Breast Cancer and the Obstetrician-Gynecologist
Authors
INTRODUCTION
Breast cancer is the most common invasive (nonskin) cancer of women. For 2007, it is estimated that there will have been 178,480 invasive breast cancers and 62,030 new cases of in situ breast cancer diagnosed in women in the USA. In addition it is estimated that there will have been 40,460 female deaths from breast cancer in 2007.1 The incidence of breast cancer seems to have peaked in 1998 and the subsequent decline since then has been attributed to the saturation of breast cancer screening and the decrease in the use of hormone replacement therapy (HRT) since the publication of the Women's Health Initiative Study in 2002.2
The American Joint Committee on Cancer lists 24 different codable histologic types of breast malignancies (Table 1), of which seven are in situ and 17 are invasive (infiltrating).3 Each specific histologic type has its own unique characteristics, biologic behavior, and prognosis when treated. In addition, the optimum treatment varies with each specific histologic type. However, for invasive and in situ breast cancer, as many as 80% of the cases are of the ductal type. Color plates 1 through 16 show typical examples of the histology and cytology of normal breast tissue, fibrocystic changes, fibroadenoma, and invasive ductal carcinoma under low- and high-power magnification. (Color plates appear at the end of the chapter.)
Table 1. Codable histologic breast malignancies
| In situ |
| Carcinoma in situ, NOS* |
| Comedocarcinoma, noninfiltrating |
| Cribriform carcinoma in situ |
| Intraductal carcinoma and lobular carcinoma in situ |
| Intraductal carcinoma, noninfiltrating, NOS |
| Lobular carcinoma in situ, NOS |
| Noninfiltrating intraductal papillary adenocarcinoma |
| Paget's disease and intraductal carcinoma of breast |
| Paget's disease, mammary |
| Invasive |
| Adenoid cystic carcinoma |
| Carcinoma, NOS |
| Carcinoma undifferentiated, NOS |
| Carcinosarcoma, NOS |
| Cribriform carcinoma, NOS |
| Infiltrating duct carcinoma, NOS |
| Inflammatory carcinoma |
| Lobular carcinoma, NOS |
| Medullary carcinoma, NOS |
| Mucinous adenocarcinoma |
| Paget's disease and infiltrating duct carcinoma of breast |
| Phyllodes tumor, malignant |
| Secretory carcinoma of breast |
| Squamous cell carcinoma, NOS |
| Tubular adenocarcinoma |
*NOS = not otherwise specified.
(Adapted from American Joint Committee on Cancer: AJCC Cancer Staging Manual, p 236. 6th edn. New York, Springer, 2002.)
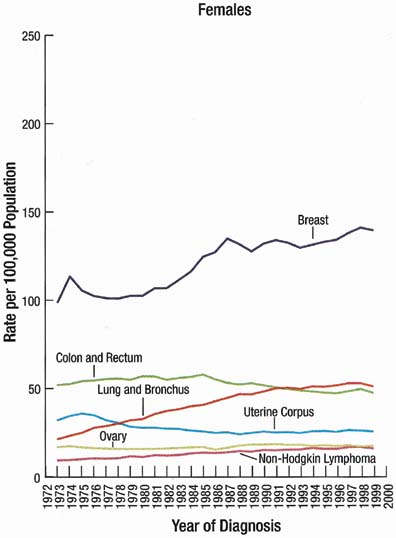
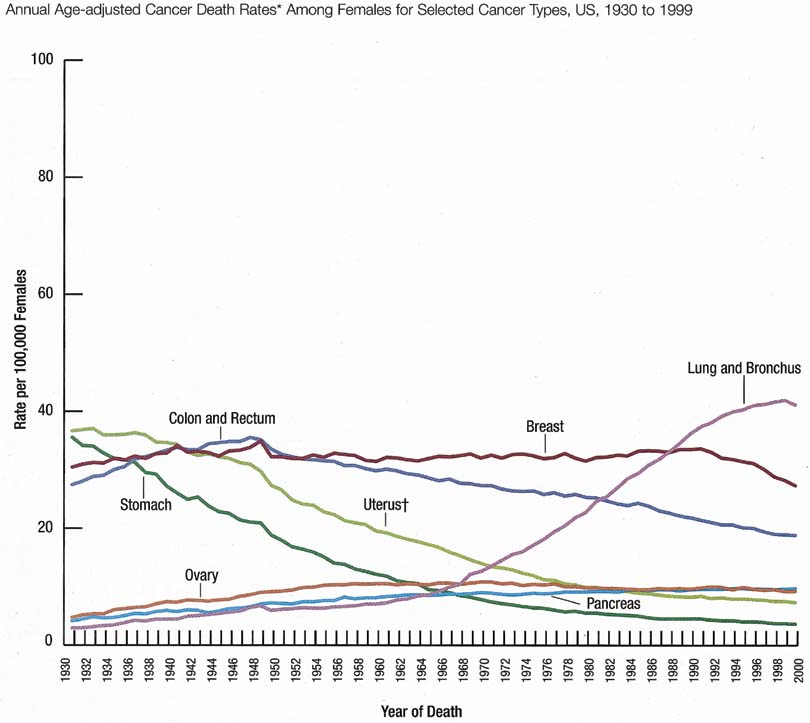
The incidence rate for breast cancer in women has increased steadily since 1977, as shown in comparison with other major invasive cancers in Figure 1.1 However, the incidence rate appears to have peaked in 1997, with a slight decrease since then. Figure 2 graphically depicts the contrasting mortality rates of the major invasive cancers.1 Possibly related to improved methods of treatment, the mortality rate for breast cancer has shown a continuous slightly downward trend of from 1940 to 1990. Probably caused by the widespread use of screening mammography, the mortality rate has decreased from 1990 to 1999. Hopefully, this trend of clinically meaningful decreasing mortality rates will continue as data for the years after 1999 become available. The 5-year survival rates by extent of breast cancer at the time of diagnosis are 86% for all stages, 97% for localized cancer, 78% for cancer with regional involvement, and 23% for metastatic cancer.2
Obstetrician-gynecologists should strive to bring about the diagnosis of breast cancer before the cancer is palpable. This goal can be effectively achieved by annual screening mammography for women beginning at age 40 years.4 Mammography can perceive small (1- to 9-mm) nonpalpable breast cancers that have the optimum curability, with more than 90% 20-year disease-specific survival using current methods of treatment (Table 2).5 Palpable cancers have a less favorable outcome. Generally, survival from palpable breast cancer correlates inversely with increasing size.6 For example, 10-to 29-mm cancers have been reported to have 67% 15-year survival (Table 3).7
Table 2. 20-year breast cancer-specific survival: mammographic appearance vs. percentage survival
Mammographic appearance | Percentage survival |
| Stellate mass without calcifications | 100 |
| Noncasting calcifications | 96 |
| Circular mass without calcifications | 93 |
| Casting calcifications | 60 |
| All 1–9-mm tumors | 93 |
(Adapted by Patricia T. Kelly, PhD, from Tabar L, Vitak B, Chen HH, et al: The Swedish Two-County Trail twenty years later. Updated mortality results and new insights from long-term follow-up. Radiol Clin North Am 38:625–651, 2000.)
Tumors 1–9-mm without nodal involvement.
