to follicle loss.28 Many of the variables that influence radiation effects equally confound the effect of a chemotherapeutic agent. In addition, effective dose is influenced by species-specific and individual metabolic patterns.
Alkylating Agents
Cyclophosphamide, melphalan, busulfan, chlorambucil, and nitrogen mustard are examples of commonly used alkylating agents that have been associated with POF.
CYCLOPHOSPHAMIDE.
Cyclophosphamide has been implicated in ovarian failure many times. In 1971, Miller and coworkers29 reported a patient with systemic lupus erythematosus who was treated for 29 months (age, 10 9/12 to 13 3/12 years) with 50 to 100 mg/day.This 46,XX individual never had secondary sex characteristics develop. Autopsy examination showed absence of oogonia and developing follicles and presence of fibroblast-like stromal cells arranged in wavy or swirling patterns. There was no evidence that maturation of any follicles had ever taken place. The authors also noted that ovarian failure after cyclophosphamide treatment had occurred in six of 33 patients with rheumatoid arthritis and four of 17 patients with systemic lupus erythematosus. The fact that the effect is seen in patients with different disorders treated with the same drug supports the hypothesis that the drug rather than the underlying disease is responsible for the ovarian effects. Data of Uldall and colleagues30 are consistent with this theory. Of 34 adult women treated with cyclophosphamide for either glomerulonephritis or systemic lupus erythematosus, 18 became amenorrheic on the average of 7 months after starting therapy; 9 of the 18 women discontinued treatment, but only one resumed menstruating after a mean follow-up period of 12 months. Warne and coworkers31 treated 22 patients (mean age, 28.2 years) with cyclophosphamide for glomerulonephritis or rheumatoid arthritis. Of the 22, 17 had definite or probable ovarian failure based on urinary estrogens, urinary gonadotropins, and ovarian biopsy. Of these 17 patients, only one patient spontaneously resumed menstruating 10 months after cessation of cyclophosphamide therapy. Therefore, cyclophosphamide causes ovarian failure in approximately half of treated adult women; age and dose are correlated with likelihood of permanent ovarian dysfunction.
The mechanism by which cyclophosphamide creates its ovarian effects is through chemically reactive intermediates created by cytochrome P-450 monooxygenase enzymes.
BUSULFAN.
In 1956, Louis and associates32 reported that four patients treated with busulfan (Myleran) for more than 3 months had amenorrhea develop. Other investigators have corroborated this finding and indicated that approximately 40% of exposed women became amenorrheic.33 Ovarian failure due to busulfan exposure appears to be age-related; however, of 10 young patients treated with high-dose busulfan preparatory to bone marrow transplant, all had ovarian failure.34
Further studies on busulfan showed that all female progeny of pregnant rats treated with busulfan had small ovaries devoid of germinal elements.35 Based on sensitivity at a particular stage of gestation, the authors concluded that busulfan exposure in utero resulted in failure of germ cell migration. That such animal work is relevant to humans is suggested by Diamond and workers.36 A 39-yearold woman with chronic granulocytic leukemia was treated with busulfan, 4 to 6 mg/day, during weeks 5 to 37 of pregnancy. She also received 6-mercaptopurine briefly and 200 rads to the spleen. Her 1077-g child had multiple anomalies, including hypoplastic ovaries characterized by compact cellular stroma, scattered clumps of pyknotic cells, and rare primordial ova. In an earlier pregnancy in which the mother was treated with only 6-mercaptopurine and radiation, a healthy child was born. Other studies of newborns exposed to busulfan in utero have shown intrauterine growth retardation and other nongonadal anomalies.33,37 However, determination of these children's gonadal function awaits puberty. There are insufficient data to make a prospective prediction.
CHLORAMBUCIL AND NITROGEN MUSTARD.
