This chapter should be cited as follows:
Whittington JR, Magann PE, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.410553
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 4
Fetal development and maternal adaptation
Volume Editor: Professor Asma Khalil, The Royal College of Obstetricians and Gynaecologists, London, UK; Fetal Medicine Unit, Department of Obstetrics and Gynaecology, St George’s University Hospitals NHS Foundation Trust, London, UK

Chapter
Amniotic Fluid: Physiology and Assessment
First published: February 2021
Study Assessment Option
By completing 4 multiple-choice questions (randomly selected) after studying this chapter readers can qualify for Continuing Professional Development awards from FIGO plus a Study Completion Certificate from GLOWM
See end of chapter for details
INTRODUCTION
Amniotic fluid is essential for growth and well-being, serving as protection for the developing fetus. Amniotic fluid is usually sterile until the onset of labor, and contains bacteriostatic properties to protect the fetus from infection.1,2 Amniotic fluid volumes are regulated with delicate balance between the maternal, fetal, and placental systems while providing an indirect measure of fetal well-being. Disorders of amniotic fluid volume have been associated with adverse pregnancy outcomes and may be due to maternal, fetal, or placental issues. Assessment of amniotic fluid with transabdominal ultrasound has become part of routine obstetric practice to guide pregnancy management. This chapter reviews amniotic fluid dynamics, normal amniotic fluid volumes, ultrasound assessment of amniotic fluid, and amniotic fluid disorders.
AMNIOTIC FLUID PHYSIOLOGY
Amniotic fluid dynamics have been described as a three-compartment system since the 1950s. Plentl et al. investigated water exchange between the maternal, fetal, and placental compartments and found that exchange increases as gestation progresses and placental size increases.3
Water makes up 98–99% of amniotic fluid. In early pregnancy, amniotic fluid has the same osmolality as maternal plasma. Therefore, this supports the concept that early amniotic fluid is a transudate of maternal plasma across the fetal skin and placental surfaces.4 Fetal skin is non-keratinized until 22 weeks, which allows amniotic fluid to pass through it easily.
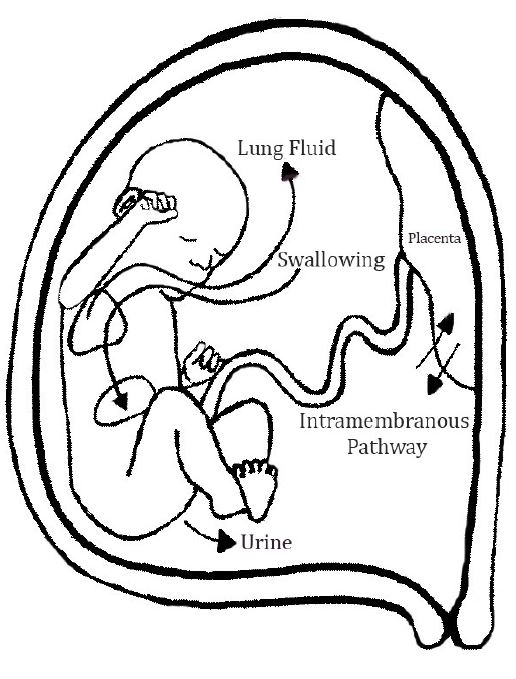
In the second half of pregnancy, amniotic fluid is regulated through pulmonary excretion, fetal urine production, fetal swallowing, intramembranous movement, and transmembranous movement (Figure 1). The intramembranous pathway involves transfer between the fetal circulation and the placenta.5 The transmembranous pathway involves transfer across the amnion and chorion; however, this is not an overall significant contributor to amniotic fluid volume.6
1
Diagram of amniotic fluid dynamics.
