This chapter should be cited as follows:
Cheshire J, Dunlop C, et al., Glob Libr Women's Med
ISSN: 1756-2228; DOI 10.3843/GLOWM.413113
The Continuous Textbook of Women’s Medicine Series – Obstetrics Module
Volume 15
The puerperium
Volume Editors:
Dr Kate Lightly, University of Liverpool, UK
Professor Andrew Weeks, University of Liverpool, UK

Chapter
The Management of Maternal Sepsis
First published: February 2021
Study Assessment Option
By answering four multiple-choice questions (randomly selected) after studying this chapter, readers can qualify for Continuing Professional Development points plus a Study Completion Certificate from GLOWM.
See end of chapter for details.
INTRODUCTION
Burden of maternal sepsis
Annually, across the globe, approximately 210 million women will become pregnant.1 Tragically, 303,000 of these women will die from causes related to their pregnancy or postpartum period.2 The vast majority of these deaths occur in low-income settings, and it is estimated that 98% could be prevented.1
Best current estimates suggest that 10.7% of maternal deaths are due to maternal sepsis, making it the third most common cause of maternal death worldwide, after postpartum hemorrhage and pre-eclampsia.3 Maternal sepsis is a highly lethal condition, as without prompt recognition and appropriate treatment, it can quickly cause death.4 The rapidly progressive nature of this condition makes it challenging to treat, especially in low-resource settings.
Even if treated, long-term morbidity from the condition is common.5,6 Antepartum and intrapartum maternal sepsis can also lead to neonatal sepsis, or other adverse neonatal outcomes such as preterm birth, admission to NICU and an increased perinatal mortality rate.7,8,9
The World Health Organization (WHO) advises that maternal infection, and subsequent sepsis, can be eliminated with good hygiene and infection control practices, as well as prompt recognition and treatment of these conditions.10 This chapter defines sepsis then describes current best management, aiming to guide the appropriate prevention, recognition and treatment of cases of maternal sepsis, and therefore reduce mortality and morbidity from this condition.
DEFINITION OF MATERNAL SEPSIS
Sepsis develops when the body’s defence reactions against infections, cause harm to its own organs.4 It is a condition that can affect all populations, including the general adult population and children.
In 2017 the definition of maternal sepsis was updated by WHO. WHO now defines maternal sepsis as “organ dysfunction resulting from infection during pregnancy, childbirth, post-abortion, or postpartum period”,4 which includes 42 days after the pregnancy has terminated, irrespective of the cause. This updated definition does not require the source of infection to be directly related to the pregnant state. Instead, any infection in the pregnant or postpartum state from which sepsis develops would be considered ‘maternal sepsis’.
This new definition reflects the change in the international discussion surrounding sepsis in the adult population. The previous understanding of sepsis, as infection plus systemic inflammatory response (SIRS), has now been updated. The most recent consensus definition on sepsis, from 2016, recommends the diagnosis of sepsis to be based on organ dysfunction secondary to infection.11
The definition of organ dysfunction is an increase in the sequential organ failure assessment (SOFA) score of more than two points.11 In practice, however, the SOFA score (Table 1) relies on the results of laboratory investigations, which makes a retrospective score and, therefore, is too slow to guide the appropriate timeliness of clinical actions required in cases of sepsis. Owing to its need for laboratory investigations, its use is also not be possible in low-resource settings.
1
The sequential organ failure assessment (SOFA) score.11
1 | 2 | 3 | 4 | |
Respiration PaO2/FIO2 (mmHg) SaO2/FIO2 | <400 221–301 | <300 142–220 | <220 67–141 | <100 <67 |
Coagulation Platelets × 103/mm3 | <150 | <100 | <50 | <20 |
Liver Bilirubin (mg/dL) | 1.2–1.9 | 2.0–5.9 | 6.0–11.9 | >12.0 |
Cardiovascular Hypotension* | MAP <70 mmHg | Dopamine ≤5 or dobutamine (any) | Dopamine >5 or norepinephrine ≤0.1 | Dopamine >15 or norepinephrine >0.1 |
Neurological Glasgow coma scale | 13–14 | 10–12 | 6–9 | <6 |
Renal Creatinine (mg/dL) Urine output (mL/dL) | 1.2–1.9 – | 2.0–3.4 – | 3.5–4.9 <500 | >5.0 <200 |
MAP, mean arterial pressure; PaO2, arterial partial pressure of oxygen; FIO2, fractional inspired oxygen; SaO2, peripheral arterial oxygen saturation.
*Vasoactive medications administered for at least 1 hour (dopamine and norepinephrine µmg/kg/min)
It is important that the definition of maternal sepsis reflects the update of international understanding on sepsis for two reasons: first, to allow for cases of maternal sepsis to be compared accurately with other groups in the adult population; and, secondly, because the normal physiological values that are measured using the SIRS criteria vary in pregnancy when compared to the general adult population.12 This latter factor can make sepsis difficult to diagnose in the pregnant and postpartum population using the prior definition.13
RISK FACTORS FOR MATERNAL SEPSIS
Whether the pregnant state itself causes an increased susceptibility towards infection is debatable, but there is evidence that the severity of infection experienced is increased14 putting expectant and postpartum women at increased risk of sepsis. Pregnant women are more susceptible to a few specific infections, namely malaria, listeriosis and HIV.14 The increase in severity and susceptibility is multifactorial. A change in the maternal immune system, physical changes (i.e. increased urinary stasis), increased risk of developing immunocompromising conditions (such as gestational diabetes), and pregnancy-specific invasive procedures and pregnancy-specific infections, i.e. chorioamnionitis, are all contributing factors.
A number of risk factors have been identified which further increase an individual’s risk of developing an infection during pregnancy and childbirth. These can be divided into social factors, medical factors and factors related to childbirth (Figure 1).5,6,15,16,17,18,19,20,21,22
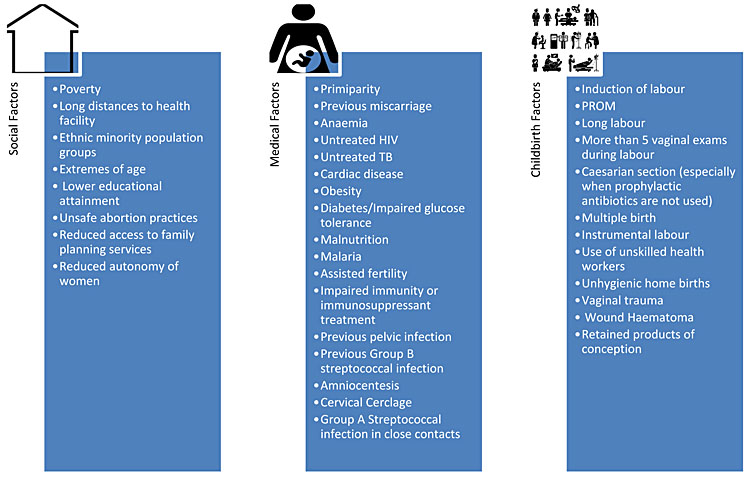
1
Factors that increase infection risk in pregnancy.
