This chapter should be cited as follows:
Osoti A, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.413763
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 10
Common obstetric conditions
Volume Editor: Professor Sikolia Wanyonyi, Aga Khan University Hospital, Nairobi, Kenya

Chapter
Placenta Previa and Placenta Abruption
First published: February 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Placenta previa and placenta abruption (abruptio placentae), the two leading and major causes of antepartum hemorrhage, result in substantial maternal and perinatal morbidity and mortality. In their severe forms, both placenta previa and placenta abruption may have long-term maternal and neonatal sequelae.
Placenta previa is the implantation of placental tissue partially or entirely within the lower segment of the uterus after 20 weeks of gestation. The abnormally implanted placenta may partially or entirely cover the cervix. Pregnancies complicated with placenta previa often present with painless vaginal bleeding after 20 weeks of gestation and are thereafter confirmed and classified on obstetric ultrasonography.
Placental abruption refers to bleeding at the decidual–placental interface of normally implanted placenta, resulting in partial or complete placental detachment prior to delivery of the fetus. The diagnosis is typically reserved for pregnancies over 20 weeks of gestation. The major clinical findings are vaginal bleeding and abdominal pain, often accompanied by hypertonic uterine contractions, uterine tenderness, and a nonreassuring fetal heart rate (FHR) pattern.
In both placenta previa and abruptio, asymptomatic cases may be diagnosed during routine obstetric ultrasonography. In general, management and outcomes depend on the severity of clinical presentation and type of placenta previa or abruptio.
PLACENTA PREVIA
Definition
Placenta previa refers to placenta which is implanted partially or completely over the lower uterine segment (over and adjacent to the internal os) after 20 weeks of pregnancy. “Previa” comes from two words: “pre” (or “prae”) meaning before, and “via” meaning way. “Previa” usually refers to anything obstructing the presenting part. Placenta previa therefore means “placenta in the way, before the baby’s presenting part”. This definition is similar to that of vasa previa which means “vessels in the way, before the baby’s presenting part”.
Epidemiology
The overall prevalence of placenta previa is estimated as 5.2 per 1000 pregnancies, with marked regional variation.1,2 The prevalence is highest among Asian studies (12.2 per 1000 pregnancies) and lower in European (3.6 per 1000 pregnancies), North American (2.9 per 1000 pregnancies) and Sub-Saharan African (2.7 per 1000 pregnancies) studies.3 The risks of placenta previa increases 1.5–5-fold following cesarean delivery and with increasing numbers of cesarean deliveries, at 1% after one cesarean delivery, 2.8% after three cesarean deliveries, and 3.7% after five cesarean deliveries.4 Placenta previa following prior cesarean sections has been associated with high and increasing risk of placenta accreta syndromes.5
Etiology and pathogenesis
Placenta previa arises from implantation by the embryo (embryonic plate) in the lower (caudad) uterine cavity (in close proximity to the cervical os). This implantation occurs as a result of defective decidual vascularization possibly from inflammation or atrophy. Due to continued placental growth, the placenta may remain at the lower segment or cover the cervical os partially or fully.
Placenta previa presents clinically as painless bleeding and is the leading single cause of major antepartum hemorrhage. Bleeding in placenta previa coincides with the development of the lower uterine segment in the third trimester. As the lower uterine segment thins in preparation for the onset of labor, placental attachment is disrupted leading to painless vaginal bleeding at the implantation site. The development of the lower segment also results in dilatation of the internal os, separation of some of the implanted placenta and subsequent bleeding. The myometrium of the lower uterine segment does not contract adequately to constrict and stop the flow of blood from the avulsed open vessels. Although thrombin released from the bleeding sites promotes uterine contractility, it also leads to a vicious cycle of bleeding–contractions–placental separation–bleeding.
Although the underlying cause of placenta previa is not known, a major risk is endometrial damage and uterine scarring.6 Other proposed hypotheses include the dropping down of the fertilized ovum and its implantation in the lower uterine segment, persistence of chorionic activity in the decidua capsularis and its contact with decidua vera of the lower uterine segment, defective decidualization and spread of the chorionic villi into the lower uterine segment, and large surface area of the placenta for example in multiple pregnancy. These pathogeneses may also explain placenta accreta syndromes and vasa previa.7 Due to lack of decidua basalis and incomplete development of the fibrinoid layer the implanting placenta may attach directly to the myometrium (accreta), invade the myometrium (increta), or penetrate the myometrium (percreta). Similarly, when sections of the placenta which undergo atrophic changes persist, they may form vasa previa.
Types of placenta previa
Based on proximity of the placental tissue to the internal cervical os, four types of placenta previa have been traditionally described.8 Type I or low lying placenta in which the placental edge is within 2 cm of but not reaching the internal cervical os. Type II or marginal placenta where the placental edge reaches the margin of but does not cover the internal cervical os, type III or partial or incomplete when the placental edge partially covers the internal cervical os (especially when closed but not entirely when fully dilated), and type IV or complete, when the placenta totally covers the internal cervical os including during full cervical dilatation. In addition, placenta previa has been described as anterior or posterior if lying in the anterior or posterior uterine wall, respectively. The majority of the placenta previa are on the posterior wall, and about one-third are placenta previa types III and IV. Clinically, placenta previa was also classified as minor (type I and II anterior) and major (type II posterior, III and IV). Other classifications systems characterized placenta previa as complete, partial, and marginal depending on how much of the internal endocervical os was covered by the placenta. Increased use of (transvaginal) ultrasonography for precise localization of the placental edge and the cervical os has led to a revision of the classification leading to elimination of the categories of “partial” and “marginal” placenta previa. The National Institutes of Health sponsored Fetal Imaging Workshop recommended two categories of placenta previa: placenta previa, when the internal os is covered partially or completely by placenta or low-lying placenta, when the placenta is implanted in the lower segment but the placental edge does not reach or cover the internal os and remains within 2 cm of the cervical os.9
Placental migration
The term “placental migration” has been used to describe placenta diagnosed as “low lying” in early pregnancy and which resolve by the third trimester. Occurring in more than 90% of cases, this is a misnomer as the placenta does not migrate but its proximal part grows toward better blood supply at the fundus (trophotropism), while the distal portions in the poorly vascularized lower segment regress and atrophy.10,11 Also, the differential growth of the lower segment relative to the upper segment due to the growing fetus may result in increased distance between distal edge of the placenta and the cervix. In general, owing to placental “migration”, any placenta previa diagnosed before 24 weeks should be confirmed by imaging studies between 28 and 32 weeks.