Table 3. 15-year breast cancer survival: correlation with tumor size
| Tumor size | Percentage survival |
| 10–14 mm | 86 |
| 15–19 mm | 72 |
| 20–29 mm | 67 |
| 30–39 mm | 46 |
(Adapted by Patricia T. Kelly, PhD, from Michaelson JS, Silverstein M, Wyatt J, et al: Predicting the survival of patients with breast carcinoma using tumor size. Cancer 95:713–723, 2002.)
Unfortunately, some cancers large enough to be palpable are not initially perceived on mammography. This probably occurs in less than 10% of cases.8 Increased mammographic density (Table 4) can obscure both nonpalpable and palpable breast cancers. Thus, women should have an annual clinical breast examination in addition to screening mammography. Ideally, the examination is performed before the mammogram so that the results of the examination are known by the radiologist/mammographer. Women with dense breasts should be preferably screened with full-field digital mammography rather than analogue mammography. The addition of ultrasound and/or MRI should be also considered in such subjects.
Table 4. The BI-RADS descriptive patterns of mammographic breast density
| The breast is almost entirely fat |
| There are scattered fibroglandular densities that could obscure a lesion on mammography |
| The breast tissue is heterogeneously dense |
| This may lower the sensitivity of mammography |
| The breast tissue is extremely dense, which lowers the sensitivity of mammography |
(American College of Radiology (ACR): Illustrated Breast Imaging Reporting and Data System (BI-RADS™), 3rd edn. Reston, VA, American College of Radiology, 1998.)
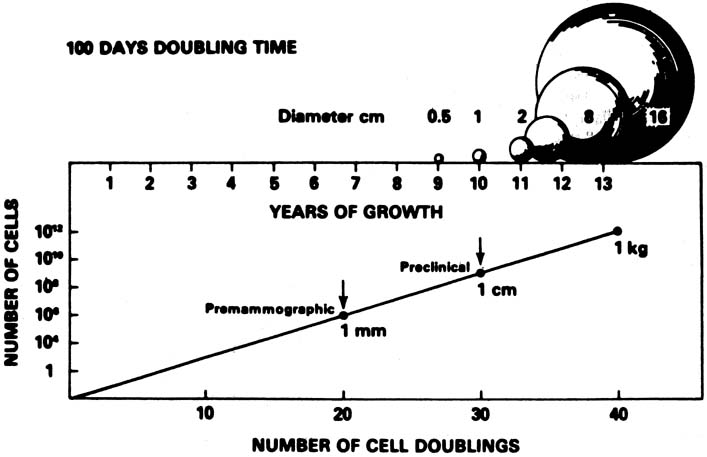
A conceptual mathematical model of the growth of a typical invasive ductal breast cancer is depicted in Figure 3.9 The growth of the cancer is based on a constant 100-day cell mass doubling time, which is the generally accepted mean rate of growth. However, estimated breast cancer doubling times have been reported in the range of 30–300 days and are not constant, but vary dependent on the specific cancer characteristics and host interaction. Nevertheless, the concept depicted is sound. There is a premammographic phase when the cancer is too small to be detected by any method currently available, followed by a preclinical phase when the cancer is too small to be palpable. Exactly when breast cancers metastasize is unknown, but the 90% 20-year disease-free survival of treated 1- to 9-mm mammographically detected cancers indicates that few had metastasized before the cancers reached a size of 1 cm. This concept underscores the importance of annual screening mammography, particularly in the 40- to 49-year-old group in which, clinically, some of the breast cancers grow aggressively with apparently shorter doubling times.
The American Cancer Society's 2007 guidelines for the early detection of breast cancer in asymptomatic women are annual mammography and clinical breast examination beginning at age 40 and clinical breast examination every 3 years for women ages 20–39.10 If a clinically suspicious breast lesion remains undiagnosed, the patient should be urgently referred to a breast specialist. If the patient has diagnosed breast cancer, optimum treatment is usually obtained at a dedicated breast center with a multidisciplinary treatment planning team (Table 5).
Table 5. A multidisciplinary treatment planning team should include the following oncologic breast specialists
| Imaging |
| Medical oncology |
| Nursing/counseling |
| Pathology |
| Plastic surgery |
| Radiation therapy |
| Social service |
| Surgery |
RISK FACTORS
Most women are concerned about the risk of breast cancer. Numerous epidemiologic studies have been published, but it is often difficult for patients, and even for physicians, to understand and appreciate the pertinent clinical meaning. Typically, a woman wants to know if she will get breast cancer, and that question is not answerable. An obstetrician-gynecologist can recommend that the patient read a clear understandable book, such as Assess Your True Risk of Breast Cancer by Patricia Kelly, PhD (An Owl Book, Henry Holt and Company, New York, NY, 2000). This book is also useful reading for obstetrician-gynecologists for insight into epidemiologic studies, statistics, relative risk, absolute risk, and statistical significance.
The clinically relevant risk factors for breast cancer are: (1) being a woman; (2) growing older; (3) gene mutations; (4) atypical ductal or lobular hyperplasia; (5) lobular carcinoma in situ (more accurately called lobular neoplasia, a tumor marker); and (6) atypical epithelial hyperplasia. Other epidemiologic risk factors are: (1) birth of first child after age 30; (2) consumption of an alcoholic beverage one or more times per day; (3) early onset of menstruation (menarche); (4) family history of breast cancer; (5) having had breast cancer; (6) increased mammographic density; (7) late ending of menstruation (menopause); (8) nulliparity; (9) postmenopausal estrogen and progestin therapy; (10) postmenopausal obesity; and (11) recent use of oral contraceptives. However, none of these epidemiologic risk factors alters clinical management. A summary of the published breast cancer relative risk data is in Table 6. Relative risk of less than 2–3 is an epidemiologic weak association without evidence of causality and, clinically, is likely to be a chance association, perhaps related to the multifactorial natural history and biologic behavior of breast cancers.
Table 6. Breast cancer relative risk factors grouped by magnitude
Relative risk < 2 | Relative risk 2–4 | Relative risk > 4 |
| Early menarche | Age > 35 first birth | Gene mutation |
| Late menarche | 1st degree relative with breast cancer | Lobular carcinoma in situ |
| Nulliparity | Radiation exposure | Ductal carcinoma in situ |
| Proliferative benign disease | Previous breast cancer | Atypical hyperplasia |
| Obesity | ||
| Alcohol use | ||
| Hormone replacement |
Summary of published levels of relative risks for breast cancer. (Brinkmann E, Morrow M: The surgeon's role in breast cancer chemoprevention. Breast Dis 12:103–112, 2001 [Table 1. p 105].)
Routine vigorous physical activity and maintenance of healthy body weight are associated with epidemiologic decreased risk of breast cancer.2
DIAGNOSIS: INVASIVE CANCER AND IN SITU CARCINOMA
Ductal carcinoma is the most common histologic type of breast cancer, accounting for as many as 80% of breast malignancies. Nonpalpable invasive ductal carcinoma is usually perceived on mammography. The typical findings are stellate solid mass or pleomorphic casting microcalcifications. However, a malignant solid mass may be circular and the calcifications may be noncasting. Ultrasound can be helpful in defining a malignant solid mass, particularly in a young woman or in any woman with mammographically dense breasts, but ultrasound is not effective in evaluating calcifications that are often not perceived on ultrasound. The definitive diagnosis of nonpalpable cancer is established by image-guided tissue core-needle biopsy, commonly with vacuum-assisted technique, or less commonly by imaging needle localization and open surgical biopsy for histologic diagnosis.