As the pregnancy progresses, the fetal renal system becomes a vital source of amniotic fluid. Fetuses with bilateral renal agenesis have essentially no amniotic fluid and fetuses with lower urinary tract obstruction develop low fluid or no fluid. Lung fluid also contributes to the amniotic fluid volume; however, in an ovine model, only half of the secreted lung fluid enters the amniotic sac, as the rest is swallowed after exiting the trachea.7 Fetal urine production and lung fluid excretion are the two main sources of inflow to the amniotic sac in the second half of gestation.8
There are two main components to outflow of amniotic fluid: fetal swallowing and intramembranous resorption. Fetuses begin to swallow as early as 16 weeks' gestational age.9 Fetal swallowing is an important regulator, as fetuses with the inability to swallow effectively (obstructed secondary to tracheal or esophageal atresia, tracheal or bowel obstruction, or the impaired ability to swallow such as neurologic abnormalities) demonstrate excess amniotic fluid volumes.10 The intermembranous pathway also serves as a means to regulate fluid levels. In an ovine model, after a large volume infusion of saline or lactated ringers, half of the excess infused fluid was transferred from the fetus via the intermembranous pathway and the other half was retained in the fetus, urine flow, and amniotic fluid.11
NORMAL AMNIOTIC FLUID VOLUME
Amniotic fluid volume is fairly constant in relation to gestational age and fetal weight in the first and early second trimesters.12 This can be explained by amniotic fluid composition in early pregnancy being largely due to the fetal skin dialysate.4 As gestation advances past 20 weeks, variations begin to occur in amniotic fluid. Many studies have been performed to determine normal amniotic fluid volume including direct measurement or dye-dilution techniques. Horsager describes direct measurement of amniotic fluid at the time of hysterotomy and accounts for blood contamination.13 Dye-dilution was originally performed by Charles and Jacoby and can be performed as long as there is adequate fluid to safely perform an amniocentesis.14 These two methodologies correlate well (r = 0.99, p < 0.001) and either can be used to measure amniotic fluid volume.15
To determine normal amniotic fluid volume, normal pregnancy must first be defined. A commonly used definition was determined by Moore, who defined normal pregnancy in the absence of twins, diabetes mellitus, hypertension, bleeding, preterm labor, fetal anomalies or rupture of the membranes. The fetus also was required to have neonatal weight between the 10th and 90th centile and a 5 minute APGAR score of greater than 6.16 More recently, a more extensive definition has been presented in a study of neonatal morbidity which used the absence of diabetes, history of venous thromboembolism, anticoagulant use, thrombophilia, chronic hypertension, placenta previa, placental abruption, non-obstetric comorbidity and the absence of asthma exacerbation or hypertensive disease of pregnancy at the time of delivery as criteria for normal pregnancy.17
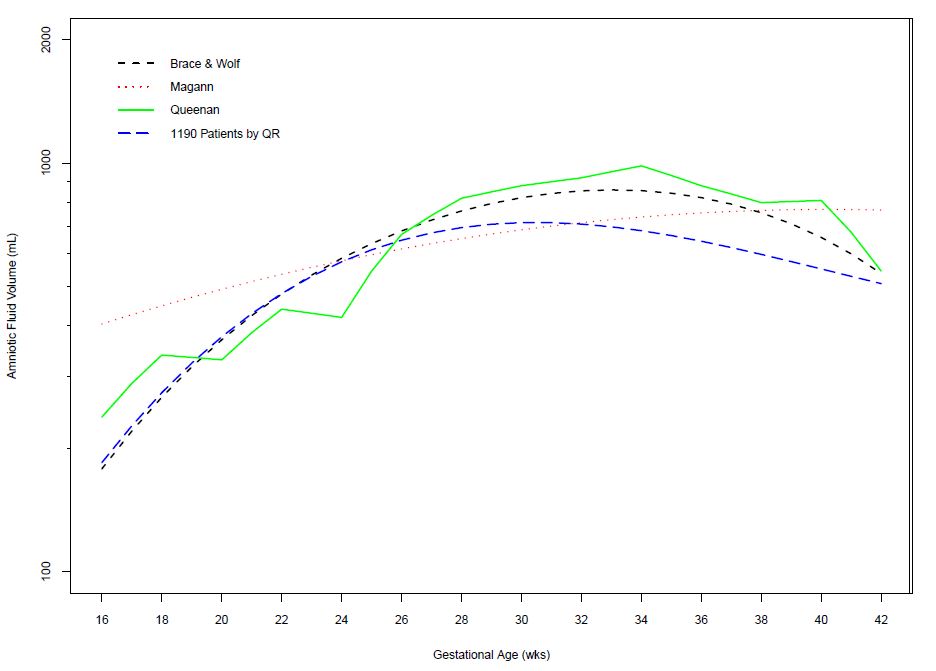
