The Steroid Hormone Receptors
Authors
INTRODUCTION
Steroid hormones exert a wide variety of effects on growth, development, and differentiation, including important regulatory and behavioral functions within the reproductive, central nervous system, and adrenal axis. These hormones act through binding to specific intracellular receptor proteins that function as both signal transducers and transcription factors to modulate expression of target genes.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Molecular cloning has revealed 48 steroid hormone and nuclear receptor genes in humans (Table 1). Sequence comparison has revealed that steroid hormone receptors belong to a diverse family of ligand-activated gene regulators that share a highly conserved structure and common mechanisms affecting gene transcription.1 The evolutionary relationship among the steroid/nuclear receptors has been deduced by the high conservation in their DNA binding domains and in their less-conserved ligand binding domains and indicates that this large group of proteins arose from a common ancestral molecule.1, 2, 3
The steroid/nuclear hormone receptor superfamily (Table 1) includes 48 receptors for the gonadal and adrenal steroids, nonsteroidal ligands such as thyroid hormones, vitamin D, retinoic acid, and fatty acids, as well as numerous "orphan" receptors whose endogenous ligands, if necessary, are either as yet unknown or being identified.4, 5 Until late 1996, only one ER was thought to mediate the physiological effects of estrogens. However, a second gene encoding a closely related, but distinct, ER, called ERβ, was first identified in rat prostate6 and later in humans.7 The original ER was renamed ERα. ERα and ERβ can form heterodimers as well as homodimers in vitro and in vivo.8 The superfamily also includes the v-erb-A and c-erb-A oncogene proteins that bind to DNA but lack a functional ligand-binding domain,9, 10, 11 and other orphan receptors, e.g., short heterodimer partner (SHP), that lack a DNA binding domain,12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 but have a ligand-binding domain. The steroid receptors are considered class I members of the nuclear receptor superfamily while the other receptors are class II receptors.12 A variety of mechanisms for achieving tissue-specific gene expression in response to steroid hormones has evolved to ensure diversity through the interaction of these receptors with other cellular proteins and gene elements.
In this review, we summarize current information on the steroid/nuclear hormone receptors, with the primary focus on receptors for the sex steroid hormones.
Table 1. Members of the steroid hormone receptor gene superfamily in mammalian tissues
Receptors with known ligand(s):
ERα32
ERβ7, 8
hAR33
PR34
GR35, 36
MR37
VDR38
TRs39
hTRβ
hTRα1
hTRα2
c-erb A140
Rev-ErbA alpha (Rev-Erb)11
c-erbA beta41
c-erbA beta-242
RARs43
alpha
beta
gamma
RXRs43, 44
alpha
beta/ear-2/H-2RIIBP
gamma
PPARs45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80
alpha
beta/delta (NUCI)
gamma
Orphan receptors
CAR81, 82, 83, 84, 85
COUP-TFI/EAR386
COUP-TFII/ARP-187
DAX-188, 89, 90, 91, 92, 93, 94
EAR295
hERR196, 97, 98, 99, 100
hERR2101, 102, 103
GCNF104, 105, 106
HNF-3107
HNF-4108, 109
hSF-1110
nur77/NGF1-B111
RTR104, 112
RZR/ROR104, 113
RORB/RZRb114
SHP115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132
TR4/TAK1133
Orphan receptors with identified ligands
FXR (farnesoid X receptor, bile)132, 134, 135
LXR (liver X receptor)61, 136, 137, 138, 139, 140, 141
PAR/PXR/SXR (human pregnane receptor)142, 143, 144, 145, 146, 147, 148
STRUCTURE OF THE STEROID HORMONE RECEPTOR PROTEIN
In order to understand how steroid hormone receptors regulate gene function, it is important to know the structure of the receptor proteins as well as the identity and cellular function of the genes that they regulate. Members of the steroid receptor superfamily share direct amino acid homology and a common structure (Fig. 1). Receptors in this superfamily contain several key structural elements which enable them to bind to their respective ligands with high affinity and specificity, recognize and bind to discrete response elements within the DNA sequence of target genes with high affinity and specificity, and regulate gene transcription.13, 14
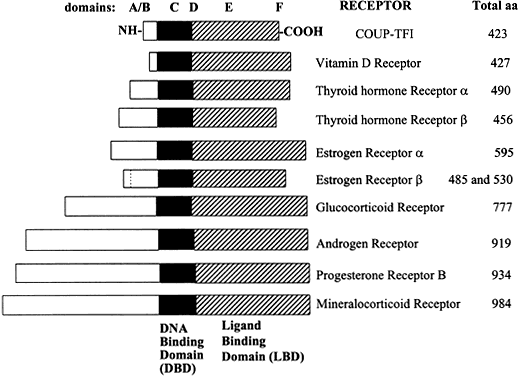
Fig. 1 Relative lengths of several members of the steroid/nuclear hormone receptor superfamily, shown schematically as linearized proteins with common structural and functional domains. Variability between members of the steroid hormone receptor family is due primarily to differences in the length and amino acid sequence of the amino (N)-terminal domain. Adapted from Wahli W, Martinez E. Superfamily of steroid nuclear receptors: Positive and negative regulators of gene expression. FASEB J 1991;5:2243-2249.
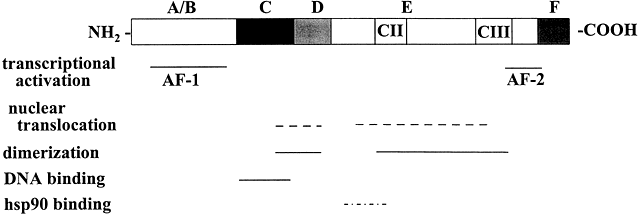
Molecular cloning of the complementary DNA (cDNA) for each of the major steroid receptors has greatly enhanced our understanding of the structure–function relationships for these molecules. The receptor proteins have five or six domains called A–F from N- to C-terminus, encoded by 8–9 exons. The receptors contain three major functional domains that have been shown experimentally to operate as independent "cassettes",13 unrestricted as to position within the molecule. The three major functional domains (Fig. 2) of the receptor are:
- A variable N-terminus (domains A and B) that confers immunogenicity and modulates transcription in a gene and cell-specific manner through its N-terminal Activation Function-1 (AF-1);
- A central DNA-binding domain (DBD, consisting of the C domain), comprised of two functionally distinct zinc fingers through which the receptor physically interacts directly with the DNA helix;
- The ligand-binding domain (LBD, domains E and in some receptors F) that contains Activation Function-2 (AF-2).
Fig. 2 Schematic representation of the common structural and functional domains of the steroid hormone receptors. The horizontal lines indicate the domains of the receptor. Adapted from Wahli W, Martinez E. Superfamily of steroid nuclear receptors: Positive and negative regulators of gene expression. FASEB J 1991;5:2243-2249.
The F domain is thought to play a role in distinguishing estrogen agonists from antagonists, perhaps through interaction with cell-specific factors.15, 16 Domain-swapping experiments in which the DBD of estrogen receptor α (ERα) was switched with that of the glucocorticoid receptor (GR), yielded a chimeric receptor that bound to specific DNA sequences bound by GR, but up-regulated transcription of glucocorticoid-responsive target genes when treated with estrogen,17 thus demonstrating the specificity of the DNA-binding domain in target gene regulation.
The amino (N)-terminal domain is hypervariable (less than 15% homology among steroid receptors) in both size and amino acid sequence, ranging in length from 25 amino acids to 603 amino acids and constituting the major source of size differences between receptors.18, 19 The AF-1 domain in this region is involved in activation of gene transcription, but does not depend on ligand binding. In rat GR, the AF-1 region is called tau 1 or enh2 and constitutes aa 108–317. Tau 1 is necessary for transcriptional activation and repression.20 Deletion of the C-terminal LBD of GR yields constitutive (hormone-independent) transcriptional activation, implying that the N-terminal regions harbor autonomous transcriptional activation functions.21
Some steroid receptors exist as isoforms, encoded by the same gene, but differing in their N-terminus. The progesterone and androgen receptors (PR and AR) exist in two distinct forms, A and B, synthesized from the same mRNA by alternate splicing. The two PR receptor isoforms differ by 128 amino acids in the N-terminal region, yielding PR-A = 90 kDa and PR-B = 120 kDa, that have strikingly differing capacities to regulate transcription.22, 23, 24 In contrast, AR-A and AR-B isoforms show minimal differences in activation of a reporter gene in response to androgen agonists or antagonists in transiently transfected cells.25
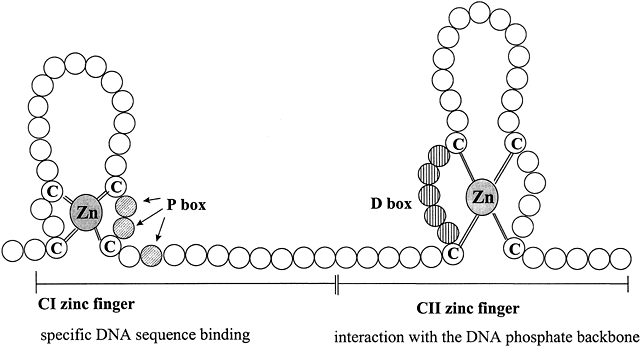
The central core or DNA-binding domain (DBD) is highly conserved and shows 60–95% homology among steroid receptors.1 The DBD varies in size from 66 to 70 amino acids, and is hydrophilic due to its high content of basic amino acids.13 The major function of this region is to bind to specific hormone response elements (HREs) of the target gene. DNA-binding is achieved through the tetrahedral coordination of zinc (Zn) by four cysteine residues in each of two extensions, that form two structurally distinct "Zn fingers" (Fig. 3).26 Zn fingers are common among gene regulatory proteins.23 Specificity of HRE binding is determined by the more highly conserved hydrophilic first Zn finger (C1),17 while the second Zn finger (C2) is involved in dimerization and stabilizing DNA binding by ionic interactions with the phosphate backbone of the DNA.18 The D box is involved in HRE half-site spacing recognition. The highly conserved DBD shared by AR, GR, mineralocorticoid receptor (MR), and PR enables them to bind to the same HRE, called the glucocorticoid response element (GRE). The more C-terminal part of the C2 Zn finger and amino acids in the hinge region are involved in receptor dimerization in coordination with amino acids in the hinge region and the LBD.
Fig. 3 Schematic diagram of type II zinc finger proteins characteristic of the DNA-binding domain structure of members of the steroid hormone receptor superfamily. Zinc fingers are common features of many transcription factors, allowing proteins to bind to DNA. Each circle represents one amino acid. The CI zinc finger interacts specifically with five base pairs of DNA and determines the DNA sequence recognized by the particular steroid receptor. The three shaded amino acids indicated by the arrows in the knuckle of the CI zinc finger are in the “P box” that allows HRE sequence discrimination between the GR and ERα. The vertically striped aa within the knuckle of the CII zinc finger constitutes the “D box” that is important for dimerization and contacts with the DNA phosphate backbone. Adapted from Tsai M-J, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 1994;63:451-483; Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet 1991;25:89-123.