Factors in the healthcare system can also increase risk of infection. Staff workload burden may be too great to allow appropriate care for all patients, or there may be a lack of staff available to cover all centers in the health system.23 Additionally, staff may lack awareness of the signs and symptoms of maternal sepsis, and how best to manage this. The health system infrastructure may be lacking, i.e. a weak medical supply chain may mean no antibiotics are available for treatment, and there may not be access to referral centers or ICUs.24 If there are inadequate sanitation practices, then attending a health facility may actually increase a women’s risk of developing an infection.25
PREVENTION OF MATERNAL INFECTION AND SEPSIS
If maternal infections can be prevented, then maternal sepsis and deaths can be averted. It is therefore essential to maximize opportunities to prevent infections, particularly in low- and middle-income country (LMIC) settings where treatment of infection and sepsis is more challenging. Preventing transmission of infections also reduces the likelihood of exposure to antibiotic resistant organisms and contributing this this global health crisis.26
Key components on the prevention of maternal sepsis
Key documents:
- “Water, sanitation and hygiene in health care facilities. Practical steps to achieve universal access to quality care.” Publication of the World Health Organization and Unicef, 2019.27https://apps.who.int/iris/bitstream/handle/10665/311618/9789241515511-eng.pdf?ua=1.
- “A Guide to the Implementation of the WHO Multimodal Hand Hygiene Improvement Strategy”. Publication of the World Health Organization, 2009.28https://www.who.int/gpsc/5may/Guide_to_Implementation.pdf.
- “WHO recommendations for prevention and treatment of maternal peripartum infections.” Publication of the World Health Organization, 2015.29https://apps.who.int/iris/bitstream/handle/10665/186171/9789241549363_eng.pdf?sequence=1.
Guidelines for infection prevention
There are key areas for infection prevention in maternity settings where guidance is available.
Water, sanitation and hygiene infrastructure
Water, sanitation and hygiene (WaSH) infrastructure is a central requirement for infection prevention in a functioning health facility, and without this the risk of hospital-acquired infections is increased. WHO has detailed guidance on how this can be reviewed, and improved where needed.26,27 Factors which must be reviewed include access to running water, sanitation services, such as toileting facilities, hand hygiene resources and healthcare waste disposal. These are not guaranteed, particularly in low-resource settings.25,27,30 Recent work by the Soapbox collaborative, an NGO working to prevent infections at the time of delivery in low-resource settings, has focused on the role of cleaners on maternity units and the importance of adequate training to ensure clean delivery areas.31 If any of these conditions are not available, then reviewing and facilitating improvements, using tools such as WASHFIT,26 can lead to improved quality of care to protect the health of women in maternity settings.
Hand hygiene
There are comprehensive guidelines and tools produced by WHO, on the central importance of good hand hygiene to prevent transmission of infection (Figure 2).32 This includes a guide to implementing the strategy in different settings.33 The five key moments for hand hygiene are before touching patient, before aseptic procedures, after contact with body fluids, after touching a patient and after touching the patient’s surroundings. Hands should be cleaned with soap and water if visibly soiled, or after toileting. Otherwise, it is appropriate to use alcohol-based handrub.34
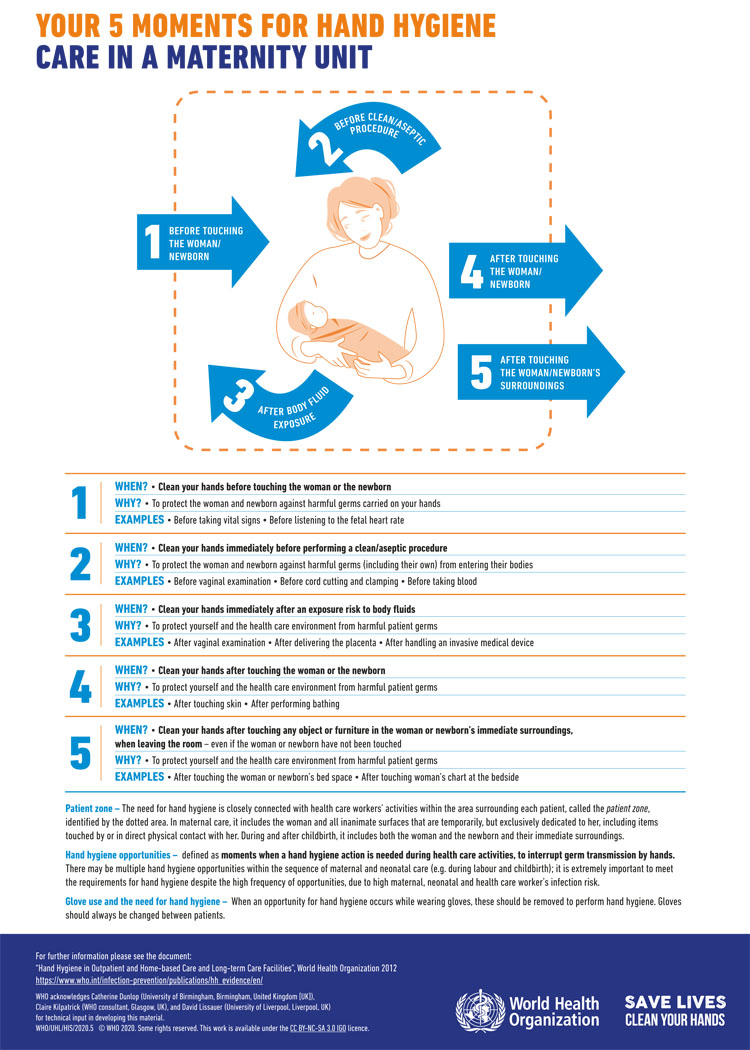
2
WHO ‘Your 5 Moments for Hand Hygiene’ poster.35 Available under Creative Commons Licence https://creativecommons.org/licenses/by-nc-sa/3.0/igo/.
Antibiotic prophylaxis
WHO has issued evidence-based guidance on the necessary actions in pregnancy and childbirth to reduce the risk to patients of developing maternal infections,29 which includes indications for antibiotic prophylaxis and treatment guidance for some infections. In addition, two recent studies have provided insight into the benefits of antibiotic prophylaxis around the time of pregnancy and childbirth.36,37 These recommendations have been compiled below in Table 2.
Is antibiotic prophylaxis recommended? | Yes | No | |
First trimester | Abortion or miscarriage surgery (MVA/EVAC/D&C) | X | |
Second or third trimester | Preterm prelabor rupture of membranes (PPROM) | X | |
Normal second or third trimester | X | ||
Preterm labor with intact amniotic membranes | X | ||
Prelabor rupture of membranes (PROM) at or near term | X | ||
First and second stage of labor | Vaginal group B streptococcus (GBS) colonization | X | |
Meconium-stained amniotic fluid | X | ||
Normal vaginal birth | X | ||
Operative vaginal birth (forceps or vacuum-assisted delivery)* | X | ||
Third stage of labor | Manual removal of the placenta | X | |
3rd or 4th degree perineal tears (torn anal sphincter, anus or rectum) | X | ||
Episiotomy | X | ||
Cesarean section | Elective or emergency cesarean section (antibiotics should be given BEFORE skin incision) | X | |
*Note: Recent trial evidence from the ANODE study has demonstrated benefit from prophylactic antibiotics during operative vaginal birth.36 WHO guidelines have not yet been updated to incorporate this new evidence. The table above reflects the current WHO guidance for operative vaginal birth.
