Amniotic Fluid: Physiology and Assessment
Authors
INTRODUCTION
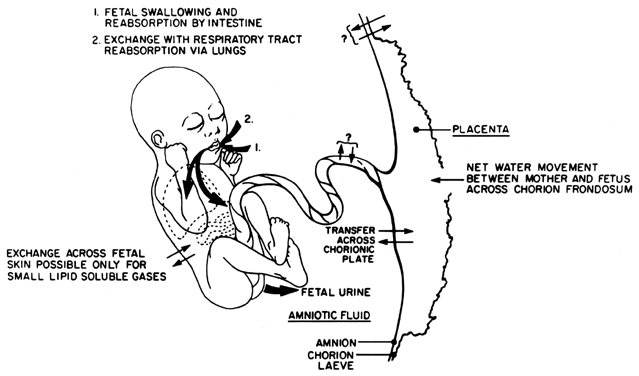
Amniotic fluid is vital to the well-being of the fetus. It cushions the fetus from injury, helps prevent compression of the umbilical cord, and allows room for it to move and grow. In addition, its bacteriostatic action helps prevent infection of the intra-amniotic environment. The quantity of amniotic fluid at any time in gestation is the product of water exchange between the mother, fetus, and placenta, and is maintained within a relatively narrow range. Disorders of this regulatory process can lead to either polyhydramnios or oligohydramnios, in which too much or too little fluid exists, respectively. These disorders may result from abnormal fetal or maternal conditions and, conversely, may be responsible for alterations of fetal well-being as well. With the advent of real-time ultrasonography, assessment of amniotic fluid has been possible, resulting in earlier recognition of abnormal conditions and possible intervention. Because precise quantification of amniotic fluid volume is not possible with ultrasonography, various techniques for both qualitative and semiquantitative assessment have been proposed. This chapter reviews the dynamics of amniotic fluid volume (Fig. 1), discusses the causality and perinatal significance of volume disturbances, and reviews the techniques of ultrasonographic assessment of amniotic fluid volume, as well as their role in the antenatal testing of high-risk fetuses.
AMNIOTIC FLUID DYNAMICS
Amniotic Fluid Production
In the first half of pregnancy, amniotic fluid is derived from fetal and possibly maternal compartments. Water and solutes freely traverse fetal skin and may diffuse through the amnion and chorion as well.1 Thus amniotic fluid in early gestation is a dialysate that is identical to the fetal and maternal plasma, but with a lower protein concentration. Active secretion of fluid from the amniotic epithelium had been previously suggested to play a role in early amniotic fluid formation, but this has not been demonstrated.
By the second trimester, the fetal skin becomes keratinized, making it impermeable to further diffusion. At this time, a fetus contributes to amniotic fluid volume and composition almost exclusively through urination. Urine has been observed in the fetal bladder as early as 11 weeks transabdominally2 and 9 weeks transvaginally.3 Because fetal urine is hypotonic (80–140 mOsm/ liter), it results in progressively hypotonic fluid (250–260 mOsm/liter near term) that contains increasing concentrations of urea, uric acid, and creatinine as the fetal kidneys mature. By term, a fetus produces on average from 500 to 700 ml/day with a slight decline in hourly fetal urine production after 40 weeks' gestation.4, 5, 6
Amniotic Fluid Elimination
Amniotic fluid is eliminated by at least three mechanisms. The primary source of elimination is through fetal swallowing, which has been observed as early as 16 weeks.7 Studies using radiolabeled red blood cells and radioactive colloid estimate that, on average, a fetus swallows from 200 to 450 ml/day at term, removing 50% of the amniotic fluid produced through fetal urination. This fluid is absorbed through the fetal gastrointestinal system and is either recycled through the kidneys or is transferred to the maternal compartment through the placenta.
A second, more debatable means of amniotic fluid removal may be by the respiratory tract. Fetal respiratory activity has been observed as early as 11 weeks' gestation.8 At term, inspiratory flow in the fetus is approximately 200 ml/kg/day, up to 600–800 ml/day.9 Because amniotic fluid is more hypotonic than fetal plasma, it is postulated that exposure of amniotic fluid to the fetal alveolar capillary bed results in net movement of water from the amniotic cavity into the fetus. Although radioisotopes have been discovered in fetal lungs after intra-amniotic instillation, this quantity has been small and inconsistent,2 leading investigators to question the actual contribution of fetal respiration to amniotic fluid removal. In fact, surface-active phospholipids originating from the fetal alveoli are found in the amniotic cavity, leading to suggestions that the fetal lungs may actually be a net contributor to amniotic fluid volume.
Amniotic fluid may also potentially be removed by continuous bulk flow (i.e., via hydrostatic and oncotic forces). Exchange of fluid may take place at the chorionic plate, where exposure of the relatively hypotonic amniotic fluid to the fetal surface of the placenta may lead to net reabsorption of water by the fetus (up to 80 ml/day). Transport across the amnion may occur through intercellular channels between amniotic epithelial cells and may be modulated by amniotic fluid prolactin levels.10 Hebertson and colleagues provided presumptive evidence for the regulatory role of the amniotic epithelium in the transport of fluid. They observed ultrastructural changes in the amnion of pregnancies complicated by disorders of amniotic fluid volume.11 Whether these changes reflect a causative role in these disorders or rather a response to long-standing fluid imbalance remains to be determined.
A final, perhaps underestimated, pathway for volume regulation may occur within the placenta itself. The large surface area of the fetal capillary/ intervillous interface could magnify small osmolar gradients between a mother and fetus, resulting in large volumes of net water transfer.12 Exchange of water at this level would influence fetal intravascular volume and potentially affect renal blood flow and urine production.
In addition to bulk flow of fluid, which occurs through pathways that are both phasic (micturition and swallowing) and nonphasic (mediated by hydrostatic and oncotic gradients), there is also bidirectional flow of water between the amniotic and maternal compartments.12, 13 This process occurs by diffusion, but with no net change in fluid volume. At term, water may leave the amniotic cavity at a rate of 400–500 ml/hour by diffusion plus bulk flow.14
NORMAL AMNIOTIC FLUID VOLUME
Amniotic fluid volume is most predictable in the first half of pregnancy, when it correlates with fetal weight. This may relate to the predominant contribution of fetal skin dialysis to amniotic fluid volume between 8 and 20 weeks. At 12 weeks' gestation, the average volume is 60 ml.2 By 16 weeks, when genetic amniocentesis is often performed, the mean volume is 175 ml.2, 15 From 20 weeks on, there is greater variance of amniotic fluid volume. Based on numerous studies using dye or para-aminohippurate dilution, radioactive isotopes, and actual collection of amniotic fluid at amniotomy, it has been determined that amniotic fluid volume increases steadily throughout pregnancy to a maximum of 400–1200 ml at 34–38 weeks; however, wide variation does exist.15, 16, 17 Despite large fluxes of fluid between the various compartments near term (500–700 ml/day through urine; 200–450 ml/day through deglutition), the net increase of amniotic fluid is only 5–10 ml/day in the third trimester. After 38 weeks, fluid volume declines by approximately 125 ml/week, to an average volume of 800 ml at 40 weeks.15, 16, 17 After 43 weeks, this volume is reduced to 250 ml.16 In some instances, this reduction may possibly reflect a shift of cardiac output away from the kidneys as a result of a relative uteroplacental insufficiency. Figure 2 provides approximate volumes at various gestational ages, based on a compilation of 12 published studies of amniotic fluid volumes.15
POLYHYDRAMNIOS
Incidence and Origin
Polyhydramnios, or hydramnios, is defined as an excessive volume of amniotic fluid relative to the gestational age. Polyhydramnios may be acute or chronic. Acute polyhydramnios is usually a fulminant second-trimester process, with fluid accumulating rapidly over a period of a few days.18 Chronic polyhydramnios has a more gradual onset and course, often presenting in the third trimester. The incidence varies, depending on whether the diagnosis is clinical or sonographic. Overall, polyhydramnios complicates approximately 0.3–1.6% of all pregnancies.18, 19, 20, 21, 22 Chronic polyhydramnios is more frequent, exceeding the incidence of acute polyhydramnios by a 50: 1 ratio.18
Risk factors for polyhydramnios may be broadly divided into maternal, fetal, placental and idiopathic origins (Table 1).
