The Prostaglandins: Basic Chemistry and Action
Authors
INTRODUCTION
CLASSIFICATION AND NOMENCLATURE
Prostaglandins, thromboxanes, and leukotrienes are enzymatically derived from essential fatty acids and constitute a unique class of polyunsaturated, hydroxylated, 20-carbon fatty acids categorized as eicosanoids.
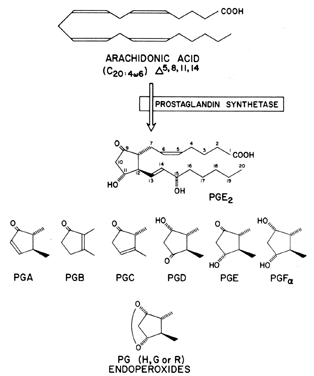
All prostaglandins are composed of a cyclopentanone nucleus with two side chains. Primary prostaglandins contain a 15-hydroxyl group with a 13,14-trans double bond (Fig. 1). Currently, three classes of prostaglandins are recognized, and these are categorized on the basis of the number of double bonds present within the prostaglandin molecule and on the fatty acid from which they are derived. Thus, prostaglandins of the 1 series have one double bond and are derived from dihomo-γ-linolenic acid, those of the 2 series have two double bonds and are derived from arachidonic acid, and those of the 3 series have three double bonds and are derived from eicosapentaenoic acid (Fig. 2). The thromboxanes and leukotrienes are also categorized by the number of double bonds in the molecule (leukotriene C4 [see Fig. 2]; TXA2, Fig. 3). Each type of prostaglandin is also allocated a group letter (e.g., A, B, C, D, E, F, G, H), in agreement with the functional substitutions in the cyclopentanone nucleus (see Fig. 1). For example, in prostaglandin F2α(PGF2α), the F indicates that the prostaglandin has two hydroxyl groups in the cyclopentanone ring (F series), the 2 indicates that it has two double bonds, and the α indicates that its hydroxyl grouping at carbon 9 is in the α configuration. Structure-function relationships and nomenclature of the prostaglandins are reviewed in greater depth elsewhere.4,5
BIOSYNTHESIS AND METABOLISM
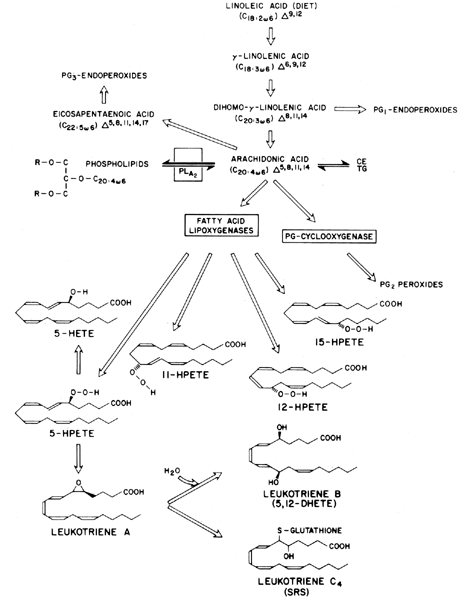
Eicosanoids exist only transiently: they are formed in a substrate-limiting environment and are rapidly metabolized. Thus, their modulation of cellular function is mostly paracrine or autocrine in fashion. The major eicosanoid precursor substrates are fatty acids, which are usually bound in the esterified form as cholesteryl esters; phospholipids; or triglycerides (see Fig. 2). The predominant prosubstrate is phospholipid, and the general consensus is that the release of free fatty acid from the prosubstrate is a major rate-limiting step in eicosanoid formation.6
Enzymes known as phospholipases split membrane-associated phospholipids by hydrolysis to give rise to many polyunsaturated free fatty acids, such as arachidonic acid, which is the main substrate for eicosanoid formation.6 Arachidonic acid is enzymatically synthesized via chain elongation and desaturation, but a major source of this fatty acid is also the diet.
The enzymes that catalyze the formation of eicosanoids exist in a substrate-limiting environment. Thus, liberation of arachidonic acid from esterified stores results in the prompt formation of these products. Release of arachidonic acid is facilitated by the hydrolytic action of phospholipases. Phospholipase C, in combination with diglyceride lipase, releases arachidonic acid from phosphatidylinositol.7 Phospholipase A2 cleaves arachidonic acid from phosphatidylcholine, as well as from phosphatidylethanolamine and phosphatidylinositol. Until recently, phospholipase A2 activity was thought to be regulated by a glucocorticoid inducible protein, originally designated lipomodulin8 but later termed lipocortin.9 Lipocortin appeared to inhibit phospholipase A2 by forming an inactive complex with the enzyme, which could be rapidly reversed by phosphorylation of lipocortin.8 This role of lipocortin as a regulator has been challenged. Lipocortin appears to be one member of a large family of calcium- and phospholipid-binding proteins,9 which only inhibits phospholipase activity in vitro when the phospholipid substrate and/or the calcium cofactor are limited.10 Currently, the role of lipocortin as a mediator of the anti-inflammatory activity of glucocorticoids is questionable.
Prostaglandin Synthetase Pathway
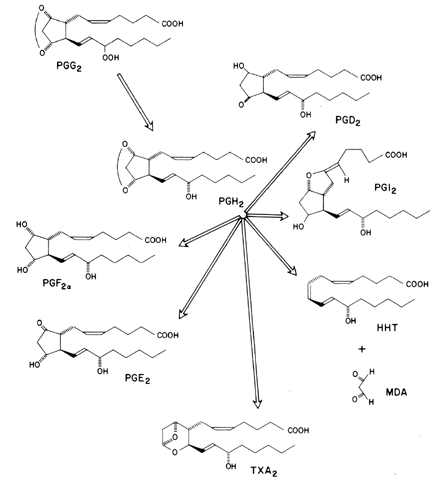
Prostaglandin synthetase (cyclooxygenase moiety) is responsible for incorporating molecular oxygen into arachidonic acid (and other fatty acids), resulting in the evolution of unstable intermediate compounds referred to as prostaglandin endoperoxides (PGG2 and PGH2 [Fig. 4]).11 Currently two PGH synthases are known, which are encoded by separate genes: one is a constitutive enzyme found in virtually all tissues, referred to as PGH synthase-1 or COX-1; the second enzyme, called PGH synthase-2 or COX-2, is inducible and is often markedly upregulated during cellular differentiation by cytokines or hormones.12,13 Histologic examination of adult COX-2-deficient mice revealed tissue abnormalities restricted to the kidneys, heart, and ovaries, with homozygous (-/-) knockout female mouse offspring found to be infertile.14
Prostaglandin cyclooxygenase is the site at which indomethacin and other nonsteroidal anti-inflammatory agents, such as aspirin, inhibit prostaglandin biosynthesis. The prostaglandin endoperoxides have a transient existence and are rapidly transformed (hydrolyzed) into more stable metabolites, such as PGD2, PGF2, PGE2, PGI2 (prostacyclin), thromboxane A, thromboxane B (TXB2), and HHT (hydroxyheptadecatrienoic acid) (see Fig. 4). The end products of prostaglandin endoperoxide metabolism appear to be enzymatically directed and depend on a number of variables: the availability of reducing agents (glutathione), molecular oxygen, or cofactors such as L-tryptophan and the presence of the metabolizing enzyme.