The hinge region or D domain is a 40–50 amino acid sequence separating the DNA-binding and ligand-binding domains that contains sequences for receptor dimerization and ligand-dependent and independent nuclear localization sequences (NLSs).27, 28 The hinge region interacts with nuclear corepressor proteins,28 and with L7/SPA, a 27 kDa protein that increases the partial agonist activity of certain antagonist-liganded steroid hormone receptors, i.e., tamoxifen-liganded ERα, RU486-occupied PR, or RU486-occupied GR.29
The carboxy (C)-terminal or ligand-binding domain (LBD) is poorly conserved, ranging in size from 218 to 264 amino acids and is hydrophobic. This region contains the ligand-binding site and dictates hormone binding specificity.30, 31 Greater structural similarity between steroid hormone ligands generally indicates greater amino acid sequence homology in the LBD. Information from the X-ray crystal structures of the LBDs of the retinoic acid receptor (RAR), thyroid hormone receptor (TR), and ERα in the presence or absence of their cognate ligands has shown that the LBD has a compact structure consisting of 12 α-helices with a “pocket” into which the ligand fits.32, 33, 34, 35 Binding of the ligand within the pocket alters the conformation of the LBD with helix 12 forming a “lid” over the pocket, trapping the ligand in a hydrophobic environment and forming a surface on the LDB with which co-activator proteins interact. Helix 12 is indispensable for AF-2 function.36, 37 For ERα, 17β-estradiol (E2) and the antiestrogen, or select ER modulator (SERM), raloxifene form different amino acid contacts within the pocket.38 This results in different positioning of helix 12 in the LBD that is thought to permit interaction with co-activators, e.g., SRC-1, in the presence of E2, but not raloxifene, or by inference antiestrogens, such as tamoxifen.38
Two human GR isoforms, GRα and GRβ, derived from the same gene by differential splicing at the C-terminus, have been reported.39 While GRα and GRβ share the first eight exons, they differ in their last two exons, i.e., exons 9α or 9β, spliced into the respective mRNA.40 GRβ was reported to localize in the cell nucleus in the absence of ligand and to block hGRα activity.41, 42
Likewise, a novel isoform of ERβ, termed ERβ2, containing an in-frame insertion of an exon of 54 nucleotides, resulting in an insertion of 18 amino acids in the LBD, was recently identified first by screening rat prostate cDNA library, and is also expressed in human cell lines.43 ERβ2 binds E2 with lower affinity (Kd = 8 nM) than ERβ1 (Kd = 1 nM). At least 10 splice variants of ERβ have been identified.44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55
Sequences within the LBD form the binding site for hsp90 that blocks the DBD in the cytosolic, nonliganded GR.40 The CII and CIII regions (Fig. 2) show homology among members of the steroid/nuclear receptor superfamily and are important in forming the ligand binding pocket.56
The C-terminal AF-2 transactivation domain is highly conserved within the nuclear receptor superfamily36 and is recognized by various transcriptional coregulators, formerly referred to as coactivators or corepressors.57, 58, 59 AF-2 is localized to the most C-terminal end of the E domain. In ERα it constitutes aa 530–553.60 A third transactivation domain called AF-2a or tau2 has been localized to the N-terminal region of the LDB of ERα36 and GR.61 Deletion experiments revealed a role for AF-2a and the DBD in targeting rat GR to the nuclear matrix,62 an interconnected ribonuclear-protein network within the nucleus that is thought to play an important role in transcription of active genes by stabilizing the assembly of the transcriptional machinery.63, 64, 65, 66, 67, 68, 69
Although individual domains of steroid/nuclear receptors can be exchanged70 and function when spliced with nonrelated transcription factors,71 forming chimeric proteins, experiments on ERα72 and GR73 show that these receptors function optimally when intact. Additionally, the N- and C- terminals of the receptor interact with each other to increase transcriptional activation.74
STRUCTURE OF STEROID HORMONE-REGULATED GENES
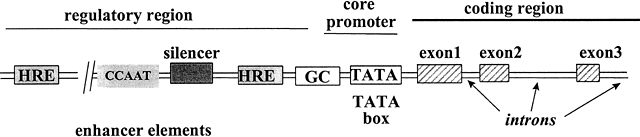
The transcription of DNA to messenger RNA (mRNA) is the most important process regulated by steroid hormones. All genes share a common basic design (Fig. 4), composed of a structural region in which the DNA encodes the specific amino acids of the protein, and a regulatory region that interacts with various proteins to control the rate of transcription. Several key elements in the regulatory region of the target gene must be activated before mRNA synthesis can occur. These elements, called ‘cis’-acting elements since they are located on the same DNA as the gene itself, are generally located near the 5' end (beginning) of the gene, and consist of four main groups: promoters, hormone-responsive enhancers, silencers, and hormone-independent enhancers.149 The promoter, essential for gene activation, sets the basal rate of transcription and controls the accuracy of transcription initiation.150 The promoter is located closest to the transcription start site and consists of two sub-elements: the TATA box and the upstream promoter. Located further upstream are one or more hormone response elements (HREs), the specific DNA-binding sites to which steroid receptors bind, conferring hormone sensitivity to the gene.151 Silencers are elements that inhibit transcription of adjacent genes in the absence of hormone activation.152 Hormone-independent enhancers are DNA sequences bound by other transcription factors that can further increase the rate of gene expression.28 The synergistic interaction between regulatory cis-acting elements permits fine-tuning of the rates of transcription of target genes in response to the local cellular and hormonal milieu.
Fig. 4 Schematic diagram of regulatory and structural regions of a steroid hormone responsive gene. Located upstream from the transcription start site are several regulatory cis-elements that interact with various factors, including steroid hormone receptors that bind to HREs, C/EBP that binds to CCAAT boxes,153 and Sp1 that binds to GC boxes.154 TFIID and the other general transcription factors bind to the TATA box, to control transcription of the target gene.149 The structural region of the gene contains nucleotides encoding the mRNA transcript that will be translated into the protein. Adapted from Tsai M-J, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 1994;63:451-483.151
Hormone response elements (HREs) are consensus 13–15-bp DNA sequences derived from alignment of genes responsive to a particular steroid hormone. HREs have two "half-sites" that each bind the C1 zinc finger of one receptor monomer. Steroid hormone receptors bind DNA as homodimers (or heterodimers, e.g., ERα/ERβ155, 156 with each monomer binding to adjacent major grooves on the same side of the DNA helix.26 Class II nuclear receptors may interact with a different class II nuclear receptor forming a heterodimer, thereby creating a more stable complex with much higher affinity that is thought to enhance transcriptional activity significantly.12 On the basis of sequence homology and functional similarity, there are three classes of hormone response elements within the steroid hormone receptor superfamily (Table 2).
Table 2. Hormone response element (HRE) binding sites for steroid/nuclear receptors. Response elements for the class II nuclear receptors are direct repeats (DR), inverted repeats (IR), or everted repeats (EvR) of the indicated half-site with the letter following the DR or IR indicating the number of nucleotides separating the half-sites, e.g., DR5 is 5’-AGGTCAnnnnnAGGTCA-3’.
| Steroid/nuclear receptor | Consensus HRE |
| AR, GR, MR, and PR ERα/ERβ Class II NR (PPAR, RAR, RXR, TR, and VDR) RXR/RAR RXR/TR RXR/VDR RXR/PPAR | GRE: 5’-AGAACAnnnTGTTCT-3’ ERE: 5’-AGGTCAnnnTGACCT-3’ DR: 5’-AGGTCA-3’ DR4, IR0, EvR6 DR3 DR1 |
The response elements for the progesterone, androgen, glucocorticoid, and mineralocorticoid receptors are closely related and are collectively referred to as the glucocorticoid response element (GRE) consisting of a palindromic (symmetrical) sequence 5’-GGTACAnnnTGTTCT-3’, where n = any nucleotide.157 AR, PR, GR, and MR show subtle differences in DNA base contact points to GREs.157 Examples of genes containing one or more GREs whose transcription is up-regulated by glucocorticoids include the much-studied mouse mammary tumor virus (MMTV) promoter158, 159 tyrosine aminotransferase,160 and enzymes involved in gluconeogenesis.161, 162, 163, 164 Examples of genes that are specifically inhibited by glucocorticoids through “negative GREs” include pro-opiomelanocortin,165 interleukin-1beta,166 gonadotropin-releasing hormone (GnRH),167 and prolactin.168
The minimal consensus estrogen response element (ERE) sequence is also palindromic, 5’-GGTCAnnnTGACC-3’,169 and differs in only two bp from the GRE.170 Extension of the length of the ERE palindrome, e.g., 5’-CAGGTCAnnnTGACCTG-3’, and the sequences immediately flanking the ERE, are important in determining the affinity with which ERα binds the ERE.171, 172, 173, 174, 175, 176, 177, 178 Examples of genes whose promoters contain functional EREs include those encoding the much studied Xenopus vitellogenin A1179 and B1 genes;180, 181 and human genes encoding pS2, a marker for human breast cancer diagnostics;182, 183, 184, 185, 186 oxytocin;187 c-fos;188 c-myc;188 TGF-α;189 prolactin;190 progesterone receptor;191 and cathepsin D.192
One of the major advances in the field of transcriptional regulation by ER has been the development of ChIP and the using of tiling arrays to identify ER binding sites throughout the human genome.193, 194, 195, 196, 197, 198, 199, 200 These studies have been performed in MCF-7 human breast cancer cells and demonstrate that with E2 treatment, ERα is recruited not only to the anticipated EREs in the 5’ promoter of known target genes, but to the 3’UTRs and at great distances from established genes in the human genome. Studies have also demonstrated chromatin looping between promoter, intron, and 3’UTR regions of genes are regulated positively and negatively by E2.193, 195
The response elements for the various class II NR, e.g., TR, RAR, RXR, and VDR, are composed of direct repeats of the half-site 5’-AGGTCA-3’ either with no space in between the half sites (DR0), or separated by a gap of 1–5 nucleotides (DR1-DR5).201 The number of nucleotides separating the half-sites determines the specificity of class II NR binding.12 Many class II NR require RXR for hormonal activation of transcription.202
GENERAL MODEL OF STEROID HORMONE RECEPTOR MECHANISM OF ACTION
(1) Arrival and entry of steroid hormones into target tissue cells
Steroid hormones are small hydrophobic and lipid-soluble molecules derived from cholesterol. They circulate in blood either free or bound (95%) to plasma carrier protein.203 Sex hormone-binding globulin (SHBG), also known as testosterone-estradiol binding globulin, TeBG, and androgen binding protein (ABP) are encoded by the same gene.204 They differ only in their glycosylation and tissue-specific expression.205 ABP is produced by the Sertoli cells of the testis and SHBG is produced by the liver and is present in the circulatory system.206 SHBG binds most gonadal steroids, and corticosteroid-binding globulin (CBG or transcortin) binds glucocorticoids and progesterone, with differing affinities. When circulating levels of steroid hormones exceed the binding capacity of their respective binding proteins, they can then bind nonspecifically, and with low affinity, to albumin, from which they can readily dissociate and enter target cells.207 The unbound and loosely albumin-bound steroids are generally believed to be the most biologically important fractions since the steroid is free to diffuse (or be actively transported) through the capillary wall and lipid plasma membrane bilayer. Extracellular binding proteins may modulate hormone response by regulating the amount of steroid available to the cell.207 The binding capacity of binding globulins has been shown to be influenced by endocrine status and other factors.203, 208
SHBG binds to a specific cell membrane receptor called sex hormone-binding globulin-receptor (SHBG-R) and activates adenylate cyclase, thus increasing intracellular cAMP.204, 206, 209, 210 Binding of SHBG to SHBG-R also transfers SHBG into the cell as a consequence of receptor-mediated endocytosis.209 The interaction of SHBG with SHBG-R was shown to be inhibited when steroids are bound to SHBG, suggesting that SHBG is an allosteric protein.209 However, if unliganded SHBG is allowed to bind to its receptor on intact cells, and an appropriate steroid hormone then is introduced, adenylate cyclase is activated and intracellular cAMP increases.208 SHBG-R inhibits the E2-induced growth of MCF-7 human breast cancer cells.210 Once the steroid hormone is in the cytoplasm, it is not yet clear whether a transport protein is required for movement of the hydrophobic steroid molecule through the aqueous cytoplasm to the receptor, regardless of whether the receptor is cytoplasmic or nuclear in location. The current model is that the steroid hormone diffuses freely in the cytoplasm.
(2) Intracellular localization of the steroid/nuclear receptors
Early attempts to isolate steroid hormone receptors led to a major controversy regarding their intracellular localization in the unliganded state. Reports published before 1984 suggested that prior to hormone treatment, steroid receptors in target tissues were located predominantly in the cytosolic fraction as large protein complexes of 300–400 kDa.211 Following hormone exposure, receptors were detected primarily in the nuclear fraction.212 It was thus initially proposed that unoccupied and untransformed receptors were located in the cytoplasm until ligand-binding, which caused their translocation into the nucleus.180 The reported presence of ER and PR in cytosol has been considered to be largely artifactual, the result of homogenization and centrifugation processes used to isolate the receptor protein.213 However, other studies demonstrate localization and even movement of ERα and ERβ between nuclear, mitochondrial, and cytoplasmic compartments.214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224, 225, 226, 227, 228, 229, 230, 231, 232
Although newly synthesized receptor proteins would be expected to contribute a small amount to cytoplasmic levels of receptor, most steroid/nuclear receptors in the unliganded state reside in the nucleus. The exceptions are the glucocorticoid and mineralocorticoid receptors, which in the unliganded state reside in the cytoplasm in association with hsp90, hsp70, and a variety of receptor-associated proteins.233 The hsp90 complex of proteins has chaperonin activity that facilitates hormone binding and subsequent proper folding of the GR.234 Upon activation by hormone-binding, and the release of hsp90 and the other GR-associated proteins, the hormone-receptor monomer is released from the complex, dimerizes, and translocates to the nucleus.235 In contrast to GR, immunohistochemical localization experiments showed that ERα,236 ERβ,6 PR,237 and AR238 are primarily nuclear in the absence of hormone treatment. All class II nuclear receptors are nuclear in the absence of ligand.12
The newly synthesized unliganded receptor is highly unstable and either moves into the nucleus, targeted by its nuclear localization signals, soon after synthesis, or associates with the hsp90 complex of cytoplasmic proteins.234 GR, MR, PR, ERα, and AR have been found in association with hsp90, although hormone-binding has not been shown to be required for translocation of these receptors to the nucleus except for GR and MR.239 Hsp90 is a general molecular chaperone involved in the folding of various proteins.240 The hsp90 dimer is thought to stabilize the receptor, protecting it from protease degradation, to block the DNA-binding domain and the nuclear localization sequence (NLS), and maintain the receptor in an inactive state until ligand-binding occurs (Fig. 5).241, 242 Hsp90 is also required for ligand binding by the steroid receptors. In addition to hsp90, unliganded steroid receptors extracted from animal tissues or mammalian cells showed that GR and PR are complexed with a number of other proteins including hsp70, FKB59, p60, p48 (Hip), and p23.243 These proteins are thought to be required for the assembly and maintenance of ligand-sensitive aporeceptor complexes.