Chlorambucil and nitrogen mustard have also been implicated in ovarian failure. Ezdinli and Stutzman38 reported that two of 31 patients became amenorrheic after chlorambucil therapy for lymphoma or leukemia. Freckman and colleagues39 noted the same complication in three patients treated for breast carcinoma with the combination of chlorambucil and prednisone;autopsy showed the absence of ovarian primordial follicles. However, all three patients were between 41 and 45 years of age. Detailed studies of four other patients treated with nitrogen mustard and chlorambucil showed menstrual irregularities 1 to 2 months after initiation of therapy in all four.40 In the three patients in whom gonadotropins were measured, follicle-stimulating hormone was increased in two; luteinizing hormone was also increased in one of the two. Autopsy performed in two cases (one with elevated follicle-stimulating hormone and luteinizing hormone, one with unmeasured gonadotropins) showed ovaries devoid of primordial follicles. The third patient whose gonadotropins were measured showed low-normal values, suggesting to the authors that amenorrhea in such cases may be related either to interference with endometrial proliferation or to disturbance in the normal “rhythmicity” of follicle growth.40
Vinca Alkaloids
Vinca alkaloids have a relatively low potential for ovarian toxicity, at least when not administered with other chemotherapeutic agents. Only a few cases of amenorrhea associated with vinblastine administration have been reported.40
Actinomycin D, Methotrexate, and Etoposide
Actinomycin D and methotrexate, used as therapy for trophoblastic disease, apparently are not associated with significant risk for POF, at least as determined by the subsequent fertility rate.41,42,43 Rustin and coworkers43 treated 177 women who wished to become pregnant; 97% achieved pregnancy. Even high-dose methotrexate (97 to 412 g) in the treatment of osteosarcoma does not appear to disrupt normal ovarian function.44 Animal studies have also shown a lack of oocyte killing by actinomycin D.8
Etoposide is also used for treatment of gestational trophoblastic disease. Basal luteinizing hormone and follicle-stimulating hormone levels were increased in approximately half of a group of 47 treated patients, primarily in those older than 40 years of age. Ovulation returned after 121 days to all women younger than 40 years but in only five of nine older than 40 years. Outcome was not related to dosage.45
Combination Chemotherapy
Morganfeld and associates46 described 13 women with Hodgkin's disease, all treated with a combination of agents: chlorambucil, vincaleukoblastine sulfate, methylhydrazine derivate, and, in a few cases, also cyclophosphamide. Six of these patients became amenorrheic, with abnormally high levels of gonadotropins. Biopsy specimens showed “thickening of the albuginea, perioophoritis, stromal fibrohyalinization, necrotic vasculitis, hemorrhage, and general disintegration of the follicular apparatus.” The other seven patients continued to have normal menses and normal gonadotropin levels. Analysis of the differences in the groups showed that the amenorrheic group received a greater total dosage of drugs.
Schilsky's group47 studied 27 women who received combination chemotherapy (mechlorethamine, vincristine, procarbazine, prednisone [MOPP])for treatment of Hodgkin's disease. Some of the patients also received radiation therapy. The median interval from completion of therapy to evaluation was 9 years, and the median age at evaluation was 30 years. Of the 24 who did not have pelvic irradiation, 11 (46%) had persistent amenorrhea develop, with elevated gonadotropins and decreased estradiol, and 13 retained normal menses. The median age at treatment of the former group was 26 years and of the latter was 20 years. The difference in outcome of the two groups was clearly related to age: eight of nine patients older than 25 years old at treatment were amenorrheic, whereas 12 of 15 younger than 25 years of age at treatment still menstruated. Dosage, stage, and morbidity of disease appeared unrelated to subsequent menstrual status. Other authors have reported ovarian failure after: (1) MVPP therapy (nitrogen mustard, vinblastine, procarbazine, prednisone)48,49,50; (2) L-phenylalanine mustard (L-PAM), cyclophosphamide, methotrexate, 5-fluorouracil (5-FU), and vincristine51; (3) steroid, vincristine, methotrexate, and 6-mercaptopurine, with or without cyclophosphamide;cranial irradiation, and intrathecal chemotherapy52; (4) L-PAM alone or with 5-FU53; and (5) cyclophosphamide, methotrexate, and fluorouracil.54
Pooling results of multiple chemotherapy regimens, approximately 50% of adult women treated with combination chemotherapy, will become amenorrheic. Ovarian dysfunction may be progressive after chemotherapy treatment, occurring more quickly in older women.54 Fertility may be reduced, even in the face of regular menses. Occurrence of pregnancy does not prove lack of damage to the germ cell pool; postpartum amenorrhea has occurred in these situations.55
Irradiation and Chemotherapy
The combination of irradiation and chemotherapy is particularly ovotoxic. Of 50 patients (15 to 36 years of age) who received total lymphoid irradiation and MOPP, only 20% had normal menses at follow-up (median interval from completion of treatment, 57 months); another 28% had only irregular menses. This contrasts with the findings in patients who had received irradiation alone or chemotherapy alone (Table 2).18 Median age was similar in the three groups.