Investigations of amniotic fluid volume by Queenan, Brace and Wolfe, and Magann have all revealed different curves of normal amniotic fluid across gestation. Queenan used dye-dilution methods, while both Brace and Wolfe and Magann et al. both used dye-dilution methods and direct measurements.18,19,20 Two of these curves support the model indicating that amniotic fluid volume increases in early gestation, remains relatively stable between 22 and 38 weeks, then declines thereafter to the end of pregnancy. However, the other shows an increase in amniotic fluid throughout gestation. Each of these curves used different statistical methods to make reference intervals for amniotic fluid at each gestational age. Recently, quantile regression was used to evaluate 1190 normal amniotic fluid volumes measured by dye-dilution and direct measurement across gestation. Ounpraseuth et al. concluded that, in comparison to other statistical techniques, quantile regression is the preferred method for analyzing amniotic fluid volumes since it is flexible and robust when data are sparse (Figure 2).21 Normal ultrasonographic amniotic fluid volumes were established by Moore and Cayle in an assessment of 791 normal singleton pregnancies between 16 and 42 weeks.22
2
Comparison of normal amniotic fluid volumes (dye determined or directly measured and quantile regression) across gestation. (Adapted from Ounpraseuth, S.T., Magann, E.F., Spencer, H.J., Rabie, N.Z., Sandlin, A.T. Normal amniotic fluid volume across gestation: Comparison of statistical approaches in 1190 normal amniotic fluid volumes. J Obstet Gynecol Res 2017;43(7):1122–31; Magann, E.F., Sandlin, A.T., Ounpraseuth, S.T. Amniotic fluid and the clinical relevance of the sonographically estimated amniotic fluid volume: oligohydramnios. J Ultrasound Med 2011;30:1573–85; Brace, R.A., Wolf, E.J. Normal amniotic fluid volume changes throughout pregnancy. Am J Obstet Gynecol 1989;161:382–8; and Queenan, J.T., Thompson, W., Whitfield, C.R., Shah, S.I. Amniotic fluid volumes in normal pregnancies. Am J Obstet Gynecol 1972;114:34–8.)
SONOGRAPHIC MEASUREMENT OF AMNIOTIC FLUID VOLUME
Ultrasound assessment of amniotic fluid volume is integral to fetal evaluation because elevated or decreased amniotic fluid is associated with increased perinatal morbidity and mortality.23,24 Amniotic fluid can be evaluated sonographically with the following methods: measurement of the amniotic fluid index (AFI), measurement of the single deepest pocket (also called the maximum vertical pocket), measurement of the 2X2 pocket, and subjective assessment. The AFI was introduced by Phelan and is defined as the summation of the vertical diameter of the largest pocket in each of the four quadrants with the maternal umbilicus as the reference point.25 The single deepest vertical pocket was introduced by Chamberlain and is the largest pocket of amniotic fluid with at least a one-centimeter horizontal diameter after assessment of the uterus.23 The two dimensional pocket was introduced by Magann and is measured by the horizontal-times-vertical measurements in the deepest pocket, measured in cm2.26 Subjective assessment involves the evaluation of amniotic fluid as normal, excessive, or decreased.27 These ultrasound assessments are simple to perform and ultrasound is readily available in resource-rich areas. In a study by Magann et al., an obstetric resident, a nurse sonographer, a maternal-fetal medicine fellow and a maternal-fetal medicine staff member utilized these four methods and compared their findings to the dye-determined amniotic fluid volume measurement for accuracy. Subjective estimates ranged from 65 to 70% correct and sonographic estimates were similar in overall accuracy at 59–67%. They concluded that neither operator experience nor method of assessment affected the accuracy of estimates of amniotic fluid volume.28
