This chapter should be cited as follows:
Wray S, Prendergast C, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.415293
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 15
The puerperium
Volume Editors:
Dr Kate Lightly, University of Liverpool, UK
Professor Andrew Weeks, University of Liverpool, UK

Chapter
Physiology of the Puerperium and Lactation
First published: February 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION AND SCOPE
The postpartum period commences after birth of the baby, and marks the end of pregnancy. The puerperium is the period of about 6 weeks, when we give special attention to the changes occurring in the mother’s body. These changes primarily include the return of the maternal organs to around pre-pregnant sizes and functions, endocrine changes as the placenta is lost, and the onset of lactation. Thought should be given early in the puerperium about contraception, and when to resume sexual relations. Local cultural or religious traditions can affect how new mothers are expected to behave in this period, but do not interfere with the normal course of the physiological events we are considering.
There are particular risks associated with the puerperal period, especially infection, hemorrhage and psychosis, which speaks to the need for continued healthcare, education and help, even when pregnancy has ended with the delivery of a healthy baby. Our goal is to give an overview of the physiological puerperal changes, and highlight any new understandings. We first discuss changes to the reproductive tract and other organs, and then the cardiovascular and endocrine systems. Maternal physical and mental well-being are then discussed under complications of the puerperium, before ending with a section on lactation.
UTERUS, CERVIX AND VAGINA
Tonic contractions help prevent postpartum hemorrhage
Immediately after delivery, tonic-like activity is required from the uterine muscle, the myometrium, to constrict the blood vessels coursing through it.1 This occlusion is the major way of reducing flow and preventing postpartum hemorrhage (PPH). Thrombosis at the ex-placental site also helps stem blood loss. It is usual for contractions to be felt as after-pains for several days postdelivery. The contractions are augmented by the hormone oxytocin stimulating the myometrium, as it is released during suckling (see below). During the first 12 hours postpartum, uterine contractions remain regular, strong, and coordinated, before their regularity and strength decrease with involution. Pain relief may be required during this period – more discomfort is experienced by multipara than in primiparous mothers.
Lochia removes waste
The puerperal uterine contractions also help in the expulsion of blood, membranes and vernix from the uterine cavity, in a vaginal discharge known as lochia.2 The red colored discharge (lochia rubra) typically lasts 3–5 days. The discharge then becomes thinner and browner, as blood contributes less to it, (lochia serosa) and by around day 10 is followed by a yellow to white discharge, composed of leukocytes and mucus but possibly also fat and cholesterol (lochia alba). The amount of lochia has been shown to be reduced in women who delivered smaller babies, had a cesarean section, and those who were not first time mothers.3 Green discolouration and malodorous lochia can be a sign of infection. A heavy discharge beyond 6 weeks can signal complications. Unusual persistence of red lochia can also indicate retained placental tissue or fetal membranes, and requires investigation.
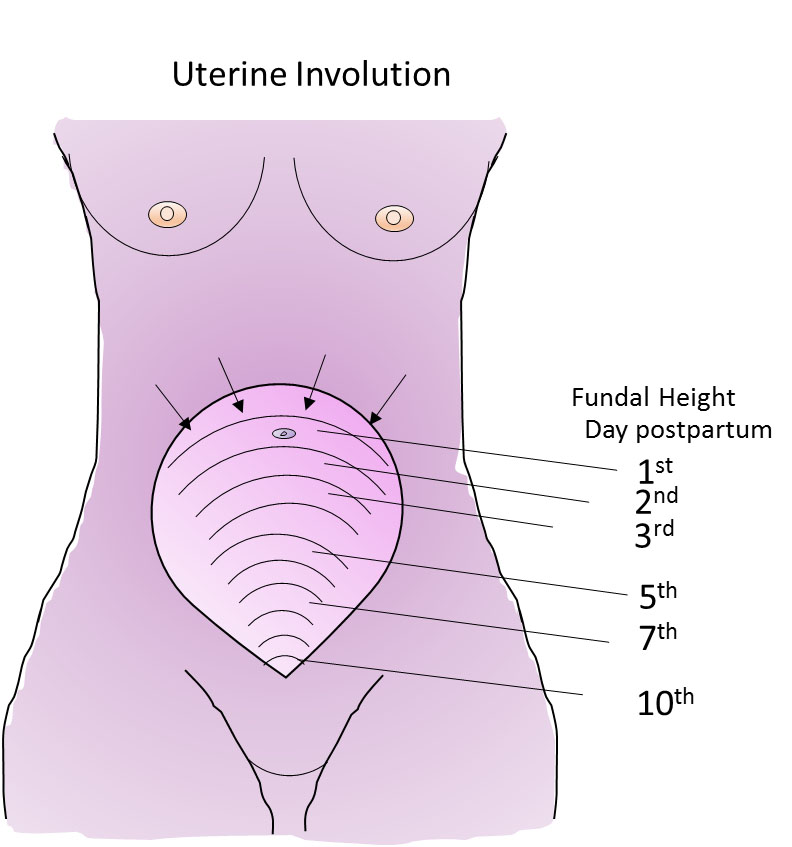
1
Fundal height as the uterus involutes.