Table 2. Menstrual Status After Treatment of Hodgkin's Disease
| Regular | Irregular | No |
Treatment | Menses (%) | Menses (%) | Menses (%) |
Total lymphoid irradiation (TLI) | 47 | 47 | 6 |
Chemotherapy | 56 | 29 | 15 |
TLI and | 20 | 28 | 52 |
chemotherapy |
|
|
|
(Adapted from Horning SJ, Hoppe RT, Kaplan HS et al:Female reproductive potential after treatment. N Engl J Med 304:1377,1981.)
Stillman and colleagues21 studied the frequency of ovarian failure in 182 girls treated for childhood malignancy with irradiation and chemotherapy. There were no cases (0 of 105) of ovarian failure in patients who had chemotherapy but either did not have abdominal radiation therapy or whose ovaries were outside the radiation field. In contrast, five (15%) of 33 children whose ovaries were at the “edge” of the field subsequently experienced ovarian failure, as did 16 (70%) of 23 whose ovaries were within the field. Similarly, radiation therapy alone is less toxic to gonads than the combination of irradiation and chemotherapy.56
Given the above, it is not surprising that of 63 patients treated with ablative radiochemotherapy before bone marrow transplantation, only five retained ovarian function. Age and particular regimen were not correlated with outcome.28 Clearly, the prognosis for normal ovarian function is much poorer if the ovaries are exposed to both chemotherapy and irradiation than if either method is used alone.
Relationship of Age to Chemotherapy-Induced Ovarian Failure
As discussed in the section on radiation, ovarian response to chemotherapeutic agents depends on many environmental and individual variables. Perhaps the most important of these is the patient's age. In general, younger women are more resistant to the deleterious ovarian effects of chemotherapeutic drugs than are older women.47,53,57 Horning and coworkers18 noted that the age of the patient was significantly and inversely related to the return of regular menses after chemotherapy. Koyama and associates)58 found that a larger total dosage of cyclophosphamide was necessary to produce permanent amenorrhea in younger women than in older women (i.e., the average dose given before amenorrhea was only 5.2 g for patients in their 40s, 9.3 g for patients in their 30s, and 20.4 g for patients in their 20s).
Ovaries of young girls seem least sensitive to alkylating and other cytotoxic agents.59,60,1,62 For example, of 17 prepubertal girls treated with multiple agents (primarily antimetabolites), 16 subsequently had normal hypothalamic-pituitary-ovarian function, whereas only seven of 11 patients of pubertal age at treatment and five of seven postmenarchal patients similarly retained normal hypothalamic-pituitary-ovarian function.52 Menses subsequently returned to the two postmenarchal patients. A slower rate of gonadal cell proliferation or differences in the distribution of the toxin may account for the increased resistance of the prepubertal ovary.63 In any case, parents of prepubertal children treated with chemotherapy alone for childhood cancer can be reassured that there is a good possibility for subsequent normal ovarian function. Although the sample size is small, there is no indication that chemotherapeutic agents other than busulfan cause ovarian failure in females exposed in utero.37
PRESERVATION OF OVARIAN FUNCTION
Perhaps oocytes in ovaries suppressed with oral contraceptives are in an analogous state to those in the prepubertal ovary. In one study of patients treated with oral contraceptives concurrently with MVPP therapy, post-therapy ovarian biopsy specimens showed normal numbers of follicles.64 However, this finding of a protective effect of oral contraceptives was not corroborated by Whitehead and coworkers.50
Another way to suppress ovarian function is with gonadotropin-releasing hormone (GnRH) agonist analogues. In one study, 44 women treated for leukemia, lymphoma, or systemic lupus erythematosus with chemotherapy and GnRH-a were compared to 55 women with similar chemotherapy and no GnRH-a(control subjects). Ninety-eight percent of the study group resumed ovarian function, whereas only 40% of the control subjects did so.65
A different approach to preserving fertility in patients about to undergo chemotherapy or radiation therapy is by removal and cryopreservation of oocytes or ovarian tissue. Early reports have shown successful pregnancies with cryopreserved oocytes,66 although not yet with transplanted or in vitro matured cryopreserved human ovarian tissue.67,68 Of course, before these approaches are offered clinically, it must be shown that malignant cells will not be transferred to the patient.