Sensitivity testing and treatment of infections
It is important to remember that pregnant women are still susceptible to infections that are not directly related to being pregnant, such as pneumonia or meningitis. For all infections, it is recommended that healthcare workers follow national guidance for antibiotic treatment. Correct antibiotic usage will depend on the organisms considered to be the most likely causative agents, and public health drives to reduce the development of antibiotic resistance globally. For these reasons it is essential that antibiotics are used appropriately, following WHO or national guidance.
Antenatal care
Finally, antenatal care should involve a combination of screening activities and preventative measures for infections that are endemic to the region. There should also be capacity for treatment of these infections if they are detected. Oral iron and folic acid supplementation is recommended for the prevention of maternal sepsis. The importance of malaria prevention and control for the pregnant population should not be forgotten in endemic areas. WHO recommends intermittent preventative treatment with sulfadoxine-pyrimethamine to be delivered during antenatal care visits.38
As well as endemic diseases, screening for common diseases in pregnancy is vital, and recommended to be conducted during routine antenatal care visits. Treatment can reduce susceptibility to infections later on in the pregnancy, and therefore save lives. This should include culture screening for asymptomatic bacteriuria for pregnant women. It is also recommended to routinely test for HIV and syphilis, and other key tests dependent on the setting. If women test positive during their antenatal care visits, then treatment should be initiated as soon as possible.38
RECOGNITION OF MATERNAL SEPSIS
Maternal sepsis is a medical emergency. Disease progression may be more rapid than in the non-pregnant state. Early recognition of the signs and symptoms is therefore crucial to enable prompt treatment to improve both maternal and fetal outcomes. Recognition of maternal sepsis can be difficult during pregnancy; however, as the typical features of maternal sepsis may not be present or may be masked by natural physiological changes that occur during pregnancy.12 For this reason, a high index of suspicion is required, with the need to pay particular attention to any concerns raised by the patients themselves or by their family members.
Maternal sepsis should be considered if there is a suspected or confirmed infection plus any sign(s) of organ dysfunction. The presence of any features of maternal infection should therefore prompt healthcare providers to consider whether there are signs of organ dysfunction and whether this could be maternal sepsis (Figure 3).
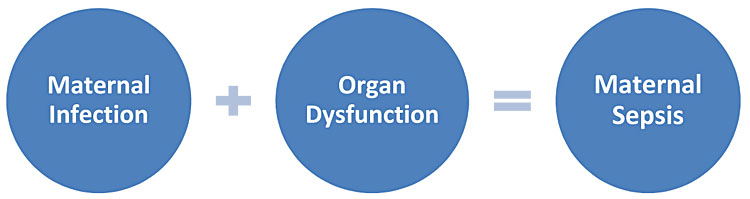
3
Components of maternal sepsis.
The role of biomarkers to identify patients with sepsis is a promising area of development within the field; however, currently no one biomarker has been identified as the gold standard.39 Biomarkers for sepsis could be used as a diagnostic tool, for staging of disease severity, for monitoring clinical response to sepsis treatment and for prognostication. Commonly used markers such as white cell count and C-reactive protein are neither specific nor sensitive.
Whilst the use of procalcitonin in guiding antibiotic therapy in patients with sepsis shows promise, NICE guidelines40 state there is currently insufficient evidence to support their routine use in guiding sepsis management, whilst the latest Surviving Sepsis Campaign (SSC) Guidelines41 found their was only low quality evidence to support its use. Further caution should be taken when considering its use within the pregnant population given the absence of an accepted normal range during pregnancy and the postoperative period.42
Serum lactate has also been suggested as a useful biomarker to guide sepsis management, with elevated lactate levels associated with poor outcome in maternal sepsis. The measurement of serum lactate is recommended as part of the latest 1-hour SSC treatment bundle,43 with a repeat measurement required if initial lactate is greater than 2 mmol/L. Furthermore, the measurement of lactate levels has been incorporated into the latest Intentional Consensus Definition of septic shock,11 which can be defined as “sepsis with persistent hypotension requiring vasopressor therapy to elevate mean arterial pressure (MAP) to greater than or equal to 65 mmHg and a lactate of greater than 2 mmol/L despite adequate fluid resuscitation”.
Maternal infections
A detailed history and thorough clinical examination will help identify common features of maternal infections. Maternal infections are any infections that develop during pregnancy, childbirth, post-abortion or postpartum period. Below are some of the features of common maternal infections (Table 3).19,20,44
3
Features of common maternal infections.
Infection | Features |
Breast abscess/mastitis | Breast pain and tenderness, erythema, painful induration, nipple discharge |
Chorioamnionitis | Abdominal pain, vaginal bleeding, offensive vaginal discharge or lochia, uterine tenderness, delayed uterine involution, fetal tachycardia >160 beats per minute, fever |
Endometritis/septic abortion | Abdominal pain, vaginal bleeding, offensive cervical discharge, uterine tenderness, delayed uterine involution, fever |
Infected wound (perineal or abdominal) | Discharge from wound, pain, erythema and swelling around wound |
Malaria | Fever, headache, muscle/joint pain, jaundice, anemia |
Meningitis | Headache, rash, photophobia, neck stiffness, confusion, fever, nausea and vomiting |
Respiratory tract infection | Productive cough, sore throat, shortness of breath/difficulty breathing, chest pain and fever |
Toxic shock syndrome (streptococcal and staphylococcal) | Nausea, vomiting, diarrhea, watery vaginal discharge, generalized maculopapular rash, conjunctival suffusion |
Urinary tract infection | Dysuria, increased frequency, urgency, abdominal/flank/back pain, rigors, fever, nausea and vomiting |
Non-specific features of infection | Lethargy, reduced appetite, fever, nausea, vomiting |
Features of suspected maternal sepsis
Optimal clinical management requires early intervention prior to the point of organ dysfunction. Abnormal maternal vital signs can herald impending organ dysfunction and the need for urgent intervention. Signs of organ dysfunction can be identified by measuring the patient’s vital signs (respiratory rate, heart rate, blood pressure, urine output, mental state). In some healthcare settings, measurement of the patient’s oxygen saturation and lactate may also be possible and provide further evidence of organ dysfunction. Clinical signs suggestive of maternal organ dysfunction are:45
- Respiratory rate greater than or equal to 25 breaths/min;
- New need for oxygen to keep saturations above 92%;
- Heart rate greater than or equal to 130 beats/min;
- Systolic blood pressure less than 90 mmHg;
- Not passed urine in over 18 hours (or less than 0.5 ml/kg/h if catheterized);
- Change in patient’s mental state or a new onset confusion;
- Serum lactate of greater than or equal to 2 mmol/L;
- Non blanching rash, mottled/ashen/cyanotic.
The presence of any of the above features along with a clinical suspicion of an infection should alert the healthcare provider to the possibility that the patient has maternal sepsis and requires urgent treatment.