The type of prostaglandin formed appears to be tissue specific. Platelets synthesize significant amounts of TXA2, a vasoconstrictor and platelet-aggregating substance. PGI2, a vasodilator and inhibitor of platelet aggregation, is produced by the arterial wall, corpus luteum, follicle, uterus, and ductus arteriosus. Two other major products of endoperoxide metabolism, PGE2 and PGF2, are produced in almost every tissue, including the follicle, uterus, and brain. PGE2 and PGF2α appear to have both antagonistic and agonistic interactions. In the oviduct, smooth-muscle PGE2 promotes relaxation, whereas PGF2α promotes contraction. In the uterus, however, both promote contractions.
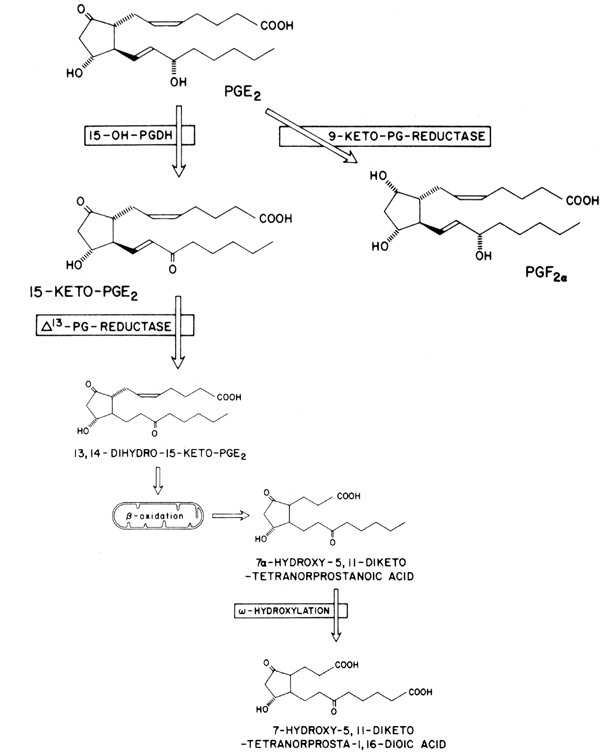
Once secreted into the peripheral circulation, most prostaglandins (with the exception of prostacyclin and TXA2) are rapidly metabolized in the lungs by an enzyme called 15-hydroxyprostaglandin dehydrogenase (15-OH-PGDH) (Fig. 5).15 This enzyme selectively oxidizes the hydroxyl group at carbon 15 into a 15-keto moiety. This step alone results in a dramatic loss of biological activity with the formation of 15-ketoprostaglandins. It has been shown that 15-hydroxyprostaglandin dehydrogenase has a short biological half-life and is subject to rapid turnover which, in some tissues, is modulated by steroid hormones.15,16 In general, highest concentrations of 15-OH-PGDH are found in the lungs, placenta, spleen, and the kidney-cortex. The brain has relatively low levels of 15-OH-PGDH activity, as do the ovary and the testis. Substrates for 15-OH-PGDH include PGE, PGF, and PGI, but the latter prostaglandin is degraded rapidly by “facile” hydrolysis. Compounds, such as lidocaine and the diuretic drugs furosemide and ethacrynic acid can inhibit 15-OH-PGDH.17 This may explain the diuretic action of the latter drugs, since PGE2 produces diuresis through a direct action in the kidney.
Following 15-OH-PGDH action, the 15-ketoprostaglandin is metabolized to the 13,14-dihydro metabolite via reduction of the double bond at position 13 by 13,14-PG reductase (see Fig. 5). This catabolic step is followed by oxidation of both α and ω side chains, and a 4-carbon fragment is lost. The terminal carbon of the ω chain is oxidized to a carboxylic acid group. The resultant compound appears to be the major urinary product, and the cyclopentane nucleus, characteristic of PGE and PGF, remains intact.
Much interest was rekindled in prostaglandin research by Moncada and colleagues, with their discovery of prostacyclin (PGI2, formerly called prostaglandin X), a highly unstable metabolite released into the peripheral circulation from the lungs and vascular endothelium of arteries. PGI2 has been referred to as an endogenous antithrombotic agent and is one of the very few prostaglandins that may be a circulating hormone.18 The major importance of PGI2 may be in the cardiovascular field, in which its ability to prevent the aggregation of platelets (and hence thrombus formation) by increasing platelet cyclic adenosine monophosphate (cAMP) biosynthesis directly opposes the actions of another novel group of compounds, the thromboxanes.
The thromboxanes are also formed by degradation of prostaglandin endoperoxides (see Fig. 4).11 Thromboxanes are synthesized in platelets and act to lower platelet cAMP formation, which then leads to aggregation of the platelets and their deposition on the vascular endothelium.19 TXA2 is a very potent venoconstricting agent, despite its very short biological half-life (30 seconds). The discovery of TXA2 and PGI2, together with a knowledge of their interactions in the control of thrombus formation and vascular tone, has led to a greater understanding of platelet function in cardiovascular physiology. For example, it has been suggested that patients who have had a heart attack may be protected against any further attacks by taking as little as one tablet of aspirin daily.20 The basis for such therapy appears to be that platelet cyclooxygenase (and subsequent TXA2 biosynthesis) recovers from aspirin inhibition at a much slower rate than does the PGI2 synthetase of the intimal regions of arterial tissue, thus imparting an antithrombotic property on aspirin. A major thrust of cardiovascular prostaglandin research appears to be the synthesis of PGI2 analogues and TXA2 antagonists, together with inhibitors of their biosynthesis or metabolism.21
Lipoxygenase Pathway
Another group of arachidonic acid metabolites receiving much attention recently are the lipoxygenase products, the hydroperoxyeicosatetraenoic acids (HPETEs) and their metabolites, the hydroxyeicosatetraenoic acids (HETEs) and the leukotrienes (see Fig. 2). HPETEs with hydroperoxide at 5-, 11-, 12-, or 15- have been described and are the precursors for the 5-, 11-, 12-, or 15-HETEs. All of these hydroxylated fatty acids can also occur spontaneously by oxygen radical attack and reduction by glutathione peroxidase.