Ideally, sonographic measurement should correctly identify patients at risk for adverse outcomes. Two prospective randomized trials have compared the use of single deepest vertical pocket versus AFI during modified biophysical profiles in complicated pregnancies. Chauhan et al. studied over 1000 women and found that AFI overestimated oligohydramnios compared to single deepest vertical pocket (17% vs. 10%); however, there was no difference in outcomes including delivery mode, NICU admission, umbilical artery pH, or APGAR score. Magann et al. studied over 500 patients and had similar outcomes, showing oligohydramnios was identified more commonly with AFI than single deepest vertical pocket resulting in increased inductions of labor (30% vs. 15%, p < 0.001) and cesarean delivery for fetal distress (13% vs. 7%, p < 0.05) without improvement in neonatal outcome. Two meta-analyses compared AFI to single deepest vertical pocket and found neither test was superior; however, AFI over-diagnoses clinically insignificant oligohydramnios. This suggested that single deepest pocket may be a superior sonographic assessment for the evaluation of oligohydramnios.29,30
Cochrane review 200831
The single deepest pocket in the assessment of amniotic fluid volume for fetal surveillance seems a better choice since the use of AFI increases the rate of diagnosis of oligohydramnios and the rate of induction without improvement in peripartum outcomes.
ASSESSMENT OF AMNIOTIC FLUID IN TWIN PREGNANCY
Twin pregnancies are at increased risk for morbidity and mortality, with a perinatal mortality rate six-fold greater than that of singleton pregnancies.32 The etiology of this increased risk is mostly accounted for by preterm birth.33 Just as amniotic fluid in assessment is important for singletons, identification of abnormal amniotic fluid volume in twin pregnancies is essential for evaluation of fetal well-being. Singleton curves for normal amniotic fluid are typically used in the evaluation of twin pregnancy. This is acceptable, as a study by Magann et al. showed that amniotic fluid volume of each sac in the third trimester of twin pregnancies is similar to amniotic fluid volume in normal singleton pregnancies.34 Magann also showed, in a series of 299 twin pregnancies (both dichorionic and monochorionic), that the single deepest pocket is constant between 17 and 37 weeks.35 Thus Morin and Lim performed a literature review with the Diagnostic Imaging Committee of the Society of Obstetricians and Gynaecologists of Canada and suggested that the single deepest pocket be used, although they note there is insufficient evidence to say AFI or single deepest pocket are more predictive of adverse perinatal outcomes.36
DISORDERS OF AMNIOTIC FLUID
Oligohydramnios
Oligohydramnios is defined as decreased amniotic fluid volume relative to gestational age and has also been defined as deficiency of amniotic fluid. Sonographically, oligohydramnios is defined as an amniotic fluid index of <5 cm or single deepest pocket <2 cm.24,25 Incidence is estimated to be 1–5% of pregnancies at term.22 Pregnancies complicated by oligohydramnios are at increased risk of morbidity and mortality. Oligohydramnios was evaluated retrospectively in 7582 fetuses in high-risk pregnancies with normal anatomy and perinatal mortality was 11% in these pregnancies versus 0.2% with normal fluid based on single deepest pocket over 2 cm.23
Oligohydramnios can be acute or longstanding and can be due to underproduction, loss, or can be idiopathic. Acute oligohydramnios usually reflects membrane rupture, whereas longstanding oligohydramnios may reflect more insidious processes. It is important to note that oligohydramnios could be indicative of an anomaly not detected until birth. Chromosomal anomalies were found in 13% of pregnancies with oligohydramnios.37 Causes of oligohydramnios are listed in Table 1.
1
Causes of oligohydramnios.