Table1. Risk factors for hydramnios
Maternal conditions | Isoimmunization |
Diabetes mellitus | |
Placental conditions | Chorioangioma |
Circumvallate placenta | |
Fetal conditions | |
Multiple gestations | Twin-to-twin transfusion syndrome |
Gastrointestinal | Esophageal atresia, duodenal or jejunal atresia, annular pancreas, midgut volvulus, diaphragmatic hernia, omphalocele, gastroschisis |
CNS lesions | Anencephaly, hydrocephalus, encephalocele, spina bifida, microcephaly, hydranencephaly |
Skeletal malformations | Arthrogryposis multiplex, osteogenesis imperfecta, thanatophoric dysplasia |
Fetal tumors | Cystic adenomatoid malformation of the lung, sacrococcygeal teratoma, cervical teratoma |
Cardiac disease | Severe congenital heart disease, fetal arrhythmias |
Genetic disorders | Down syndrome, trisomy 13 and 18, Pena-Shokeir syndrome, multiple congenital anomalies, myotonia dystrophica |
Fetal renal and endocrine disorders | Vasopressin insufficiency |
Hematologic disorders | Homozygous α-thalassemia, fetomaternal hemorrhage |
Intrauterine infections | Rubella, syphilis, toxoplasmosis, parvovirus |
Miscellaneous | Nonimmune hydrops fetalis, fetal retroperitoneal fibrosis |
Idiopathic |
(Modified from Cardwell MS: Polyhydramnios: A review. Obstet Gynecol Surv 42:612, 1987. Copyright by Williams & Wilkins, 1987)
Diabetes mellitus is the most common maternal factor, occurring in approximately 25% of cases.18 The exact mechanism for polyhydramnios with diabetes is unclear. It may represent fetal polyuria secondary to fetal hyperglycemia. However, van Otterlo and colleagues, measuring fetal urinary output by ultrasonography, found no increase in urine output in 12 of 13 diabetic pregnancies complicated by polyhydramnios.5 Alternatively, fetal glycosuria may lead to an increase in amniotic fluid osmolality, resulting in water transfer from the fetal compartment to maintain osmolar equilibrium. Pedersen, however, found no association between amniotic fluid glucose concentration and volume.23
Isoimmunization is another, albeit decreasing, cause of polyhydramnios. The proposed inciting mechanism is extramedullary hematopoiesis in response to fetal anemia, which results in portal hypertension and hypoalbuminemia. The decrease in colloid oncotic pressure, as well as hydrostatic venous engorgement, leads to extravasation of fluid into the interstitium of the placenta.24 How this extravascular fluid results in hydramnios is unclear. The extracellular fluid could possibly be transferred across the placenta and membranes into the amniotic cavity. Alternatively, the interstitial fluid in the placenta could perhaps interfere with water transfer between the fetal and maternal compartments, resulting in fetal volume overload, polyuria, and ultimately polyhydramnios.
Fetal conditions have been observed in approximately 20% of polyhydramnios cases.18 Fetal malformations of the central nervous system (CNS) comprise almost 50% of fetal anomalies, with anencephaly being the most common.20 The postulated mechanisms for polyhydramnios due to CNS malformations include centrally-mediated reduction in fetal swallowing,25 fetal polyuria resulting from insufficient production of vasopressin from the fetal pituitary,26 and transudation of fluid across the uncovered meninges.24 Gastrointestinal anomalies constitute the second leading structural fetal cause. Any gastrointestinal obstruction proximal to the ligament of Treitz, such as duodenal or esophageal atresia, may interfere with the effective removal of amniotic fluid by the alimentary tract.24
Fetal circulatory disturbances account for approximately 7% of fetal anomalies responsible for hydramnios.20 Structural cardiac malformations and persistent fetal arrhythmias may result in right and left heart failure. Presumably, the resulting increase in venous pressure causes an elevation in hydrostatic pressure in the fetal capillaries, with transudation of fluid into the interstitial space. This mechanism would occur systemically in the fetus, leading to the characteristic appearance of nonimmune hydrops (subcutaneous edema, ascites, pleural and pericardial effusions), as well as in the placenta, resulting in polyhydramnios.
Other circulatory disturbances can also result in polyhydramnios. In twin-to-twin transfusion syndrome, the recipient twin becomes plethoric and may develop hydramnios, either through volume overload, increased renal blood flow, and polyuria,27 or through a hydropic placenta. The donor twin becomes anemic, often leading to oligohydramnios and the “stuck twin” syndrome. Placental chorioangiomas and sacrococcygeal teratomas are other abnormalities in which large arteriovenous shunts may lead to high-output cardiac failure and ultimately polyhydramnios.24, 28
Inadequate fetal respiratory activity secondary to anomalies may prevent fluid absorption at the alveolar/capillary interface, leading to polyhydramnios. Examples include compressing tumors, such as cystic adenomatoid malformations, displaced abdominal contents, such as congenital diaphragmatic hernia, and thoracic wall abnormalities, such as thanatophoric dysplasia.
Polyhydramnios not associated with an identifiable cause is labeled “idiopathic” and accounts for 30–60% of cases.18, 20, 24, 29 Further research is necessary to identify other as yet undetermined causes. One such possibility is a disorder of intra-amniotic prolactin regulation by the chorion and decidua. Under normal circumstances, prolactin may be partially responsible for control of water homeostasis in the intra-amniotic environment. In vitro studies on human amnion have shown reduced diffusion of water in response to ovine prolactin administered on the fetal side of the membrane.10 Hence, an overproduction of decidual prolactin may impair diffusional flow of water away from the amniotic compartment, leading to polyhydramnios.
Clinical Presentation
The maternal signs and symptoms of polyhydramnios are usually caused by the overdistended uterus and its compressing effect on intrathoracic and intra-abdominal organs. Elevation of the diaphragm can result in dyspnea and occasionally respiratory distress.30 Back and abdominal discomfort are also frequent complaints, as are nausea and vomitting.18 Edema of the lower extremities may result from compression of the inferior vena cava.
