The Endometrial Cycle
Authors
INTRODUCTION
Morphologically, the endometrium is one of the most dynamic target tissues in women. Its cyclic structural changes mirror changes in metabolic functions, and both are regulated by ovarian estradiol and progesterone. Because of this interplay of structure, function, and ovarian hormonal stimuli, the endometrium is considered one of the most sensitive indicators of the hypothalamic-pituitary-ovarian hormonal axis. As a result, morphologic evaluation of the endometrium is used in diagnostic evaluation of infertile patients to determine whether ovulation is occurring (Fig. 1).
Fig. 1. Schematic representation of steroid hormone-morphologic interactions during the endometrial cycle. Estradiol promotes endometrial proliferation, whereas after ovulation, progesterone converts estradiol-primed endometrium into secretory tissue. Postovulatory estradiol amplifies the progesterone effect, and after withdrawal of both estradiol and progesterone, the endometrial mucosa breaks down and regenerates within the period of menstruation.1 |
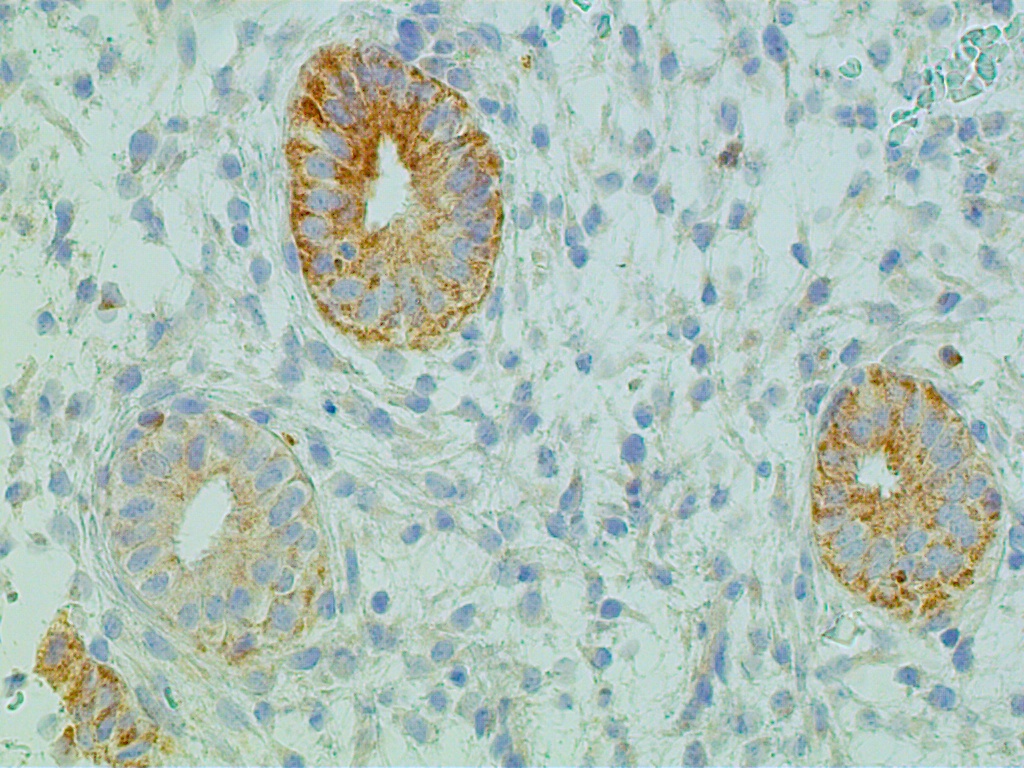
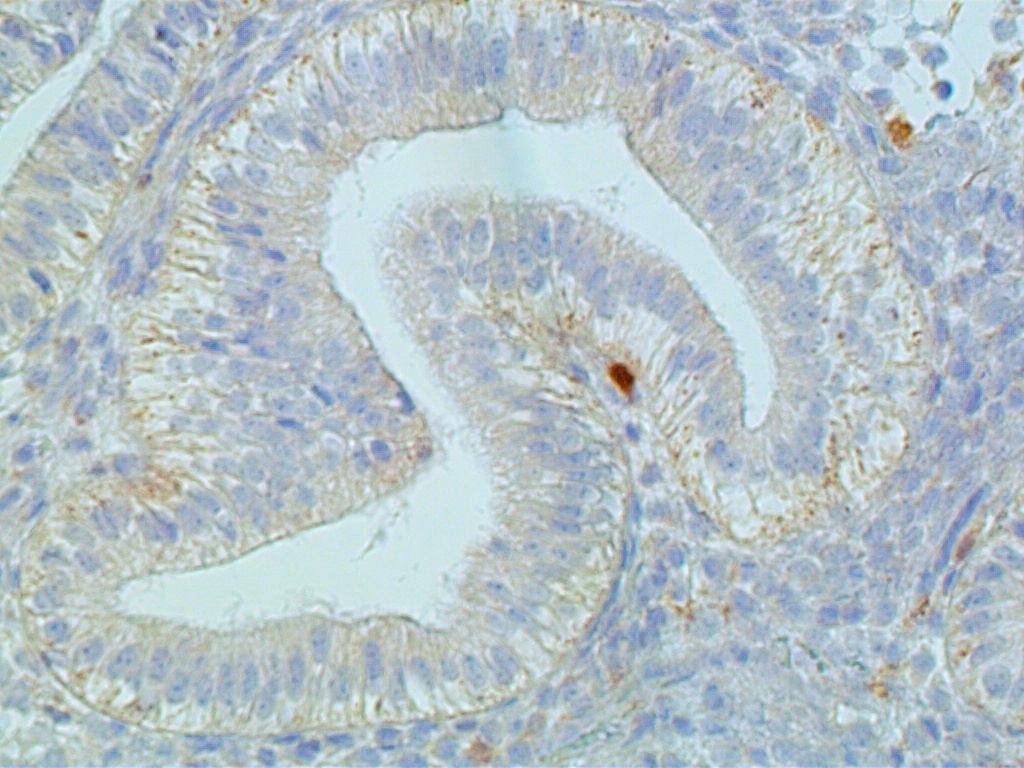
Steroid hormone control of endometrial, epithelial, stromal, and presumably endothelial cells is mediated by estrogen receptors and progesterone receptors. These steroid receptors are specific proteins concentrated exclusively in the nuclei of both endometrial epithelial and stromal cells, as well as the endothelial cells of stromal capillaries. (Fig. 2). They have high affinity to bind estradiol and progesterone, respectively.2
Fig. 2. Receptors are localized to epithelial and stromal cell nuclei as well as the endothelial lining of capillaries (arrow). |
This chapter contains a review of the technical procedures for handling endometrial tissues and a discussion of the morphologic aspects of the endometrium, focusing on the interpretation and understanding of the physiomorphology of the endometrial cycle.
OBTAINING SPECIMENS
Method
To ensure a good specimen for morphologic interpretation, a biopsy sample should be taken from both the anterior and the posterior endometrium and fixed immediately in 10% buffered formalin. In current practice, the device that is most often used is the Pipelle endometrial aspirator. To ensure a maximum amount of tissue for morphological reading, the specimen should be placed on a piece of lens paper or some other adhesive tissue and then immersed in the fixative. By this means, all of the tissue fragments remain tightly attached to the lens paper, rather than floating in the fixative, and no tissue will be lost for histologic examination. In premenopausal women with regular menstrual cycles, histological preparations include the upper portion of the functional layer of the endometrium. This is necessary, for in most instances morphological changes occur in the functionalis as opposed to the basalis layer, and, by inference, provide a clinically useful diagnosis. In cases in which little or no tissue is obtained but the endometrium was penetrated with the aspirator, a repeat procedure should be performed. If the repeat aspiration still yields little tissue, one can assume severe endometrial atrophy or obstructing endometrial polyp. Taking an endovaginal ultrasonography of the uterus may solve this dilemma. If the aspirator is 'blocked' at the lower uterine segment (internal os), traction may be applied on the uterus with either a single-toothed tenaculum or preferably an Emmet tenaculum placed about half a centimetre into the anterior endocervical canal. This will help pass over the area of resistance. The pathology requisition should contain all pertinent information, including date of last menstrual period.
