Surgical Management of Intractable Pelvic Hemorrhage
Authors
INTRODUCTION
The background of ligation of the hypogastric (internal iliac) arteries for control of pelvic hemorrhage is not clear. Medical historians attribute the procedure to different surgeons in diverse specialties. In the United States, the operation was reported before 1900. Since then, diverse articles about this procedure have appeared sporadically; its usefulness has been demonstrated in many areas dealing with the pelvis and its contents.
Ligation of the hypogastric arteries can be a lifesaving procedure for patients with intractable hemorrhage from pelvic viscera. This is especially true in the field of obstetrics and gynecology, in which hemorrhage remains a major cause of mortality. Some physicians may be reluctant to perform hypogastric artery ligation for fear of injury to the pelvic viscera by interruption of the blood supply. With rare exceptions, this reluctance is unwarranted. Short- and long-term effects of hypogastric artery ligation generally are salutary. In contrast, so-called conservative procedures, such as vaginal packing, suturing of the vaginal vault, and supracervical hysterectomies, frequently fail to control hemorrhage. Before hypogastric artery ligation was considered a potential approach to the problem of pelvic hemorrhage, time, blood, and, alas, lives were lost. The technique of hypogastric artery ligation is acquired easily and should be practiced by the obstetrician and gynecologist.
In recent years, a major advance to the treatment of postpartum hemorrhage has been recognized world wide by gynecologists dealing with this problem. This is the B-Lynch "brace" suture, first described by the second author of this chapter in 1997 when five cases were reported.1 Since that time, more than 1500 cases have been reported to the website (Christopherbl@aol.com) with less than ten failures. The operation is based on the principal of providing compression to the uterus when it is in an atonic state and maintaining that compression.
ANATOMY FOR INTERNAL ILIAC ARTERY LIGATION
Topographic Anatomy and Surgical Landmarks
The intra-abdominal arrangement of the iliac vascular system can be projected on the abdominal surface. Beginning at a point ½ inch below and to the left of the umbilicus, draw a line inferolaterally so as to bisect a line running medially and inferiorly from the anterosuperior iliac spine to the middle of the symphysis pubis. The upper one-third of the umbilicospinal line traces the course of the common iliac artery before its bifurcation. The distal two-thirds of the same line delineates the external iliac artery; a line dropped medioinferiorly from this junction to the pelvic floor suggests the course of the hypogastric artery. The external bony landmark to the level of the bifurcation of the common iliac artery is the anterosuperior iliac spine. In most instances a line between both anterosuperior iliac spines bisects the points of bifurcation.
Internally, the aorta generally bifurcates into the common iliac arteries at the level of the fourth lumbar vertebra. The common iliac arteries, in turn, divide into the external and internal iliac (hypogastric) arteries. The external iliac artery courses along the psoas muscle laterally and ventrally to the leg, where it becomes the femoral artery. The hypogastric artery drops medioinferiorly along the border of the psoas muscle into the pelvis. An internal bony landmark for the level of the aortic bifurcation is the sacral promontory (lumbosacral articulation).
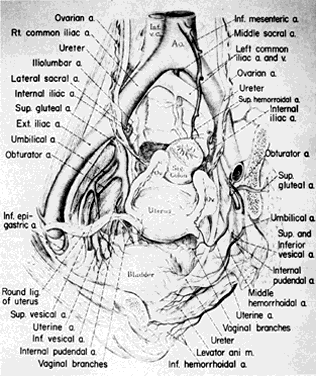
Once the hypogastric artery reaches the pelvis, it divides into the so-called anterior and posterior divisions. Those in turn divide into many branches, which are designated collectively as the hypogastric axis (Table 1). Figure 1 illustrates the gross anatomy.
Table 1. Branches of internal iliac artery
Posterior Division | Anterior Division | |
Parietal | Parietal | Visceral |
Iliolumbar a. | Obturator a. | Umbilical a. (fetal) |
|
| Superior vesical a. |
Lateral sacral a. | Internal pudendal a. | Inferior vesical a. |
|
| Middle hemorrhoidal a. |
Superior gluteal a. | Inferior gluteal a. | Uterine a. |
|
| Vaginal a. |
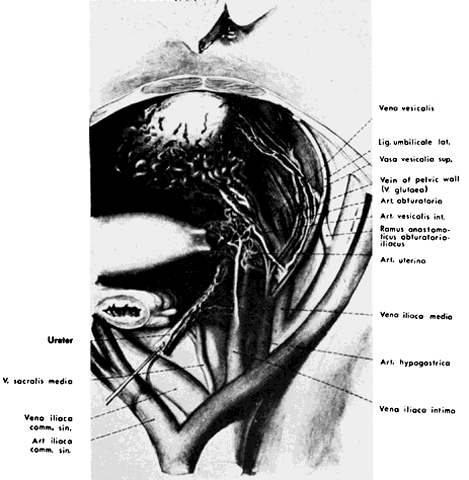
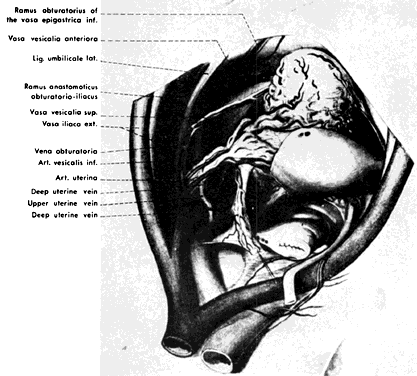
The hypogastric arteries have important relationships to neighboring anatomic structures. Knowledge of these relationships facilitates locating the arteries, as well as dissecting and ligating them. These relationships, listed below, are illustrated in Figure 2, Figure 3 and Figure 4.
|
|
- Anteromedially, the hypogastric (internal iliac) artery is covered by peritoneum; it is a retroperitoneal structure. On the right side of the pelvis at this point, the terminal end of the ileum and cecum may overlie the peritoneum.
- The ureter lies anterior to the hypogastric artery (retroperitoneal, attached to the undersurface of the peritoneum).
- Posterolateral to the hypogastric artery are the external iliac vein and the obturator vein.
- Posteromedial to the hypogastric artery is the hypogastric vein.
- Lateral to the hypogastric artery are the psoas muscles, major and minor.
Collateral Circulation
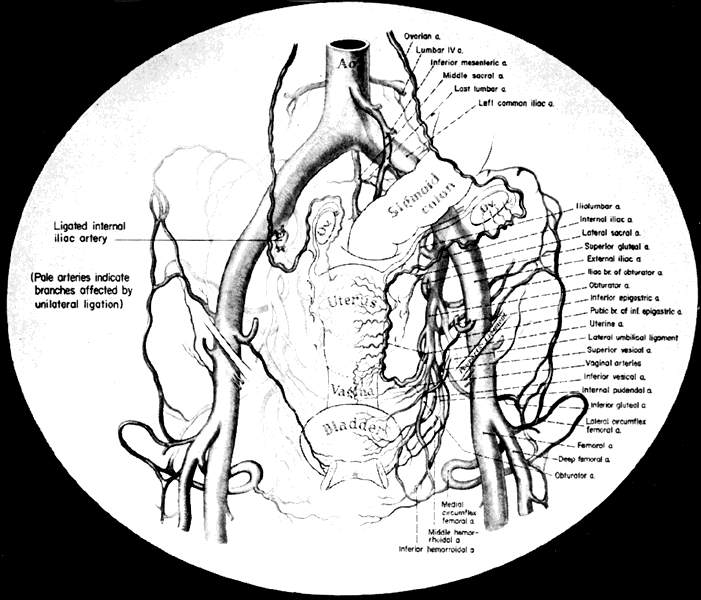
The collateral circulation of the pelvis has been the subject of discussion for at least a century. Gray's Anatomy (1870 edition) mentioned the many anastomoses. Anastomoses occur in each hemipelvis, horizontally and vertically across the pelvis.2, 3 The vertical system functions to a greater extent than the horizontal, especially after bilateral ligation. Figure 5 is a schematic representation of what might occur after unilateral ligation.
Three major vertical anastomoses exist in each hemipelvis: (1) lumbar-iliolumbar, (2) middle sacral, and (3) superior hemorrhoidal-middle hemorrhoidal. Bilateral ovarian-uterine anastomoses are another important vertical link. Anastomoses also occur between the inferior epigastric and medial circumflex femoral arteries, the circumflex and perforating branches of the deep femoral artery and the inferior gluteal artery, and the superior gluteal artery and the posterior branches of the lateral sacral artery.
The horizontal anastomoses are the branches of the vesical artery from each side and the pubic branches of the obturator artery from each side. Both systems are outlined in Table 2.
Table 2. Major pelvic anastomoses
- Ovarian artery (branch of aorta) with the uterine artery
- Superior hemorrhoidal artery (branch of inferior mesenteric) with middle hemorrhoidal artery
- Middle hemorrhoidal artery with inferior hemorrhoidal (branch of internal pudendal from hypogastric)
- Obturator artery with inferior epigastric artery (branch of external iliac)
- Inferior gluteal artery with circumflex and perforating branches of deep femoral artery
- Superior gluteal artery with lateral sacral artery (posterior branches)
- Lumbar arteries with iliolumbar artery
Horizontal
Variations in the Pelvic Circulation
The forgoing anatomic descriptions suffice for practical purposes. Dissection of the branches of the hypogastric artery, however, frequently reveals variation in the number of branches, the relative size of the branches, and the length and diameter of the right and left hypogastric arteries.3, 4 Figure 6 illustrates some of the documented variations in the branches of the hypogastric artery. Table 3 lists the collateral circulation of the branches of the hypogastric artery.
