This chapter should be cited as follows:
Paizis K, Jarvis E, Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.414973
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 8
Maternal medical health and disorders in pregnancy
Volume Editor:
Dr Kenneth K Chen, Alpert Medical School of Brown University, USA
Originating Editor: Professor Sandra Lowe

Chapter
Kidney Physiology and Acute Kidney Disease in Pregnancy
First published: December 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Pregnancy is associated with structural, metabolic, synthetic, and functional changes in the kidney and renal tract that are central to successful pregnancy outcomes.
In women with compromised function, this can be associated with adverse pregnancy outcomes for both mother and baby. Pre-pregnancy counseling, good contraceptive education to avoid unwanted or ill-advised pregnancies, and management by a multidisciplinary team during pregnancy may help optimize outcomes, alleviate anxiety, and dispel some of the myths surrounding pregnancy in women with more severe renal disorders. Acute renal failure in pregnancy has also been better recognized and managed over the last decade. This chapter discusses the normal physiological changes seen in pregnancy, management of pregnant women with acute and chronic kidney disease including dialysis and renal transplantation, and contraception for women with renal disease.
PHYSIOLOGY
Structural
Kidneys increase in size by 1–1.5 cm in pregnancy due to an increase in vascular and interstitial volume.1,2,3 Physiological dilatation of the urinary system (pelvis, calyx, and ureter) is thought to be secondary to hormonal smooth muscle relaxation and compression from the gravid uterus. This dilatation is normally observed in the mid trimester and increases with increasing gestation. It is seen more commonly in primigravid and multiple pregnancies. Right-sided dilatation is more common than left, possibly as a consequence of compression of the ureter by the right ovarian and iliac veins at the pelvic brim and dextra-rotation of the uterus, providing some protection to the left ureter.4,5 In the third trimester it is uncommon for renal pelvis volume to exceed 15 mm.6 These structural changes may take up to 6–12 weeks to resolve postpartum.
Renal function and creatinine
Renal blood flow increases by 150% during pregnancy.7 There is a relaxation of both efferent and efferent arterioles and a resultant increase in glomerular filtration. These changes are most likely mediated via relaxin, progesterone, and nitric oxide.8 The increase in glomerular filtration reduces serum creatinine (sCr) compared to prepregnancy levels. Two recent systematic reviews have attempted to provide gestation-specific measures of renal function. SCr levels >76 mmol/l (0.86 mg/dl) in the first trimester, >72 mmol/l (0.81 mg/dl) in the second trimester, and >77 mmol/l (0.87 mg/dl) in the third trimester are considered abnormal and a sign of renal impairment. In healthy women, sCr reaches a nadir in the 2nd trimester and starts to rise from 32 weeks onwards.9,10
A pictorial summary of the renal structural, physiological, and hormonal changes in pregnancy is demonstrated in Figure 1.11
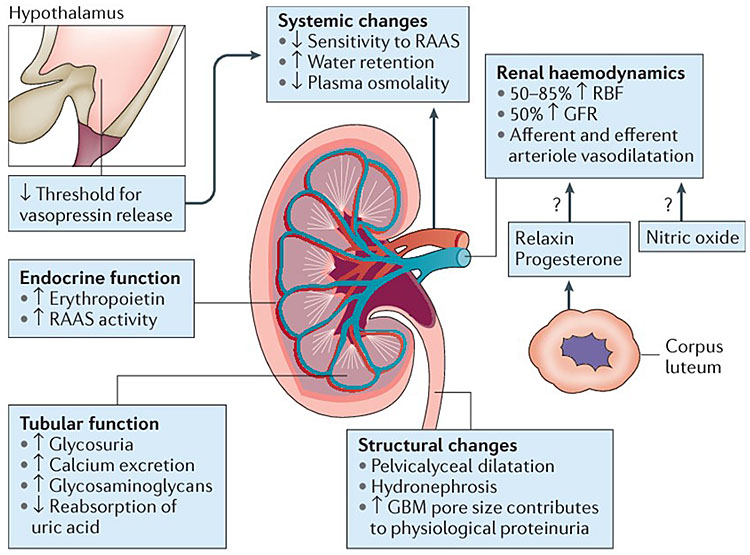
1
Physiological changes in the kidney during pregnancy. Reproduced from Wiles, Nelson-Piercy and Bramham. Reproductive health and pregnancy in women with chronic kidney disease. Nat Rev Nephrol, 2018,11 with permission.
Assessment of Renal Function in Pregnancy
Outside of pregnancy, formulae such as the Modification of Diet in Renal Disease (MDRD) or the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation provide a better estimation of kidney function than sCr. These equations should not be used in pregnancy as they have been shown to underestimate renal function.12,13 Cystatin C has also been shown to underestimate renal function in pregnancy. The Cockcroft–Gault formula both overestimates and underestimates renal function depending what body weight is used (prepregnancy or pregnancy.8
Inulin clearance is accepted as the gold standard measurement of glomerular filtration rate in the nonpregnant and pregnant population. In practice, inulin clearance measurements are complex and impractical and often reserved for use in research settings.14,15 Twenty-four-hour urine collection for estimation of creatinine clearance has been shown to correlate with inulin clearance16 when collected accurately and using the recommended collection technique; in pregnancy this involves lying in the left lateral position before and after collection to minimize urine pooling in dilated ureters.17 Practically this is difficult to do in pregnancy, and accuracy is hampered by incomplete collections.15,18 For the above reasons sCr is used for assessment of renal function. Reference ranges for creatinine levels in each trimester can be found in Table 1.
1
Normal reference values in pregnancy. These values are only a guide due to differences in laboratory methods worldwide.
Prepregnancy | First trimester | Second trimester | Third trimester | |
Sodium (mmol/l) | 135–145 | 134–142 | 129–142 | 130–142 |
Potassium (mmol/l) | 3.5–5.5 | 3.4–4.5 | 3.4–4.5 | 3.4–4.8 |
Chloride (mmol/l) | 100–110 | 95–105 | 95–105 | 95–105 |
Bicarbonate (mmol/l) | 22–30 | 18–24 | 18–24 | 18–24 |
Creatinine (mmol/l) | 59–79 | 25–76 | 25–72 | 25–77 |
Albumin (g/l) | 36–48 | 35–48 | 32–43 | 28–40 |
GFR (ml/min) | 106–132 | 131–166 | 135–170 | 117–182 |
Serum osmolality (mOsmol/l) | 275–295 | 265–280 | 264–278 | 264–279 |
Uric acid mmol/l | 0.15–0.35 | 0.12–0.25 | 0.14–0.29 | 0.18–0.37 |
Urinary protein (mg/24 h) | <150 | <300 | <300 | <300 |
Spot pr/creat (mg/mmol) | <15 | <30 | <30 | <30 |
Urinary calcium (mg/24) | 112 | 200–300 | >300 | >300 |
pH | 7.38–7.42 | 7.4–7.45 | 7.4–7.45 | 7.4–7.45 |
PaO2 (mmHg) | 90–100 | 93–100 | 90–98 | 92–107 |
PaCO2 (mmHg) | 38–42 | 27–32 | 27–32 | 27–32 |
Uric acid (mmol/l) | 0.15–0.40 | <0.3 | <0.3 | 0.31–0.36 |
Proteinuria
Proteinuria increases in pregnancy to an upper limit of 300 mg/24 hours, which is double the upper limit in the nonpregnant population (≤150 mg/24 hours). Factors that contribute to increase proteinuria in pregnancy include increase in glomerular blood flow and increase in glomerular permeability (Figure 2).2
The assessment of proteinuria in pregnancy is discussed in the accompanying chapter: Screening for Hypertension and Proteinuria in pregnancy.