Fig. 5 Schematic diagram of the mechanism of steroid hormone receptor action in a target cell. A target cell contains the steroid hormone receptor(s) required to respond to a given steroid hormone. A. Most steroid hormones that enter the cell travel in the bloodstream either in free state or loosely bound to serum albumins. Estrogens and androgens are bound to steroid hormone binding globulin (SHBG) with high affinity. The cell membrane contains a SHBG receptor linked to adenylate cyclase. Activation of adenylate cyclase by the SHBG receptor or peptide hormones such as LH generate cAMP which activates the protein kinase A (PKA) phosphorylation cascade. As described in the text, PKA can activate ERα and other steroid hormone receptors in the absence of ligand binding. Most receptors in the steroid/nuclear receptor superfamily reside in the nucleus in the newly synthesized or unliganded state. The exception is the glucocorticoid receptor (GR) which is complexed with the hsp90 chaperonin complex in the cytoplasm. Hsp90 and its accompanying proteins are thought to stabilize the receptor until hormone-binding occurs. Although receptors for progesterone, estrogens, and androgens have also been found in complexes with the primarily cytosolic hsps, the function of these associations is unclear. In the case of estrogens, progestins, and androgens, the hormones freely enter the nucleus and bind to their cognate receptor. Binding of hormone ligand to the receptor “activates” the receptor by inducing alterations in the conformation of the receptor protein. This allows the receptors to form homodimers and bind to specific hormone response elements (HREs, see Table 2 for specific sequences) in the DNA in the regulatory region of the target gene. B. Once bound to the HRE, the liganded steroid hormone receptor induces a DNA bend and, in the presence of agonist ligand, recruits coactivator proteins. The HRE-bound receptor also interacts with other DNA-bound transcription factors, as shown for the interaction of Sp1 with ER. Certain coactivator proteins have histone acetyltransferase (HAT) activity that acetylates lysine residues in histones H3 and H4. This results in a “loosening” of chromatin structure that facilitates the binding and transcriptional activity of RNA polymerase II at the transcription start site. Adapted from Naar AM, Beaurang PA, Robinson KM, Oliner JD, Avizonis D, Scheek S, Zwicker J, Kadonaga JT, Tjian R. Chromatin, TAFs, and a novel multiprotein coactivator are required for synergistic activation by Sp1 and SREBP-1a in vitro. Genes Dev 1998;12:3020-3031;244 Zwijsen RML, Buckle RS, Hijmans EM, Loomans CJM, Bernards R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D. Genes Dev 1998;12:3488-3498.245 C. In addition to genomic activity, recent studies have shown that many NR, as reviewed in the text, including ERα, are located in complexes of proteins in caveolae, including caveolin-1 (Cav-1) and c-src, in the plasma membrane.246 Binding of E2 to PM-associate ERα or to GPR30, a membrane ER, activates intracellular signaling cascades and cross-talks with EGFR and IGF-R. ERα and ERβ are also present in mitochondria,247 although their roles in this organelle remain to be defined.
The role of the hsp90 complex in ER function is not yet clear. Purification of ERα after chemical crosslinking in intact MCF-7 human breast cancer cells revealed one ERα monomer complexed with two molecules of hsp90 and one molecule of p59 (FKBP52), but no hsp70 or 40 kDa cyclophilin.248 Another study showed hsp90 was not required for ligand-dependent transcriptional activation by ERα.249 Although hsp70 was shown to be required for purified, recombinant ERα to bind EREs in vitro,250 other experiments imply that hsp70 targets inappropriately folds nuclear proteins251, 252 and is not required for ERα–ERE binding.253
(3) Entry of steroid/nuclear receptors into the nucleus
Steroid hormone receptors within living cells are dynamic.254 They shuttle between the cytoplasm and the nucleus. The hormone receptor enters the nucleus by two processes: passive diffusion through the “ever opened” central channel of the nuclear pore or active transport that is mediated by interaction of the NLSs on the receptor proteins with the NLS receptor–hsp90 complex.255 The NLS-steroid hormone receptor-NLS receptor-hsp90 complex binds to the nuclear pore complex via nucleoporins in an ATP-dependent process.256, 257 The receptor is then trapped by binding to intranuclear components.255 Steroid hormone receptor complexes have been demonstrated in association with nuclear membranes and with chromatin components including histones, nonhistone basic proteins, DNA, and ribonucleoproteins,258 and with nuclear matrix.259, 260
The TR, VDR, RAR, RXR, and other class II nuclear receptors do not form high molecular weight complexes, but are believed to enter the nucleus directly and become tightly associated with chromatin.202 However, recent experiments may cause rethinking of this model. The subcellular localization of human TRβ1 fused at its N-terminus to green fluorescent protein (GFP) was followed in living cells.261 In the absence of thyroid hormone T3, more GFP-TRβ1 was present in the cytoplasm, and when the cells were treated with T3, the GFP-TRβ1 was predominantly localized in the nucleus.261 Since GFP-TRβ1 bound T3 and DNA in a manner identical to wild type TRβ1 and since T3 treatment moved all of the GFP-TRβ1 into the cell nucleus, the authors concluded that their findings reflect the behavior of endogenous TR.261 With the exception of the v-erb A oncogene product and some of the orphan receptors that function constitutively, or are activated by growth factor-mediated phosphorylation, unliganded class II nuclear receptors (NR) are not transcriptionally active until liganded. This is because the class II NR including RAR, RXR, and TR are constitutively bound by corepressor proteins that silence transcription until the appropriate ligand is bound.262, 263, 264
(4) Ligand-dependent activation of steroid/nuclear receptors
The ligand-binding domain of the receptor may act as a repressor of receptor function since deletion of the LBD from the glucocorticoid and progesterone receptors causes constitutive gene activation.265, 266 Ligand-binding to the receptor stimulates dissociation of the receptor-hsp90 complex151 which facilitates conformational changes in the receptor (activation) that exposes the DNA-binding domain and promotes dimerization of the receptor. Binding of the hormone to the receptor may be only one of several factors that activates or transforms the receptor, enabling it to bind as a dimer to specific hormone response elements located adjacent to or sometimes at a distance from the transcription start site of the regulated gene.
It is important to note that Type II nuclear receptors, e.g., TR, VDR, RAR, RXR, and the orphan receptors, do not interact with hsp90. These receptors are bound to DNA in the absence of ligand and are associated with corepressor proteins, e.g., NCoR and SMRT.262, 263, 264 Corepressor proteins NCoR and SMRT are associated with a complex of proteins that have histone deacetylase activity that are believed to repress gene expression by maintaining chromatin in a more condensed conformation.262, 263
Hormone-dependent phosphorylation of steroid hormone receptors may play an important role in binding of the receptor to its specific response element on the gene and subsequent activation of transcription. PR, GR, ERα, and VDR are all phosphorylated after binding to their respective ligands.267
(5) Ligand-independent activation of steroid hormone receptors
Although steroid and nuclear receptors are classically activated by ligand binding and are subsequently phosphorylated, a second mode of activation in the absence of ligand has been detected for certain receptors. Phosphorylation of certain steroid hormone/nuclear receptors in response to cell-membrane activated signaling cascades activates the receptor in the absence of the cognate ligand.268, 269 Examples of peptide hormones and growth factors that activate steroid receptors by triggering intracellular phosphorylation cascades include dopamine, epidermal growth factor (EGF), insulin, and insulin-like growth factor (IGF).269 Activation of β2-adrenergic receptors by the anti-inflammatory, anti-asthmatic drugs salbutamol and salmeterol was recently demonstrated to activate GR, resulting in nuclear translocation and transactivation of a GRE-driven reporter gene.270 Signal transduction took place through activation of a cAMP-cascade mediated by the cell membrane β2-adrenergic receptor. Likewise activation of the protein kinase A (PKA) pathway stimulated transcription by the MR in a ligand-independent manner.271 Interestingly, PR appears to be refractory to activation induced by phosphorylation cascades.269
ChIP studies have demonstrated that ERα and ERβ are associated with some gene promoters in various cell types in the absence of E2 treatment.196, 272, 273, 274, 275, 276, 277 More recently, proteomic studies of proteins associated with unliganded ERα identified the deleted breast cancer-1 gene product DBC-1 (KIAA1967) to be a direct ligand-independent binding partner of ERα in the nucleus of MCF-7 human breast cancer cells.278 Functional analyses revealed that DBC-1 was a principal determinant of unliganded ER protein levels and survival activity in human breast cancer cells.
(6) Binding of steroid/nuclear receptors to HREs and DNA bending
The sequences specifically recognized by the various steroid/nuclear receptors were described in Table 2. When steroid/nuclear receptors bind their cognate HRE, the DNA is deformed, causing a bend in the DNA. DNA bending appears to be important in cellular processes mediated by multiprotein complexes, including transcription in both prokaryotes,279 and eukaryotes.280, 281, 282, 283 DNA bending is thought to facilitate interactions between components of the transcription complex bound to different sites and to promote DNA looping to allow single proteins to contact multiple DNA elements. ERα binding to an ERE results in a bend of the DNA toward the major groove.284, 285 Other steroid receptors including GR286 and PR287 also induce DNA bending. Similarly, Class II NR including TR and RXR induce DNA bending.288 More recently, NR-mediated transcriptional activation has been demonstrated to involve “chromatin kissing” in which E2-induced ERα activation induces rapid interchromosomal interactions among subsets of ERα-bound transcription units, with a dramatic reorganization of nuclear territories requiring nuclear actin/myosin-I transport machinery, dynein light chain 1 (DLC1), histone lysine demethylase (LSD1), and a specific subset of transcriptional coactivators and chromatin remodeling complexes.289
That the topology of DNA is important for steroid hormone receptor recognition of HREs is reinforced by the observation that the nonhistone chromosomal protein HMG-1, which recognizes irregular DNA structure, enhances the binding of PR, ERα, AR, and GR to their respective response elements in vitro, but has no effect on the binding of TR, RAR, RXR, or VDR to their target sequences.287, 288, 290 Moreover, co-expression of HMG-1 or HMG-2 increased PR-mediated transcription in transiently transfected mammalian cells by seven to 10-fold without altering the basal promoter activity of target reporter genes.291
(7) Direct regulation of gene transcription by steroid hormone receptors
Initiation of transcription is a complex event occurring through the cooperative interaction of multiple factors at the target gene promoter (Fig. 4). When bound to the specific HRE on the DNA, the hormone-receptor complex interacts with basal transcription factors and with other proteins to stabilize basal transcription factor binding and promote the assembly of the transcription initiation complex. Once the transcription initiation complex is in place, the enzyme RNA polymerase II is recruited to the transcription start site where it begins transcribing the DNA sequence into mRNA.
(a) Interaction of steroid/nuclear receptors with basal transcription factors
In order for RNA polymerase II to initiate transcription, basal transcription factors TFIIA, TFIIB, TFIID, TFIIE, TRIIF, and TFIIH must assemble on the core promoter and phosphorylate the CTD of RNA pol II.292, 293, 294, 295, 296, 297, 298 TFIID consists of the TATA box binding protein (TBP) and at least eight tightly associated factors (TAFs) of 18–250 kD.299 Since the amount of RNA polymerase II is limited in the nucleus, genes must compete for it by assembling an appropriate set of cis-elements, HREs, and binding sites for sequence-specific transcription factors, such as AP-1,300 CREB,301 TFIIB, and Sp1.302 The net result is the synthesis of new messenger RNAs (mRNAs) which move into the cytoplasm and are translated into new proteins that may alter cell function (acting in an intracrine manner) or may be modified further and secreted by the cell to act as endocrine, autocrine, or paracrine factors.