Risk factors for placenta previa
Multiple factors that increase the risk of defective decidualization and, therefore, the placenta previa4,12,13,14,15,16 include:
- Placenta previa increases with increasing parity. Grand multiparas have higher (5%) risk compared with nulliparous (0.2%).
- Advanced maternal age: placenta previa occurs in an estimated 1% of deliveries among women aged 35 years and 2% of deliveries above 40 years. Compared to younger women, those more than 35 years and 40 years of age have more than a 4- and 9-fold greater risk for placenta previa, respectively.
- Asian women have the highest rates of placenta previa compared to white and black women, 4.5, 3.3 and 3.0 per 1000 births, respectively.
- Prior cesarean delivery: the risk of placenta previa increases with the number of cesarean sections from an estimated 0.9% after one, 1.7% after two, 3% after three and up to 10% after four or more cesarean deliveries due to endometrial scarring.
- Other uterine surgeries like curettage, myomectomy have a slightly elevated risk of previa. Also, induced abortion and prior abortion due to endometrial scaring and inflammation increase the risk.
Factors that increase placental surface area as result of decreased uteroplacental oxygenation and increase the risk of placenta previa include:
- Cigarette smoking (2–3-fold increase) because carbon monoxide hypoxemia causes compensatory placental hypertrophy and more surface decidual vasculopathy;
- Maternal cocaine use (4-fold increase);
- High altitude;
- Multiple gestation is associated with 30–40% increase in placenta previa as result of larger placental surface area;
- Male fetuses have large placental surface area and delayed implantation;
- Prior placenta previa increases the risk of subsequent previa four to eightfold;
- Other factors statistically associated with placenta previa include fetal malpresentation, preterm labor, preterm prelabor rupture of membranes, intrauterine fetal growth restriction, congenital anomalies, amniotic fluid embolism, prior infertility treatment, and abnormally elevated prenatal screening of maternal serum α-fetoprotein.
The diagnosis of placenta previa is based on history, clinical examination findings and supporting imaging studies. Increasingly, however, routine ultrasonography has resulted in earlier diagnosis of asymptomatic cases without or prior to clinical presentation.
History
Vaginal bleeding
The hallmark of placenta previa is painless per vaginal bleeding after 20 weeks of pregnancy, which occurs in more than 70% of the cases. Less than 10% of patients with placenta previa are asymptomatic. Although bleeding may be provoked by labor, pelvic examination, or sexual intercourse, often no predisposing factor is identified. The initial bleeding “herald or sentinel bleed” is seldom profuse and is typically followed by major bleeding. Some (10–20%) patients with placenta previa may have uterine contractions and such (painful) vaginal bleeding which may be confused with placenta abruptio.
Although the painless hemorrhage often occurs near the end of the second trimester or in the third trimester, placenta previa classically presents with painless third-trimester bleeding. About one-third of women bleed before 30 weeks, one-third between 30 and 36 weeks, and the rest after 36 weeks. Bleeding prior to 30 weeks is associated with increased maternal and perinatal mortality and morbidity including blood transfusion. The bleeding may stop spontaneously and recur in labor.
Other findings on history will depend on the severity of the bleeding. For example, patients may be hemodynamically stable, present with mild hypotension or present in shock from severe hemorrhage.
Clinical examination
General clinical examination
The findings on clinical examination depend on severity of the bleeding and may range from stable with no pallor and normal or minimally altered vital signs to clinical features of shock and raised shock index.
Abdominal examination
The size of the uterus may be disproportionate to the gestational age resulting in a higher symphysiofundal height compared to the gestational age. Placenta previa may prevent the fetus from establishing normal polarity resulting in abnormal fetal lie and malposition/malpresentations and unengaged presenting part. The uterus may be relaxed, soft and non-tender compared to findings of placenta abruptio. There may also be associated multiple pregnancy or uterine leiomyomata. Fetal heart sound is usually present, unless there is severe bleeding and placental separation when the fetal heart rate tracing may have repetitive late decelerations or other non-reassuring fetal heart rate patterns. Rarely performed, placenta previa can be suggested by Stallworthy’s sign, where the fetal heart rate slows down and soon recovers promptly on pressing the head down into the pelvis.17 This is more evident with posterior placenta previa.
Other clinical findings depend on the severity of the bleeding. For example, in extremely severe cases there may be absence of fetal heart tones due to intrauterine fetal exsanguination.
Pelvic examination
The only permitted routine pelvic examination is inspection of the vulva and clothing to ascertain continued bleeding, amount of blood loss, and the color of the blood. In placenta previa, the blood is bright red as the bleeding occurs from the separated uteroplacental sinuses close to the cervical opening and escapes out immediately. Digital or speculum examination can provoke further placental separation and massive fatal hemorrhage. Speculum examination and not digital vaginal examination should be performed in an operation theater when cesarean delivery can be performed immediately. However, experienced staff can safely conduct a careful speculum examination for mild cases, stable patients, if there is lack of immediate ultrasound for placental localization.
Diagnosis
The clinical presentation of painless and often recurrent vaginal bleeding after 20 weeks of pregnancy is often diagnostic of placenta previa unless proven otherwise. In such women placenta previa can only be excluded after imaging studies.