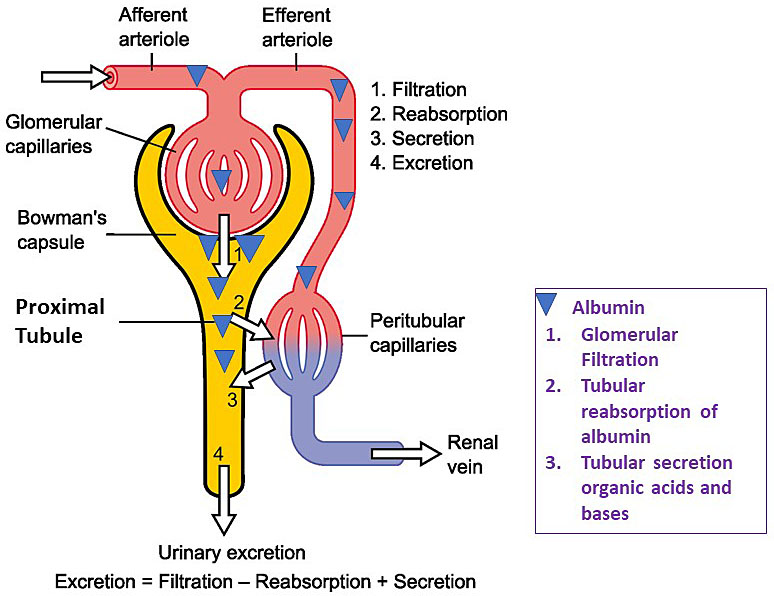
2
Physiology of the nephron: glomerular and tubular handling of protein. https://commons.wikimedia.org/wiki/File:Physiology_of_Nephron.png Madhero88, CC BY 3.0 <https://creativecommons.org/licenses/by/3.0>, via Wikimedia Commons. Modified to reflect filtration of albumin.
Proteinuria and renal disease in pregnancy
Acute and chronic kidney disease are discussed in detail in the following sections. All women with chronic kidney disease (non-proteinuric and proteinuric) should have baseline proteinuria assessment at the beginning of pregnancy; this information can be critical later in pregnancy. Proteinuria present before 20 weeks' gestation is consistent with underlying kidney disease and may often be the only manifestation of this. Increase in proteinuria after 20 weeks can have many differentials, including worsening of underlying kidney disease, pre-eclampsia (PE), or physiological increase in proteinuria from baseline due to increase glomerular filtration and glomerular permeability (discussed in the previous section). Some studies in diabetic nephropathy have shown a 2–5 times increase in proteinuria in the 3rd trimester when compared to the 1st trimester or prepregnancy values.19,20 This situation poses a difficult diagnostic challenge an approach to this, and will be discussed in more detail in the sections on acute and chronic kidney disease.
Gestational proteinuria is defined as new-onset proteinuria in pregnancy that is not associated with features of pre-eclampsia, underlying renal disease, or urinary tract infection after 20 weeks of pregnancy. The true incidence of gestational proteinuria is unknown. Reports in the literature vary from 2 to 13.4%.21,22 Some studies have shown an increase in the development of pre-eclampsia (30–50%),23,24 while others have shown a relationship with the risk factors of pre-eclampsia (increase BMI, twin pregnancies, maternal age, and nulliparity).22 The underlying pathogenesis is not known; Holston showed a correlation with lower circulating angiogenic factors and gestational proteinuria.25 Angiogenic factors are important for the integrity of the glomerular basement, and a reduction in these levels may offer an insight into the pathogenesis.26 In women with gestational proteinuria, regular review for development of pre-eclampsia is advised. Women with gestational proteinuria should be followed up postpartum to ensure resolution of proteinuria. If this does not resolve by 6 months postpartum or, if worsening of renal function and proteinuria occur, further investigation for underlying renal disease by a nephrologist is recommended.24,27 Time to resolution of proteinuria has been shown to correlate with degree of proteinuria and, in some women, can take up to 48 months to completely resolve.28
Electrolytes and Acid–Base Balance
Serum electrolytes
Pregnancy is associated with numerous changes in tubular function that impact volume regulation, electrolyte balance, acid–base equilibrium and serum osmolality. Normal ranges for serum electrolytes are altered in pregnancy; see Table 1.9,10,29,30,31,32,33,34
Volume regulation
An increase in plasma volume in pregnancy is driven by activation of the renin–angiotensin–aldosterone system (RAAS), which increases sodium and water reabsorption and results in total body sodium increase of 1000 mmol.2,35,36 This sodium balance is in regulated by a complex balance between promoters of sodium wasting and promoters of sodium absorption. Raised GFR, progesterone, and atrial natriuretic peptide all increase sodium excretion37,38 whilst aldosterone and deoxycorticosterone promote sodium reabsorption.39,40 Progesterone is an antagonist of aldosterone; it competitively inhibits the binding of aldosterone to its receptor in the kidney,38and this decreases sodium reabsorption by the kidney. Progesterone also prevents potassium losses that would have occurred with the increase in sodium reabsorption;41 as with sodium there is a net increase in potassium stores.2 Aldosterone levels are significantly increased in pregnancy, despite a significant increase in plasma volume. The regulation of aldosterone production in pregnancy is not well understood. Estrogen increases hepatic synthesis while vascular endothelial growth factor (VEGF) stimulates adrenal production of aldosterone. Despite this increase in total body sodium, serum levels of sodium and potassium fall in pregnancy—sodium by 4 mmol/l and potassium by 0.25 mmol (see Table 2). This decrease is due to increase in water retention. In pregnancy there is a resetting of the plasma osmostat and antidiuretic hormone (ADH) release occurs at lower serum osmolality.42,43 The net effect of this is water retention and reduction of serum osmolality, by 8–10 mmol/l.
Acid–base balance
There is an increase in acid production in pregnancy due to increases in metabolic rate. Despite this, pH is higher as there is a respiratory alkalosis due to a progesterone-mediated increase in minute ventilation.41 Serum bicarbonate levels are lower due to mixed metabolic acidosis and respiratory alkalosis.32
PREGNANCY-RELATED ACUTE KIDNEY INJURY (PRAKI)
Definition and diagnosis
The definition of AKI in pregnancy is controversial. RIFLE (Risk, Injury, Failure, Loss, End Stage) and AKIN (Acute Kidney Injury Network) criteria have been used outside the pregnancy setting to diagnose and grade the severity of AKI. The use of these criteria in pregnancy has not been validated. Consequently, there are less robust definitions used in pregnancy for the diagnosis of AKI. These include doubling of serum creatinine (often baseline creatinine is unknown), creatinine level >87–95 mmol/l (0.67–1.0 mg/dl), creatinine rise >26 mmol/l (>0.3 mg/dl), or urine output <20 ml/h for >12 h.8
Incidence and outcomes
Worldwide the incidence of prAKI has fallen primarily due the reduction in septic abortions and puerperal sepsis related to improvements in antenatal care.44,45 In low- and middle-income countries, the incidence of AKI is still high (0.2–1.8%)44,45 but is falling with an incidence of 1/18,000 pregnancies in developed nations.46 Recent data from Canada and the USA have demonstrated increasing rates of AKI. In Canada rates increased from 1.6/10,000 deliveries in 2003 to 2.3/10,000 deliveries in 2007.47 In the USA, prAKI rates increased over a 10-year period from 2.4/10,000 deliveries to 6.3/10,000 deliveries from 1999–2001 to 2010–2011.48 Potential explanations for this include better recognition of prAKI and an older maternal population with co-morbidities,48,49,50 fluid restrictions in the management of women with pre-eclampsia, and increased numbers of women with underlying hypertension and chronic kidney disease. PrAKI most commonly presents in the 3rd trimester and postpartum period.8
PrAKI is associated with an increase in both maternal and fetal mortality and morbidity.45,51 This is not surprising as AKI is often a reflection of the severity of the underlying clinical condition. Women may require dialysis support, inotropes for management of sepsis and hypotension, and regular monitoring of fluid electrolyte status. Many of these resources are not readily available in low- and middle-income countries. This contributes to poorer fetal and maternal outcomes with a higher risk of maternal death (odds ratio [OR]: 4.5), cesarean section (OR: 1.49), disseminated intravascular coagulation (OR: 3.41), placental abruption (OR: 3.13), and increase in the risk of hemorrhage and longer ICU stay. There was also an increase in fetal complications, with an increase in stillbirth and neonatal death (OR: 3.39) and increase in premature delivery and higher risk of intrauterine growth restriction.51,52
Etiology
PrAKI can be due to obstetric or nonobstetric causes and can be divided into prerenal, renal, and postrenal (Table 2). Pre-eclampsia (PE) and Hemolysis Elevated Liver enzymes and Low Platelets (HELLP) are the commonest cause of prAKI.53,54 Sepsis and hemorrhage are also common causes worldwide.52
2
Common causes of acute kidney injury in pregnancy, incidence in pregnancy, and risk of AKI.