Table 3. Collateral circulation of branches of hypogastric arteries
Posterior Trunk | Anterior Trunk | ||
Branch | Collaterals | Branch | Collaterals |
Iliolumbar a. | Gluteal a. | Internal pudendal a. | External pudendal a. |
| Lumbar a. |
| Inferior gluteal a. |
| Lateral circumflex a. |
|
|
| Deep circumflex a. | Obturator a. | Middle circumflex a. |
| Spinal a. |
| Inferior epigastric a. |
|
|
| Iliolumbar a. |
|
|
| Inferior gluteal a. |
Lateral sacral a. | Spinal a. |
|
|
| Superior gluteal a. |
|
|
| Inferior gluteal a. | Inferior gluteal a. | Lateral circumflex a. |
| Middle sacral a. |
| Middle circumflex a. |
|
|
| First perforating a. |
Superior gluteal a. | Lateral sacral a. |
|
|
| Deep circumflex a. | Umbilical a. | Vesical a. |
| Inferior gluteal a. |
| Ureteric a. |
| Lateral circumflex a. |
|
|
|
| Uterine a. | Ovarian a. |
|
| Middle hemorrhoidal a. | Superior hemorrhoidal a. |
|
|
| Inferior hemorrhoidal a. |
The variations are relatively unimportant; however, some specific exceptions must be mentioned:
- Obstruction of the distal ureter by aberrant branches of the anterior division4: The obstruction usually is situated in the lower one third of the ureter at a point midway between the bladder and the brim of the pelvis. Hydroureter and dilatation of the renal calyces may occur in such cases; they can be demonstrated by an excretory urogram. Significant clinical symptoms are pain and discomfort in the loin on the affected side. Infection secondary to stasis of urine is likely to be accompanied by chills, fever, nausea, and hematuria. Therapy consists of surgical resection of the aberrant vessel (Fig. 7).
- Failure of the distal hypogastric (umbilical) artery to atrophy: During fetal life, the hypogastric artery ascends from the pelvis along the bladder and onto the back of the anterior abdominal wall, where after joining its contralateral artery, it passes into the umbilicus as the umbilical artery. At the time of birth, its function normally ceases; atrophy converts the distal hypogastric artery into a fibrous cord called the lateral umbilical ligament. In the absence of atrophic changes, however, ureteral obstruction may develop.
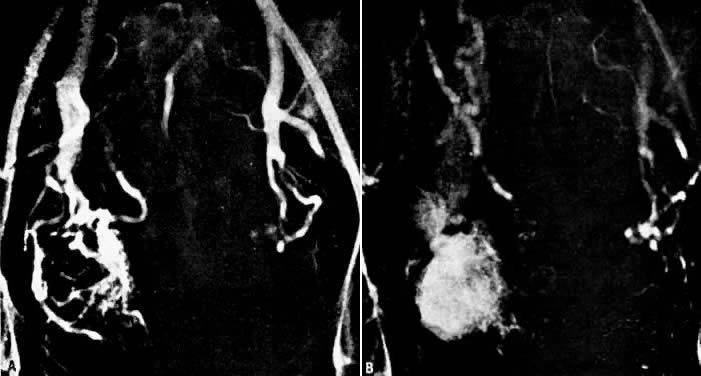
- Fistulous channels between branches of the hypogastric arteries and veins: Most patients with this rare condition have one or more of the following signs and symptoms:
- Pelvic pain in the area of the fistula radiating to the back, vagina, or down the posterior aspect of the leg on the same side
- A pulsatile mass with a bruit detectable vaginally or abdominally
- Edema of the legs
- Possible cardiac decompensation.
- Pelvic pain in the area of the fistula radiating to the back, vagina, or down the posterior aspect of the leg on the same side
In the pregnant patient with this fistula, antepartum bleeding or abnormal enlargement of the uterus is probable. Severe, catastrophic hemorrhage has been reported in the postpartum period or after abortion. Percutaneous femoral aortography establishes the diagnosis. Therapy is achieved via surgery5 (Figs 8 and 9).
PHYSIOLOGY OF INTERNAL ILIAC ARTERY LIGATION
At one time, ligation of the hypogastric system was regarded as equivalent to shutting off all blood to the area. Fortunately, this is not true. If it were, it is likely that the procedure would not be harmless. In reality, the hypogastric artery distal to the point of ligation is never emptied of blood.6 The anastomotic system functions immediately after ligation. What does occur is the virtual abolition of the arterial pulse pressure. This is associated with reduced mean blood pressure and rate of blood flow in the collateral system. As a result, the trip-hammer effect of arterial pulsations is abolished. In effect, ligation changes the distal portion of the artery so that the net pressure is equivalent to that in a vein. Clots remain in situ and are not dislodged by arterial pulsation.
INDICATIONS FOR LIGATION OF THE INTERNAL ILIAC ARTERY(IES)
Active
Conventional methods of therapy for pelvic hemorrhage, such as packing, suturing, and the use of hemostatic agents frequently are ineffective. Active indications for surgical intervention include the following conditions in which rapid and life-threatening hemorrhage can occur:
- Intraperitoneal hemorrhage in the immediate postoperative period: Loss of a ligature around the uterine artery can result in profuse hemorrhage. Frequently, the severed artery is retracted within a hematoma. Hypogastric artery ligation stops the bleeding at a point proximal to its source.
- Conization of the cervix: Postoperative infection or failure to use lateral hemostatic sutures may precipitate massive bleeding. Packing and suturing may prove totally ineffective.
- Lacerations of the cervix, lower uterine segment, and upper vagina: It may be impossible to suture every bleeding point. The only alternative to hypogastric artery ligation would be hysterectomy and partial vaginectomy, with resultant loss of childbearing function.
- Uterine atony: Many patients with uterine atony are near death at the time of operation. Hypogastric artery ligation is a quick and rational alternative to hysterectomy, allowing preservation of reproductive potential.
- Placenta previa: Implantation of the placenta into the lower uterine segment may interfere with its ability to contract. Bleeding may be copious. Hysterectomy is the only alternative.
- Rupture of the uterus: If rupture occurs in the lower uterine segment and extends cephalad, the uterine artery and its branches may tear. Although hysterectomy often is necessary, it sometimes fails to control bleeding from the branches of the uterine artery, which may have retracted.
- Advanced endometrial carcinoma: Blood supply to the tumor will be diminished. The ovarian vessels should be ligated at the same time.
- Late-stage carcinoma of the cervix: Hemorrhage may be exsanguinating before or after radiation therapy. The ovarian vessels also should be ligated.
- Hemorrhage from vaginal angles during or after abdominal hysterectomy: With good reason, the vaginal angles are characterized as “coffin corners.” Mass sutures placed in this area frequently incorporate a ureter.
- Vaginal vault bleeding after vaginal hysterectomy: Postsurgical infection predisposes a patient to vaginal vault bleeding. Bleeding may occur several days after vaginal hysterectomy. Occasionally, the tissue is too necrotic to suture, and packing is useless.
- Placenta accreta: The uterus may be saved by hypogastric artery ligation.
- Abdominal pregnancy with placental implantation in broad ligament area: Disruption of the placental site can precipitate fatal hemorrhage because a mechanism does not exist for the vessels to clamp down. Hypogastric artery ligation may be crucial to patient survival.
- Miscellaneous: Other surgical indications are fracture of the pelvis, gunshot wounds, and spontaneous rupture of pelvic veins associated with pregnancy.
Prophylactic
Certain operative conditions are associated with hemorrhage. Operative time frequently is consumed in controlling bleeding areas. Early ligation of both hypogastric arteries allows the surgical procedure to progress in a relatively dry field and saves time.
Prophylactic surgical intervention is indicated in the following conditions and procedures:
- Intraligamentous pregnancy: The placental implantation is between the leaves of the broad ligament. Ligation before removal is advisable.
- Intraligamentous leiomyoma: An intraligamentous leiomyoma often must be removed by manually peeling it away from the broad ligament. Hypogastric ligation decreases bleeding from the base of the broad ligament.
- Pelvic inflammatory disease: Operation for pelvic inflammatory disease often is difficult, and bleeding is profuse. Ligation may decrease the constant ooze.
- Extensive endometriosis: Adhesions are frequently dense. Diminished blood supply facilitates the operative procedure.
- Wertheim's hysterectomy: Ligation of the hypogastric arteries should be an integral part of Wertheim's operation.
- Hysterectomy among Jehovah's Witnesses: Patients belonging to the sect known as Jehovah's Witnesses and to certain other religious sects refuse to receive blood transfusions. Every effort should be made to minimize blood loss.
- Multiple leiomyoma: The “key” fibroid may present at the corporocervical junction, distorting the branches of the uterine artery. Hypogastric artery ligation may diminish the risk in this area.
- Bleeding, necrotic rectovaginal fistula: Even though cure may not be possible, personal comfort and hygiene may be improved because of the decreased blood supply that sometimes follows hypogastric artery ligation.
- Myomectomy: Blood loss at the time of myomectomy is reduced. Future menstrual function is not impaired.
- Groin dissection and vulvovaginectomy: When the deep lymph nodes are removed, ligation of the hypogastric arteries reduces blood loss during the remaining procedure.
- Abruptio placentae with atony or Couvelaire uterus, or both: If hypofibrinogenemia is not present, uterine blood loss can be reduced effectively by ligation of the hypogastric arteries.
