This chapter should be cited as follows:
Bloomfield V, Allen L, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.418463
The Continuous Textbook of Women’s Medicine Series – Gynecology Module
Volume 2
Adolescent gynecology
Volume Editor: Professor Judith Simms-Cendan, University of Miami, USA

Chapter
Management of Adnexal Masses in Adolescents
First published: March 2023
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Adnexal lesions in adolescents are common and can represent ovarian or tubal pathology. A thoughtful approach to history taking and physical exam is required to guide investigations. Through this chapter, we describe common adnexal pathologies in adolescents, and highlight key management principles focused on expectant management and ovarian preservation whenever appropriate.
CLINICAL PRESENTATION
The clinical presentation of adnexal masses varies within the adolescent population based on size, underlying pathology, and other patient factors. Common symptoms include complaints of pelvic pain or bulk symptoms, such as pelvic pressure or abdominal fullness.1,2,3,4,5 Depending on the size of the adnexal mass, adolescents may describe increased abdominal girth or bloating.6,7
In the event of acute pathology, such as adnexal torsion, cyst hemorrhage or rupture, patients may present with sudden onset pelvic pain and associated symptoms such as nausea, vomiting, or fever. Although uncommon, hormone secreting tumors may present with symptoms of hormone excess, such as abnormal uterine bleeding with estrogen-secreting tumors, or virilization with androgen-secreting tumors.8
A comprehensive history, physical exam, and appropriate investigations, as outlined below, are necessary to help identify the underlying etiology.
EVALUATION
Patient history
In addition to taking a thorough history of the presenting complaint, past medical and surgical history, clinicians should review pubertal development, menstrual and gynecologic history. Although endometriomas are less common amongst the adolescent population, symptoms of cyclic and acyclic pelvic pain, dyschezia, dysuria, and dyspareunia should be explored. In addition, clinicians should inquire about symptoms suggestive of an underlying malignant process, such as unintended weight loss, fevers, or night sweats.
All adolescents should be interviewed in confidence to elicit an accurate social and sexual history. As a minimum, this should include questions surrounding a patient’s home environment, education, use of alcohol, tobacco and other substances, sexual activity including contraceptive use, mood disorders, and safety.
Physical exam
An assessment of vital signs, including height and weight, should be collected and compared to age-appropriate normal values. Tanner staging of breast and pubic hair allows for assessment of pubertal development.9
A thorough abdominal exam should assess for presence of a pelvic or abdominal mass, and signs of peritonitis, which may suggest adnexal torsion, hemorrhagic cyst, ruptured cyst, tubovarian abscess, or ectopic pregnancy. A pelvic exam is deferred in younger, non-sexually active adolescents; however, a rectal exam is often well tolerated and allows for assessment of pelvic masses. Speculum and bimanual exams are possible in older or sexually active adolescents. Single digit bimanual exam assesses for cervical motion tenderness and evaluates the size, mobility, and tenderness of the adnexa. On exam, malignant masses are often solid and fixed on palpation with irregular surfaces and may be bilateral.
Laboratory investigations
For sexually active adolescent patients, urine βhCG and vaginal or cervical swabs should be included to rule out pregnancy and infectious etiologies, such as a tubo-ovarian abscess, respectively. A complete blood count should be ordered to assess for anemia in the context of a hemorrhagic cyst or ectopic pregnancy, which can lead to hemoperitoneum. Elevated white blood cell count can be suggestive of infectious etiologies. The platelet count may also be elevated as an acute phase reactant in malignancy or inflammatory process.
Pre-operative tumor markers can be considered as part of the assessment of an adnexal mass if suspicious for malignancy (Table 1).10,11,12 Clinicians should be aware that normal tumor markers do not exclude the risk of malignancy, and have not been validated in pediatric patients.10,11,12 In a study by Rogers et al. abnormal tumor markers differentiated a malignant mass with a high negative predictive value of 94.4%, but poor positive predictive value of 17.1%.13 If a malignant process is identified on pathology, tumor markers can be followed during systemic treatment (such as chemotherapy) to monitor for clinical response, and during post-treatment surveillance as a sign of disease recurrence.10,11,12
1
Tumor markers for adnexal masses in adolescent patients.
