This chapter should be cited as follows:
Aref A, Napolitano R, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.409583
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 10
Common obstetric conditions
Volume Editor: Professor Sikolia Wanyonyi, Aga Khan University Hospital, Nairobi, Kenya

Chapter
Polyhydramnios
First published: February 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Polyhydramnios (which can be sometimes referred to as hydramnios) is an excessive accumulation of amniotic fluid. Polyhydramnios may be associated with an increased risk of adverse pregnancy outcomes, such as preterm birth, placental abruption and fetal anomalies.1 The incidence in a general obstetric population usually ranges from 0.2 to 1.6%.1,2 In the past, the diagnosis of polyhydramnios was made clinically with detection of a symphyseal fundal height (SFH) measured in centimeters 2 cm larger than the respective gestational age measured in weeks or above the 90th centile according with a specific norm,3,4 or the occurrence of exaggerated pregnancy symptoms. However, ultrasound assessment of the amniotic fluid volume is the most accurate method of diagnosis. There are various methods of assessing the amniotic fluid volume. This assessment can be a subjective assessment or a quantitative estimation using the maximal vertical pocket (MVP), amniotic fluid index (AFI), two-diameter pocket or three-dimensional measurements.2 Currently, it is widely accepted that polyhydramnios is defined as an MVP greater than 8 cm, or an AFI of 25 cm or greater.1,5,6 Polyhydramnios can further be classified according to the severity into mild, moderate and severe, with severe polyhydramnios in the second trimester being associated with significant perinatal morbidity due to prematurity and risk of aneuploidy.7,8 Other authors classify polyhydramnios according to the etiological causes whether idiopathic, or due to maternal or fetal causes. This is because the outcome and pregnancy management vary according to the causes.
ETIOLOGY AND PATHOPHYSIOLOGY
The amniotic fluid volume depends on a balance between its production and its removal. In early pregnancy, there is little fetal contribution to the amniotic fluid. Later in pregnancy, the two primary sources of amniotic fluid are the fetal kidneys and lungs. The primary sources of amniotic fluid removal are fetal swallowing and absorption into the fetal blood perfusing the surface of the placenta. The relative contribution of each of these mechanisms varies markedly over the course of the pregnancy.1,3 For instance, a fetus close to term will produce between 500 and 1200 ml urine and swallow between 210 and 760 ml of amniotic fluid per day. Even small changes in this equilibrium can result in significant alterations in amniotic fluid volume at this stage which reflects the association between fetal gastrointestinal tract obstruction and severe cases of polyhydramnios.6
Furthermore, uteroplacental unit function contributes to amniotic fluid balance. If uteroplacental insufficiency occurs in association with fetal growth restriction, the fetus redistributes the blood flow to critical areas such as the heart, the brain and the suprarenal glands, at the expense of renal perfusion. This reduced blood flow to the kidneys results in decreased renal output, leading to oligohydramnios. This explains the presence of oligohydramnios in cases of placental insufficiency. This is evident in most severe cases of fetal growth restriction when signs of decompensation are present, mainly occurring before 32 weeks of gestation.
Recently, a more modern concept recognizes that amniotic fluid is dynamic and can rapidly change due to normal maternal physiological processes (hydration, activity and rest phase, position, etc.). It is unlikely that such factors would affect the ultrasound observations; however, they may alter the diagnosis near the upper and lower thresholds of normality.
The incidence of polyhydramnios ranges between 0.2 and 1.6%, with differences in the reported rates due to variations in diagnostic criteria. Causes of polyhydramnios can be idiopathic (60%; where no fetal or maternal causes can be identified such as in cases of fetal macrosomia not associated with maternal diabetes) or due to maternal or fetal causes (Table 1).8,9
1
Causes of polyhydramnios.