- Miscellaneous: Hemorrhage from bladder tumors before cystectomy and abdominoperineal resection are indications for prophylactic surgical intervention.
LIGATION OF THE INTERNAL ILIAC ARTERY: SURGICAL TECHNIQUE
General Considerations
Either a midline or a transverse abdominal incision may be used. The surgeon should not use an unfamiliar incision. The transverse incision may take more time, especially when patients are obese. Visualization is better from the opposite side of the pelvis. To work on the contralateral side, the surgeon may elect to change sides during the operation.
In most situations, bilateral ligation is preferable to unilateral ligation. Not only is hemostasis more secure, but also any doubt about a possible return to the operating room is removed. Although it is possible to perform the operation by the extraperitoneal approach, the intra-abdominal approach is preferable except in cases of extreme obesity.
Some surgeons advocate complete transection of the hypogastric vessel between two ligatures. This has no practical or physiologic advantage. On the contrary, its practice may lead to injury of the underlying veins. If such an injury should occur in the course of the operation, applying pressure with a gauze sponge or suturing with an atraumatic needle and fine suture material usually suffices to repair the defect. If this should fail, however, the vein itself can be ligated above and below the defect. Incorporation of the previously tied artery into the suture in the vein adds strength and security as well as a splinting effect.
The choice of suture material depends on the preference of the surgeon. Number 1-0 chromic catgut, double-strand 2-0 black silk, and umbilical artery tape all have been used. Wet umbilical tape is particularly advisable for older patients, who are more likely to have arteriosclerosis. Two ties should be placed firmly but gently in continuity approximately 0.5 cm apart and 0.5–1 cm below the bifurcation.
Transabdominal Approach
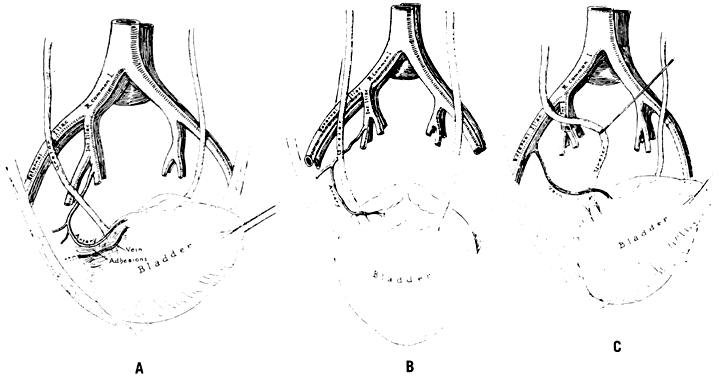
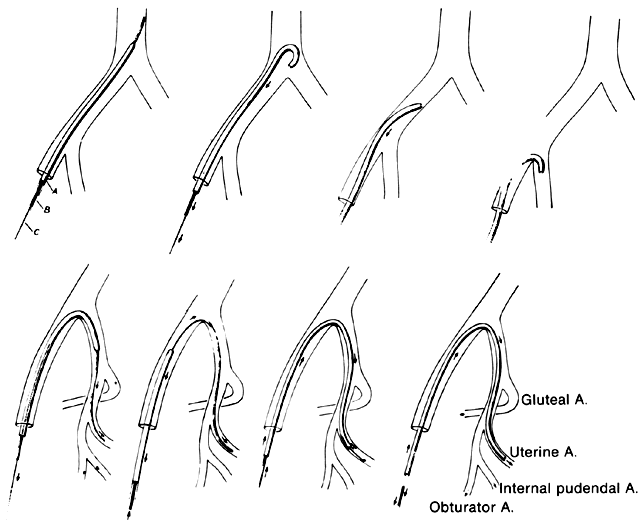
The abdomen is opened and the viscera packed away in the usual manner. Identification of the bifurcation of the common iliac artery is made by the two bony landmarks: the sacral promontory and an imaginary line drawn through both anterosuperior iliac spines. A longitudinal incision into the posterior parietal peritoneum is made. If the uterine corpus is present, this incision can be started in the peritoneum on the posterior surface of the round ligament at the junction of the middle and medial thirds. The incision is extended proximally for almost 10 cm. If the uterine corpus is absent, the incision can be started over the external iliac artery and carried proximally to the level of the bifurcation. Another method is to incise into the peritoneum directly over the bifurcation. The incision then is carried distally a few inches. All these incisions have one feature in common: They result in the formation of a medial and lateral peritoneal flap. The ureter is always on the medial flap and may be visualized, reflected, and protected with ease. The ureter normally crosses the common iliac artery from lateral to medial at a point just proximal to the bifurcation.
Once the peritoneum is opened, loose areolar tissue must be wiped away with firm, gentle motions in the direction of the vessels, not across them. Small pieces of dental cotton on long, curved forceps are effective. The fingers also may be used. When the areolar tissue has been removed, the bifurcation comes into view. Correct orientation begins with digital palpation of the bounding pulse. The vein may be visualized posteriorly. The ureter should be located on the medial peritoneal flap. The bifurcation feels like an inverted Y. The branch coming off at right angles is the hypogastric (internal iliac) artery. It courses medially and inferiorly to the palpating finger. The continuing branch is the external iliac artery. It courses laterally and superiorly out over the psoas muscles to the leg, where it becomes the femoral artery.
The surgeon must accurately identify these two branches. There is no room for error. Should the external iliac artery be ligated, the leg that receives its blood supply from this vessel soon will become cold, numb, and pale. Loss of the limb can follow. If the external branch is ligated, the sutures can be cut; however, if the artery has been transected, repair often is difficult.
After identification, the hypogastric artery should be elevated from the vein by Mixter forceps or the forceps designed by Reich and Nechtow3 (Fig. 10). The artery often is firmly adherent to the underlying vein; caution is advised. The point of the instrument should be directed toward the midline and placed at the border of the artery, and the forceps tips should be spread open gently. At the same time, the forceps should be “nudged” medially. When the artery is lifted off the vein, the external branch should be reexamined and reidentified. After confirmation, ligatures should be passed beneath the artery and tied gently but firmly. The artery should not be transected.
The peritoneum should be closed with interrupted 3-0 plain catgut because a continuous suture can kink the ureter. The procedure on the left pelvic wall may be slightly more complicated because it frequently is necessary to mobilize the sigmoid flexure at the “white line” to obtain adequate exposure.
Extraperitoneal Approach
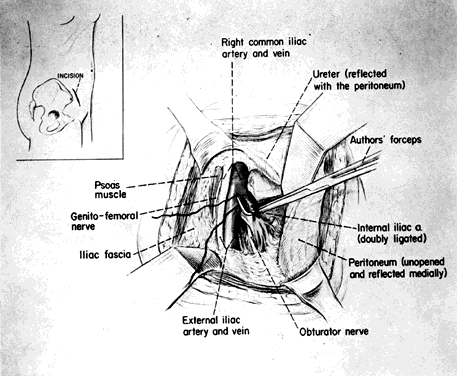
The skin incision in the inguinal area parallels the course of the external oblique muscle. It runs 6–8 cm in length in a line 3–5 cm medial to the anterosuperior iliac spine. After the fat and subcutaneous tissues are dissected away, a muscle-splitting incision bares the peritoneum. This is gently reflected medially, exposing the posterior surface; the ureter is reflected medially and the vessels laterally. Ligation is performed as previously described. Closure is the same as for a herniorrhaphy (Fig. 11).
Midline Extraperitoneal Approach
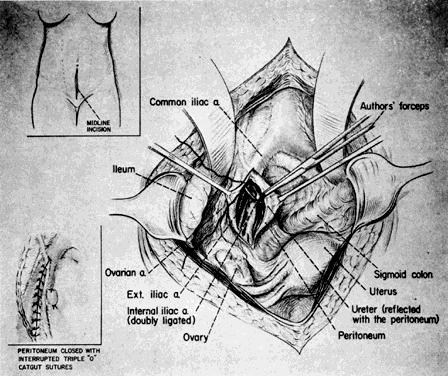
A midline extraperitoneal approach to the aorta is advocated by some. One authority extended its use to bilateral ligation of the hypogastric arteries. A midline abdominal incision is made. After the anterior sheath of the rectus muscle is exposed and opened below the level of the umbilicus, dissection caudal to the semilunar line of Douglas is performed, and the peritoneal and preperitoneal fat are separated. The peritoneum and its contents are reflected to the right (or left), thus exposing the retroperitoneal structures7 (Fig. 12).
Concomitant Ovarian Vessel Ligation
If the ovarian vessels are also to be ligated, the tube and ovary are picked up with Babcock's forceps and put on a stretch away from the origin of the infundibulopelvic ligament. The ovarian artery and vein may be palpated as cordlike structures beneath the peritoneum. Numerous varicosities may obscure the area, but selection of a free space in the peritoneum is crucial. The varicosities can be pushed away by the thumb and first finger. Once the clear spot has been found, a suture ligature (chromic catgut on an atraumatic curved noncutting needle) is passed and tied. A second, parallel ligature completes the procedure.
Pitfalls
The major pitfall associated with ligation of the hypogastric artery is delay. When hemorrhagic shock is irreversible, this operation will not overcome it. Inadequate transfusion is another pitfall in the therapy of patients with severe hemorrhage. Blood loss is often seriously underestimated.
Failure to remember that the vaginal artery is a separate branch of the hypogastric artery rather than a branch of the uterine artery may lead the surgeon into the pitfall of an unnecessary and ineffective hysterectomy for control of bleeding. Injury to the external iliac artery from retractors or mistaken ligation of this vessel can lead to loss of the entire lower limb.