Ca-125 | CEA | AFP | βhCG | LDH | Estradiol | Inhibin | Testosterone | |
Germ cell tumors | ||||||||
Choriocarcinoma | X | |||||||
Endodermal sinus tumors | X | X | ||||||
Dysgerminoma | X | |||||||
Immature teratoma | X | X | ||||||
Mixed germ cell tumors | X | X | X | |||||
Polyembryonal | X | X | ||||||
Epithelial tumors | X | X | ||||||
Sex cord stromal tumors | ||||||||
Granulosa cell tumors | X | X | ||||||
Sertoli-Leydig tumors | X | X |
Ca-125, Cancer antigen 125; CEA, carcinoembryonic antigen; AFP, α fetal protein; βhCG, beta human chorionic gonadotropin.
Imaging
Transabdominal ultrasound imaging is first line for adolescents with adnexal masses. In older or sexually active adolescents, transvaginal imaging can be considered. Whenever possible, ultrasound imaging should be reviewed by radiologists with expertise in pediatric and adolescent gynecologic imaging. Lesions should be characterized based on origin, size, and presence of solid and cystic components. Blood flow to the ovary, as well as within the adnexal mass, can be assessed with Doppler studies.
If ultrasound imaging is inconclusive or there is a high suspicion of malignancy, further imaging with magnetic resonance or computed tomography can be considered to better characterize the lesion or for staging purposes.
POTENTIAL PATHOLOGIES
Functional ovarian cysts
Follicular cysts account for approximately 20 to 50% of ovarian masses in adolescents.1,7,14 These cystic lesions represent failure of the mature follicle to ovulate and involute. Similarly, cystic structures can form when fluid accumulates within the corpus luteum. As part of the natural history of follicular and corpus luteal cysts, cyst walls become vascularized before regressing. Transformation into a hemorrhagic cyst is possible and will often present with acute onset pelvic pain in the luteal phase of the menstrual cycle.15
On imaging, it is important to note that simple ovarian cysts up to 3 cm in diameter typically represent normal ovarian follicles. Simple, follicular cysts appear to be unilocular with smooth, thin walls.15 Corpus luteum cysts have a characteristic thickened wall, with circumferential blood flow and can reach up to 8 cm.16 A fine network of linear or curvilinear echos, in keeping with a ‘cobweb’ or reticular pattern, is suggestive of a hemorrhagic cyst (Figure 1). Echos may simulate septations, but do not typically extend across the entire cyst like a true septum.15,17 Presence of free fluid within the pelvis can suggest follicular cyst rupture, or intra-abdominal hemorrhage from a hemorrhagic cyst.
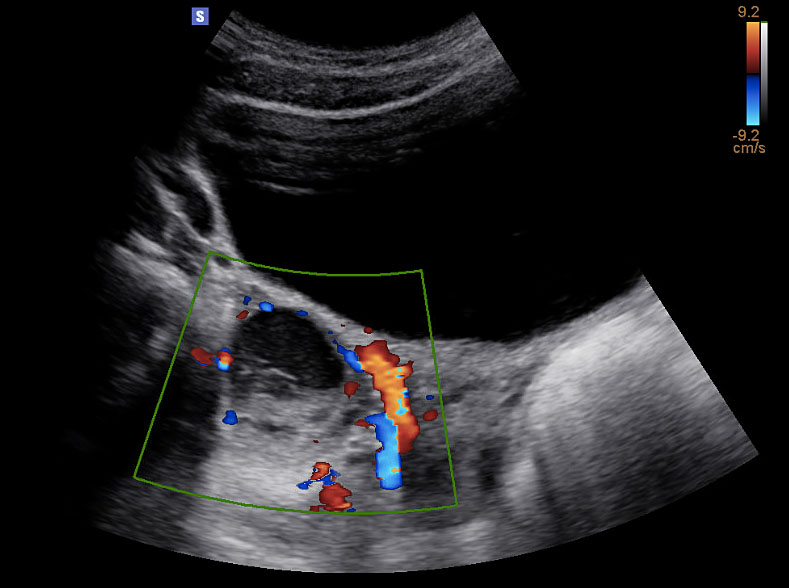
1
Ultrasound image (transverse view) of hemorrhagic ovarian cyst. Reticular pattern with retracting clot. Doppler waveforms document blood flow to the ovary. Image published with patient permission.