1. | Idiopathic causes 60% |
2. | Maternal causes
|
3. | Fetal causes
|
In cases when polyhydramnios is due to fetal anomalies, the outcome depends on the underlying pathology. Severe polyhydramnios in the second trimester has a significant association with perinatal mortality rates due to prematurity or aneuploidy.7,8 Polyhydramnios has been reported to increase the risk for placental abruption and postpartum hemorrhage as a result of over distension or rapid deflation of the uterus. The most common cause of severe polyhydramnios are fetal anomalies (often associated with an underlying genetic abnormality or syndrome), while idiopathic factors, maternal diabetes, and multiple gestation are more often associated with milder cases.10,11
Polyhydramnios can be caused by fetal anomalies in most organ systems. The most common structural anomalies associated with polyhydramnios are those that interfere with fetal swallowing and/or the absorption of amniotic fluid. Decreased swallowing may be due to a primary gastrointestinal obstruction (e.g. duodenal, esophageal, or intestinal atresia), neuromuscular disorders (e.g. anencephaly), or due to secondary gastrointestinal tract obstruction as in cases of massive unilateral dysplastic kidneys that may cause fetal bowel obstruction. Also, increased amniotic fluid may be attributed to increased urine production due to decreased gastrointestinal absorption secondary to bowel compression, or tumor secretion of prostaglandins leading to hypercalcemia-induced polyuria.11,14
The presence of fetal growth restriction in cases of polyhydramnios can be suggestive of trisomy 18 where other sonographic markers of trisomy 18 are typically present. Excess amniotic fluid in this syndrome may be related to difficulty swallowing or to intestinal abnormalities.13
Increased urine production may occur in high fetal cardiac output states (e.g. fetal anemia due to alloimmunization, parvovirus infection, fetomaternal hemorrhage, and hemolysis) or, rarely in syndromes such as fetal Bartter syndrome.14
In the rare Bartter syndrome, which is usually an autosomal recessive tubular disorder associated with intrauterine presentation of renal tubular hypokalemic alkalosis. The fetus usually develops polyuria and later polyhydramnios during the second half of pregnancy. Infants usually exhibit postnatal polyuria and persistent renal salt wasting, requiring life-long treatment. A severe but transient form of Bartter syndrome has been attributed to mutations associated with the X chromosome that appear to be essential for fetal renal salt reabsorption and maintenance of normal amniotic fluid homeostasis.11,14
In monochorionic multiple gestation, polyhydramnios/oligohydramnios sequence is diagnostic of twin–twin transfusion syndrome (TTTS). The mechanism for polyhydramnios in pregnancies complicated by maternal diabetes is unclear, but theoretically may be owing to fetal osmotic diuresis secondary to fetal hyperglycemia. This contributes to mild degrees of polyhydramnios in most of the cases. Maternal diabetes, especially if poorly controlled can lead to fetal macrosomia (estimated fetal weight on ultrasound above 4.5 kg or >95th centile according with a specific norm).12,15
CLINICAL EVALUATION