INFECTIOUS AGENTS
Mumps virus has been implicated by several authors as the cause of POF and infertility.697071 In animal test systems, mumps causes acute cytolytic damage of the ependymal cells lining the aqueduct of Sylvius, the lateral ventricles, and the foramen of Monroe. These infections are characterized by an inflammatory response.72 Therefore, it is theoretically possible that mumps might damage sensitive ovarian tissues either directly, by oocyte cytolysis, or indirectly, by secondary effects of the inflammatory reaction.
In 1975, Morrison and colleagues69 reported three cases of mumps oophoritis believed by the authors to be responsible for POF. One patient, exposed as a 39-week fetus, subsequently showed primary amenorrhea and atrophic ovaries. Her mother had secondary amenorrhea and atrophic ovaries develop as a result of the same episode of mumps parotiditis, which was also associated with abdominal pain. A third, unrelated, patient had mumps develop at age 13 years and subsequently showed secondary amenorrhea and menopausal symptoms. Other authors have found that patients attending an infertility clinic are more likely to report a previous mumps infection than are control subjects.70 Conversely, sterility is obviously rare after mumps oophoritis.73,74,75 There is no evidence that other viruses cause POF, although diagnosis of viral oophoritis is obviously difficult. Pelvic tuberculosis is associated with ovarian failure in a minority of cases.
GALACTOSEMIA
Galactosemia is an autosomal-recessive disorder in which the enzyme galactose-l-phosphate uridyl transferase is deficient in many tissues. Consequently, galactose-l-phosphate, galactose, and galactitol levels are increased. If untreated, such patients fail to thrive and show hepatomegaly, cataracts, and psychomotor retardation. However, even with early therapy(avoidance of galactose-containing foods from early infancy or restriction of maternal lactose ingestion during pregnancy), central nervous system dysfunction is still common as is ovarian failure.76,77,78 Despite treatment with low-galactose diets since 1 day of age, 71% of female patients in one study had abnormal ovarian function.78 Endocrine studies have shown hypergonadotropic hypogonadism. Therefore, a direct toxic effect of galactose or its metabolites on ovarian parenchyma, perhaps during fetal life, has been postulated.
This hypothesis was tested by Chen and colleagues,79 who fed rats a 50% galactose diet at various prenatal and postnatal stages. They found a substantially reduced number of oocytes, particularly in rats exposed during the premeiotic stages of oogenesis (early and midgestation). This reduction may result from disturbance of germ cell migration, proliferation, or differentiation. However, reduction of maternal galactose intake during pregnancies at risk for galactosemic fetuses has not prevented fetal oocyte damage.
A single epidemiologic study of the age of menopause in 15 women, two carriers of the galactosemia gene and 13 carriers of the Duarte variant, showed a decrease of 5 years.80 However, a survey of 33 obligate galactosemia carriers did not confirm this trend.81
SMOKING AND POLYCYCLIC AROMATIC HYDROCARBONS
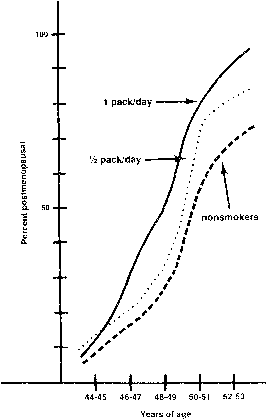
An inverse dose-response relationship between number of cigarettes smoked per day and age at menopause has been claimed based on epidemiologic studies.82,83,84,85 That is, at any given age between 44 and 53 years, a woman who smokes one pack a day is more likely to have undergone menopause than a woman who smokes either one-half-pack a day or no cigarettes at all (Fig. 3).83 This effect is said to be independent of body mass and other confounding factors.83,86 Although not proved, follicle density is decreased in current or past cigarette smokers; therefore, oocyte depletion is the most likely mechanism.87,88 The active ingredient responsible for earlier menopause, if such exists, is unknown. However, one logical possibility is the polycyclic aromatic hydrocarbons (e.g., benzo(a)pyrene). Found in cigarette smoke, polycyclic aromatic hydrocarbons are “toxic to and destructive to oocytes in several animal test systems.”9 Apparently, these compounds are not directly toxic but become so after being metabolized by microsomal enzyme systems to reactive electrophilic intermediates. These products then bind covalently to tissue macromolecules(DNA, ribonucleic acid [RNA], and proteins) to disrupt normal cellular function. Ovaries of mice and rats have the microsomal enzyme system necessary to metabolize polycyclic aromatic hydrocarbons; whether humans do also is unknown. Although it advances the age at menopause perhaps as much as 5 years, cigarette smoking is unlikely to be a major etiologic factor in POF in young women.