Diagnosis of Polyhydramnios
The diagnosis of polyhydramnios had formerly been a clinical one, retrospectively based on the presence of more than 2000 ml of amniotic fluid at the time of delivery or membrane rupture. Antenatal suspicion was raised by difficulty in palpating fetal parts, distant fetal heart sounds by unamplified auscultation, a tense uterine wall, and disproportionate growth of the fundal height. Historically, amniography was used to qualitatively assess amniotic fluid volume. This method was subsequently supplanted by static ultrasonographic imaging, which was used to calculate total intrauterine volume (TIUV). However, inaccuracies in measurement as well as the advent of real-time ultrasonography led to the abandonment of TIUV. Real-time ultrasonography is now the primary means of amniotic fluid volume assessment; however, strict ultrasonographic criteria have never been uniformly adopted. Chamberlain and colleagues arbitrarily defined polyhydramnios as a fluid pocket of at least 8 cm in vertical and transverse diameters.31 Using this criterion, the incidence of polyhydramnios in a select high-risk referral population was 3.2%. Those patients with polyhydramnios had a higher incidence of major congenital anomalies (4%), macrosomia (33%), and perinatal mortality (3.3%) compared to a control group with normal amniotic fluid volume. More recently, the amniotic fluid index (AFI), which is discussed in more detail later in this chapter, has replaced the largest vertical pocket in many ultrasound units. An AFI of greater than 20 cm was arbitrarily defined as excessive amniotic fluid volume.32 An alternative to the semi-quantitative techniques mentioned above is simply the subjective impression of increased amniotic fluid volume.19, 29 Subjective criteria have included the displacement of the fetus from the anterior uterine wall by amniotic fluid, as well as the presence of “floating extremities.”29 Simply put, if there appears to be excessively abundant fluid, it probably is. Bottoms and colleagues, using subjective criteria, found that the sensitivity and positive predictive value in detecting infants large for gestational age were similar to the 8-cm largest vertical pocket rule.33
Perinatal Complications
The increased perinatal morbidity and mortality associated with polyhydramnios are due to both an increase in congenital/genetic anomalies and preterm births. Perinatal mortality used to approach 100% with acute polyhydramnios18; however, with aggressive repetitive amniocentesis, survivors have been reported.30, 34, 35 Chronic polyhydramnios tends to have a better prognosis, especially if idiopathic in origin. Perinatal mortality has ranged from 34% to 69% in older studies.18, 20, 22 However, Chamberlain and colleagues quoted a 3.3% mortality when the diagnosis was made sonographically.31 Some of the variation in survival may be a function of diagnostic criteria differences and prenatal therapy, as well as improved survival of both preterm and anomalous infants.
Polyhydramnios may be complicated by preterm labor in up to 26% and premature rupture of membranes in up to 19% of cases.20 Both may occur as a result of overdistention of the uterus. Malpresentations are also encountered more frequently, as a result of both the abundance of amniotic fluid in which the fetus may maneuver and the earlier gestational age at the time of delivery.19 Other intrapartum complications may include placental abruption due to rapid decompression of the uterus at the time of rupture of membranes, dysfunctional labor patterns, and postpartum hemorrhage as a result of uterine atony.19, 20, 22
Clinical Management
Treatment of polyhydramnios may be medical or surgical or both. The method chosen will depend on the etiology, severity, clinical symptoms, and gestational age at diagnosis, as well as the presence and type of associated anomalies.
If the diagnosis is made on the basis of ultrasonographic findings, an attempt should be made to establish the cause. In cases that are not acute or severe and are not associated with a fetal malformation, patients should be rescanned periodically to assess the progression or improvement of the fluid volume. Some reports have documented gradual resolution of polyhydramnios, either spontaneously or as a result of treating the underlying cause (e.g., control of hyperglycemia, intrauterine transfusion of the anemic fetus). These pregnancies progressed uneventfully after resolution of the polyhydramnios, with no adverse sequelae observed.19, 36
In the absence of rapidly progressive polyhydramnios or maternal symptoms, management is expectant. If a patient experiences increasing dyspnea, back pain, or preterm labor, hospitalization for possible tocolysis and amniocentesis should be considered.
Medical management, including salt restriction, diuretics, and intra-amniotic vasopressin has not proved beneficial.24 Indomethacin has been suggested as a therapeutic modality to reduce the amniotic fluid volume, because it has been observed to decrease urinary output in neonates being treated for patent ductus arteriosus. A reduction in amniotic fluid has been observed in one series of eight patients with hydramnios treated with indomethacin, as documented by decreasing fundal height measurements and largest vertical fluid pocket by ultrasonography.37 This observation further confirms the important contribution of fetal urination in overall amniotic fluid dynamics. Although case reports and early studies suggested the therapeutic benefit of indomethacin in the treatment of polyhydramnios, it is not typically used in the third trimester, due to its recognized affects of in-utero narrowing of the fetal ductus arteriosus, which can result in pulmonary hypertension postnatally.38
Therapeutic amniocentesis, or amnioreduction, is an effective modality for acute decompression of the tense and distended uterine cavity. It is typically performed for relief of maternal symptoms or preterm labor. It should be performed under ultrasonic guidance to avoid fetal contact, using a long 20 gauge amniocentesis needle which is often connected via plastic tubing to a suction bottle. Amnioreduction is usually accomplished over 30–45 minutes, although no ideal time period for drainage has been established. During this time, uterine contractions may occur, which can be uncomfortable for the patient. Typically, these contractions will abate spontaneously within 24 hours after the procedure has been completed. The quantity of amniotic fluid that should be removed has also not been established and may be dependent on gestational age, severity, and rapidity of reaccumulation. Volumes aspirated in various reports have ranged from 200 to 4000 ml.22, 30, 34, 35 There has been concern that too rapid or too extensive a decompression could result in placental separation.22, 24, 30, 34, 35 Amniocentesis may need to be repeated initally 2–3 times in the first week, followed by weekly amnioreduction or as clinically indicated. Periodic evaluation of maternal electrolytes and serum protein may need to be assessed if frequent amniocenteses are required18 although no studies have demonstrated the efficacy of such surveillance.
OLIGOHYDRAMNIOS
Incidence and Origin
Oligohydramnios is defined as a decrease in the volume of amniotic fluid, relative to the gestational age. The incidence in an unselected population without membrane rupture ranges from 0.4% to 19%, depending on the criteria used for diagnosis and the study population.33, 39, 40 Oligohydramnios onset may be either acute or chronic. Acute onset is most commonly the result of membrane rupture, whereas chronic oligohydramnios may reflect a structural abnormality of the fetal urinary tract or a pathophysiologic response to chronic or intermittent fetal hypoxemia. Risk factors for oligohydramnios are shown in Table 2.
Table 2. Risk factors for oligohydramnios
Chronic and/or intermittent fetal hypoxemia
Fetal growth restriction
Postterm pregnancy
Repetitive cord compression
Fetal anomalies
Renal agenesis
Renal anomalies (e.g., multicystic dysplastic kidneys, polycystic kidneys)
Posterior urethral valves
Bilateral ureteropelvic junction obstruction
Non-steroidal anti-inflammatory medications
Twin-to-twin transfusion
Premature rupture of membranes
Pathogenesis
Spontaneous premature rupture of membranes (PROM) is the most common cause of acute oligohydramnios. The incidence of rupture of membranes before term is approximately 1–2%. Vintzileos and colleagues found that 35% of patients with PROM did not demonstrate a vertical amniotic fluid pocket greater than 2 cm, and this figure did not vary with gestational age.41
Chronic oligohydramnios may be the product of major fetal anomalies or prenatal hypoxia. The importance of the contribution of fetal urine to amniotic fluid volume is demonstrated by several fetal anomalies in which there is either obstruction of the urinary tract or bilateral renal agenesis/dysfunction. These anomalies are associated with decreased amniotic fluid formation.