For 5-HETE there is further metabolism to the leukotrienes (see Fig. 2). The leukotrienes are named for their conjugated “triene” and for being first identified in leukocytes. Leukotriene B4 is a potent chemotactic agent for polymorphonuclear leukocytes.22 Leukotriene C4 and its metabolite leukotriene D4 are identical to the slow-reacting substance of anaphylaxis.23,24,25
Lipoxygenase products have demonstrated both inhibitory and stimulating activities in several systems. 12-Lipoxygenase products appear to be stereospecific inhibitors of prostaglandin-induced platelet aggregation.26 15-HETE inhibits the 5-lipoxygenase of polymorphonuclear leukocytes and thus the formation of the vasoactive (LTC and LTD) and inflammatory (LTB) leukotrienes.27 5-HETE, but not 11-, 12-, or 15-HETE, promotes luteinizing hormone (LH) release from cultured pituitary cells.28 However, gonadotroph-enriched rat pituitary cells metabolize arachidonic acid to 11-, 12-, and 15-HETE, as well as many cyclooxygenase products, but not to 5-HETE. It has been suggested that as-yet unidentified pituitary cells may produce 5-HETE.29
ACTIONS OF PROSTAGLANDINS
Prostaglandins exhibit a wide range of biological effects, and their actions are among the most varied of any naturally occurring compounds. Despite this observation, this group of lipids displays a marked structure-activity specificity, which is determined mainly by cyclopentanone ring substitutions and the degree of unsaturation of the prostanoic acid side chains. The cellular response to prostaglandins is mediated by their interaction with plasma membrane receptors.
PG receptors were initially classified on the basis of functional activities of natural and synthetic agonists, and antagonists were classified into the following categories: DP, EP, FP, IP, and TP. The first letter denotes the prostaglandin type, and the letter P stands for “prostanoid.”30 Later, studies by binding analysis and molecular cloning confirmed the presence of distinct receptor types as well as three or four subtypes of EP (EP1–4). Plasma membrane prostaglandin receptors belong to the superfamily of G-protein-coupled receptors characterized by seven transmembrane-spanning regions.31 Intracellular second messengers of prostaglandin receptors include cAMP, protein kinase C, and calcium.30,31 The various receptors show remarkable specificity for the eicosanoid, with at least a 100-fold preference for the ligand. At high concentrations, PGE2 and PGF2α interact with the DP receptor; similarly, PGF2α will activate the EP receptor, whereas PGD2 and PGE2 will interact with the FP receptor at high concentrations. Most tissues contain a mixture of receptors, which appears to be the basis for the often opposite effects of a particular prostaglandin at different doses.
Role of Prostaglandins in Gonadotropin Secretion
Recent data implicating a physiologic role of prostaglandins in the regulation of gonadotropin-releasing hormone (GnRH) secretion have been published.32,33 A number of reports now suggest that prostaglandins, particularly PGE2, exert stimulatory influences on gonadotropin release, an effect that appears to be mediated by an action at the level of the hypothalamus. PGE2 has been shown to stimulate the release of GnRH from the hypothalamus, and pretreatment of animals with antisera to neutralize endogeneous GnRH prevents the PGE2-induced release of LH.34 In addition, PGE1 and PGE2 appear to be the most potent stimulators of growth hormone release from cultured adenohypophyseal cells.35 PGB, PGA1, PGA2, PGB2, PGF1, and PGF2 are also active stimulators, but their effects are not seen at physiologic doses. In general, prostaglandins appear not to stimulate gonadotropin secretion by a direct action on the pituitary.
Further evidence suggesting that prostaglandins might affect gonadotropin secretion (by acting directly on the hypothalamus) is supplied from studies using prostaglandin synthetase inhibitors, such as indomethacin, which apparently reduce gonadotropin secretion.36 That prostaglandins act by way of hypothalamic-releasing factors is evident on the basis of two observations:
- Pretreatment of female and male rats with antiserum to LH-releasing hormone (LHRH) impaired the ability of PGE2 (100 mg per rat) to increase LH secretion.36
- Direct administration of PGE2 into the ventricle of the brain of the rat mimicked the intravenous effect of prostaglandins on the stimulation of gonadotropin secretion.36
Additional findings provide compelling support for the concept that PGE2 acts at the level of the median eminence to elicit release of LHRH. This contention is further corroborated by the finding that median eminence tissue contains greater amounts of endogenous prostaglandins than basal hypothalamic regions. Data obtained from in vitro incubations of median eminence fragments obtained from male rats have shown that norepinephrine and dopamine stimulate the simultaneous release of LHRH and PGF2α. This effect is blocked by indomethacin, suggesting that intraneuronally produced prostaglandins are the mediators of catecholamine-stimulated LHRH release. These interesting findings are discussed in greater detail in a review by Ojeda and co-workers.36
Ovulation and Prostaglandins
After the discovery that indomethacin and aspirin (inhibitors of prostaglandin synthesis) could block ovulation,33,37 it was suggested that prostaglandins were involved in the ovarian follicular rupture process. This contention was further strengthened by the finding that intraovarian injection of PGF2α antiserum also inhibited ovulation.38 There is now a substantial amount of evidence indicating that follicular prostaglandin formation is enhanced during ovulation and that this elevation is dependent on gonadotropins.39
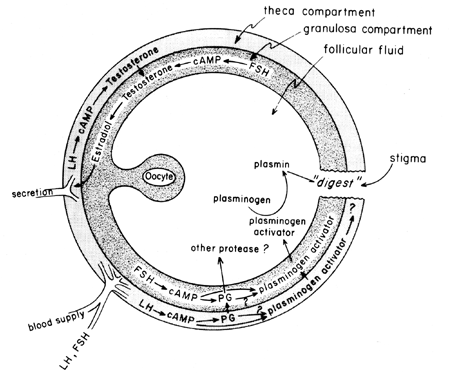
The midcycle surge of gonadotropins stimulates follicular eicosanoid biosynthesis by a cAMP-mediated process that is dependent on gene activation, but independent of steroidogenesis. LH appears to be the dominant physiologic pituitary gonadotropin responsible for the induction of ovulation, and it seems likely that the effects of LH on follicular rupture may be mediated by leukocytes that secrete proteolytic enzymes, oxygen radicals, and prostaglandins. Indomethacin, for instance, will block ovulation normally induced by large doses of human chorionic gonadotropin in vivo. Prostaglandins may mediate the stimulatory effects of LH on “ovulatory enzymes,” such as protease or collagenase.40 There is also the possibility that prostaglandins may elicit a contractile response in the follicle wall,41 which is now known to contain contractile elements, such as myosin and actin. Plasminogen activator or some other protease appears to be intrinsically involved in follicle rupture,42 and it is evident that secretion of this protein is associated with the LH-induced rise in follicular prostaglandin biosynthesis, although it has been pointed out that these two events may not be interdependent. A possible mechanism of prostaglandin action in follicular rupture is shown in Figure 6.
|
Role of Prostaglandins in Luteal Function: Luteolysis and Menstruation
The mechanism by which the human corpus luteum regresses 10 to 12 days after its formation is a mystery. Since the early finding that PGF2α was luteolytic in the rat and many other subprimate species,43 a major research effort has been made to investigate the possibility of menstrual regulation with PGF2α.PGF2α induces functional regression of the corpus luteum by a receptor-mediated process, independent initially of changes in ovarian or luteal blood flow.32 Within minutes, PGF2α depletes ascorbic acid,44 uncouples the occupied LH receptor from adenylate cyclase, and decreases transport of gonadotropin from capillaries to the luteal cell.