Polyhydramnios can be suspected by finding a uterine size that is larger for gestational age by SFH measurement than the norm. The increase in amniotic fluid volume is usually asymptomatic; however, the pregnant woman may experience persistent shortness of breath, uterine irritability and contractions, and abdominal discomfort when uterine distention is severe. In some other cases, it can present clinically by the associated complications or consequences it causes like maternal respiratory compromise, preterm labor, premature rupture of membranes (PROM), with consequent risk of umbilical cord prolapse, preterm delivery, fetal malposition, macrosomia, placental abruption, prolonged labor, or postpartum uterine atony. In case of the above symptoms or sudden increase in the SFH measurement according with specific norms, the woman should be referred for ultrasound assessment.3,4,5
There is no specific threshold beyond which an increased SFH is associated with polyhydramnios. WHO, in its 2018 recommendation on SFH measurement, stated that it is a commonly practiced method for fetal growth assessment, in order to detect intrauterine growth restriction (IUGR) and has also the potential to detect multiple pregnancy, macrosomia, polyhydramnios and oligohydramnios.3 For fetuses growing normally (from 24 weeks of gestation), the SFH measurement in centimeters should correspond to the number of weeks of gestation, with an allowance of a 2-cm difference either way. This cut-off value of more than 2 cm can be used as a low-cost method for detecting polyhydramnios in areas where ultrasound (the most accurate screening tool) is resource-intensive and not widely available.3 A more evidence-based criterion for diagnosis is to consider a SFH greater than the 90th centile according with prescriptive charts developed in low-risk women. The international standards for SFH measurements are freely available online, although the suggested cut-off to improve outcome is under evaluation.4
DIAGNOSIS
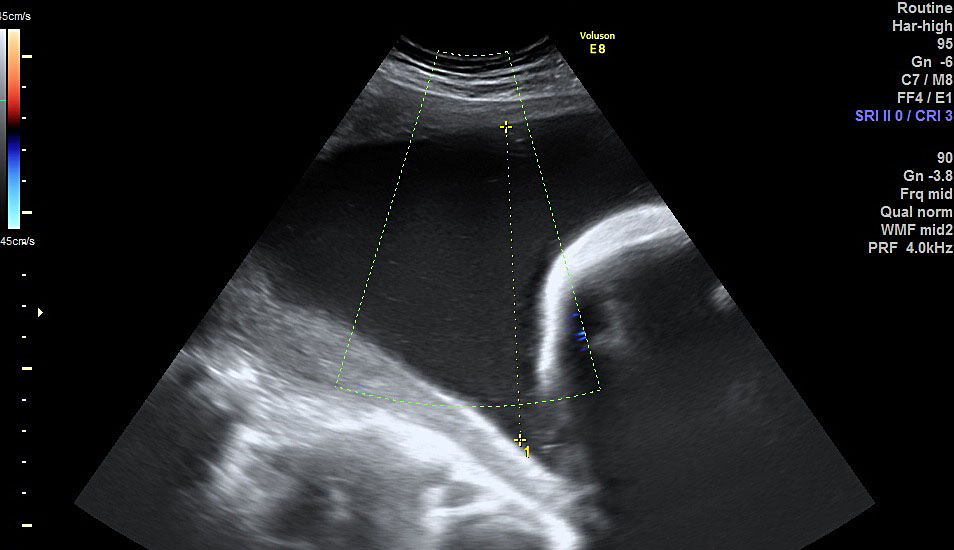
The current main modality of diagnosing polyhydramnios is ultrasound assessment of amniotic fluid volume whether subjectively or quantitative assessment. The quantitative assessment is more reproducible and can be either measuring the MVP ≥8 cm or AFI ≥25 cm, at any gestation (the use of charts for the measurements is not validated). To measure the AFI, the uterus is divided into four quadrants using the umbilicus as a reference. The probe is held longitudinally to the mother and at right angles to the sagittal plane of the patient’s abdomen (Figure 1). The deepest vertical pool in each quadrant that contains no fetal parts or umbilical cord is measured sequentially and cumulatively. For measuring the MVP, the uterus is divided into four quadrants. The amniotic fluid volume is measured vertically in the deepest amniotic fluid pocket (Figure 2).16 Both methods are universally used for diagnosis. There is no consensus on which one should be the method of choice. They have been reported as having similar reproducibility.17 The use of AFI seems not to be associated with a better outcome and therefore the Society of Obstetricians and Gynaecologists of Canada recommend to use the MVP as the preliminary ultrasound quantitative assessment.5,18 However, in view of the more detailed association with chromosomal abnormality, we suggest that the AFI should be calculated routinely in any case of suspected polyhydramnios or MVP >8 cm.10 Invasive methods (such as dye dilution) have not been proved to be of clinical significance. Some authors categorize patients with polyhydramnios into mild (MVP 8–11 cm or AFI 25–<30 cm), moderate (MVP 12–15 cm or AFI 30–<35 cm) and severe (MVP ≥16 cm or AFI ≥35 cm).