Chronic hypoxemia |
Fetal anomalies |
Other etiologies |
Regardless of the etiology, fetuses affected by oligohydramnios remain at increased risk of adverse outcomes. This increased risk stems from cord accidents, fetal lung hypoplasia, malformations, and contractures if prolonged and severe.38 For pregnancies with isolated oligohydramnios before 37 weeks, adverse pregnancy outcomes are related significantly to iatrogenic prematurity.39 Oligohydramnios places the term fetus at increased risk for cesarean delivery, 5-min APGAR less than 7, and low birth weight.40
When oligohydramnios is discovered, rupture of membranes should be ruled out clinically. Ultrasound, combined with testing of the vaginal fluid (either nitrazine, ferning, or with monoclonal antibodies to detect placental alpha-microglobulin-1 (PAMG-1, Amni Sure International, LLC, Cambridge, MA)) can be used to confirm rupture of membranes. When diagnosis of rupture of membranes is unclear, an indigo carmine test can be undertaken; however, indigo carmine has been in shortage in the United States and other dyes have not been well studied in pregnancy.41 In addition to ruling out rupture, anatomic evaluation of the fetus should be undertaken along with an assessment of placental function and fetal growth. If oligohydramnios has been prolonged, fetal pulmonary status should be assessed due to risk of lethal fetal pulmonary hypoplasia.
Management of oligohydramnios depends on the suspected cause and gestational age at discovery. In patients with preterm prelabor rupture of membranes, antibiotics are recommended to extend latency, steroids are recommended for fetal lung maturity, and delivery is recommended at 34 weeks' gestation as long as no signs of placental abruption or infection are present prior to this point.42 Antenatal testing is recommended for patients with oligohydramnios until delivery is undertaken due to the increased risk of adverse outcomes. If oligohydramnios is discovered at term, delivery is typically recommended. In a systematic review of 12 studies including 35,999 women, induction in pregnancies with isolated oligohydramnios was associated with increased risk of cesarean delivery (OR 2.07).43
Oligohydramnios is associated with a higher incidence of small for gestational age infants, post-maturity syndrome, distress in labor, cesarean delivery, low umbilical artery pH and APGAR scores and higher perinatal mortality. This highlights the importance of ultrasonographic assessment of amniotic fluid volume when evaluating pregnancies at risk for adverse outcomes. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists recommend patients with pregnancies affected by oligohydramnios be referred to a specialist obstetrician.44
Polyhydramnios
Polyhydramnios, also called hydramnios, is defined as increased amniotic fluid for gestational age. Using actual fluid volume, polyhydramnios is defined as an amniotic fluid volume of greater than 2 liters.45 Sonographically, it is defined as a single deepest pocket greater than 8 cm or amniotic fluid index greater than 24 or 25 cm.23,46,47 Others define polyhydramnios as subjectively increased amniotic fluid volume or above the 95th centile for gestational age.22,28,48 Polyhydramnios affects 0.41–1.08% of pregnancies.49,50 Patients may present with increased weight, increased uterine fundal height, dyspnea and edema. Pregnancies complicated by polyhydramnios are at increased risk of respiratory distress, hypoglycemia, macrosomia, preterm birth, nonreactive non-stress tests, perinatal morbidity and congenital anomalies.51Antenatal testing can include either non-stress test or biophysical profile, neither has been shown to be superior.
Polyhydramnios can be due to decreased absorption of the amniotic fluid, overproduction of amniotic fluid, or idiopathic. Decreased absorption is typically due to obstruction of fetal swallowing; this can be due to anatomic abnormalities such as tracheal or bowel obstruction or neurologic abnormalities. Overproduction can be due to fetal hyperglycemia and subsequent polyuria with maternal diabetes. It can also be due to fetal arrhythmias, fetal anemia, and twin-twin transfusion syndrome. Chromosomal abnormalities and TORCH infections are also causes of polyhydramnios. Idiopathic hydramnios accounts for the majority of polyhydramnios and is seen in approximately 65% of cases.52
Polyhydramnios is defined for twins the same as for singleton gestations. Polyhydramnios has increased incidence in twin pregnancies, affecting up to 1 in 6 twin gestations.