It is the patient herself who usually notices, often coincidentally, what she perceives as a mass. Then she presents to her physician, often an obstetrician-gynecologist, for confirmation that it is a palpable dominant mass and for diagnosis. A palpable dominant breast mass is defined as a three-dimensional distinct mass that is different from the remainder of the breast tissue and from the tissue of the other breast. The definitive diagnosis of the mass can be established by fine-needle aspiration cytology, tissue core-needle biopsy histology, or less commonly by open surgical biopsy.
Ductal carcinoma in situ (DCIS) is most commonly nonpalpable and perceived on screening mammography as malignant calcifications, usually pleomorphic and of the casting type. The definitive diagnosis is usually established by stereotactic vacuum-assisted core biopsy or by open surgical biopsy with imaging needle localization. Tissue histology, usually of the entire lesion, is essential to be certain that there is not associated invasive ductal carcinoma.
Invasive lobular carcinoma spreads diffusely with a typical histologic Indian file pattern. Thus, invasive lobular carcinoma is often not apparent, either by palpation or by imaging, until the cancer is at an advanced stage. Lobular carcinoma in situ (LCIS) is thought to be a tumor marker with associated increased risk of eventual invasive carcinoma that usually is of the ductal type when it does occur and may even occur in the contralateral breast. The presence of high grade cytology and necrosis significantly increases the risk associated with LCIS.
REFERRAL AND CONTINUING FOLLOW-UP
If an obstetrician-gynecologist is not trained and experienced in an appropriate diagnostic technique, the patient with a palpable dominant breast mass or with mammography findings suspicious for malignancy (BI-RADS categories 4 and 5; Table 7)11 should be expeditiously referred to a breast center or a breast specialist if a breast center is not available. It is important to continue to follow-up the patient to be certain she is seen in referral and that a definitive diagnosis is established. If the patient delays or refuses the referral, her noncompliance should be clearly documented in her medical record and the patient informed, by certified mail if necessary, of the potentially life-threatening seriousness of her condition and inaction.
Table 7. The BI-RADS mammographic assessment categories
| Category 0 | Need additional imaging evaluation |
| Category 1 | Negative |
| Category 2 | Benign finding |
| Category 3 | Probably benign finding: short interval follow-up suggested |
| Category 4 | Suspicious abnormality: biopsy should be considered |
| Category 5 | Highly suggestive of malignancy: appropriate action should be taken |
(American College of Radiology (ACR): Illustrated Breast Imaging Reporting and Data System (BI-RADS™), 3rd edn. Reston, VA, American College of Radiology, 1998.)
As a primary health care provider for women, the obstetrician-gynecologist should continue to be available to the patient, her spouse/partner, and her family for counseling, compassionate explanations of the diagnostic and treatment options, and caring and understandable answers to their questions and concerns.
TREATMENT OPTIONS
Equivalent long-term breast cancer survivals can be achieved by either breast-conserving therapy (lumpectomy, axillary lymph node dissection, and irradiation) or modified radical mastectomy.12 Radical mastectomy, which was The Standard for more than 70 years, is no longer indicated.13, 14
Breast-Conserving Surgery (BCS)
Breast-conserving therapy is the preferred treatment for stages I and II (up to a size of 5 cm) breast cancer.15Table 8 describes the American Joint Committee on Cancer staging of breast cancer.3 The absolute and relative contraindications for breast conserving therapy are listed in Table 9. The most common reason for selecting modified radical mastectomy instead of breast conserving therapy for stages I and II breast cancers is the patient's own choice. The medical contradictions are uncommon. Perhaps as many as 10% of women with stage I and 30% with stage II are not candidates for breast-conserving therapy.16 However, some of these unfavorable candidates and some women with cancers larger than stage II can benefit from pre-operative neoadjuvant chemotherapy or endocrine therapy, which can potentially decrease the size of the cancer and make a recommendation for breast-conserving therapy appropriate.17, 18
Table 8. TNM staging of breast cancer 2002
| DEFINITION OF TNM | |
| Primary Tumor (T) | |
| Definitions for classifying the primary tumor (T) are the same for clinical and for pathologic classification. If the measurement is made by physical examination, the examiner will use the major headings (T1, T2, or T3). If other measurements, such as mammographic or pathologic measurements, are used, the subsets of T1 can be used. Tumors should be measured to the nearest 0.1 cm increment. | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| Tis (DCIS) | Ductal carcinoma in situ |
| Tis (LCIS) | Lobular carcinoma in situ |
| Tis (Paget's) | Paget's disease of the nipple with no tumor |
Note: Paget's disease associated with a tumor is classified according to the size of the tumor. | |
| T1 | Tumor 2 cm or less in greatest dimension |
| T1mic | Microinvasion 0.1 cm or less in greatest dimension |
| T1a | Tumor more than 0.1 cm but not more than 0.5 cm in greatest dimension |
| T1b | Tumor more than 0.5 cm but not more than 1 cm in greatest dimension |
| T1c | Tumor more than 1 cm but not more than 2 cm in greatest dimension |
| T2 | Tumor more than 2 cm but not more than 5 cm in greatest dimension |
| T3 | Tumor more than 5 cm in greatest dimension |
| T4 | Tumor of any size with direct extension to (a) chest wall or (b) skin, only as described below |
| T4a | Extension to chest wall, not including pectoralis muscle |
| T4b | Edema (including peau d'orange) or ulceration of the skin of the breast, or satellite skin nodules confined to the same breast |
| T4c | Both T4a and T4b |
| T4d | Inflammatory carcinoma |
| Regional lymph nodes (N) | |
| Clinical | |
| NX | Regional lymph nodes cannot be assessed (e.g., previously removed) |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis to movable ipsilateral axillary lymph node(s) |
| N2 | Metastases in ipsilateral axillary lymph nodes fixed or matted, or in clinically apparent* ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastasis |
| N2a | Metastasis in ipsilateral axillary lymph nodes fixed to one another (matted) or to other structures |
| N2b | Metastasis only in clinically apparent* ipsilateral internal mammary nodes and in the absence of clinically evident axillary lymph node metastasis |
| N3 | Metastasis in ipsilateral infraclavicular lymph node(s) with or without axillary lymph node involvement, or in clinically apparent* ipsilateral internal mammary lymph node(s) and in the presence of clinically evident axillary lymph node metastasis; or metastasis in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement |
| N3a | Metastasis in ipsilateral infraclavicular lymph node(s) |
| N3b | Metastasis in ipsilateral internal mammary lymph node(s) and axillary lymph node(s) |
| N3c | Metastasis in ipsilateral supraclavicular lymph node(s) |
| Pathologic (pN)a | |
| pNX | Regional lymph nodes cannot be assessed (e.g., previously removed, or not removed for pathologic study) |
| pN0 | No regional lymph node metastasis histologically, no additional examination for isolated tumor cells (ITC) |
Note: Isolated tumor cells (ITC) are defined as single tumor cells or small cell clusters not greater than 0.2 mm, usually detected only by immunohistochemical (IHC) or molecular methods but which may be verified on H&E stains. ITCs do not usually show evidence of malignant activity e.g., proliferation or stromal reaction. | |
| pN0(i−) | No regional lymph node metastasis histologically, negative IHC |
| pN0(i+) | No regional lymph node metastasis histologically, positive IHC, no IHC cluster greater than 0.2 mm |
| pN0(mol−) | No regional lymph node metastasis histologically, negative molecular findings (RT-PCR)b |
| pN0(mol+) | No regional lymph node metastasis histologically, positive molecular findings (RT-PCR)b |