ETHANOL
Rats who consume a 5% ethanol liquid diet manifest decreased ovarian function (e.g., no estrous cycles and decreased ovarian and uterine weights compared with control subjects).89 Serum alcohol levels in such rats average 249 mg/dL, a level consistent with severe intoxication if found in humans who are ordinarily social drinkers. Ovarian suppression is apparently not caused by caloric deprivation alone.
However, whether ethanol is directly ovotoxic or rather suppresses the hypothalamic-pituitary axis is unknown. Inhibition of estrus has also been reported yin mice whose water contained 20% ethanol. Conversely, moderate alcohol consumption is associated with later menopause in humans.90
MISCELLANEOUS
Lead, mercury, manganese, and cadmium have toxic effects on the ovaries of rodents and nonhuman primates. Women exposed to high doses of lead have shown menstrual disturbances and decreased fertility; however, the site of effect, relationship to age at exposure, and toxic dose are not known.63,91
Coulam and colleagues92 have observed POF secondary to spontaneous bilateral ovarian hemorrhages. The cause of the hemorrhages could not be ascertained, but they might have been associated with an autoimmune process.
Transient and permanent amenorrhea after uterine embolization for treatment of fibroids has been reported. However, most patients so treated have not experienced ovarian failure, and this outcome seems most likely in perimenopausal women.93
Finally, McDonough and coworkers94 invoked an environmental cause for gonadal dysgenesis in a woman with a normal chromosome complement(46,XX) and a monozygotic twin with normal ovarian function. Because the twins were identical genetically, the authors presumed that their divergent ovarian function was caused by an environmental factor. Severe rubeola in childhood in the affected twin was considered, but other unknown environmental insults were also possible.
REFERENCES
Mattison DR, Nightingale MS, Shiromizu K: Effects of Toxic Substances on Female Reproduction. Research Needs for Evaluation of Health Effects of Toxic Chemical Dumps. NIEHS, October 27-28, 1981 |
|
Brent RL: Radiations and other physical agents. In Wilson JG, Fraser FC(eds): Handbook of Teratology, pp 153–223, Vol 1. New York, Plenum Press, 1977 |
|
Committee on the Biological Effects of Ionizing Radiation: The Effects on Populations of Exposure to Low Levels of Ionizing Radiation: 1980, pp 477–499. Washington, DC, National Academy Press, 1980 |
|
Ash P: The influence of radiation on fertility in man. Br J Radiol 53: 271, 1980 |
|
Pedersen RA, Manigia F: Ultraviolet light induced unscheduled DNA synthesis by resting and growing mouse oocytes. Mutat Res 49: 425, 1978 |
|
Mandl AM: The radiosensitivity of germ cells. Biol Rev 39: 288, 1964 |
|
Baker TG: Radiosensitivity of mammalian oocytes with particular reference to the human female. Am J Obstet Gynecol 110: 746, 1971 |
|
Dobson RL, Felton JS: Female germ cell loss from radiation and chemical exposures. In Mattison DR (ed): Reproductive Toxicology, Progress in Clinical and Biological Research, pp 175–190, Vol 117. New York, Alan R Liss, 1983 |
|
Mattison DR: How Xenobiotic compounds can destroy oocytes. Contemp Obstet Gynecol 15: 157, 1980 |
|
Jacox HW: Recovery following human ovarian irradiation. Radiology 32: 538, 1939 |
|
Peck SW, McGreer JT, Kretzschmar NR et al: Castration of the female by irradiation. Radiology 34: 176, 1940 |
|
Blot WJ, Sawada H: Fertility among female survivors of the atomic bombs of Hiroshima and Nagasaki. Am J Hum Genet 24: 613, 1972 |
|
Seigel DG: Frequency of live births among survivors of Hiroshima and Nagasaki atomic bombings. Radial Res 28: 278, 1966 |
|
Tabuchi A: Fetal disorders due to ionizing radiation. Hiroshima J Med Sci 13: 125, 1964 |