Maternal diabetes is linked to polyhydramnios and macrosomia. Patients with known diabetes and polyhydramnios have been found to have increased hemoglobin A1C (a marker of poor control).53
When polyhydramnios is discovered, it is recommended that a review of maternal history is undertaken, maternal glucose tolerance assessed, fetal anatomy be evaluated, and fetal growth be assessed. After 24 weeks, antenatal testing is recommended although the optimal type of testing or interval between assessments are not clear, as there is no current consensus.54 Additionally, optimal timing of delivery for patients with polyhydramnios has not been established.55
SUMMARY
Amniotic fluid is a highly complex system that is reflective of maternal or fetal health or both. When assessing fetal well-being, evaluation of the amniotic fluid is an essential part of that assessment. Practitioners should be familiar with the normal amniotic fluid volume and dynamics thereof and be familiar with the preferred methods of sonographic assessment. If abnormalities of amniotic fluid exist, further workup should be undertaken to elicit the cause.
PRACTICE RECOMMENDATIONS
- When obstetric ultrasonography is performed, an assessment of amniotic fluid is an integral part of the fetal assessment.
- The single deepest pocket is the preferred method for evaluating oligohydramnios and for evaluation of twin gestations.
- Disorders of amniotic fluid volume can be predictive of adverse perinatal outcome; careful assessment of the mother and fetus is recommended.
- When either oligohydramnios or polyhydramnios is discovered after fetal viability, antenatal testing is indicated.
CONFLICTS OF INTEREST
Dr Everett Magann is the author of an UpToDate article on Assessment of Amniotic Fluid Volume.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Rehbinder EM, Lodrup Carlsen KC, Staff AC, et al. Is amniotic fluid of women with uncomplicated term pregnancies free of bacteria? Am J Obstet Gynecol 2018. Doi: 10.1016/j.ajog.2018.05.028. PMID: 29852156. | |
Nazir MA, Pankuch GA, Botti JJ, et al. Antibacterial activity of amniotic fluid in the early third trimester. Its association with preterm birth and delivery. Am J Perinatol 1987;4:59–62. | |
Plentl AA. The dynamics of the amniotic fluid. Ann N Y Acad Sci 1959;75:746–61. | |
Beall MH, van den Winjgaard J, van Gemert MJ, et al. Amniotic fluid water dynamics. Placenta 2007;28:816–23. | |
Moore TR. Amniotic fluid dynamics reflect fetal and maternal health and disease. Obstet Gynecol 2010;116:3:759–765. | |
Gilbert WM, Brace RA. The missing link in amniotic fluid volume regulation: intramembranous absorption. Obstet Gynecol 1989;74:748–54. | |
Brace RA, Wlodek ME, Cock ML, et al. Swallowing of lung liquid and amniotic fluid by the ovine fetus under normoxic and hypoxic conditions. Am J Obstet Gynecol 1994;171:764–70. | |
Ross MG, Brace RA. Amniotic fluid biology- basic and clinical aspects. J Mat Fet Med 2001;10:2–19. | |
Pritchard JA. Fetal swallowing and amniotic fluid volume. Obstet Gynecol 1966;28(5):606–10. | |
Kimble RM, Harding JE, Kolbie A. Does gut atresia cause polyhydramnios? Pedatr Surg Int 1998;13(2–3):115–7. | |
Brace RA, Moore TR. Transplacental, amniotic, urinary, and fetal fluid dynamics during very-large-volume fetal intravenous infusions. Am J Obstet Gynecol 1991;164(3):907–16. | |
Monie IW. The volume of the amniotic fluid in the early months of pregnancy. Am J Obstet Gynecol 1953;66(3):616–25. | |
Horsager R, Nathan L, Leveno KJ. Correlation of measured amniotic fluid volume and sonographic predictions of oligohydramnios. Obstet Gynecol 1994;83(6):955–8. | |