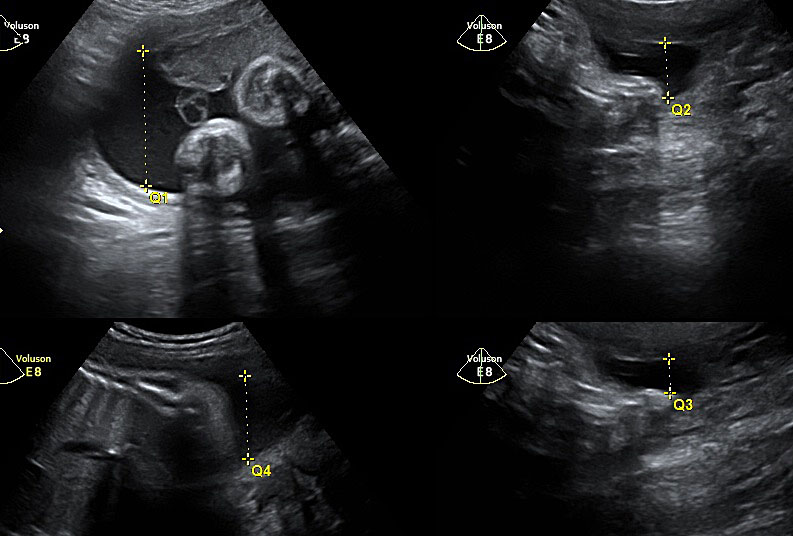
1
Measurement of amniotic fluid index.
2
Measurement of maximal vertical pocket.
The next step in diagnosis of polyhydramnios after evaluation of severity, is a comprehensive maternal and fetal evaluation with the aim of identifying whether the cause is fetal, maternal or idiopathic. A detailed medical history should be obtained to evaluate the heritable diseases associated with polyhydramnios. Screening for maternal diabetes is appropriate if not previously performed.19 Furthermore, a detailed sonographic evaluation should be performed to look for fetal structural anomalies or fetal hydrops. Chromosomal analysis should be offered when congenital anomalies are detected, as this information is likely to affect management. Amniocentesis for karyotype in all cases of severe polyhydramnios is not routinely recommended; however, if the AFI is >30 cm and genetic laboratory investigation is available to explore for microarray mutation, karyotype by invasive methods should be offered. In severe polyhydramnios with a normal karyotype and ultrasound findings of anomalies, microarray or gene sequencing may detect a genetic abnormality of clinical significance. For example, 22q11.2 micro deletion syndrome is associated with polyhydramnios and hypo-plastic thymus as the only sonographic findings. Also, Noonan’s syndrome is often associated with polyhydramnios, as well as other abnormalities, and can be diagnosed by gene sequencing.20,21
If fetal hydrops is identified, the next step is evaluation for an immune or nonimmune etiology. In cases of fetal hydrops associated with polyhydramnios, evaluation of potential fetal anemia includes assessment of peak systolic velocity of the middle cerebral artery with a value >1.5 multiples of the median, suggesting moderate or severe anemia, irrespective of the cause. Work-up for fetal anemia also includes testing for fetomaternal hemorrhage by Kleihauer-Betke test and screening for maternal antibodies to D, C, Kell, Duffy, and Kidd antigens to determine maternal antibody production against the fetal red blood cells. When evaluating significant ultrasound anomalies (placental calcifications, brain abnormality, etc.) which can be predictive of infection, fetal infections such as cytomegalovirus, toxoplasmosis, syphilis, and parvovirus B19 should be considered. In monochorionic multiple gestation, polyhydramnios in one of the twins can be a sequence suggestive of TTTS which entails a referral to fetal medicine specialist.
MANAGEMENT
Management depends mainly on the treatment of the underlying cause and the relief of the maternal symptoms caused by the excessive amniotic fluid accumulation (Figure 3). In uncontrolled maternal diabetes mellitus, a multidisciplinary approach is important with the involvement of maternal medicine specialists, diabetologists, and dieticians to optimize the maternal blood sugar control.19 However, management of diabetes in pregnancy is beyond the scope of this chapter.