| Acute kidney injury (AKI) | Clinical |
| Prerenal | |
| Hyperemesis gravidarum | 1st to 2nd trimester, hypovolemia, postural drop |
| Sepsis | Hypotension, tachycardia, shock, fever, raised inflammatory markers |
| Postpartum hemorrhage | History of blood loss peripartum, low Hb; if severe can have TMA picture on blood film |
| Placental abruption | PV blood loss, low Hb, pain over uterus, hypotension due to hypovolemia |
| Renal | |
| Acute tubular necrosis | In setting of severe hypotension and shock, can take days to weeks to recover—severe cases lead to acute cortical necrosis which is nonrecoverable |
| Pre-eclampsia | Hypertension, proteinuria, liver and renal abnormalities, IUGR >20 weeks' gestation |
| HELLP | Hypertension (70%), proteinuria, TMA picture on blood film, impaired coagulation, platelet recovery after 72 hours, >20 weeks' gestation |
| Acute fatty liver of pregnancy | Acute liver dysfunction, raised coagulation, high ammonia, encephalopathy, lactic acidosis, hypoglycemia, absence of ketones in urine, high serum cholesterol |
| Thrombotic thrombocytopenic purpura (TTP) | TMA on blood film, platelet count <30,000, neurological symptoms and signs, normal clotting, LDH >1000, low ADAMTS-13 activity (<10%), Antepartum—2nd to 3rd trimester |
| Atypical hemolytic uremic syndrome (HUS) | TMA on blood film, Platelet count >30,000, creatinine >200 mmol/l, normal clotting, ADAMSTS-13 activity normal, LDH >1000, postpartum; disorder of complement regulation |
| Lupus nephritis | Active urinary sediment with glomerular red blood cells and proteinuria, low complement C3, C4 levels, positive ANA and ds DNA |
| Acute interstitial nephritis | History of NSAID, antibiotic use, rash, inactive urinary sediment with no proteinuria, normal BP |
| First presentation chronic kidney disease | Small kidneys on ultrasound |
| Nephrotoxic medication | Aminoglycosides (gentamicin, vancomycin) NSAIDs |
| Contrast nephropathy | Contrast—CT scan |
| Postrenal | |
| Obstruction | Hydronephrosis on renal ultrasound may be hard to distinguish from physiological hydronephrosis; calculi, compression from uterus, damage during surgical procedure, sloughed papillae in diabetic nephropathy. |
Hb, hemoglobin; TMA, thrombotic microangiopathy, hemolysis, fragmented red cells, raised LDH, low haptoglobins, thrombocytopenia; PV, per vagina; PE, pre-eclampsia; IUGR, intrauterine growth retardation; ADAMTS-13, ADAMTS-13, ADAM metallopeptidase with thrombospondin type 1 motif 13; NSAIDs, nonsteroidal anti-inflammatory drugs; CT, computerized tomography.
History, examination, and investigations
A good clinical history and examination are of utmost importance in determining the underlying etiology, and will help target investigations and identify and prioritize treatment. Septic abortions and hypovolemia secondary to hyperemesis are more common in the 1st trimester. PE, HELLP, and hemorrhage are more common in the 2nd and 3rd trimesters. Acute fatty liver of pregnancy (AFLP) and hemolytic-uremic ayndrome (p-aHUS) are rare cause of prAKI in late pregnancy or postpartum.55 The presence of systemic symptoms such as arthralgias and rashes is an alert to underlying autoimmune diseases, whilst fevers may be an alert to underlying infection. A medication history may help identify potential nephrotoxic medications and family history, and may be important in the diagnosis of inherited renal diseases and pregnancy-associated atypical p-aHUS.
Physical examination can reveal important signs such as fever, hypertension or hypotension, rashes, and synovitis in autoimmune disease and volume status (hyper- or hypovolemia). If available it is important and critical to compare current serum creatinine levels and perform urine analysis and spot protein/creatinine ratio with prepregnancy or early pregnancy values. Fresh microscopy of a mid-stream urine specimen is important in ruling out infection and allows examination for the presence of red blood cells and casts, which may be a sign of underlying glomerulonephritis.56 The presence of both hematuria and proteinuria in the absence of infection is suspicious of underlying primary glomerular disease.
Renal ultrasound is not always required, but is useful in excluding obstruction is associated with dilatation of the ureters and renal pelvis,57 and may also be helpful in identifying chronic kidney disease where the kidney size is small (<8 cm) and the renal cortex appears echogenic.58 A renal biopsy may be required in women with significant prAKI where the diagnosis is unclear, to identify potential treatable causes such as interstitial nephritis or acute glomerulonephritis.
A summary of basic investigations (Table 3) and a practical approach to prAKI (Figure 3) can be found below.
3
Investigations in AKI of pregnancy.
Test | Association |
Full blood examination (FBE) |
|
Urea, creatinine, and electrolytes |
|
Liver function tests |
|
LDH |
|
MSU, casts, and RBC morphology |
|
Proteinuria |
|
INR and APTT |
|
ADAMTS-13 |
|
ANA, ENA, dsDNA |
|
Complement |
|
Renal ultrasound |
|
Hb, hemoglobin; PE, pre-eclampsia; HELLP, hemolysis, elevated liver enzymes, low platelets; TTP, thrombotic thrombocytopenic purpura; p-aHUS, pregnancy-associated hemolytic, uremic syndrome; AFLP, acute fatty liver of pregnancy, LDH, lactate dehydrogenase; RBC, red blood cells; ADAMTS-13, ADAM metallopeptidase with thrombospondin type 1 motif 13; ANA, antinuclear antibody; ENA, extractable nuclear antibody; dsDNA, double-stranded DNA.
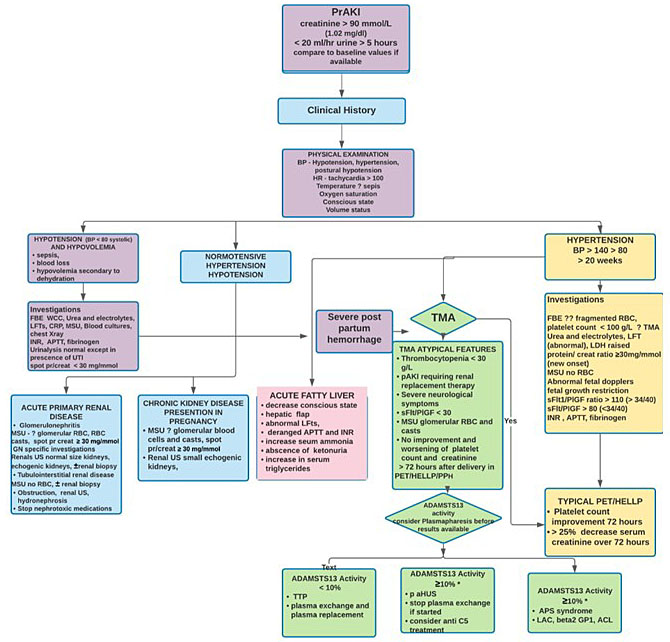
3
Diagnostic approach to pAKI. BP, blood pressure; HR, heart rate; FBE, full blood count; WCC, white cell count; LFTs, liver function tests; CRP, C-reactive protein; MSU, mid-stream urine; INR, international normalized ratio; APTT, activated partial thromboplastin time; UTI, urinary tract infection; RBC, red blood cells; US, ultrasound; TMA, thrombotic microangiopathy; sFlt/PlGF, soluble FMS-like tyrsosine kinase/placental growth factor; PPH, postpartum hemorrhage; ADAMSTS-13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member13; TTP, thrombotic thrombocypenic purpura; p-aHUS, pregnancy hemoytic uremic syndrome; C5, complement 5; APS, antiphospholipid syndrome; LAC, lupus anticoagulant; ACL, anticardilipin. *ADAMS TS13 cutoff is 20% in some laboratories.
Thrombotic microangiopathy (TMA)
TMA is a pathological definition; it relates to the presence of anemia (Hb <10 g/dl), fragmented red blood cells (schistocytes), thrombocytopenia (<100 g/l), undetected haptoglobins, and raised lactate dehydrogenase (LDH), with evidence of end organ damage such a AKI, neurological, or liver involvement.55 In pregnancy, HELLP and PE are the commonest causes of TMA.59 Thrombotic thrombocytopenic purpura (TTP) and pregnancy-associated atypical hemolytic-uremic syndrome (p-aHUS) are less common causes of TMA in pregnancy and the postpartum period. These disorders can be precipitated by pregnancy, and often this may be the first clinical presentation of these rare conditions.60,61 The measurement of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS-13) activity is a particularly useful investigation in TMA, because activity (<10%) is consistent with the diagnosis of TTP.62 There are no specific diagnostic criteria for p-aHUS, which is a disorder of unregulated complement activation and is a diagnosis of exclusion when presenting for the first time in pregnancy.55 Targeted therapies are now available for TTP (plasma exchange and steroids to remove and decrease production of inhibiting antibodies, plasma infusion to replace metalloproteinase),55 and p-aHUS (eculizumab, a human monoclonal antibody, to complement 5 [C5] that has dramatically improved the prognosis of aHUS)63. Early initiation of treatment may significantly impact both maternal and fetal outcome. Often plasma exchange and plasma infusion may need to be initiated prior to confirmation of a diagnosis in the setting of severe or worsening end organ involvement55 Catastrophic anti-phospholipid syndrome (CAPS) and severe systemic lupus erythematosus (SLE) can present as a TMA of pregnancy;64 a clinical history of autoimmune may help guide diagnosis.