| pN1 | Metastasis in 1 to 3 axillary lymph nodes, and/or in internal mammary nodes with microscopic disease detected by sentinel lymph node dissection but not clinically apparent** |
| pN1mi | Micrometastasis (greater than 0.2 mm, none greater than 2.0 mm) |
| pN1a | Metastasis in 1 to 3 axillary lymph nodes |
| pN1b | Metastasis in internal mammary nodes with microscopic disease detected by sentinel lymph node dissection but not clinically apparent** |
| pN1c | Metastasis in 1 to 3 axillary lymph nodes and in internal mammary lymph nodes with microscopic disease detected by sentinel lymph node dissection but not clinically apparent.** (If associated with greater than 3 positive axillary lymph nodes, the internal mammary nodes are classified as pN3b to reflect increased tumor burden) |
| pN2 | Metastasis in 4 to 9 axillary lymph nodes, or in clinically apparent* internal mammary lymph nodes in the absence of axillary lymph node metastasis |
| pN2a | Metastasis in 4 to 9 axillary lymph nodes (at least one tumor deposit greater than 2.0 mm) |
| pN2b | Metastasis in clinically apparent* internal mammary lymph nodes in the absence of axillary lymph node metastasis |
| pN3 | Metastasis in 10 or more axillary lymph nodes, or in infraclavicular lymph nodes, or in clinically apparent* ipsilateral internal mammary lymph nodes in the presence of 1 or more positive axillary lymph nodes; or in more than 3 axillary lymph nodes with clinically negative microscopic metastasis in internal mammary lymph nodes; or in ipsilateral supraclavicular lymph nodes |
| pN3a | Metastasis in 10 or more axillary lymph nodes (at least one tumor deposit greater than 2.0 mm), or metastasis to the infraclavicular lymph nodes |
| pN3b | Metastasis in clinically apparent* ipsilateral internal mammary lymph nodes in the presence of 1 or more positive axillary lymph nodes; or in more than 3 axillary lymph nodes and in internal mammary lymph nodes with microscopic disease detected by sentinel lymph node dissection but not clinically apparent** |
| pN3c | Metastasis in ipsilateral supraclavicular lymph nodes |
| Distant metastasis (M) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| STAGE GROUPING | |||||||||||
| Stage 0 | Tis | N0 | M0 | Stage IIB | T2 | N1 | M0 | Stage IIIB | T4 | N0 | M0 |
| Stage I | T1* | N0 | M0 | T3 | N0 | M0 | T4 | N1 | M0 | ||
| Stage IIA | T0 | N1 | M0 | Stage IIIA | T0 | N2 | M0 | T4 | N2 | M0 | |
| T1* | N1 | M0 | T1* | N2 | M0 | Stage IIIC | Any T | N3 | M0 | ||
| T2 | N0 | M0 | T2 | N2 | M0 | Stage IV | Any T | Any N | M1 | ||
| T3 | N1 | M0 | |||||||||
| T3 | N2 | M0 | |||||||||
*Clinically apparent is defined as detected by imaging studies (excluding lymphoscintigraphy) or by clinical examination or grossly visible pathologically.
aClassification is based on axillary lymph node dissection with or without sentinel lymph node dissection. Classification based solely on sentinel lymph node dissection without subsequent axillary lymph node dissection is designated (sn) for “sentinel node,” e.g., pN0(i+) (sn).
bRT-PCR: reverse transcriptase/polymerase chain reaction.
**Not clinically apparent is defined as not detected by imaging studies (excluding lymphoscintigraphy) or by clinical examination.
*T1 includes T1mic
Note: Stage designation may be changed if post-surgical imaging studies reveal the presence of distant metastases, provided that the studies are carried out within 4 months of diagnosis in the absence of disease progression and provided that the patient has not received neoadjuvant therapy.
(American Joint Committee on Cancer: AJCC Cancer Staging Manual, pp 227–228, 6th edn. New York, Springer, 2002.)
Table 9. Medical contraindications to breast-conserving therapy
| Absolute |
| Pregnancy, because of contraindicated radiation therapy |
| Two or more cancers in different breast quadrants |
| Diffuse “malignant” microcalcifications |
| Prior radiation therapy to the breast area |
| Persistent surgical margins involved with cancer |
| Relative |
| Collagen vascular disease |
| Multiple gross cancers in one quadrant |
| Diffuse indeterminate calcifications |
| A large cancer in a small breast |
| Large pendulous breast, because of difficulties with radiation therapy |
(Adapted from Morrow M, Strom EA, Bassett LW, et al: Standard for breast conserving therapy in the management of invasive breast carcinoma. CA Cancer J Clin 52:277–300, 2002.)
An open surgical biopsy of a suspicious dominant mass should be performed with the same careful technique as lumpectomy for known cancer.19 Otherwise, re-excision of the area of the tumor bed is usually necessary.20 As many as 50% of women referred to a cancer center who had their cancer diagnosed by open surgical biopsy required re-excision of the area of the biopsy because the biopsy margins were positive (cut across cancer).20 Definitive diagnosis of cancer by fine-needle aspiration cytology or tissue core-needle biopsy histology should eliminate the need for most re-excisions if the open surgical biopsy follows National Surgical Adjuvant Breast and Bowel Project (NSAPB) technique for lumpectomy.19
Axillary lymph node clearance involves removal of the levels I,II and III lymph nodes (Table 10 shows the description of the levels of axillary lymph nodes). If the patient has known invasive cancer associated with pathological axillary nodes, then axillary lymph node dissection can be performed at the same time as her lumpectomy. Sentinel node biopsy (SNB) using the dual localization (radioactive isotope tracer and blue dye) approach has recently become the gold standard technique of evaluating the axilla in patients with clinically node-negative invasive breast cancer. THE SNB can be evaluated intraoperatively by frozen section or molecular analysis and immediate axillary node clearance is performed if the SNB is positive for malignancy. The technique of SNB however requires special equipment and a learning curve of experience for the surgeon (Fig. 4). Although the sentinel node biopsy is well-established,21, 22, 23 however, the technique is currently available in a limited number of breast centers worldwide. Advantages of the SNB include a lower risk of developing lymphedema (a major complication of axillary lymph node clearance that requires prolonged treatment, often with limited success) and shorter hospitalization.
Table 10. Description of the surgical levels of axillary lymph nodes
| Level I: | Lateral to the lateral border of the pectoralis minor muscle |
| Level II: | Under the insertion of the pectoralis minor, between the medial and lateral borders of the pectoralis minor muscle and the interpectoral (Rotter's) node |
| Level III: | Medial to the medial border of the pectoralis minor muscle, including the nodes designated as apical |
(Adapted from American Joint Committee on Cancer: AJCC Cancer Staging Manual, 6th edn, p 224. New York, Springer, 2002.)
Radiation therapy (irradiation) is an essential component of breast-conserving therapy and is usually performed in an oncologic radiation therapy center. The radiation oncologist directs the technique and dosage of the therapy, which is individualized to the particular medical circumstances of each breast cancer patient. Usually a total dose of whole breast irradiation of 4500–5000 centigrays is administered in fractions using opposed tangential fields. The course of irradiation is usually administered in daily fractions (5 days per week) for 3–6 weeks. A boost dose of irradiation to the tumor bed increases the target dosage to 6000–6500 centigrays. Careful individualized radiation therapy planning and modern equipment minimize the adverse effects of irradiation, particularly the uncommon but devastating long-term sequellae.24 Radiation therapy has been demonstrated to control the local recurrence of invasive breast cancer surgically treated with lumpectomy.14, 25
A state-of-the-art (as of 2002) comprehensive clinical review of breast-conserving therapy for invasive breast cancer, authored by multidisciplinary authorities, has been published.26 This work provides details and documentation for obstetrician-gynecologists in clinical practice who seek further information about breast-conserving therapy.