Postoperative Care
Special care is not necessary. Large hematomas or collections of serosanguineous fluid can be drained through separate stab wounds. Usually this is unnecessary. Antibiotics are not indicated after ligation of the arteries. Their use is dictated only by the presence of infection. Early ambulation is advisable in all cases. Bladder atony will not develop; an indwelling catheter may be necessary to facilitate adequate assessment of urinary output.
SPECIAL CONSIDERATIONS
Injury to Vital Structures
Opinions endorsing the safety of the procedure are overwhelming. The available data suggest that this operation does not result in necrosis of vital pelvic structures. One report to the contrary is by Tajes,8 who cited a case of his own in which this operation resulted in necrosis of the buttocks. Tajes also reviewed two previously reported cases: in one case, the bladder mucosa sloughed; in the other, scrotal necrosis ensued.
Maintenance of Reproductive Function
It has not always been possible to follow young patients for whom this operation has been performed. More important, many patients do not understand the exact nature or extent of their operation. A patient may remember only that she was sick and bleeding, that she was operated on, and that she recovered. The following consultation case illustrated this point:
A 17-year-old para-1 hemorrhaged profusely after a Kielland's forceps assisted delivery. Examination revealed that the cervix was avulsed from the vaginal mucosa along the entire posterior aspect. Attempts to suture this were unsuccessful, and bleeding persisted. Reexamination revealed an opening in the left vaginal fornix, extending into the abdominal cavity. The patient was taken to the operating room. At laparotomy, an opening in the posterior vagina was observed to extend into the left broad ligament. The peritoneum was opened, the hypogastric artery ligated, and the laceration closed. The opposite hypogastric artery was not ligated. Eight weeks later, the vagina had healed and menses had returned. The patient recalled nothing of the event.
The incidence of postoperative amenorrhea is not known. It is common for menses to resume after the operation. There have been reports of normal pregnancy and delivery occurring after bilateral hypogastric artery ligation, although it is impossible to say how frequently this occurs. It is entirely reasonable to believe that reproductive capacity is not lost after this operation, provided that the patient has a normal uterus. It is important to remember that pituitary necrosis (Sheehan's syndrome discussed elsewhere in these volumes) can affect the ability to reproduce after postpartum hemorrhage, especially if blood replacement has been delayed or inadequate, hemorrhage has been severe, and shock profound. Fortunately, this is not a common occurrence.
Failures
Occasionally ligation of the hypogastric arteries fails to stem pelvic hemorrhage. The reason for this is not clear, but some suggestions are (1) massive necrosis after infection with destruction of the vessels; (2) the presence of large, aberrant branches feeding blood to the area; (3) dislodgment of clots when blood pressure rises; and (4) concomitant severe venous bleeding; however, these circumstances are rare.
TECHNIQUES OF SURGICAL MANAGEMENT FOR INTRACTABLE OBSTETRIC HEMORRHAGE
NEW DEVELOPMENTS IN THERAPEUTIC OPTIONS
The type of surgical intervention depends upon several factors, paramount of which is the experience of the surgeon. Other factors include parity and desire for future children, the extent of the hemorrhage, the general condition of the patient and place of confinement. Women at high risk of postpartum hemorrhage should not be delivered in isolated units or units ill-equipped to manage sudden, life-threatening emergencies. Immediate access to specialist consultant care, blood products and intensive care are essential.
The B-Lynch Suture Compression Technique
The procedure was first performed and described by Mr Christopher B-Lynch, a consultant obstetrician, gynecological surgeon, Fellow of the Royal College of Obstetricians and Gynaecologists of the UK and Fellow of the Royal College of Surgeons of Edinburgh, based at Milton Keynes General Hospital National Health Service (NHS) Trust (Oxford Deanery, UK), during the management of a patient with a massive postpartum hemorrhage in November 1989. This patient refused consent to an emergency hysterectomy!9 Table 4 provides an audit summary of five case histories of other patients with severe life-threatening postpartum hemorrhage managed with this technique.
The Principle
The suture aims to exert continuous vertical compression on the vascular system. In the case of postpartum hemorrhage from placenta previa, a transverse lower segment compression suture is effective.
See Figures 13A (i and ii), 13B and 13C.
Surgeon’s position. In outlining the steps involved, we assume that the surgeon is right-handed and standing on the right-hand side of the patient. A laparotomy is always necessary to exteriorize the uterus. A lower segment transverse incision is made or the recent lower segment Cesarean section suture (LSCS) removed to check the cavity for retained placental fragments and to swab it out.
Test for the potential efficacy of the B-Lynch suture before performing the procedure. The patient is placed in the Lloyd Davies or semi-lithotomy position (frog leg). An assistant stands between the patient’s legs and intermittently swabs the vagina to determine the presence and extent of the bleeding. The uterus is then exteriorized and bimanual compression performed. To do this, the bladder peritoneum is reflected inferiorly to a level below the cervix (if it has been taken down for a prior LSCS, it is pushed down again). The whole uterus is then compressed by placing one hand posteriorly with the ends of the fingers at the level of the cervix and the other hand anteriorly just below the bladder reflection. If the bleeding stops on applying such compression, there is a good chance that application of the B-Lynch suture will work and stop the bleeding.
Even in the presence of coagulopathy, bimanual compression will control diffuse bleeding points. If this test is successful, the application of the suture will also succeed. However, application of the B-Lynch suture is not a substitute for the medical treatment of coagulopathy, which should take place along with the operative intervention.
| Fig. 13a-c Summary of the application of the B-Lynch procedure |
Suture application. Given that the test criteria for the B-Lynch suture placement are met, the uterus remains exteriorized until application of the suture is complete. The senior assistant takes over in performing compression and maintains it with two hands during the placement of the suture by the principal surgeon.
- First stitch relative to the low transverse Cesarean section/hysterotomy wound. With the bladder displaced inferiorly, the first stitch is placed 3 cm below the Cesarean section/hysterotomy incision on the patient’s left side and threaded through the uterine cavity to emerge 3 cm above the upper incision margin approximately 4 cm from the lateral border of the uterus (Fig. 13A(i)).
- The fundus. The suture is now carried over the top of the uterus and to the posterior side. Once situated over the fundus, the suture should be more or less vertical and lie about 4 cm from the cornu. It does not tend to slip laterally toward the broad ligament because the uterus has been compressed and the suture milked through, ensuring that proper placement is achieved and maintained (Fig. 13A).
- The posterior wall. The location on the posterior uterus where the suture is placed through the uterine wall is actually easy to surface mark posteriorly. It is on the horizontal plane at the level of the uterine incision at the insertion of the uterosacral ligament (Fig 13B).
- Role of the assistant. As the operation proceeds, the assistant continues to compress the uterus as the suture is fed through the posterior wall into the cavity. This will enable progressive tension to be maintained as the suture begins to surround the uterus. Assistant compression will also help to pull the suture material through to achieve maximum compression, without breaking it, at the end of the procedure. Furthermore, it will prevent suture slipping and uterine trauma. The suture now lies horizontally on the cavity side of the posterior uterine wall.
- The fundus. As the needle pierces the uterine cavity side of the posterior wall, it is placed over the posterior wall, bringing the suture over the top of the fundus and onto the anterior right side of the uterus. The needle re-enters the cavity exactly in the same way as it did on the left side, that is 3 cm above the upper incision and 4 cm from the lateral side of the uterus through the upper incision margin, into the uterine cavity and then out again through 3 cm below the lower incision margin (Fig. 13A(ii)).
- Later role of the assistant. The assistant maintains the compression as the suture material is milked through from its different portals to ensure uniform tension and no slipping. The two ends of the suture are put under tension and a double throw knot is placed for security to maintain tension after the lower segment incision has been closed by either the one- or two-layer method.
- Relation to the hysterotomy incision. The tension on the two ends of the suture material can be maintained while the lower segment incision is closed, or the knot can be tied first, followed by closure of the lower segment (Fig. 13C). If the latter option is chosen, it is essential that the corners of the hysterotomy incision be identified and stay sutures placed before the knot is tied. This ensures that, when the lower segment is closed, the angles of the incision do not escape it. Either procedure works equally well. It is important to identify the corners of the uterine incision to make sure no bleeding points remain unsecured, particularly when most of these patients are hypotensive with low pulse pressure at the time of the B-Lynch suture application.
- Post-application and hysterotomy closure. It is probable that the maximum effect of suture tension lasts for only about 24–48 h. Because the uterus undergoes its primary involutionary process in the first week after vaginal or Cesarean section delivery, the suture may have lost some tensile strength, but hemostasis would have been achieved by that time. There is no need for delay in closing the abdomen after the application of the suture. The assistant standing between the patient’s legs swabs the vagina again and can confirm that the bleeding has been controlled.