Uterine involution is highly dynamic
If we are impressed at how much the uterus has grown during the 9 months of pregnancy, we should be even more impressed with how quickly it reverses this growth. The uterus decreases from a weight of around 1 kg to less than 100 g. The uterus returns to around its pre-pregnant state in just 6 weeks. Despite this, the turnover of muscle proteins, connective tissue and cellular remodeling occurring during involution is relatively under-appreciated and under-researched, which perhaps can be said of the whole puerperium, compared with pregnancy. Much has been made of how to assess the rate of involution by palpation and tape measure to determine the distance between the symphysis pubis and uterine fundus. Deviation from accepted normal values could indicate an abnormality and the need for clinical investigation. Studies over the past 20 years or so have indicated that there is so much normal variation between women, and error in the measurements, that these manual measurements of involution are rarely worth doing..4,5 Sonographic data are more reliable, but not warranted for routine use. Palpation, however, showing an increase in fundal height needs further evaluation. Delayed involution may be a sign of retained products of conception or endometritis, or a high uterus due to pelvic hematoma or abscess.
The reduction in muscle mass and fiber size in involution, is not just stimulated by the large changes in the hormones that were supporting pregnancy (see below) but also the loss of mechanical stimulation, stretch.6 There is catabolism of the muscle as its mass reduces, and the extracellular matrix is remodeled by metalloproteinases. Coordinated apoptosis and proliferation results in the myometrium returning to its non-pregnant state.
The endometrium regenerates in 3–6 weeks, and menstruation can occur within this time. The decidua has been cast off in the lochia, and new endometrial growth occurs from the basal layers. The mechanisms governing endometrial puerperal repair in women are not well understood. It is thought that imperfect repair is a cause of future fertility problems and recurrent miscarriages. Recent scientific studies, however, have indicated the involvement of a major pathway important in cell–cell communication, which involves gene regulation mechanisms that control multiple cell differentiation processes during embryonic and adult life – the Notch signaling pathway.7
Cervix and vagina: the rigors of labor take their toll on the cervix and vagina
The repair of the cervix during the puerperium is vital to stop infection and hemorrhage. Its remodeling and return to a rigid non-pregnant state from its flaccid postdelivery state, is vital to future term pregnancies, and involves physiological, biochemical and biophysical changes. The internal os of the cervix should have closed by second week postpartum. The external os may remain somewhat open for many weeks.
Given the extensive collagenous composition of the cervix, it is metalloproteinases and collagenases, and then extracellular matrix protein synthesis, along with cells of the immune and inflammation systems, that play the predominant role in this reconstruction of the cervix.8 Interestingly, a recent proteomic analysis of mouse postpartum cervix, identified four pathways that were significantly differentially upregulated during remodeling and warrant further investigation: intermediate filaments, actin-binding proteins, hypoxia-induced proteins, and proteins involved in immune modulation and/or wound healing.9
The vagina and vulva will initially be edematous, and enlarged but return to their usual state over the first few weeks of the puerperium. The vaginal walls will weaken slightly with each pregnancy, contributing to the age-related risk of genital prolapse.
Tears and episiotomies, depending upon their size, will heal within the next 2–3 weeks but obviously need to be kept clean and dry, and reassurance given to the mother about healing. Pelvic pain resolves in most women by 6 weeks. Pain on intercourse during the first weeks and months of the puerperium is likely due to tears and episiotomies, as well as the trauma to the vagina during delivery. There is a correlation between the extent of perineal trauma and the presence and intensity of dyspareunia for 3–6 months postpartum. Maternal exhaustion and sleep deprivation may also impact on sexual function. A recent cohort study of women in Holland found that most women quickly resume sexual activity after childbirth; 74.2% by 6 weeks postpartum.10 An earlier prospective study of Australian women found it took 12 weeks for 78% to have commenced intercourse.11
URINARY SYSTEM
There are three main conditions that affect the puerperium and the urinary system: urinary retention, incontinence and diuresis. Urinary retention is common, and the bladder can easily become over-distended in days 1–2 of the puerperium. The reasons for this are physiological, neurological, and mechanical. Physiologically, there is a persistent reduction in the bladder’s smooth muscle tone. This starts in pregnancy and has an associated increased postvoid residual volume. Neurologically, epidural analgesia temporarily further impairs bladder function, and micturition reflexes may be inhibited with suturing of the peritoneum. Mechanically, edema in the urethra, or urethral compression from postdelivery vulval edema, can contribute to retention. The overfilled bladder can lead to overflow incontinence, which is why within a few hours of delivery women are encouraged to try to pass urine. Emptying the bladder will also prevent its mechanical interference with uterine contractions. Inappropriate or delayed diagnosis and management of puerperal urinary retention can lead to bladder dysfunction, urinary tract infection, and catheter-related complications. The dilation in the renal calyces that occurs with pregnancy reverses by the end of the puerperium.