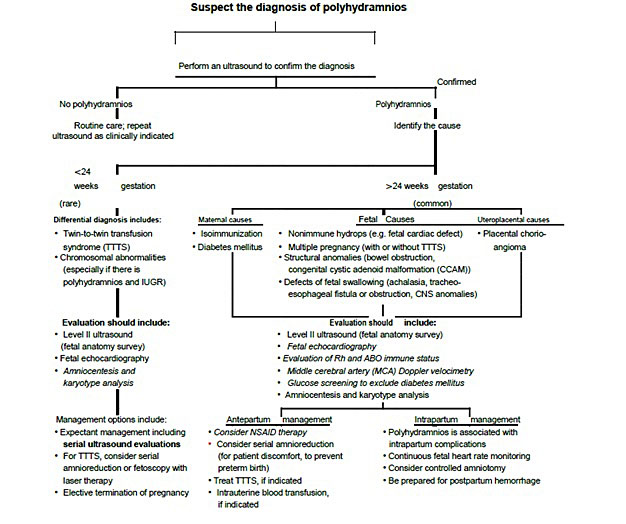
3
Algorithm for the diagnosis of polyhydramnios from Norwitz ER. Obstetric Clinical Algorithms: Management and Evidence Textbook with permission from John Wiley and Sons.
Involvement of maternal–fetal medicine specialists is needed in cases of suspected fetal anomaly, severe or worsening polyhydramnios or when there is a reduced fetal movement. In TTTS, referral and management should be in a fetal medicine center with multidisciplinary team involvement of a fetal medicine specialist. The most effective procedure to treat TTTS is fetoscopic laser photocoagulation of the anastomotic vessels. Serial amnioreductions, which involve draining the amniotic fluid from around the recipient twin, can be considered as a non-optimal treatment in cases where there is no access to laser fetoscopy. This procedure may improve circulation in the donor twin and may need to be performed multiple times during the pregnancy.22
Polyhydramnios is associated with increased risk of preterm delivery. Thus, recently evaluation of the cervical length to determine the need for administration of steroids to promote fetal lung maturity is advisable in case of symptomatic women.
AMNIOREDUCTION
Amnioreduction can be offered as an intervention only if polyhydramnios is both severe and symptomatic (associated with significant maternal discomfort or risk of severe preterm labor). The main goal of the intervention is to relieve maternal discomfort and prolong pregnancy to a gestational age that can be appropriate for delivery of healthy fetus. This is mainly carried out by a fetal medicine specialist under ultrasound guidance and through a removal of amniotic fluid via amniocentesis to obtain normal AFI values (usually <2 liters). Medical amnioreduction through administration of a prostaglandin synthetase inhibitor is no longer advisable. Ultrasound-guided needle placement is the standard for therapeutic amnioreduction. Transplacental passage should be avoided if possible. A surgical site is created with appropriate skin scrubs and sterile towels. Typically, the site chosen for amnioreduction is ventral to the fetus. Before needle insertion, sonographic calipers may be used to calculate the approximate depth to which the needle should be advanced. The needle is observed within the two-dimensional image generated by the transducer as it moves into the target location. Both a slower technique using a three-way stopcock with a 50 ml syringe or a more rapid method using a vacuum-assisted drainage system are available for amniodrainage. The overall risk of complications such as preterm labor, premature rupture of membranes, and of placental abruption is relatively small (~1.5%). Sometimes, the procedure needs to be repeated in order to normalize the AFI to normal (<25 cm) or until maternal discomfort is relieved. Serial amniodrainages can be technically difficult and the risk of the aforementioned complications increases with each procedure. Amnioreduction performed beyond 28 weeks of gestation can be delayed as much as possible to minimize the risk of preterm delivery associated with the procedure. Administration of intramuscular steroids is advised prior to the procedure to promote fetal lung maturation.23
DELIVERY AND LABOR MANAGEMENT
There is insufficient evidence in the literature to recommend induction of labor for isolated polyhydramnios. However, induction of labor is indicated when polyhydramnios is a part of the clinical picture such as uncontrolled maternal diabetes or associated with other obstetric or fetal conditions that require induction of labor. There are no worldwide national guidelines or large studies to guide management decisions. Mild polyhydramnios can be simply monitored and treated conservatively. Practice recommendations usually advise that induction of labor by artificial rupture of the membranes (ARM) should be controlled, performed by an obstetrician and with consent to proceed to lower-segment cesarean section if required (increased risk of cord prolapse). Although there is no absolute contraindication to use of oxytocin or prostaglandins in cases of polyhydramnios, these agents should be used with caution. There is a marked increase in the incidence of postpartum hemorrhage related to atony in patients with polyhydramnios. Theoretically, use of uterine stimulants may increase this risk; however, prostaglandins for cervical ripening and oxytocin for induction are widely used.