Timing
The best way to prove or disprove that ovulation has taken place is to take an endometrial sample on cycle day 22 or later, preferably at the onset of uterine bleeding. By obtaining samples at the time of early uterine bleeding, the pathologist will be able to determine whether the bleeding is caused by the breakdown of postovulatory, secretory endometrium; by focal necrosis of the endometrium associated with anovulation; by other pathologic states; or by hormone administration. Also, during the period of bleeding, both the external os and the isthmus (lower uterine segment) are widened, facilitating penetration of the biopsy forceps into the endometrial cavity. The final argument in favor of taking samples at the onset of bleeding is that endometrium of the first 2 days of menstruation is relatively easy to recognize histologically. In contrast, secretory-phase endometrium often demonstrates subtle changes and, in many cases, combinations of morphologic changes, resulting in most instances in errors of 4–5 days. The pathologist can improve this to 2–3 days, however, by acquiring expertise in endometrial dating (all cases of normal endometria are to be dated regardless of reasons for sampling), and by basing the dating on those endometrial morphologic alterations that represent the most advanced phase of the menstrual cycle. For example, if an endometrial biopsy contains changes consistent with postovulatory days (POD) 2, 3 and 4, the pathologist should report the diagnosis as 'POD 4 or 18th-day secretory endometrium' assuming that ovulation has taken place on the 14th day of an idealized endometrial cycle of 28 days total. In any case, one should not average cycle days and report 'POD 3 or 17th-day secretory endometrium'. Endometrial biopsies are not to be taken at the onset of bleeding in the following two conditions: if luteal phase defect (LPD) is suspected clinically and is desired to be confirmed histologically, when the biopsy should be taken between POD 7 (21st) and POD 9 (23rd) cycle days to demonstrate a 3–4 day delay in endometrial maturation; or if there are asynchrony of gland/stromal development and dissimilar maturation in different regions of the endometrial specimen. These changes are presumably due to the poor development of the corpus luteum. According to most investigators, measurements of serum progesterone levels rather than histological dating of the endometrium provide for the best assessment of LPD (see discussion later in this chapter). In LPD, circulating progesterone levels are decreased and not sufficient to promote full secretory differentiation of the endometrium. A repeat biopsy taken during the same period of the following cycle will further confirm an abnormally short corpus luteum life span.
In cases of clinical membranous dysmenorrhea, the endometrial biopsy should be taken on cycle days 5–10. In these instances, the histologic specimens contain large fragments (casts) of endometrium, often with focal Arias-Stella reaction, or star-shaped glands with dense stroma alternating with foci of normal menstrual endometrium. Such a condition, also called irregular shedding, is presumably associated with a persistent corpus luteum from a recent or remote intrauterine or ectopic pregnancy and with relatively increased blood progesterone levels. Because of the stabilizing effect of progesterone on lysosomal enzymes and prostaglandins, menstrual breakdown may be delayed, prolonged, and extensive.
MORPHOPHYSIOLOGIC INTERPRETATION OF THE ENDOMETRIAL CYCLE
The major morphologic criteria useful for dating the endometrium throughout the cycle are presented in Fig. 3. In routine dating, the pathologist should avoid bias by evaluating the histologic section before reading the clinical information. After examining the specimen, the pathologist should attempt to correlate histology with clinical history. In the most common terminology for dating the endometrial biopsy, day 1 is used as the first day of bleeding, and this is used in Fig. 3 as the starting point.3 Endometrial cycle length is often described as an idealised 28 days in duration, but this may be slightly longer or shorter even in normal women. These physiologic variations occur in the preovulatory phase, as tight programming of postovulatory events fixes the postovulatory interval at about 14 days. It is not necessary to date the endometrium during the proliferative phase. During this period, daily morphologic alterations are not sufficiently distinctive to provide adequate benchmarks for accurate dating. Also, because proliferation precedes the ovulatory period, dating proliferative endometrium gives the clinician no relevant information on whether ovulation is occurring. The daily changes in the endometrium during the postovulatory period were, in the past, considered significant enough from one day to another to provide accurate evaluation of the endometrial cycle.
Fig. 3. Major morphologic criteria for dating the endometrium during the menstrual cycle.4 |
Dating the postovulatory endometrium as described originally by Noyes et al 4 has been used routinely for evaluating the corpus luteum function in patients with a variety of infertility problems, and, in particular, luteal phase defect (LPD). The reliability of endometrial histological dating as a diagnostic tool in cases of infertility has recently been challenged for lacking precision and inter- and intra-observer agreement variability.5, 6 More specifically, it has been shown that many of the histological features used for endometrial dating are more irregularly distributed over time, or occur in more variable combinations, than perviously believed. This suggests that distinctiveness exists in the responsiveness of the glands and stroma (presumably because they have different biological functions) to circulating sex steroids. This is understandable, for the menstrual cycle is a dymanic succession of biological phenomena with inherent variability. As a result, in routine practice, the longer lasting histological changes which evolve during the postovulatory period provide the most accurate endometrial dating. Finally, one has to realize that the day of ovulation is also subject to variation, for a given patient may ovulate on day 13, 14 or 15 or even later of a given menstrual cycle.
To circumvent the limitations of the Noyes et al histology criteria,4 it is recommended to use three- rather than two-day endometrial stromal delay development in assessing LPD. It has been suggested, furthermore, to use two parameters, e.g. endometrial biopsy and serum progesterone determinations for the routine evaluation of endometrial response in fertility treatments including IVF programs.6 In a study assessing the accuracy of dating the postovulatory endometrium, the following histological features were the most reliable: glands/stromal breakdown (menstrual endometrium); basal location of gland cell nuclei (POD 5–6 and later in the cycle); subnuclear cytoplasmic vacuolization (early secretory phase); serrated glandular configuration (mid-secretory phase); epithelial mitoses (early phase); and periarteriolar predecidual reaction (mid-secretory phase).6
Menstrual Period
In most women with normal-length cycles, the menstrual period lasts 4 ± 1 days. During this period, the endometrial lining undergoes rapid degeneration and regeneration. Both phenomena are presumably independent of hormonal influence, as estradiol and progesterone levels are low.2 On cycle days 28 and 1, the endometrium is thick, red, and soft. The stroma beneath the surface epithelium contains blood lakes, fragmented stromal cells, and inflammatory exudate. These rapidly become generalized, resulting in a rough, friable, hemorrhagic surface. When seen in cross-section, the functional layer of the endometrium occupies the upper two thirds of the entire thickness; the lower third or basal layer changes very little during the endometrial cycle.
On cycle day 2, the functional layer becomes disorganized, containing predecidual stromal cells admixed with epithelial glandular cells; both cellular systems undergo extensive apoptotic cell death initiated by a rapid decline in circulating estrogen and progesterone in the preceding days. These systemically induced events are diffuse throughout the endometrial mucosa of the body and fundus regions. The isthmic endometrium is not significantly sensitive to cyclic hormonal stimuli and is not used for dating purposes. The menstrual fluid is made up of autolyzed tissue admixed with a heavy polymorphonuclear exudate, red blood cells, and proteolytic enzymes. One of the latter is blood protease plasmin, which prevents clotting of menstrual blood. Plasminogen activators, which convert plasminogen into plasmin, are found in and released from degenerated endometrial vascular endothelium.7 Anovulatory bleeding, in contrast to menstrual bleeding, is associated with thrombosis of dilated vessels and patchy, non-uniform, secondary focal tissue breakdown. The background endometrium is largely intact and most commonly shows hyperplastic changes of unopposed estrogens.