It is important to note that the presence of a pyrexia is no longer considered to be evidence of having maternal sepsis, as a temperature on its own is not a sign of organ dysfunction, merely a sign that the patient may have an underlying infection.46 The presence of hypothermia has a stronger association with adverse outcomes.47
Early warning scores
Maternity departments should have a standard method in place for recording patient’s vital signs during their inpatient stay. If available, patient’s vital signs should be recorded on a modified early obstetric warning score (MEOWS) or similar chart.46 Figure 4 illustrates an example of a paper based MEOWS chart. These charts track patient’s vital signs over time and trigger a clinical action when the vital signs become deranged and have been shown to help identify patients at risk of deterioration. Their use in obstetrics has been associated with a reduction in mortality.48 These charts will only work well, however, when a complete set of observations are taken on a regular basis. Missing or irregular observations reduce the ability to recognize deteriorating patients.49
Early warning scores have been demonstrated to outperform the SOFA and qSOFA scores as tools to identify high risk patients outside of the intensive care setting; however, the low sensitivities of each of these methods may limit their use as a predictive tool for adverse outcomes.50,51,52,53,54
To be effective early warning scores should be accompanied by a clear escalation policy to guide healthcare providers on the need for further action to optimize patient care. A decision making tool can help guide healthcare providers on when to act in patients with maternal sepsis.45,55 A decision tool suitable for low resource settings has been developed and successfully trialed56 and been shown to help improve the recognition and management of maternal sepsis.
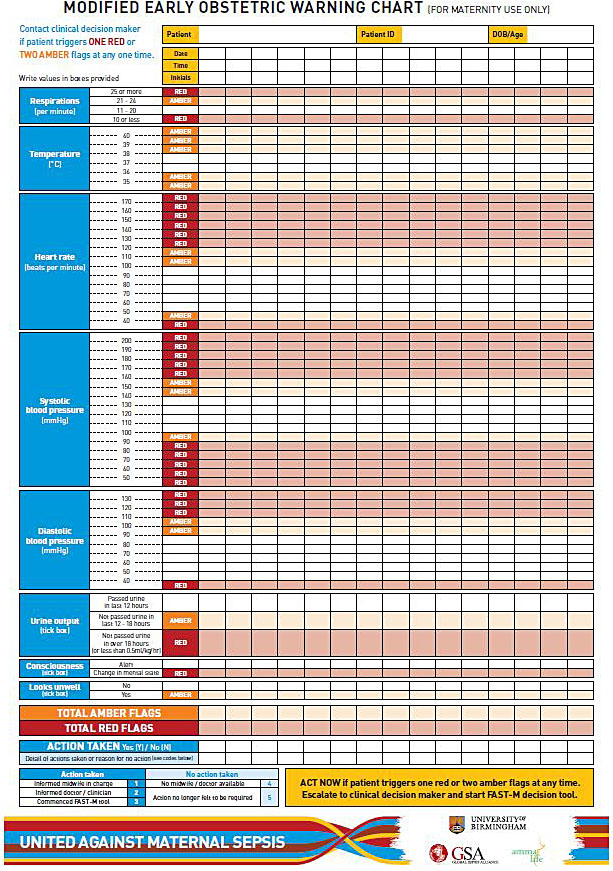
4
Example of a paper based MEOWS chart.
Laboratory investigations
Whilst not routinely available in all healthcare facilities, laboratory Investigations can be useful in determining whether there is any biochemical evidence of organ dysfunction. Evidence of an acute kidney injury (raised serum urea, creatinine and reduced eGFR), elevated serum lactate, hematologic derangements (low platelets) or derangement of synthetic liver function (prolonged clotting, raised bilirubin) in the presence of a suspected infection should alert the healthcare provider to the possibility of maternal sepsis. Other laboratory investigations such as full blood count, serum electrolytes, serum C reactive protein and serum glucose may also be measured, if available, to aid diagnosis and management.46 Serial lactate measurements may be used to monitor treatment progression with early lactate clearance indicative of improved survival.57
MANAGEMENT OF MATERNAL SEPSIS
Suspicion of maternal sepsis should prompt an immediate review by a senior clinician.46 Treatment should not be delayed whilst awaiting this review. The aim of sepsis management is to maintain perfusion of vital organs whilst identifying and treating the source of the infection. For patients with sepsis, prompt treatment is crucial is associated with reduced mortality.58
Use of a sepsis care bundle
Care bundles are a set of evidence-based components which when performed together and reliably, improve patient outcomes. Completion of each element of the bundle is necessary to achieve the desired outcome and not completing one of the elements may therefore affect the overall patient outcome. In high-income countries the use of sepsis care bundles for the immediate management of sepsis has been shown to reduce mortality and improve patient outcomes.59,60 The SSC bundles43,61 and the UK Sepsis Trusts’ Sepsis 6 bundle62 have been adopted worldwide to good effect.60,63 In low resource settings, however, use of these sepsis care bundles is often not possible due to resource limitations. Currently no maternal sepsis care bundle exists, however, work is underway to test a maternal sepsis care bundle, the “FAST-M” maternal sepsis care bundle (Figure 5)56 that is specifically aimed at treating maternal sepsis in low resource settings.
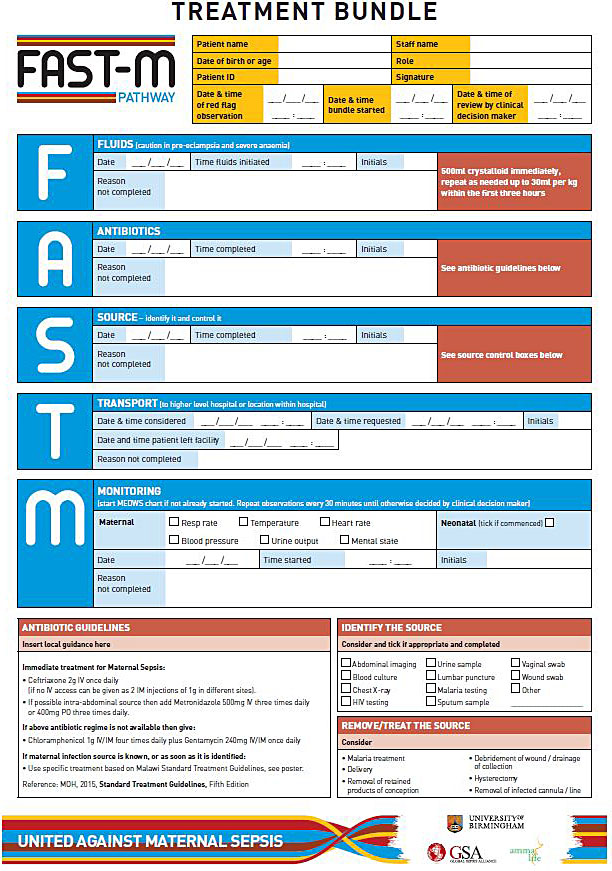
5
Example of a paper-based clinical treatment tool to guide healthcare practitioners through the recommended steps for managing a patient with maternal sepsis.
Key components in the management of maternal sepsis
Key documents:
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43 : 304–77. Doi: 10.1007/s00134–017–4683–6.
- National Institute for Health and Care Excellence (NICE). Sepsis: recognition, assessment and early management, 2016. https://www.nice.org.uk/guidance/ng51/evidence/full-guideline-2551523297.
- Society for Maternal-Fetal Medicine (SMFM), Plante LA, Pacheco LD, Louis JM, Louis JM. SMFM Consult Series #47: Sepsis during pregnancy and the puerperium. Am J Obstet Gynecol 2019;220(4):B2–10.