Leak of urine often accompanies laughing, straining or coughing in the puerperal woman (stress incontinence), but this will naturally resolve in most women. It is assumed to occur due to the stretching of the base of the bladder during both pregnancy and delivery. Pelvic floor exercises will help. Specialist help should be provided if the problem persists. Women should also know that pain or burning on micturition can be signs of a urinary infection in the bladder.
It is normal for there to be diuresis starting around day 2 and lasting 3–4 days, as fluid and salt balance return to non-pregnant values. There will be a fall in plasma volume and hematocrit may therefore rise, unless there was excessive blood loss during delivery (see Table 1 below).
GASTROINTESTINAL TRACT
Although gastrointestinal symptoms are common postnatally, most are mild and resolve spontaneously. The new mother may experience thirst, hemorrhoids, hunger, flatulence or constipation, but all pass. In some cases stool softeners and topical anesthetic may be helpful. The most serious condition at this time and beyond is fecal incontinence. The muscles and nerves in the pelvic floor that control bowel movements and the anal sphincter will have been stretched and perhaps damaged during delivery. It is also the case that where forceps or vacuum delivery modes have been used, or a significant tear extends backwards, damage to nerves and muscle can occur. Although embarrassing, fecal incontinence usually resolves after several months, but needs specialist attention if it persists. Cases which do not resolve may require surgical repair.
SKIN, HAIR AND JOINTS
The skin, hair, and nails rapidly reverse any changes that occurred in pregnancy. The dark pigmentation that occurs in the vulva, abdominal wall and face in some women, passes. Any edema of pregnancy is quickly dissipated. Stretch marks (striae gravidarum), become less apparent as the puerperium progresses. Most women experience hair loss over the first few months postpartum, which although potentially upsetting, is only restoring growth to non-pregnant levels. The loosening of ligaments and muscles, especially the diastasis recti, that has occurred with pregnancy, gradually reverse in the puerperium. Exercise and sporting activity should be reintroduced during the early postpartum period. This may be within a week following a normal vaginal delivery but delayed by a few weeks following a cesarean section delivery. The American College of Obstetricians and Gynecologists advises postpartum women should get at least 150 minutes of moderate-intensity aerobic activity every week.12 The general benefits of exercise on health and well-being underlie this advice, especially as a way to help increase mood, lose weight and strengthen muscle.13,14
RESPIRATORY SYSTEM
Although respiratory problems may be common in pregnancy, the respiratory system is little affected by the puerperium. Abnormal breathing patterns should alert clinicians to postpartum complications, such as infection.
ENDOCRINE CHANGES
Marked endocrine changes in the puerperal period affect physiology and emotions. Significant modification of the maternal endocrine system occurs to enable a successful pregnancy. The hormones estrogen and progesterone are both produced by the corpus luteum during the first few weeks of gestation, but the placenta takes over production soon after implantation. Levels of both these hormones rise steadily throughout pregnancy but after parturition and delivery of the placenta, the levels drop rapidly.15 Cortisol and prolactin are other hormones that exhibit a dramatic concentration rise during pregnancy. The placenta produces corticotropin-releasing hormone (pCRH), which stimulates placental release of cortisol. This process is independent of the usual hypothalamic–pituitary–adrenal axis (HPA axis) that generates cortisol in non-pregnant individuals. At parturition, there is a marked further increase in cortisol concentrations, which is believed to help mature the fetal systems ready for birth, but delivery of the placenta removes pCRH and plasma CRH levels return to normal within 15 hours postpartum. Cortisol levels fall rapidly in the first days and weeks postpartum.16 Prolactin is secreted by the anterior pituitary gland, and by the end of gestation, levels are 10–20 times higher than non-pregnant levels.15 The actions of prolactin are inhibited by the presence of progesterone, but once progesterone levels drop, prolactin stimulates milk production. Suckling causes further release of prolactin and levels will remain elevated until such time as breastfeeding stops. If a woman does not breastfeed, prolactin levels go back to normal within 2–3 weeks.
Parturition is accompanied by a large amount of oxytocin secretion from the posterior pituitary. In addition to its peripheral roles in myometrial contractility during labor, and milk ejection during lactation, oxytocin has a critical central role in the development of maternal behavior: attachment and bond formation.17 Both animal and human studies point to the role of oxytocin in the initiation of maternal behavior. There is recent suggestion that administration of exogenous oxytocin (Pitocin), as a means to augment labors, is negatively correlated with oxytocin release in breastfeeding mothers and a difficulty with suckling behavior in newborn infants.18,19
The dramatic hormonal changes that occur in the puerperium are linked to the development of mood disorders, ranging from the ‘baby blues’ to postpartum depression and postpartum psychosis. There is recent evidence linking the sudden withdrawal of estradiol or abnormally low levels of oxytocin with development of these maternal mental health issues.20,21
OVARIAN FUNCTION AND CONTRACEPTION
Mothers should receive contraceptive counseling. In non-lactating women, the first menstruation occurs 45–64 days postpartum and the first ovulation occurs between 45 and 94 days postpartum.22 Between 20 and 80% of first menses are anovulatory. However, the earliest ovulations reported were at 25–27 days postpartum, meaning that women who do not breastfeed their infants, risk conceiving again rapidly, unless alternative methods of contraception are used. In women who breastfeed, the return of ovulation and menses are delayed, potentially for long periods of time. Suckling suppresses the pulsatile release of gonadotropin-releasing hormone, which in turn inhibits the pulsatile release of luteinizing hormone, leading to depression of ovarian activity.23 There is a direct relationship between the intensity of breastfeeding and the length of time before ovulation resumes.24 Reducing breastfeeding duration or frequency and introducing supplementary feeds are all associated with earlier return of ovulation.25 The Lactational Amenorrhea Method of contraception (LAM) is effective during the first 6 months postpartum and has the benefit of being low cost and available to all. However, mothers should receive contraceptive counseling to ensure they are aware of the need for alternative contraception as soon as they reduce the intensity and frequency of the feeds.