Charles D, Jacoby HE. Preliminary data on the use of sodium aminohippurate to determine amniotic fluid volumes. Am J Obstet Gynecol 1966; 15, 95(2):266–9 PMID:. | |
Magann EF, Whitworth NS, Files JC, et al. Dye-dilution techniques using aminohippurate sodium: do they accurately reflect amniotic fluid volume? J Matern Fetal Neonatal Med 2002;11(3):167–70. | |
Moore TR. Superiority of the four-quadrant sum over the single-deepest-pocket technique in ultrasonographic identification of abnormal amniotic fluid volumes. Am J Obstet Gynecol 1990;163(3):762–7. | |
Chauhan SP, Rice MM, Grobman WA, et al. Neonatal morbidity of small- and large for-gestational age neonates born at term in uncomplicated pregnancies. Obstet Gynecol 2017;130(3):511–9. | |
Queenan JT, Thompson W, Whitfield CR, et al. Amniotic fluid volumes in normal pregnancies. Am J Obstet Gynecol 1972;114(1):34–8. | |
Brace RA, Wolf EJ. Normal amniotic fluid volume changes throughout pregnancy. Am J Obstet Gynecol 1989;161(2):382–8. | |
Magann EF, Bass JD, Chauhan SP, et al. Amniotic fluid volume in normal singleton pregnancies. Obstet Gynecol 1997;90(4 Pt 1):524–8. | |
Ounpraseuth ST, Magann EF, Spencer HJ, et al. Normal amniotic fluid volume across gestation: Comparison of statistical approaches in 1190 normal amniotic fluid volumes. J Obstet Gynecol Res 2017;43(7):1122–31. | |
Moore TR, Cayle JE. The amniotic fluid index in normal human pregnancy. Am J Obstet Gynecol 1990;162(5):1168–73. PMID: 2187347. | |
Chamberlain PF, Manning FA, Morrison I, et al. Ultrasound evaluation of amniotic fluid volume. I. The relationship of marginal and decreased amniotic fluid volumes to perinatal outcome. Am J Obstet Gynecol 1984;150:245–9. | |
Chamberlain PF, Manning FA, Morrison I, et al. Ultrasound evaluation of amniotic fluid volume. II. The relationship of increased amniotic fluid volume to perinatal outcome. Am J Obstet Gynecol 1984;150:250–4. | |
Phelan JP, Smith CV, Broussard P, et al. Amniotic fluid volume assessment with the four-quadrant technique at 36–42 weeks gestation. | |
Magann EF, Nolan TE, Hess LW, et al. Measurement of amniotic fluid volume: accuracy of ultrasonography techniques. Am J Obstet Gynecol 1992;167:1533–7. | |
Goldstein RB, Filly RA. Sonographic estimation of amniotic fluid volume. Subjective assessment versus pocket measurements. J Ultrasound Med 1988;7(7):363–9. | |
Magann EF, Perry KG, Jr, Chauhan SP, et al. The accuracy of ultrasound evaluation of amniotic fluid volume in singleton pregnancies: the effect of operator experience and ultrasound interpretative technique. J Clin Ultrasound 1997;25:249–53. | |
Magann EF, Doherty DA, Field K, et al. Biophysical profile with amniotic fluid volume assessments. Obstet Gynecol 2004;104:5–10. | |
Magann EF, Chauhan SP, Doherty DA, et al. The evidence for abandoning the amniotic fluid index in favor of the single deepest pocket. Am J Perinatol 2007;24(9):549–55. | |
Nabhan AF, Abdelmoula YA. Amniotic fluid index versus single deepest vertical pocket as a screening test for preventing adverse pregnancy outcome. Cochrane Database of Systematic Reviews 2008, Issue 3. Art. No.: CD006593. DOI: 10.1002/14651858.CD006593.pub2. | |
Ghai V, Vidyasagar D. Morbidity and mortality factors in twins. Clin Perinatol 1988;123. | |
Gardner MO, Goldenberg RL, Cliver SP, et al. The origin and outcome of preterm twin pregnancies. Obstet Gynecol 1995;85(4):553–7. | |
Magann EF, Whitworth NS, Bass JD, et al. Amniotic fluid volume of third-trimester diamniotic twin pregnancies. Obstet Gynecol 1995;85:957–60. | |
Magann EF, Doherty DA, Ennen CS, et al. The ultrasound estimation of amniotic fluid volume in diamniotic twin pregnancies and prediction of peripartum outcomes. Am J Obstet Gynecol 2007;196(6):570 e1–6; discussion 570 e6–8. | |