Ovarian neoplasms
Ovarian neoplasms account for 30–50% of adnexal masses within the adolescent population.7 Neoplasms are distinguished histologically depending on their cell type such as germ cell, epithelial, sex cord-stromal, and others.18 A detailed review of ovarian malignancies is beyond the scope of this chapter. While ovarian masses represent the most common gynecologic neoplasm in the adolescent population, the proportion of malignant ovarian neoplasms is low at approximately 4%.19 Amongst adolescents, ages 10 to 19, the incidence of malignant ovarian tumor is 1.072 per 100,000 patients per year.19
Germ cell tumors
Amongst pediatric and adolescent patients, germ cell tumors (GCTs) are the most common ovarian neoplasms. GCTs account for 55–70% of ovarian neoplasms in adolescents.20 GCTs arise from primordial ovarian germ cells and can include any of the germ cell layers, including ectoderm, mesoderm, or endoderm resulting in heterogenous lesions. GCTs may demonstrate rapid growth and patients may describe a sudden increase in abdominal girth or discomfort over a short period of time.20 Patients with GCTs most commonly present with abdominal pain and can also describe bulk-related symptoms, or hormone-related symptoms. Rarely, an auto-immune syndrome, N-methyl-D-aspartate receptor encephalitis, has been associated with ovarian teratomas.21,22
Approximately 80–95% of GCTs are benign, and include mature cystic teratomas and gonadoblastoma.23,24 Malignant GCTs include immature teratoma, dysgerminoma, mixed GCTs, embryonal tumor, choriocarcinoma, and polyembryoma. In a large population-based study of children, adolescent and adult patients, immature teratoma was the most common malignant GCTs (39%), followed by dysgerminoma (32%) and mixed germ cell tumor types (28%).23 Malignant GCTs are commonly limited to a single ovary, representing stage 1A disease. More advanced disease, stage 1B or greater, with bilateral ovarian involvement occurs rarely, in 10–17% of cases.25
As noted in Table 1, certain tumor markers, including LDH, AFP, and βhCG can be elevated in malignant GCTs. Ultrasound imaging is key as several GCTs have characteristic findings. Ultrasound findings suggestive of mature cystic teratomas include a unilocular cystic mass (Figure 2a), hyperechoic nodule with distal acoustic shadowing (Figure 2b), presence of fat and calcifications and dot-dash pattern (sometimes referred to as the ‘dermoid mesh’).26
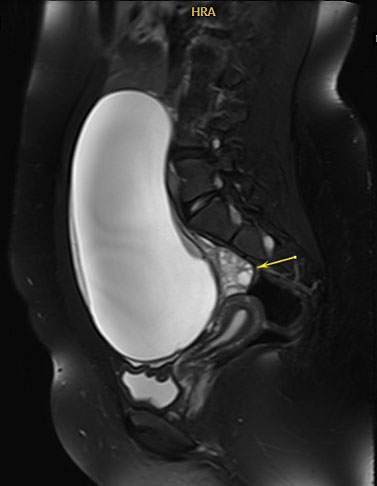
2a
Magnetic resonance imaging of unilocular cystic mass with involved ovary displaced posteriorly (arrow). Final pathology consistent with mature cystic teratoma. Image published with patient permission.