|
Ray GR, Trueblood HW, Enright LP et al: Oophoropexy: A means of preserving ovarian function following pelvic megavoltage radiotherapy for Hodgkin's disease. Radiology 96: 175, 1970 |
|
Thomas PRM, Winstanly D, Peckhamm et al: Reproductive and endocrine function in patients with Hodgkin's disease: Effects of oophoropexy and irradiation. Br J Cancer 33: 226, 1976 |
|
International Commission on Radiological Protection: Radiosensitivity and Spatial Distribution of Dose. ICRP Publication No. 14. Elmsford, NY, Pergamon Press, 1969 |
|
Horning SJ, Hoppe RT, Kaplan HS et al: Female reproductive potential after treatment. N Engl J Med 304: 1377, 1981 |
|
Lushbaugh CC, Casarett GW: The effects of gonadal irradiation in clinical radiation therapy: A review. Cancer 37: 1111, 1976 |
|
Shalet SM, Beardwell CG, Morris Jones PH et al: Ovarian function following abdominal irradiation in childhood. Br J Cancer 33: 655, 1976 |
|
Stillman RJ, Schinfeld JS, Schiff et al: Ovarian failure in long-term survivors of childhood malignancy. Am Obstet Gynecol 139:62, 198l |
|
Baker TG: The sensitivity of rat, monkey, and human oocytes to X radiation in organ culture. In Sikov MR, Mahlum DD (eds): Radiation Biology of the Fetal and Juvenile Mammal. U.S. Atomic Energy Commission Division of Technical Information, Oak Ridge, Tennessee, 1969 |
|
Burrow GN, Hamilton HB, Hrubec Z: Study of adolescents exposed in utero to the atomic bomb, Nagasaki, Japan. JAMA 192: 357, 1965 |
|
Blot WJ, Shimzu Y, Kate H et al: Frequency of marriage and live birth among survivors prenatally exposed to the atomic bomb. Am J Epidemiol 102: 128, 1975 |
|
Mondorf L, Faber M: The influence of radiation on human fertility. J Reprod Fertil 15: 165, 1968 |
|
Meyer MB, Merz T, Diamond EL: Investigation of the effects of prenatal X-ray exposure of human oogonia and oocytes as measured by later reproductive performance. Am J Epidemiol 89: 619, 1969 |
|
Rubin A, Erdman WJ II: Microwave exposure of the human female pelvis during early pregnancy and prior to conception. Am J Phys Med 38: 219, 1959 |
|
Meirow D: Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol 169: 123, 2000 |
|
Miller JJ III, Williams GF, Leissring JL: Multiple late complications of therapy with cyclophosphamide, including ovarian destruction. Am J Med 50: 530, 1971 |
|
Uldall PR, Kerr DNS, Tacci D: Sterility and cyclophosphamide. Lancet l:693, 1972 |
|
Warne GL, Fairly KF, Hobbs JB et al: Cyclophosphamide-induced ovarian failure. N Engl J Med 289: 1159, 1973 |
|
Louis J, Limarzi L, Best WR: Treatment of chronic granulocytic leukemia with Myleran. Arch Intern Med 97: 229, 1956 |
|
Sinalley RV, Wall RL: Two cases of busulfan toxicity. Ann Intern Med 64: 154, 1966 |
|
Teinturier C, Hartmann O, Valteau-Couanet D et al: Ovarian function after autologous bone marrow transplantation in childhood: High-dose busulfan is a major cause of ovarian failure. Bone Marrow Trans 22: 989, 1998 |
|
Heller RH, Jones HW Jr: Production of ovarian dysgenesis in the rat and human by busulfan. Am J Obstet Gynecol 89: 414, 1964 |
|
Diamond I, Anderson MM, McCreadie SR: Transplacent-al transmission of busulfan (Myleran) in a mother with leukemia. Pediatrics 25: 85, 1960 |
|
Simpson JL, Gelbus MS, Martin AO et al: Genetics in Obstetrics and Gynecology, pp 247–265. New York, Grune & Stratton, 1982 |
|
Ezdinli EZ, Stutzman L: Chlorambucil therapy for lymphomas and chronic lymphocytic leukemia. JAMA 191: 444, 1965 |
|
Freckman HA, Fry HL, Mendez FL et al: Chlorambucil prednisone therapy for disseminated breast carcinoma. JAMA 189: 23, 1964 |
|
Sobrinho LG, Levine RA, DeConti RC: Amenorrhea in patients with Hodgkin's disease treated with antineoplastic agents. Am J Obstet Gynecol 109: 135, 1971 |