During cycle days 1 and 2, synthesis of nuclear deoxyribonucleic acid (DNA) is near zero level in the secretory functional layer of the endometrium. These findings indicate that the cellular components of the functional layer undergo irreversible cell injury before being expelled during the menstrual period. On cycle days 2 and 3, the functional layer gradually becomes cleaved off from the underlying basal layer, resulting in a thin, denuded basal layer with a ragged surface onto which residual basal gland stumps open. Loss of the bulk of endometrial mucosa explains why scant tissue is obtained in endometrial biopsy or dilation and curettage (D & C) during the second part of the menstrual period.
Starting on day 2 and for the subsequent 2 days, proliferation of the basal gland epithelium begins in the areas of denudation. The surface of the endometrium is re-epithelialized as the residual glandular epithelium spreads over the denuded surface. Another source of resurfacing epithelium is the surface epithelium of peripheral regions of the endometrial cavity, such as the lower uterine segment and peritubal ostia, which remain intact during the menstrual period. The subsequent development of interanastomoses between converging epithelial proliferations leads ultimately to complete reconstruction of a new surface epithelium by cycle day 5. Complete re-epithelialization of the surface coincides with cessation of bleeding.
DNA tracing studies have shown that increased DNA synthesis is confined to the basal layer of the body and fundus of the uterus and the adjacent isthmic and peritubal-ostial endometrial mucosa, all of which remain intact during menstruation. This increase occurs only after complete denudation of the basal layer by cycle day 3. The postmenstrual endometrium is repaired by cellular migration and replication of surface epithelial cells.2 The resurfacing cells are flattened and spindle-shaped, with intracellular microfilamentous-microtubular systems and pseudopodial projections. These features are consistent with ameboid contraction-expansion-mediated motility. Typically, the regenerative surface epithelium is devoid of cells involved in DNA synthesis or mitoses (Fig. 4).
Fig. 4. Regenerative endometrium. Surface epithelial cells supported by tightly packed stromal cells. Both lack DNA synthesis as evidenced by negative immunostaining reaction for Ki-67 (x250).
|
Endometrial stromal cells modulate the growth, steroid hormone action, and functional differentiation of the epithelial cells.2 In vitro experiments have demonstrated production of basement membrane-like material with heavy desmosomal attachments by epithelial (but not stromal) cells. The basement membrane provides anchorage and participates in the control of proliferation and migration of epithelial cells as well as in their differentiations. An essential component of epithelial regeneration is provided by stromal cells which aggregate in cellular 'balls' beneath the regenerative surface epithelium (Fig. 5).
Fig. 5. Menstrual endometrium, cycle days 3 and 4. A. Note dense stromal cell aggregates beneath the regenerative surface epithelium (arrow) (x150). |
Tenascin, an extracellular matrix protein, is immunolocalized around proliferative endometrial glands and is believed to enhance epithelial cell migration and proliferation during periods of postmenstrual repair by inhibiting cell attachments to fibronectin.2
After the initial epithelial spread, cell division and migration operate simultaneously until a confluent surface layer has been regenerated by cycle day 5. The sudden increase in nucleic acid synthesis and a very short DNA-synthesis phase of the regenerative cells result in accelerated tissue turnover. These characteristics of migration and accelerated tissue turnover explain the spectacularly rapid wound healing capability of the human endometrium. DNA and ultrastructural data do not support the concept that the regenerative endometrium derives directly from persistent secretory spongiosa or stromal fibroblasts of the endometrium.
The mechanisms of induction of endometrial proliferation at the end of menstruation do not seem to require estradiol. Indeed, during cycle days 3 and 4, despite increased DNA activity, plasma levels of estrogens and progesterone receptors are low and unchanged from the premenstrual values. By immunostaining, the regenerative surface epithelium and underlying stromal cells are seen to be depleted of receptors for estradiol and progesterone (Fig. 6).
Fig. 6. Regenerative post-menstrual endometrium. Immunostaining for ER is negative in regenerative surface epithelium (arrow) and the immediately underlying stromal cells. In contrast, the endometrial glands and stromal cells in the deeper layer immunostained strongly for ER and PR (not shown). The findings suggest that the early phase of regeneration is independent of hormonal influence (x250). |
Experimental endometrial regeneration in the rabbit has shown that proliferation kinetics and morphologic alterations of the regenerative but estrogen-deprived atrophic endometrium associated with ovariectomy are similar to those in animals with intact ovaries.2 It is likely that the tumor suppressor PTEN-positive endometrial glands which appear to persist in the basalis during menstruation may also contribute to post-menstrual endometrial regeneration.8 In contrast, cycle days 7 through 12 are characterized by a sudden increase in the incorporation of nuclear thymidine and mitoses in the stromal, vascular, and gland cell components of the regenerated endometrium. These changes are accompanied by an increase in plasma levels of estrogens and progesterone receptors and a slight decrease in serum pituitary hormones, alterations that are consistent with target cell sensitivity and response to preovulatory estradiol.
Proliferative Phase
The preovulatory endometrium demonstrates proliferative changes in the glands, stromal cells, and vascular system (Fig. 7).
Fig. 7. Proliferative endometrium. The glands have relatively narrow lumens, pseudostratified nuclei and mitotic figures, and the stroma is cellular and slightly edematous (x250). |
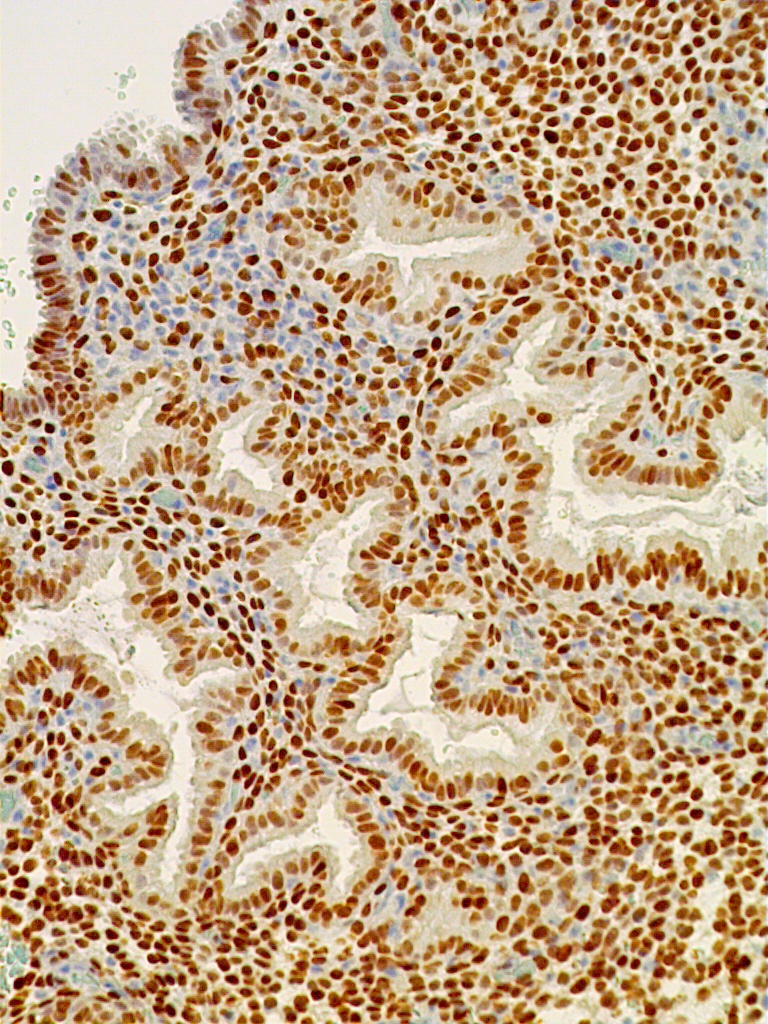
The glands acquire numerous mitoses and gradually become longer and larger, with convoluted shapes. The stroma becomes vascularized. These changes take place under the stimulatory action of estradiol, which stimulates the DNA-promoter enzyme, thymidylate synthetase.2 This in turn stimulates ribonucleic acid (RNA) polymerase activity, DNA synthesis, and mitosis. Endometrial growth during the pre-ovulatory phase is further substantiated by immunohistochemical tracing methods. The most often used proliferation markers are the DNA S-phase Ki-67 (Mib-1 molecule), the anti-apoptotic bcl-2, and the tumor suppressor gene p53. All of these can be identified with variable intensity and distribution in the proliferative endometrium (Fig. 8 A-C).