Fluids
Restoration of organ perfusion is a key part of maternal sepsis treatment; however, the optimal volume and timing of intravenous fluid therapy in sepsis is not currently known. The aim of fluid resuscitation should be to restore organ perfusion and achieve hemodynamic stability. Several studies have provided conflicting evidence around the benefits and risks of aggressive early goal directed fluid resuscitation.64,65,66,67,68,69
Despite being considered safe,70 the lack of a clear benefit of using albumin compared to crystalloid71,72,73,74 coupled with its increased expense makes crystalloid solutions the fluid of choice in the initial resuscitation in patients with sepsis.41,46
In light of available evidence in the non-pregnant population the SSC recommend that if hypotensive or lactate is greater than or equal to 4 mmol/L, initial fluid resuscitation should follow a fluid bolus regimen using intravenous balanced crystalloids or saline.43 Initial fluid resuscitation should consist of fluid boluses up to a total of 30 ml/kg of crystalloid over 3 hours with frequent reassessment of the patient’s hemodynamic status in between boluses.41 It is recommended that each fluid bolus consists of 500 ml crystalloid over less than 15 minutes.46 Reassessment of the patient’s hemodynamic status in between boluses should take the form of a thorough clinical examination and assessment of the patient’s vital signs to avoid a sustained positive fluid balance which has been shown to worsen mortality in sepsis.75,76 The use of passive leg raising may be used to determine the patient’s fluid responsiveness and need for further fluid boluses.77 Fluid responders will demonstrate an increase in cardiac output after 2–3 minutes of passive leg raise, whilst those who do not demonstrate an increase in cardiac output may benefit from vasopressor therapy. Passive leg raising should not be used to guide fluid therapy in the third trimester due to compression of the inferior vena cava. In these patients small boluses of 250–500 ml should be administered; if there is a corresponding increase in cardiac output, further fluid administration is likely required. If measurement of lactate is available, normalization of lactate in patients with raised lactate can be used to guide fluid resuscitation. A recent trial comparing the role of a fluid resuscitation strategy targeting normalization of capillary refill time versus one targeting normalization of serum lactate found no survival benefit in using capillary refill time to guide fluid resuscitation.78
If there is no improvement in the patient’s hemodynamic status despite adequate fluid resuscitation, the need for vasopressor support should be considered, aiming for a target mean arterial pressure (MAP) of greater than or equal to 65 mmHg.41,77 Norepinephrine is the recommended first line vasopressor for use in maternal sepsis.77 The target MAP should be individualized based on determining overall organ perfusion.
Care must be taken in children under 16 years of age where aggressive fluid therapy has been demonstrated to worsen survival.79 Fluid management in patients with maternal sepsis and pre-eclampsia is complicated due to their increased risk of developing pulmonary edema and should therefore be managed by healthcare workers with adequate experience in this clinical situation.
Antibiotics
Antibiotics are the cornerstone of sepsis management and early antibiotic administration has been shown to improve patient outcomes and reduce mortality.80,81 For every hour delay in antibiotic administration, the mortality risk from sepsis increases (OR 1.04 per hour; 95% CI 1.03–1.06; p <0.001).80 It is therefore recommended that empiric broad spectrum antibiotics are given at their maximum recommended dose within 1 hour of recognition of sepsis (Table 4).41,46 Appropriate antibiotic selection can be particularly difficult in low and middle income settings where bacterial, parasitic, viral and fungal causes must all be considered, particularly in HIV endemic regions.82 In cases of maternal sepsis secondary to malaria, antimalarial therapy should be given.
Microbiological diagnosis of the causative agent is crucial to targeting the specific organism and minimizing potential antibiotic resistance. Blood cultures should be taken prior to administration of antibiotics; however, administration of antibiotic treatment should not be delayed if cultures are not readily available. Once the pathogen is identified and sensitivities established, the antibiotic regimen should be narrowed accordingly.
4
Suggested empirical antibiotic regimens for treatment of bacterial maternal sepsis, after pregnancy (adapted from RCOG Green Top Guidelines No. 64b).20
Source of Sepsis | Organisms | Antimicrobial | In case of allergy | Notes |
Mastitis OR cesarean section wound infection OR intravenous cannula site infection | MSSA MRSA Streptococci | Flucloxacillin* + clindamycin *If MRSA use vancomycin instead of flucloxacillin | Vancomycin + clindamycin Clindamycin/teicoplanin are alternatives in MRSA | Trough level vancomycin 5–20 mg/l needed for mastitis cases |
Endometritis | Gram-negative anaerobes Streptococci | Cefotaxime + metronidazole + gentamicin (gentamicin immediately and once only) | Gentamicin + clindamycin + ciprofloxacin | |
Acute pyelonephritis | Gram-negatives Occasionally caused by staphylococci and streptococci | Cefotaxime + gentamicin (gentamicin once only) | Gentamicin + ciprofloxacin | EBSLs: gentamicin + meropenem |
Unknown origin | MRSA Streptococci Gram-negatives Anaerobes | Meropenem + clindamycin + gentamicin (gentamicin usually once only) | Clindamycin + gentamicin + metronidazole + ciprofloxacin | Gram-negative organism possibilities include ESBL producers and pseudomonas Carbapenems contraindicated in severe penicillin allergy |
Toxic shock syndrome | Staphylococci Streptococci | Flucloxacillin* + clindamycin + gentamicin (gentamicin once only) *For MRSA use vancomycin instead of flucloxacillin | Vancomycin + clindamycin + gentamicin (gentamicin immediately and once only) OR Linezolid + gentamicin (gentamicin once only) | Essential to include an antitoxin agent; i.e. clindamycin or linezolid Consider intravenous immunoglobulin |
ESBL, extended-spectrum beta-lactamase; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
All regimens are suggestions. Sensitivities and local guidelines may vary and should be consulted. Complex cases and unusual allergies require consultation with a microbiologist.
Rationalize the therapy if able once the causative organism is known
Source identification and control
Often a thorough history taking and clinical examination is sufficient to identify the source of infection. If the source is not immediately clear, further investigations should be tailored according to the patient’s history and clinical findings. Where available, blood and urine cultures should be obtained along with relevant swabs to help guide antibiotic susceptibility. In areas where malaria is prevalent consider rapid testing for malarial parasites. If no clear source of infection is identified, abdominal imaging and involvement of the surgical team should be considered.
Source control in maternal sepsis is very important and healthcare practitioners should consider the need for evacuation of retained products of conception, removal of any indwelling foreign devices such as indwelling catheters or lines, or the need for laparotomy or drainage of any collections, as antibiotics alone in these cases will be inadequate.