Application after normal vaginal delivery. If laparotomy is required for the management of atonic postpartum hemorrhage, hysterotomy is necessary to apply the B-Lynch suture. Hysterotomy will also allow exploration of the uterine cavity, exclude retained products of conception, evacuate large blood clots and diagnose abnormal placentation and decidual tears, damage and bleeding. B-Lynch suture application or any modification of it (see below) without hysterotomy or re-opening of the Cesarean section wound runs the potential risk of secondary postpartum hemorrhage. Therefore, confirmation that the uterine cavity is completely empty is essential. Furthermore, hysterotomy ensures that the correct application of the suture provides maximum and even distribution of the compressive effect during and after application of the B-Lynch suture (Figs 13 and 14). Also, it avoids blind application of the suture and the possibility of obliteration of the cervical and/or uterine cavities that may lead to clot retention, infected debris, pyometria, sepsis and morbidity.9, 11, 12, 13
Application for abnormal placentation. The B-Lynch suture may be beneficial in cases of placenta accreta, percreta and increta. In a patient with placenta previa, a figure-of-eight or transverse compression suture to the lower anterior or posterior compartment or both is applied to control bleeding. If this is not completely successful, then, in addition, the longitudinal Brace suture component may be applied for further/complete hemostasis.9
| Fig. 14 Thein vivoeffect of correct applicationof the B-Lynch surgical technique seen immediatelyafter successful suture application. No congestion,no ischemia and no ‘shouldering’ of the sutures atthe fundus |
POSTOPERATIVE FOLLOW-UP
Three patients from the original series had laparoscopy postoperatively for sterilization, suspected pelvic inflammatory disease or appendicitis. One patient who had a history of ileostomy for surgical reasons had laparotomy 10 days after her B-Lynch suture for suspected intestinal obstruction (unpublished data, B-Lynch). Magnetic resonance imaging and hysterosalpingography were performed on one patient, showing no intraperitoneal or uterine sequelae14 (Fig. 15A–C). No complications have been observed in the five patients of the first published series15 (see Table 4). Moreover, all have succeeded in further pregnancy and delivery.16, 17
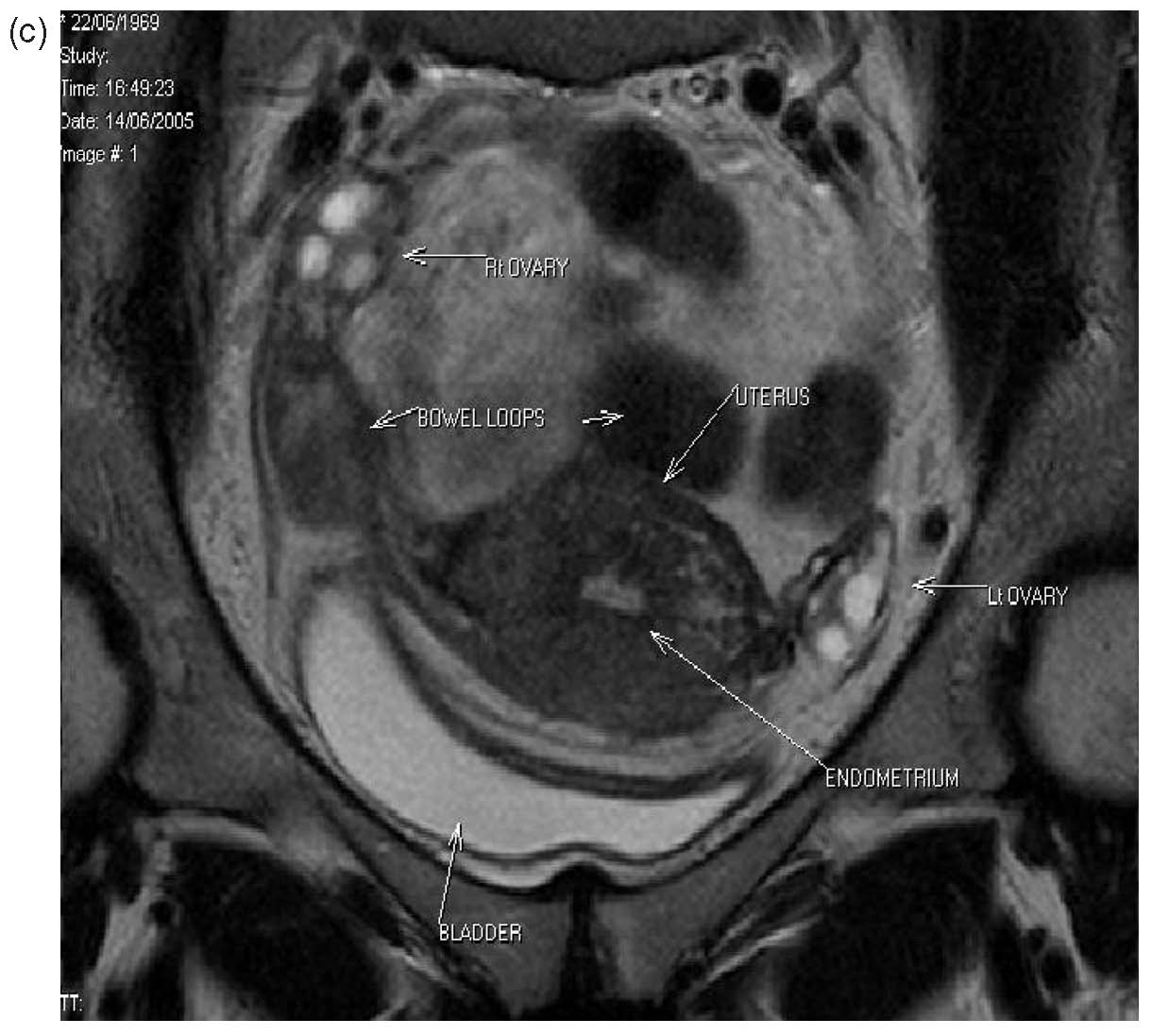 |
| Fig. 15a-c Normal MRI 6 months after massive postpartum hemorrhage treated by B-Lynch surgicaltechnique followed by uneventful spontaneous vertex vaginal delivery 22 months later. (a) Sagittal viewshowing normal endometrial cavity and treated Cesarean incision site; (b) coronal view, with no uterinecavity synechiae18; (c) view at level of incision for Cesarean section, showing well-healed features |
| Fig. 16 The Hayman uterine compression suturewithout opening the uterine cavity11 |
| Fig. 17 The Cho multiple square suturescompressing anterior to posterior uterine walls12 |
Table 5 The B-Lynch surgical technique: clinical points
1.
2. 7. 11. | User-friendly suture material monocryl No.1 mounted on 90-cm curved ethigard blunt needle (codeW3709) (Ethicon, Somerville, NJ). Other rapidly absorbable sutures can be used according to the surgeon’s preference. A good length and needle are essential18 |
Table 6 The Hayman uterine compression suture:clinical points
1. 2. | Lower uterine segment or uterine cavity not opened |
Table 7 The Cho multiple square sutures: clinicalpoints
1.
2. 3. 6. | Multiple full-thickness square sutures applied, probably time-consuming if many square sutures required |
WORLD-WIDE REPORTS
The current level of application of the B-Lynch suture world-wide includes over 1300 successful cases; of these, there are only 19 failures. The Indian subcontinent has the largest number of reported successful applications, over 250, followed by Africa, South America, North America, Europe and other countries. The 17 reported failures were because of delay in application, poor technique, defibrination and inappropriate material. Various suture materials have been used. However, the monocryl suture (code WC3709) is recommended because it is user- and tissue-friendly with uniform tension distribution and is easy to handle.19 Holtsema and colleagues recently opined, in a review, that the B-Lynch technique for postpartum hemorrhage should be an option for every gynecologist.20 Wohlmuth and colleagues published outcome of a large series with a 91% success rate (world-wide cumulative success rate 98%).21
CONCLUSION
Of the compression suturing techniques described above, the B-Lynch procedure has been recommended by the 2000–2002 Triennial Confidential Enquiry into Maternal Deaths in the United Kingdom,22 The Royal College of Obstetricians and Gynaecologists in the UK, and the Cochrane Database of systematic reviews. To date, no serious adverse outcomes have been associated with the B-Lynch surgical technique.9, 16, 19, 21, 23 Furthermore, the latest 2000–2002 Triennial Confidential Enquiry states that no deaths were reported in women who had had interventional radiology or B-Lynch suture in the management of postpartum hemorrhage.22
It is important to remember that, if a patient is a known or appreciated risk for postpartum hemorrhage, then the elective delivery should be performed in the day time, with prearranged co-operation between the imaging department and the obstetric team. Theater staff should be alerted in time so that conservative surgery can be carried out quickly if needed. Patients at particular risk are those with obesity, cardiomyopathy, coagulopathy, abnormal placentation, polyhydramnios and specific religious convictions contraindicating blood transfusion.
PLACEMENT OF LIGATURES IN STEPWISE DEVASCULARIZATION
The essential requirements are not simple and may not be available in every unit. First, there is a need for a competent obstetrician who is conversant and competent at pelvic gynecological procedures, and who has a working knowledge of the pelvic anatomy, including the vascular and neurological supply of the pelvic organs. Second, there is a need for an obstetric anesthetist, as well as a vascular and/or gynecological cancer surgeon on standby. Finally, provisions must be available for admission postoperatively to the intensive care unit.
This set of requirements takes full account of the extraordinarily generous blood supply to the uterus and the pelvic organs (see Fig. 18). The surgical approach starts with ligature of the uterine artery and its distribution to the uterus, preferably as it emerges from crossing over the ureter or as it approaches the uterine wall to penetrate and establish its division.24 This could be carried out unilaterally or bilaterally about 2 cm from the uterine angle at Cesarean section or where the lower segment is opened after conservative surgery for postpartum hemorrhage has failed (Fig. 19).
| Fig. 18 Placement of ligatures in the process of stepwise devascularization, including ligature of thedescending uterine and vaginal arteries |
| Fig. 19 The complex vascular distribution to the pelvic organs. In this procedure of stepwisedevascularization, the patient must be in the Lloyd Davis or modified lithotomy position, with one of theassistants able to access and swab the vagina to assess bleeding control |
It is absolutely essential to remember that the internal iliac (hypogastric artery) gives off independent branches that descend to the cervix and vagina (vaginal branch), respectively. Devascularization can be achieved by independent ligation sutures applied bilaterally to the cervix and/or vagina. The ovarian vascular supply to the uterus is also ligated, either unilaterally or bilaterally. Unilateral or bilateral ligature of the internal iliac artery may become necessary as a further step to control massive postpartum hemorrhage. A skilful surgeon should aim to ligate the anterior division of the internal iliac artery in order to achieve further devascularization of the uterus without compromising blood supply to the posterior division. However, ligation of the internal iliac directly could be done unilaterally or bilaterally without devascularizing the pelvic organs16, 25. This may save time, life and organ.