A review of the pertinent literature reveals many conflicting reports on the effects of PGF2α in humans. Some studies have recorded transient declines in circulating levels of progesterone by PGF2α,45 although other studies have failed to demonstrate such an effect in normally ovulating women.46,47 In view of the existence of putative PGF2α receptors in the human corpus luteum,48 it was reasoned that the failure to achieve luteolysis with intravenous infusions of PGF2α was perhaps due to rapid pulmonary metabolism. Subsequent studies involving the direct injection of PGF2α into the human corpus luteum induced progesterone withdrawal, the nadir of which coincided with the onset of early menses.49 These findings suggest that endogenous ovarian prostaglandins (or some other luteolytic agent) may be required for luteal regression. This seems feasible in view of the observation that human luteal function appears to be independent of uterine control, as neither hysterectomy50 nor congenital absence of the uterus, vagina, or fallopian tubes affects ovarian cyclicity.51 Human luteal tissue is certainly capable of producing prostaglandins, as shown by in vitro experiments52; however, attempts to correlate increased PGF2α levels in the ovary with luteal regression in the late luteal phase have not been successful.53 It seems that the only time ovarian PGF2α production is enhanced in the human menstrual cycle is shortly after ovulation.54 It is also apparent, however, that concentrations of PGF2α are always higher in ovarian stromal tissue through early, mid, and late luteal phases.
In contrast to the in vivo effects of PGF2α, in vitro studies have demonstrated both transient and sustained luteolytic effects in human luteal tissue.55,56,57 It is the general consensus that an early event in PGF2α-induced luteolysis is the abrogation of gonadotropin-sensitive cAMP production derived from studies in the rat corpus luteum.58,59 This abrogation is likely to be by a direct inhibition of adenyl cyclase by increases in intracellular Ca2+.60 Recent evidence from the field of neuroscience indicates that prostaglandins stimulate the release of intracellular Ca2+ by increasing the hydrolysis of phosphoinositol to diacylglycerol and inositol triphosphate.61 Consistent with findings in laboratory animals, it was shown that regression of the human corpus luteum is also associated with a loss of LH receptors.62
It is well known that the newly formed corpus luteum of many species is refractory to the lytic action of PGF2α63; this observation also appears to extend to the human corpus luteum.64 Henderson and McNatty65 suggested that PGF2α receptors in the newly formed corpus luteum may somehow be masked by occupied LH receptors, and that gradual dissociation of LH from its receptor may increase the susceptibility of the corpus luteum to PGF2α. An alternative hypothesis was offered by other investigators,65 who suggest that the susceptibility of the human corpus luteum to PGF2α may be dependent on ovarian noradrenaline levels, which increase during the luteal phase. This increase in catecholamines appears to permit the antigonadotropic effect of PGF2α on human corpus luteum.66
Prostaglandins in the Uterus
Unlike the myometrium, which mainly produces PGI2, the nonpregnant endometrium predominantly produces PGF2α and PGE2. The synthesis of prostaglandins is greater in the glandular epithelium than in the stroma of the endometrium, and during the secretory phase than during the proliferative phase of the cycle.67,68 Both PGF2α and PGE2, through their interaction with specific receptors, are known to stimulate myometrial contractility, leading to an increase in intracellular Ca2+. Such a mechanism of prostaglandin-induced uterine hypercontractility has been implicated in the pathogenesis of primary dysmenorrhea. Elevated levels of PGF2α and PGE2 have been identified in the endometrium and menstrual fluid of women with dysmenorrhea, and antiprostaglandin agents have been shown to result in a marked reduction in pain in these patients.
Various prostaglandins are also produced in the uterine cervix, and receptor sites for both PGE and PGF are present in the cervix.69 The increased production of prostaglandins, or their local administration, is associated with cervical ripening in pregnant women.69
Prostaglandins and Parturition
A number of observations indirectly implicate the involvement of prostaglandins in parturition. It is known, for example, that the administration of indomethacin or other nonsteroidal anti-inflammatory drugs, such as aspirin, prolongs gestation.69 Moreover, prostaglandins, particularly PGF2α, are known to be potent stimulators of uterine contractility and induce cyclic contractions of the gravid uterus. Elevated prostaglandin concentrations are associated with the onset of spontaneous labor in humans. Furthermore, the period of time preceding the onset of contractions is characterized by plasma PGF2α levels that are equivalent to those of nonpregnant women. Immunization against PGF2α also delays the onset of parturition.70 Prostaglandins are now used to induce early labor and abortion; indeed, prostaglandin is the drug of choice for accomplishing midtrimester abortions.69,71
Based on extensive studies in sheep, it appears that prostaglandins synthesized locally in the uterus initiate labor by directly acting on the myometrium to promote uterine contractions. A decrease in local, placental progesterone, combined with an increase in estrogen, results in the release of Ca2+ from lysosomes that have become fragile. This Ca2+ release stimulates prostaglandin synthesis by increasing phospholipase activity. Oxytocin appears to facilitate, rather than initiate, the estrogen and progesterone effects.72 Fetal membranes, decidua vera, and myometrium may all participate in the formation of prostaglandins.73,74
A recent proposal is that prostaglandin production by intrauterine tissue is tonically inhibited during human pregnancy, and that such inhibition is progressively removed as term approaches. Although the mechanisms effecting the tonic inhibition of prostaglandin synthesis have not been established, several antiphospholipase peptides have been found in human placenta, amnion,75 and chorion.76 Another possibility is that prostaglandin synthesis is stimulated in parallel with removal of synthesis inhibition. Potential signals for increased synthesis of prostaglandins are several growth factors and platelet-activating factor. Fetal lung and kidney tissues secrete platelet-activating factor, which is capable of stimulating prostaglandin production by human amnion.77
Prostaglandins and the Ductus Arteriosus
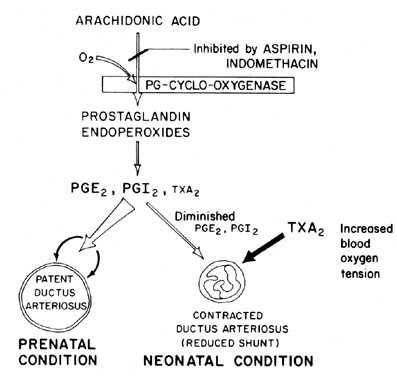
The maintenance of patency (relaxation of vasodilation) of the ductus arteriosus is pivotal in controlling the oxygenation of tissues in the fetus. After birth, the ductus arteriosus normally becomes constricted or loses its patency. The mechanism by which this closure occurs is unclear, although blood oxygen tension is believed to be an important factor (Fig. 7). The primary function of the ductus arteriosus in the fetus is to maintain some degree of left-to-right arterial blood shunting, thereby controlling the amount of venous return to the lungs. Closure of the ductus at term is an important physiologic process that, if incomplete, leads to respiratory distress and cyanosis/hypoxia—syndromes that are responsible for the high morbidity and mortality in many premature infants suffering from patent ductus arteriosus. For this reason, there is a good deal of interest in the mechanism(s) of controlling ductus arteriosus function. This interest is particularly acute in the field of prostaglandin research, in which there is now a considerable amount of evidence suggesting the involvement of prostaglandins in controlling ductus arteriosus patency at term.