It should be noted that the Faculty of Sexual and Reproductive Healthcare (FSRH) guidelines on Contraception after Pregnancy (2017),26 indicate that there are restrictions on the use of combined hormonal contraception in the first 3–6 weeks postpartum due to the increased risk of venous thromboembolism.
CARDIOVASCULAR SYSTEM
Significant changes in cardiovascular parameters occur in the puerperium and beyond. Major adaptations to the maternal cardiovascular system occur during pregnancy, which are necessary to ensure adequate blood supply to the placenta and fetus. By the third trimester of pregnancy, heart rate, stroke volume and cardiac output are increased; there is an increased plasma volume and decreased peripheral vascular resistance. Table 1 demonstrates that significant changes occur to these parameters immediately after parturition. While much of the cardiovascular system has returned to normal by 6 weeks after birth, cardiac output does not normalize until 24 weeks after birth, and peripheral vascular resistance remains elevated and is still higher than normal at 6 weeks.
There is considerable variation in the scientific literature regarding the extent and time courses of some of these hemodynamic changes (particularly in relation to stroke volume and cardiac output). Much of the variation is related to the posture of the mother when measurements were made,27 the use of invasive techniques (that have subsequently been superseded by more reliable, non-invasive techniques such as Doppler echocardiography) and whether non-pregnant, pre-conception or postpartum values are used for the control/reference group. Recent meta-analyses and comprehensive reviews can help clarify these data.28,29,30
It is important to note that hypertension may appear for the first time during the puerperium. One study has shown that 12% of previously normotensive women developed raised diastolic blood pressure in the first few days following parturition, possibly due to raised plasma volume.31 Women who exhibited hypertension during pregnancy may be even more at risk in early puerperium and should therefore be carefully monitored. On average, time to normalization of blood pressure is 5.4 ± 3.7 weeks after a pregnancy complicated with hypertensive disorders of pregnancy (HDP), but roughly 20% of women remained hypertensive at 6 months.32 A recent national cohort study of >1 million women noted that rates of postpregnancy hypertension in the first year after birth are 12–25 fold higher in women whose pregnancy was complicated by HDP than in women who had a normotensive pregnancy.33 In the long term, 14–32% of women (depending on age) with HDP in their latest pregnancy, develop hypertension in the decade after the birth, compared with 4–11% in women with a normotensive pregnancy.33
COAGULATION SYSTEM
Maternal blood in the puerperium is in a hypercoagulable state, putting mothers at risk of thromboembolism. Multiple changes occur to the coagulation system during pregnancy (Table 1). Levels of clotting factors are increased (fibrinogen, factor VII, VIII, IX, X and von Willebrand factor) and the natural anticoagulants are reduced (protein S and tissue plasminogen activator).34,35 All these aid the functioning of the placenta and prevent postpartum hemorrhage. In early puerperium, the blood remains in a hypercoagulable state and therefore mothers are still at an increased risk of thromboembolism (deep vein thrombosis and pulmonary embolism). Venous thromboembolism remains a leading cause of maternal death in the UK, with no decrease in mortality rates over the past 20 years.36 Indeed, as the maternity population is getting older, obesity levels rise and increasing numbers of cesarean sections carried out, the risk of thromboembolism will only increase.
As the plasma volume increases at a greater rate than red blood cell volume in pregnancy, a physiological hemodilution occurs. Conversely, decreases in plasma volume in the puerperium, lead to an elevated hematocrit.
1
Summary of the maternal cardiovascular and coagulation changes that occur in late pregnancy and during the puerperium.