Steroid receptors interact with basal transcription factors TFIIB, TBP, and various TAFs of TFIID.303 ERα and PR interact directly with TFIIB.304 ERα interacts directly with TBP using both AF-1 and AF-2 as interaction surfaces.305 ERα interacts with human TFIID component TAFII30 through the LBD in a ligand-independent manner. The functional relevance of ERα–hTAFII30 interaction is indicated by the inhibition of ERα-mediated transactivation by monoclonal antibodies to hTAFII30.306 Studies indicate that steroid and nuclear receptors use different domains for interaction with basal transcription factors.303
(b) Interaction of steroid/nuclear receptors with coactivators (coregulators that increase transcriptional activity)
Steroid hormone receptors interact with multiple proteins both when bound to DNA or in solution in vitro. Early experiments showed that overexpression of one type of steroid hormone receptor could inhibit, or “squelch” the activation of transcription mediated by a different steroid hormone receptor, hinting that steroid receptors compete for limited amounts of a factor(s) required for transcription.307 Over the past 4 years, at least 15 different coactivators have been identified (Table 3). These proteins have also been termed receptor interacting proteins (RIPs) and RAPs (receptor associated proteins); however, not all RIPs are coactivators. By definition, coactivators are considered to interact directly with the steroid/nuclear receptor and enhance transcription.308, 309, 310 Thus, the first coactivators for steroid receptors to be discovered, the SWI/SNF proteins,311 may not be strictly coactivators, but instead may serve as bridging factors that interact between coactivators and basal transcription factors. Several coactivators have “general” transcriptional activator function, since they enhance transcription by different types of transcription factors, including steroid receptors.
It is clear that steroid receptors can interact with a number of different coactivators. Some of these coactivators, i.e., SRC-1, CBP, and TIF2, have been demonstrated to play a critical role in ligand-activated transcription.309, 310 Coactivator proteins contain one or more copies of a NR binding motif, also called the NR box, consisting of the aa = LXXLL, which physically interacts with the steroid/nuclear receptors. Steroid receptors show different affinities for the various coactivators312 and use different amino acids to contact the coactivators.313 Coactivators SRC-1, ACTF, and CREB/p300/CBP have histone acetyltransferase (HAT) activity314, 315 providing the mechanism for enhanced transcription.263 Many transcriptional regulatory proteins have intrinsic HAT activity.316, 317 There is a complex ‘histone code’ regulating gene transcription.318, 319 HATs acetylate lysine residues on the N-terminal tails of histones H3 and H4 in chromatin, resulting in a weaker association of histones with DNA, thus altering nucleosomal conformation and stability in a manner that facilitates transcriptional activation by RNA polymerase II.320 SRC-1, CBP/p300, CREB, and other coactivators are believed to form a ternary complex with liganded steroid receptors to increase the rate of hormone-responsive gene transcription.321 Thus, several HAT activities may be tethered to hormone-activated receptors on the promoter, yielding synergistic transactivation. It is important to note that not all genes are affected by histone acetylation, and steroid and nuclear receptors show different affinities of interaction with coactivators.322
The current model suggests that different target cells express different levels of coactivators and corepressors which, along with the amount of receptor protein and ligand, allows fine-tuning of target gene transcription in response to steroid hormones.323 Northern blot analysis confirmed the idea that different rat tissues324 and cell lines325 express different amounts of the mRNA for p300, CBP, SRC-1, RIP140, SMRT, and NCoR.
Table 3. Examples of coactivator proteins that interact with steroid hormone receptors. These proteins have been identified in yeast and mammalian two hybrid screening, during purification, in immunoprecipitation assays, and by cross-linking studies. Some, but not all, have been shown to stimulate transcription in cell systems309, 310, 326
| Names | Interacts with SR/NR | Effect of SR ligand on direct interaction | Effect of co-expression on transcription | Other information | References |
| ACTR/ SRC-3/ RAC3/ p/CIP/ AIB1 | ERα RXRα TR | Requires agonist ligand | Stimulates E2-dependent transcription | · AIB1 expression elevated in human breast and ovarian cancers327 |
|
| ARA70 (ELE1α)
· truncated variant ELE1β | AR ERα GR PPAR | Androgens and antiandrogens promote AR-ARA70 interaction – also genistein and RU486330
| Stimulates AR transcription with DHT or E2 | · No intrinsic HAT activity · Interacts with p/CAF that has HAT activity · Interacts with TFIIB · Highest ELE1α and ERE1β expression in testis | |
| CBP/p300/p270 | AR, ERα, GR, PPARg, RAR, RXR, TR, HNF4 | Requires agonist ligand, except for AR | · Stimulates transcription | · Intrinsic histone acetyltransferase activity · Interacts with SRC-1 · CBP/p300 is also a cofactor for AP-1, c-myb, STAT1, E1A, p53, and Myo-D · Helps recruits RNA Pol. II holoenzyme to the promoter · CBP interacts with TFIIB | 334, 335, 336, 337, 338, 339, 340, 341, 342, 343
|
| RIP140
| ERα TR, RXR, PPARα PPARγ | Requires agonist ligand; antagonists tamoxifen and ICI 164,384 block interaction with ERα | · Stimulates ERα-induced transcription · Inhibits PPAR and RXR activities | · Identical to ERAP140344 | |
| SRC-1/NoA-1 | AR, ERα, ERβ1, PR, GR, TR, RARβ, RXRα, PPARγ, HNF4
| · Requires agonist ligand · Antagonist inhibits interaction | Stimulates PR, GR, and ERα-induced transcription | · Intrinsic HAT activity · Interacts with p300/CBP, TBP, and TFIIB · Targeted gene disruption resulted in viable and fertile mice, but with decreased growth and development of target organs, e.g., uterus, testis, and mammary gland, and a compensatory increased expression of TIF2
| 349, 350, 351, 338, 343, 352, 353
|
| SWI/SNF | ER, GR, RAR, HNF-4 |
| Stimulates transcription | · Chromatin remodeling complex in yeast with human homologues
| |
| TIF1α | ERα, ERβ, PR, RXR, VDR | Requires agonist ligand | Stimulates transcription – requires agonist ligand for ERα, but for ERb 4-OHT acts as an agonist | · Interacts with heterochromatin proteins, including hSNF2β of the SWI/SNF complex and TIF1β |
|
| TIF2/GRIP1/ | ERα, GR, AR, PR, RAR, RXR, TR, VDR, HNF4 | Requires agonist ligand | Stimulates ERα, AR, PR, TR, RAR, and RXR but not GR, or VDR | · 40% sequence homology to SRC-1 | 343, 363, 364, 365, 366, 367, 368
|
(c) Microarrays have identified hormone-regulated genes in cells and animal tissues
A major advance in the past 8 years, since the initial publication of this chapter, has been the development of microarrays of human, mouse, and rat gene and the identification of genes regulated by steroid hormone action using these assays. Time course assays have identified primary and secondary estrogen target genes regulated by ERα in MCF-7 breast cancer cells369, 370, 371, 372, 373, 374, 375, 376 and by ERα and ERβ in U2OS human osteoblast cells.156, 200, 373, 374, 377, 378, 379
(8) Indirect regulation of gene transcription by interaction of steroid/nuclear receptors with other transcription factors
Steroid receptors interact directly with different transcription factors and alter target gene transcription without the steroid receptor interacting directly with DNA. The best studied example of this “transcriptional cross-talk” is the interaction of the AP-1 transcription factor with GR.380 Depending on the cell type381 and the composition of the AP-1 complex, GR synergizes with AP-1 (Jun-Jun homodimer) or suppresses the Fos-Jun heterodimer.382In vitro assays demonstrate that ERα interacts with the Fos-Jun AP1 heterodimer and that raloxifene and tamoxifen are more potent agonists than E2 at AP-1 sites.383
The antiestrogen tamoxifen activates ERα-mediated induction of promoters regulated by AP-1 sites including the human collagenase gene.384 This contrasts with the inability of tamoxifen to activate transcription from promoters bearing classical EREs. Tamoxifen agonism at AP-1 sites is cell type specific, i.e., occurring in cell lines of uterine, but not of breast, origin. The DBD of ERα is required for tamoxifen activation at AP-1 sites. Conversely, the AP-1 components cJun and cFos inhibited E2-dependent ERα-stimulated reporter gene activity in transiently transfected MCF-7 or CV-1 cells transfected with ERα.385 DNA binding experiments revealed that ERα-ERE binding was inhibited by the cJun protein and that ERα inhibited cJun-DNA binding.385
E2 regulates gene transcription in opposite ways through ERα and ERβ from an AP1 site: with ERα, E2 activated transcription, whereas with ERβ, E2 inhibited transcription.386 Moreover, in contrast to ERα, the antiestrogens tamoxifen, raloxifene, and ICI 164,384 were potent transcriptional activators with ERβ at an AP1 site. Thus, the two ERs differ in how they respond to ligand and response element, suggesting that ERα and ERβ may play different roles in gene regulation.386
Another transcription factor with which ERα interacts directly to activate gene transcription is Sp1.387 A number of estrogen-responsive genes are activated by ERα-Sp1 interaction including cathepsin D,388 RARα,389 VEGF,390 and c-fos.391 ERα and Sp1 physically interact in a manner that requires both the N-terminal and C-terminal regions of ERα.392 The interaction of the Sp1 and ERα and the resulting increase in Sp1-DNA binding is observed in the presence or absence of E2, whereas transactivation of promoter-reporter constructs is E2-dependent. These results indicate that transcriptional activation requires more than ERα-Sp1 interaction and increased DNA binding. A likely interpretation is that coactivators are required.
NF-kB also interacts directly with ERα, ERβ, and GR and inhibition of NF-kB has been demonstrated to increase ERβ and GR transcriptional activity in cells.393
(9) Repression of target gene transcription by steroid/nuclear receptors
Binding of the hormone-receptor complex can also repress transcription of target genes, although the precise mechanisms are not as well elucidated as transcriptional activation. One mechanism by which a steroid receptor represses gene transcription is by binding to the same, or an overlapping, DNA binding site as that used by a different activator protein, thus competitively blocking activator binding to DNA. One example of this mechanism is the mutual inhibition by GR and AP-1 on the proliferin gene promoter that contains a composite GRE-AP-1 binding site.382
Competition for limiting amounts of coactivators is another mechanism by which steroid hormones inhibit gene expression. The mutual inhibition of AP-1 and steroid receptor transactivation, including AR, ERα, GR, and PR,380 is that they compete for limiting amounts of the coactivators CBP/p300 in the nucleus.394
Likewise, GR and ERα also block NF-kB-mediated transactivation.380 GR and other steroid receptors interact directly with NF-kB, and NF-kB inhibits GR, ERα, and PR-mediated transcription. GR and NF-kB compete for limited amounts of the coactivators CBP and SRC-1.395
Another mechanism for repression by steroid receptors is by binding to the HRE and recruiting corepressor proteins that quench the activity of activators bound to the promoter. The corepressors N-CoR and SMRT263 were first identified by their binding to unliganded TR, RAR, and RXR (Table 4). Tethering of these corepressors to the DNA by their interaction with the DNA-bound TR, RAR, or RXR recruits other components of the corepressor complex that mediate the actual events of transcriptional silencing.396 Binding of TR or RAR ligands causes dissociation of NCoR, but only when the receptors are bound to DNA. Unlike coactivator complexes that have endogenous HAT activity, corepressors do not appear to have enzymatic activity. Corepressors NCoR and SMRT appear to function by recruiting histone deacetylase (HDAC)-containing multiprotein repression complexes to the promoter.263 Deacetylation of chromatin helps maintain a condensed state of nucleosomal structure that blocks the binding of components of the RNA polymerase II transcription initiation complex.
Table 4. Examples of corepressor proteins that interact with steroid/nuclear receptors. These proteins have been identified in yeast and mammalian two hybrid screening, during purification, in immunoprecipitation assays, and by cross-linking studies.396, 326 In general, interaction of steroid and nuclear receptors with these proteins represses gene transcription in cell systems
| Name | Interacts with | Effect of SR ligand | Effect of co-expression on transcription | Other information | References |
| NCoR | TR, RAR, RXR, PR, ERα
| 4-OHT-ERα | · Increased transcriptional activity of unliganded PR397 | · NCoR expression reduced in TAM-resistant breast cancer cells398 |
|
| SMRT | TR, RXR, RAR, ERα | Unliganded class II NR; antagonist-liganded PR | · Overexpression strongly reduced basal and 4-OHT-stimulated gene expression with no effect on E2 activity400 | · HDAC1 is associated with SMRT
|
(10) What is the mechanism of repression of target gene transcription achieved by hormone antagonists?