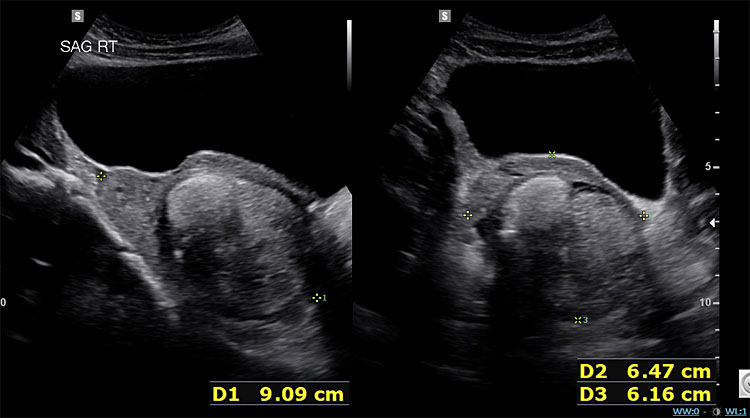
2b
Ovarian neoplasm with echogenic nodule and distal acoustic shadowing. Final pathology in keeping with mature teratoma. Image published with patient permission.
Epithelial neoplasms
Epithelial neoplasms are the second most common neoplasm in adolescent patients, accounting for 20 to 30% of ovarian neoplasms in adolescents and women under 25.27 Neoplasms are commonly benign (70%), and less likely borderline (20%) or malignant (<10%).27 Benign serous and mucinous cystadenomas are typically slow growing and can attain large sizes (up to 40 cm). On imaging, uni- or multilocular thin-walled cysts are noted without enhancing septations or solid components (Figure 3). Mucinous tumors may appear to have difference densities of cystic fluid due to mucin layering.6
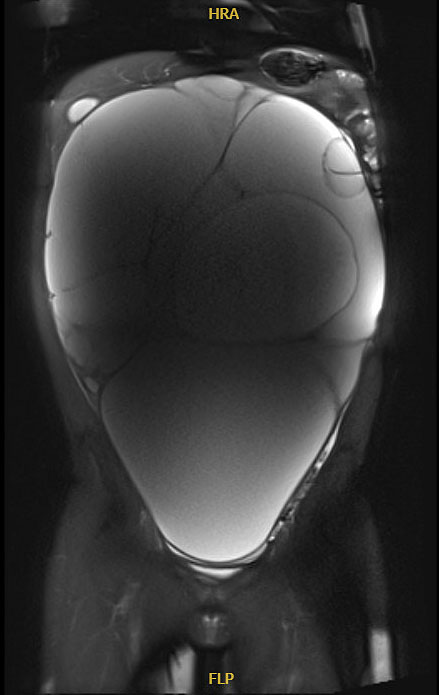
3
Magnetic resonance imaging of massive multicystic lesion with thin internal septations. Final pathology in keeping with serous cystadenoma. Image published with patient permission.
Sex-cord stromal tumors
Sex-cord stromal tumors develop from the sex cord cells (Sertoli or granulosa cell), stromal cells (fibroma, thecoma), or both. Sex-cord stromal tumors can be benign or malignant, and are uncommon in the adolescent population (<10% of ovarian neoplasms in children and adolescents).8 Similar to other neoplasms, patients often present with pelvic pain and discomfort.10 However, some SCST produce hormones, including estrogen or androgens, and patients may present with symptoms of hormone excess or virilization. Examples of hormonal-related symptoms include precocious puberty (or rapid pubertal tempo), irregular vaginal bleeding, acne, hirsutism, or voice deepening.7,8,24
Endometriomas
Endometriomas are benign ovarian masses of ectopic endometrial tissue. Ovarian endometriomas remain a less common ovarian neoplasm in the general adolescent population, and prevalence is poorly defined.28 However, emerging incidence suggests endometriomas may be present in up to one third of adolescent patients with surgically documented endometriosis.28 It is important to take a thorough history of pelvic pain symptoms, including associated symptoms of dysmenorrhea, non-menstrual (non-cyclic) pain, dysuria, and dyschezia, to help identify at-risk patients. On ultrasound imaging, endometriomas often present as homogenous with ground-glass echoes with no solid component. Lesions can be uni- or multilocular.28
Other adnexal pathology
Lesions within the adnexa can represent both congenital and acquired etiologies. Para-ovarian cystic lesions within the adnexa are frequently mesonephric remnants, such as Hydatid of Morgagni cysts. These cysts do not typically cause clinical symptoms, but can present with intermittent, severe abdominal pain with associated nausea and vomiting with torsion of the cyst or ipsilateral adnexa.