Late pregnancy | Early puerperium | Late puerperium | |
Cardiovascular | |||
Heart rate | Falls after delivery but remains elevated compared to normal39,40 | ||
Stroke volume | Normal by 4–12 weeks29 Normal by 24 weeks39 | ||
Cardiac output | Initial sharp increase, then falls28,39,40,43 Returns to normal29 | Elevated29 | |
Blood pressure | Initial elevation (1 h), pre-labor values by 24 h43 Rises over first 4 days31 | Normal by 6–8 weeks Normal by 4–12 weeks28 | |
Plasma volume | Elevated30 | Initial decrease due to blood loss but rises again 2–5 days postpartum44 | Normal by 6 weeks30 |
Peripheral vascular resistance | Elevated29 | Still higher than normal at 6 weeks29 | |
Coagulation | |||
Hematocrit | Decreased45 | Elevated45 | |
Fibrinogen | |||
Clotting factors (VII, VIII, IX, X, XII,VWF) | Some remain elevated48 | Normal by 8 weeks48 | |
Protein S (anticoagulant factor) | Not normalized by 5 weeks46 | ||
Tissue plasminogen activator (tPA) (Anticoagulant factor) | Elevated34 | Falls34 | |
Platelet count | Decreased49 Decreased but within normal range46 | Rises but still low49 Normal46 | Normal at 4–8 weeks49 Normal46 |
Fibrinolytic activity | Normal by 3 weeks47 Normal by 4–6 weeks50 |
LACTATION AND BREAST FEEDING
The major physiological event of the puerperium is the establishment of lactation
The World Health Organization (WHO) now recommends exclusive breast feeding for 6 months, as there is increasing evidence for short- and long-term health benefits. However, recent numbers indicate that only 40% of mothers worldwide attain this goal (WHO and Unicef, 2017)52.
Physiology of lactation
Preparation for lactation occurs during puberty and pregnancy, well before puerperium and the initial latch of the newborn infant. It is important to appreciate the structure and cellular components of the lactating breast in order to fully understand the physiology of lactation (see Figure 2). During puberty, increasing estrogen and progesterone levels stimulate development of new alveolar buds from which milk-secreting mammary gland lobules will evolve. During pregnancy, the volume of breast tissue increases and formation of new alveolar-lobular structures continues alongside further maturation of the milk-producing apparatus. Estrogen, progesterone and prolactin are necessary for such development in pregnancy; however, other factors including placental lactogen and growth hormone also play a role. Little or no milk is produced during pregnancy because high levels of progesterone and estrogen block the secretory activity of the cells in the alveoli. During labor and lactation, further growth and differentiation occurs increasing the glandular component of the breast.
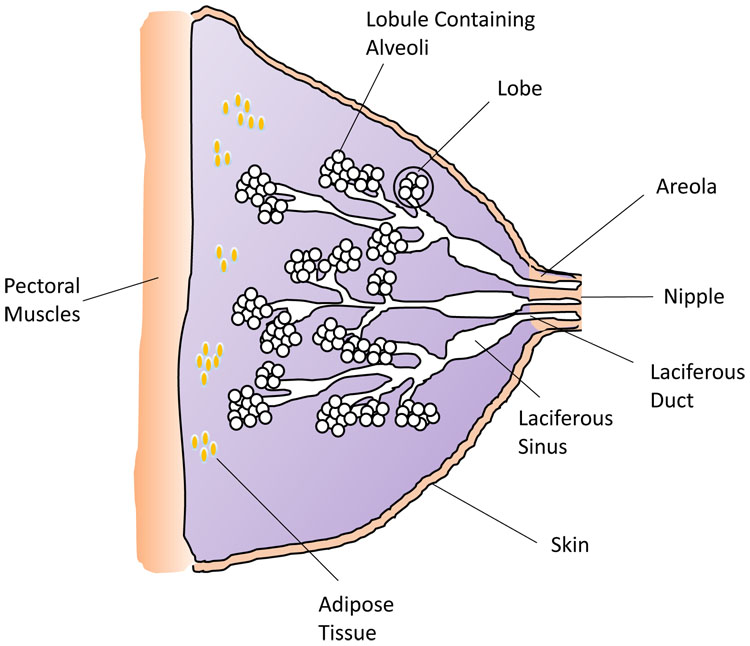
2
Diagram of the breast structure and tissues. (Credit: H Wallace)
Milk production
Prolactin stimulates mammary gland ductal growth, epithelial cell proliferation and induces milk production and secretion. After parturition, removal of the placenta stimulates a significant drop in progesterone, estrogen, and human placental lactogen,53 coinciding with an increase in prolactin, cortisol and insulin. Prolactin concentration increases rapidly by the tactile stimulation of the nipple-areolar complex with suckling of the nipple, stimulating nerve endings and subsequent release from the anterior pituitary regulated by the hypothalamus. Suckling, via neural connections, inhibits dopamine secretion which normally inhibits prolactin, thereby removing inhibition to allow increased prolactin secretion and stimulation of milk production.
Prolactin stimulates mammary gland ductal growth, epithelial cell proliferation and induces milk production and secretion. Within the alveoli, milk proteins are packed into secretory vesicles and milk is released into the lumen of the alveoli by exocytosis and budding.54 Colostrum is the first milk produced by mothers during the first 4 days postpartum, and differs to mature milk in that it has more of an immunological function. It contains high levels of the antibody immunoglobulin A (IgA) and leukocytes, protecting the baby from infection and developmental factors including growth modulators. From day 5, the nutritional constituents of the milk increase and by 2 weeks the main components include protein, fat and lactulose. The milk also contains micronutrients including vitamins A, B and D, and microbiota to aid the establishment of the baby’s initial intestinal microbiome.