|
This evidence is based on the following series of observations:
The ductus arteriosus in the fetus with cardiopulmonary deterioration and hyaline membrane disease can be closed by administration of prostaglandin synthetase inhibitors.78
Indomethacin can induce contraction of the hypoxic vessel, as demonstrated by its effects in animals near term.79
Administration of PGE2 (and PGE1) induces relaxation (loss of patency) of isolated fetal lamb ductus arteriosus preparations (circular strips) in a low oxygen environment (PO2 less than 14 mmHg).80
It seems likely that prostaglandins of the E series, together with prostaglandin antagonists and prostaglandin-synthetase blockers (aspirin), may prove to be desirable nonsurgical treatments for preterm infants with potentially fatal patent ductus arteriosus. Recent evidence suggests a balance between ductus patency and constriction that is maintained by synthesis of dilating prostaglandins (PGI2 and PGE2) and constricting prostaglandin (TXA2). In the lamb fetus, both the ductus arteriosus and the lung synthesize PGI2 and PGE2; then, as term approaches, the lung shifts toward TXA2 synthesis.81 Further information on prostaglandin involvement in the control of ductus arteriosus function is available.82
POTENTIAL PARALLEL ROLES OF OXYGEN RADICALS AND EICOSANOIDS IN REPRODUCTION REPRODUCTION
Recent studies suggest that reactive oxygen species may serve a physiologic role in reproduction. In some cases, the effects of oxygen radicals are identical to those of prostaglandins, notably their luteolytic properties.
Reactive oxygen species include singlet oxygen, superoxide anion, hydrogen peroxide, and the hydroxyl radical. Both the superoxide anion and the extremely reactive hydroxyl radical, which is derived from hydrogen peroxide in the presence of superoxide and iron, produce cell damage.83 Major sites of action of oxygen radicals are the cell membrane and DNA, and one significant effect is the nonenzymatic peroxidation of polyunsaturated fatty acids in the membrane phospholipids.84,85 The formation of lipoperoxides increases membrane rigidity, alters membrane permeability, and impairs anchoring of the cytoskeleton.86 Membrane peroxidation also increases eicosanoid synthesis by stimulating phospholipase A2 activity.87
Reactive oxygen species are produced in the reproductive system. An important source is leukocytes, which are known to infiltrate the preovulatory follicle after LH exposure88 and the corpus luteum during regression.89 Phagocytic leukocytes have a membrane-associated oxidase that, upon activation, produces oxygen radicals in amounts sufficient to injure or kill cells.90 Activation of macrophages can be initiated by the cytokine interferon-γ and by antigen presentation.91 Also, activated macrophages produce tumor necrosis factor-α and interleukin-1, which can stimulate release of oxygen radicals from neutrophils.91 It is therefore very likely that immune-cell-derived oxygen radicals are generated in the ovary, because in the corpus luteum interferon-γ induces antigens,92 macrophages produce tumor necrosis factor-γ,93 and neutrophils are resident.
Another source of oxygen radicals may be endothelial cells. Both cytokines and ischemia reperfusion stimulate endothelial-cell production of oxygen radicals.94 Ischemia in the follicle and the corpus luteum has been well documented,32,95,96 which implicates ovarian endothelial cells as a potential source of oxygen radicals. Ovarian parenchymal cells also may be a source of oxygen radicals. The steroidogenic enzymes involved in hydroxylation and side-chain cleavage produce oxygen radicals. Any or all of the above sources may account for the production of superoxide seen in the ovary97 and in luteal membranes.98
Mechanisms for protection from oxygen radicals are also present in the reproductive system. Both transforming growth factor-β, which prevents macrophage activation,99,100 and adenosine, which prevents activation of neutrophils, are produced in the ovary.101 Also, the enzymes capable of detoxifying oxygen radicals, such as superoxide dismutase, catalase, and glutathione peroxidase, can be found. In the corpus luteum, LH induces superoxide dismutase and peroxidase.97,102 In addition, significant levels of the antioxidant vitamins E and C can be found in the ovary.
One role that oxygen radicals may play in reproduction may be as the final mediators of luteolysis. Plasma membranes from regressing corpora lutea produce superoxide and show increased lipid peroxidation.98,103 In cultured luteal cells, oxygen radicals produce an abrupt and complete abrogation of LH-sensitive adenylate cyclase activity and progesterone synthesis.104 These actions are similar to those seen with PGF2α, but the magnitude of the oxygen radical effect is much greater and is not mediated by this eicosanoid.104 Oxygen radicals also may play a role in follicular atresia, ovulation, and oocyte function. Oxygen radicals are produced at ovulation and in the oocyte.105,106 Oxygen radicals also induce uterine contractions and coincide with the production of prostaglandins. Thus, it appears that reactive oxygen species can be generated in reproductive tissue and that these species may play a regulatory role that parallels that of the prostaglandins.
SUMMARY
The many actions of the prostaglandins in reproductive physiology are truly remarkable, as is our rapidly expanding knowledge of these effects. Our knowledge remains far from complete, however, and much more research is needed to fully elucidate the role(s) of prostaglandins in many physiologic processes, particularly in areas such as luteolysis, where the hope is that these biologically active lipids may provide a method for regulating menstruation and fertility. Other important fields of clinical significance that deserve further attention are parturition and ductus arteriosus function.
Further developments in prostaglandin research are likely to include the clinical application of more selective inhibitors, antagonists, and long-acting superpotent agonist analogues of prostaglandins.
Supported by NIH Grant HD-10718.