Increased proliferation leads to a considerable thickening of the endometrial mucosa. It is interesting to note that the endometrium demonstrates geographic variations in its response to hormonal stimuli. Maximum DNA synthesis is observed in the fundus and body of the uterus, whereas the isthmic and cornual regions contain comparatively lower values. Also, nuclear DNA activity is higher in the upper third than in the lower two thirds of the functional layer. This zonal variation in sensitivity of endometrial tissue to hormonal influence may be related to different physiologic functions: the upper functional layer facilitates implantation and nutrition of the blastocyst, whereas the lower functional layer is involved in the secretory activity and provides for the integrity of the endometrial mucosa. Whether differences in hormonal responses are due to dissimilar vascular supply of the upper and lower layers or to the intrinsic, heterogeneous nature of the endometrial tissue in terms of receptor content, or to both, remains to be determined. Maximum DNA synthesis during the midproliferative phase of the cycle (i.e. cycle days 8–10) correlates with the maximum number of mitoses in both the stromal and epithelial endometrial cellular elements, as well as with the midproliferative peak of plasma estradiol and nuclear estradiol receptors. Increased nuclear DNA synthesis and mitotic activity in gland cells correlate with high levels of nucleolar organizer regions. These are loops of DNA, transcribed to ribosomal RNA, and are proliferation indicators. An increase in estradiol-mediated DNA activity during the early and midproliferative phases and its decrease by cycle days 11 through 14 may reflect development of a refractory state secondary to long-standing estradiol stimulation. According to animal studies, DNA synthesis decreases rather than increases after 2 days of estrogen administration. Inhibition of nucleic acid synthesis is apparently not related to loss of estradiol receptors or nuclear translocation of estradiol receptors, but rather, presumably, to accumulation of the chalone-like inhibitors of DNA synthesis. This hypothesis is attractive, but it remains to be tested.
Recent biochemical and immunohistochemical studies have identified several peptides suspected to be involved in the autocrine or paracrine control of endometrial growth.2 Epidermal growth factor is produced by both epithelial and stromal cells and is likely to stimulate alone or with estradiol stromal/epithelial proliferation.9 Insulin-like growth factors also promote endometrial cellular mitosis, including that of decidual cells. It is likely that epidermal growth factor mediates the mitogenic action of estradiol. The transforming growth factor-β; mainly inhibits cellular proliferation and promotes differentiation of the endometrium, acting via autocrine and paracrine mechanisms. It is increased in the secretory phase and particularly in early pregnancy decidua and may enhance placental calcium transport. Cytokines are multifactorial immunomodulators acting by autocrine, paracrine, or endocrine mechanisms on proliferation and differentiation of cells of the immune system.
In addition to tissue proliferation, estradiol promotes the development of free and bound ribosomes, mitochondria, golgiosomes and primary lysosomes in gland cells and presumably in stromal cells. Biochemically, these organelles each provide for protein matrix, energy, and synthesis of various enzymes. Some of these enzymes, including glucose-6-phosphatase, hexokinase, pyruvate kinase, and lactate dehydrogenase, are involved in carbohydrate metabolism.2 Other important proteins thought to be produced by estradiol are estradiol receptors and progesterone receptors. Concentrations of estradiol receptors and progesterone receptors increase in both the blood and the endometrium during the proliferative phase of the cycle (see Fig. 2). Another characteristic feature of proliferative-surface and gland-lining cells is an increase in the number of cilia and microvilli. These decrease considerably during the secretory phase, suggesting that endometrial ciliogenesis and microvillogenesis are estrogen dependent.2 Additional evidence for this concept is provided by observations of even more cilia and microvilli in hyperplastic endometria, whereas progestational therapy leads to their disappearance and decrease, respectively. Ciliated cells are especially numerous around gland openings. It has been suggested that this peculiar distribution and strong-forward and slow-recovery ciliary beat pattern facilitate mobilization and distribution of endometrial secretions during the luteal phase of the cycle.
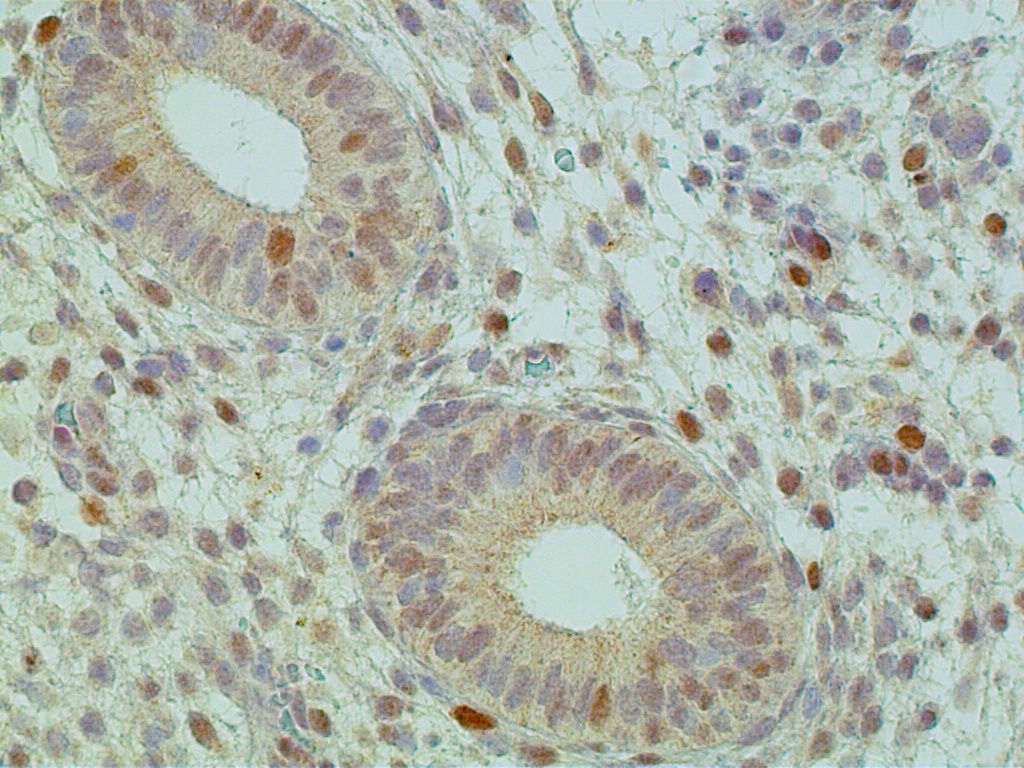
Intracytoplasmic filaments serve as a cytoplasmic 'skeleton' in gland and stromal cells. Gland cells have cytokeratin- and vimentin-positive intracytoplasmic filaments, whereas endometrial stromal cells stain strongly for vimentin, smooth muscle-related antigens and CD-10 (Fig. 9).
Fig. 9. Proliferative endometrium, cycle day 13. Interglandular stromal cells strongly and diffusely immunostain for CD-10 (x250).
|
Lymphoid aggregates resembling follicles may be seen in the endometrial stroma, particularly in the basal layer and during the proliferative phase of the cycle. They are made of T-cells, macrophages, and B-cells, and stain for IgA, IgM, or IgG. They are unlikely to play a significant role, if any, in the local secretory immune system. Indeed, endometrial epithelial cells synthesize negligible amounts of immunoproteins,2 and IgG-containing plasma cells are absent in normal endometrium. The observations are consistent with the sterile nature of normal endometrium.