Now only for historical reasons and in very resource-limited settings, a double set-up technique can be diagnostic. In this procedure, the patient is set in the operating room with the surgical team ready for an immediate cesarean section. A digital vaginal examination is then performed. A finger is passed around the cervix through all the fornices to assess for bogginess suggestive of placental tissue. If the presenting part and not bogginess is felt clearly through all the fornices, the finger is gently introduced into the cervical canal to evaluate for the placenta (firm) and blood clots (soft and friable). Once placenta previa is confirmed a cesarean section is immediately performed. If the placenta previa is ruled out, then artificial rupture of membranes and membrane sweeping is performed, and labor augmented as the other common cause of bleeding in this case is likely to be placenta abruptio. With the growing availability of obstetric ultrasound, double set-up examination is rarely necessary.
Imaging studies
The imaging studies for placental location can be obstetric ultrasonography (transabdominal, transvaginal or transperineal) and magnetic resonance imaging. Ultrasonography is the initial imaging study for confirmation or ruling out placenta previa. Transabdominal sonography is confirmatory of placenta previa in 96% of cases. Precision is improved by emptying the maternal urinary bladder. False positive results may be due to a full bladder or myometrial contractions. Poor imaging could be due to maternal obesity and posterior placenta (poorly visualized as a result of acoustic shadow from the fetal presenting part), lack of anatomical landmark posteriorly (compared to anterior uterovesical angle) below which placenta is defined.
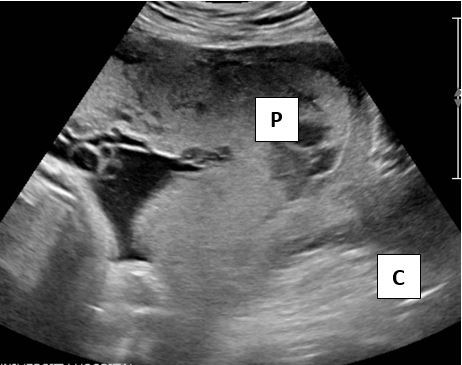
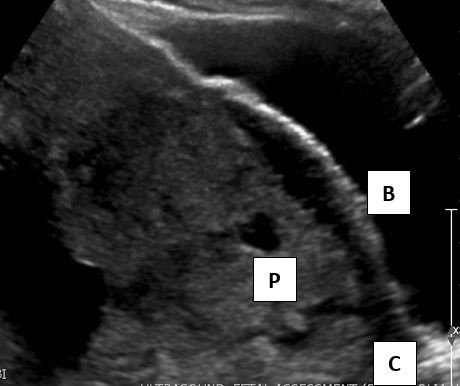
Transvaginal sonography (TVS) is safe and superior to the transabdominal ultrasound.18 Due to the proximity of the transducer to the uterus and higher frequencies, TVS has a superior resolution, does not require a full bladder and has nearly 100% accuracy (Figures 1 and 2).
1
Images showing total or complete placenta previa. Sonogram shows placenta (P) covering the cervix (C).
2
Sonogram of anterior placenta previa at 36 weeks’ gestation. The placental margin (P) extends downward toward the cervix (C) and close to the urinary bladder (B).
Transperineal sonography (TPS) is also accurate with a positive-predictive value of 98%, and negative-predictive value of 100%.19,20 TPS is more acceptable to patients compared to TVS. In addition, color Doppler ultrasound shows diffuse vascular lakes with turbulent flow in the hypoechoic areas near the cervix. Three-dimensional power Doppler showing hypervascularity at the uterine serosa bladder junction is diagnostic.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is superior to ultrasonography in visualizing placental abnormalities especially posterior placenta previa and placental accreta syndromes. Placenta previa appears as dark intraplacental bands on T2-weighted images. However, MRI is more time consuming, not portable, not widely available and expensive.
If undiagnosed preoperatively, placenta previa may be diagnosed or confirmed at cesarean section.
The differential diagnoses of placenta previa include abruptio placentae, local cervical lesions (polyps, carcinoma), circumvallate placenta, vasa previa or heavy show.
Management
Any pregnant woman with any amount of vaginal bleeding after 20 weeks’ gestation should be admitted. Admission is necessary to establish a diagnosis, and to undertake a comprehensive evaluation of maternal hemodynamic status and fetal well-being. In addition, admission is recommended because the severity of subsequent bleeding may be unpredictable. Further management of placenta previa is determined by fetal viability and gestational age, presence of labor, and severity of bleeding.
General management principles
At admission, the general condition of the patient is evaluated, and the degree of pallor vital signs, fetal heart rate established.
Two large-gauge intravenous lines are placed, intravenous normal saline started and blood samples taken for blood grouping and cross matching, estimation of hemoglobin/hematocrit and, if available, complete blood count, coagulation profile including prothrombin time and partial prothrombin time, International Normalized Ratio (INR), fibrinogen, and D-dimers levels. If transfusion is anticipated, blood urea nitrogen, creatinine, and electrolytes may also be evaluated.
A bedside clotting time can be performed by placing blood into a plain (red-top) tube and put aside. The blood should clot within 6 minutes, and delayed clotting is suggestive of coagulopathy. A standard Rhesus immunoglobulin dose of 300 mg is administered to Rh negative and indirect Coombs test negative women, unless the qualitative Kleihauer-Betke stain suggests the need for additional doses of Rh immunoglobulin. Rhesus negative women may also undergo a quantitative rosette test or flow cytometry to assess the degree of fetal–maternal hemorrhage.
For all cases of obstetric hemorrhage, use of evidence-based standards like obstetric safety bundles and obstetric hemorrhage protocols are highly encouraged.