REFERENCES
Elattar TMA: Prostaglandins: Physiology, biochemistry, pharmacology and clinical applications. J Oral Pathol 7: 239, 1978 |
|
Hall AK, Behrman HR: Prostaglandins: Biosynthesis, metabolism and mechanism of cellular action (review). In Lee JB (ed): Prostaglandins (Comprehensive Endocrinology). New York, Elsevier/North-Holland, 1980 |
|
Ramwell PW, Leovey EMK, Sintetos AL: Regulation of the arachidonic acid cascade. Biol Reprod 16: 70, 1977 |
|
Nelson NA: Nomenclature and structure of prostaglandins. J Med Chem 17: 911, 1973 |
|
Lands WEM: The biosynthesis and metabolism of prostaglandins. Ann Rev Physiol 41: 633, 1979 |
|
Kunze H, Vogt W: Significance of phospholipase A2 for prostaglandin function. Ann NY Acad Sci 180: 122, 1971 |
|
Bell RL, Kennerly DA, Stanford N et al: Diglyceride lipase: A pathway for arachidonate release from human platelets. Proc Natl Acad Sci USA 76: 3238, 1979 |
|
Hirata F, Notsu Y, Matsuda K et al: Modulation of neuroreceptor functions by lipomodulin, a phospholipase inhibitory protein. Adv Exp Med Biol 175: 187, 1984 |
|
Whitehouse BJ: Lipocortins, mediators of the anti-inflammatory actions of corticosteroids? J Endocrinol 123: 363, 1989 |
|
Davidson FF, Dennis EA: Biological relevance of lipocortins and related proteins as inhibitors of phospholipase A2. Biochem Pharmacol 38: 3645, 1989 |
|
Nugteren DH, Hazelhof E: Isolation and properties of intermediates in prostaglandin biosynthesis. Biophys Biochem Acta 326: 448, 1973 |
|
Wu KK: Inducible cyclooxygenase and nitric acid synthase. Adv Pharmacol 33: 179, 1995 |
|
Smith WL: Prostanoid biosynthesis and mechanism of action. Am J Physiol 263: F181, 1992 |
|
Dinchuk JE, Car BD, Foct RJ et al: Renal abnormalities and altered inflammatory response in mice lacking cyclooxygenase: II. Nature 378: 406, 1995 |
|
Hansen HS: 15-Hydroxy-prostaglandin dehydrogenase: A review. Prostaglandins 12: 647, 1976 |
|
Ham EA, Cirillo VJ, Zanetti ME et al: Estrogen-directed synthesis of specific prostaglandins in uterus. Proc Natl Acad Sci USA 72: 1420, 1975 |
|
Tai H-H, Hollander CS. Kinetic evidence of a distinct regulatory site on 15-hydroxyprostaglandin dehydrogenase. In Samuelsson B, RP (ed): Advances in Prostaglandin and Thromboxane Research, Vol 1, p 171. New York, Raven Press, 1976 |
|
Moncada S, Korbut R, Bunting S et al: Prostacyclin is a circulating hormone. Nature 273: 767, 1978 |
|
Needleman P, Minkes M, Raz A: Thromboxanes: Selective biosynthesis and distinct biological properties. Science 193: 163, 1976 |
|
Jaffe EA, Weksler RB: Recovery of endothelial cell prostaglandin I2 production after inhibition by low doses of aspirin. J Clin Invest 53: 532, 1979 |
|
Johnson RA, Morton DR, Nelson NA: Nomenclature for analogues of prostacyclin I2. Prostaglandins 15: 737, 1978 |
|
Ford-Hutchinson AW, Bray MA, Dorg MV et al: Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 286: 264, 1980 |
|
Falkenheim SF, Macdonald FH, Huber MM et al: Effect of the 5-hydroperoxide of eicosatetraenoic acid and inhibitors of the lipoxygenase pathway on the formation of slow reaction substance by rat basophilic leukemia cells: Direct evidence that slow reacting substance is a product of the lipoxygenase pathway. J Immunol 125: 163, 1980 |
|
Morris HR, Taylor GW, Piper PJ et al: Structure of slow-reacting substance of anaphylaxis from guinea pig lung. Nature 285: 104, 1980 |
|
Lewis RA, Austen KF, Drazen JM et al: Slow reacting substances of anaphylaxis: Identification of leukotrienes C-1 and D from human and rat sources. Proc Natl Acad Sci USA 77: 3710, 1980 |
|
Croset M, Largarde M: Stereospecific inhibition of PGH2 -induced platelet aggregation by lipoxygenase products of icosaenoic acids. Biochem Biophys Res Commun 112: 878, 1983 |
|
Vanderhoek JY, Bryant RW, Bailey JM: Inhibition of leukotriene biosynthesis by the leukocyte product 15-hydroxy-5,8,11,13-eicosatetraenoic acid. J Biol Chem 255: 10064, 1980 |
|
Naor Z, Vanderhoek JY, Linder HR et al: Arachidonic acid products as possible mediators of the action of gonadotrophin-releasing hormone. In Samuelsson B, Paoletti R, Ramwell PW (eds): Advances in Prostaglandin, Thromboxane, and Leukotriene Research, Vol 12, p 259. New York, Raven Press, 1983 |
|
Vanderhoek JY, Kiesel L, Naor Z et al: Arachidonic acid metabolism in gonadotroph-enriched pituitary cells. Prostaglandins Leukot Med 15: 375, 1984 |
|
Coleman RA, Smith WL, Narumiya S: International union of pharmacology classification of prostanoid receptors: Properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46: 205, 1994 |
|
Thierauch K-H, Dinter H, Stock G: Prostaglandins and their receptors: I. Pharmacologic receptor description, metabolism and drug use. J Hypertension 11: 1315, 1993 |
|
Behrman HR: Prostaglandins in hypothalamo-pituitary and ovarian function. Ann Rev Physiol 41: 685, 1979 |
|
Orczyk G, Behrman HR: Ovulation blockade by aspirin or indomethacin: In vivo evidence for a role of prostaglandins in gonadotropin secretion. Prostaglandins 1: 3, 1972 |
|
Lindner HR, Zor U, Bauminger S et al: The use of prostaglandin synthetase inhibitors in analyzing the role of prostaglandins in reproductive physiology. In Robinson J, Vane JR (eds): Prostaglandin Synthesis Inhibitors: Their Effects on Physiological Function and Pathological States, pp 271–287. New York, Raven Press, 1974 |
|
Labrie F, Pelletier G, Borgeat P et al: Mode of action of hypothalamic regulatory hormones in the adenohypophysis. In Martini L, Ganong WG (eds): Frontiers in Neuroendocrinology, pp 63–93. New York, Raven Press, 1976 |
|
Ojeda SR, Naor Z, Negro-Vilar A: The role of prostaglandins in the control of gonadotropin and prolactin secretion (review). Prostaglandins Med 5: 249, 1979 |
|
O'Grady JP, Caldwell BV, Auletta EJ et al: The effects of an inhibitor of prostaglandin synthesis (indomethacin) on ovulation, pregnancy and pseudopregnancy in the rabbit. Prostaglandins 1: 97, 1972 |