Postpartum hemorrhage (PPH) can precipitate prAKI in the setting of hypovolemia and hypotension. A less common scenario is TMA following PPH.65 If severe AKI is present with significant oliguria, anuria, or dialysis dependence, renal cortical necrosis (irreversible ischemia of the renal cortex) should be ruled out. A diagnosis of renal cortical necrosis avoids further unnecessary investigations and treatments such as plasma exchange.55
A combination of clinical and laboratory features is used to guide and determine the underlying etiology of TMA (Table 4). It is important to remember that conditions can co-exist; both TTP and p- aHUS can be complicated by PE and HELLP.66,67 In such complex cases it is always critical to involve multidisciplinary teams early in the management of these women.
4
Clinical and laboratory features of thrombotic microangiopathy in pregnancy.
Clinical and laboratory | PE/HELLP | TTP | P-aHUS | PPH | CAPS |
Commonest presentation | 2nd and 3rd trimester | 2nd and 3rd trimester | Postpartum | Postpartum | All trimesters and postpartum |
Hypertension | +++ | +/− | ++ | − | +/− |
Neurological symptoms | + | +++ | +/− | − | ++ |
Hemolysis | ++ | +++ | +++ | +/− | ++ |
Proteinuria | +++ | +/− | +++ | − | +/− |
Thrombocytopenia | ++ | +++ | ++/+++ | ++/+++ | ++ |
AKI | + | ++ | +++ 70% dialysis dependent | + | ++/+++ |
LFT derangement | ++ | +/− | +/− | +/− | ++ |
Coagulopathy | Yes | No | No | Yes | No |
ADAMTS-13 activity | ≥10% | <10% | ≥10% | ≥10% | ≥10% |
Recovery | 72 hours from nadir | Worse unless treated | Worse unless treated | Recovery 48–72 hours | Worse unless treated |
Treatment | Supportive | Plasmapheresis and plasma infusion +/− steroids | Anti-C5 treatment | Supportive | Anticoagulation +/− anti-CD20 |
HELLP, hemolysis, elevated liver enzymes; AKI, acute kidney injury; LFTS, liver function tests; ADAMTS-13, ADAM metallopeptidase with thrombospondin type 1 motif 13; anti-C5 treatment, antibody to complement 5; anti-CD 20, antibody to CD 20-positive B cells.
Acute lupus nephritis and glomerulonephritis
Lupus nephritis can present acutely in pregnancy and, when the first presentation is in the second and third trimesters, can often be difficult to distinguish from PE as often many of the clinical features are similar.68 A history of arthralgias, rashes, mouth ulcers, and alopecia may be helpful, and physical examination may reveal signs of synovitis and malar rash. Examination of the urine may reveal glomerular red blood cells and casts in addition to proteinuria; antinuclear antibodyies and double-stranded DNA may be positive and titres increased; complement levels that normally increase in pregnancy tend to fall in active lupus nephritis (complements 3 and 4 are consumed), and inflammatory markers are often raised (Table 5).
5
Differentiation of lupus nephritis and pre-eclampsia by clinical and laboratory features.
Lupus nephritis | Pre-eclampsia | |
Timing | All trimesters and postpartum | 2nd and 3rd trimesters |
clinical | Athralgias, rash, synovitis, hypertension | Hypertension, headache |
Urine | Active renal sediment, hematuria (glomerular), casts, proteinuria | Proteinuria |
Laboratory | Worsening renal function, thrombocytopenia | Worsening renal function, thrombocytopenia |
Serology | Increased dsDNA, low C3, C4, increased CRP | Normal C3 C4 levels |
Treatment | Immunosuppression | Delivery |
Renal Biopsy | Lupus nephritis | Endotheliosis, acute tubular necrosis |
PlGF | ≥100 pg/ml TRIAGE test | Low <12 pg/ml TRIAGE test |
sFlt/PlGF | ≤38 | ≥85 |
dsDNA, double-stranded DNA; C3, complement 3; C4, complement 4; PlGF, placental growth factor; sFlt-1, soluble fms-like tyrosine kinase-1.
Antiangiogenic markers
Pre-eclampsia (PE) is an imbalance between pro- (PlGF, placental growth factor) and anti-angiogenic factors (sFlt-1 soluble fms-like tyrosine kinase-1),69 which leads to vascular endothelial activation and inflammation.70 sFlt-1/PlGF ratio and PlGF assay are useful tools in the prediction and diagnosis of PE.71,72 Small studies have used the sFlt/PlGF ratio to differentiate PE from active lupus nephritis.73,74 Larger studies are needed to determine the role of these assays and differentiation of underlying clinical conditions that may be misdiagnosed as PE.
Management
Management of prAKI requires a number of simultaneous steps to avoid worsening of maternal, and potentially fetal, condition.
General principles of management include:
Recognition of AKI
- Early involvement of multidisciplinary team if available; this may include maternal fetal medicine specialist, obstetric medical physician and nephrologist, intensive care physicians, and neonatologists
- Consideration of transfer to a tertiary referral center
Emergency management
Volume assessment and fluid resuscitation
Volume assessment is crucial in the acute management of prAKI. Hypovolemia is associated with a decrease in renal and placental perfusion. Volume depletion is commonly seen following obstetric hemorrhage, sepsis, and in severe hyperemesis. Clinical signs of hypovolemia include decreasing daily weight, hypotension, tachycardia, significant postural drop of >20 mmHg, and low jugular venous pressure (JVP).75 More invasive monitoring is used in the intensive care setting: measurement of central venous pressure and pulmonary capillary wedge pressure have been replaced by pulse pressure variation (PPV)76 and stroke volume variation (SVV).77 These and other dynamic assessments represent more accurate assessments of volume status and reserved for women that are critically ill. A recent small study used ultrasound to measure inferior vena cava to aortic diameter (IVC/Ao diameter) at the transhepatic level to assess volume status and guide fluid management in pregnant women.78 This promising, simple, noninvasive, and inexpensive tool may increase the accuracy of bedside volume assessment and add to clinical assessment which can at times be difficult.
Recognition and correction of hypovolemia may prevent worsening of prAKI and improve placental perfusion. The aims of fluid replacement are to achieve euvolemia, restore normotension, and maintain a urine output >30 ml/hour.79 The choice of fluid replacement is determined by the clinical situation and includes blood, blood products, and crystalloid and colloid solutions. Crystalloids are preferred over colloids as they are cheaper and more readily available.80 Albumin has been shown to have comparable safety to saline and can be used in combination with crystalloids.81 Colloids such as hydroxyethyl starch (HES) should be avoided as they have been associated with increased risk of renal failure.82 A large study in a critically ill nonpregnant population demonstrated better renal outcomes and decrease mortality when balanced crystalloid solutions (lactated Ringer’s or Plasma-Lyte A) were used compared to normal saline. If available, balanced crystalloid solutions should be used in the critically ill.83 The exact volume required will depend on many factors and should be assessed by response in blood pressure, urine output, and daily weight; the more invasive measures discussed above may be used in the intensive care setting.
Volume overload is often associated with clinical signs of hypertension, peripheral edema, raised JVP, and often evidence of left ventricular failure. Oliguria may also be present. Fluid restriction and loop diuretics are the treatment of volume overload. Higher doses of diuretics may be required when there is significant renal impairment. Renal replacement therapy may need to be started if there is oliguria and worsening renal function.
In HELLP and PE, intravascular volume is often reduced and vascular permeability increased.84 Overzealous fluid resuscitation increases the risk of pulmonary edema, and is further increased when oliguria is present. Hypoalbuminemia is also a contributory factor. If significant intravascular depletion is suspected and oliguria is present in combination with renal impairment, a trial of 300 ml of crystalloid can be given followed by clinical assessment, if there is no response in urine output and no evidence of fluid overload, a further bolus of 300 ml of fluid can be given under close supervision.