Conservative management
Conservative management may be safe and extend the pregnancy by an average of 4 weeks after the sentinel bleeding. Antenatal corticosteroid, such as intramuscular betamethasone 12 mg every 24 hours for two doses or dexamethasone 6 mg every 12 hours for four doses, to enhance fetal lung maturation should be administered to patients who are at less than 36 weeks’ gestation, if delivery is not imminent. During conservative care, patients should be evaluated continuously by monitoring of vulval pads to assess for increased bleeding, and serial growth fetal ultrasonography every 2–3 weeks and regular hemoglobin check. Patients should receive supplementary hematinics or blood products if anemic. Patients on conservative management who are stable should remain admitted until at least 48 hours of no bleeding. Home management can be considered for stable asymptomatic cases, if there is a controlled setting at home, limited activity, adequate support and access to transport to a nearby hospital. Any subsequent significant bleeding patients are readmitted until delivery. Women on conservative management may benefit from pelvic rest, reduced strenuous physical activity, and avoidance of sexual intercourse. Unless contraindicated, tocolytics may be considered, but only during administration of antenatal corticosteroids.
Delivery
Delivery is the appropriate management for patients with placenta previa at or beyond 36 weeks’ gestation or severe bleeding, fetal compromise at any gestational age.
All women with placenta previa (types II, III, IV) are delivered by cesarean section, while asymptomatic women with low lying placenta more than 2 cm from the cervical os can undergo normal labor and delivery. In both cases, there is increased risk of primary postpartum hemorrhage from lower uterine segment atony.
Cesarean section for placenta previa should be performed by the most experienced team including obstetric, anesthetic and neonatal team members because of the substantial risk of intraoperative hemorrhage and adverse neonatal outcome. If alert, patients should be counseled and consent obtained for additional interventions like uterine compression sutures, e.g. tamponade sutures like B-Lynch and hysterectomy. Other support systems required may include urology, massive blood transfusion specialists, critical care, and interventional radiology for placenta previa and accreta.
When morbidly adherent placenta is suspected, uterine artery catheterization can be performed before the delivery by interventional radiology. Whereas regional or spinal-epidural anesthesia can be safely performed for nonurgent cases and is preferred due to lower blood loss, general anesthesia is often recommended primarily for unstable cases and when additional procedures are required, regional anesthesia can be converted to general anesthesia.
In severe cases and limited experience, a vertical skin incision provides optimal exposure. Otherwise a transverse incision may be adequate.
Cesarean section is often performed via a lower uterine segment incision due to vast experience, direct access to bleeding sinuses, and placenta accreta. However, these incisions are associated with substantial hemorrhage from anterior blood vessels, anterior placenta and the fetus. In general, the incision should be away from the placenta if placenta previa accreta is suspected/confirmed. If the placenta is anterior, the umbilical cord is clamped immediately to prevent excessive blood loss. A vertical incision is preferable in cases of premature fetus or transverse lie.
A classical cesarean section or high vertical uterine incision may be performed to expedite delivery, avoid or limit placental disturbance during delivery, reduce fetal hemorrhage and leave accreta placenta in situ and preserve uterus. However, reduced access makes it difficult to control bleeding. In addition, classical cesarean section is associated with increased hemorrhage and uterine rupture in subsequent pregnancies. After delivery of the fetus, and spontaneous separation of the placenta, the uterine incision is closed and any excess hemorrhage controlled by standard procedures such as uterotonics, tranexamic acid, bimanual uterine massage, balloon tamponade, compression B-Lynch, Hackethal or Cho sutures, uterine artery or internal iliac artery ligation, uterine artery or internal iliac artery embolization or hysterectomy depending on the skill set and availability of required resources. Patients with placenta previa should be screened for morbidly adherent placenta (MAP), if there is high suspicion, and a cesarean section should be performed without manipulation of the placenta. In addition, hemorrhage from MAP can be managed conservatively by resection or leaving placenta in situ, or through cesarean hysterectomy.5,21,22,23,24
Complications
In general, up to one-third of patients with early onset of bleeding from placenta previa complete 36 weeks of gestation leading to good maternal and perinatal outcomes. Although maternal mortality is less than 1% and perinatal mortality is less than 5%, following placenta previa, there can be significant morbidity for both the woman and fetus or neonate.
Maternal complications
Women with placenta previa have higher risk of postpartum hemorrhage due to atony of lower uterine segment and bleeding at the placental implantation site. As a result, they are more likely to receive additional uterotonics for example high-dose oxytocin, misoprostol, ergometrine, carbetocin, Carboprost (15-methyl prostaglandin F2α) and/or surgical interventions like suturing of the placental bed, B-Lynch suture, hysterectomy. In addition to antepartum, intrapartum and postpartum hemorrhages, other complications of placenta previa include cesarean delivery and associated complications. Catastrophic maternal hemorrhage from placenta previa may result in end organ dysfunction such as acute tubular necrosis and Sheehan syndrome following pituitary infarction, blood transfusion, septicemia, thrombophlebitis, disseminated intravascular coagulation, prolonged hospitalization, and maternal death. Prior uterine incision and placenta previa have an increased likelihood of MAP (as placenta accreta, increta, and percreta) and its management including cesarean hysterectomy.
Neonatal complications
Preterm birth represents the greatest source of morbidity for the fetus. In addition, about one-third (35%) of infants whose mothers receive blood transfusion also require transfusion. In severe cases, there may be fetal anoxia and intrauterine or early neonatal death. Preterm infants who survive severe intrauterine hypoxia may suffer long-term neurocognitive and developmental disability.
PLACENTA ABRUPTIO
Placenta abruptio or abruptio placentae, of the causes of bleeding during pregnancy, refers to the partial or total premature separation of the normally implanted placenta before delivery of the fetus, and after 20 weeks’ gestation.