During labor, clinical and ultrasound assessment to confirm maintenance of fetal vertex presentation may be needed. The excess amniotic fluid allows greater fetal mobility so conversion to a breech, compound, or transverse presentation may occur. Spontaneous rupture of membranes can cause sudden severe uterine decompression with risk of cord prolapse or abruption and therefore early admission to maternity units of such women is advised. The fetal heart rate is monitored continuously by cardiotocography as these pregnancies are at increased risk of abnormalities. In case of AFI >30 cm the risk of esophageal atresia is higher and therefore prophylactic nasogastric tube passage in the neonate before breastfeeding is recommended to avoid regurgitation.
PRACTICE RECOMMENDATIONS
- Polyhydramnios means an excessive accumulation of the amniotic fluid; it can be associated with an increased risk of adverse pregnancy outcomes, such as preterm birth, placental abruption and fetal anomalies.
- The amniotic fluid volume depends on a balance between its production (mainly by fetal kidneys and lungs) and its uptake or removal (by fetal swallowing or absorption into the fetal surface).
- Polyhydramnios can be idiopathic, or due to fetal or maternal causes. Severe polyhydramnios (AFI >30 cm) in the second trimester being associated with significant perinatal morbidity due to prematurity or aneuploidy.
- The current main modality of diagnosing polyhydramnios is ultrasound assessment of amniotic fluid volume whether subjectively or quantitative assessment using either the single MVP ≥8 cm or AFI ≥25 cm. A quantitative estimation should be performed to differentiate between mild, moderate, or severe polyhydramnios (AFI < 30, between 30 and 35, and >35 cm, respectively).
- A comprehensive maternal and fetal evaluation including detailed fetal anatomic ultrasound evaluation to identify the cause of polyhydramnios (whether fetal, or maternal or idiopathic cause) is essential to guide the further management.
- Management depends mainly on the treatment of the underlying cause and the relief of the maternal symptoms caused by the excessive amniotic fluid.
- Treatment should be individualized and in a multidisciplinary team with involvement of a fetal medicine specialist and may require amnioreduction to control symptoms in severe cases and to prolong pregnancy in preterm cases.
- There is no clear evidence or guidelines regarding timing of delivery or management of labor, but labor should be managed with continuously monitored fetal heart rate by cardiotocography and rupture of membranes should be managed considering the risk of cord prolapse.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Dashe JS, McIntire DD, Ramus RM, et al. Hydramnios: anomaly prevalence and sonographic detection. Obstet Gynecol 2002;100(1):134–9. PMID: 12100815. | |
Phelan JP, Ahn MO, Smith CV, et al. Amniotic fluid index measurements during pregnancy. J Reprod Med 1987;32(8):601–4. PMID: 3309290. | |
WHO recommendation on Symphysis-fundal height measurement (https://extranet.who.int accessed on 19/8/2018). | |
Papageorghiou AT, Ohuma EO, Gravett MG, et al. International standards for symphysis-fundal height based on serial measurements from the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project: prospective cohort study in eight countries. BMJ 2016;355:i5662. PMID: 27821614. | |
Lim KI, Butt K, Naud K, et al. Amniotic Fluid: Technical Update on Physiology and Measurement. J Obstet Gynaecol Can 2017;39(1):52–58. PMID: 28062025. | |