A variety of mechanisms is likely to be involved in hormone antagonist action. First, the antagonist activity of an antihormone may depend on the cell or tissue type. For instance, antiestrogen-liganded ERα binds EREs with high affinity,175 but in certain cell types, e.g., MCF-7 human breast cancer cells, transcription is not activated,402 whereas in endometrial cells, tamoxifen stimulates transcription.403 The proposed mechanism for these observations involves the inability of antiestrogen-liganded ERα to interact with co-activator proteins, e.g., SRC-1,404 TIF1α405 that have HAT activity that “loosens” chromatin structure to allow assembly of the transcription initiation complex. Another possibility is that antiestrogen-liganded ERα may recruit corepressor proteins to the promoter, thus inhibiting chromatin changes that promote a “loosening” of nucleosomal structure.323, 406 Indeed, NCoR interacts with the tamoxifen metabolite 4-hydroxytamoxifen (4-OHT)-liganded ER.407 Breast cancer cells that are resistant to tamoxifen-inhibition of cell proliferation show reduced NCoR levels compared to tamoxifen-sensitive cells, suggesting a mechanism whereby cells become resistant to tamoxifen.407 Similarly, NCoR and SMRT repress the agonist activity of antiprogestin RU486-liganded PR or tamoxifen-liganded ERα.408
Actions of NR in the plasma membrane
While most of the effects of steroid hormones are mediated through their interaction with their cognate receptors and subsequent effects on target gene transcription, certain rapid effects of steroid hormones are incompatible with a transcriptional mechanism. Specific binding sites for androgen, estrogen, progesterone, glucocorticoid, and vitamin D receptors have been reported in the plasma membrane of various target cell types.409, 410, 411, 412, 413, 414, 415, 416, 417, 418, 419, 420, 421, 422, 423, 424, 425, 426, 427, 428, 429, 430, 431, 432, 433, 434, 435, 436, 437, 438, 439, 440, 441, 442, 443, 444
Extranuclear activities of GR and MR
GR can bind to cytoskeletal structures445 and glucocorticoids stimulate the rapid-onset of polymerization of actin in a non-genomic manner that involves decreased intracellular cAMP.446 GR has also been found in mitochondria in hepatic cells where it may activate mitochondrial gene expression,447 and in leukemic cells, sensitivity to glucocorticoids-induced apoptosis appears to be regulated by translocation of GR into mitochondria.448 Rapid stress-induced changes in male amphibian reproductive behavior appear to be mediated by corticosteroid receptors within neuronal membranes that may be identical to CBP.447 MRs are also present in limbic neuronal plasma membranes and have been suggested to be important in mediating the initial stress response.449
Membrane PR
One of the membrane steroid receptors that have been well-characterized to date is the membrane progesterone receptor that was initially characterized in amphibian oocytes, fish, and in spermatids.436, 437, 438, 450, 451, 452, 453, 454 There are three subtypes of membrane PR: α, β, and γ, and each has seven transmembrane domains and is a GPCR that is linked to inhibition of adenylate cyclase.436, 437, 455, 456 The bioactivity and roles of mPRs remains controversial since mPRs did not bind progesterone, activate ERK1/2 (MAPK), p38 MAPK, or change Ca+2 signaling in MDA-MB-231 cells.457 In Xenopus, the classical intracellular PR also mediates these rapid, non-genomic responses.458 A novel progesterone receptor that mediates rapid changes in Ca+2 conductance has been demonstrated in human sperm plasma membrane.459 A high affinity progesterone-binding membrane protein of 200 kDa was described in pig liver.460 The single-transmembrane protein PGMRC1 (MW 26–28 kDa) was first purified from pig livers and has subsequently been identified in a variety of other tissues.456 PGMRC1 can bind to other molecules including heme, cholesterol metabolites, and proteins.456 The mechanism by which the synthetic progestin R5020 upregulates c-ErbB2 and c-ErbB3 levels in PR-negative T47D-YB human breast cancer cells involves membrane progesterone receptor and studies have demonstrated that the stimulatory effect of progestins on breast cancer cell proliferation is mediated by activation of MAPK in a transcription-independent manner.461, 462, 463, 464, 465, 466 In addition to membrane progesterone receptor action, metabolites of progesterone and deoxycorticosterone act as positive allosteric modulators of the gamma-aminobutyric acid (GABA) A receptor complex in the cortex of rat brain.467 This means that these hormones bind to an effector site and not the GABA binding site and increase the affinity of GABA binding to the (GABA)A receptor. There is clearly a need for further research to elucidate the roles of membrane PRs in mediating progestin activity in various tissues.
Nongenomic estrogen action
E2 has “nongenomic, extranuclear, or membrane-initiated” effects, i.e., independent of ER-mediated transcription, that occur within minutes after E2 administration.413 Nongenomic estrogen action has been reviewed.223, 421, 468
The best characterized system for the study of endogenous membrane ERα is the rat GH3/B6 pituitary tumor cell line in which ~10% of ERα was localized in the plasma membrane.415, 419, 469, 470, 471, 472, 473 Exposure of GH3 pituitary cells to E2 elicited a rapid (within 5 minute) release of prolactin (PRL) in a manner that cannot be accounted for by genomic effects of estradiol mediated through nuclear ER. Additionally, binding sites for estrogen with different biochemical properties from the classical nuclear receptor have been reported in the endoplasmic reticulum of uterine tissues.474
Estradiol appears to have both genomic and nongenomic effects in the brain.475 The cardiovascular protective effects of estradiol are thought to be mediated at least in part by nongenomic ER and involve increased intracellular cAMP,476, 477 inhibition of Ca2+ influx,476, 478 and stimulation of NO release.413, 479, 480, 481, 482, 483 Estradiol has been shown to exert direct beneficial effects on human oocytes during in vitro maturation and these effects are at least partly due to steroid action at the oocyte surface.484
A comparison of nuclear and membrane localization of recombinant ERα and ERβ in transfected CHO cells (considered to be ER null) showed that both ERα and ERβ were expressed predominantly in the nucleus with ~5% of each ER subtype located in the cell membrane.485 E2 treatment of these transfected CHO cells activated Gαq and Gαs proteins in the membrane and rapidly stimulated corresponding inositol phosphate production and adenylate cyclase activity.485 Interestingly, membrane ERα and ERβ showed a distinct difference in their activities: c-Jun N-terminal kinase activity was stimulated by E2 in ERβ-expressing CHO, but was inhibited in CHO-ERα cells.485 Further studies from this research group have demonstrated that plasma membrane receptors work as dimers,486 that ERα interacts with caveolin-1 (Cav-1) which interacts with G proteins, Src, Grb7, Raf, Ras, MEK, and the EGFR at the plasma membrane of MCF-7 cells, and triggers the release of HB-EGF into the culture medium that, in turn, activates the EGFR and downstream signaling leading to activation of ERK1/2 (MAPK) and PI3K/AKT signaling cascades418, 421 that, in turn, phosphorylate ERα.223 Palmitoylation of ERα is important for its membrane localization.425, 487
In endothelial cells (EC), E2 rapidly increased intracellular cAMP,476 inhibited Ca2+ influx,478 stimulated Ca2+ release from internal stores,488 and stimulated nitric oxide (NO) production.489 In MCF-7 cells, E2 rapidly increased PIP2-phospholipase C activity,490 mobilized intracellular Ca2+, and activated the MAPK488 and PI3K/AKT pathways.491 Immunohistochemical techniques have visualized membrane ERα in pituitary cells.415 Since ERα lacks a transmembrane domain, how it gets to the plasma membrane (PM) has been controversial, but it appears to involve palmitoylation.492 In MCF-7 cells, the adaptor protein Shc shuttles ERα from the nucleus to the PM where ERα interacts with the IGF-1 receptor (IGF-1R).493 Similarly, another scaffold protein, called MNAR (modulator of nongenomic activity of ER) was reported to mediate ERα-cSrc interaction and MAPK signaling.494, 495, 496 A role for a membrane caveolae-localized ERα variant (ERα46) in rapid NO release via PI3K/Akt activated endothelial nitric oxide synthase (eNOS) in EC has been reported.416, 480, 497, 498
Direct interactions between plasma membrane-associated ERα and Gαi have been implicated in eNOS activation and NO production in COS-7 cells transfected with ERα and specific Gαi proteins.2 E2 stimulated the direct interaction of ERα with Gαi2via ERα-striatin interaction in MCF-7 and other cells.3 Likewise, E2 rapidly activates the G protein-coupled signal cascades in MCF-7 and other breast cancer cell lines.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
Estradiol was reported to activate the angiotensin II receptor AT1 in ER-negative SKBr3 breast cancer cells.499
GPR30, a PM protein, was reported to bind E2 with high affinity (Kd = 2.7 nM) resulting in activation of adenylate cyclase.224, 439, 500, 501, 502 Importantly, tamoxifen and ICI 182,780 also bind GPR30 with high affinity and mimic the effects of E2.439 Phytoestrogens, e.g., genistein, and endocrine disruptors, e.g., bisphenol A and nonylphenol, also bound and activated GPR30 with an affinity comparable to E2.441 Overexpression of GFP-tagged GPR30 in COS7 cells revealed the GFP-GPR30 in the plasma membrane and Golgi and endogenous GPR30 had a similar distribution in MCF-7, SKBr, and MDA-MB-231 breast cancer cell.224 Overexpression of FLAG-GPR30 in HeLa cells revealed that physiological concentrations (10 nM–1 μM) of E2 stimulated FLAG-GPR30 translocation from the plasma membrane to the cytoplasm and that intracellular Ca2+ was elevated within several seconds after the addition of E2 in cells expressing FLAG-GPR30.502 Although the role of GPR30 in MCF-7 and SKBr3 breast cancer cells has been questioned,421 it appears likely that GPR30 may be a novel membrane estrogen receptor in a cell type-specific manner, e.g., thyroid,503 endometrial,504 ovarian,505 and breast506, 507 cancer cells. Indeed, GPR30 expression in primary human breast tumors was recently demonstrated to correlate with Her2/neu expression and metastases, i.e., the opposite of ERαexpression.506, 507
MECHANISMS FOR ASSURING TISSUE-SPECIFICITY OF GENE EXPRESSION
Diversity of tissue responses to steroid hormone action, despite conservation of structure and function, is achieved through a variety of mechanisms. Some receptors are expressed in only a limited number of cell types, e.g., the gonadal steroid receptors, while others are found in a large number of cell types, e.g., the glucocorticoid and thyroid hormone receptors. Other tissue-specific gene regulatory proteins (transcription factors, coactivators, and corepressors) are involved in the modulation of gene transcription by steroid/nuclear receptors. Cell-specific post-translational modification of receptors is another mechanism to assure tissue-diversity of hormone responses.269 Multiple receptor isoforms for hormones may also account for tissue-specific gene expression.508 Receptor isoforms have been shown to arise through alternative splicing of mRNA from a single gene (PR and AR), from multiple genes (ER), or a combination of both (TR).39
Interactions with other transcription factors, coactivators, and corepressors, appear to play a significant role in determining specificity. Thus, even though receptors for several different hormones, i.e., AR, GR, MR, and PR, can bind a common HRE sequence, they may exhibit different interactions with other DNA-bound factors and coactivators, hence achieving different modulation of target gene expression.509
REGULATION OF RECEPTOR NUMBERS
The half-life of steroid hormone receptors ranges from 2–4 h for ERα510, 511 and 4 h for AR,512 to 7–10 h for PR513 and 19 h for GR.514 The relatively long half-life of the steroid hormone receptors strongly suggests that the receptor proteins are recycled before eventual degradation.515
Steroid hormones generally autoregulate their receptor levels.516 Desensitization or down-regulation of receptor numbers, measured by decreased ligand binding capacity, occurs in response to exposure to high levels of ligand, and involves the reduction in receptor mRNA levels, thus decreasing the number of available receptors. The receptor gene may be negatively regulated by the hormonal ligand itself via its receptor protein interacting with specific HREs in the gene.517 Up-regulation or self-priming may occur in an analogous fashion. Additionally, steroid hormones can regulate receptor levels for other hormones, e.g., E2 increases PR levels in estrogen-responsive tissues.518 Progesterone, in return, can not only down-regulate its own receptors, but also ERα519 and ERβ.520 This increase or decrease in receptor levels in homologous or heterologous regulation can be due to alterations in receptor gene transcription and/or decay rates for receptor mRNA and/or protein.Binding of the cytosolic GR complex to very long 3'-untranslated regions of its receptor mRNA has been reported to cause premature degradation.520
TARGETED GENE DISRUPTION (GENE "KNOCK-OUT") MOUSE STUDIES
Steroid and nuclear hormone receptors have been detected in virtually every major organ and tissue in the mammalian body, including the brain. Much new information on the location and function of the steroid hormone receptors has been derived from recently developed techniques that allow manipulation of a specific mouse gene in vitro to generate targeted gene disruption of the gene for a given receptor, creating homozygous “gene knockout” mice. The technique disrupts the linear gene by inserting an antibiotic resistance gene, e.g., neomycin, into one coding region (one exon) of the gene. The mutant DNA is inserted into genomic DNA by homologous recombination in mouse embryonic stem cells to generate transgenic mice. Thus, the mRNA for the targeted gene is truncated or nonsensical. This technique has resulted in ERα, ERβ, MR, PR, and GR, "knockout" mice.521
(1) ERα knockout mice (ERKO)
To the surprise of the investigators and others, loss of ERα expression was not lethal and had no effects on the ratio of male:female mice born.522 ERKO mice survived to adulthood and developed grossly normal external genitalia, but both sexes were infertile.523 Females have hypoplastic uteri and hyperemic ovaries with no corpora lutea.307 Serum levels of estradiol in the ERKO females are more than 10-fold higher than those in the wild type, consistent with a syndrome of hormone insensitivity.524, 525, 526 ERKO females have 10-fold higher circulating E2 and elevated LH, but not FSH.527 Ovarian histology is abnormal.528 Mammary glands of adult ERKO female mice lack branching and terminal end bud formation.529 Disruption of ERα signaling in ERKO mice leads to an obese phenotype.530, 531 Maternal behavior as measured by retrieving of pups was reduced.525 In some cases, pups were killed by the ERKO females, which was not seen in wild-type animals.527 Aggression toward other females was increased and female-typical lordosis behavior was reduced.527
Adult ERKO males exhibit a number of alterations in reproductive tract histology including atrophied, degenerated seminiferous tubules, and diluted, infertile sperm.532 The mice exhibit decreased sperm counts and significantly lower testicular weight than wild-type (wt) males.527, 528, 529 The reproductive capacity of sperm from ERKO males is significantly compromised in in vitro fertilization experiments. ERKO males appear to have normal mounting behavior toward wt females, but exhibit an almost complete lack of intromission and ejaculation. ERKO males are consistently less aggressive than wt mice.533 These findings indicate that ERα gene expression during development plays a major role in the organization of male-typical aggressive and emotional behaviors in addition to simple sexual behaviors.533, 534, 535, 536, 537
E2 protected both wild-type and ERKO female mice in response to carotid arterial injury, indicating that ERα may not be required for the protective actions of E2 in the vascular system.538 The rapid effects of E2 on vascular tissue, e.g., rapid changes in vasomotor tone, available nitric oxide, and the resting potential of smooth muscle cells, have been demonstrated to be mediated by a membrane form of ER.421
There has been one reported human case with an ERα mutation.539 The patient, a male, exhibited severe osteoporosis and insufficient closure of the epiphyseal growth plates.