|
Armstrong DT, Grinwich DL, Moon YS et al: Inhibition of ovulation in rabbits by intrafollicular injection of indomethacin and prostaglandin F antiserum. Life Sci 14: 129, 1974 |
|
Marsh JM, Yang NST, LeMaire WJ: Prostaglandin synthesis in rabbit graafian follicles in vitro: Effect of luteinizing hormone and cyclic-AMP. Prostaglandins 7: 269, 1974 |
|
Beers WH, Strickland S, Reich E: Ovarian plasminogen activator: Relationships to ovulation and hormone regulation. Cell 6: 387, 1975 |
|
Zor U, Lamprecht SA: Mechanism of prostaglandin action in endocrine glands. In Litwack G (ed): Biochemical Actions of Hormones, Vol 4, pp 85–133. New York, Academic Press, 1977 |
|
Strickland S, Beers WH: Studies on the role of plasminogen activator in ovulation. J Biol Chem 251: 5694, 1976 |
|
Horton EW, Poyser NL: Uterine luteolytic hormone: A physiological role for prostaglandin F2α. Physiol Rev 56: 595, 1976 |
|
Musicki B, Kodaman PH, Aten RF et al: Endocrine regulation of ascorbic acid transport and secretion in luteal cells. Biol Reprod 54: 399, 1996 |
|
Wentz AC, Jones GS: Transient luteolytic effect of PGF2α in the human. Obstet Gynecol 42: 172, 1973 |
|
Turksoy NR, Safaii HS: Immediate effect of prostaglandin F2α during the luteal phase of the menstrual cycle. Fertil Steril 26: 634, 1975 |
|
Lehmann F, Peters F, Breckwoldt M et al: Plasma progesterone levels during infusion of prostaglandin F2α in the human. Prostaglandins 1: 269, 1972 |
|
Powell WS, Hammarstrom S, Samuelsson B et al: Prostaglandin F2α receptor in human corpus luteum. Lancet 1: 1120, 1974 |
|
Korda AR, Shutt DA, Smith ID et al: Assessment of possible luteolytic effect of intra-ovarian injection of prostaglandin F2α in the human. Prostaglandins 9: 443, 1975 |
|
Belig CG, Marcus SL, Markham SM: Functional activity of the corpus luteum following hysterectomy. J Clin Endocrinol Metab 30: 30, 1970 |
|
Fraser IS, Baird DT, Hobson BM et al: Cyclical ovarian function in women with congenital absence of the uterus and vagina. J Clin Endocrinol Metab 36: 634, 1973 |
|
McDougall AN, Maule Walker FM, Watson J: The effect of cloprostenol on human luteal steroid and prostaglandin secretion in vitro. Br J Pharmacol 60: 425, 1976 |
|
Challis JRG, Calder AA, Dilley S et al: Production of prostaglandins E and Fα by corpora lutea, corpora albicantes and stroma from the human ovary. J Endocrinol 68: 401, 1976 |
|
Swanston IA, McNatty KP, Baird DT: Concentration of prostaglandin F2α and steroids in the human corpus luteum. J Endocrinol 73: 115, 1977 |
|
McNatty KP, Henderson KM, Sawers RS: Effects of prostaglandin F2α on the production of progesterone by human granulosa cells in tissue culture. J Endocrinol 67: 231, 1975 |
|
Henderson KM: Influence of 16-aryloxy-prostaglandins on the production of progesterone by human granulosa cells in vitro. J Endocrinol 71: 259, 1976 |
|
Dodson KS, Watson J: Effects of cloprostenol (ICI 80996) and flurprostenol (ICI 81008) on progesterone production by human luteal tissue superfused in vitro. Prostaglandins Med 2: 239, 1979 |
|
Thomas JP, Dorflinger LJ, Behrman HR: Mechanism of the rapid antigonadotropic action of prostaglandins in cultured luteal cells. Proc Natl Acad Sci USA 75: 1344, 1978 |
|
Hall AK, Robinson J: Functional luteolysis in the pseudopregnant rat: Effects of PGF2α and 16-aryloxy-PGF2α in vitro. J Endocrinol 81: 157, 1979 |
|
Dorflinger LJ, Albert PJ, Williams AT et al: Calcium is an inhibitor of luteinizing hormone-sensitive adenylate cyclase in the luteal cell. Endocrinology 114: 1208, 1984 |
|
Nishizuka Y: Turnover of inositol phospholipids and signal transduction. Science 225: 1365, 1984 |
|
McNeilly AS, Kerin J, Swanston IA et al: Changes in the binding of human chorionic gonadotropin/luteinizing hormone, follicle-stimulating hormone and prolactin to human corpora lutea during the menstrual cycle and pregnancy. J Endocrinol 87: 31, 1980 |
|
Behrman HR, Luborsky-Moore JL, Pang CY et al: Mechanism of PGF2α action in functional luteolysis. In Channing CP, Marsh J, Sadler WA (eds): Ovarian Follicular and Corpora Luteum Function, pp 557–575. New York, Plenum Press, 1979 |
|
Bennegard B, Dennefors B, Hamberger L: Interaction between catecholamines and prostaglandin F2α in human luteolysis. Acta Endocrinol 106: 532, 1984 |
|
Henderson KM, McNatty KP: A possible interrelationship between gonadotropin stimulation and prostaglandin F2α inhibition of steroidogenesis by granulosa-luteal cells in vitro. J Endocrinol 73: 71, 1977 |
|
Dennefors BL, Sjogren A, Hamberger L: Progesterone and adenosine 3',5'-monophosphate formation by isolated human corpora lutea of different ages: Influence of human chorionic gonadotropin and prostaglandins. J Clin Endocrinol Metab 55: 102, 1982 |
|
Rees MCP, Anderson ABM, Demers LM et al: Endometrial and myometrial prostaglandin release during the menstrual cycle in relation to menstrual blood loss. J Clin Endocrinol Metab 58: 813, 1984 |
|
Smith SK, Kelly RW: Effect of platelet-activating factor on the release of PGF2α and PGE2 by separated cells of human endometrium. J Reprod Fertil 82: 271, 1988 |
|
Goldberg VJ, Ramwell PW: Role of prostaglandins in reproduction. Physiol Rev 55: 325, 1975 |
|
Csapo AI: The fetus and birth. Ciba Found Symp 47: 159, 1977 |
|
Karim SMM: Prostaglandins and Reproduction. Baltimore, University Park Press, 1975 |
|
Okazaki T, Casey ML, Okita JR et al: Initiation of human parturition: XII. Biosynthesis and metabolism of prostaglandins in human fetal membranes and uterine decidua. Am J Obstet Gynecol 139: 373, 1981 |
|
Novy MJ, Liggins GC: Role of prostaglandins, prostacyclin, and thromboxanes in the physiologic control of the uterus and in parturition. Semin Perinatol 4: 45, 1980 |
|