TREATMENT OF PELVIC HEMORRHAGE WITH INTERNAL ILIAC ARTERY EMBOLIZATION
Advancements in arteriographic techniques have not been fully appreciated or widely used in gynecologic practice. The emergence of arteriography as a well-defined subspecialty of radiology has led to the development and refinement of catheterization techniques that assist in the control of pelvic hemorrhage. Even relatively small arteries, such as the uterine branches of the internal iliac vessels, can be catheterized in the presence of massive retroperitoneal hematomas that distort normal anatomic findings. As a result, therapeutic embolization is effective in arresting massive pelvic hemorrhage caused by trauma (obstetric or otherwise) or cancer. To date, however, the technique has not been widely used to control postpartum hemorrhage.
A number of investigators confirm the efficacy of embolization techniques for control of bleeding related to obstetric and gynecologic problems. Mitty and colleagues reported that nine patients with obstetric hemorrhage from uterine, vaginal, or placental tears were successfully treated with surgical gelatin sponge embolization.26 Greenwood and co-workers described nine patients (eight obstetric, one gynecologic) who also were successfully treated with embolization therapy.27 Other series or single case reports document the use of uterine artery or internal iliac embolization in the management of hemorrhage after abortion, vaginal hemorrhage, abdominal pregnancy, vascular malformations, and trophoblastic disease.28, 29, 30, 31, 32 For the most part, success rates have been high, with little or no procedurally related morbidity and no mortality. Other investigators have reported on the use of internal iliac artery embolization to treat continued hemorrhage after successful hypogastric artery ligation, pointing out the relatively high failure rate of this technique as reported in the surgical literature.33, 34
Internal Iliac Artery Embolization
For the most part, the common femoral artery approach can be used to catheterize and embolize the iliac vessels. The use of modern steerable catheters as well as hydrophilic wires and catheters permits catheterization of the anterior divisions of both internal iliac arteries by a single femoral approach (Fig. 20). Other approaches that may be necessary in particularly problematic circumstances include the bilateral femoral approach, which enables the internal iliac artery to be catheterized on the contralateral side, or the brachial artery approach, which can provide access to both internal iliac arteries and their divisions. The disadvantage of the latter approach, however, is the fairly long catheter course from the arm to the pelvis.
Diagnostic Arteriography
Diagnostic arteriography should be carried out before any embolization procedure. An injection at the aortic bifurcation is ideal for visualization of all pelvic vessels. This overview permits the examiner to identify the overall branching pattern of the pelvic vessels. This is particularly necessary because the arrangement of the internal iliac artery branches varies considerably. After pelvic arteriography, selective internal iliac arteriography is carried out with at least two radiographic views of the vessel. The importance of multiple views of the pelvis cannot be overstated. The complexity and overlap of multiple vessels almost always requires several projections to identify vessels and to clarify their course, position, and distribution.
Although the major angiographic sign of bleeding is extravasation of contrast material, many authors do not report this sign in all patients with pelvic bleeding. Indeed, we and others find visualization of morphologic arterial changes, such as enlargement, tortuosity, or compression of the vessel by hematoma, to be helpful signs, especially if extravasation is not present. Among the 12 patients (including a number of actively bleeding patients) in whom we have performed uterine artery embolization, we have not noted active extravasation of contrast material. Rather, our decision to embolize was based on clinical circumstances and knowledge of pelvic anatomy (see below).
In our estimation, superselective catheterization of vessels is also important for the successful performance of embolization. We have found that the uterine artery can be catheterized (in most cases bilaterally) without too much difficulty.
Embolization Agents
A number of materials can be used for embolization, including Gianturco coils, surgical gelatin pledgets, polyvinyl alcohol foam particles (200–1000 μm), and liquids (e.g., tissue adhesives, alcohol). For management of bleeding, we strongly prefer the use of particulate material. In general, surgical gelatin pledgets cut to appropriate sizes of 1–5 mm provide ideal hemostasis and allow the vessel to recanalize within 2–3 weeks after the threat of hemorrhage has stopped. For more permanent embolizations, such as in uterine arteriovenous malformations, small particles or liquids have been used, but these should be injected with extreme caution because they can produce undesirable side effects in adjacent branch vessels as well as significant tissue necrosis.
Position of Embolization
In most cases of uterine bleeding, superselective uterine artery embolization is the ideal approach. Superselective catheterization and embolization permits complete occlusion of uterine vessels while preserving other normal pelvic arterial structures. In nonuterine bleeding, such as bleeding from vaginal or pelvic sidewall sources, bilateral embolization of the anterior division of the hypogastric artery with surgical gelatin pledgets is the most effective therapy. Some authors prefer anterior division embolization for all circumstances. Despite the fact that specific prospective comparisons have not been made between superselective embolization and less-selective anterior division embolization, we believe that the superselective approach is superior to less-selective embolization techniques.
Embolization of the appropriate or selected branch should be performed to the point of complete vessel occlusion, with production of vascular stasis in the blind segment as judged by frequent angiographic injections of contrast material.
Complications
Complications of pelvic embolization predominantly consist of inadvertent embolization of nontarget vessels. Fortunately, the pelvis is so richly supplied by collateral vessels (see above) that the use of medium-sized (1–4 mm) occluding agents will rarely result in ischemia unless the (gluteal) branches of the posterior division are occluded.
Other complications have also been reported, including occasional ischemic sciatic neuropathy from overzealous embolization of pelvic artery branches with very small particles. Moreover, the use of liquids or small particles also can produce rectal ischemia (through hemorrhoidal branches) or, rarely, areas of bladder ischemia. For these reasons, all patients must be observed carefully after embolization procedures. Despite the above caveats, pelvic embolization is very well tolerated if appropriately performed by skilled interventional radiologists.
THE NORTHWESTERN EXPERIENCE WITH PELVIC EMBOLIZATION FOR UTERINE BLEEDING
Our experience with selective transcatheter embolotherapy for uterine hemorrhage includes 12 patients treated between 1987 and 1995. They ranged in age from 16 to 42 years, and all had failed conservative therapy; most had received transfusions and one patient had von Willebrand's disease. Seven patients had postpartum uterine hemorrhage, and five had uterine vascular malformations. In the postpartum group, two had cervical artery lacerations after dilatation and curettage, one had placenta accreta after a cesarean section, one had placental subinvolution with hemorrhage 2 weeks after cesarean section, one had a uterine artery pseudoaneurysm at a cesarean section suture line, one had active bleeding from the uterine fundus after cesarean section, and one had uterine atony with coagulopathy. Among the patients with uterine vascular malformations, two had congenital arteriovenous malformations, and three had complications of gestational trophoblastic disease (choriocarcinoma, invasive mole, or hydatidiform mole).
Our technique for uterine artery embolization utilized the femoral approach. Bilateral uterine artery embolization was used in ten patients; unilateral uterine artery embolization was used in two patients. All patients were embolized with either surgical gelatin pledgets or polyvinyl alcohol foam particles (300–700 μm). Two patients had minor complications: One had a femoral artery false aneurysm treated successfully with ultrasound-guided compression; the other patient had transient fever for 3 days after embolization, which was believed to be related to endometritis. No ischemic complications were observed. Of the 12 patients, 11 were cured of hemorrhage; the remaining patient with uterine atony had recurrent bleeding at 8 days, necessitating a hysterectomy. Of the 11 patients who retained their uterus, one patient became pregnant 8 months after embolization. The following six case reports illustrate the significant benefits of pelvic embolization for uterine hemorrhage.
CASE REPORTS
Case 1.