Chronic or intermittent fetal hypoxia may also result in reduced amniotic fluid volume. Chronic low-grade fetal hypoxia may be a consequence of long-standing uteroplacental insufficiency or maternal hypoxia, whereas prenatal cord compression may lead to either prolonged or repetitive episodes of acute hypoxia of varying intensity and duration. Corroborative evidence for this pathophysiologic process leading to oligohydramnios exists in both animal and human models.
ANIMAL MODEL.
A redistribution of fetal cardiac output has been noted in pregnant ewes made hypoxic.42 An increased proportion of cardiac output was shunted to the brain, myocardium, and adrenal glands (high-priority organs), with a decrease in blood flow to the gut, musculature, spleen, lungs, and kidneys (low-priority organs). Under ordinary circumstances, this decreased renal perfusion would ultimately result in reduction of fetal urine production and oligohydramnios.
HUMAN MODEL.
Support for redistribution of cardiac output away from the fetal kidneys as the operative mechanism is also provided by several human observations. Wladimiroff and Campbell measured hourly human fetal urine production rate (HFUPR) by measuring bladder volume on two occasions 1 hour apart.6 The increase in bladder volume was equated with the hourly production rate. A normal curve of HFUPR versus gestational age in 92 normal pregnancies from 30 to 41 weeks was established. Subsequently, 62 pregnancies at risk for chronic uteroplacental insufficiency were studied; 47% had HFUPRs below the fifth percentile limit. Of the 29 fetuses with decreased HFUPR, 18 (62%) had a birthweight less than the 10th percentile for gestational age. Additionally, all nine subjects that delivered infants whose weights were less than the fifth percentile had HFUPRs below the normal range.
Oligohydramnios has been demonstrated in 3–54% of prolonged pregnancies, reflecting differences in sonography criteria used.32, 43, 44, 45, 46 The pathogenesis is presumably similar to the mechanism causing a growth-retarded fetus, because it is clinically recognized that prolonged pregnancy is associated with uteroplacental insufficiency in approximately 10% of cases.
However attractive the theory of redistribution of flow is, there may be additional operative mechanisms. In an animal model subjected to hypoxemia, glomerular filtration was maintained despite decreased renal blood flow.47 It was also observed that one or more episodes of fetal stress could result in secretion of vasopressin as well as catecholamines.47, 48 Secretion of vasopressin could result in significant antidiuresis and reduction of fetal lung secretion, resulting in further diminution of amniotic fluid.49
A final, hypothesized cause of unexplained oligohydramnios is amniotic rupture with an intact chorion.50 The amniotic fluid could potentially be reabsorbed into the extra-amniotic space, resulting in no loss of fluid per vaginam. Corroborative evidence for this process exists in amniotic band syndrome, in which it is theorized that the fetus is partially extruded into the extra-amniotic space. To date, no experimental confirmation of this pathway for oligohydramnios has been provided.
Regardless of the precise mechanism, the presence of oligohydramnios in the absence of structural anomalies or membrane rupture suggests an altered normal physiological process. Its presence also increases the risk for prenatal cord compression. Unfortunately, it is not known what prognostic significance one should attribute to the observation, particularly in a pre-term fetus.
Clinical Presentation and Diagnosis
Before widespread use of real-time ultrasonography, the diagnosis of oligohydramnios was occasionally suspected on the basis of “soft” clinical signs, including easily palpated fetal parts, inadequate fundal height growth, and difficulty in ballottement of the fetal head. Absence of amniotic fluid at the time of artificial rupture of the membranes is also strongly suggestive of oligohydramnios and, in the absence of a sonographic diagnosis, may be the first indication of its presence.
Although ultrasonography has provided a means of assessing the volume of amniotic fluid, a consensus of criteria for sonographic diagnosis of oligohydramnios has not been achieved. In early reports, amniotic fluid volume was assessed subjectively, allowing for differences according to gestational age. Subsequent investigators attempted to quantify amniotic fluid volume by various techniques. Data presented in these studies are listed in Table 3 and summarized below.
Table 3. Ultrasonographic assessment of oligohydramnios
Author | Population | Largest pocket criteria | Oligohy- | Abnormal endpoint | Sensitivity(%) | Specificity(%) | Positive predictive value (%) | Negative predictive value (%) |
Crowley(1980)46 | >42 weeks | Subjective | 35 | Cesarean for “fetal distress” | 100 | 68 | 12 | 100 |
Manning (1981)51 | Suspected IUGR | <1 cm | 24 | SGA | 84 | 97 | 90 | 95 |
Philipson (1983)40 | Unselected | Subjective | 4 | SGA | 16 | 97 | 40 | 91 |
Bottoms (1986)33 | Unselected | Subjective | 19 | SGA | 32 | 83 | 23 | 89 |
Hill (1983)39 | Unselected | <1 cm | 0 | SGA | 0 | 100 | — | 98 |
| Subjective | 1 | SGA | 50 | 100 | 100 | 99 | |
Haddick (1984)52 | Retrospective SGA pregnancy | <1 cm | 4 | SGA | 4 | — | — | — |
Mercer (1984)53 | Retrospective unselected | <1 cm | 3 | Apgar 5 <7 | — | — | 8 | — |
Chamberlain(1984)54 | Referred for biophysical profile | Vertical | 3 | SGA | 13 | 98 | 25 | 95 |
|
|
| ||||||
Phelan (1985)45 | >41 weeks | <1 cm | 3 | Apgar 5 <7 | 25 | 98 | 29 | 97 |
Postmaturity | 3 | 97 | 17 | 83 | ||||
Bastide (1986)55 | Referred for biophysical profile | Vertical <1 cm | 0.7 | SGA | — | — | 37 | — |
Patterson (1987)56 | Risk for malnutrition | Average <3.2 cm | 15 | SGA | 40 | 91 | 50 | 86 |
Hashimoto (1987)43 | >42 weeks | “Volume” <60 | 46 | Postmature | 90 | 64 | 36 | 97 |
Rutherford (1987)57 | “High risk” pregnancy | Sum of 4 vertical quadrants | 8 | Cesarean for “fetal distress” | 30 | 93 | 11 | 98 |
Silver (1987)44 | Mean of 3 vertical <3.8 cm | 54 | Delivery for nonreassuring fetal heart rate trace | 79 | 55 | 41 | 87 | |
Varma (1988)58 | “At risk” pregnancy | Vertical <2 cm | 3 | SGA | — | — | 39 | 92 |
Subjective Ultrasonographic Evaluation of Amniotic Fluid Volume
Crowley used subjective criteria to evaluate amniotic fluid volume in pregnancies after 42 weeks, looking for the presence or absence of anechoic space between fetal limbs and uterine wall, as well as between limbs and the fetal trunk.46 Philipson and colleagues used the subjective criteria of paucity of amniotic fluid, crowding of the fetal parts, and “poor fluid/fetal interspace.”40 Projecting these criteria to a theoretical study population in predicting small for gestational age (SGA) infants, the sensitivity was 15.5% and positive predictive value was 39.6%. Bottoms and associates subsequently compared a five-tiered subjective evaluation (oligohydramnios, decreased, normal, increased, hydramnios) to an objective measurement of maximum vertical pocket diameter, the latter measured with the transducer held at right angles to the sagittal plane of the maternal abdomen.33 Using SGA infants as an abnormal end point, the sensitivity and positive predictive values were similar between the two techniques (32% vs.31%, and 83% vs. 82%, respectively). Similarly, Goldstein and Filly also demonstrated good correlation between subjective and objective evaluations of amniotic fluid volume.59
Semi-Quantitative Ultrasonographic Assessment of Amniotic Fluid Volume
In 1981, the concept of the "1 cm rule" was introduced in a selected high-risk patient population.33 Subjects included were those with suspected fetal growth restriction (FGR) based on fundal height measurements. Volume was arbitrarily classified as decreased if the largest fluid pocket measured less than 1 cm in broadest dimension. Of these subjects, 24% were found to have decreased amniotic fluid volume, and 90% of these were delivered of babies that were found to be SGA. Conversely, 84% of SGA infants were observed to have had decreased fluid. However, subsequent studies were less optimistic, showing both a lower prevalence and sensitivity of' oligohydramnios as a predictor of IUGR.33, 39, 40, 52
The 1 cm rule was re-evaluated in 1984.54 Amniotic fluid volume was recorded in all patients referred for a biophysical profile by measuring the vertical and transverse dimension of the largest amniotic fluid pocket, with the transducer oriented perpendicular to the contour of the uterus. Vertical diameters less than 1 cm were classified as decreased, 1–2 cm as marginal, and greater than 2 cm to less than 8 cm as normal. It was found that 0.9% had decreased and 2% had marginal amniotic fluid. As a result of improved sensitivity in detecting FGR by including the marginal category (5.5% for decreased, 13.2% for decreased plus marginal groups), it was suggested that the 1 cm rule might be too stringent. Subsequently, the amniotic fluid component of the biophysical profile was modified to a 2 cm cutoff in two perpendicular planes.