Romero R, LaFreniere D, Hobbins JC, Mitchell MD: A product from human decidua inhibits prostaglandin production by human amnion. Prostaglandins Leukot Med 30: 29, 1987 |
|
Huang KS, Wallner BP, Mattaliano RJ et al: Two human 35 kd inhibitors of phospholipase A2 are related to substrates of pp 60 v-src and of the epidermal growth factor receptor/kinase. Cell 46: 191, 1986 |
|
Wilson T, Liggans GC, Aimer CP, Skinner SJM: Partial purification and characterization of two compounds from amniotic fluid which inhibit phospholipase activity in human endometrial cells. Biochem Biophys Res Commun 131: 22, 1985 |
|
Billah MM, Johnston JM: Identification of phospholipid platelet-activating factor (1-0-alkyl-2-acetyl-sn-glycero-3-phosphocholine) in human amniotic fluid and urine. Biochem Biophys Res Commun 113: 51, 1983 |
|
Sharpe GL, Larsson KS, Thalme B: Studies on closure of the ductus arteriosus: XIII. In utero effect of indomethacin and sodium salicylate in rats and rabbits. Prostaglandins 9: 585, 1975 |
|
Heymann MA, Rudolph AM: Effects of prostaglandins and blockers of prostaglandin synthesis on the ductus arteriosus: Animal and human studies. In Coceani F, Olley PM (eds): Advances in Prostaglandin and Thromboxane Research, Vol 4, p 363. New York, Raven Press, 1978 |
|
Olley PM, Coceani F, Rowe RD: Role of prostaglandin E1 and E2 in the management of neonatal heart disease. In Coceani F, Olley PM (eds): Advances in Prostaglandin and Thromboxane Research, Vol 4, p 345. New York, Raven Press, 1978 |
|
Printz MP, Friedman WF, Skidgel RA: Prostaglandin biosynthetic pathways in fetal lamb ductus arteriosus and lung: Relationship to ductal patency and closure. In Samuelsson B, Paoletti R, Ramwell PW (eds): Advances in Prostaglandin, Thromboxane, and Leukotriene Research, Vol 12, p 483. New York, Raven Press, 1983 |
|
Prostaglandins and perinatal medicine. In Coceani F, Olley PM (eds): Advances in Prostaglandins and Thromboxane Research, Vol 4. New York, Raven Press, 1978 |
|
Fridovich I: Biological effects of the superoxide radical. Arch Biochem Biophys 247: 1, 1986 |
|
Roberts P, Newby A, Hallett M, Campbell A: Inhibition by adenosine of reactive oxygen metabolite production by human polymorphonuclear leukocytes. Biochem J 227: 669, 1985 |
|
Cronstein B, Kubersky S, Weissmann G, Hirschhorn R: Engagement of adenosine receptors inhibits hydrogen peroxide (H2 02) release by activated human neutrophils. Clin Immunol Immunopathol 42: 76, 1987 |
|
Lippman R: Free radical-induced lipoperoxidation and aging. In Miquel J, Quintanilha AT, Weber H (eds): Handbook of Free Radicals and Antioxidants in Bio-medicine, Vol I, p 187. Boca Raton, CRC Press, 1989 |
|
Recknagel R, Glende E: The carbon tetrachloride hepatotoxicity model: Free radicals and calcium homeostasis. In Miquel J, Quintanilha AT, Weber H (eds): Handbook of Free Radicals and Antioxidants in Biomedicine, Vol III, p 3. Boca Raton, CRC Press, 1989 |
|
Cavender J, Murdoch W: Morphological studies of the microcirculatory system of periovulatory ovine follicles. Biol Reprod 39: 989, 1988 |
|
Adams E, Hertig A: Studies on the human corpus luteum: I. Observations on the ultrastructure of development and regression of luteal cells during the menstrual cycle. J Cell Biol 41: 696, 1969 |
|
Gabig T, Kipnes R, Baboir B: Solubilization of the O2 -forming activity responsible for the respiratory burst in human neutrophils. J Biol Chem 253: 6663, 1978 |
|
Nathan C, Murray H, Wiebe M, Rubin B: Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med 158: 670, 1983 |
|
Fairchild D, Pate J: Interferon-gamma induction of major histocompatibility complex antigens on cultured bovine luteal cells. Biol Reprod 40: 453, 1989 |
|
Bagavandoss P, Kunkel S, Wiggins R, Keyes P: Tumor necrosis factor-α (TNF-a) production and localization of macrophages and T lymphocytes in the rabbit corpus luteum. Endocrinology 122: 1185, 1988 |
|
Matsubara T, Ziff M: Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol 137: 3295, 1986 |
|
Pang C, Behrman H: Relationship of luteal blood flow and corpus luteum function in pseudopregnant rats. Am J Physiol 237: E30, 1979 |
|
Pang C, Behrman H: Acute effects of PGF2α on ovarian and luteal blood flow, luteal gonadotropin uptake in vivo and gonadotropin binding in vitro. Endocrinology 108: 2239, 1981 |
|
Laloraya M, Kumar-G P, Laloraya M: Changes in the levels of superoxide anion radical and superoxide dismutase during the estrous cycle of Rattus norvegicus and induction of superoxide dismutase in rat ovary by lutropin. Biochem Biophys Res Comm 157: 146, 1988 |
|
Sawada M, Carlson J: Superoxide radical production in plasma membrane samples from regressing corpora lutea. Can J Physiol Pharmacol 67: 465, 1989 |
|
Tsunawaki S, Sporn M, Ding A, Nathan C: Deactivation of macrophages by transforming growth factor-β. Nature 334: 260, 1988 |
|
Yoshida R, Murray H, Nathan C: Agonist and antagonist effects of interferon A and B on activation of human macrophages. J Exp Med 167: 1171, 1988 |
|
Soodak L, MacDonald G, Behrman H: Luteolysis is linked to LH-induced depletion of ATP in vivo. Endocrinology 122: 187, 1988 |
|
Agrawal P, Laloraya M: Induction of peroxidase in corpora lutea of rat ovary by lutropin. Biochem J 166: 205, 1977 |
|
Sawada M, Carlson J: Association of lipid peroxidation during luteal regression in the rat and natural aging in the rotifer. Exp Gerontol 20: 179, 1985 |
|
Behrman H, Preston S: Luteolytic actions of peroxide in rat ovarian cells. Endocrinology 124: 2895, 1989 |
|
Turner E, Hager L, Shapiro B: Ovothiol replaces glutathione peroxidase as a hydrogen peroxide scavenger in sea urchin eggs. Science 242: 939, 1988 |
|
Somers C, Battaglia D, Shapiro B: Localization and developmental fate of ovoperoxidase and proteoliaisin, two proteins involved in fertilization envelope assembly. Dev Biol 131: 226, 1989 |