Endometrial Angiogenesis
The two main mechanisms of endometrial vessel formation have been suggested to occur via intussusception and sprouting.10 The former consists of internal division or splitting of pre-existent vessels. In the latter, endothelial cell activation, degradation of basement membrane, migration and proliferation lead to formation of tubules, stabilization of pericytes, and extracellular matrix formation. Pericytes may, in fact, play a significant role in angiogenesis and regulation of blood flow. The cycling endometrium requires repeated, rapid, short-term proliferation and rapid arrest of neovascularisation. Angiogenesis in the endometrium is regulated and controlled by a multitude of promoters and inhibitors, all of which are believed to be under the influence of estradiol and progesterone during the menstrual cycle. The most important promoter of endothelial growth via mitosis is the vascular endothelial growth factor (VEGF).11 A leading angiogenic inhibitor is thromboplastin-1 which is stimulated by progesterone and is responsible for inhibiting excessive growth of the endometrial capillary system during the postovulatory phase of the cycle.12
Secretory Phase
During the postovulatory, or secretory, phase the estradiol-primed endometrium is under progestagenic stimulation and undergoes secretory differentiation.2 The first morphologic event indicating ovulation occurs on POD 2 (postovulation day 2) with the appearance of small, cylindrical vacuoles occupying the base of some of the gland cells in the functional layer. Because similar changes may be produced by estrogens alone in the absence of ovulation, variably sized subnuclear vacuoles in a mitotically active endometrium are not considered specific to ovulation. The most reliable histologic alterations that are considered specific to ovulation are seen on the POD 3 or 17th day of the cycle.5, 6 These include unifromly developed subnuclear glycogen vacuoles in gland lining cells and palisading of gland cell nuclei. Both phenomena involve every cell in a given gland (Fig. 10). TAG-72 or B72.3, a mucin-like glycoprotein, is exclusively found in postovulatory secretory gland cells; it serves as an immunomarker of ovulation.
Fig. 10. Secretory endometrium. Day 17 (POD 3) secretory endometrial glands with S-shaped configuration, subnuclear vacuolization, and palisading of nuclei in middle of lining epithelium (x450).
|
At the transmission electron microscopic level, ovulation may be recognized by the appearance of giant mitochondria and the so-called nucleolar channel system in gland cells.1 Nucleolar channel systems are unique to women and occur only during the postovulatory period (Table 1). They are presumably produced by the infolding of the nuclear membranes under progesterone stimulation.
Table 1. Morphologic evidence of ovulation
MORPHOLOGY | POSTOVULATORY DAY |
| Nucleolar channel system* in gland cells | 0–11 |
| Subnuclear vacuolization with nuclear palisading of gland cells | 3–4 |
| Stromal edema with ferning of glandular epithelium | 8–9 |
| Perivascular and stromal predecidualization | 9–14 |
Cycle Days | |
| Diffuse predecidual and glandular necrosis, inflammation and vascular thrombosis | 1–2 |
| Inflammatory exudate, aggregates of stromal cells (stromal balls) with or without hypertrophic surface epithelial cells, diffuse | 2–4 |
*at ultrastructural level |
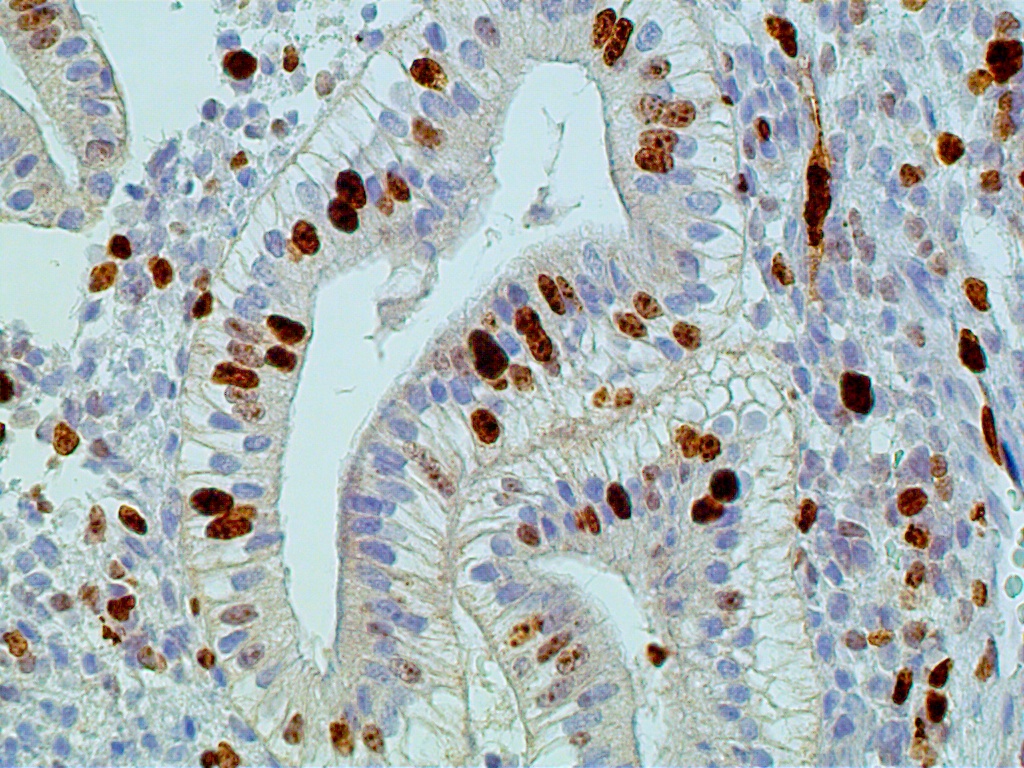
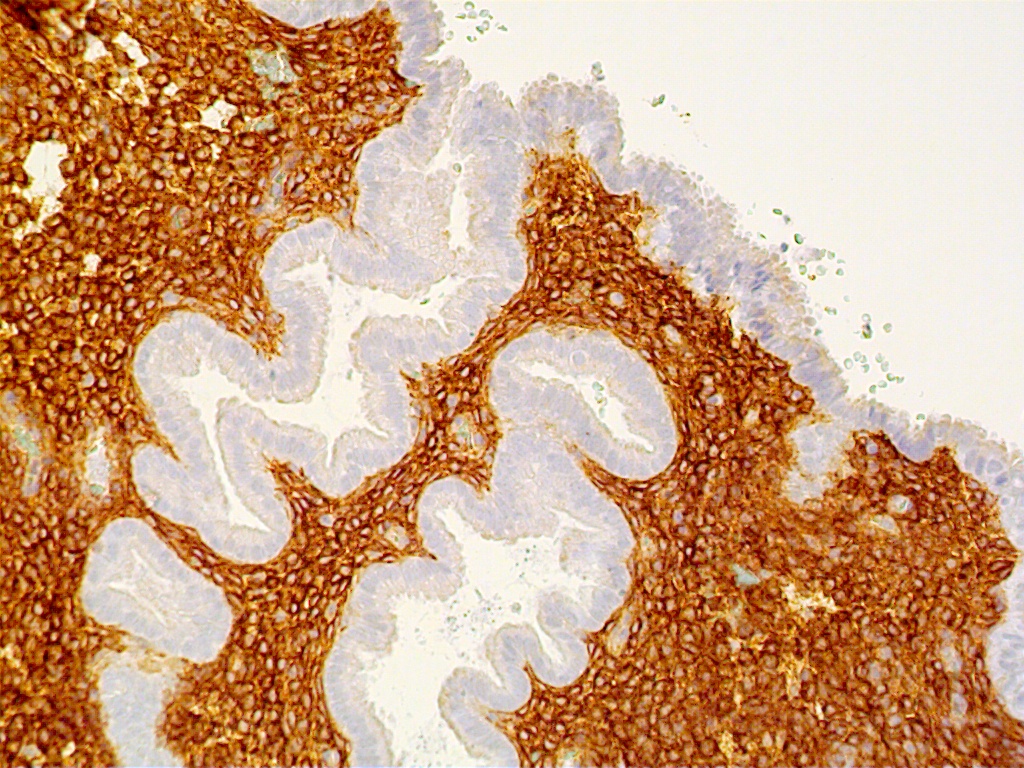
During the first four postovulatory days, both the glandular and stromal cells are engaged in DNA synthesis and mitotic activity although to a much lower degree than is seen in the preovulatory and proliferative endometrium (Fig. 11 A). Similarly, other proliferative markers such as bcl-2 and tumor suppressor gene p16 are only rarely seen, if at all (Fig. 11 B and C).