In addition to the 2 cm rule, other objective techniques of amniotic fluid volume have been evaluated. Patterson and colleagues measured the vertical and two horizontal dimensions of the largest fluid pocket and calculated a mean value of the three dimensions.56 Only pockets that were free of umbilical cord and extremities were included. By using a receiver operating characteristic curve, a statistical tool that is employed to maximize sensitivity and specificity, a cutoff of 3.2 cm was established. Using this value, 15% of a study population “at risk for fetal malnutrition” was abnormal. The 3.2-cm cutoff was 40% sensitive and 91% specific, with a 50% positive predictive value and 86% negative predictive value for detecting SGA infants. Observed differences in average fluid volume were more likely to be due to true differences between patients and not due to measurement error; measurement of the average dimension of the largest amniotic fluid pocket had an interpatient variability that was fourfold higher than the intraobserver variability. In contrast, use of maximum vertical diameter had an intraobserver variability that was higher than the interpatient variability. The authors concluded that average amniotic fluid volume was more reproducible than the largest vertical diameter and would be a superior screening test to identify malnourished fetuses.
In 1987, Phelan and colleagues introduced the four-quadrant technique of amniotic fluid volume assessment.32 Using 353 gravidas at 36–42 weeks who were referred for an external cephalic version or postdate pregnancy from 36 to 42 weeks, the largest vertical diameter in each quadrant was measured and summed. For all measurement, the transducer was held in a sagittal plane perpendicular to the floor. This number, in centimeters, was termed the amniotic fluid index (AFI). Between 36 and 40 weeks, the average AFI was 12.4 ± 4.6 cm. Whereas two standard deviations below and above the mean would have resulted in statistical cutoffs of 3.7 and 22.1 cm, respectively, the authors used threshold values of 5 cm to arbitrarily define decreased amniotic fluid and 20 cm for excessive fluid. Further investigation by these authors demonstrated a significant increase in meconium-stained fluid, cesarean section, and low Apgar scores in subjects with AFI less than 5 cm.57
Moore and Cayle subsequently assessed the amniotic fluid index in 791 normal pregnancies between 16 and 42 weeks.60 Since significant differences were observed between the preterm, term, and postdates pregnancies, a finding that is consistent with physiologic fluid changes that occur throughout gestation, the data was stratified by week of gestation. The mean AFI value at each week of pregnancy, as well as the 90–95% confidence intervals, was calculated (Table 4). This study demonstrated the importance of establishing gestation-specific norms for the AFI, rather than a single cutoff value. Interestingly, the 2.5th percentile AFI value at each gestational age was greater than the 5 cm threshold established by Phelan.32 Therefore, the use of the 2.5th percentile cutoff would result in a more frequent diagnosis of oligohydramnios.
Table 4. Amniotic fluid index values in normal pregnancy (in mm)
Amniotic fluid index percentile values | ||||||
Week | 2.5th | 5th | 50th | 95th | 97.5th | n |
16 | 73 | 79 | 121 | 185 | 201 | 32 |
17 | 77 | 83 | 127 | 194 | 211 | 26 |
18 | 80 | 87 | 133 | 202 | 220 | 17 |
19 | 83 | 90 | 137 | 207 | 225 | 14 |
20 | 86 | 93 | 141 | 212 | 230 | 25 |
21 | 88 | 95 | 143 | 214 | 233 | 14 |
22 | 89 | 97 | 145 | 216 | 235 | 14 |
23 | 90 | 98 | 146 | 218 | 237 | 14 |
24 | 90 | 98 | 147 | 219 | 238 | 23 |
25 | 89 | 97 | 147 | 221 | 240 | 12 |
26 | 89 | 97 | 147 | 223 | 242 | 11 |
27 | 85 | 95 | 146 | 226 | 245 | 17 |
28 | 86 | 94 | 146 | 228 | 249 | 25 |
29 | 84 | 92 | 145 | 231 | 254 | 12 |
30 | 82 | 90 | 145 | 234 | 258 | 17 |
31 | 79 | 88 | 144 | 238 | 263 | 26 |
32 | 77 | 86 | 144 | 242 | 269 | 25 |
33 | 74 | 83 | 143 | 245 | 274 | 30 |
34 | 72 | 81 | 142 | 248 | 278 | 31 |
35 | 70 | 79 | 140 | 249 | 279 | 27 |
36 | 68 | 77 | 138 | 249 | 279 | 39 |
37 | 66 | 75 | 135 | 244 | 275 | 36 |
38 | 65 | 73 | 132 | 239 | 269 | 27 |
39 | 64 | 72 | 127 | 226 | 255 | 12 |
40 | 63 | 71 | 123 | 214 | 240 | 64 |
41 | 63 | 70 | 116 | 194 | 216 | 162 |
42 | 63 | 69 | 110 | 175 | 192 | 30 |
(Moore TR, Cayle JE: The amniotic fluid index in normal human pregnancy. Am J Obstet Gynecol 162:1168, 1990)
Comparison of Ultrasonographic Assessment of Amniotic Fluid Volume
To date, no single method to assess amniotic fluid volume has proved to be the most valuable clinically. Difficulty in comparing fluid assessment methods arises from differences in the population tested, the abnormal end point chosen, and the variety of ultrasonographic criteria. The 2 cm rule traditionally had been most widely used, predominantly as a component of the biophysical profile. Recently, however, the amniotic fluid index has appeared with increasing frequency in the literature and in clinical practice. The AFI, by measuring all four quadrants, would appear to more accurately assess serial changes in fluid volume over time, compared to a single vertical pocket, which might be subject to greater variation due to fetal positioning. Additionally, by using gestation-specific norms, the AFI may more accurately reflect abnormalities in fluid volume compared to the 2 cm rule. However, the AFI has not been evaluated as extensively in identifying the fetus at risk for IUGR, cord compression, and abnormal perinatal outcome. By comparison, the use of subjective criteria, which may be less dependent on fetal positioning in serial testing, relies more on a gestalt of fluid volume than on any one measurement value. As a result, the experience of the examiner may be more critical in determining if the amniotic fluid is appropriate for the gestational age, as the same subjectively normal amniotic fluid volume at 42 weeks might be decreased for 34 weeks. Additionally, subjective criteria may vary from individual to individual, making interobserver communication and statistical comparisons more difficult to express. At the author's institution, the amniotic fluid volume is initially assessed subjectively. If it is normal, no AFI or largest vertical pocket is measured. However, if the fluid subjectively appears decreased, an AFI is calculated.