Milk ejection
The second mechanism vital to successful lactation is the release of the hormone oxytocin, which is involved in the milk ejection or let down reflex. The release of oxytocin occurs in a similar way to prolactin, but is mediated by an independent neuroendocrinological pathway (Figure 3). Infant suckling leads to afferent signals to the hypothalamus, which in turn triggers release of oxytocin from the posterior pituitary gland in a pulsatile manner.12 Oxytocin then travels in the bloodstream and, in turn, stimulates the contractile myoepithelial cells in the alveolus. (Figure 3). The resulting contraction forces milk into the ducts from the alveolar lumens and out through the nipple. Oxytocin can also be released in response to various sensory inputs including hearing a baby cry. It also has a psychological effect, which includes inducing a state of calm, and reducing stress and anxiety. It may also enhance feelings of affection between mother and child, an important factor in bonding.
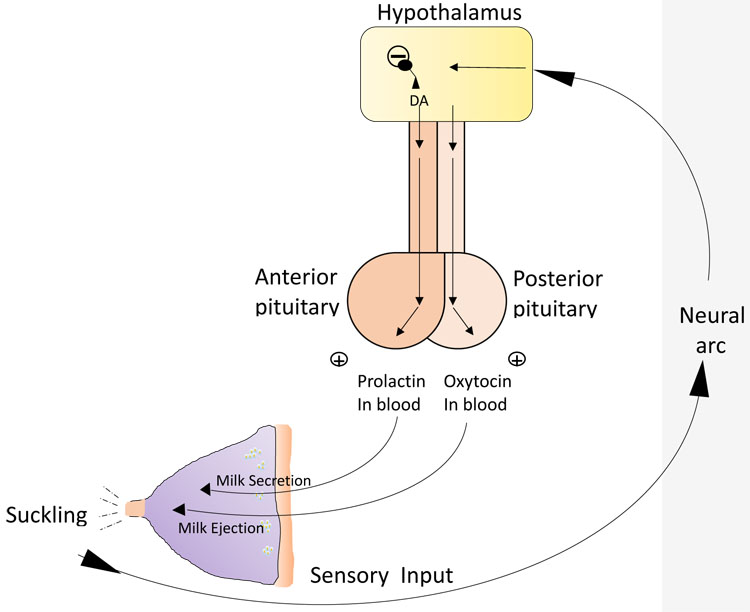
3
Relationship between pituitary hormones and milk secretion and ejection. (Drawing credit: H Wallace)
Maintenance of lactation
Milk expulsion by day 3 is critical for the establishment of successful breastfeeding. Subsequent regular removal of milk and stimulation of the nipple is essential to maintain the level of milk production. If milk is not removed, accumulation of a feedback inhibitor of lactation will lead to a fall in milk production and will initiate mammary involution. If breast milk is removed, the inhibitor is also removed, and secretion will resume. The physiological significance of the feedback inhibitor is to regulate the volume of milk produced to meet the needs of the baby; this is determined by how much milk the baby takes.
Pathophysiology and management of breastfeeding
Milk ejection can be inhibited by emotional stress. The process of lactation and breastfeeding can be negatively affected by factors that prevent normal breast development leading to insufficient breast tissue, or the production and/or secretion of milk which may be due to a hormone imbalance for example. For lactation to continue, necessary levels of hormone secretion must be maintained. Inadequate milk production may also be due to inefficient calorie intake to meet demands, and maternal nutrition affects both the quality and quantity of breast milk. The milk ejection reflex can be affected by incorrect fixing and suckling of the nipple or the infant’s inability to latch. Poor attachment or infrequent feeding can lead to breast engorgement and the development of mastitis, causing inflammation and swelling of the nipple which can also become infected.
Milk ejection can be inhibited by the emotional stress of the mother, which explains why the anxiety caused by the desire to breastfeed correctly can have an inhibitory effect. There can also be a perception of insufficient milk supply which, along with pain caused by incorrect suckling, can lead to the mother choosing to stop breastfeeding.55 This emphasizes the importance of creating a supporting environment for new mothers providing encouragement from the healthcare team.
Clinical significance
An understanding of the physiology of lactation, from puberty to postpartum is essential to maximizing the chances of successful breastfeeding. Several aspects of the lactation process still remain to be explored and understood; however, new approaches and large scale clinical trials will continue to improve both mother and child’s health. One recent study has demonstrated the use of laser Doppler perfusion monitoring as a tool for investigating the hemodynamics of the lactating breast.56
The baby friendly initiative is a global partnership between the UK, WHO and Unicef aimed at supporting infant feeding. The program aims to support breastfeeding based on ‘extensive and resounding evidence’ that breastfeeding saves lives.
CONCLUSIONS
To overlook the importance of the first few weeks in the postpartum period is to endanger mothers and their babies.