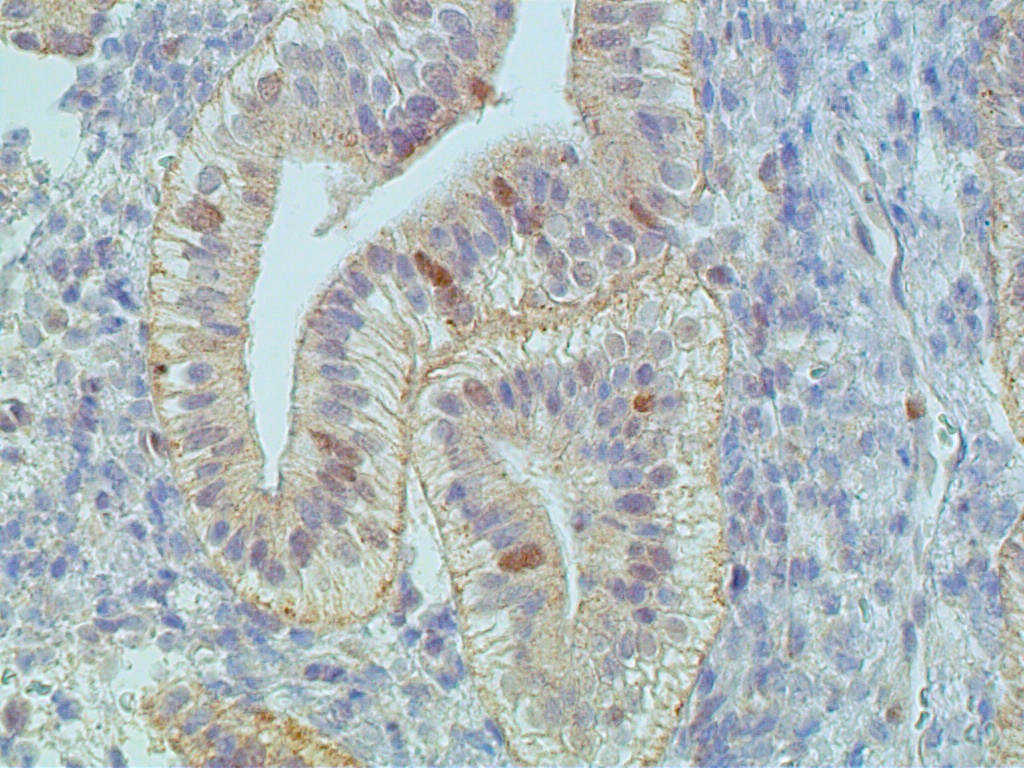
The glands are engaged in intracellular but not yet in active extracellular secretion of glycoproteins. On POD 5 and 6 (cycle days 19 and 20), the intracellular secretory products are extruded into the glandular space by apocrine-type secretion. This is characterized by protrusions and eventual detachment of the apical portion of cells containing glycoproteins. Transudation of plasma from circulating blood in the endometrial mucosa also contributes to uterine secretory fluids. The peak of intraglandular secretions on POD 7 (cycle day 21) coincides with the time of implantation of the free blastocyst if fertilization has taken place in this cycle. Nucleic acid synthesis by gland cells ceases as apocrine secretory activity is initiated by POD 5 (cycle day 19) (Fig. 12 A-C).
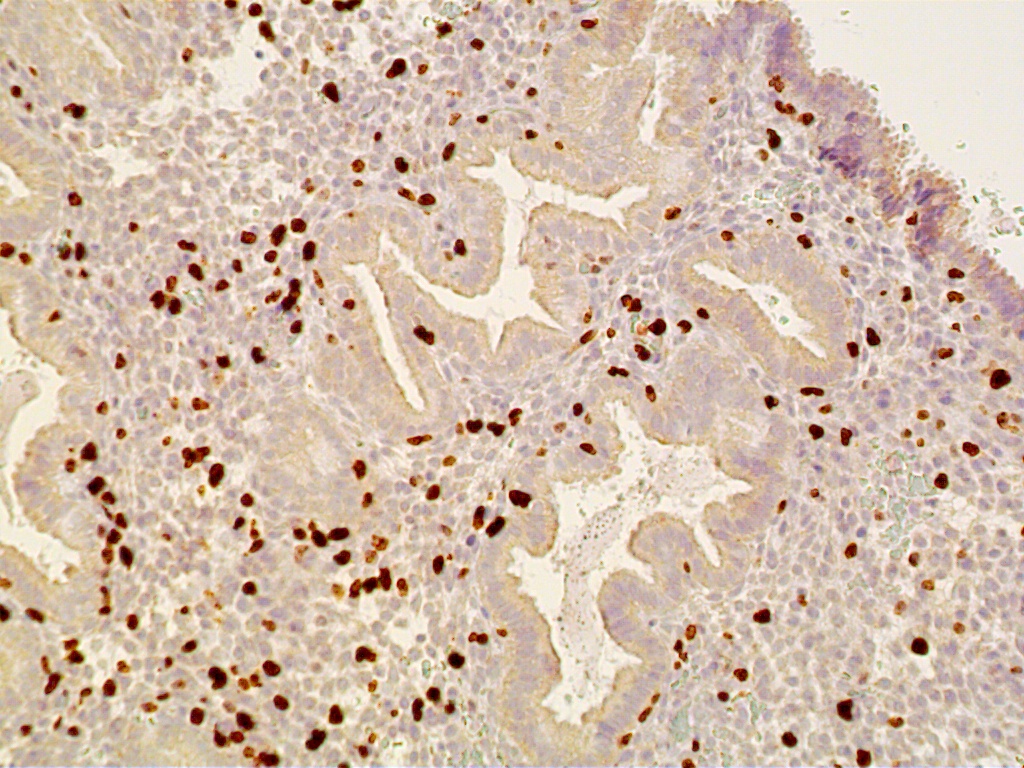 Fig. 12. Postovulatory day 11 (cycle day 25).
Fig. 12. Postovulatory day 11 (cycle day 25).
Fig. 12A. Immunostaining for the proliferation marker Ki-67 is confined to predecidual stromal cells as DNA synthesis is shut off within secretory-type endometrial gland cells (x150).
Fig. 12B. Immunostaining for ER is present in both gland and stromal predecidual cells but of less intensity than in proliferative endometrim (x150).