(2) ERβ knockout (BERKO)
Mice lacking ERβ develop normally and are indistinguishable grossly and histologically from their litter mates.524 Breeding experiments with young, sexually mature females show that they are fertile and exhibit normal sexual behavior, but have fewer and smaller litters than wild-type mice. Superovulation experiments indicate that this reduction in fertility is the result of reduced ovarian efficiency. The mutant females have normal mammary gland development and normal lactation. Adult male mice show no overt abnormalities and reproduce normally. Older mutant males display signs of prostate and bladder hyperplasia. The investigators concluded that ERβ is essential for normal ovulation efficiency but is not essential for female or male sexual differentiation, fertility, or lactation.524 BERKO mouse mammary glands showed abnormal epithelial growth, overexpression of Ki67 and severe cystic breast disease as the mice aged.540 BERKO females showed increased anxiety541 and learning deficits.542 Both ERα and ERβ may protect against colorectal cancer in a mouse model.543
(3) PR knockout mice (PRKO)
Mice carrying a null mutation of the progesterone receptor gene exhibit several reproductive abnormalities, including anovulation, attenuated lordotic behavior, uterine hyperplasia, and lack of mammary gland development.544 There were no effects on the viability or sexual differentiation of homozygous PR gene disrupted mice.544 The females homozygous for PR disruption were completely infertile while males exhibited no apparent effects on fertility. Serum LH levels in PRKO mice were found to be elevated by approximately 2-fold over basal (metestrus) values in wild-type mice.545 By contrast, basal FSH levels were not different in PRKO and wild-type mice. Basal levels of E2 and progesterone in serum were likewise similar in the two groups, as were hypothalamic LHRH concentrations. Basal PRL levels were slightly higher in PRKO versus wild-type mice. These results confirm the essential role of progesterone receptors in the regulation of hypothalamic and/or pituitary processes that govern gonadotropin secretion.545
(4) GR knockout
Most of the mice homozygous for disruption of GR die shortly after birth due to severe lung atelectasis.546 Additional defects were found in the adrenals, liver, brain, bone marrow, and thymus as well as in the feedback-regulation of the HPA-axis.547 However, disruption of the ability of GR to dimerize is not lethal.548
(5) MR knockout
MR−/− mice, obtained by targeted gene disruption, died between days 8–13 after birth after exhibiting signs of pseudohypoaldosteronism and the pups died from dehydration by renal sodium and water loss.549 The MR−/− mice showed severe dehydration, hyperkalemia, hyponatremia, and high plasma levels of renin, angiotensin II, and aldosterone.550 The MR knockout mice showed significant increases in the expression level of several renal angiotensin system components: renin, angiotensinogen, angiotensin II receptor (AT1), but no alteration in angiotensin-I converting enzyme was detected in the kidney.550
(6) Androgen receptor insensitivity
A natural deficiency of androgen receptor occurs in the Tfm mouse551 that has an androgen receptor mutation that results in androgen insensitivity syndrome (AIS) that is an X-linked inherited disease.521 Various mutations in the AR in humans have been shown to cause AIS.552 Tfm mice (the males) exhibit complete infertility.521
Male androgen receptor knockout (ARKO) mice are phenotypically female with an 80% reduction in testes size, and serum testosterone concentrations are lower than in wild-type (wt) mice.553 The male ARKO mice have reduced spermatogenesis and cancellous bone volumes and the female ARKO have reduced fertility.553 The mice also have specific skeletal muscle defects.554 Interestingly, none of the male or female ARKO mice exposed to N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN) developed bladder cancer, whereas dihydrotestosterone (DHT) treatment of castrated ARKO or wt mice increased bladder cancer incidence by 25 and 50%, thus implicating roles for androgens and AR in the development of bladder cancer.555 Creation of transgenic mice with conditional knockout of AR only in prostate epithelia (pes-ARKO) revealed that these mice lacked external phenotypic differences seen in the ARKO mice, but that they showed increased prostate epithelial cell proliferation.556
METHODS FOR THE MEASUREMENT OF RECEPTORS IN TISSUES
Steroid hormone receptors are present in relatively low numbers in target tissues. Their instability when isolated from cells and tendency to adhere to the surfaces of test tubes requires special consideration in handling under experimental and clinical conditions. The basic principles of measurement of nuclear hormone receptors are the same as for plasma membrane receptors.557 Multi-point titration and sucrose density gradient analyses are commonly used for measurement of steroid hormone receptors in tissue samples.558 Tissue homogenates containing receptors are incubated with increasing amounts of radioactively labeled steroids of high specific activity in the presence and absence of corresponding excess unlabeled steroid or incubating with a fixed concentration of labeled steroid and increasing amount of corresponding unlabeled steroid.559 Tritiated or radioiodinated steroids are most commonly used because they are available in high specific activities. The binding in the presence of corresponding excess unlabeled steroid hormone is nonspecific binding, which is unsaturable and generally represents binding to nonreceptor sites. Nonspecific binding is subtracted from total binding (the amount bound in the absence of unlabeled steroid) to obtain saturable and high-affinity specific binding. By appropriately varying the incubation conditions, kinetic and equilibrium binding constants, specificity of binding, and other properties can be determined by titration analysis. Receptor-bound and free hormones are separated by dextran-coated charcoal559 or precipitation of steroid-receptor complex with protamine sulfate560 or absorption to hydroxyapatite561 followed by centrifugation and scintillation counting. For clinical samples, ER and PR status in breast tumor samples are quantitated by monoclonal antibody-based methods using a commercially available kit from Abbott Laboratories.562 One important clinical application of this method is the use of ERα and PR status as an indication for antiestrogen therapy.563
Scatchard analysis is the most universal method for calculating binding affinity (KD) and number (binding capacity) of steroid hormone receptors.564 The shape of Scatchard plots can be linear or curvilinear with upward or downward concavity. The interpretation and calculation of binding constants is the same as for membrane receptors.557
Ligand binding affinity and specificity for steroid hormone receptors
The apparent dissociation constants for steroid hormones binding to their cognate receptors is in the range of 10-11 to 10-9 M, which is close to circulating steroid hormone concentrations. The affinity of nonrelated steroid hormones to the receptor is generally about 100 times lower than the appropriate steroid hormone. However, if the levels of these nonrelated hormones are greatly elevated due to some physiological or pathological process, then significant binding and biological response to them can be expected.
Ligand binding specificity is also influenced by interaction with coactivators. In the presence of coactivator ARA70, E2 bound to the AR and induced AR-responsive transcriptional activity more than 30-fold in DU145 human prostate cancer cells.331 In contrast, the synthetic estrogen diethylstilbestrol (DES) had no effect. The significance of this newly discovered ability of E2 to activate AR is strengthened by finding patients with Reifenstein partial-androgen-insensitive syndrome with a single mutation in the AR that makes the receptor nonresponsive to E2-stimulation in the presence of ARA70. These data suggest that testosterone/dihydrotestosterone are not the only ligands for the AR.565
MOLECULAR BIOLOGY TECHNIQUES FOR STUDY OF STEROID HORMONE RECEPTORS
Prior to the development of cloning techniques, steroid hormone receptor structures were analyzed by painstaking methods of classical protein chemistry. Protein purification required processing of large amounts of tissues and sequencing was laborious and slow. Ligand-binding characteristics were described by Scatchard analysis and binding displacement curves, as described above.
Molecular biological techniques have revolutionized the study of hormone receptor biology, providing a wealth of information regarding the structure and mechanism of action of the steroid and nuclear hormone receptors. Common techniques used by molecular biologists include Southern, northern, and western blotting, used to detect the presence and size of specific DNA, RNA, and protein sequences, respectively.566 Molecular cloning of DNA provides a technique whereby a single segment of DNA can be isolated from a large population of genes, purified to homogeneity and amplified to produce sufficient quantities for structural and functional analysis. Receptors are now routinely cloned from RNA extracted from small amounts of hormone-responsive tissues or cells. When it is possible to predict a certain degree of homology of DNA sequence of the hormone receptor with other known proteins, specific DNA segments can be amplified using polymerase chain reaction (PCR), with the cDNA as template and highly conserved sequences from related proteins as primers.567 Alternatively, specific enzymes may be used to copy, cut and splice together pieces of DNA that are inserted into circular plasmid DNA or bacteriophage viruses, introduced into bacteria and allowed to divide many times. The greatly amplified DNA is then isolated, purified and the nucleotide sequence of the cloned DNA segment is determined. The cDNA clone can be transcribed into mRNA and used to confirm biological activity in in vivo and in vitro functional expression systems and the amino acid sequence of the encoded protein can be readily determined. The cDNA can be tagged with radioactivity or a fluorescent marker and used to detect the presence or describe the distribution of receptor mRNA in various tissues and endocrine states using solution hybridization and in situ hybridization methods.