Epidemiology
Placenta abruptio contributes to about one-third of all antepartum hemorrhages, occurs in about 0.5–1% of all pregnancies, causes fetal death in about 1 of every 420–830 deliveries and results in 10% of preterm births.25,26 Although some studies show increases others show decreases or plateau in incidence and/or prevalence of abruptio placenta.25 The highest incidence of placenta abruption occurs at 24–26 weeks’ gestation.27
Classification
Several categories of abruption placenta are described. Some are more severe with greater risk of adverse consequences.
Placenta abruptio can be, retroplacental, between the placenta and myometrium; marginal, subchorionic, between the placenta and membranes; or preplacental, subamniotic, between the placenta and the amniotic fluid.25,27 Retroplacental abruptio, the most significant category, is described as severe if 30–40% of the surface area is involved. If this severe form contains more than 60 mL of blood, it results in 50% fetal mortality as result of compromised fetal oxygenation and perfusion. A retroplacental abruption in which there is no obvious discernible external bleeding is also called concealed abruptio placenta. Occurring at the edge of the placenta, marginal placenta abruptio results in lifting of the placental edge away from the uterus. In severe cases, marginal placenta abruptio may extend from the edge to the rest of the placenta. Preplacental placental abruptio are often without clinical significance.
Severity of placenta abruptio has been graded using the Sher Severity system into three grades.28 Grade 1 have less than 100 mL of uterine bleeding, non-tender uterus, and reassuring fetal status; grade 2 have tender uterus, non-reassuring fetal status, and concealed hemorrhage, and grade 3 the most severe form is characterized by fetal demise, maternal shock, and extensive concealed hemorrhage. Grade 3A have no coagulopathy compared to grade 3B who have coagulopathy. Severity based on placental surface detachment of below 20%, 20–40% and above 40% has been associated no fetal danger, imminent fetal distress and imminent fetal demise, respectively.29
Pathogenesis
The pathophysiology of placenta abruption varies on whether the abruptio is spontaneous (majority of cases) or traumatic.25,30 Preceded by impaired trophoblastic invasion and atherosis, inflammation or infection, spontaneous placenta abruption is thought to start when a decidual spiral artery ruptures into the decidua basalis causing a retroplacental hematoma. The hematoma may expand causing further disruption of more spiral vessels and extension of placental abruptio and compression of the underlying placenta. In traumatic abruptio placenta, placental separation may result from varying degrees of trauma from such sources as motor vehicle accidents, physical assault or fall. In these cases, the placental separation is thought to result from deceleration injury as well as deformation of the elastic myometrium around an inelastic placenta. In spontaneous abruptio the source of bleeding is usually maternal due to separation within the maternal decidua, while the placental villi are unaffected. Fetal–maternal hemorrhage is likely to occur in traumatic abruption when there is placental tear.
Placenta abruptio can also be acute or chronic in onset. Chronic abruption placenta begins early in pregnancy and may be associated with oligohydramnios as part of chronic abruption-oligohydramnios sequence (CAOS).30,31,32,33
Risk factors
Factors associated with higher risk placenta abruptio4,12,13,14,15,16,25,27,34 include:
- Increasing maternal age. The incidence of placenta abruption increases with maternal age and is for example 2.3 times more likely among women above 40 years compared with those aged 35 years or younger
- Race or ethnicity. Severe grades of abruption are more common in African-American and white women versus Asian or Latin-American women at 1 : 200, 1 : 300 and 1 : 350, respectively.
- Genetic and family history. Severe grades of abruptio placenta are associated with increased risk of abruptio among sisters. High levels of homocysteine can lead to thrombosis and damage of the spiral arteries leading to placental abruption. Hyperhomocystinemia results from mutations in the methylenetetrahydrofolate reductase gene, which prevents normal remethylation. This can be reduced by folate and pyridoxine supplementation.
- Hypertensive disorders in pregnancy. The risk of placental abruption is up to two-fold higher in women with gestational hypertension, pre-eclampsia, chronic hypertension, or chronic hypertension with superimposed pre-eclampsia.
- Preterm premature rupture of membranes (PROM). The risk of abruption is up to 5% following PROM.
- Cigarette smoking is associated with two-fold risk for abruption.
- Cocaine use is associated with severe grades of abruptio that may result in stillbirth.
- Lupus anticoagulant and thrombophilias. There is mixed evidence on the association between lupus anticoagulant and thrombophilias and placental abruption
- Incidence of abruptio placenta is higher in women with fibroids especially those at placental implantation site and myoma volume exceeding 200 mL.
- Prior placenta abruption. Women with prior abruptio especially of severe grade have higher risk of recurrent abruptio, which increases with the number of previous placenta abruptio.
- There are conflicting data regarding increase risk among women of great parity.
Diagnosis
The diagnosis of placenta abruptio is based on history, clinical examination findings and may be supported by imaging studies, laboratory, and postpartum histopathologic studies.
History
The typical clinical presentation of abruptio placenta is sudden onset of abdominal (uterine) pain and vaginal bleeding. Other symptoms are associated with severity of bleeding. However, in nearly 20% of cases, placenta abruptions are concealed and, therefore, do not present with vaginal bleeding. Thus, the amount and severity of vaginal bleeding may not correlate with the grade or severity of abruptio placenta. Posteriorly located abruptio placenta may present with varying degrees of back pain. In severe cases of abruptio placenta, there may fetal compromise hence loss of fetal movement.
Examination
General examination findings correlate with severity of abruptio irrespective of the amount of vaginal bleeding. Patients may be stable or present in shock. Those with hypertensive disorders in pregnancy may present with elevated blood pressures despite substantial blood loss.
Abdominal examination typically reveals uterine tenderness and irritability with or without uterine contractions. Increased uterine tone may obscure uterine contractions. Fetal status can be reassuring, nonreassuring or in severe cases where more than half of the placenta separates, fetal death may occur. In cases of massive concealed abruption patients may have severe pain, a hard uterus, fetal demise and coagulopathy which may manifest with easy bruisability or epistaxis.