Ectopic pregnancies, tubo-ovarian abscesses and hydrosalpinx can also occur within the adolescent population. Full review of these pathologies is beyond the scope of this chapter.
PRE-OPERATIVE RISK STRATIFICATION OF A SUSPICIOUS MASS
Accurate pre-operative risk assessment of malignancy remains an active area of investigation within pediatric and adolescent gynecology. As described below, it is important to differentiate between benign and malignant masses pre-operatively to allow for appropriate surgical planning. Several risk-stratification tools, such as Risk of Malignancy Index (RMI), International Ovarian Tumor Analysis (IOTA), and Ovarian-Adnexal Reporting and Data System (O-RADS), have been developed and validated in adult populations; however, to date, these scores have not been evaluated in the adolescent population.29,30
Several ultrasound characteristics predictive of malignancy have been identified. Size of the neoplasm can be used to risk stratify adnexal lesions as benign or malignant. Literature varies with studies suggesting size thresholds of 5 to 8 cm. Oltmann et al. retrospectively reviewed 424 patients who underwent surgical management of ovarian lesions and found a size cut-off of 8 cm predicted malignancy with an odds ratio of 19.0.31 However, while size alone offers a high negative predictive value (100%), it has poor positive predictive value (21%).13 Presence of solid or solid-cystic components on ultrasound predict malignancy with an odds ratio of 10.13 and 3.62, respectively. Combining lesion size (≥ 8 cm) with presence of complex features on ultrasound (including thick septations, solid nodules, or papillary components) increases the positive predictive value of malignancy to 37%.13
Further efforts have endeavored to improve the predictive value of ultrasound. Stankovic et al. published the Decision Tree System, which provides an algorithm for management of adnexal masses in pediatric and adolescent patients.32 This algorithm uses clinical presentation and ultrasound findings, such as morphology and the ovarian crescentic sign, to risk stratify lesions. Authors report use of the algorithm predicts malignancy with a positive predictive value of 86% and negative predictive value of 99% for surgically managed patients. Unfortunately, efforts to validate this decision algorithm within a Canadian population did not replicate such a robust positive predictive value; Goldberg et al. reported the algorithm positively predicted a malignancy in 30% of patients, with a negative predictive value of 98%.33
Ongoing efforts are required to develop and prospectively evaluate more complex algorithms, with the goal of generating a sensitive and specific prediction tool.
MANAGEMENT
Management considerations of adnexal masses within an adolescent population are outlined below. A fulsome discussion of the management of ectopic pregnancies, tubo-ovarian abscesses, and endometriomas is beyond the scope of this chapter and is described elsewhere.
Within the adolescent population, expectant management is the mainstay of treatment for ovarian cysts in the absence of an indication for intervention (for example, ovarian cyst complicated by torsion or features suspicious for malignancy). As noted above, the majority of functional ovarian cysts will resolve within 2 months, and hemorrhagic cysts will resolve within 3 months.3 Resolution should be documented with a follow-up ultrasound approximately 2–3 months after presentation. Expectant management with analgesia as needed is recommended. Suppression with combined hormonal contraception will not impact the natural history of an existing ovarian cyst. However, combined hormonal contraception containing 30 μg of ethinyl estradiol can be considered to suppress the hypothalamic-pituitary-ovarian axis to prevent development of physiologic cysts in the future.34 In adolescents with uncomplicated ovarian cysts, signs and symptoms of ovarian torsion should be reviewed as a reason to present for urgent care.