Once the molecular structure of the receptor protein is known, the structure-function relationships of certain amino acid sequences relative to those of other receptors in the steroid hormone receptor family are investigated using domain-swapping and site-directed mutagenesis methods. In domain-swapping experiments, hybrid mRNA transcripts can be produced from a receptor cDNA in which a key functional element, such as the ligand-binding domain, is replaced with the nucleotide sequence from the same region of another closely related receptor.568 With this technique, it has been possible to determine the functional significance and specificity of the various regions of the steroid receptor proteins.568 Site-directed mutagenesis has been used to determine the identity of amino acid residues that are important for DNA and ligand binding of ERα.569
The electrophoretic mobility shift assay (EMSA), also called gel mobility shift assay, is the most common technique to examine the binding of a given steroid/nuclear receptor to DNA.570 For EMSA, a preparation of a particular steroid/nuclear receptor is incubated with a short (20–400 bp) fragment of [32P]-labeled double-stranded DNA. The DNA may be a synthetic construct of a particular HRE or a fragment from the promoter of a particular gene that is thought to contain a HRE. The protein-DNA reaction mixture is then separated by a nondenaturing polyacrylamide gel electrophoresis. The free [32P]-labeled DNA migrates to the bottom of the gel, while the migration of the [32P]-labeled DNA that is bound by the receptor is slowed. The gel is dried and exposed to X-ray film or placed in a Phosphorimager cassette to detect where the [32P] is located. The free, unbound [32P]-labeled DNA appears at the bottom and the receptor-bound DNA is located toward the middle or top of the gel. Addition of a 50–200-fold molar excess of cold competitor DNA is used to determine the specificity of the receptor-DNA complex. Addition of an antibody to a particular steroid/nuclear receptor is used to confirm the identity of the protein in the protein-DNA complex. The antibody may either block the receptor-DNA binding, leaving an empty spot on the autoradiograph; “supershift” the receptor-DNA complex by binding stably to it and thus further retarding its mobility in the electrophoretic field; or have no effect, indicating that the epitope recognized by the antibody is not available under the assay conditions. EMSA is useful to compare the relative binding affinities of a given receptor for various HREs in vitro.177
Chromatin immunoprecipitation (ChIP) is used to examine the association of specific proteins with specific regions of the genome by using specific antibodies that recognize a specific protein or a specific modification, e.g., phosphorylation or acetylation, of a protein. ChIP is used in molecular endocrinology research to examine the time or ligand-dependency of direct interaction of NR with a region of DNA in a cell or tissue.571, 572 This technique is primarily used to examine the binding of a NR to a known region containing a HRE in cultured cells. For ChIP analysis of NR-DNA interaction in cells, the cells are treated with vehicle versus ligand for a short period of time (20–60 min) followed by cross-linking of protein-protein and protein-DNA in live cells with formaldehyde. After cross-linking, the cells are lysed and whole cell extracts are sonicated to shear the DNA to ~500 bp lengths. The proteins together with cross-linked DNA are subsequently immunoprecipitated with specific antibodies, e.g., to ERα.272, 273, 403, 572, 573, 574, 575, 576, 577, 578, 579 The protein-DNA cross-links in the immunoprecipitated extract are reversed by heating and the DNA fragments are purified and PCR amplified using primers flanking the region of interest and a nonspecific region, e.g., far upstream of the promoter or ERE-containing region.580 “Re-ChIP” is used to identify proteins that simultaneously interact with the first protein/gene region.156, 277, 581, 582, 583, 584
To examine how a particular ligand, steroid receptor, and HRE affect transcription, transient transfection experiments are performed in cultured mammalian cell.402 For these experiments, the cell must either contain the steroid receptor of interest or the receptor must be introduced into the cell by means of an expression vector encoding the cDNA of the receptor with a strong viral promoter, e.g., CMV or RSV, to ensure its expression. The reporter vector contains a reporter gene not expressed in normal mammalian cells. Examples of reporter genes are chloramphenicol acetyltransferase (CAT),585 luciferase,402 and green fluorescent protein (GFP).586 The reporter and hormone receptor expression plasmids are transfected into the mammalian cells using calcium phosphate co-precipitation, electroporation, or by means of lipid-mediated transfer, e.g., Lipofectamine.402 Following a recovery period, the cells are treated with hormone for 12–48 h. The cells are then lysed and reporter gene activity is measured. As a control, cells are usually co-transfected with a β-galactosidase or Renilla reporters as an indication of the percent efficiency with which the cells have taken up the plasmid DNA.587 This technique allows quantitation of how different ligands and DNA sequences affect the transcriptional response of a given steroid receptor. In addition, co-transfection of the cells with expression plasmids for coactivators or corepressors allows determination of the functional consequence of these proteins.588
EFFECTS OF STEROID HORMONES ON BEHAVIOR
Effects of steroid hormones on behavior
Steroid hormones exert profound effects on mood, mental state, and memory by acting on both "classical" monoamine and neuropeptide transmitter mechanisms in the brain.589, 590 In women, estrogen is thought to protect against depression and delay the onset of schizophrenia,591, 592, 593 and Alzheimer's disease,594, 595, 596, 597, 598, 599 although other studies indicate that estrogens may increase the risk of the development of dementia.600 Estradiol has been implicated in the physiologic control of eating in laboratory animals and thus may play a role in anorexia nervosa, bulimia, and other eating disorders.601 Estrogens and progestins have been shown to regulate the synthesis and release of brain neurotransmitters including norepinephrine, dopamine, serotonin, gonadotropin releasing hormone, beta-endorphin, corticotropin releasing factor, and prolactin.589 A comparison of pre- and postmenopausal women to “stress reactivity to math and speech tasks” showed a higher elevation in systolic blood pressure in postmenopausal women compared with premenopausal women in response to the stressor, indicating that estrogens “blunt” the stress response, thus offering a hypothesis for the increased risk of cardiovascular disease in postmenopausal women.602, 603 Estrogens have proven mood-elevating and antidepressant properties and prior to the termination of the Women’s Health Initiative (WHI) hormone replacement therapy (HRT) trials,604, 605 HRT was commonly used to treat these and other menopausal symptoms.606
Estrogens607 and progestins608 play a key role in female rodent sexual receptivity609 and in behavior.610, 611, 612 Studies with ER and PR knockout (ERKO and PRKO) mice showed that the female mice exhibited reduced521, 533, 608, 613, 614 or exhibited no lordosis,521 respectively, and that PR-regulation of dopamine synthesis in the brain is involved in this lack of responsiveness to male advances.609 PRKO male mice showed reduced mount frequencies compared to wild-type mice, indicating a role for progestins in regulating male sexual response.615, 616
The sexual orientation of homosexual (gay) men is a biological variation of human sexuality/sexual preference that has been postulated to be dictated by testosterone action on the prenatal and possibly postnatal brain.617 Whether homosexual males are “hyper-masculinized” by high or “under-masculinized” by low prenatal testosterone remains uncertain.618 Likewise, the etiology of female (lesbian) homosexuality is undefined, but appears to involve prenatal and/or postnatal hormone exposure, at least in “butch” lesbians.618 Differences in auditory system responses of lesbian and bisexual females compared to heterosexual women were suggested to be caused by alterations in brain structures that had been partially masculinized by exposure to high levels of prenatal androgen.619 Correlations of auditory responses with sexual orientation in males and females were most often consistent with the hypothesis that male homosexuals were undermasculinized and female homosexuals overmasculinized, presumably due to prenatal testosterone exposure.620 Experiments in female zebra finches showed that early estrogen treatment or injection with an estrogen synthesis inhibitor resulted in a preference for females over males as mates.621 These results suggest that sexual partner preference may result from hormone actions in the developing brain of these birds, but the implications on human sexual orientation are uncertain.
It is still unclear to what extent cross-gender identity (transsexuals) is due to pre- and perinatal organizing effects of sex hormones on the brain.622 A study of early onset, adult male-to-female (MTF) and female-to-male (FTM) transsexuals, who were not yet hormonally treated, showed pronounced gender differences, and that both transsexual groups occupied a position in between the two control groups, indicating a pattern of performance away from their biological sex.623 Prenatal androgen exposure has been implicated in transsexualism and the ratio of the 2nd to the 4th (2D:4D) digit lengths has been suggested to be negatively correlated to prenatal androgen exposure.624 A recent study reported that the right-hand 2D:4D in MFT is higher than in control males but similar to that observed in control females, but no differences were seen in FMT relative to control females.624 The authors concluded that these data support a biological etiology of male-to-female transsexualism, implicating decreased prenatal androgen exposure in MFT.624
In animal models, androgens play a critical role in engendering neural sexual dimorphism by masculinizing the nervous system of males.625 Usually, the androgen must act early in life, often during the fetal period, to masculinize the nervous system and behavior.625 Interestingly, androgens appear to work by being converted to estrogens and binding to ER, since ERKO male mice show reduced masculinization535 and aromatase knockout (ArKO) mice have decreased fertility and deficits in male sexual behavior including mount, intromission, and ejaculation.626 In aging men, decreased testosterone has been associated with symptoms including depression, anxiety, irritability, insomnia, weakness, diminished libido, impotence, poor memory, reduced muscle and bone mass, and diminished sexual body hair.627 However, there is great interindividual variability, and the connection between serum testosterone levels and clinical psychiatric signs and symptoms is not clear-cut, since other hormonal changes are implicated as well.627 Previous studies suggested that postmenopausal women have “androgen deficiency” resulting in decreased sexual desire, and that administration of testosterone improves a number of parameters of sexuality in the testosterone-treated postmenopausal women.628 However, more recent results indicate no benefit of testosterone therapy, highlighting the concern that conversion of androgens to estrogens may increase breast cancer risk.629
STEROID HORMONE RECEPTORS IN DISEASE STATES
Clinical manifestations of abnormal steroid hormone receptor function have been demonstrated to involve variation in receptor numbers and the ability to stimulate transcription of certain genes. Hormone resistance, the failure of tissues to respond to normal circulating hormone levels, has been shown in a variety of endocrine systems to be due to the genetic lack or functional defect in steroid hormone receptors. The gene for the human androgen receptor has been isolated, cloned, and mapped to a locus on the X chromosome.630 Deletions in all or part of this gene have been shown in several individuals with complete androgen insensitivity.631, 632 The lack of functional androgen receptors results in testicular feminization.633 Hypocalcemic (Type II) rickets results from a single gene mutation in the steroid hormone binding domain of the human vitamin D receptor gene.634, 635
Sex steroid hormones have been implicated in the pathogenesis of breast, uterine, ovarian, prostate, thyroid, and other cancers. Expression of mutant forms of ERα may be an important factor in some cases of breast cancer.636 Determination of steroid hormone receptor expression, i.e., ERα and PR, in breast tumors, is critical to the selection of appropriate therapy. Hormone-dependent tumors tend to have elevated levels of steroid hormone receptors.637 One study found that 50% of the 60 breast tumors sampled expressed both ERa and ERβ, and that the ERα+/ERβ+ phenotype was associated with node positive status.638 It has long been established that elevated levels of ERα and ERβ are highly associated with poor histological differentiation in breast cancer and may serve as a predictor of responsiveness to endocrine therapy as well as a prognostic indicator of metastatic disease.637 The combined presence of ER and PR in a breast tumor provides an additional indication of the probability of prolonged survival following antiestrogen therapy.639, 640, 641, 642
Association between single-nucleotide polymorphisms (SNPs) in ER genes and disease have been demonstrated in several cases.643
Steroid hormone receptor analysis has limited value in the selection of patients with endometrial cancer for endocrine therapy because both normal and malignant uterine tissues contain significant levels of both estrogen and progesterone receptors.644 Although the results of different studies disagree, progesterone receptor expression appears a better prognostic indicator than ERα expression in ovarian cancer.645 At present, the relationship between steroid-receptor status and response to endocrine therapy in ovarian cancer remains to be established.