Pelvic examination is not recommended unless placenta previa is completely ruled out.
Imaging studies
Obstetric sonography
While ultrasound may identify chronic cases of placenta abruptio, it can not differentiate placenta from fresh clots and, therefore, has limited to no role in detecting acute bleeding from placental separation. If positive, early cases of abruptio are hyperechoic or isoechoic compared to placenta. The clots may become hypoechoic and then sonolucent 2 weeks after the acute phase. Jello sign refers to the “wobble or jiggle” of the clots when bounced by the transducer.27,35 The sensitivity of sonography is estimated at only 25%, and negative findings do not exclude abruptio placenta. About half of patients with abruptio abruptions produce have no findings on ultrasound. The main role of ultrasound, however, is to rule out placenta previa.
Magnetic resonance imaging
When patients are stable and diagnosis is uncertain, MRI may be considered as it is highly sensitive for placental abruption.
Other investigations
Laboratory tests that may support the diagnosis of abruptio placenta include elevated serum levels of D-dimers. Leakage across the placenta may lead to elevated maternal serum levels of α-fetoprotein and β-human chorionic gonadotropin. Inhibin A levels may or may not be increased.
Cardiotocography may demonstrate uterine contractions and abnormal fetal heart rate such as variable and late decelerations, poor variability, prolonged bradycardia, or a sinusoidal pattern due to fetal hypoxia and asphyxia. Rh negative women may undergo Indirect Coombs Test and Kleihauer-Betke testing prior to administration of Rh immunoglobulin.
Differential diagnosis
The differential diagnoses of abruptio placenta include bleeding from placenta previa, vasa previa, heavy show or local causes. Other differential diagnoses include uterine rupture, chorioamnionitis, preterm labor and other sources of abdominal pain.
Management
Similar to cases of placenta previa, women with any amount of vaginal bleeding or suspected to have placenta abruptio after 20 weeks’ gestation should be admitted for individualized timely care to ascertain fetal and maternal well-being and avert adverse maternal and perinatal outcomes. Management of placenta abruptio is determined by severity of hemorrhage, maternal hemodynamic stability, fetal status and gestational age, and presence of labor.
General management principles
At admission, the general condition of the patient is assessed and maternal vital signs including fetal heart rate measured and monitored continuously or as often as is feasible to detect any early deterioration. One or two large-gauge intravenous lines are placed for collection of blood samples for a baseline complete blood count including platelet count, blood grouping and cross-match, baseline blood urea nitrogen and electrolytes, coagulation profile, and a bedside clotting time. In severe cases, the patient is catheterized to monitor urine output. Early involvement of the multidisciplinary care team may be required for central venous pressure line insertion and monitoring, massive blood transfusion specialists or renal team and neonatal care team as appropriate. Rhesus immunoglobulin is administered to Rh negative women if indicated. For all cases of obstetric hemorrhage, use of evidence-based standards like obstetric safety bundles and hemorrhage protocols to optimize maternal and neonatal outcomes are highly encouraged.
Conservative management
Pregnancies complicated with abruptio placenta below 34 weeks may be managed conservatively if mother and fetus are stable. Conservative management may be safe and extend the pregnancy by an average of 4 weeks after the sentinel bleeding. Antenatal corticosteroid for example intramuscular betamethasone 12 mg every 24 hours for two doses or dexamethasone 6 mg every 12 hours for four doses to enhance fetal lung maturation should be administered to patients who are at less than 36 weeks’ gestation, if delivery is not imminent. During conservative care, patients should be evaluated continuously by monitoring of vulval pads to assess for increased bleeding, and serial growth fetal ultrasonography every 2–3 weeks and regular hemoglobin check. Patients should receive supplementary hematinics or blood products if anemic. Patients on conservative management who are stable should remain admitted until at least 48 hours of no bleeding. Home management can be considered for stable asymptomatic cases, if there is a controlled setting at home, limited activity, adequate support and access to transport to a nearby hospital. Any subsequent significant bleeding patients are readmitted until delivery. Women on conservative management may benefit from pelvic rest, reduced strenuous physical activity, and avoidance of sexual intercourse. Unless contraindicated, tocolytics may be considered only during administration of antenatal corticosteroids. When conservative care is offered, plans should be put in place for emergency delivery or delivery at or near term.
Delivery
The timing and route of delivery is determined by fetal and maternal stability. Maternal or fetal compromise mandates immediate delivery, usually by cesarean. Immediately delivery is recommended if the patient is unstable patients, there is non-reassuring fetal status or after 34 weeks of gestation. In severe cases, delivery should be realized via emergency cesarean section unless the patient is in advanced labor. For vaginal delivery, early amniotomy and, if needed, oxytocin can be administered to expedite delivery. Vaginal route, unless contraindicated or the mother is hemodynamically unstable, is recommended for all cases of fetal death.
Complications
Although maternal mortality remains low (0.5–1%), the fetal mortality is very high (20–70%) depending on severity of separation, the underlying cause, and gestational age.
Maternal complications
Patients with placental abruption may present with hypovolemic shock which may lead to acute tubular necrosis, acute cortical necrosis or acute kidney injury and consumptive coagulopathy due to procoagulant consumption from intravascular activation of clotting factors. Women with consumptive coagulopathy have elevated fibrinogen, and prolonged prothrombin time and partial thromboplastin time. These complications are more frequent in women with massive, and especially concealed, abruption. Extravasation of blood into uterine myometrium and subserosa due to uteroplacental apoplexy results in couvelaire uterus. Blood may also extravasate into the broad ligaments, ovaries, and free in the peritoneal cavity. Although a concern due to uterine hypotony, couvelaire uterus is not an indication for hysterectomy. Severe grades of abruptio placenta may lead to Sheehan syndrome which may affect all pituitary hormones leading to varying degrees of failed lactation, amenorrhea, breast atrophy, loss of pubic and axillary hair, hypothyroidism, and adrenal cortical insufficiency.