Hyperkalemia
Hyperkalemia is commonly seen in prAKI. High potassium levels can be life threatening and may cause cardiac arrythmias, conduction defects, and muscle weakness. The rapidity of the increase, absolute level of potassium, and ECG changes of toxicity determine the urgency of treatment.85 A practical approach to the management of hyperkalemia is summarized in Figure 4.
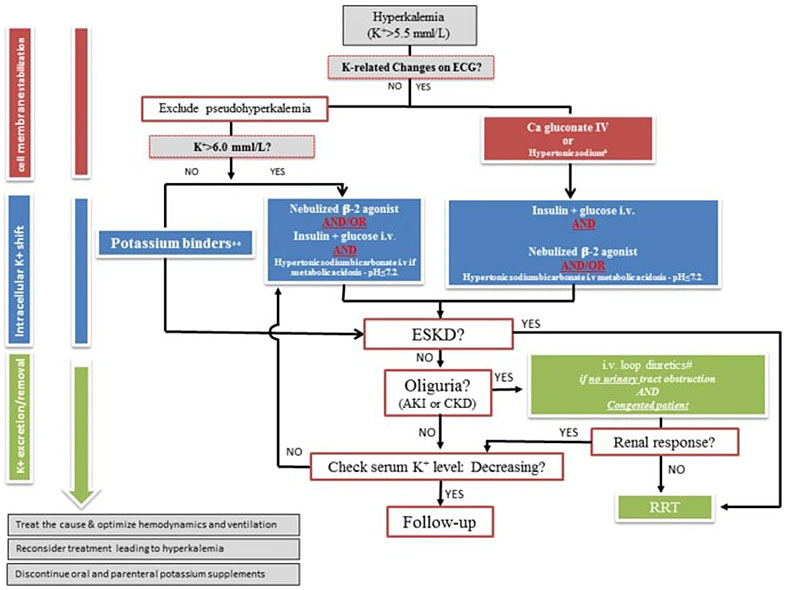
4
A practical approach to the management of hyperkalemia. Springer Open. ESKD, end-stage kidney disease; AKI, acute kidney injury; CKD, chronic kidney disease; RRT, renal replacement therapy.
Management of potassium ≥6.0 mmol/l
- Stop medications associated with hyperkalemia – beta blockers, nonsteroidal inflammatory drugs (NSAIDs), potassium-retaining diuretics (spironolactone, amiloride)
- Establish IV access
- Continuous ECG monitoring86
- 12-lead ECG – ECG changes associated with hyperkalemia include peaked T- waves, broadening of P-waves, widening of the QRS complex, and broad sinusoidal complexes
- Identify high risk
- Potassium ≥6.5 mmol/l
- Rapid increase in potassium
- Oliguria (unable to excrete potassium)
- If ECG changes are present, administer calcium gluconate intravenously (IV) (10 ml 10% solution): onset of action 1–5 minutes and duration of action 30 minutes.87
- Monitor potassium levels 1, 2 , 4 , 6 hours then 24 hourly depending on severity
Lowering of potassium ≥6.0 mmol/l
- Insulin/dextrose
IV insulin/dextrose: 50 ml 50% dextrose with 5–10 units short-acting insulin given as a bolus over 5–10 minutes. This increases movement of potassium into cells.88 Onset of action 15–30 minutes, duration of action 2–6 hours. Blood glucose levels should be monitored at 15 minutes and hourly for 6 hours.89 The risk of hypoglycemia is greater when renal impairment exists, as the half life of insulin is prolonged.89,90 Expected potassium decrease 0.5–1.5 mmol/l. - Sodium bicarbonate
Correction of acidosis when pH <7.2: 100 ml of 8.4% sodium bicarbonate as an infusion over 30–60 minutes. Sodium bicarbonate delivers a high sodium load and should be used cautiously in the setting of volume overload and oliguria. Onset of action 1 hour. - Salbutamol
Nebulized salbutamol 10 mg (higher does than regular nebulized salbutamol used in asthma). Onset of action 15–30 minutes and duration 2–6 hours. Synergistic effect when combined with insulin/dextrose, but may not always be effective when used as a single agent.91 Expected potassium decrease 0.5–1 mmol/l. Intravenous salbutamol can be used: 500 mcg slow infusion over 30 minutes.85 Salbutamol can cause reactive tachycardia, especially when given IV.
Treatment of mild hyperkalemia: potassium <6.0 mmol/l and no ECG changes
- Sodium polystyrene sulphonate (Resonium)
15–30 g orally or 30–60 gm rectally as an enema (retain for 60 minutes) every 8 hours. Onset of action 2 hours with maximum effect 4–6 hours. Resonium is not a treatment for acute hyperkalemia92 due to its delayed onset and increase in gastrointestinal side effects.93 Potassium is expected to decrease by 2 mmol/l. There is limited experience with new potassium-binding resins such as sodium zirconium cyclosilicate and patiromer94 in pregnancy. - Loop diuretics
A trial of loop diuretics in the setting of oliguria and volume overload may increase excretion of potassium.95 Frusemide can be used at 20–40 mg IV; higher doses can then be used if there is there has been no response at these low doses. Frusemide doses up to 120–250 mg can be trialed but, if there is no response in urine output, specialist input should be urgently sought. Potassium lowering is less predictable with loop diuretics.
Management of acidosis when pH <7.2
Acidosis can be treated with sodium bicarbonate, but this is only an interim measure.
- Sodium bicarbonate
100 ml of 8.4% sodium bicarbonate as an infusion over 30–60 minutes. Sodium bicarbonate delivers a high sodium load and should be used cautiously in the setting of volume overload and oliguria. Onset of action 1 hour.96,97
Renal replacement therapy/dialysis
The main indications for renal replacement therapy are volume overload, refractory hyperkalemia or metabolic acidosis, or severe uremic symptoms (pericarditis, neuropathy, or encephalopathy).98 The threshold for initiation of dialysis needs to take into account the stage of pregnancy; early initiation may potentially improve fetal outcomes although evidence is limited and most of the data have been extrapolated fromm the chronic kidney disease population.99 In women who are hemodynamically unstable, continuous renal replacement therapy avoids hypotensive episodes that can occur with intermittent hemodialysis and may better mainta in utero placental perfusion.100
Monitoring and optimization of fetal health
Fetal outcomes are intricately linked to underlying maternal health. Acute renal injury in pregnancy is often a reflection of a major pregnancy-related medical condition. Recognition of the severity of the condition and optimization of maternal health will optimize fetal outcomes. In general, maternal health takes priority. Because fetal well-being is dependent on adequate uteroplacental blood flow, prompt treatment of hypotension, hypovolemia, and acidosis is crucial.99 Frequent monitoring of fetal heart rate once viability is reached, uteroplacental Dopplers, and early delivery on obstetric grounds may be required.101 Magnesium sulfate for neonatal neuroprotection and corticosteroids, depending on the gestational age and clinical scenario, may be indicated prior to delivery.101
Long-term follow-up following prAKI
Although renal recovery is generally good following prAKI, follow-up is often poor. In women requiring dialysis, 6–10% were still on dialysis 6 months postpartum. There also appears to be a risk of end-stage kidney disease requiring dialysis long term (2.4%),51 which is similar to nonpregnant AKI. PrAKI has been associated with an increased risk of PE and adverse fetal outcomes in subsequent pregnancies, despite return to normal creatinine levels.3 All women with prAKI should have long-term follow-up with annual review of blood pressure and urinalysis. Referral to a nephrologist should be considered if there is persistent proteinuria or renal impairment 6 months postpartum.102 In the nonpregnant population, AKI is also a risk factor for cardiovascular disease.103 Future studies looking at long-term cardiovascular and renal outcomes will hopefully provide better insight into optimal postpartum management.
PRACTICE RECOMMENDATIONS
- In pregnancy, kidney size increases and there is physiological increase in size of the renal pelvis. The right renal pelvis is often more dilated than the left; physiological dilatation is normally not greater than 15 mm. Physiological dilatation should always improve postpartum.
- Pregnancy is associated with significant salt and water retention due to activation of the renin–angiotensin–aldosterone system.
- Increase in renal blood flow results in increased glomerular filtration and creatinine levels fall in pregnancy, reaching a nadir in the second trimester.
- Serum creatinine levels >76 mmol/l (86 mg/dl) in the first trimester, >72 mmol/l (0.81 mg/dl) in the second trimester, and >77 mmol/l (0.87 mg/dl) in the third. trimester are considered abnormal and a sign of renal impairment.
- Baseline proteinuria increases in pregnancy: the accepted upper limit of normal is 300 mg/24 hours.