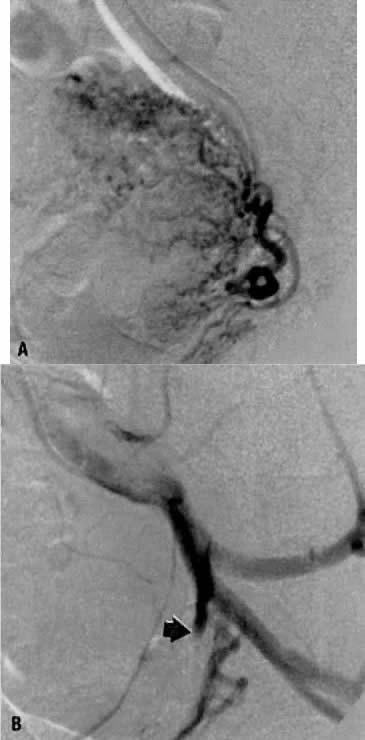
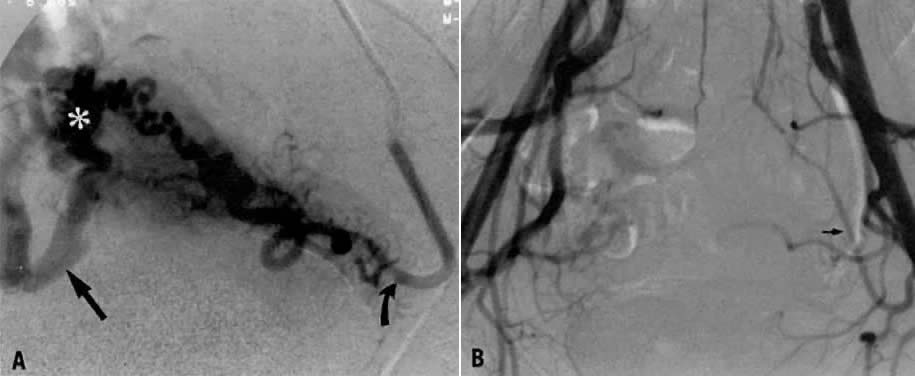
This 35-year-old woman (gravida 2, para 2) had brisk and unremitting hemorrhage after forceps delivery of a healthy term infant. A cervical laceration was found and sutured; however, the patient continued to bleed; 5 units of whole blood were administered. Despite uterine and vaginal packing, bleeding continued. In an effort to avoid hysterectomy, arteriography and embolization were requested. Selective left uterine arteriography (Fig. 18A), performed from the right common femoral approach, showed the typical appearance of the uterine artery and its branches postpartum. Although no extravasation was seen, the brisk hemorrhage (presumably from a descending cervical branch of the uterine artery) prompted the use of embolization with surgical gelatin pledgets. This intervention resulted in complete occlusion of the uterine artery (Fig. 18B). The right uterine artery also was embolized, resulting in prompt cessation of bleeding.
Case 2.
This 26-year-old woman (gravida 2, para 1) underwent dilatation and curettage for a missed abortion and bled briskly after the procedure; the bleeding did not respond to conventional measures. To preserve reproductive function, embolization was carried out bilaterally. Figure 19 represents the left (Fig. 19A) and right (Fig. 19B) uterine artery injections, both of which were performed from a right common femoral approach. Of note is the typical spiral endometrial artery pattern of the right uterine artery. Here also, no specific bleeding was demonstrated, but bilateral selective uterine embolization with surgical gelatin produced complete occlusion of the left (Fig. 19C) and right (Fig. 19D) uterine arteries with prompt cessation of the hemorrhage.
Case 3.
This 23-year-old woman (gravida 1, para 1) had an uneventful term cesarean section 2 months earlier. Approximately 2 weeks before hospital admission, intermittent vaginal bleeding developed, with each episode producing approximately 100 mL of blood loss. Physical examination showed no abnormality, and a dilatation and curettage did not correct the problem. Ultimately, hysteroscopy revealed a small pulsatile mass in the lower uterine segment which was believed to be arterial in nature. An arteriovenous malformation was suspected.
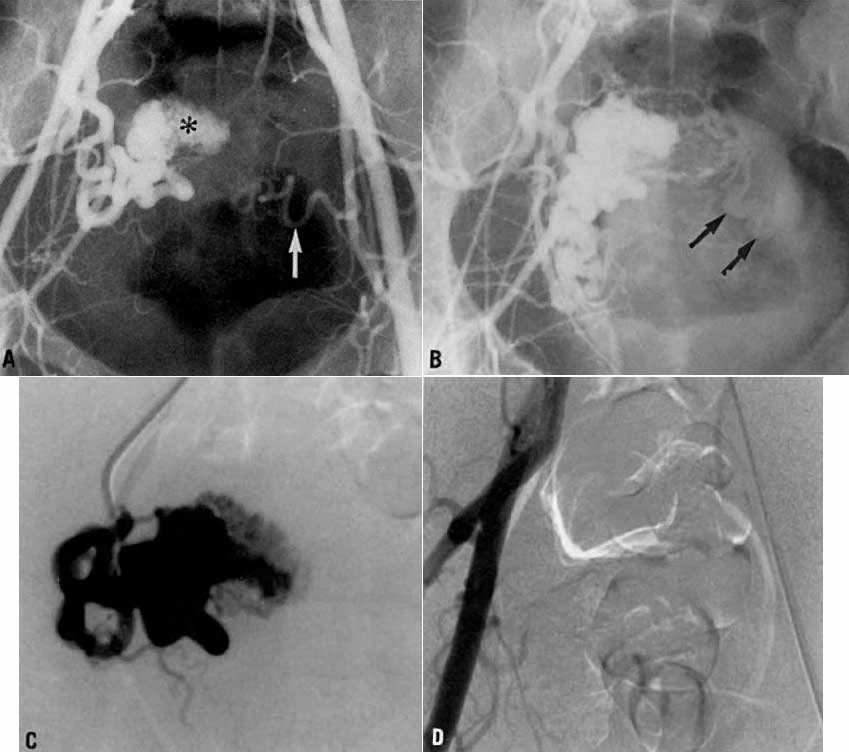
Transvaginal ultrasound with color Doppler showed a focal arterial aneurysm with a turbulent flow pattern within it located on the left side of the uterus (Fig. 20A). Selective left uterine arteriography showed the lesion to be a false aneurysm of the uterine artery seen both in the early (Fig. 20B) and late (Fig. 20C) phases of left uterine arteriography. Embolization with surgical gelatin yielded complete occlusion of the left uterine artery (Fig. 20D). The abnormality did not fill, and the patient had an uneventful postoperative course with no further episodes of bleeding and elimination of the abnormality as evidenced by a follow-up transvaginal ultrasound taken 2 weeks later.
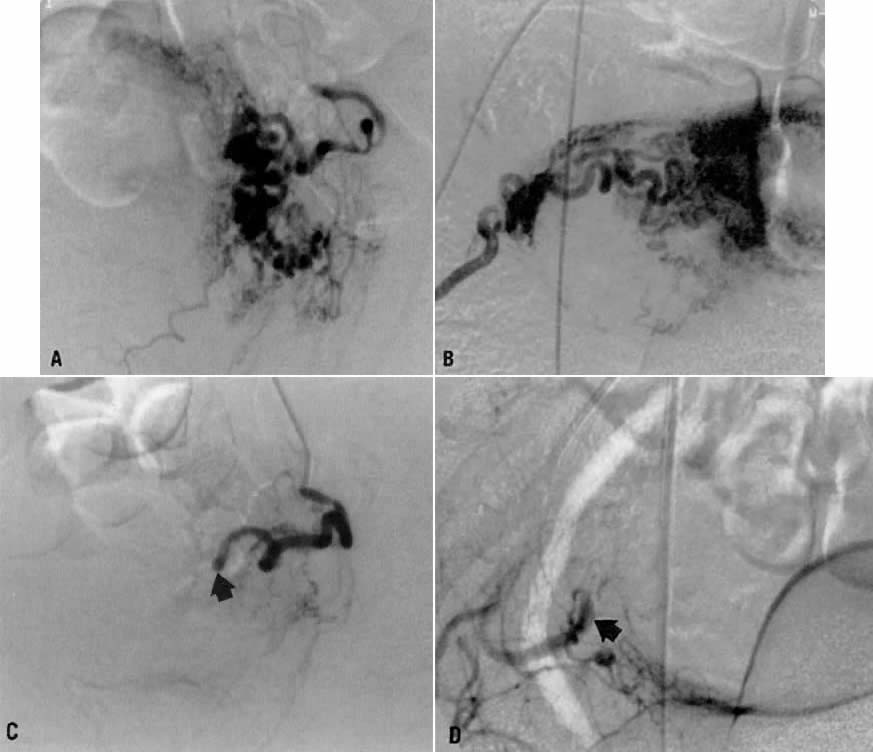
Case 4.
This 30-year-old woman (gravida 3, para 3) required cesarean section at 42 weeks. Despite what appeared to be complete removal of the placenta, the patient continued to bleed from the placental site after surgery, and placenta accreta was considered a diagnostic possibility. To avoid a hysterectomy and to preserve reproductive function, uterine embolization was requested. Anteroposterior pelvic arteriography (Fig. 21A) showed enlargement of the right uterine artery with persistence of contrast enhancement in the late phase (Fig. 21B). Selective right uterine arteriography (Fig. 21C) also showed enlargement of the right uterine artery with typical postpartum changes of the spiral endometrial arteries and dense staining in the late phase (Fig. 21D). Both right and left uterine arteries were embolized successfully and without complication. This intervention produced prompt cessation of bleeding.
Case 5.
In this 36-year-old patient, bleeding occurred after successful chemotherapy for gestational trophoblastic disease (hydatidiform mole). Transfusion was required on two occasions. Figure 22A shows selective left uterine artery injection, demonstrating the uterine artery with supply of a hypervascular fundal mass with early venous drainage. The right uterine artery was normal. The left uterine artery was embolized with polyvinyl alcohol foam particles (300–500 μm). This intervention produced complete occlusion of the left uterine artery. A pelvic arteriogram (Fig. 22B) shows occlusion of the left uterine artery and no evidence of a hypervascular mass.
Case 6.