|
Lewis JL: Chemotherapy of gestational choriocarcinoma. Cancer 30: 1517, 1972 |
|
Pastorfide GB, Goldstein DR: Pregnancy after hydatidiform mole. Obstet Gynecol 42: 67, 1973 |
|
Rustin GJS, Bagshawe KD, Newlands ES et al: Cytotoxic drugs and sterility. Lancet 1: 1316, 1981 |
|
Shamberger RC, Rosenberg SA, Seipp CA et al: Effects of high-dose methotrexate and vincristine on ovarian and testicular functions in patients undergoing postoperative adjuvant treatment of osteosarcoma. Cancer Treat Rep 65: 739, 1981 |
|
Matsui H, Seki K, Sekiya S et al: Reproductive status in GTD treated with etoposide. J Reprod Med 42: 104, 1997 |
|
Morganfeld MC, Goldberg V, Parisier H et al: Ovarian lesions due to cytostatic agents during the treatment of Hodgkin's disease. Surg Gynecol Obstet 134: 826, 1972 |
|
Schilsky RL, Sherins RJ, Hubbard SM et al: Long-term follow-up of ovarian function in women treated with MOPP chemotherapy for Hodgkin's disease. Am J Med 71: 552, 1981 |
|
Chapman RM, Sutcliffe SB, Malpas JS: Cytotoxic-induced ovarian failure in women with Hodgkin's disease: I. Hormone function. JAMA 242: 1877, 1979 |
|
Woxman JHX, Terry YA, Wrigley PFM et al: Gonadal function in Hodgkin's disease: Long-term follow-up of chemotherapy. Br Med J 285: 1612, 1982 |
|
Whitehead E, Shalet SM, Blackledge G et al: The effect of combination chemotherapy on ovarian function in women treated for Hodgkin's disease. Cancer 52: 988, 1983 |
|
Rose DP, Davis TE: Ovarian function in patients receiving adjuvant chemotherapy for breast cancer. Lancet 1: 1174, 1977 |
|
Siris ES, Leventhal BG, Vaitukaitis JL: Effects of childhood leukemia and chemotherapy on puberty and reproductive function in girls. N Engl J Med 294: 1143, 1976 |
|
Fisher B, Sherman B, Rockette H et al: l-Phenylalanine mustard (L-PAM)in the management of premenopausal patients with primary breast cancer: Lack of association of disease-free survival with depression of ovarian failure. Cancer 44: 847, 1979 |
|
Bines J, Oleske DM, Cobleigh MA: Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol 14: 1718, 1996 |
|
Chapman RM: Gonadal injury resulting from chemotherapy. In Mattison DR(ed): Reproductive Toxicology. Progress in Clinical and Biological Research, Vol 117, pp 149–161. New York, Alan R. Liss, 1983 |
|
Ahmed SR, Shalet SM, Campbell RHA et al: Primary gonadal damage following treatment of brain tumors in childhood. J Pediatr 103: 562, 1983 |
|
Shamberger RC, Sherins RJ, Ziegler JL et al: Effects of postoperative adjuvant chemotherapy and radiotherapy on ovarian function in women undergoing treatment for soft tissue sarcoma. J Natl Cancer Inst 67: 1213, 1981 |
|
Koyama H, Wada T, Nishizawa Y et al:Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer 39: 1403, 1977 |
|
Arneil GC: Cyclophosphamide and the prepubertal testis. Lancet 2: 1259, 1972 |
|
DeGroot GW, Falman C, Winter JSD: Cyclophosphamide and the prepubertal gonad: A negative report. J Pediatr 84: 123, 1974 |
|
Pensisi AJ, Grushkin CM, Lieberman E: Gonadal function in children with nephrosis treated with cyclophosphamide. Am J Dis Child 129: 315, 1975 |
|
Lentz RD, Bergstein J, Steffes MW et al: Postpubertal evaluation of gonadal function following cyclophosphamide therapy before and during puberty. J Pediatr 91: 385, 1977 |
|
Mattison DR: Ovarian toxicity: Effects on sexual maturation, reproduction and menopause. In Clarkson TW, Nordberg GF, Sager PR (eds):Reproductive and Developmental Toxicity of Metals, pp 317–342. New York, Plenum Press, 1983 |
|
Chapman RM, Sutcliffe SB: Protection of ovarian function by oral contraceptives in women receiving chemotherapy for Hodgkin's disease. Blood 58: 849, 1981 |
|
Blumenfeld Z: Ovarian rescue/protection from chemotherapeutic agents. J Soc Gynecol Investig 8: S60, 2001 |