The variability in definitions of ultrasound-based oligohydramnios was highlighted in a clinical commentary by Magann and colleagues, which included a plea for future studies that correlate amniotic fluid volume assessment to clinically relevant perinatal outcomes.61 Fischer and colleagues assessed postdate women and compared various ultrasound criteria for oligohydramnios with a composite perinatal outcome.62 The largest pocket in each quadrant was measured in two perpendicular planes. Indices evaluated included the largest vertical pocket, largest transverse pocket, AFI, largest pocket product (vertical x transverse), sum of all pocket measurements, and the sum of the pocket products. They found that the largest vertical pocket, the AFI, and the sum of all pockets were significantly different between the normal and abnormal perinatal outcome groups. Using receiver operating characteristic curves to establish optimal threshold values, a vertical pocket of 2.7 was ideal in identifying abnormal perinatal outcome. No optimal AFI cutoff based could be established based on the ROC curve.
Chauhan and colleagues performed a prospective randomized clinical trial comparing the AFI to the largest vertical pocket.63 They randomly assigned 1080 high risk gravidas to be followed with weekly nonstress tests and either an AFI or largest vertical pocket. They defined oligohydramnios as either an AFI of 5 cm or less, or the absence of a fluid pocket measuring at least 2 x 1 cm. Women followed by the AFI was significantly more likely to be diagnosed with oligohydramnios than those in the largest vertical pocket group (17% vs. 10%, p = 0.002). However, there was no difference between the two fluid assessment techniques with regards to cesarean delivery for non-reassuring fetal heart rate testing, Apgar score, umbilical artery pH <7.1, or admission to the neonatal intensive care unit. The authors concluded that using the AFI increases the number of interventions for oligohydramnios without improving perinatal outcome. They also observed that both techniques of amniotic fluid assessment are poor diagnostic tests for predicting adverse perinatal outcome.
Inconsistencies in Ultrasonographic Assessment of Amniotic Fluid Volume
Difficulties arise when comparing various criteria for oligohydramnios. One variable not often addressed in studies is the inclusion or exclusion of fluid pockets that contain loops of umbilical cord. With oligohydramnios, the umbilical cord makes up an increased proportion of fluid pockets. Some studies excluded any pocket that contained cord, while others measured the dimensions of fluid that surrounded cord.
A frequently overlooked but critically important issue is that of transducer positioning. In some reports, the transducer was held at right angles to the uterine contour,54, 58 whereas in others the plane of the ultrasound was perpendicular to the floor or sagittal plane of the abdomen.32, 33 Many studies did not indicate how the transducer was oriented. Orientation is critical in evaluating vertical diameter. If the transducer is held perpendicular to the uterine contour, a view from the lateral aspect of the uterus might falsely create a vertical pocket on the ultrasonography screen. For the sake of consistency, it is recommended that the transducer be oriented longitudinally and perpendicular to the plane of the floor (the plane in which the fluid has layered), thereby minimizing differences if the subject is laterally displaced.
Clinical Significance of Oligohydramnios
The literature suggests that oligohydramnios does increase the risk in a fetus with no major anomalies. However, the clinical significance of oligohydramnios differs between studies, depending on criteria used and end points evaluated. Overall, decreased amniotic fluid is associated with a higher incidence of SGA infants (less than the 10th percentile for gestational age), postmaturity syndrome, variable and late decelerations in labor, cesarean section for nonreassuring fetal heart rate tracing, lower umbilical artery pH, lower Apgar scores, and higher perinatal mortality. Second-trimester oligohydramnios is especially associated with adverse perinatal outcomes, as a result of both pulmonary hypoplasia and lethal congenital anomalies .64
The relative degree to which the increased morbidity results from either the underlying condition producing the oligohydramnios or from a direct effect of the reduced fluid (i.e., umbilical cord compression) has not been determined. However, there is some suggestion that part of the risk of cord compression may be reversible, as indicated by studies in which fluid was removed versus those in which fluid was replaced (amnioinfusion) to determine clinical effect. Gabbe and colleagues noted that removal of amniotic fluid from the amniotic cavity of fetal monkeys resulted in variable decelerations secondary to cord compression.65 This pattern resolved after amnioinfusion. Confirmation of this finding in humans has been demonstrated by Miyazaki and associates, who observed a significant diminution of intrapartum variable decelerations in 5 1% of patients treated by amnioinfusion through an intrauterine pressure catheter.66 Nageotte and co-workers observed a significantly lower rate of variable decelerations and higher cord pH values in patients who had PROM and underwent prophylactic amnioinfusion.67
Pulmonary hypoplasia, as measured by low wet lung weights, low lung DNA content, and low radial alveolar counts, can occur after PROM and oligohydramnios in the very preterm gestation (<24 weeks).68 It may result from limitation of lung expansion secondary to prolonged external compression, inhibition of fetal respiratory movements, and lack of fluid circulation into the terminal alveoli, which may require growth factors contained in amniotic fluid that are critical for alveolar development.69 In one study of PROM in which pulmonary hypoplasia was observed, the majority of cases were less than 26 weeks at the time of membrane rupture, suggesting that the developing terminal air sacs are more susceptible to the damaging effects of oligohydramnios.70 Further, prolonged oligohydramnios increases the risk of Potter's sequence, which, in addition to pulmonary hypoplasia, includes fetal skeletal and facial deformities due to prolonged external compression.69
Antepartum Management of Spontaneous Oligohydramnios
In the absence of membrane rupture or fetal urinary obstruction, there is no known direct treatment for antepartum oligohydramnios. Oligohydramnios in the absence of major congenital anomalies may be a marker for prior fetal adjustment to chronic uteroplacental insufficiency or partial cord occlusion, as well as a predisposing factor for cord compression. Therefore, it is generally recommended that, depending on gestational age, these patients be either followed closely with serial antenatal testing (nonstress test, biophysical profile) including assessment for the presence of variable decelerations, or else delivered. However, when oligohydramnios is present, there is no consensus at this time about a critical diagnosis-to-delivery interval. In the presence of marked oligohydramnios at term, delivery should be initiated within 24 to 48 hours after diagnosis, or earlier in the presence of associated findings such as spontaneous variable decelerations. Further study is indicated to determine the benefits of such an approach on both short- and long-term morbidity.