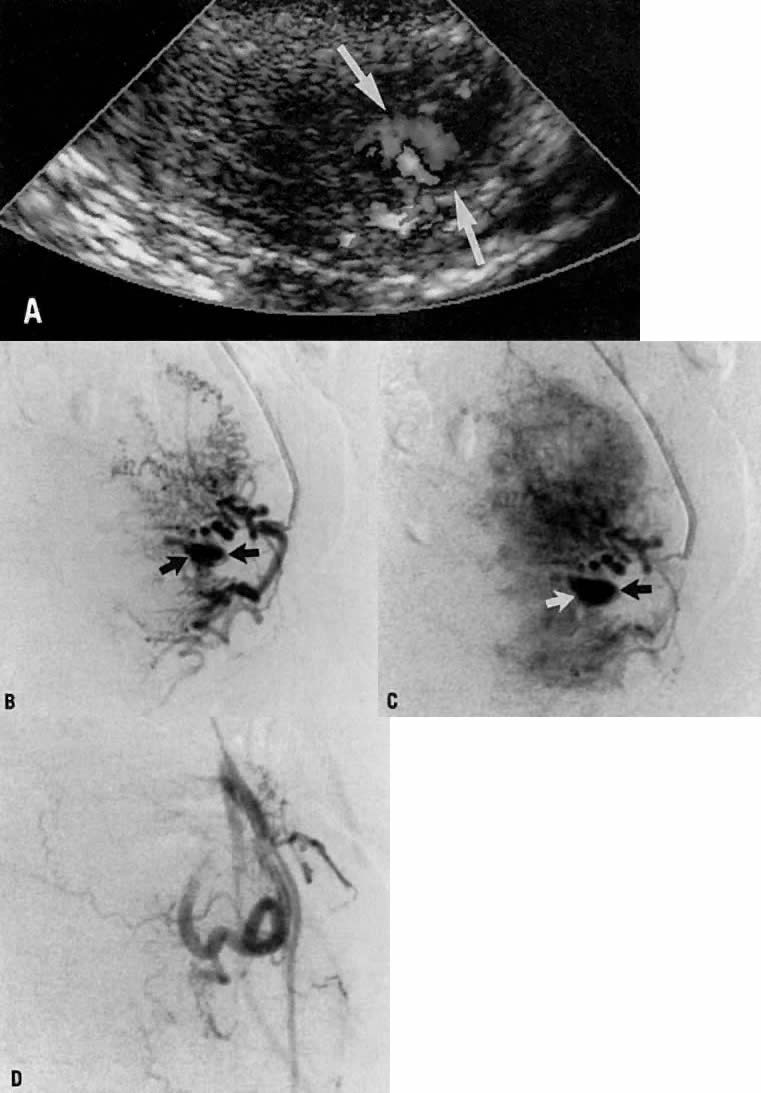
This 16-year-old patient (gravida 3, para 0) had experienced three spontaneous first-trimester abortions, all with significant hemorrhage. After the third abortion, hysteroscopy demonstrated multiple enlarged pulsatile uterine arteries in the uterine fundus; a clinical diagnosis of uterine arteriovenous malformation was made. Figure 23A is an anteroposterior view of the pelvis, demonstrating an enlarged right uterine artery supplying a hypervascular mass. Note the normal left uterine artery. Figure 23B shows the late arterial phase, in which there is early venous drainage.
Pelvic embolization was requested. Figure 23C shows subselective right uterine arteriography with tortuous dilated vessels supplying the mass. The malformation was embolized with polyvinyl foam particles (300–500 μm) and surgical gelatin pledgets. Complete occlusion of the right uterine artery was achieved. Because of the hypervascular nature of the mass, the left uterine artery also was embolized with polyvinyl alcohol particles. Figure 23D shows a right common iliac artery injection with obliteration of the arteriovenous malformation after embolization. Six months after embolization, the patient became pregnant again and had an uneventful first trimester; however, because of her persistent and overwhelming concerns about the possibility of bleeding, she insisted on and underwent an elective hysterectomy for termination of pregnancy. Pathologic examination of the uterus showed a thrombosed arteriovenous malformation without evidence of new vessels: Clearly, the embolization had been completely successful in obliterating the malformation. Unfortunately, the patient did not benefit from the therapy.
REFERENCES
B-Lynch C, Coker A, Lawal AH et al: The B-Lynch surgical technique for the control of massive postpartum haemorrhage:an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol 104:372-5, 1997 |
|
Burchell RC, Olson G: Internal iliac artery ligation: Aortograms. Am J Obstet Gynecol 94: 117, 1966 |
|
Reich WJ, Nechtow MJ, Keith LG: Supplementary report on hypogastric artery ligation in prophylactic and active treatment of hemorrhage in pelvic surgery. Int Surg 44: 1, 1965 |
|
Hyams J: Aberrant blood vessels as a factor in lower ureteral obstruction. Surg Gynecol Obstet 48: 474, 1929 |
|
Benson RC, Dotter CT, Peterson CG et al: Congenital arteriovenous fistula and pregnancy. Am J Obstet Gynecol 92: 672, 1965 |
|
Burchell RC, Mengert WF: Internal iliac artery ligation: A series of 200 patients. J Int Fed Obstet Gynecol 7: 85, 1969 |
|
Shumacker HB Jr: Midline extraperitoneal exposure of the abdominal aorta and iliac arteries. Surg Gynecol Obstet 135: 791, 1972 |
|
Tajes RV: Ligation of the hypogastric arteries and its complications in resection of cancer of rectum. Am J Gastroenterol 26: 612, 1956 |
|
B-Lynch C, Cowen M.J. A new non-radical surgical treatment of massive post partum hemorrhage. Contemp Rev Obstet Gynaecol 1997; March:19–24 |
|
Chez RA, B-Lynch C. The B-Lynch suture for control of massive post partum hemorrhage. Contemp Obstet Gynaecol 1998;43:93–8 |
|
Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of post partum hemorrhage. Obstet Gynecol 2002; 99:502–6 |
|
Cho JH, Jun HS, Lee CN. Hemostatic suturing technique for uterine bleeding during cesarean delivery. Obstet Gynecol 2000;96: 129–3 |
|
Ochoa M, Allaire AD, Stitely ML. Pyometra after hemostatic square suture technique. Obstet Gynecol 2002;99:506–9 |
|
Ferguson JE, Bourgeois FJ, Underwood PB, B-Lynch C. Suture for post partum hemorrhage. Obstet Gynecol 2000;95:1020–2 |
|
Drife J. Management of primary post partum haemorrhage. Br J Obstet Gynaecol 1997;104: 275–7 |
|
El-Hammamy E, B-Lynch C. A worldwide review of the uses of the uterine compression suture techniques as alternative to hysterectomy in the management of severe post-partum haemorrhage. J Obstet Gynaecol 2005;25:143–9 |
|
Tsitpakidis C, Lalonde A, Danso D, B-Lynch C. Long term anatomical and clinical observations of the effects of the B-Lynch uterine compression suture for the management of post partum hemorrhage – ten years on. J Obstet Gynaecol 2006; in press |
|
Wu HH, Yeh GP. Uterine cavity synechiae after hemostatic square suturing technique. Obstet Gynecol 2005;105:1176–8 |
|
Price N, B-Lynch C. Technical description of the B-Lynch suture for treatment of massive hemorrhage and review of published case. Int J Fertil Womens Med 2005;50:148–63 |
|
Holtsema H, Nijland R, Huisman A, Dony J, van den Berg PP. The B-Lynch technique for post partum haemorrhage: an option for every gynaecologist. Eur J Obstet Gynaecol Reprod Biol 2004;115:39–42 |
|
Wohlmuth C, Gumbs J, Quebral-Ivie J. B-Lynch suture, a case series. Int J Fertil Womens Med 2005;50:164–73 |
|
Department of Health. Why Mothers Die: Report on Confidential Enquiries into Maternal Deaths in the United Kingdom 2000–2002 Triennial Report. London: RCOG Press, 2004: 94–103 |
|
Allam MS, B-Lynch C. The B-Lynch and other uterine compression suture techniques. Int J Gynaecol Obstet 2005;89:236–1 |
|
O’Leary JA. Uterine artery ligation in the control of post-caesarean hemorrhage. J Reprod Med 1995;40:189–93 |
|
Clarke SL, Koonings P, Phelan JP. Placenta accreta and prior cesarean section. Obstet Gynecol 1985;66:89–92 |
|
Mitty HA, Sterling KM, Alvarez M, Gendler R: Obstetric hemorrhage: Prophylactic and emergency arterial catheterization and embolotherapy. Radiology 188: 183, 1993 |
|
Greenwood LH, Glickman MG, Schwartz PE et al: Obstetric and nonmalignant bleeding: Treatment with angiographic embolization. Radiology 164: 155, 1987 |
|
Pearl ML, Braga CA: Percutaneous embolization in control of life-threatening pelvic hemorrhage from gestational trophoblastic disease. Obstet Gynecol 80: 571, 1992 |
|
Haseltine FP, Glickman MG, Marchesi S et al: Uterine embolization in a patient with postabortal hemorrhage. Obstet Gynecol 63: 78S, 1984 |
|
Vogelzang RL, Nemcek AA, Skirtic Z et al: Uterine arteriovenous malformations: Primary treatment with therapeutic embolization. J Vasc Interventional Radiol 2: 517, 1991 |
|
Kivikoski AI, Martin C, Weyman P et al: Angiographic arterial embolization to control hemorrhage in abdominal pregnancy: A case report. Obstet Gynecol 71: 456, 1988 |
|
Rosenthal DM, Colapinto R: Angiographic arterial embolization in the management of postoperative vaginal hemorrhage. Am J Obstet Gynecol 151: 227, 1985 |
|
Clark SL, Phelan JP, Yeh SY et al: Hypogastric artery ligation for obstetric hemorrhage. Obstet Gynecol 66: 353, 1985 |
|
Evans S, McShane P: The efficacy of internal iliac artery ligation in obstetric hemorrhage. Surg Gynecol Obstet 160: 250, 1985 |