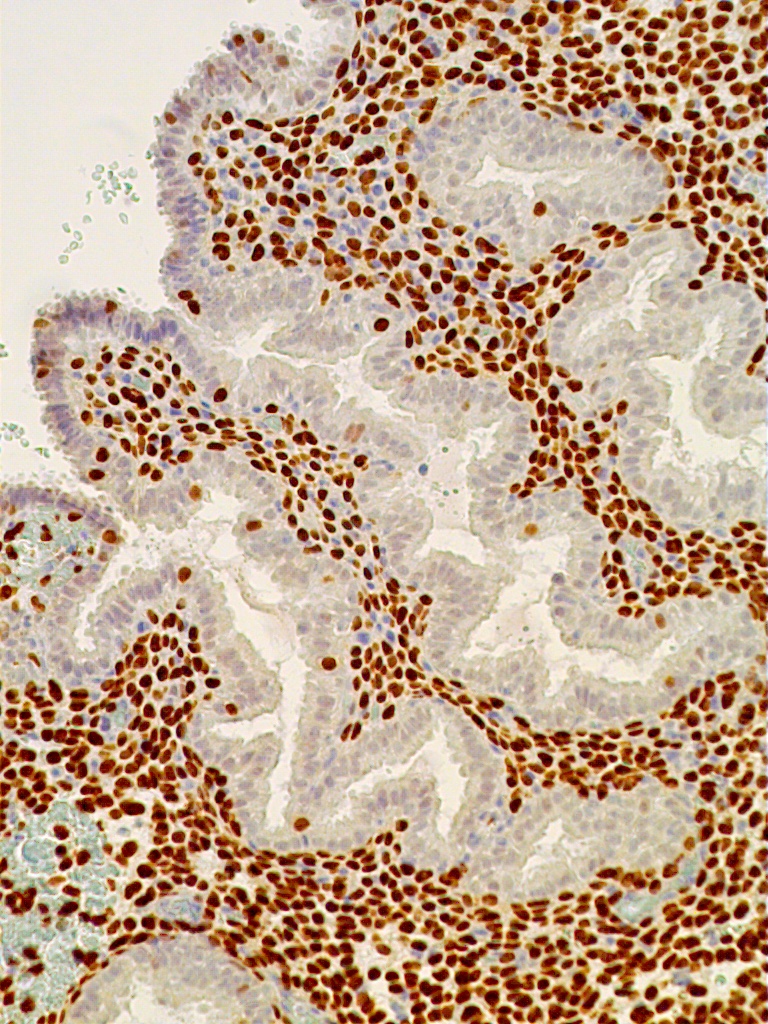
Fig. 12C. C. Immunostaining for PR is confined exclusively to predecidual stromal cells indicating arrest in E2-dependent glandular growth (x150).
This correlates with total lack of mitoses in the glands during the mid and late periods of the secretory phase. Inhibition of mitosis has been attributed to rising levels of postovulatory progesterone, which antagonizes the action of estradiol by decreasing estrogen receptors and by increasing the progesterone-specific enzyme 17 β-hydroxydehydrogenase.2 This enzyme converts estradiol into estrone, which leaves the target cell with negligible stimulatory effect on the nucleus. As a result, an increase in 17 β-hydroxydehydrogenase lowers the concentration of estradiol and its products (i.e. specific receptors for estradiol) in the tissue and increases estrone sulfotransferase. It has been suggested that in mice, progesterone induces the epithelial cells to enter a nondividing (G0) stage of the cell cycle during decidualization.
For accurate dating purposes from cycle day 21 (POD 7) onwards, the pathologist relies on changes in the stroma, rather than in the glands. These include edema, coiling of spiral arterioles, and predecidualization of the stroma. These alterations are possibly mediated by prostaglandin F2α (PGF2α) and prostaglandin E (PGE2). Indeed, estradiol stimulates the production of PGF2α, whereas progesterone stimulates the synthesis of both PGF2α and PGE2 in vitro.2 The prostaglandin PGE2 presumably promotes capillary permeability and stromal edema via histamine, whereas vascular endothelial growth factor is a potent mitogen of endothelial cells. Endometrial vascular proliferation at the implantation site is related to the blastocyst rather than to histamine or PGE2. The blastocyst has a unique biologic property that is shared only with tumor cells producing the so-called angiogenesis factor, a substance capable of inducing growth of new capillaries. Receptors for human chorionic gonadotropin and luteinizing hormone are present in vascular smooth muscle cells and endothelium, suggesting their possible role in regulating blood flow.
PGF2α, Ki-67, and other peptides are responsible for the predecidual transformation and growth of spindle-shaped stromal cells (Fig. 12A). Although decidualized cells have receptors for estradiol, its role in this process, if any, is not clear. This change consists of cytonuclear enlargement with tetraploid nuclei resulting in plump, liver-like epithelioid cells. Predecidualization (not pseudodecidualization) is accompanied by an increase in nuclear DNA synthesis, mitotic activity, and the formation of a pericellular laminin substance.2 The latter is typical of epithelial cells and is believed to be related to the midsecretory phase peak of estradiol plasma levels. Although progesterone plasma levels are also elevated during this period of the cycle, progesterone-dependent 17β-hydroxydehydrogenase appears to be a specific enzyme only for the endometrial gland cells. Consequently, predecidual cells are relatively independent of the growth-inhibitory effect of progesterone.
Predecidual cells are of stromal origin (Fig. 13). They have mesenchymal-related (e.g. CD-10, vimentin, desmin), not epithelial-related (e.g. cytokeratin) antigens. They represent precursor forms of gestational decidual cells (decidua vera). Because they develop after implantation, they are not involved in the implantation process per se. The cells have several metabolic functions related either to pregnancy or, if conception has not occurred, to menstrual breakdown of the endometrium. For example, prolactin is produced by decidual cells and is related to osmoregulation of amniotic fluid. After implantation, the decidual cells appear to control the invasive nature of the normal trophoblast, for a lack of decidual reaction in the endometrium is accompanied by deep myometrial implantation of the placenta (i.e. placenta accreta, increta, percreta).
Fig. 13. POD 11 (cycle day 25). The predecidual cells of the compacta layer of the functionalis (below the surface epithelium) display a strong and diffuse immunostaining reaction for CD-10 (x150).
|
Decidual tissue during early gestation is rich in lymphocytes, natural killer cells and macrophages. The latter are observed near nidation sites, are of the major histocompatibility complex (MHC) class II type, and have the potential to present fetal antigens to T-lymphocytes. In addition, they secrete monokines and have immunosuppressive activity.13
In the nongestational endometrium, predecidual cells are engaged in phagocytosis and digestion of extracellular collagen matrix. These cellular activities may contribute to menstrual breakdown of the endometrium. Uterine decidual response may be induced experimentally both in vivo and in vitro in the prepubertal rat maintained on progesterone by PGF2α.2 Indomethacin, an inhibitor of prostaglandin synthesis, prevents decidualization, providing supporting evidence for the concept of prostaglandin-mediated decidual reaction. The presence of progesterone, however, appears to be a prerequisite for decidualization, for without it, no such reaction is observed in vitro. Predecidual transformation of stromal cells begins around the arterioles on POD 9 cycle day 23 and later involves the stroma under the surface epithelium, producing the compacta layer by POD 11.
By POD 12 (cycle day 26), the predecidual stromal reaction under the surface epithelium coalesces with predecidual cells around the arterioles forming large sheets of predecidua. One day later, this confluence may involve the upper two thirds of the functional layer. In addition to marked predecidualization, the stroma on POD 12 (cycle day 26) and POD 13 (cycle day 27) is associated with a progressively increasing number of extravasated polymorphonuclear leukocytes and the so-called endometrial granulocytes (Körnchenzellen), or K-cells.1 These granulocytes have a granular, eosinophilic, cytoplasmic substance and resemble eosinophils except for having a single, round (nonlobulated) nucleus. They are members of the large granular lymphocyte series. They are particularly numerous during the first trimester of pregnancy and may play a role in placentation. By POD 14 (cycle day 28), the spiral arterioles become dilated, and fissures appear in the compacta layer. These fissures contain edematous fluid, red blood cells, and acute inflammatory exudate.