- The criteria for diagnosis of pregnancy acute kidney injury include: doubling of serum creatinine (often baseline creatinine is unknown), creatinine level >87–95 mmol/l (0.67–1.0 mg/dl), creatinine rise >26 mmol/l (>0.3 mg/dl), or urine output <20 ml/h for >12 h.
- PE and HELLP are the commonest causes of thrombotic microangiopathy in pregnancy. In cases with atypical or concerning clinical or laboratory features, it is best to involve nephrologists/hematologists/obstetric physicians early in the disease course as early intervention may significantly impact long-term outcomes.
- Levels of potassium >6 mmol/l should be treated as an emergency. IV access, ECG monitoring, and potassium lowering with dextrose/insulin and Ca gluconate may be required.
- In all women with pregnancy, acute kidney injury should be followed up to ensure that renal function and proteinuria return to normal. Referral to a nephrologist for investigation and management should be considered if proteinuria and renal function have not normalized 6 months postpartum.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Bailey RR, Rolleston GL. Kidney length and ureteric dilatation in the puerperium. J Obstet Gynaecol Br Commonw 1971;78(1):55–61. PubMed PMID: 5557675. Epub 1971/01/01. | |
Odutayo A, Hladunewich M. Obstetric nephrology: renal hemodynamic and metabolic physiology in normal pregnancy. Clin J Am Soc Nephrol 2012;7(12):2073–80. PubMed PMID: 22879432. Epub 2012/08/11. | |
Tangren JS, Wan Md Adnan WAH, Powe CE, Ecker J, Bramham K, Hladunewich MA, et al. Risk of preeclampsia and pregnancy complications in women with a history of acute kidney injury. Hypertension 2018;72(2):451–9. PubMed PMID: 29915020. PMCID: PMC6074052. Epub 2018/06/20. | |
Rasmussen PE, Nielsen FR. Hydronephrosis during pregnancy: a literature survey. Eur J Obstet Gynecol Reprod Biol 1988;27(3):249–59. PubMed PMID: 3280355. Epub 1988/03/01. | |
Fried AM, Woodring JH, Thompson DJ. Hydronephrosis of pregnancy: a prospective sequential study of the course of dilatation. J Ultrasound Med 1983;2(6):255–9. PubMed PMID: 6876256. Epub 1983/06/01. | |
Wadasinghe SU, Metcalf L, Metcalf P, et al. Maternal physiologic renal pelvis dilatation in pregnancy: Sonographic reference data. J Ultrasound Med 2016;35(12):2659–64. PubMed PMID: 27821653. Epub 2016/11/09. | |
Lindheimer MD, Davison JM, Katz AI. The kidney and hypertension in pregnancy: twenty exciting years. Semin Nephrol 2001;21(2):173–89. PubMed PMID: 11245779. Epub 2001/03/14. | |
Bramham Ke, Hall MMe, Nelson-Piercy Ce. Renal disease in pregnancy. 2nd edn. Cambridge University Press 2018. | |
Harel Z, McArthur E, Hladunewich M, et al. Serum creatinine levels before, during, and after pregnancy. JAMA 2019;321(2):205–7. PubMed PMID: 30644975. PMCID: PMC6439761. Epub 2019/01/16. | |
Wiles K, Bramham K, Seed PT, et al. Serum creatinine in pregnancy: A systematic review. Kidney Int Rep 2019;4(3):408–19. PubMed PMID: 30899868. PMCID: PMC6409397. Epub 2019/03/23. | |
Wiles KS, Nelson-Piercy C, Bramham K. Reproductive health and pregnancy in women with chronic kidney disease. Nat Rev Nephrol 2018;14(3):165–84. PubMed PMID: 29355168. Epub 2018/01/23. | |
Koetje PM, Spaan JJ, Kooman JP, et al. Pregnancy reduces the accuracy of the estimated glomerular filtration rate based on Cockroft-Gault and MDRD formulas. Reprod Sci 2011;18(5):456–62. PubMed PMID: 21079240. Epub 2010/11/17. | |
Smith MC, Moran P, Ward MK, et al. Assessment of glomerular filtration rate during pregnancy using the MDRD formula. BJOG 2008;115(1):109–12. PubMed PMID: 17970797. Epub 2007/11/01. | |
Lopes van Balen VA, van Gansewinkel TAG, de Haas S, et al. Maternal kidney function during pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2019;54(3):297–307. PubMed PMID: 30288811. PMCID: PMC6772153. Epub 2018/10/06. | |
Ahmed SB, Bentley-Lewis R, Hollenberg NK, et al. A comparison of prediction equations for estimating glomerular filtration rate in pregnancy. Hypertens Pregnancy 2009;28(3):243–55. PubMed PMID: 19440935. PMCID: PMC3811128. Epub 2009/05/15. | |
Sims EA, Krantz KE. Serial studies of renal function during pregnancy and the puerperium in normal women. J Clin Invest 1958;37(12):1764–74. PubMed PMID: 13611044. PMCID: PMC1062863. Epub 1958/12/01. | |
Lindheimer MD. L24. The meaning of proteinuria in preeclampsia. Pregnancy Hypertens. 2011 Jul-Oct;1(3–4):252. PubMed PMID: 26009055. Epub 2011/07/01. | |
Cote AM, Firoz T, Mattman A, et al. The 24-hour urine collection: gold standard or historical practice? Am J Obstet Gynecol 2008;199(6):625 e1–6. PubMed PMID: 18718568. Epub 2008/08/23. | |
How HY, Sibai B, Lindheimer M, et al. Is early-pregnancy proteinuria associated with an increased rate of preeclampsia in women with pregestational diabetes mellitus? Am J Obstet Gynecol 2004;190(3):775–8. PubMed PMID: 15042013. Epub 2004/03/26. | |
Purdy LP, Hantsch CE, Molitch ME, et al. Effect of pregnancy on renal function in patients with moderate-to-severe diabetic renal insufficiency. Diabetes Care 1996;19(10):1067–74. PubMed PMID: 8886551. Epub 1996/10/01. | |
Kattah A, Milic N, White W, et al. Spot urine protein measurements in normotensive pregnancies, pregnancies with isolated proteinuria and preeclampsia. Am J Physiol Regul Integr Comp Physiol 2017;313(4):R418-R24. PubMed PMID: 28747409. PMCID: PMC5668620. Epub 2017/07/28. | |
Macdonald-Wallis C, Lawlor DA, Heron J, et al. Relationships of risk factors for pre-eclampsia with patterns of occurrence of isolated gestational proteinuria during normal term pregnancy. PLoS ONE 2011;6(7):e22115. PubMed PMID: 21789220. PMCID: PMC3138774. Epub 2011/07/27. | |
Rezk M, Abo-Elnasr M, Al Halaby A, et al. Maternal and fetal outcome in women with gestational hypertension in comparison to gestational proteinuria: A 3-year observational study. Hypertens Pregnancy 2016;35(2):181–8. PubMed PMID: 26909553. Epub 2016/02/26. | |
Morikawa M, Yamada T, Minakami H. Outcome of pregnancy in patients with isolated proteinuria. Curr Opin Obstet Gynecol 2009;21(6):491–5. PubMed PMID: 19633554. Epub 2009/07/28. | |
Holston AM, Qian C, Yu KF, et al. Circulating angiogenic factors in gestational proteinuria without hypertension. Am J Obstet Gynecol 2009;200(4):392 e1–10. PubMed PMID: 19168169. PMCID: PMC2679962. Epub 2009/01/27. | |
Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 2003;111(5):707–16. PubMed PMID: 12618525. PMCID: PMC151905. Epub 2003/03/06. | |
Brown MA, Magee LA, Kenny LC, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 2018;72(1):24–43. PubMed PMID: 29899139. Epub 2018/06/15. | |
Berks D, Steegers EAP, Molas M, et al. Resolution of hypertension and proteinuria after preeclampsia. Obstet Gynecol 2009;114(6):1307–14. PubMed PMID: 19935034. Epub 2009/11/26. | |
Gronowski AM. Handbook of Clinica Laboratory Testing during Pregnancy. Totowa, N.J. Humana, Oxford: Blackwell, 2004. | |
Larsson A, Palm M, Hansson LO, et al. Reference values for clinical chemistry tests during normal pregnancy. BJOG 2008;115(7):874–81. PubMed PMID: 18485166. Epub 2008/05/20. | |