Morin L, Lim K. Ultrasound in twin pregnancies. J Obstet Gynaecol Can 2011;33:643–56. | |
Stoll C, Alembik Y, Roth MP, et al. Study of 224 cases of oligohydramnios and congenital malformations in a series of 225,669 consecutive births. Community Genet 1998;1:71–7. | |
Oyelese Y. Placenta, umbilical cord, and amniotic fluid: the not-less-important accessories. Clin Obstet Gynecol 2012;55:307–23. | |
Melamed N, Pardo J, Milstein R, et al. Perinatal outcome in pregnancies complicated by isolated oligohydramnios diagnosed before 37 weeks of gestation. Am J Obstet Gynecol 2011;205(3);241 e1–6. | |
Ashok BA, Manjushri W. Low amniotic fluid index at term as a predictor of adverse perinatal outcome. The Journal of Obstetrics and Gynecology of India. 2014;64(2):120–3. | |
Ireland K, Rodriguez E, Acosta O, et al. Intra-amniotic dye alternatives for the diagnosis of preterm prelabor rupture of membranes. Obstet Gynecol 2017;129(6):1040–5. | |
American College of Obstetricians and Gynecologists (2018) OG practice bulletin no 188. | |
Shrem G, Nagawkar SS, Hallak M, et al. Isolated oligohydramnios at term as an indication for labor induction: a systematic review and meta-analysis. Fetal Diagn Ther 2016;40(3):161–73. | |
RANZCOG. Maternal suitability for models of care, and indications for referral within and between models of care (C-Obs 30). 2012. | |
Magann EF, Morton ML, Nolan TE, et al. Comparative efficacy of two sonographic measurements for the detection of aberrations in the amniotic fluid volume and the effect of amniotic fluid volume on pregnancy outcome. Obstet Gynecol 1994;85:959–62. | |
Baron C, Morgan MA, Garite TJ. The impact of amniotic fluid volume assessed intrapartum on perinatal outcome. Am J Obstet Gynecol 1995:175:164–7. | |
Phelan JP, Ahn MO, Smith CV, et al. Amniotic fluid index measurements during pregnancy. J Reprod Med 1987;32:601–4. | |
Chauhan SP, Doherty DD, Magann EF, et al. Amniotic fluid index vs single deepest pocket technique during modified biophysical profile: a randomized clinical trial. Am J Obstet Gynecol 2004;191:661–7, discussion 7–8. | |
Queenan JT, Gadow EC. Polyhydramnios: chronic versus acute. Am J Obstet Gynecol 1970;108:349–55. | |
Golan A, Wolman I, Langer R, et al. Fetal malformations associated with chronic polyhydramnios in singleton pregnancies. Eur J Obstet Gynecol Reprod Biol 1992;47:185–8. | |
Ott WJ. Reevaluation of the relationship between amniotic fluid volume and perinatal outcome. Am J Obstet Gynecol 2005;192:1803–9, discussion 9. | |
Panting-Kemp A, Nguyen T, Chang E, et al. Idiopathic polyhydramnios and perinatal outcome. Am J Obstet Gynecol 1991;181. | |
Idris N, Wong SF, Thomae M, et al. Influence of polyhydramnios on perinatal outcome in pregestational diabetic pregnancies. Ultrasound Obstet Gynecol 2010;36:338–43. | |
O’Neill E, Thorp J. Antepartum evaluation of the fetus and fetal well being. Clin Obstet Gynecol 2012;55:722–30. | |
Dubil EA, Magann EF. Amniotic fluid as a vital sign for fetal wellbeing. Australas J Ultrasound Med 2013;16(2):62–70. | |
Moore TR. Clinical assessment of amniotic fluid. Clin Obstet Gynaecol 1997;40:303–13. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can now automatically receive 2 Continuing Professional Development credits from FIGO plus a Study Completion Certificate from GLOWM for successfully answering 4 multiple choice questions (randomly selected) based on the study of this chapter.
Medical students can receive the Study Completion Certificate only.
(To find out more about FIGO’s Continuing Professional Development awards programme CLICK HERE)