Modified Radical Mastectomy
Modified radical mastectomy (total mastectomy and axillary lymph node dissection) removes the entire breast, including the overlying skin and the axillary lymph nodes, usually levels I and II, en bloc. The major modification is the preservation of the pectoralis major muscle, which facilitates improved wound healing and, potentially, allows reconstruction. Modified radical mastectomy is performed for stages I and II breast cancer for which breast-conserving therapy is contraindicated (see Table 9), for selected cancers larger than stage II, and for patient preference when equal therapeutic results and breast cancer survival can be achieved with either breast-conserving therapy or modified radical mastectomy.
Breast Reconstruction
Most women who require or request mastectomy are candidates for breast reconstruction.16 This option should be presented to the patient when she is making her treatment choice. If she is interested in reconstruction, she should be referred, before scheduling her definitive surgery, to a plastic surgeon experienced in the techniques of both prosthetic reconstruction and autologous tissue reconstruction. Both prosthetic and autologous tissue reconstructions can be immediate (during the same operation as the modified racial mastectomy) or delayed (performed at a later date). Immediate or delayed breast reconstruction following conventional non-skin sparing mastectomy (NSSM) often results in prominent scars on the new breast and a paddle of skin that is of a different color and texture. Skin-sparing mastectomy (SSM) preserves most of the overlying skin during an immediate breast reconstruction (IBR) thus leading to a superior aesthetic outcome. It also reduces the need for contralateral breast adjustment in order to achieve symmetry. In selected cases, the nipple-areola complex can be also preserved. Breast reconstruction can be achieved using a breast prosthesis, the latissimus dorsi (LD) myocutaneous flap (usually plus a breast prosthesis) (Fig. 5; Fig. 6), deep inferior epigastric perforator (DIEP) free flap, transverse rectus abdominis myocutaneous (TRAM) flap, or superior/inferior gluteal artery perforator (S-GAP or I-GAP) free flaps. The choice of the reconstruction method depends upon the patient's body habitus, co-morbidity, smoking history, size and shape of her breasts, her preference and the surgeon's experience. TRAM is the most common autologous tissue reconstruction. Data from 1994 to 1995 reveal that only 8.3% of mastectomy patients had breast reconstruction.16 This low utilization is influenced by: (1) patient age and comorbidity; (2) income; (3) geographic location; (4) type of hospital; and (5) tumor size (for example, women aged 50 or younger had a more than four-fold higher rate of reconstruction than older women); (6) the need for postmastectomy RT and (7) the availability of surgical expertise.
RADIATION THERAPY
Radiation therapy (RT) is an essential component of breast-conserving surgery (BCS) and has been described. Indications for post-mastectomy RT include involvement of more than three nodes, positive surgical margins and/or tumors larger than 5 cm. Although radiation therapy is effective in local control of breast cancer, the long-term (10-year and 20-year) morbidity especially ischemic heart disease remains a concern.24 Modern techniques of accurate planning and radiation delivery will reduce long-term complications. Partial breast irradiation which can be given intra-operatively or post-operatively through special catheters following BCS seems to be a promising alternative to whole-breast RT in selected cases and is currently under investigation.
Radiation therapy, or irradiation, is a common component of the treatment of stages III and IV, often called advanced breast cancer. The exact irradiation technique and dosage is individualized to each case. Irradiation of the axilla should be avoided whenever possible in view of the high rate of complications such as lymphedema and restriction of shoulder movement. Radiation therapy can be also used to treat local recurrences and metastases such as bony and brain deposits.
ADJUVANT SYSTEMIC THERAPY
Adjuvant systemic therapy for breast cancer began with various surgical hormonal ablations, such as oophorectomy and adrenalectomy. In the 1960s, various single-agent chemotherapies were used and reported. Clinical trails were performed in the 1970s. Multidrug chemotherapy, often called polychemotherapy, was found to be more effective than the single-drug therapies.17, 27, 28
Chemotherapy
In 2005, the Early Breast Cancer Trialists Collaborative Group updated their meta-analysis of 194 unconfounded clinical trials of multidrug therapies.29 Combinations of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF), and later of cyclophosphamide, doxorubicin (Adriamycin is the proprietary name of doxorubicin), and 5-fluorouracil (CAF) achieved significant reductions in the 5 year breast cancer recurrence rates and the 15 year mortality rates. Allocation to about 6 months of anthracycline-based polychemotherapy (e.g., with FAC or FEC) reduces the annual breast cancer death rate by about 38% (SE 5) for women younger than 50 years of age when diagnosed and by about 20% (SE 4) for those of age 50-69 years when diagnosed, largely irrespective of the use of tamoxifen and of oestrogen receptor (ER) status, nodal status, or other tumour characteristics. Such regimens are significantly (2p = 0.0001 for recurrence, 2p < 0.00001 for breast cancer mortality) more effective than CMF chemotherapy.29 Anthracyline and/or taxane based regimens are the most frequently used chemotherapy protocols in the adjuvant setting. Taxanes are particularly indicated for node positive breast cancer.29
Table 11 lists classifications of systemic therapeutic agents with examples. The recommendations for adjuvant therapy for node-negative breast cancer are given in Table 12. The recommendations for adjuvant therapy for node-positive breast cancer are given in Table 13. Molecular signatures such as Oncotype DX and Mammaprint are increasingly being used to refine chemotherapy recommendations in premenopausal women with node negative ER+ve breast cancer smaller than 2 cm. For example a recurrence score of 18 or less (range 0–100) indicates that the potential benefit from adjuvant chemotherapy is too small to justify its use in such cases.
Table 11. Systemic chemotherapeutic agents in use for breast cancer with examples
| Antimetabolites |
| Methotrexate |
| 5-Fluorouracil |
| Alkylating agents |
| Cyclophosphamide |
| Antitumor antibiotics |
| Doxorubicin (an anthracycline) |
| Mitotic spindle inhibitors (taxanes) |
| Paclitaxel |
| Docetaxel |
(Adapted from Russell CR: Adjuvant systemic therapy for breast cancer. In Hindle WH, (ed): Breast Care, a Clinical Guidebook for Women's Health Care Providers. New York, Springer, 1999.)
Table 12. Recommendations for Adjuvant Therapy for Node-Negative Breast Cancer
Condition | Standard therapy | Consideration |
| <1 cm, ER+, low histologic grade | Tamoxifen or none | |
| 1–2 cm, ER+, low histologic grade | ||
| Premenopausal | Chemotherapy and/or tamoxifen | Aromatase inhibitors can be considered after ovarian ablation |
| Postmenopausal | Aromatase inhibitors or tamoxifen | |
| >2 cm or ER− | ||
| Premenopausal | ||
| ER+ | Chemotherapy followed by tamoxifen | Aomatase inhibitors can be considered after ovarian ablation |
| ER− | Chemotherapy | |
| Postmenopausal | ||
| ER+ | Aromatase inhibitors or tamoxifen | Chemotherapy should be considered |
| ER− | Chemotherapy |
(Russell CR: Adjuvant systemic therapy for breast cancer. In Hindle WH (ed): Breast Care, a Clinical Guidebook for Women's Health Care Providers. New York, Springer, 1999.)