PRACTICE RECOMMENDATIONS
Good community aftercare, and awareness in mothers of what is normal and what is a danger sign, are needed. Help with breastfeeding, contraception and mental well-being will pay dividends.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Wray S. Insights into the uterus. Experimental Physiology 2007;92(4):621–31. | |
Fletcher S, Grotegut CA, James AH. Lochia patterns among normal women: a systematic review. J Womens Health (Larchmt) 2012;21(12):1290–4. | |
Oppenheimer LW, Sherriff EA, Goodman JD, et al. The duration of lochia. Br J Obstet Gynaecol 1986;93(7):754–7. | |
Cluett ER, Alexander J, Pickering RM. What is the normal pattern of uterine involution? An investigation of postpartum uterine involution measured by the distance between the symphysis pubis and the uterine fundus using a paper tape measure. Midwifery 1997;13(1):9–16. | |
Keirse MJ. Discovering the Holy Grail in postpartum uterine involution. Birth 2011;38(1):80. | |
Wray S. The effects of metabolic inhibition on uterine metabolism and intracellular pH in the rat. The Journal of Physiology 1990;423:411–23. | |
Strug MR, Su RW, Kim TH, et al. RBPJ mediates uterine repair in the mouse and is reduced in women with recurrent pregnancy loss. FASEB J 2018;32(5):2452–66. | |
Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends in endocrinology and metabolism: TEM 2010;21(6):353–61. | |
Stanley RL, Ohashi T, Gordon J, et al. A proteomic profile of postpartum cervical repair in mice. J Mol Endocrinol 2018;60(1):17–28. | |
Lagaert L, Weyers S, Van Kerrebroeck H, et al. Postpartum dyspareunia and sexual functioning: a prospective cohort study. Eur J Contracept Reprod Health Care 2017;22(3):200–6. | |
McDonald EA, Brown SJ. Does method of birth make a difference to when women resume sex after childbirth? BJOG 2013;120(7):823–30. | |
ACOG. Exercise after pregnancy.: American College of Obstetricans and Gynecologists, 2019 [Available from: https://www.acog.org/Patients/FAQs/Exercise-After-Pregnancy?IsMobileSet=false.] | |
Amorim AA, YM L. Diet or exercise, or both, for weight reduction in women carrying excess weight after childbirth. Cochrane, 2013 [Available from: https://www.cochrane.org/CD005627/PREG_diet-or-exercise-or-both-for-weight-reduction-in-women-carrying-excess-weight-after-childbirth.] | |
NHS. Exercise in Pregnancy 2017 [Available from: https://www.nhs.uk/conditions/pregnancy-and-baby/pregnancy-exercise/?tabname=labour-and-birth.] | |
Kodogo V, Azibani F, Sliwa K. Role of pregnancy hormones and hormonal interaction on the maternal cardiovascular system: a literature review. Clin Res Cardiol 2019;108(8):831-846. | |
Dickens MJ, Pawluski JL. The HPA Axis During the Perinatal Period: Implications for Perinatal Depression. Endocrinology 2018;159(11):3737–46. | |
Kim S, Strathearn L. Oxytocin and Maternal Brain Plasticity. New Dir Child Adolesc Dev 2016;2016(153):59–72. | |
Jonas K, Johansson LM, Nissen E, et al. Effects of intrapartum oxytocin administration and epidural analgesia on the concentration of plasma oxytocin and prolactin, in response to suckling during the second day postpartum. Breastfeed Med 2009;4(2):71–82. | |
Olza Fernandez I, Marin Gabriel M, Malalana Martinez A, et al. Newborn feeding behaviour depressed by intrapartum oxytocin: a pilot study. Acta Paediatr 2012;101(7):749–54. | |
Douma SL, Husband C, O'Donnell ME, et al. Estrogen-related mood disorders: reproductive life cycle factors. ANS Adv Nurs Sci 2005;28(4):364–75. | |
Moura D, Canavarro MC, Figueiredo-Braga M. Oxytocin and depression in the perinatal period-a systematic review. Arch Womens Ment Health 2016;19(4):561–70. | |
Jackson E, Glasier A. Return of ovulation and menses in postpartum nonlactating women: a systematic review. Obstetrics and Gynecology 2011;117(3):657–62. | |
McNeilly AS. Neuroendocrine changes and fertility in breast-feeding women. Prog Brain Res 2001;133:207–14. | |
Perez A, Vela P, Masnick GS, et al. First ovulation after childbirth: the effect of breast-feeding. American Journal of Obstetrics and Gynecology 1972;114(8):1041–7. | |
Gray RH, Campbell OM, Apelo R, et al. Risk of ovulation during lactation. Lancet 1990;335(8680):25–9. | |
Gynecologists TFoSRHotRCoO. Contraception After Pregnancy 2017 [Available from: https://www.fsrh.org/standards-and-guidance/documents/contraception-after-pregnancy-guideline-january-2017/] | |
Atkins AJ, Watt JM, Milan P, et al. The influence of posture upon cardiovascular dynamics throughout pregnancy. European Journal of Obstetrics, Gynecology and Reproductive Biology 1981;12(6):357–72. | |
Melchiorre K, Sharma R, Thilaganathan B. Cardiac structure and function in normal pregnancy. Curr Opin Obstet Gynecol 2012;24(6):413–21. | |