Patients with hemorrhagic cysts may present with severe pelvic pain and may have evidence of hemoperitoneum on imaging. The acute presentation of a hemorrhagic cyst can be difficult to differentiate from adnexal torsion; careful review of imaging with radiology colleagues will help to accurately diagnose hemorrhagic cysts and prevent unnecessary surgical intervention. Supportive care with adequate analgesia is recommended as clinical symptoms are expected to resolve without surgical intervention. While hemoperitoneum warrants a thorough work up and cautious surveillance, it is not an absolute indication for operative management. A hemodynamically stable patient can safely be managed medically with analgesia and serial assessment of hemodynamic stability and hemoglobin.
Surgical management is reserved for patients with acute presentations, such as ovarian torsion, large or persistent masses, and lesions with features concerning for malignancy. The goal of any surgical intervention is to relieve symptoms, achieve accurate diagnosis, and preserve ovarian function whenever appropriate.
For cystic lesions with benign features requiring surgical intervention, ovary-sparing therapy with the goal of preserving ovarian tissue and function is gold standard even in the situation of ovarian torsion. Cystectomy is preferred to aspiration given high rates of recurrence.3 Patients should be counseled that surgical pathology will be reviewed, and in the event of an unexpected malignant finding, a second procedure involving oophorectomy and possible staging may be required for management. When skill and resources allow, use of minimally invasive laparoscopic techniques are safe in the adolescent population and optimize post-operative recovery.35 However, given the goal of preserving ovarian function, use of open technique, such as a mini-laparotomy with controlled cyst drainage, may minimize trauma and use of electrocautery on ovarian tissue.
For suspected malignancies, intervention usually includes surgical staging with possible exploration, pelvic washings, unilateral salpingoopherectomy, sampling of suspected pathological lymph nodes, peritoneal sampling, and omental biopsy.12 Multidisciplinary collaboration amongst Pediatric and Adolescent Gynecology, Pediatric Surgery, Gynecologic Oncology and Pediatric Oncologists, ideally before surgical intervention, informs appropriate investigations and decision making as to the most appropriate surgical approach and treatment.
CONCLUSIONS
Adnexal lesions in adolescents commonly represent functional ovarian cysts, which can often be managed expectantly. Ovarian neoplasms are also common and are most often benign. Careful history, physical exam, and ultrasound imaging is key to achieve diagnosis. When surgical intervention is indicated, ovarian-sparing surgery is preferred when pre-operative investigations suggest a benign etiology. Further efforts are needed to improve diagnostic algorithms to prevent unnecessary oophorectomies within this patient population.
PRACTICE RECOMMENDATIONS
- During medical interactions, all adolescents should be interviewed in confidence to elicit an accurate social and sexual history with questions surrounding home environment, education, use of alcohol and substances, sexual activity, mood disorders, and safety.
- Swabs for sexually transmitted infections and urine βhCG should be offered to all sexually active teens.
- Transabdominal ultrasound imaging is first line for adolescents with adnexal masses. In older or sexually active adolescents, transvaginal imaging can be considered.
- Functional cysts, including follicular cysts, account for approximately 20–50% of ovarian masses in adolescents.
- Ovarian neoplasms account for 30–50% of adnexal masses within the adolescent population. The rate of malignancy is low at approximately 4%.
- Within the adolescent population, expectant management is the mainstay of treatment for ovarian cysts in the absence of an indication for intervention such as torsion or concern for malignancy.
- For suspected malignancies, intervention usually includes surgical staging with possible exploration, pelvic washings, unilateral salpingoopherectomy, sampling of suspected pathological lymph nodes, peritoneal sampling and omental biopsy. Multidisciplinary collaboration informs appropriate investigations and decision making as to the most appropriate surgical approach and treatment.