NB. Herceptin for 18 months should be considered in all patients with HER-2 positive tumors who also require chemotherapy.
Table 13. Recommendations for adjuvant therapy for node-positive breast cancer
| Estrogen receptor states | Standard therapy | Consideration |
| Premenopausal | ||
| ER+ | Chemotherapy followed by tamoxifen | Aromatase inhibitors after ovarian ablation is an alternative to tamoxifen |
| ER− | Chemotherapy | |
| Postmenopausal | ||
| ER+ | Chemotherapy and/or aromatase inhibitors or tamoxifen | Consider extended adjuvant endocrine therapy |
| ER− | Chemotherapy |
(Russell CR: Adjuvant systemic therapy for breast cancer. In Hindle WH, (ed): Breast Care, a Clinical Guidebook for Women's Health Care Providers. New York, Springer, 1999.)
NB. Herceptin should be considered in all patients with HER-2 positive breast cancer requiring chemotherapy.
Breast cancer chemotherapy is complex and is being continuously updated. It is critical that the chemotherapy be individualized and adapted to each patient's particular circumstances. The choice of specific medications used and the dosage and treatment schedule are the purview of the patient's medical oncologist. The actual treatment plan varies with the clinical experience and familiarity of the medical oncologist with a multitude of different chemotherapy options. Furthermore, if the patient's medical situation qualifies, then current chemotherapy clinical trials are often available under local, regional, or national institutional guidelines. The role of the obstetrician-gynecologist is that of supportive follow-up and availability for the patient's consultation.
Hormonal Therapy
Tamoxifen is the most commonly used hormonal therapy. Unless the patient has had a hysterectomy, an obstetrician-gynecologist is directly involved in the follow-up of a woman using tamoxifen. Multiple studies have shown that tamoxifen has an estrogenic effect on the endometrium, with more than a two-fold increased incidence of endometrial carcinoma in women using tamoxifen. Therefore, women using tamoxifen need to be followed-up by the same guidelines as women using unopposed estrogen therapy. Any abnormal uterine bleeding should urgently be investigated and an endometrial biopsy performed for diagnosis. If there is no abnormal uterine bleeding, a woman using tamoxifen should be followed-up in the routine manner with annual mammograms, clinical breast examinations, and pelvic examinations. It is important that a woman using tamoxifen know that the occurrence of any abnormal uterine bleeding is a critical indication for an urgent appointment with her obstetrician-gynecologist.
Oncologists for women with DCIS, node-negative invasive carcinoma, node-positive invasive carcinoma, and metastatic invasive carcinoma often prescribe tamoxifen 20 mg daily as a single or divided dose.30, 31, 32 Recent clinical trials have shown that aromatase inhibitors (anastrozole, letrozole and exemestane) are superior to Tamoxifen in postmenopausal women with ER+ invasive breast cancer in adjuvant, neoadjuvant and metastatic settings.33 Adjuvant hormonal therapy is usually given for 5 years, because there is evidence of no further benefit after 5 years of treatment.30 In postmenopausal women with ER+ invasive breast cancer who have already completed 5 years of tamoxifen, adjuvant endocrine therapy may be extended with an aromatase inhibitor such as letrozole for further 4 years especially if the tumor was node positive (extended adjuvant).33 Furthermore, switching endocrine therapy from Tamoxifen to an aromatase inhibitor after 2–3 years seems to be superior to taking tamoxifen alone for 5 years in such patients.33 Tamoxifen may be also indicated for the prevention of breast cancer in selected women at high risk of developing ER+ breast cancer and women with identified BRCA-1 and/or BRCA-2 genetic mutations.34, 35 However, BRCA gene mutations probably account for only 5% of all breast cancers.36
The nonneoplastic side-effects of tamoxifen compared with placebo therapy are given in Table 14. Tamoxifen therapy has additional benefits of reducing postmenopausal osteoporotic bone loss, reduction of hip, spine, and wrist fractures, and reduction in cholesterol and low-density lipoproteins. Compared with tamoxifen, aromatase inhibitors are less likely to cause endometrial cancer and DVT but are more likely to cause bone fractures (due to increased bone loss) and hypercholesterolemia.37 There is insufficient evidence to support the routine use of adjuvant endocrine therapy in patients with pure DCIS.38
Table 14. Side-effects of tamoxifen compared with placebo therapy
| Percent of patients reporting the symptom | ||
| Toxicity | Placebo (n = 1439) | Tamoxifen (n = 1422) |
| Hot flashes | 46.2 | 62.4* |
| Fluid retention | 28.6 | 30.9* |
Vaginal discharge | 14.2 | 28.4* |
Irregular menses | 17.6 | 23.6* |
Nausea | 22.6 | 23.3* |
Skin rash | 14.3 | 17.7* |
Diarrhea | 12.9 | 9.8* |
Vascular thrombi | 0.2 | 1.5* |
*Statistically significant difference.
(Russell CR: Adjuvant systemic therapy for breast cancer. In Hindle WH, (ed): Breast care, a Clinical Guidebook for Women's Health Care Providers. New York, Springer, 1999.)
Tamoxifen belongs to a group of medications called selective estrogen receptor modulators (SERMs). Other SERMs undergoing investigation are droloxifene, idoxifene, raloxifene, tibolone, and toremifene. Only tamoxifen is Food and Drug Administration (FDA)-approved for the treatment of breast cancer. Raloxifene is FDA-approved for the prevention of osteoporosis. Preliminary investigations suggest that raloxifene is as effective as tamoxifen for the prevention of both DCIS and invasive breast cancer. In addition, it appears that raloxifene does not have an estrogenic effect on the endometrium, as does tamoxifen. Breast cancer clinical trials of raloxifene are underway, and if the results are as conclusive as those for tamoxifen, then FDA approval of raloxifene for the treatment of breast cancer will be sought.
Biological Therapy
HER-2 over-expression is implicated in the pathogenesis of breast cancer and represents a key marker and determinant of patient outcome. Herceptin (Trastuzumab, TZ) is a recombinant humanized monoclonal antibody which targets HER-2. Introduction into clinical practice has significantly improved the natural history of HER-2 over-expressing tumors and has altered the standard of care for these women. The clinical utility of TZ was first established in the management of HER-2 over-expressing metastatic breast cancer (MBC), with improvements recognized in both the quality and quantity of life. Prospective randomized controlled trials have consistently demonstrated the efficacy of TZ for early breast cancer (EBC) in the adjuvant setting with significant improvements in disease free and overall survival. Emerging roles for TZ include neo-adjuvant therapy and the treatment of progressive disease. TZ is well-tolerated and safe, however, associated cardiac dysfunction remains a significant clinical concern. Appropriate patient selection and monitoring for cardiac dysfunction are required.39
Other examples of biological therapy include bevacizumab and lapatinib. The former is a recombinant humanized monoclonal antibody against vascular endothelial growth factor and has been approved as first-line therapy for metastatic breast cancer (mBC). Lapatinib is a dual inhibitor of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER-2) tyrosine kinases. It was approved by the FDA in 2007. It is indicated for use in combination with capecitabine for the treatment of patients with advanced breast cancer or metastatic breast cancer (MBC) whose tumors overexpress HER-2 (ErbB2) and who have received previous treatment that included an anthracycline, a taxane, and herceptin.