Decisions regarding the timing of delivery in patients with maternal sepsis are complex and can depend on many factors including the maternal condition, gestational age and fetal condition. Hemodynamic stabilization of the mother should take priority before operative intervention is considered as attempting delivery in a hemodynamically unstable mother increases both maternal and fetal mortality. Do not attempt delivery in preterm pregnancies unless an intrauterine infection is suspected.19 If delivery is required, a general anesthetic for cesarean section is considered the safer option.83
The decision to give antenatal corticosteroids for fetal lung maturity in the woman with sepsis, if preterm delivery is anticipated, should be considered with caution.19 For women with chorioamnionitis who are likely to deliver preterm, WHO conditionally recommends that antenatal steroids should not be given, and additionally should be avoided in cases of ongoing systemic infection, including tuberculosis. This conditional recommendation is made to avoid exacerbating the maternal infection, but benefits and harms will need to be weighed up in the specific context.84
Transfer to higher level care
The need to transfer the patient to a higher level of care should be considered in all patients receiving treatment for maternal sepsis. This decision should usually be made by a senior clinician as part of their review. Early multidisciplinary involvement with intensive care physicians is recommended.19,20,46,85
The decision to transfer should be considered early on given that transferring a patient to higher level care can often be difficult to arrange in low resource settings. The decision to transfer should not stop the patient from receiving initial sepsis treatment with antibiotics and fluids whilst transfer arrangements are being made. All reasonable attempts should be made to stabilize the patient prior to transfer.
The need to transfer a patient with maternal sepsis should not be a one off decision and should be reviewed daily as part of their on-going clinical care.
The following features should prompt healthcare workers to consider the need for transfer:
- A case of maternal sepsis presenting outside of an acute hospital setting, e.g. in a health center
- The patient requires higher level of care than the facility can provide (e.g. radiological investigations, the need for surgical intervention, etc.)
- Persistent maternal hypotension or a non-improving serum lactate despite adequate fluid resuscitation requiring vasopressor support
- Decreased levels of consciousness requiring airway protection or mechanical ventilation
- Multi-organ failure.
Monitoring
Patients undergoing treatment for maternal sepsis should receive on-going regular monitoring of their vital signs to evaluate their response to treatment and help guide further management.46
The failure of a patient’s vital signs to improve despite initial sepsis treatment, should prompt consideration of the need to transfer the patient to a higher level care setting where interventions such as imaging, hemodynamic support or surgical intervention may be more readily available. In patients with persistent symptoms despite adequate treatment, the possibility of an abdominal collection should be considered and the need for abdominal imaging or an exploratory laparotomy reviewed. In patient’s being treated for maternal sepsis during the intrapartum period, fetal monitoring is recommended.
Care of the neonate
Following the delivery of a patient being treated for maternal sepsis, monitoring of the neonate’s vital signs is recommended to alert healthcare providers to developing signs of neonatal sepsis. Risk factors for early-onset neonatal infection include prelabor rupture of membranes, preterm birth following spontaneous labor, prolonged rupture of membranes, confirmed or suspected chorioamnionitis and maternal group B streptococcal colonization.86 Clinical features of early neonatal sepsis can be non-specific, but include poor feeding, grunting, irritability or altered muscle tone, pallor, tachycardia and increased work of breathing.86,87 If there are any risk factors for early-onset neonatal infections or any clinical indicators for neonatal sepsis an urgent clinical assessment must be performed and the need for antibiotic treatment considered.
Antibiotic stewardship
The ongoing need for antibiotic therapy should be reviewed daily by a senior clinician.41 Clinical progress such as resolution of shock or improvement in clinical biomarkers may guide decisions to de-escalate antibiotic therapy. Typically a 7–10 day course of antibiotics in the absence of unresolved source control issues is normally sufficient.88,89 Failure to respond to antibiotic treatment as evidenced by persistent of organ dysfunction and infection beyond 48–72 hours should prompt healthcare practitioners to consider an alternate source of infection and review the current antibiotic regimen.90
PRACTICE RECOMMENDATIONS
- Water, sanitation and hygiene (WaSH) infrastructure is essential to reduce risk of hospital-acquired infections. Ongoing monitoring and leadership in these areas should be a management focus, especially in low resource settings.
- Healthcare practitioners should practice the WHO recommended five moments of hand hygiene, to reduce risk of hospital acquired infections for patients in their care.
- Healthcare practitioners should follow the WHO recommendations for prevention of maternal infections. Importantly, these include antimicrobial prophylaxis for cases of preterm pre-labor rupture of membranes, confirmed vaginal group B streptococcus colonization in labor, cesarean sections, manual removal of placenta and 3rd or 4th degree tears.
- Antenatal care is a key opportunity to screen for infections and treat conditions that may later increase the pregnant woman’s risk of maternal sepsis
- Treatment of suspected maternal infections or maternal sepsis should follow local antimicrobial guidance, to ensure therapy treats the most likely organisms, and does not contribute to the global issue of antimicrobial resistance.
- The presence of any features of maternal infection should prompt healthcare providers to consider whether there are signs of organ dysfunction and whether this could be maternal sepsis.
- Maternity departments should have a standard method in place for recording patient’s vital signs during their inpatient stay. If available, patient’s vital signs should be recorded on a modified early obstetric warning score (MEOWS) or similar chart.
- A detailed history taking and thorough clinical examination should help identify the source of maternal infection.
- The presence of any features of maternal sepsis should prompt healthcare workers to commence treatment for maternal sepsis immediately.
- An immediate senior clinical review should be arranged, however, treatment should not be delayed whilst awaiting the review.
- The aim of fluid therapy is restoration of the patient’s hemodynamic status. Fluid therapy should be in the form of a balanced crystalloid and given in 500 ml boluses over 15 minutes each with reassessment of hemodynamic status between boluses. Initial fluid therapy should not exceed 30 ml/kg over 3 hours. If the patient remains hemodynamically unstable, the use of vasopressors should be considered.
- Broad-spectrum intravenous antibiotics should be administered within 1 hour of maternal sepsis recognition.
- The need for ongoing antibiotic therapy should be clinically reviewed on a daily basis.
- If the source of infection is not immediately clear following history and examination, additional radiological investigations or surgical intervention may be required.
- The need to transfer the patient to a higher level of care should be considered early on in all patients receiving treatment for maternal sepsis. The decision to transfer a patient should not stop that patient from receiving initial sepsis treatment with antibiotics and fluids whilst transfer arrangements are being made.
- Patients undergoing treatment for maternal sepsis should receive ongoing regular monitoring of their vital signs to evaluate their response to treatment and help guide further management.
- In patients being treated for maternal sepsis during the intrapartum period, fetal monitoring is recommended.