|
Porcu E, Fabbri R, Damiano G et al: Clinical experience and applications of oocyte cryopreservation. Mol Cell Endocrinol 169: 33, 2000 |
|
Oktay K, Karlikaya GG, Aydin BA: Ovarian cryopreservation and transplantation: Basic aspects. Mol Cell Endocrin 169: 105, 2000 |
|
Hovatta O: Cryopreservation and culture of human primordial and primary ovarian follicles. Mol Cell Endocrinol 169: 95, 2000 |
|
Morrison JC, Givens JR, Wiser WL et al: Mumps oophoritis: A cause of premature menopause. Fertil Steril 26: 655, 1975 |
|
Prinz W, Taubert H-D: Mumps in pubescent females and its effect on later reproductive function. Gynaecologia 167: 23, 1968 |
|
Cramer DW, Welch WR, Cassells S et al: Mumps, menarche, menopause, and ovarian cancer. Am J Obstet Gynecol 147: 1, 1983 |
|
Kurent JE, Sever JL: Infectious diseases. In Wilson JG, Fraser FC (eds):Handbook of Teratology, Vol I, pp 225–259. New York, Plenum Press, 1977 |
|
Brooks H: Involvement of the ovary in epidemic parotitis. JAMA 60: 359, 1913 |
|
Renard P, Celers J: Viral infections with urogenital manifestations. In Debré R, Celers J (eds): Clinical Virology: The Evaluation and Management of Human Viral Infections, pp 705–714. Philadelphia, WB Saunders, 1970 |
|
Stevenson RE: The Fetus and Newly Born Infant, 2nd ed, pp 199–276. St Louis, CV Mosby, 1977 |
|
Hoefnagel D, Wurster-Hili D, Child EL: Ovarian failure in galactosemia. Lancet 2: 1197, 1979 |
|
Kaufman FR, Kagut MD, Donnell GH et al: Hypergonadotrophic hypogonadism in female patients. N Engl J Med 304: 994, 1981 |
|
Waggoner DD, Buist NRM, Donnell GN: Long-term prognosis in galactosaemia: Results of a survey of 350 cases. J Inherit Metab Dis 13: 802, 1990 |
|
Chen Y-T, Mattison DR, Feigenbaum L et al: Reduction in oocyte number following prenatal exposure to a diet high in galactose. Science 214: 1145, 1981 |
|
Cramer DW, Harlow BL, Barbieri RL et al: Galactose-l-phosphate uridyl transferase activity associated with age at menopause and reproductive history. Fertil Steril 51: 609, 1989 |
|
Kaufman FR, Devgan S, Donnell GN: Results of a survey of carrier woman for the galactosemia gene. Fertil Steril 60: 727, 1993 |
|
Daniell HW: Osteoporosis of the slender smoker. Arch Intern Med 136: 298, 1976 |
|
Jick H, Porter J, Morrison AS: Relation between smoking and age of natural menopause. Lancet 1: 1354, 1977 |
|
Bailey A, Robinson D, Vessey M: Smoking and age of natural menopause. Lancet 2: 722, 1977 |
|
Cramer DW, Harlow BL, Xu H et al: Cross-sectional and case-controlled analyses of the association between smoking and early menopause. Maturitas 22: 79, 1995 |
|
Daniell HW: Smoking, obesity, and the menopause. Lancet 2: 373, 1978 |
|
Mattison DR: The effects of smoking on fertility from gametogenesis to implantation. Environ Res 28: 410, 1982 |
|
Westhoff C, Murphy P, Heller D: Predictors of ovarian follicle number. Fertil Steril 74: 624, 2000 |
|
Bo WJ, Brueger WA, Rudeen PK et al: Ethanol-induced alterations in the morphology and function of the rat ovary. Anat Rec 202: 255, 1982 |
|
Torgerson DJ, Thomas RE, Campbell MK, Reid DM: Alcohol consumption and age of maternal menopause are associated with menopause onset. Maturitas 26: 21, 1997 |
|
Vermonde-Van Eck GJ, Meigs JW: Changes in the ovary of the Rhesus monkey after chronic lead intoxication. Fertil Steril 11: 223, 1960 |
|
Coulam CB, Field CS, Kempers RD: Spontaneous bilateral ovarian hemorrhage as a cause of premature ovarian failure. Mayo Clin Proc 56: 762, 1981 |
|
Amato P, Roberts AC: Transient ovarian failure: A complication of uterine artery embolization. Fertil Steril 75: 438, 2001 |
|
McDonough PC, Tho PT, Byrd JR: Twins discordant for 46,XX gonadal dysgenesis. Fertil Steril 28: 251, 1977 |