Oligohydramnios resulting from congenital urinary tract obstruction (e.g., posterior urethral valves) may be treated, especially in a preterm gestation, by sonographically directed procedures that divert urine from the bladder to the amniotic cavity.71 In a fetus with preserved renal function based on serial analysis of urinary electrolytes obtained from vesicocentesis, vesico-amniotic shunting may be a useful temporizing measure to decompress the bladder, alleviate oligohydramnios, and prevent pulmonary hypoplasia.72, 73Intrapartum Management
Oligohydramnios increases the risk of cord compression during labor; consequently, the fetus should be followed closely for variable decelerations. Persistent, moderate or severe variable decelerations may be ameliorated with the use of amnioinfusion during labor.
SUMMARY
Amniotic fluid is the product of complex and dynamic fetal and placental physiologic processes. Disruption of the fine balance may result in overproduction or underproduction of fluid. Therefore, alterations in amniotic fluid volume serve as important markers of both in utero developmental defects as well as physiological responses to fetal hypoxemia and other metabolic disturbances such as maternal/fetal hvperglycemia. Both polyhydramnios and oligohydramnios may be associated with either major congenital anomalies or adverse perinatal outcomes. Although the ultrasonographic diagnostic criteria have yet to be firmly established, it is apparent that both subjective and objective criteria have been used successfully to identify these conditions. Polyhydramnios, particularly when severe and detected early in gestation, can be treated antenatally with serial amniocenteses. Oligohydramnios with intact membranes, especially when severe and in the absence of anomalies, is usually managed by delivery; however, further research is indicated to delineate management guidelines.
Amniotic fluid volume remains an important component of any obstetric ultrasonographic examination. Standardized criteria for the diagnosis of polyhydramnios and oligohydramnios, which may be gestational age dependent, are necessary to improve comparisons between studies and to improve communication among those performing and interpreting sonographic assessments of amniotic fluid volume.
REFERENCES
Lind T, Kendall A, Hytten FE: The role of the fetus in the formation of amniotic fluid. J Obstet Gynaecol Br Commonw 79: 289, 1972 |
|
Abramovich DR: Fetal factors influencing the volume and composition of liquor amnii. J Obstet Gynaecol Br Commonw 77: 865, 1970 |
|
Bronshtein M, Yoffe N, Brandes JM et al: First and early second-trimester diagnosis of fetal urinary tract anomalies using transvaginal sonography. Prenat Diagn 10:653, 1990. |
|
Abramovich DR, Garden A, Jandial L et al: Fetal swallowing and voiding in relation to hydramnios. Am J Obstet Gynecol 54: 15, 1979 |
|
van Otterlo LC, Wladimiroff JW, Wallenburg HCS: Relationship between fetal urine production and amniotic fluid volume in normal pregnancy and pregnancy complicated by diabetes. Br J Obstet Gynaecol 84: 205, 1977 |
|
Wladimiroff JW, Campbell S: Fetal urine-production rates in normal and complicated pregnancy. Lancet 1: 151, 1974 |
|
Pritchard JA: Fetal swallowing and amniotic fluid volume. Obstet Gynecol 28: 606, 1966 |
|
Boddy K, Dawes GS: Fetal breathing. Br Med Bull 31: 3, 1975 |
|
Duenhoelter JH, Pritchard JA: Fetal respiration: Quantitative measurements of amniotic fluid inspired near term by human and rhesus fetuses. Am J Obstet Gynecol 125: 306, 1976 |
|
Leontic EA, Schruefer JJ, Andreassen B et al: Further evidence for the role of prolactin on human fetoplacental osmoregulation. Am J Obstet Gynecol 133: 435, 1979 |
|
Hebertson RM, Hammond ME, Bryson MJ: Amniotic epithelial ultrastructure in normal, polyhydramnic, and oligohydramnic pregnancies. Obstet Gynecol 68: 74, 1986 |
|
Seeds AE: Current concepts of amniotic fluid dynamics. Am J Obstet Gynecol 138: 575, 1980 |
|
Wallenburg HCS: The amniotic fluid. I. Water and electrolyte homeostasis. J Perinat Med 5: 193, 1977 |
|
Gillibrand PN: The rate of water transfer from the amniotic sac with advancing pregnancy. J Obstet Gynaecol Br Commonw 76: 530, 1969 |
|
Brace RA, Wolf EJ: Normal amniotic fluid volume changes throughout pregnancy. Am J Obstet Gynecol 161: 382, 1989 |
|
Elliott PM, Inman WHW: Volume of liquor amnii in normal and abnormal pregnancy. Lancet 2: 835, 1961 |
|
Queenan JT, Thompson W, Whitfield CR et al: Amniotic fluid volumes in normal pregnancies. Am J Obstet Gynecol 114: 34, 1972 |
|
Queenan JT, Gadow EC: Polyhydramnios: Chronic versus acute. Am J Obstet Gynecol 108: 349, 1970 |
|
Zamah NM, Gillieson MS, Waiters JH et al: Sonographic detection of polyhydramnios: A five-year experience. Am J Obstet Gynecol 143: 523, 1982 |
|
Jacoby HE, Charles D: Clinical conditions associated with hydramnios. Am J Obstet Gynecol 94: 910, 1966 |
|
Murray SR: Hydramnios: A study of 846 cases. Am J Obstet Gynecol 88: 65, 1964 |
|
Barry AP: Hydramnios. Obstet Gynecol 11: 667, 1958 |
|
Pedersen J: Glucose content of the amniotic fluid in diabetic pregnancies. Acta Endocrinol 15: 342, 1954 |
|
Wallenburg HCS, Wladimiroff JW: The amniotic fluid. II. Polyhydramnios and oligohydramnios. J Perinat Med 6: 233, 1977 |
|
Pritchard JA: Deglutition by normal and anencephalic fetuses. Obstet Gynecol 25: 289, 1965 |
|
Benirschke K, McKay DG: The antidiuretic hormone in fetus and infant. Obstet Gynecol 1: 638, 1953 |
|
Kirshon B: Fetal urine output in hydramnios. Obstet Gynecol 73: 240, 1989 |
|
Cousins L, Benirschke K, Porreco R et al: Placentomegaly due to fetal congestive failure in a pregnancy with a sacrococcygeal teratoma. J Reprod Med 25: 142, 1980 |
|
Alexander ES, Spitz HB, Clark RA: Sonography of polyhydramnios. AJR 138: 343, 1982 |
|
Queenan JT: Recurrent acute polyhydramnios. Am J Obstet Gynecol 106: 625, 1970 |
|
Chamberlain PF, Manning FA, Morrison I et al: Ultra-sound evaluation of amniotic fluid volume. II. The relationship of increased amniotic fluid volume to perinatal outcome. Am J Obstet Gynecol 150: 250, 1984 |
|
Phelan JP, Smith CV, Broussard P et al: Amniotic fluid volume assessment with the four-quadrant technique at 36–42 weeks’ gestation. J Reprod Med 32: 540, 1987 |
|
Bottoms SF, Welch RA, Zador IE et al: Limitations of using maximum vertical pocket and other sonographic evaluations of amniotic fluid volume to predict fetal growth: Technical or physiologic? Am J Obstet Gynecol 155: 154, 1986 |