Meah VL, Cockcroft JR, Backx K, et al. Cardiac output and related haemodynamics during pregnancy: a series of meta-analyses. Heart 2016;102(7):518–26. | |
de Haas S, Ghossein-Doha C, van Kuijk SM, et al. Physiological adaptation of maternal plasma volume during pregnancy: a systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017;49(2):177–87. | |
Walters BN, Thompson ME, Lee A, et al. Blood pressure in the puerperium. Clin Sci (Lond) 1986;71(5):589–94. | |
Podymow T, August P. Postpartum course of gestational hypertension and preeclampsia. Hypertension in Pregnancy 2010;29(3):294–300. | |
Behrens I, Basit S, Melbye M, et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ (Clinical research ed) 2017;358:j3078. | |
Cerneca F, Ricci G, Simeone R, et al. Coagulation and fibrinolysis changes in normal pregnancy. Increased levels of procoagulants and reduced levels of inhibitors during pregnancy induce a hypercoagulable state, combined with a reactive fibrinolysis. European Journal of Obstetrics, Gynecology and Reproductive Biology 1997;73(1):31–6. | |
Katz D, Beilin Y. Disorders of coagulation in pregnancy. Br J Anaesth 2015;115(Suppl 2):ii75–88. | |
Knight M, Bunch K, Tuffnell D, et al. Saving Lives, Improving Mothers’ Care – Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2014–162018. Available from: https://www.ndph.ox.ac.uk/files/news/mbrrace-uk-maternal-report-2018-v0-6-05-oct-2018.pdf/@@download. | |
Aguilar HN, Mitchell BF. Physiological pathways and molecular mechanisms regulating uterine contractility. Human reproduction update 2010;16(6):725–44. | |
Robson SC, Boys RJ, Hunter S, et al. Maternal hemodynamics after normal delivery and delivery complicated by postpartum hemorrhage. Obstetrics and Gynecology 1989;74(2):234–9. | |
Duvekot JJ, Peeters LL. Maternal cardiovascular hemodynamic adaptation to pregnancy. Obstet Gynecol Surv 1994;49(12 Suppl):S1–14. | |
Robson SC, Dunlop W, Hunter S. Haemodynamic changes during the early puerperium. Br Med J (Clin Res Ed) 1987;294(6579):1065. | |
Capeless EL, Clapp JF. When do cardiovascular parameters return to their preconception values? American Journal of Obstetrics and Gynecology 1991;165(4 Pt 1):883–6. | |
Robson SC, Hunter S, Boys RJ, et al. Serial study of factors influencing changes in cardiac output during human pregnancy. The American Journal of Physiology 1989;256(4 Pt 2):H1060–5. | |
Robson SC, Dunlop W, Boys RJ, et al. Cardiac output during labour. Br Med J (Clin Res Ed) 1987;295(6607):1169–72. | |
Ueland K. Maternal cardiovascular dynamics. VII. Intrapartum blood volume changes. American Journal of Obstetrics and Gynecology 1976;126(6):671–7. | |
Milman N, Byg KE, Agger AO. Hemoglobin and erythrocyte indices during normal pregnancy and postpartum in 206 women with and without iron supplementation. Acta Obstetricia et Gynecologica Scandinavica 2000;79(2):89–98. | |
Bremme K, Ostlund E, Almqvist I, et al. Enhanced thrombin generation and fibrinolytic activity in normal pregnancy and the puerperium. Obstetrics and Gynecology 1992;80(1):132–7. | |
Dahlman T, Hellgren M, Blomback M. Changes in blood coagulation and fibrinolysis in the normal puerperium. Gynecologic and Obstetric Investigation 1985;20(1):37–44. | |
Stirling Y, Woolf L, North WR, et al. Haemostasis in normal pregnancy. Thromb Haemost 1984;52(2):176–82. | |
Reese JA, Peck JD, McIntosh JJ, et al. Platelet counts in women with normal pregnancies: A systematic review. Am J Hematol 2017;92(11):1224–32. | |
Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost 2003;29(2):125–30. | |
Wright JG, Cooper P, Astedt B, et al. Fibrinolysis during normal human pregnancy: complex inter-relationships between plasma levels of tissue plasminogen activator and inhibitors and the euglobulin clot lysis time. Br J Haematol 1988;69(2):253–8. | |
World Health Organisation UNICEF. Tracking progress for breastfeeding policies and programmes: Global breastfeeding scorecard 2017 [Available from: https://www.who.int/nutrition/publications/infantfeeding/global-bf-scorecard-2017/en/.] | |
Kulski JK, Smith M, Hartmann PE. Perinatal concentrations of progesterone, lactose and alpha-lactalbumin in the mammary secretion of women. The Journal of Endocrinology 1977;74(3):509–10. | |
Truchet S, Honvo-Houeto E. Physiology of milk secretion. Best Pract Res Clin Endocrinol Metab 2017;31(4):367–84. | |
Li J, Kendall GE, Henderson S, et al. Maternal psychosocial well-being in pregnancy and breastfeeding duration. Acta Paediatr 2008;97(2):221–5. | |
van der Hoek M, den Haan L, Kaspers A, et al. Cutaneous perfusion of the human lactating breast: a pilot study with laser Doppler perfusion monitoring. Physiol Meas 2019;40(5):05NT01. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)