Neonatal complications
Abruptio placenta may lead to preterm birth and related sequelae, non-reassuring fetal status or even worse fetal demise, and CAOS. About 10–15% of fetal survivors of placenta abruption suffer neurological sequelae.
Conclusion
Placenta previa and abruptio remain the major leading causes of antepartum hemorrhage, with varying degrees of maternal and perinatal morbidity and mortality. Painless or painful vaginal bleeding is the hallmark of placenta previa versus abruption. Identification of risk factors may further guide the most likely diagnosis and antecedent complications. Patients with suspected placenta abruptio or placenta should be admitted for comprehensive evaluation and stabilization. Delivery is planned at or near term for stable patients but earlier for cases for severe bleeding.
HUMAN RESOURCES
There is need for continued training and mentorship of midwives and other cadres of obstetric care team on management protocols, algorithms and referral processes for patients with placenta previa and abruption. For quick clinical decision-making, bedside ultrasound training and resources should be available for labor ward staff to help triage patients with suspected placenta abruptio or previa. Centers of excellence in management of placenta previa and morbidly adherent placenta are needed to avert adverse outcomes associated with lower level of skill and experience. Local and regional blood transfusion services and well equipped and trained local laboratory personnel are required and essential for averting adverse maternal and perinatal outcomes associated with placenta previa and abruptio.
IMPLICATIONS FOR HEALTH SYSTEMS IN LOW-RESOURCE COUNTRIES
Pregnant women with vaginal bleeding require prompt and comprehensive maternal and fetal evaluation. Therefore, all women with suspected placenta abruptio and placenta previa should have clear documentation on the evaluation, potential final diagnosis and management plan. For unstable and preterm pregnancies referral to more equipped facilities should be made early and clearly documented.
Health systems should be equipped to identify risk factors for these pregnancy complications and where possible administer prevention interventions like folic acid and folate supplementation and reducing unnecessary (primary) cesarean sections. In line with the recent World Health Organization Antenatal Care Guidelines, routine one obstetric ultrasound after 20 weeks may identify subclinical cases of placenta previa or abruptio.
In tertiary and better-equipped health facilities, all pregnant women with placenta previa and abruption should be closely monitored while aiming at timely delivery and identification of potential complications from severe bleeding such as acute kidney injury and coagulopathy. Furthermore, when delivery is indicated anticipation and management of potential intrapartum including intraoperative complications should be prioritized.
Health facilitie,s where women with placenta abruptio and previa deliver, should undergo regular auditing of care and outcomes in order to increase identification and management. For example where morbidly adherent placenta is not diagnosed until intrapartum or postpartum, such reports can improve the quality of ultrasound imaging and reporting. In addition, if severe maternal outcomes arise from lack of blood products, blood transfusion infrastructure can be strengthened to improve outcomes.
Just like antepartum and intrapartum care, postpartum care after deliveries complicated with placenta previa and abruption is critical in preventing further deterioration of maternal condition and newborn care. Therefore, facilities should be equipped with maternal critical care and newborn intensive care units to handle postpartum and newborn complications. Where these services are not available, clear referral mechanisms must be identified. Counseling of mothers and their families should be prioritized especially where severe morbidity and mortality occurs. Debriefing mechanism for health care providers should also be availed.
Finally, advocacy, awareness and legal framework are required to increase awareness and accountability when taking care of women with placenta previa and or abruption. Different platforms that emphasize vaginal bleeding as a danger sign during pregnancy and the need for prompt care may reduce the first of three delays in averting maternal and/or perinatal mortality. Women and families should know their rights and advocate for proper physical and financial access to skilled health care provision. Also, because preterm birth or severe maternal hemorrhage may be accompanied by huge financial costs, health systems should consider cost alleviation.
In conclusion, health facilities offering care for pregnant women with placenta previa and abruptio must be should be equipped to evaluate, diagnose, refer and comprehensively take care of the patients from diagnosis to the postpartum period. Health systems should consider the supportive care from laboratory and blood transfusion services as well as cost implication following severe morbidity or mortality related to placenta abruptio or previa. Community participation, advocacy and awareness are key to addressing the first and second delays in care. Audits and reviews after deliveries complicated by placenta previa and abruptio can help identify and address factors associated with the third delay.
PRACTICE RECOMMENDATIONS
- Facilities taking care of pregnant women with vaginal bleeding after 20 weeks of gestation must be equipped with resources to diagnose the most likely cause of bleeding. Where this is not possible such patients must be promptly referred to better-equipped facilities.
- Facilities taking care of pregnant women with placenta previa and/or abruption must offer every woman and her family with clear plan of management from initial point of contact to the postpartum period including when referral may be required.
- Pregnant women with vaginal bleeding after 20 weeks of gestation require psychosocial support, education, communication, and financial support especially after prolonged hospitalization and related costs.
- Facilities taking care of pregnant women with vaginal bleeding after 20 weeks of pregnancy who do not have critical care services must have clear referral mechanism and resources.