Hill LM, Breckle R, Thomas ML, et al. Polyhydramnios: ultrasonically detected prevalence and neonatal outcome. Obstet Gynecol 1987;69(1):21–5. PMID: 3540761. | |
Touboul C, Boileau P, Picone O, et al. Outcome of children born out of pregnancies complicated by unexplained polyhydramnios. BJOG 2007;114(4):489–92. PMID: 17309542. | |
Many A, Hill LM, Lazebnik N, et al. The association between polyhydramnios and preterm delivery. Obstet Gynecol Obstet Gynecol 1995;86(3):389–91. PMID: 7651648. | |
Dorleijn DM, Cohen-Overbeek TE, Groenendaal F, et al. Idiopathic polyhydramnios and postnatal findings. J Matern Fetal Neonatal Med 2009;22(4):315–20. PMID: 19085623. | |
Pri-Paz S, Khalek N, Fuchs KM, et al. Maximal amniotic fluid index as a prognostic factor in pregnancies complicated by polyhydramnios. Ultrasound Obstet Gynecol 2012;39(6):648–53. PMID: 21898637. | |
Shani H, Sivan E, Cassif E, et al. Maternal hypercalcemia as a possible cause of unexplained fetal polyhydramnios: a case series. Am J Obstet Gynecol 2008;199(4):410.e1–5. PMID: 18928992. | |
Lee SM, Jun JK, Lee EJ, et al. Measurement of fetal urine production to differentiate causes of increased amniotic fluid volume. Ultrasound Obstet Gynecol 2010;36(2):191–5. PMID: 20069667. | |
Khan S, Donnelly J. Outcome of pregnancy in women diagnosed with idiopathic polyhydramnios. Aust N Z J Obstet Gynaecol 2017;57(1):57–62. PMID: 28251633. | |
Sieck UV, Ohlsson A. Fetal polyuria and hydramnios associated with Bartter's syndrome. Obstet Gynecol 1984;63(3 Suppl):22S–24S. PMID: 6366663. | |
Papageorghiou AT, Ohuma EO, Altman DG, et al., International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet 2014;384(9946):869–79. Erratum in: Lancet 2014;384(9950):1264. PMID: 25209488. | |
Papageorghiou AT, Sarris I, Ioannou C, et al., Ultrasound methodology used to construct the fetal growth standards in the INTERGROWTH-21st Project. International Fetal and Newborn Growth Consortium for the 21st Century. BJOG 2013;120 Suppl 2:27–32. PMID: 23841904. | |
Sande JA, Ioannou C, Sarris I, et al. Reproducibility of measuring amniotic fluid index and single deepest vertical pool throughout gestation. Prenat Diagn 2015;35(5):434–9. PMID: 25297394. | |
Nabhan AF, Abdelmoula YA. Amniotic fluid index versus single deepest vertical pocket as a screening test for preventing adverse pregnancy outcome. Cochrane Database Syst Rev 2008;(3):CD006593. PMID: 18646160. | |
Crimmins S, Mo C, Nassar Y, et al. Polyhydramnios or excessive fetal growth ere markers for abnormal perinatal outcome in euglycemic pregnancies. Am J Perinatol 2018;35(2):140–145. PMID: 28838004. | |
Sagi-Dain L, Sagi S. Chromosomal aberrations in idiopathic polyhydramnios: A systematic review and meta-analysis. Eur J Med Genet 2015;58(8):409–15. PMID: 26186913. | |
Morris RK, Meller CH, Tamblyn J, et al. Association and prediction of amniotic fluid measurements for adverse pregnancy outcome: systematic review and meta-analysis. BJOG 2014;121(6):686–99. PMID: 24738894. | |
Roberts D, Neilson JP, Kilby MD, et al. Interventions for the treatment of twin-twin transfusion syndrome. Cochrane Database Syst Rev 2014;(1):CD002073. PMID: 24482008. | |
Dickinson JE, Tjioe YY, Jude E, et al. Amnioreduction in the management of polyhydramnios complicating singleton pregnancies. Am J Obstet Gynecol 2014;211(4):434.e1–7. PMID: 24881825. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)