DUCTAL CARCINOMA IN SITU
Ductal carcinoma in situ (DCIS) is a heterogeneous disease, in terms of its radiological characteristics, histological morphology and molecular attributes. This diversity is reflected in its natural history and influences optimal treatment strategy. Before the advent and use of mammography, DCIS accounted for approximately 2% of the diagnosed breast malignancies. In 2007, the incidence of diagnosed DCIS is estimated to be somewhat more than 25% of that of invasive carcinoma.1 In a breast center with a large volume of screening mammography, DCIS can account for more than 30% of the diagnosed malignancies. Plates 17 and 18 show the histological features of low- and high-grade DCIS.
Until the acceptance of breast conserving therapy for invasive cancer, the treatment of DCIS was total mastectomy without axillary node dissection. Adjuvant chemotherapy is not indicated for DCIS. Most cases of localized DCIS are currently treated with lumpectomy (with tumor-free margins) followed by adjuvant radiation therapy.12, 25 Extensive and/or multicentric lesions are treated with total mastectomy (+/- immediate breast reconstruction) combined with the SNB in case there is an occult invasive malignancy. The SNB may be also considered in selected high risk cases treated with BCS (e.g., large high grade lesions +/- mass on imaging).38 Tamoxifen may be indicated in selected cases following BCS.34, 37, 38. Without RT following BCS, the risk of local recurrence is approximately 1% per year; however, approximately 50% of the recurrences are invasive carcinoma. There is no consensus as to the optimum treatment of DCIS, particularly for low-grade lesions less than 1 cm and those with clear surgical margins. Adjuvant RT may be safely omitted in selected cases of BCS for small low-grade lesions treated with adequate local excision.38 The details and specific treatment of the spectrum of DCIS continues to be debated by authorities in the field. A state-of-the-art (as of 2008), comprehensive, clinical review of the management of DCIS authored by leading experts has been recently published.38. The authors of this report highlight that a significant proportion of DCIS lesions behave in a clinically benign fashion and do not progress to invasive disease and therefore, a reliable identification of these patients could allow treatment to be less radical or safely omitted. Management should be tailored to the individual within the context of a multidisciplinary team. Approaches such as biological profiling and molecular analysis represent an opportunity to improve our understanding of the tumor biology of this condition and rationalize its treatment.38
CHEMOPREVENTION AND SURVEILLANCE
Based on an almost 50% decreased incidence of contralateral breast cancer during the study period demonstrated in the NSABP clinical trial,31 a prevention trial for women at high risk for breast cancer was undertaken. The results of the NSABP P-1 prevention trial documented a 45% reduction in clinically diagnosed breast cancer for women treated with tamoxifen 20 mg daily for 5 years.35 The incidence of estrogen receptor-positive cancers was reduced by 67%.33 Long-term clinical trails, such as for 15–20 years, will be required to confirm that the cancers were indeed prevented; that is, they did not occur. It is possible that the cancers did not become large enough to be diagnosable during the 5 years of tamoxifen therapy. The fact that most breast cancers are present for many years before the time of mammographic and clinical diagnosis (see Fig. 3) makes the expected outcome of long-term studies uncertain. Knowing the endpoint of disease-specific survival would be the most informative. Raloxifene was also reported to decrease the incidence of breast cancer as a secondary end-point in clinical trials. In the Study of Tamoxifen and Raloxifene (STAR) trial, postmenopausal women at increased risk of breast cancer received either tamoxifen (20 mg/day) or raloxifene (60 mg/day) over 5 years.40 There were an equal number of cases of invasive breast cancer in women assigned to tamoxifen and raloxifene. There were fewer cases of DCIS in the tamoxifen group than in the raloxifene group. There were fewer cases of uterine cancer and thromboembolic events with raloxifene than with tamoxifen. There is no evidence however that chemoprevention with tamoxifen or raloxifen improves survival and therefore their overall use for this indication has been limited. Aromatase inhibitors haven been recently reported to decrease the incidence of ER +ve cancer in the contralateral breast by 75% (greater reduction than tamoxifen). There is no evidence however that chemoprevention with tamoxifen or raloxifene improves survival and therefore their overall use for this indication has been limited. Aromatase inhibitors have been recently reported to decrease the incidence of ER +ve cancer in the contralateral breast by 75% (greater reduction than tamoxifen). Anastrozole and tamoxifen as chemopreventative agents are being currently investigated in a randomized controlled trial (IBIS II).
There are data to support the importance of routine annual obstetrician-gynecologists follow-up, including mammography, clinical breast examination, and pelvic examination, for women with breast cancer. Postmenopausal uterine bleeding should be expeditiously evaluated because, particularly for women using tamoxifen, the possibility of endometrial cancer is a significant concern.
ESTROGEN AND ESTROGEN/PROGESTIN THERAPY
Because the breasts are a component of the female reproductive system and their development and physiologic function (lactation) requires the coordinated and balanced action of female (and other) hormones, controversy continues as to the role of estrogen and progesterone in the initiation and promotion of breast cancer. Furthermore, with women's fear of breast cancer and the media's dramatization of breast cancer-related news, obstetrician-gynecologists will be called on to respond and put seemingly urgent latest news in perspective.
Human estrogen was identified in 1929 and used clinically in 1938. Conjugated equine estrogen (CEE), which is the most commonly prescribed estrogen in the United States, was marketed in 1942; subsequently, more than 3700 studies have reported on CEE. Figure 7 graphically summarizes the risk estimates of breast cancer for ever-users compared with never-users of estrogen replacement therapy (ERT).41 Figure 8 is a similar graph of risk estimates of breast cancer comparing ever-users with never-users of hormone replacement therapy (HRT).41 Figure 9 summarizes the relative risks for breast cancer deaths with HRT.42 Although there are occasional outliers, most of the mean values have wide confidence intervals, and few reach epidemiologic statistical significance. No obvious trends are apparent. The results of The Women's Health Initiative Study, a randomized controlled primary prevention trial (planned duration, 8.5 years) in which 16608 postmenopausal women aged 50–79 years with an intact uterus at baseline were recruited by 40 US clinical centers in 1993–1998, were published in 2002. The study showed that the use of HRT was associated with an increased risk of developing breast cancer. Surprisingly however, it demonstrated that HRT had no protective effect on the cardiovascular system but to the contrary, it could even promote the development of a cardiovascular event.43 Therefore it is currently recommended that HRT use is avoided when possible and it is used in lowest possible dose for the shortest possible period when indicated.
CONCLUSION
Obstetrician-gynecologists play an important role in the detection of breast abnormalities, the diagnosis of breast cancer, and the continuing follow-up of women treated for breast cancer. They should see that breast cancer screening for women aged 40 years and older includes annual mammograms and clinical breast examinations. Furthermore, obstetrician-gynecologists should be a readily available source to their patients of accurate information and caring counsel about breast cancer and breast-related concerns.
If a woman presents with a dominant breast mass that remains undiagnosed or does not respond to treatment, she should be expeditiously referred to a breast specialist for triple assessment (clinical examination, breast imaging and needle biopsy). The American College of Obstetricians and Gynecologists guidelines for referral are: (1) explain to the patient that she needs further care; (2) provide names of qualified physicians from whom the patient can receive care; (3) answer the patient's questions; and (4) document these steps and include a detailed description of the clinical findings in the medical record.44 This is sound advice.