- Audits following deliveries complicated with placenta previa and/or abruption can identify gaps in care and reduce future adverse pregnancy outcomes.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Faiz AS, Ananth CV. Etiology and risk factors for placenta previa: an overview and meta-analysis of observational studies. J Matern Fetal Neonatal Med 2003;13(3):175–90. | |
Gallagher P, Fagan CJ, Bedi DG, et al. Potential placenta previa: definition, frequency, and significance. AJR Am J Roentgenol 1987;149(5):1013–5. | |
Cresswell JA, Ronsmans C, Calvert C, et al. Prevalence of placenta praevia by world region: a systematic review and meta-analysis. Trop Med Int Health 2013;18(6):712–24. | |
Getahun D, Oyelese Y, Salihu HM, et al. Previous cesarean delivery and risks of placenta previa and placental abruption. Obstet Gynecol 2006;107(4):771–8. | |
Jauniaux E, Chantraine F, Silver RM, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: Epidemiology. Int J Gynaecol Obstet 2018;140(3):265–73. | |
Silver RM. Abnormal Placentation: Placenta Previa, Vasa Previa, and Placenta Accreta. Obstet Gynecol 2015;126(3):654–68. | |
Sisson EM, Dixon DL, Dow AW. A Trial of Blood-Pressure Reduction in Black Barbershops. N Engl J Med 2018;379(2):200. | |
Oppenheimer L, COMMITTEE MFM. Diagnosis and management of placenta previa. J Obstet Gynaecol Can 2007;29(3):261–6. | |
Reddy UM, Abuhamad AZ, Levine D, et al., Participants FIWI. Fetal imaging: Executive summary of a Joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists, American College of Radiology, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound Fetal Imaging Workshop. Am J Obstet Gynecol 2014;210(5):387–97. | |
Heller HT, Mullen KM, Gordon RW, et al. Outcomes of pregnancies with a low-lying placenta diagnosed on second-trimester sonography. J Ultrasound Med 2014;33(4):691–6. | |
Cho JY, Lee YH, Moon MH, et al. Difference in migration of placenta according to the location and type of placenta previa. J Clin Ultrasound 2008;36(2):79–84. | |
Aliyu MH, Lynch O, Wilson RE, et al. Association between tobacco use in pregnancy and placenta-associated syndromes: a population-based study. Arch Gynecol Obstet 2011;283(4):729–34. | |
Taylor VM, Peacock S, Kramer MD, et al. Increased risk of placenta previa among women of Asian origin. Obstet Gynecol 1995;86(5):805–8. | |
Macones GA, Sehdev HM, Parry S, et al. The association between maternal cocaine use and placenta previa. Am J Obstet Gynecol 1997;177(5):1097–100. | |
Hendricks MS, Chow YH, Bhagavath B, et al. Previous cesarean section and abortion as risk factors for developing placenta previa. J Obstet Gynaecol Res 1999;25(2):137–42. | |
Ananth CV, Smulian JC, Vintzileos AM. The association of placenta previa with history of cesarean delivery and abortion: a metaanalysis. Am J Obstet Gynecol 1997;177(5):1071–8. | |
Stallworthy J. The dangerous placenta. Am J Obstet Gynecol 1951;61(4):720–37. | |
Leerentveld RA, Gilberts EC, Arnold MJ, et al. Accuracy and safety of transvaginal sonographic placental localization. Obstet Gynecol 1990;76(5 Pt 1):759–62. | |
Rani PR, Haritha PH, Gowri R. Comparative study of transperineal and transabdominal sonography in the diagnosis of placenta previa. J Obstet Gynaecol Res 2007;33(2):134–7. | |
Hertzberg BS, Bowie JD, Carroll BA, et al. Diagnosis of placenta previa during the third trimester: role of transperineal sonography. AJR Am J Roentgenol 1992;159(1):83–7. | |
Sentilhes L, Kayem G, Chandraharan E, et al., Panel FPADaMEC. FIGO consensus guidelines on placenta accreta spectrum disorders: Conservative management. Int J Gynaecol Obstet 2018;140(3):291–8. | |
Jauniaux E, Bhide A, Kennedy A, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. Int J Gynaecol Obstet 2018;140(3):274–80. | |
Jauniaux E, Ayres-de-Campos D, Panel FPADaMEC. FIGO consensus guidelines on placenta accreta spectrum disorders: Introduction. Int J Gynaecol Obstet 2018;140(3):261–4. | |
Allen L, Jauniaux E, Hobson S, et al., Panel FPADaMExpert C. FIGO consensus guidelines on placenta accreta spectrum disorders: Nonconservative surgical management. Int J Gynaecol Obstet 2018;140(3):281–90. | |
Tikkanen M. Placental abruption: epidemiology, risk factors and consequences. Acta Obstet Gynecol Scand 2011;90(2):140–9. | |
Ananth CV, Keyes KM, Hamilton A, et al. An international contrast of rates of placental abruption: an age-period-cohort analysis. PLoS One 2015;10(5):e0125246. | |
Oyelese Y, Ananth CV. Placental abruption. Obstet Gynecol 2006;108(4):1005–16. | |
Sher G, Statland BE. Abruptio placentae with coagulopathy: a rational basis for management. Clin Obstet Gynecol 1985;28(1):15–23. | |
Nkwabong E, Tiomela Goula G. Placenta abruption surface and perinatal outcome. J Matern Fetal Neonatal Med 2017;30(12):1456–9. | |
Ananth CV, Oyelese Y, Prasad V, et al. Evidence of placental abruption as a chronic process: associations with vaginal bleeding early in pregnancy and placental lesions. Eur J Obstet Gynecol Reprod Biol 2006;128(1–2):15–21. | |
Kurata Y, Kido A, Minamiguchi S, et al. MRI findings of chronic abruption-oligohydramnios sequence (CAOS): report of three cases. Abdom Radiol (NY) 2017;42(7):1839–44. | |
Kobayashi A, Minami S, Tanizaki Y, et al. Adverse perinatal and neonatal outcomes in patients with chronic abruption-oligohydramnios sequence. J Obstet Gynaecol Res 2014;40(6):1618–24. | |
Elliott JP, Gilpin B, Strong TH, et al. Chronic abruption-oligohydramnios sequence. J Reprod Med 1998;43(5):418–22. | |
Ananth CV, Smulian JC, Demissie K, et al. Placental abruption among singleton and twin births in the United States: risk factor profiles. Am J Epidemiol 2001;153(8):771–8. | |
Glantz C, Purnell L. Clinical utility of sonography in the diagnosis and treatment of placental abruption. J Ultrasound Med 2002;21(8):837–40. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)