- Following the delivery of a patient being treated for maternal sepsis, monitoring of the neonate’s vital signs is recommended to alert healthcare providers to developing signs of neonatal sepsis.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
Graham W, Woodd S, Byass P, et al. Diversity and divergence: the dynamic burden of poor maternal health. Lancet 2016;388(10056):2164–75. | |
Alkema L, Chou D, Hogan D, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet 2016;387(10017):462–74. | |
Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Heal 2014;2(6):e323–33. | |
Bonet M, Nogueira Pileggi V, Rijken M, et al. Towards a consensus definition of maternal sepsis: results of a systematic review and expert consultation. Reprod Health [Internet] 2017;14(1):67. Available from: http://reproductive-health-journal.biomedcentral.com/articles/10.1186/s12978-017-0321-6. | |
Van Dillen J, Zwart J, Schutte J, et al. Maternal sepsis; epidemiology, etiology and outcome. Curr Opin Infect Dis 2010;23:249–54. | |
Acosta CD, Harrison DA, Rowan K, et al. Maternal morbidity and mortality from severe sepsis : a national cohort study. BMJ Open Access 2016;6:e012323. doi: 10.1136/bmjopen-2016–012323. | |
Acosta CD, Bhattacharya S, Tuffnell D, et al. Maternal sepsis: A Scottish population-based case-control study. BJOG An Int J Obstet Gynaecol 2012;119(4):474–83. | |
Knowles SJ, O’Sullivan NP, Meenan AM, et al. Maternal sepsis incidence, aetiology and outcome for mother and fetus: A prospective study. BJOG An Int J Obstet Gynaecol 2015;122(5). | |
The Alliance for Maternal and Neonatal Health Improvement (AMANHI) Mortality Study Group. Articles Population-based rates, timing, and causes of maternal deaths, stillbirths, and neonatal deaths in south Asia and sub-Saharan Africa : a multi-country prospective cohort study. Lancet Glob Heal 2018;1297–308. | |
World Health Organization. Maternal Mortality; Fact sheet. | |
Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315(8):801. | |
Bauer ME, Bauer ST, Rajala B, et al. Maternal Physiologic Parameters in Relationship to Systemic Inflammatory Response Syndrome Criteria. Obstet Gynecol 2014;124(3):535–41. | |
Frankling C, Finfer S, Lissauer D, et al. The dark ages of maternal sepsis: time to be enlightened. Br J Anaesth 2018;120(4):626–8. | |
Kourtis AP, Read JS, Jamieson DJ. Pregnancy and Infection. N Engl J Med 2014;2211–8. | |
Thaddeus S, Maine D. Too far to walk: maternal mortality in context. Soc Sci Med 1994;38(8):1091–110. | |
Acosta CD, Kurinczuk JJ, Lucas DN, et al. Severe Maternal Sepsis in the UK, 2011–2012: A National Case-Control Study. PLoS Med 2014;11(7). | |
Acosta CD, Knight M, Lee HC, et al. The Continuum of Maternal Sepsis Severity: Incidence and Risk Factors in a Population-Based Cohort Study. PLoS One 2013;8(7):1–8. | |
GLOWM The Global Library of Women’s Medicine. Maternal Sepsis Prevention Recognition Treatment [Internet]. Available from: http://www.glowm.com/pdf/Maternal_Sepsis_WallChart_WEB.pdf. | |
Royal College of Obstetricians and Gynaecologists. Bacterial Sepsis in pregnancy: Green top guideline No.64a. 2012; | |
Royal College of Obstetricians and Gynaecologists. Bacterial Sepsis following pregnancy: Green top guideline No.64b. 2012; | |
Centre for Maternal and Child Enquiries (CMACE). Saving mothers’s lives: reviewing maternal deaths to make motherhood safer: 2006–08. The Eighth Report on Condiential Enquiries into Maternal Deaths in the United Kingdom. BJOG 2011;118(1):1–203. | |
Lewis G (Editor). Saving mothers’ lives: reviewing maternal deaths to make motherhood safer 2003–2005. The seventh report on confidential enquires into maternal deaths in the United Kingdom. The Confidential Enquiry into Maternal and Child Health (CEMACH). London; 2007. | |
Kyei-Nimakoh M, Carolan-Olah M, McCann TV. Access barriers to obstetric care at health facilities in sub-Saharan Africa – a systematic review. Syst Rev 2017;6(1):110. | |
World Health Organisation. The WHO health systems framework [Internet]. [cited 2017 Jun 11]. Available from: http://www.wpro.who.int/health_services/health_systems_framework/en/. | |
Benova L, Cumming O, Gordon BA, et al. Where There Is No Toilet: Water and Sanitation Environments of Domestic and Facility Births in Tanzania. PLoS One 2014;9(9):e106738. | |
World Health Organisation, UN Children’s Fund. Water and Sanitation for Health Facility Imprvement Tool (WASH FIT): A Practical guide for improving quality of care throug water, sanitation and hygiene in health care facilities. 2018. | |
World Health Organisation, Unicef. Water, sanitation and hygiene in health care facilities. Practical steps to achieve universal access to quality care. 2019. | |
World Health Organisation. Guide to Implementation. A Guide to the Implementation of the WHO Multimodal Hand Hygiene Improvement Strategy. 2009. | |
World Health Organisation, Human Reproduction Programme. WHO recommendations for prevention and treatment of maternal peripartum infections. 2015. | |
Campbell OMR, Benova L, Gon G, et al. Getting the basic rights – the role of water, sanitation and hygiene in maternal and reproductive health: A conceptual framework. Trop Med Int Heal 2015;20(3):252–67. | |
Cross S, Gon G, Morrison E, et al. An invisible workforce : the neglected role of cleaners in patient safety on maternity units. Glob Health Action [Internet]. 2019;0(0). Available from: https://doi.org/10.1080/16549716.2018.1480085. | |
World Health Organisation. Cleaner care is safer care; Tools and Resources [Internet]. 2017 [cited 2017 Sep 25]. Available from: http://www.who.int/gpsc/5may/tools/en/. | |
World Health Organization. Guide to Implementation: A Guide to the Implementation of the WHO Multimodal Hand Hygiene Improvement Strategy. Geneva, Switzerland; 2009. | |
World Health Organisation. Hand Hygiene Technical Reference Manual. 2009. | |
World Health Organisation. Your 5 Moments for Hand Hygiene [Internet]. 2009 [cited 2018 Feb 1]. Available from: http://www.who.int/gpsc/5may/Your_5_Moments_For_Hand_Hygiene_Poster.pdf?ua=1. | |
Knight M, Chiocchia V, Partlett C, et al. Prophylactic antibiotics in the prevention of infection after operative vaginal delivery (ANODE): a multicentre randomised controlled trial. Lancet 2019;393:2395–403. | |
Lissauer D, Wilson A, Hewitt C, et al. A Randomized Trial of Prophylactic Antibiotics for Miscarriage Surgery. N Engl J Med 2019;380:1012–21. | |
World Health Organisation. WHO recommendations on antenatal care for a positive pregnancy experience. 2016. | |
Cohen J, Vincent J-L, Adhikari NKJ, et al. Sepsis: a roadmap for future research. Lancet Infect Dis 2015;15(5):581–614. | |
National Institute for Health and Care Excellence (NICE). Procalcitonin testing for diagnosing and monitoring sepsis (ADVIA Centaur BRAHMS PCT assay, BRAHMS PCT Sensitive Kryptor assay, Elecsys BRAHMS PCT assay, LIAISON BRAHMS PCT assay and VIDAS BRAHMS PCT assay): NICE Diagnostic Guidance [DG18]. London; 2015. | |
Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43(3):304–77. | |
Levin G, Rottenstreich A. The role of procalcitonin in the diagnosis and management of infections in the field of obstetrics and gynecology. Acta Obstet Gynecol Scand 2018;97(9):1148–1148. | |
Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med 2018;1–4. | |
World Health Organization. Managing complications in pregnancy and childbirth: a guide for midwives and doctors – 2nd edn. Geneva; 2017. | |