CONFLICTS OF INTEREST
Author(s) statement awaited.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Kirkham YA, Lacy JA, Kives S, et al. Characteristics and management of adnexal masses in a canadian pediatric and adolescent population. J Obstet Gynaecol Can 2011;33(9):935–43. doi:10.1016/s1701-2163(16)35019-8. | |
Salvador S, Scott S, Glanc P, et al. Guideline No. 403: Initial Investigation and Management of Adnexal Masses. J Obstet Gynaecol Can 2020;42(8):1021–1029.e3. doi:10.1016/j.jogc.2019.08.044. | |
Northridge JL. Adnexal Masses in Adolescents. Pediatr Ann 2020;49(4):e183–7. doi:10.3928/19382359-20200227-01. | |
Kelleher CM, Goldstein AM. Adnexal masses in children and adolescents. Clin Obstet Gynecol 2015;58(1):76–92. doi:10.1097/GRF.0000000000000084. | |
Hermans AJ, Kluivers KB, Wijnen MH, et al. Diagnosis and treatment of adnexal masses in children and adolescents. Obstet Gynecol 2015;125(3):611–5. doi:10.1097/AOG.0000000000000665. | |
Amies Oelschlager AM, Gow KW, Morse CB, et al. Management of Large Ovarian Neoplasms in Pediatric and Adolescent Females. J Pediatr Adolesc Gynecol 2016;29(2):88–94. doi:10.1016/j.jpag.2014.07.018. | |
Deligeoroglou E, Eleftheriades M, Shiadoes V, et al. Ovarian masses during adolescence: clinical, ultrasonographic and pathologic findings, serum tumor markers and endocrinological profile. Gynecol Endocrinol 2004;19(1):1–8. doi:10.1080/09513590410001712895. | |
de Faria FW, Valera ET, Macedo CRPD, et al. Comment on: Consensus recommendations from the EXPeRT/PARTNER groups for the diagnosis and therapy of sex cord stromal tumors in children and adolescents. Pediatr Blood Cancer 2022:e29650. doi:10.1002/pbc.29650. | |
StatPearls, 2022. | |
van Heerden J, Tjalma WA. The multidisciplinary approach to ovarian tumours in children and adolescents. Eur J Obstet Gynecol Reprod Biol 2019;243:103–10. doi:10.1016/j.ejogrb.2019.10.032. | |
Lawrence AE, Fallat ME, Hewitt G, et al. Understanding the Value of Tumor Markers in Pediatric Ovarian Neoplasms. J Pediatr Surg 2020;55(1):122–5. doi:10.1016/j.jpedsurg.2019.09.062. | |
Spinelli C, Pucci V, Buti I, et al. The role of tumor markers in the surgical approach of ovarian masses in pediatric age: a 10-year study and a literature review. Ann Surg Oncol 2012;19(6):1766–73. doi:10.1245/s10434-012-2249-y. | |
Rogers EM, Casadiego Cubides G, Lacy J, et al. Preoperative risk stratification of adnexal masses: can we predict the optimal surgical management? J Pediatr Adolesc Gynecol 2014;27(3):125–8. doi:10.1016/j.jpag.2013.09.003. | |
Kirkham YA, Kives S. Ovarian cysts in adolescents: medical and surgical management. Adolesc Med State Art Rev 2012;23(1):178–91, xii. | |
Levine D, Patel MD, Suh-Burgmann EJ, et al. Simple Adnexal Cysts: SRU Consensus Conference Update on Follow-up and Reporting. Radiology 2019;293(2):359–71. doi:10.1148/radiol.2019191354. | |
Jain KA. Sonographic spectrum of hemorrhagic ovarian cysts. J Ultrasound Med 2002;21(8):879–86. doi:10.7863/jum.2002.21.8.879. | |
Jermy K, Luise C, Bourne T. The characterization of common ovarian cysts in premenopausal women. Ultrasound Obstet Gynecol 2001;17(2):140–4. doi:10.1046/j.1469-0705.2001.00330.x. | |