|
Denehy TR, Hollander DI, Dembner A et al: Acute polyhydramnios. Int J Gynecol Obstet 28: 181, 1989 |
|
Pitkin RM: Acute polyhydramnios recurrent in successive pregnancies. Obstet Gynecol 48: 42S, 1976 |
|
Hill LM: Resolving polyhydramnios: A sign of improved fetal status. Am J Perinatol 5: 61, 1988 |
|
Cabrol D, Landesman R, Muller J et al: Treatment of polyhydramnios with prostaglandin synthetase inhibitor (indomethacin). Am J Obstet Gynecol 157: 422, 1987 |
|
Moise KJ, Huhta JC, Sharif DS et al: Indomethacin in the treatment of premature labor: Effects on the fetal ductus arteriosus. N Engl J Med 319: 327, 1988 |
|
Hill LM, Breckle R, Wolfgram KR et al: Oligohydramnios: Ultrasonically detected incidence and subsequent fetal outcome. Am J C Obstet Gynecol 147: 407, 1983 |
|
Philipson EH, Sokol RJ, Willjams T: Oligohydramnios: Clinical associations and predictive value for intrauterine growth retardation. Am J Obstet Gynecol 146: 271, 1983 |
|
Vintzileos AM, Campbell WA, Nochimson DJ et al: Degree of oligohydramnios and pregnancy outcome in patients with premature rupture of the membranes. Obstet Gynecol 66: 162, 1985 |
|
Cohn HE, Sacks EJ, Heymann MA et al: Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol 120: 817, 1974 |
|
Hashimoto B, Filly RA, Belden C et al: Objective method of diagnosing oligohydramnios in the postterm pregnancies. J Ultrasound Med 6: 81, 1987 |
|
Silver RK, Dooley SL, Tamura RK et al: Umbilical cord size and amniotic fluid volume in prolonged pregnancy. Am J Obstet Gynecol 157: 716, 1987 |
|
Phelan JP, Platt LD, Yeh SY et al: The role of ultrasound assessment of amniotic fluid volume in the management of the postdate pregnancy. Am J Obstet Gynecol 151: 304, 1985 |
|
Crowley P: Non quantitative estimation of amniotic fluid volume in suspected prolonged pregnancy. J Perinat Med 8: 249, 1980 |
|
Robillard JE, Weitzman RE, Burmeister L et al: Developmental aspects of the renal response to hypoxemia in the lamb fetus. Circ Res 48: 128, 1981 |
|
Ross MG, Ervin MG, Leake RD et al: Isovolemic hypotension in ovine fetus: Plasma arginine vasopressin response and urinary effects. Am J Physiol 250: E564, 1986 |
|
Ross MG, Ervin G, Leake RD et al: Fetal lung liquid regulation by neuropeptides. Am J Obstet Gynecol 150: 421, 1984 |
|
Hickok DE, McClean J, Shepard TH et al: Unexplained second trimester oligohydramnios: A clinical-pathologic study. Am J Perinatol 6: 8, 1989 |
|
Manning FA, Hill LM, Platt LD: Qualitative amniotic fluid volume determination by ultrasound: Antepartum detection of intrauterine growth retardation. Am J Obstet Gynecol 139: 254, 1981 |
|
Hoddick WK, Callen PW, Filly RA et al: Ultrasonographic determination of qualitative amniotic fluid volume in intrauterine growth retardation: Reassessment of the 1 cm rule. Am J Obstet Gynecol 149: 758, 1984 |
|
Mercer LJ, Brown LG, Petres RE et al: A survey of pregnancies complicated by decreased amniotic fluid. Am J Obstet Gynecol 149: 355, 1984 |
|
Chamberlain PF, Manning FA, Morrison I et al: Ultrasound evaluation of amniotic fluid volume. I. The relationship of marginal and decreased amniotic fluid volumes to perinatal outcome. Am J Obstel Gynecol 150: 245, 1984 |
|
Bastide A, Manning F, Harman C et al: Ultrasound evaluation of amniotic fluid: Outcome of pregnancies with severe oligohydramnios. Am J Obstet Gynecol 154: 895, 1986 |
|
Patterson RM, Prihoda TJ, Pouliot MR: Sonographic amniotic fluid measurement and fetal growth retardation: A reappraisal. Am J Obstet Gynecol 157: 1406, 1987 |
|
Rutherford SE, Phelan JP, Smith CV et al: The four-quadrant assessment of amniotic fluid volume: An adjunct to antepartum fetal heart rate testing. Obstet Gynecol 70: 353, 1987 |
|
Varma TR, Bateman S, Patel RH et al: Ultrasound evaluation of amniotic fluid: Outcome of pregnancies with severe oligohydramnios. Int J Gynecol Obstet 27: 185, 1988 |
|
Goldstein RB, Filly RA: Sonographic estimation of amniotic fluid volume: Subjective assessment versus pocket measurements. J Ultrasound Med 7: 363, 1988 |
|
Moore TR, Cayle JE: The amniotic fluid index in normal human pregnancy. Am J Obstet Gynecol 162: 1168, 1990 |
|
Magann EF, Isler CM, Chauhan SP, Martin JN: Amniotic fluid volume estimation and the biophysical profile: A confusion of criteria. Obstet Gynecol 96:640, 2000 |
|
Fischer RL, McDonnell M, Bianculli KW, Perry RL, Hediger ML, Scholl TO: Amniotid fluid volume estimation in the postdate pregnancy. A comparison of techniques. Obstet Gynecol 81:698,1993 |
|
Chauhan SP, Doherty DD, Magann EF, Cahanding F, Moreno F, Klausen JH: Amniotic fluid index vs single deepest pocket technique during modified biophysical profile: A randomized clinical trial. Am J Obstet Gynecol 191:661,2004 |
|
Moore TR, Longo J, Leopold GR et al: The reliability and predictive value of an amniotic fluid scoring system in severe second-trimester oligohydramnios. Obstet Gynecol 73: 739, 1989 |
|
Gabbe SG, Ettinger BB, Freeman RK et al: Umbilical cord compression associated with amniotomy: Laboratory observations. Am J Obstet Gynecol 126: 353, 1976 |
|
Miyazaki FS, Nevarez F: Saline amnioinfusion for relief of repetitive variable decelerations: A prospective randomized study. Am J Obstet Gynecol 153: 301, 1985 |
|
Nageotte MP, Freeman RK, Garite TJ et al: Prophylactic intrapartum amnioinfusion in patients with preterm rupture of membranes. Am J Obstet Gynecol 153: 557, 1985 |
|
Thibeault DW, Beatty EC, Hall RT et al: Neonatal pulmonary hypoplasia with premature rupture of fetal membranes and oligohydramnios. J Pediatr 107: 273, 1985 |
|
Lawrence S, Rosenfeld CR: Fetal pulmonary development and abnormalities of amniotic fluid volume. Semin Perinatol 10: 142, 1986 |
|
Nimrod C, Varela-Gittings F, Machin G et al: The effect of very prolonged membrane rupture on fetal development. Am J Obstet Gynecol 148: 540, 1984 |
|
Harrison MR, Golbus MS, Filly RA et al: Management of the fetus with congenital hydronephrosis. J Pediatr Surg 17: 728, 1982 |
|
Johnson MP, Bukowski TP, Reitleman C, Isada NB, Pryde PG, Evans MI: In utero surgical treatment of fetal obstructive uropathy: A new comprehensive approach to identify appropriate candidates for vesicoamniotic shunt therapy. Am J Obstet Gynecol. 170:1770, 1994 |
|
Kumar S, Fisk NM:Distal urinary obstruction. Clin Perinatol. 30:507,2003 |