The cyclic endometrium, as with many other tissue systems in the body, is subject to two fundamental types of cell death: apoptosis and necrosis. Apoptosis, or programmed, single cell death plays a complementary but opposite role to mitosis in normal tissue homeostasis. It is considered to be the major process responsible for cell death in various physiologic cell-turnover and differentiation events in both the embryo and the adult.14 Histologically, apoptosis is recognized by multiple fragments of condensed, shrunken nuclear material and cytoplasm of single cells. These alterations were traditionally called 'polydust' before the true origin of this nuclear debris was recognized. These apoptotic bodies are subsequently engulfed by adjacent cells. An inflammatory reaction composed of leukocytes is generally absent in association with apoptotic bodies. Apoptosis is seen in gland cells and in predecidual cells, both in vivo and in vitro.14 The process is likely to be initiated by increased levels of tumor suppressor p53 and transforming growth factor-α due to severe (irreversible) DNA damage.15 Whether apoptosis is produced by a single mechanism of endonuclease activation leading to the hallmark of apoptosis, which is internucleosomal DNA cleavage and subsequently DNA fragmentation, is not clear. In vitro experiments in the rodent suggest that apoptosis in endometrial stromal cells, including their decidual variants, is induced by transforming growth factor-α via an autocrine or paracrine mechanism. The extent to which apoptosis contributes to endometrial degeneration and gestational decidual regression is not clear. Also unclear is whether apoptosis is more or less important than tissue necrosis. Indeed, the endometrium demonstrates morphologic evidence of coagulative tissue necrosis in response to vasoconstriction of basal arteries and ischemia. The necrotic tissue with increased hydrolytic enzyme activity is characterized by collapse of the upper two thirds of the functional layer. It contains fragmented tissue, with swelling of the cells' nuclei and membrane disruption. The final event is heterolysis of dead cells due to the action of inflammatory cells invading the necrotic tissue.
Electron microscopy combined with enzyme-tracing studies have shown that in the absence of conception, the endometrium undergoes gradual involution, degeneration, and necrosis during the last 5 days of the cycle.7 During the proliferative phase, estradiol stimulates the development of Golgi complex-derived primary lysosomes, many of which contain acid phosphatase, a potent lytic enzyme (see Fig. 10). Primary lysosomes are present in the epithelial, stromal, and endothelial cells of the functional layer of the endometrium.2 During the first half of the luteal phase, lytic enzymes, including acid phosphatase, are confined to membrane-bound lysosomes. Their release is presumably inhibited by the membrane-stabilizer effect of progesterone. The sudden decrease in estradiol and progesterone levels causes a failure in the membrane integrity of acid phosphatase-containing lysosomes. As a result, the lysosomal enzyme is released into enzyme-free autophagic bodies filled with sequestered intracellular elements. The destructive action of the acid hydrolases leads to digestion of the incorporated cytoplasmic elements, producing empty vacuoles. Thus, portions of the cytoplasmic substance are removed by lysosomal autodigestion. It also has been suggested that the gradual increase in lysosome-membrane permeability may result in direct intracellular and intercellular diffusion of the lytic enzymes, including type II collagenase found in predecidual cells. Relaxin, which is found in gland and decidualized cells, stimulates collagenase and plasminogen activators, contributing to tissue breakdown. It destroys the glandular and stromal cells as well as the vascular endothelium. Also, matrix metalloproteins cause breakdown of integrin-mediated adherence on the endothelium to its basement membrane and the exocellular matrix, and play an important role in the menstrual breakdown of the endometrium including its vascular system. Vascular luminal surface membrane injury promotes platelet deposition, release of prostaglandins, vascular thrombosis, disruption of basement membrane and eventually tissue necrosis and bleeding.7 Endometrial bleeding affects mainly the capillary and venous vascular systems of the functionalis layer of the endometrium.
A cytoplasmic pore-forming protein, perforin, which is present in the cytoplasmic granules of cytotoxic T-lymphocytes and natural killer cells, has been demonstrated in the human endometrium.16 Perforin-positive cytotoxic lymphocytes (CD3, CD56, and CD8) become numerous during the second phase of the postovulatory phase of the menstrual cycle, and their colonization is progesterone mediated. Perforin forms pores on the target cell membrane, enhancing passage of cytotoxic molecules that in turn produce or contribute to target cell death. Their maximum accumulation before menses and their participation in the regression of nongestational corpus luteum and deciduoma in the rodent may suggest that perforin-positive cytotoxic cells may play an important role in the menstrual breakdown of the endometrium.16 PGF2α and PGE2 increase significantly in the secretory endometrium by the 25th day of the cycle and reach maximum concentrations during the menstrual period, but PGF2α increases to a much greater degree than does PGE2. It has been speculated that the high levels of the potent vasoconstrictors endothelin, which acts on spiral arterioles, and PGF2α, which is seen during menstruation, may stimulate the onset of bleeding via vasoconstriction of spiral arterioles at the endometrial-myometrial junction and the expulsion of degenerated endometrium through myometrial contractions, respectively.17
In conclusion, the healthy human endometrium is unique for no other tissue systems demonstrate such rapid, hormonal-mediated tissue growth followed by monthly enzymatic degeneration, shedding, bleeding, hemostasis, and regeneration. These complex cellular and molecular events take place in the functionalis layer of the endometrium following withdrawal of both E2 and P. Despite earlier suggestions, they are not necessarily sufficiently temporarily distinct and discriminated enough to allow a high degree of reproducibility in accurately dating the endometrium by histology. However, by obtaining endometrial tissue at the onset or within three days of anticipated menses, and by acquiring experience with dating, all normal-appearing endometrial specimens allow for a relatively reliable assessment of the hypothalamo-ovarian axis.
REFERENCES
Mutter GL and Ferenczy A. Anatomy and Histology of the Uterine Corpus. In: RJ Kurman (ed), Blaustein’s Pathology of the Female Genital Tract, 5th Edition, Springer, New York, 2002. pp. 383-420 |
|
Giudice LC, Ferenczy A: The endometrial cycle: Morphologic and biochemical events. In Adashi EY, Rock JA, Rosenwaks Z (eds): Reproductive Endocrinology, Surgery, and Technology, pp 271–300. New York, Raven Press, 1995 |
|
Noyes RW: Normal phases of the endometrium. In: Norris HJ, Hertig AT, Abell MR (eds): The Uterus, pp 110–135, Baltimore, Williams & Wilkins, 1973 |
|
Noyes RW, Hertig AT, Rock J: Dating the endometrial biopsy. Fertil Steril 1: 3-25, 1950 |
|
Murray JM, Meyer WR, Zaino RJ, et al: A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril 81: 1333-1343, 2004 |
|
Fadare O, Zheng W: Histologic dating of the endometrium; accuracy, reproducibility and practical value. Adv Anat Pathol 12: 39-46, 2005 |
|
Ferenczy A: Pathophysiology of endometrial bleeding. Maturitas 45: 1-14, 2003 |
|
Mutter GL, Tan AI, Baak JPA, Kust A, Zhou X-P, Eng C: Molecular identification of latent precancers in histologically normal endometrium. Cancer Res 61: 4311-4314, 2001 |
|
Cooke PS, Buchanan DL, Kurita T, et al: Role of stromal-epithelial interactions in hormonal responses of the uterus. In: Glasser SR, Alpin JD, Giudice LC, et al (eds): The Endometrium, pp 151-166, London, Taylor and Francis, 2002 |
|
Weston G, Rogers PAW: Endometrial angiogenesis. Baillière’s Clin Obstet Gynecol 14: 919-936, 2000 |
|
Print C, Valtola R, Evans A, Lessan K, Malik S, Smith S: Soluble factors from human endometrium promote angiogenesis and regulate the endothelial cell transcriptome. Human Reprod 19: 2356-2366, 2004 |
|
Kamphaus GD, Colorado PC, Panka DJ, et al: Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem 275: 1209-1215, 2000 |
|
Mizuno M, Aoki J, Kimbara T: Functions of macrophages in human decidual tissue in early pregnancy. Am J Reprod Immunol 31: 180-188, 1994 |
|
Stewart BW: Mechanisms of apoptosis: Integration of genetic, biochemical, and cellular indicators. J Natl Cancer Inst 86: 1286-1296, 1994 |
|
Moulton BC: Transforming growth factor-beta stimulates endometrial stromal apoptosis in vitro. Endocrinology 134: 1055-1060, 1994 |
|
Hameed A, Fox WM, Kurman RJ et al: Perforin expression in human cell-mediated luteolysis. Int J Gynecol Pathol 14: 151-157, 1995 |
|
Markee JE: Menstruation in intraocular endometrial transplants in the Rhesus monkey. Contrib Embryol 28: 219-308, 1940 |