Klajnbard A, Szecsi PB, Colov NP, et al. Laboratory reference intervals during pregnancy, delivery and the early postpartum period. Clin Chem Lab Med 2010;48(2):237–48. PubMed PMID: 19943809. Epub 2009/12/01. | |
Lim VS, Katz AI, Lindheimer MD. Acid-base regulation in pregnancy. Am J Physiol 1976;231(6):1764–9. PubMed PMID: 990114. Epub 1976/12/01. | |
Lind T, Godfrey KA, Otun H, et al. Changes in serum uric acid concentrations during normal pregnancy. Br J Obstet Gynaecol 1984;91(2):128–32. PubMed PMID: 6696858. Epub 1984/02/01. | |
Cunningham FG. Waltham M. Normal reference ranges for laboratory values in pregnancy. Up to Date. 2019 https://www.uptodate.com/contents/normal-reference-ranges-for-laboratory-values-in-pregnancy? | |
Atherton JC, Bielinska A, Davison JM, et al. Sodium and water reabsorption in the proximal and distal nephron in conscious pregnant rats and third trimester women. J Physiol 1988;396:457–70. PubMed PMID: 3411501. PMCID: PMC1192055. Epub 1988/02/01. | |
Lumbers ER, Pringle KG. Roles of the circulating renin-angiotensin-aldosterone system in human pregnancy. Am J Physiol Regul Integr Comp Physiol 2014;306(2):R91–101. PubMed PMID: 24089380. Epub 2013/10/04. | |
Irons DW, Baylis PH, Davison JM. Effect of atrial natriuretic peptide on renal hemodynamics and sodium excretion during human pregnancy. Am J Physiol 1996;271(1 Pt 2):F239–42. PubMed PMID: 8760268. Epub 1996/07/01. | |
Landau RL, Lugibihl K. Inhibition of the sodium-retaining influence of aldosterone by progesterone. J Clin Endocrinol Metab 1958;18(11):1237–45. PubMed PMID: 13587641. Epub 1958/11/01. | |
Bentley-Lewis R, Graves SW, Seely EW. The renin-aldosterone response to stimulation and suppression during normal pregnancy. Hypertens Pregnancy 2005;24(1):1–16. PubMed PMID: 16036386. PMCID: PMC4458140. Epub 2005/07/23. | |
Nolten WE, Lindheimer MD, Oparil S, et al. Desoxycorticosterone in normal pregnancy. I. Sequential studies of the secretory patterns of desoxycorticosterone, aldosterone, and cortisol. Am J Obstet Gynecol 1978;132(4):414–20. PubMed PMID: 212954. Epub 1978/10/15. | |
McAuliffe F, Kametas N, Costello J, et al. Respiratory function in singleton and twin pregnancy. BJOG 2002;109(7):765–9. PubMed PMID: 12135212. Epub 2002/07/24. | |
Schrier RW. Body fluid volume regulation in health and disease: a unifying hypothesis. Ann Intern Med 1990;113(2):155–9. PubMed PMID: 2193561. Epub 1990/07/15. | |
Lindheimer MD, Barron WM, Davison JM. Osmoregulation of thirst and vasopressin release in pregnancy. Am J Physiol 1989;257(2 Pt 2):F159–69. PubMed PMID: 2669525. Epub 1989/08/01. | |
Prakash J, Pant P, Prakash S, et al. Changing picture of acute kidney injury in pregnancy: Study of 259 cases over a period of 33 years. Indian J Nephrol 2016;26(4):262–7. PubMed PMID: 27512298. PMCID: PMC4964686. Epub 2016/08/12. eng. | |
Liu Y, Bao HD, Jiang ZZ, et al. Pregnancy-related acute kidney injury and a review of the literature in China. Intern Med 2015;54(14):1695–703. PubMed PMID: 26179522. Epub 2015/07/17. eng. | |
Stratta P, Besso L, Canavese C, et al. Is pregnancy-related acute renal failure a disappearing clinical entity? Ren Fail 1996;18(4):575–84. PubMed PMID: 8875682. eng. | |
Mehrabadi A, Liu S, Bartholomew S, et al. Hypertensive disorders of pregnancy and the recent increase in obstetric acute renal failure in Canada: population based retrospective cohort study. BMJ: British Medical Journal 2014;349:g4731. | |
Mehrabadi A, Dahhou M, Joseph KS, et al. Investigation of a rise in obstetric acute renal failure in the United States, 1999–2011. Obstet Gynecol. 2016;127(5):899–906. PubMed PMID: 27054929. eng. | |
Mehrabadi A, Liu S, Bartholomew S, et al. Hypertensive disorders of pregnancy and the recent increase in obstetric acute renal failure in Canada: population based retrospective cohort study. BMJ 2014;349:g4731. PubMed PMID: 25077825. PMCID: PMC4115671. Epub 2014/08/01. eng. | |
Lunn MR, Obedin-Maliver J, Hsu CY. Increasing incidence of acute kidney injury: also a problem in pregnancy? Am J Kidney Dis 2015;65(5):650–4. PubMed PMID: 25577135. Epub 2015/01/13. eng. | |
Liu Y, Ma X, Zheng J, et al. Pregnancy outcomes in patients with acute kidney injury during pregnancy: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2017;17(1):235. PubMed PMID: 28720086. PMCID: PMC5516395. Epub 2017/07/20. eng. | |
Szczepanski J, Griffin A, Novotny S, et al. Acute kidney injury in pregnancies complicated with preeclampsia or HELLP syndrome. Front Med (Lausanne) 2020;7:22. PubMed PMID: 32118007. PMCID: PMC7020199. Epub 2020/03/03. | |
Mehrabadi A, Liu S, Bartholomew S, et al. Hypertensive disorders of pregnancy and the recent increase in obstetric acute renal failure in Canada: population based retrospective cohort study. BMJ 2014;349:g4731. PubMed PMID: 25077825. PMCID: PMC4115671. Epub 2014/08/01. | |
Huang C, Chen S. Acute kidney injury during pregnancy and puerperium: a retrospective study in a single center. BMC Nephrol 2017;18(1):146. PubMed PMID: 28460634. PMCID: PMC5412057. Epub 2017/05/04. eng. | |
Fakhouri F, Scully M, Provot F, et al. Management of thrombotic microangiopathy in pregnancy and postpartum: report from an international working group. Blood 2020;136(19):2103–17. PubMed PMID: 32808006. Epub 2020/08/19. | |
Hamadah AM, Gharaibeh K, Mara KC, et al. Urinalysis for the diagnosis of glomerulonephritis: role of dysmorphic red blood cells. Nephrol Dial Transplant 2018;33(8):1397–403. PubMed PMID: 29156008. Epub 2017/11/21. | |
Webb JA. Ultrasonography and Doppler studies in the diagnosis of renal obstruction. BJU Int 2000;86(Suppl 1):25–32. PubMed PMID: 10961272. Epub 2000/08/29. | |
Ahmed S, Bughio S, Hassan M, et al. Role of ultrasound in the diagnosis of chronic kidney disease and its correlation with serum creatinine level. Cureus 2019;11(3):e4241. PubMed PMID: 31131164. PMCID: PMC6516621. Epub 2019/05/28. | |
Bayer G, von Tokarski F, Thoreau B, et al. Etiology and outcomes of thrombotic microangiopathies. Clin J Am Soc Nephrol 2019;14(4):557–66. PubMed PMID: 30862697. PMCID: PMC6450353. Epub 2019/03/14. | |
Moatti-Cohen M, Garrec C, Wolf M, et al. Unexpected frequency of Upshaw-Schulman syndrome in pregnancy-onset thrombotic thrombocytopenic purpura. Blood 2012;119(24):5888–97. PubMed PMID: 22547583. Epub 2012/05/02. | |
Fakhouri F, Roumenina L, Provot F, et al. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. J Am Soc Nephrol 2010;21(5):859–67. PubMed PMID: 20203157. PMCID: PMC2865741. Epub 2010/03/06. | |
Scully M, Yarranton H, Liesner R, et al. Regional UK TTP registry: correlation with laboratory ADAMTS 13 analysis and clinical features. Br J Haematol 2008;142(5):819–26. PubMed PMID: 18637802. Epub 2008/07/22. | |
Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 2013;368(23):2169–81. PubMed PMID: 23738544. Epub 2013/06/07. | |
Silver RM. Catastrophic antiphospholipid syndrome and pregnancy. Seminars in Perinatology 2018;42(1):26–32. PubMed PMID: 29179957. Epub 2017/11/29. | |
Frimat M, Decambron M, Lebas C, et al. Renal cortical necrosis in postpartum hemorrhage: A case series. Am J Kidney Dis 2016;68(1):50–7. PubMed PMID: 26786299. Epub 2016/01/21. eng. | |