The Uk Sepsis Trust. Clinical Professional Resources. 2018. | |
National Institute for Health and Care Excellence (NICE). Sepsis: recognition, assessment and early management. 2016. | |
Remick DG, Xioa H. Hypothermia and sepsis. Front Biosci 2006;11:1006–13. | |
Shields LE, Wiesner S, Klein C, et al. Use of Maternal Early Warning Trigger tool reduces maternal morbidity. Am J Obstet Gynecol 2016;214(527):e1–6. | |
Downey CL, Tahir W, Randell R, et al. Strengths and limitations of early warning scores: A systematic review and narrative synthesis. Int J Nurs Stud 2017;76:106–19. | |
Churpek MM, Snyder A, Han X, et al. Quick Sepsis-related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores for Detecting Clinical Deterioration in Infected Patients outside the Intensive Care Unit. Am J Respir Crit Care Med 2017;195(7):906–11. | |
Goulden R, Hoyle M-C, Monis J, et al. qSOFA, SIRS and NEWS for predicting inhospital mortality and ICU admission in emergency admissions treated as sepsis. Emerg Med J 2018;35(6):345–9. | |
Song J-U, Sin CK, Park HK, et al. Performance of the quick Sequential (sepsis-related) Organ Failure Assessment score as a prognostic tool in infected patients outside the intensive care unit: a systematic review and meta-analysis. Crit Care 2018;22(1):28. | |
Liu Y-C, Luo Y-Y, Zhang X, et al. Quick Sequential Organ Failure Assessment as a prognostic factor for infected patients outside the intensive care unit: a systematic review and meta-analysis. Intern Emerg Med 2019; | |
Lo RSL, Leung LY, Brabrand M, et al. qSOFA is a Poor Predictor of Short-Term Mortality in All Patients: A Systematic Review of 410,000 Patients. J Clin Med 2019;8(1):61. | |
Suleiman I, Vousden N, Shennan AH. Recognition and Management of Maternal Sepsis in Low and Middle-Income Countries: What do we know and where are the Gaps? Open access. Glob Womens Heal 2018;1(1):1–5. | |
Evaluation of the FAST-M maternal sepsis bundle: a feasibility study. | |
Nguyen HB, Rivers EP, Knoblich BP, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med 2004;32(8):1637–42. | |
Seymour CW, Gesten F, Prescott HC, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med 2017. | |
Damiani E, Donati A, Serafini G, et al. Effect of performance improvement programs on compliance with sepsis bundles and mortality: a systematic review and meta-analysis of observational studies. PLoS One 2015;10(5):e0125827. | |
Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010;38(2):367–74. | |
The Surviving Sepsis Campaign. Hour-1 Bundle. | |
Nutbeam T, Daniels R, Keep J, UK Sepsis Trust. Toolkit : Emergency Department management of Sepsis in adults and young people over 12 years- 2016. The Royal College of Emergency Medicine; National Institute for Health and Care Excellence (NICE); 2016. | |
Daniels R, Nutbeam T, McNamara G, et al. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J 2011;28(6):507–12. | |
Rivers E, Nguyen B, Havstad S, et al. Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. N Engl J Med 2001;345(19):1368–77. | |
Mouncey PR, Osborn TM, Power GS, et al. Trial of Early, Goal-Directed Resuscitation for Septic Shock. N Engl J Med 2015;372(14):1301–11. | |
ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, Delaney A, Bailey M, Bellomo R, et al. Goal-Directed Resuscitation for Patients with Early Septic Shock. N Engl J Med 2014;371(16):1496–506. | |
ProCESS Investigators, Yealy DM, Kellum JA, et al. A Randomized Trial of Protocol-Based Care for Early Septic Shock. N Engl J Med 2014;370(18):1683–93. | |
Jaehne AK, Rivers EP. Early Liberal Fluid Therapy for Sepsis Patients Is Not Harmful: Hydrophobia Is Unwarranted but Drink Responsibly. Crit Care Med 2016;44(12):2263–9. | |
Andrews B, Semler MW, Muchemwa L, et al. Effect of an Early Resuscitation Protocol on In-hospital Mortality Among Adults With Sepsis and Hypotension. JAMA 2017;318(13):1233–40. | |
SAFE Study Investigators, Finfer S, McEvoy S, et al. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med 2011;37(1):86–96. | |
Rochwerg B, Alhazzani W, Sindi A, et al. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med 2014;161(5):347–55. | |
Caironi P, Tognoni G, Masson S, et al. Albumin Replacement in Patients with Severe Sepsis or Septic Shock. N Engl J Med 2014;370(15):1412–21. | |
Jiang L, Jiang S, Zhang M, et al. Albumin versus Other Fluids for Fluid Resuscitation in Patients with Sepsis: A Meta-Analysis. PLoS One 2014;9(12):e114666. | |
Xu J-Y, Chen Q-H, Xie J-F, et al. Comparison of the effects of albumin and crystalloid on mortality in adult patients with severe sepsis and septic shock: a meta-analysis of randomized clinical trials. Crit Care 2014;18(6):702. | |
de Oliveira FSV, Freitas FGR, Ferreira EM, et al. Positive fluid balance as a prognostic factor for mortality and acute kidney injury in severe sepsis and septic shock. J Crit Care 2015;30(1):97–101. | |
Acheampong A, Vincent J-L. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care 2015;19:251. | |
Society for Maternal-Fetal Medicine (SMFM), Plante LA, Pacheco LD, et al. SMFM Consult Series #47: Sepsis during pregnancy and the puerperium. Am J Obstet Gynecol 2019;220(4):B2–10. | |
Hernández G, Ospina-Tascón GA, Damiani LP, et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs. Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomised Clinical Trial. JAMA 2019;321(7):654–64. | |
Maitland K, Kiguli S, Opoka RO, et al. Mortality after Fluid Bolus in African Children with Severe Infection. N Engl J Med [Internet] 2011[cited 2017 Sep 6];364(26):2483–95. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1101549. | |
Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis 2017;1–10. | |
Liu VX, Fielding-Singh V, Greene JD, et al. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am J Respir Crit Care Med 2017;196(7):856–63. | |
Becker JU, Theodosis C, Jacob ST, et al. Surviving sepsis in low-income and middle-income countries: new directions for care and research. Lancet Infect Dis 2009;9:577–82. | |
Elton R, Chaudhari S. Sepsis in Obstetrics. Contin Educ Anesth Crit Care Pain 2015; | |
World Health Organisation. WHO recommendations on interventions to improve preterm birth outcomes. Geneva; 2015. | |
Knight M, Kenyon S, Brocklehurst P, et al. Saving Lives, Improving Mothers’ Care – Lessons learned to inform future maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–12. Oxford; 2014. | |
National Institute for Health and Care Excellence (NICE). Neonatal infection (early onset): antibiotics for prevention and treatment (CG149). 2012. | |
Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet 2017;390:1770–80. | |
Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63(5):e61–111. | |
Pugh R, Grant C, Cooke RP, Dempsey G. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev 2015;(8):CD007577. | |
Dünser MW, Festic E, Dondorp A, et al. Recommendations for sepsis management in resource-limited settings. Intensive Care Med 2012;38(4):557–74. |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can automatically receive 2 Continuing Professional Development points plus a Study Completion Certificate from GLOWM for successfully answering four multiple-choice questions (randomly selected) based on the study of this chapter. Medical students can receive the Study Completion Certificate only.
(To find out more about the Continuing Professional Development awards programme CLICK HERE)