All women with a newly diagnosed breast cancer should be referred to and managed in designated breast cancer centers that adopt a multi-disciplinary and evidence-based approach to management.
COLOR PLATES
REFERENCES
Jemal A, Siegel R, Ward E et al: Cancer statistics, 2007. CA Cancer J Clin 57(1):43-66, 2007 |
|
Li CI, Daling JR: Changes in breast cancer incidence rates in the United States by histologic subtype and race/ethnicity, 1995 to 2004. Cancer Epidemiol Biomarkers Prev 16(12):2773-80, 2007 |
|
American Cancer Society: Cancer Facts and Figures 2003. Atlanta, GA, American Cancer Society, 2003 |
|
Michaelson JS, Halpern E, Kopans DB: Breast cancer: Computer simulation method for estimating optimal intervals for screening. Radiology 212:551-560, 1999 |
|
Tabar L, Vitak B, Chen HH et al: The Swedish Two-County Trial twenty years later. Updated mortality results and new insights from long-term follow-up Radiol Clin North Am 38:625-651, 2000 |
|
Ries L, Henson D, Harras A: Survival from breast cancer according to tumor size and nodal status. Surg Oncol Clin North Am 3:35-50, 1994 |
|
Michaelson JS, Silverstein M, Wyatt J et al: Predicting the survival of patients with breast carcinoma using tumor size. Cancer 95:713-723, 2002 |
|
Baker LH: Breast cancer detection demonstration project: Five-year summary report. Cancer 32:194-225, 1982 |
|
Wertheimer MD, Costanza ME, Dodson TF et al: Increasing the effort toward breast cancer detection. JAMA 255:1311-1315, 1986 |
|
Smith RA, Cokkinides V, Eyre HJ: American Cancer Society guidelines for the early detection of cancer, 2003. CA Cancer J Clin 53:27-43, 2003 |
|
American College of Radiology (ACR): Illustrated Breast Imaging Reporting and Data System (BI-RADStm), 3rd edn. Reston, VA, American College of Radiology, 1998 |
|
Fisher B, Bauer M, Margolese R et al: Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with and without radiation in the treatment of breast cancer [NSABP B-06]. N Engl J Med 312:665-673, 1985 |
|
Fisher B, Jeong J-H, Anderson S et al: Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation [NSABP B-04]. N Engl J Med 347:567-575, 2002 |
|
Fisher B, Redmond C, Fisher ER et al: Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with and without radiation [NASBP B-04]. N Engl J Med 312:674-681, 1985 |
|
NIH Consensus Conference: Treatment of early-stage breast cancer JAMA 265:391, 1991 |
|
Patani N, Mokbel K: Oncological and aesthetic considerations of skin-sparing mastectomy. Breast Cancer Res Treat 111(3):391-403, 2008 |
|
Russell CA: Adjunctive systemic therapy for breast cancer. In: Hindle W (ed): Breast Care, A Clinical Guidebook for Women's Primary Health Care Providers. New York, Springer, 1999 |
|
Fisher B, Brown A, Mamounas E et al: Effect of preoperative chemotherapy on local regional disease in women with operable breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15:2483-2493, 1997 |
|
Margolese R, Poisson R, Shibata H et al: The technique of segmental mastectomy (lumpectomy) and axillary dissection: A syllabus from the National Surgical Adjuvant Breast Project workshops. Surgery 102:828-34, 1987 |
|
Cox CE, Reintgen DS, Nicosia SV et al: Analysis of residual cancer after diagnostic breast biopsy: An argument for fine-needle aspiration cytology. Ann Surg Oncol 2:201-206, 1995 |
|
Tafra L, Lannin DR, Swanson MS et al: Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg 233:51-59, 2001 |
|
Singh Ranger G, Mokbel K: The evolving role of sentinel lymph node biopsy for breast cancer. Eur J Surg Oncol Rev 29(5):423-5, 2003 |
|
Veronesi U, Paganelli G, Viale G et al: Sentinel-lymph-node biopsy as a staging procedure in breast cancer: update of a randomised controlled study. Lancet Oncol 7(12):983-90, 2006 |
|
Early Breast Cancer Trialists' Collaborative Group: Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: An overview of the randomised trials Lancet 355:1757-1770, 2000 |
|
Fisher B, Anderson S, Bryant J et al: Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347(16):1233-41, 2002 |
|
Morrow M, Strom EA, Bassett LW et al: Standard for breast conserving therapy in the management of invasive breast carcinoma. CA Cancer J Clin 52:277-300, 2002 |
|
Cole BF, Gelber RD, Gelber S et al: Polychemotherapy for early breast cancer: an overview of the randomised clinical trials with quality-adjusted survival analysis. Lancet 358:277-286, 2001 |
|
5th International Conference on Adjuvant Therapy of Primary Breast Cancer: St. Gallen, Switzerland. Anticancer Drugs 6:1-96, 1995 |
|
Early Breast Cancer Trialists' Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 365:1687–1717, 2005 |
|
Fisher B, Dignam J, Bryant J et al: Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors [NSABP B-14]. J Natl Cancer Inst 88:1529-1542, 1996 |
|
Fisher B, Costantino J, Redmond C et al: A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor positive tumors. N Engl J Med 320:479-484, 1989 |
|
Fisher B, Dignam J, Bryant J et al: Five versus more than five years of tamoxifen for lymph node-negative breast cancer: Updated findings from the National Surgical Adjuvant Breast and Bowel project B-14 randomized trial. J Natl Cancer Inst 93:684-690, 2001 |
|
Herold CI, Blackwell KL: Aromatase inhibitors for breast cancer: proven efficacy across the spectrum of disease.Clin Breast Cancer 8(1):50-64, 2008 Review. |
|
Fisher B, Land S, Mamounas E et al: Prevention of invasive breast cancer in women with ductal carcinoma in situ: An update of the National Surgical Adjuvant Breast and Bowel Project [NSABP B-24] experience. Semin Oncol 28:400-418, 2001 |
|
Fisher B, Costantino JP, Wickerham DL et al: Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 90:1371-1388, 1998 |
|
Easton DF, Bishop DT, Ford D et al: Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. Am J Hum Genet 52:678-701, 1993 |
|
Fisher B, Dignam J, Wolmark N et al: Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet 353:1993-2000, 1999 |
|
Patani N, Cutuli B, Mokbel K: Current management of DCIS: a review. Breast Cancer Res Treat 111(1):1-10, 2008 |
|
Patani N, Mokbel K: Herceptin and breast cancer: An overview for surgeons. Surg Oncol. In Press, 2008 |
|
Vogel VG: The NSABP Study of Tamoxifen and Raloxifene (STAR) trial. Expert Rev Anticancer Ther 9(1):51-60, 2009 |
|
Bush TL, Whiteman M, Flaws JA: Hormone replacement therapy and breast cancer: a qualitative review. Obstet Gynecol 98:498-508, 2001 |
|
Nanda K, Bastian LA, Schulz K: Hormone replacement therapy and the risk of death from breast cancer: a systematic review. Am J Obstet Gynecol 186:325-334, 2002 |
|
Rossouw JE, Anderson GL, Prentice RL et al: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 288(3):321-33, 2002 |
|
American College of Obstetricians and Gynecologists: ACOG Committee on Gynecologic Practice, Committee Opinion. Role of the obstetrician-gynecologist in the diagnosis and treatment of breast disease American College of Obstetricians and Gynecologists, Washington DC, No. 186, September 1997 |