Meinhold-Heerlein I, Fotopoulou C, Harter P, et al. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch Gynecol Obstet 2016;293(4):695–700. doi:10.1007/s00404-016-4035-8. | |
Brookfield KF, Cheung MC, Koniaris LG, et al. A population-based analysis of 1037 malignant ovarian tumors in the pediatric population. J Surg Res 2009;156(1):45–9. doi:10.1016/j.jss.2009.03.069. | |
Zalel Y, Piura B, Elchalal U, et al. Diagnosis and management of malignant germ cell ovarian tumors in young females. Int J Gynaecol Obstet 1996;55(1):1–10. doi:10.1016/0020-7292(96)02719-1. | |
Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol 2009;66(1):11–8. doi:10.1002/ana.21756. | |
Hsu MH, Huang CC, Hung PL, et al. Paraneoplastic neurological disorders in children with benign ovarian tumors. Brain Dev 2014;36(3):248–53. doi:10.1016/j.braindev.2013.04.009. | |
Smith HO, Berwick M, Verschraegen CF, et al. Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol 2006;107(5):1075–85. doi:10.1097/01.AOG.0000216004.22588.ce. | |
Hassan E, Creatsas G, Deligeorolgou E, et al. Ovarian tumors during childhood and adolescence. A clinicopathological study. Eur J Gynaecol Oncol 1999;20(2):124–6. | |
Pectasides D, Pectasides E, Kassanos D. Germ cell tumors of the ovary. Cancer Treat Rev 2008;34(5):427–41. doi:10.1016/j.ctrv.2008.02.002. | |
Malde HM, Kedar RP, Chadha D, et al. Dermoid mesh: a sonographic sign of ovarian teratoma. AJR Am J Roentgenol 1992;159(6):1349–50. doi:10.2214/ajr.159.6.1442421. | |
Aggarwal A, Lucco KL, Lacy J, et al. Ovarian epithelial tumors of low malignant potential: a case series of 5 adolescent patients. J Pediatr Surg 2009;44(10):2023–7. doi:10.1016/j.jpedsurg.2009.06.027. | |
Shim JY, Laufer MR. Adolescent Endometriosis: An Update. J Pediatr Adolesc Gynecol 2020;33(2):112–9. doi:10.1016/j.jpag.2019.11.011. | |
Andreotti RF, Timmerman D, Strachowski LM, et al. O-RADS US Risk Stratification and Management System: A Consensus Guideline from the ACR Ovarian-Adnexal Reporting and Data System Committee. Radiology 2020;294(1):168–85. doi:10.1148/radiol.2019191150. | |
Timmerman D, Valentin L, Bourne TH, et al. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet Gynecol 2000;16(5):500–5. doi:10.1046/j.1469-0705.2000.00287.x. | |
Oltmann SC, Garcia N, Barber R, et al. Can we preoperatively risk stratify ovarian masses for malignancy? J Pediatr Surg 2010;45(1):130–4. doi:10.1016/j.jpedsurg.2009.10.022. | |
Stanković ZB, Sedlecky K, Savić D, et al. Ovarian Preservation from Tumors and Torsions in Girls: Prospective Diagnostic Study. J Pediatr Adolesc Gynecol 2017;30(3):405–12. doi:10.1016/j.jpag.2017.01.008. | |
Goldberg HR, Kives S, Allen L, et al. Preoperative Risk Stratification of Adnexal Masses in the Pediatric and Adolescent Population: Evaluating the Decision Tree System. J Pediatr Adolesc Gynecol 2019;32(6):633–8. doi:10.1016/j.jpag.2019.07.005. | |
Group ECW. Ovarian and endometrial function during hormonal contraception. Hum Reprod 2001;16(7):1527–35. doi:10.1093/humrep/16.7.1527. | |
Winton C, Yamoah K. Ovarian torsion and laparoscopy in the paediatric and adolescent population. BMJ Case Rep 2020;13(5). doi:10.1136/bcr-2019-232610. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)