Huerta A, Arjona E, Portoles J, et al. A retrospective study of pregnancy-associated atypical hemolytic uremic syndrome. Kidney Int 2018;93(2):450–9. PubMed PMID: 28911789. Epub 2017/09/12. eng. | |
Pourrat O, Coudroy R, Pierre F. Differentiation between severe HELLP syndrome and thrombotic microangiopathy, thrombotic thrombocytopenic purpura and other imitators. Eur J Obstet Gynecol Reprod Biol 2015;189:68–72. PubMed PMID: 25879992. Epub 2015/03/25. eng. | |
Zhao C, Zhao J, Huang Y, et al. New-onset systemic lupus erythematosus during pregnancy. Clin Rheumatol 2013;32(6):815–22. PubMed PMID: 23358829. Epub 2013/01/30. | |
Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111(5):649–58. PubMed PMID: 12618519. PMCID: PMC151901. Epub 2003/03/06. | |
Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350(7):672–83. PubMed PMID: 14764923. Epub 2004/02/07. | |
Leanos-Miranda A, Campos-Galicia I, Isordia-Salas I, et al. Changes in circulating concentrations of soluble fms-like tyrosine kinase-1 and placental growth factor measured by automated electrochemiluminescence immunoassays methods are predictors of preeclampsia. J Hypertens 2012;30(11):2173–81. PubMed PMID: 22902831. Epub 2012/08/21. | |
Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med 2016;374(1):13–22. PubMed PMID: 26735990. Epub 2016/01/07. | |
Hirashima C, Ogoyama M, Abe M, et al. Clinical usefulness of serum levels of soluble fms-like tyrosine kinase 1/placental growth factor ratio to rule out preeclampsia in women with new-onset lupus nephritis during pregnancy. CEN case reports 2019;8(2):95–100. PubMed PMID: 30565047. PMCID: PMC6450983. Epub 2018/12/20. | |
de Jesus GR, de Jesus NR, Levy RA, et al. The use of angiogenic and antiangiogenic factors in the differential diagnosis of pre-eclampsia, antiphospholipid syndrome nephropathy and lupus nephritis. Lupus 2014;23(12):1299–301. PubMed PMID: 25228732. Epub 2014/09/18. | |
McGee S, Abernethy WB, 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA 1999;281(11):1022–9. PubMed PMID: 10086438. Epub 1999/03/23. | |
De Backer D, Heenen S, Piagnerelli M, et al. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med 2005;31(4):517–23. PubMed PMID: 15754196. Epub 2005/03/09. eng. | |
Marik PE, Cavallazzi R, Vasu T, et al. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med 2009;37(9):2642–7. PubMed PMID: 19602972. Epub 2009/07/16. eng. | |
Menon LP, Balakrishnan JM, Wilson W, et al. Caval aortic index: A novel tool for fluid assessment in obstetric emergencies. J Emerg Trauma Shock 2020;13(1):50–3. PubMed PMID: 32395050. PMCID: PMC7204966. Epub 2020/05/13. | |
Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical care (London, England). 2004;8(4):R204–12. PubMed PMID: 15312219. PMCID: PMC522841. Epub 2004/08/18. | |
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120(4):c179–84. PubMed PMID: 22890468. Epub 2012/08/15. | |
Martin GS, Bassett P. Crystalloids vs. colloids for fluid resuscitation in the Intensive Care Unit: A systematic review and meta-analysis. J Crit Care 2019;50:144–54. PubMed PMID: 30540968. Epub 2018/12/13. | |
Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA 2013;309(7):678–88. PubMed PMID: 23423413. Epub 2013/02/21. | |
Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med 2018;378(9):829–39. PubMed PMID: 29485925. PMCID: PMC5846085. Epub 2018/02/28. | |
Brown MA, Zammit VC, Lowe SA. Capillary permeability and extracellular fluid volumes in pregnancy-induced hypertension. Clin Sci (Lond) 1989;77(6):599–604. PubMed PMID: 2691173. Epub 1989/12/01. | |
Depret F, Peacock WF, Liu KD, et al. Management of hyperkalemia in the acutely ill patient. Ann Intensive Care 2019;9(1):32. PubMed PMID: 30820692. PMCID: PMC6395464. Epub 2019/03/02. | |
Mattu A, Brady WJ, Robinson DA. Electrocardiographic manifestations of hyperkalemia. Am J Emerg Med 2000;18(6):721–9. PubMed PMID: 11043630. Epub 2000/10/24. | |
Robert T, Joseph A, Mesnard L. Calcium salt during hyperkalemia. Kidney Int 2016;90(2):451–2. PubMed PMID: 27418095. Epub 2016/07/16. | |
Ho K. A critically swift response: insulin-stimulated potassium and glucose transport in skeletal muscle. Clin J Am Soc Nephrol 2011;6(7):1513–16. PubMed PMID: 21700825. Epub 2011/06/28. | |
Pierce DA, Russell G, Pirkle JL, et al. Incidence of hypoglycemia in patients with low eGFR treated with insulin and dextrose for hyperkalemia. Ann Pharmacother 2015;49(12):1322–6. PubMed PMID: 26416951. Epub 2015/09/30. | |
Coca A, Valencia AL, Bustamante J, et al. Hypoglycemia following intravenous insulin plus glucose for hyperkalemia in patients with impaired renal function. PLoS ONE 2017;12(2):e0172961. PubMed PMID: 28245289. PMCID: PMC5330504. Epub 2017/03/01. | |
Mandelberg A, Krupnik Z, Houri S, et al. Salbutamol metered-dose inhaler with spacer for hyperkalemia: how fast? How safe? Chest 1999;115(3):617–22. PubMed PMID: 10084465. Epub 1999/03/20. | |
Evans EE, Haines RF. The agglutination of ion exchange resin particles coated with polysaccharide. J Bacteriol 1954;68(1):130–1. PubMed PMID: 13183915. PMCID: PMC357348. Epub 1954/07/01. | |
Harel Z, Harel S, Shah PS, et al. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med 2013;126(3):264 e9–24. PubMed PMID: 23321430. Epub 2013/01/17. | |
Paton DM. Patiromer: a significant advance in the management of hyperkalemia. Drugs Today (Barc) 2015;51(12):695–703. PubMed PMID: 26798850. Epub 2016/01/23. | |
Reyes AJ. Loop diuretics versus others in the treatment of congestive heart failure after myocardial infarction. Cardiovasc Drugs Ther 1993;7(6):869–76. PubMed PMID: 8011561. Epub 1993/12/01. | |
Jaber S, Jung B. Time to treat metabolic acidosis in ICU with sodium bicarbonate? Maybe. Anaesth Crit Care Pain Med 2018;37(6):499–500. PubMed PMID: 30573204. Epub 2018/12/24. | |
Jaber S, Paugam C, Futier E, et al. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet 2018;392(10141):31–40. PubMed PMID: 29910040. Epub 2018/06/19. | |
Gonzalez Suarez ML, Kattah A, Grande JP, et al. Renal Disorders in Pregnancy: Core Curriculum 2019. Am J Kidney Dis 2019;73(1):119–30. PubMed PMID: 30122546. PMCID: PMC6309641. Epub 2018/08/21. | |
Hladunewich MA, Hou S, Odutayo A, et al. Intensive hemodialysis associates with improved pregnancy outcomes: a Canadian and United States cohort comparison. J Am Soc Nephrol 2014;25(5):1103–9. PubMed PMID: 24525032. PMCID: PMC4005313. Epub 2014/02/13. eng. | |
Hall DR, Conti-Ramsden F. Acute kidney injury in pregnancy including renal disease diagnosed in pregnancy. Best Pract Res Clin Obstet Gynaecol 2019;57:47–59. PubMed PMID: 30661950. Epub 2019/01/22. | |
Vinturache A, Popoola J, Watt-Coote I. The changing landscape of acute kidney injury in pregnancy from an obstetrics perspective. J Clin Med 2019;8(9). PubMed PMID: 31500091. PMCID: PMC6780924. Epub 2019/09/11. | |
Unverdi S, Ceri M, Unverdi H, et al. Postpartum persistent proteinuria after preeclampsia: a single-center experience. Wien Klin Wochenschr 2013;125(3–4):91–5. PubMed PMID: 23334478. Epub 2013/01/22. | |
Odutayo A, Wong CX, Farkouh M, et al. AKI and Long-term risk for cardiovascular events and mortality. J Am Soc Nephrol 2017;28(1):377–87. PubMed PMID: 27297949. Epub 2016/06/13. eng. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)