Glucocorticoid resistance results from an inability of glucocorticoids to exert their effects in their target tissues.646 Glucocorticoid resistance is associated with elevated ACTH and cortisol, with an attendant increase in adrenal androgens and steroids with salt-retaining activity. Clinical manifestations of glucocorticoid resistance vary from asymptomatic to different degrees of hypertension and/or hypokalemic alkalosis and/or hyperandrogenism. In women, the excess androgens can result in acne, hirsutism, male type baldness, menstrual irregularities, oligoanovulation, and infertility; in men, it may lead to infertility; and in children to precocious puberty. Different molecular defects in the GR gene, resulting in various defects in the receptor protein, alter the functional characteristics and/or concentrations of GR and cause glucocorticoid resistance.647 It is postulated that acquired tissue-specific glucocorticoid resistance may play a role in the origin and pathogenesis of depression, steroid-resistant asthma, autoimmune disorders, and AIDS.648, 649 Glucocorticoid receptor mutations have been suggested to play a role in the pathogenesis of leukemia, hereditary glucocorticoid resistance, and Nelson's syndrome.648 Corticosteroid-resistant asthma has recently been found associated with decreased GR and increased AP-1-DNA binding in peripheral blood mononuclear cells as compared to corticosteroid-sensitive asthma.650 These findings indicate that variations in the GR may play a central role in a wide variety of diseases.651
Hypertension and pseudohypoaldosteronism are associated with diminished or complete loss of high-affinity aldosterone receptors in select target tissues in some, but not all patients.652 MR induces the transcription of specific genes which regulate apical amiloride-sensitive epithelial sodium channels located in the apical membrane of epithelial cells from the distal colon and kidney collecting duct.653
Estrogens play a central role in the immune response and immune-mediated diseases and cells involved in the immune response, namely thymocytes, macrophages, and endothelial cells, express ERα.654 Certain pathological states are characterized by the production of abnormal levels of antibodies to steroid hormone receptors. Although the autoimmune disorder systemic lupus erythematosus (SLE) was postulated to result from anti-estrogen receptor action,655 and one study showed that men with autoimmune disorders, including SLE, or related diseases such as mixed connective disease and Sjogren's syndrome, had increased serum levels of antibodies to estrogen receptors compared to women,656 another report showed that peripheral blood monocytes of SLE patients showed a similar Kd and number of specific [3H]E2 binding sites compared to normal values.657 Hormonal regulation of B cell function was recently reviewed.658 Similarly, ERα variants do not appear to play a role in SLE.659
ENDOCRINE DISRUPTORS
Endocrine disruptors (ED) are chemicals present in the environment that are thought to disrupt endogenous hormone action by acting either as hormone agonists or antagonists in vivo. ED have been postulated to play a role in the increased incidence of breast cancer in the US since 1940660, 661 and decreased sperm counts662 as well as reproductive tract abnormalities in wildlife species.663 In the years following World War II, the production and use of a variety of hydrocarbons as pesticides, herbicides, food preservatives, and plastics has increased.664 A growing body of evidence indicates that some of these compounds, whether as food or aquatic contaminants, mimic, antagonize, or indirectly alter the activity of estrogens in vivo.665, 666, 667, 668 Such compounds are collectively referred to as “environmental estrogens” (EE) or “xenoestrogens”. Amongst the chemicals thought to be xenoestrogens are various organochloride pesticides, e.g., dieldrin; polychlorinated biphenyls (PCBs), a family of 209 related compounds widely used as industrial coolants; alkylphenolic polyethoxylates (APEOs: 4-nonylphenol [NP] and 4-octylphenol [OP]), used as nonionic surfactants in detergents, paints, herbicides, and pesticides; bisphenol A, used in manufacturing plastics; and the heavy metal cadmium.669 EE are also natural dietary components, e.g., the phytoestrogen coumestrol.433 Because they are lipophilic, EE can enter the human body by ingestion or adsorption through the skin and mucosal membranes. Certain EE, e.g., OP and NP,670 are present in water from sewage treatment plants and bioaccumulate in aquatic species and in animal fat.660 Bisphenol A is a contaminate in canned foods671 and was found in human saliva after dental work.672 However, the level of human exposure to these agents and whether these levels are sufficient to cause harmful effects remains controversial.142, 673, 674, 675, 676, 677, 678, 679, 680 Long-term exposure to exogenous estrogens is thought to increase the relative risk of not only breast, but also liver, ovarian, and uterine tumors.681, 682, 683, 684, 685 Because EE lack structural similarity to natural estrogens, i.e., estradiol (E2), having in common only a phenol ring,686 it is not immediately obvious which compounds have estrogenic or antiestrogenic activity. Although all EE stimulate cell proliferation, not all EE induce estrogen target genes, indicating a divergence in the molecular pathways regulating cell proliferation and estrogen end-product induction. The US Environmental Protection Agency (EPA) is currently funding considerable research on endocrine disruptors.
SUMMARY
The past 20 years have seen remarkable progress in our understanding of the mechanism of action of steroid hormones and receptor function. The development of molecular cloning, targeted gene disruption, microarrays, and ChIP analysis of NR-chromatin interaction has greatly facilitated the molecular understanding of steroid receptor action. We know that steroid hormones and other ligands mediate their biological activities by binding to a superfamily of related receptors that share a common modular structure. The steroid receptor superfamily includes not just receptors for steroid hormones and thyroid hormones, but also a diverse set of other gene regulators, some of which are called orphan receptors and whose ligand, if necessary, is, at present, unknown. Tremendous progress has been made in understanding the way hormone-liganded-steroid receptors interact with specific binding sites in hormone-regulated genes and the role of receptor interaction with other nuclear transcription factors in the regulation of hormone target gene transcription. Another significant advance is the identification of coactivators and corepressors as proteins that interact directly with steroid/nuclear receptors, but not with DNA, that aid the receptors in modulating target gene expression. The nucleosome remodeling activity of these coactivators and corepressors and their associated proteins has revealed the importance of chromatin structure in hormone-induced gene transcription. The importance of multiple levels of “cross-talk” between cell-membrane-bound receptors, acting via second messenger phosphorylation cascades, and nuclear hormone receptors, and between different classes of transcriptional enhancer proteins, indicates the overall complexity involved in specific gene regulation. Finally, recent developments in the analysis of abnormal receptor structure and function have enhanced our potential for clinical diagnosis and treatment of numerous endocrine disorders.
REFERENCES
Laudet V 1997 Evolution of the nuclear receptor superfamily: early diversification from an ancestral orphan receptor. J Mol Endocrinol 19:207-226 |
|
Thornton JW 2001 Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci U S A 98:5671-5676. |
|
Thornton JW, Need E, Crews D 2003 Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science 301:1714-1717 |
|
Willson TM, Moore JT 2002 Genomics versus orphan nuclear receptors--a half-time report. Mol Endocrinol 16:1135-1144. |
|
Benoit G, Malewicz M, Perlmann T 2004 Digging deep into the pockets of orphan nuclear receptors: insights from structural studies. Trends in Cell Biology 14:369-376 |
|
Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-A 1996 Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925-5930 |
|
Mosselman S, Polman J, Dijkrms R 1996 ERb: Identification and characterization of a novel human estrogen receptor. FEBS Lett 392:49-53 |
|
Ogawa S, Inoue S, Watanabe T, Hiroi H, Orimao A 1998 The complete primary structure of human estrogen receptor beta (hER beta) and its heterodimerization with ER alpha in vivo and in vitro. Biochem Biophys Res Commun 243:122-126 |
|
Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM 1986 The c-erb-A gene encodes a thyroid hormone receptor. Nature 324:641-646 |
|
Harding HP, Lazar MA 1995 The monomer-binding orphan receptor Rev-Erb represses transcription as a dimer on a novel direct repeat. Mol Cell Biol 15:4791-4802 |
|
Harding HP, Lazar MA 1993 The orphan receptor Rev-ErbA alpha activates transcription via a novel response element. Mol Cell Biol 13:3113-3121 |
|
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM 1995 The nuclear receptor superfamily: the second decade. Cell 83:835-839 |
|
Evans RM 1988 The steroid and thyroid hormone receptor superfamily. Science 240:889-895 |
|
Evans RM 2005 The Nuclear Receptor Superfamily: A Rosetta Stone for Physiology. Mol Endocrinol 19:1429-1438 |
|
Montano MM, Muller V, Trogaugh A, Katzenellenbogen BS 1995 The carboxy-terminal F domain of the human estrogen receptor: Role in the transcriptional activity of the receptor and the effectiveness of antiestrogens as estrogen antagonists. Mol Endocrinol 9:814-825 |
|
Koide A, Zhao C, Naganuma M, Abrams J, Deighton-Collins S, Skafar DF, Koide S 2007 Identification of Regions within the F Domain of the Human Estrogen Receptor {alpha} that Are Important for Modulating Transactivation and Protein-Protein Interactions. Mol Endocrinol 21:829-842 |
|
Green S, Chambon P 1987 Oestradiol induction of a glucocorticoid-responsive gene by a chimaeric receptor. Nature 325:75-78 |
|
O'Malley BW 1990 The steroid receptor superfamily: More excitement predicted for the future. Mol Endocrinol 4:363-369 |
|
O'Malley BW 2005 A Life-Long Search for the Molecular Pathways of Steroid Hormone Action. Mol Endocrinol 19:1402-1411 |
|
Ingiguez-Lluhi JA, Lou DY, Yamamoto KR 1997 Three amino acid substitutions selectively disrupt the activation but not the repression function of the glucocorticoid receptor N terminus. J Biol Chem 272:4149-4156 |
|
Godowski PJ, Rusconi S, Miesfeld R, Yamamoto KR 1987 Glucocorticoid receptor mutants that are constitutive activators of transcriptional enhancement. Nature 325:365-368 |
|
Owen GI, Jennifer K. Richer LT, Takimoto G, Horwitz KB 1998 Progesterone regulates transcription of the p21(WAF1) cyclin- dependent kinase inhibitor gene hrough Sp1 and CBP/p300. J Biol Chem 273:10696-10701 |
|
Richer JK, Lange CA, Wierman AM, Brooks KM, Tung L, Takimoto GS, Horwitz KB 1998 Progesterone receptor variants found in breast cells repress transcription by wild-type receptors. Breast Cancer Res Treat 48:231-241 |
|
Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB 2002 Differential Gene Regulation by the Two Progesterone Receptor Isoforms in Human Breast Cancer Cells. J Biol Chem 277:5209-5218 |
|
Gao T, McPhaul MJ 1998 Functional activities of the A and B forms of the human androgen receptor in response to androgen receptor agonists and antagonists. Mol Endocrinol 12:654-663 |
|
Klug A, Schwabe JWR 1995 Zinc fingers. FASEB J 9:597-604 |
|
Picard D, Kumar V, Chambon P, Yamamoto KR 1990 Signal transduction by steroid hormones: nuclear localization is differentially regulated in estrogen and glucocorticoid receptors. Cell Regul 1:291-299 |
|
Safer JD, Cohen RN, Hollenberg AN, Wondisford FE 1998 Defective release of corepressor by hinge mutants of the thyroid hormone receptor found in patients with resistance to thyroid hormone. J Biol Chem 273:30175-30182 |
|
Jackson TA, Richer JK, Bain DL, Takimoto GS, Tung l, Horwitz KB 1997 The partial agonist activity of antagonist-occupied steroid receptors is controlled by a novel hinge domain-binding coactivator L7/SPA and the corepressors N-CoR or SMRT. Mol Endocrinol 11:693-705 |
|
Beato M, Candau R, Chavez S, Mows C, Truss M 1996 Interaction of steroid hormone receptors with transcription factors involves chromatin remodelling. J Steroid Biochem Molec Biol 56:47-59 |
|
Beato M, Sanchez-Pacheco A 1996 Interaction of steroid hormone receptors with the transcription initiation complex. Endocrine Rev 17:587-609 |
|
Walter P, Green S, Greene GL, Krust A, Birnert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, Chambon P 1985 Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci USA 82:7889-7893 |
|
Chang CS, Kokontis J, Liao ST 1988 Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 240:324-326 |
|
Misrahi M, Atger M, d'Auriol L, Loosfelt H, Meriel C, Fridlansky F, Guiochon-Mantel A, Galibert F, Milgrom E 1987 Complete amino acid sequence of the human progesterone receptor deduced from cloned cDNA. Biochem Biophys Res Commun 143:740-748 |
|
Weinberger C, Hollenberg SM, Ong ES, Harmon JM, Brower ST, Cidlowski J, Thompson EB, Rosenfeld MG, Evans RM 1985 Identification of human glucocorticoid receptor complementary DNA clones by epitope selection. Science 228:740-742 |
|
Weinberger C, Hollenberg SM, Rosenfeld MG, Evans RM 1985 Domain structure of human glucocorticoid receptor and its relationship to the v-erb-A oncogene product. Nature 318:670-672 |
|
Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM 1987 Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237:268-275 |
|
Baker AR, McDonnell DP, Hughes M, Crisp TM, Mangelsdorf DJ, Haussler MR, Pike JW, Shine J, BW OM 1988 Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci U S A 85:3294-3298 |
|
Lazar MA 1993 Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 14:184-193 |
|
Sap J, Munoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennstrom B 1986 The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature 324:635-640 |
|
Koenig RJ, Warne RL, Brent GA, Harney JW, Larsen PR, Moore DD 1988 Isolation of a cDNA clone encoding a biologically active thyroid hormone receptor. Proc Natl Acad Sci U S A 85:5031-5035 |
|
Hodin RA, Lazar MA, Wintman BI, Darling DS, Koenig RJ, Larsen PR, Moore DD, Chin WW 1989 Identification of a thyroid hormone receptor that is pituitary-specific. Science 244:76-79 |
|
Giguere V 1994 Retinoic acid receptors and cellular retinoid binding proteins: complex interplay in retinoid signaling. Endocr Rev 15:61-79 |
|
Glass CK 1994 Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocrine Rev 15:391-407 |
|
Issemann I, Green S 1990 Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347:645-650 |
|
Schmidt A, Endo N, Rutledge SJ, Vogel R, Shinar D, Rodan GA 1992 Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acids. Mol Endocrinol 6:1634-1641 |
|
Gearing KL, Gottlicher M, Teboul M, Widmark E, Gustafsson JA 1993 Interaction of the peroxisome-proliferator-activated receptor and retinoid X receptor. Proc Natl Acad Sci U S A 90:1440-1444 |
|
Sher T, Yi HF, McBride OW, Gonzalez FJ 1993 cDna cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry 32:5598-5604 |
|
Green S, Wahli W 1994 Peroxisome proliferator-activated receptors: finding the orphan a home. Mol Cell Endocrinol 100:149-153 |
|
Mukherjee R, Jow L, Noonan D, McDonnell DP 1994 Human and rat peroxisome proliferator activated receptors (PPARs) demonstrate similar tissue distribution but different responsiveness to PPAR activators. J Steroid Biochem Mol Biol 51:157-166 |
|
Greene ME, Blumberg B, McBride OW, Yi HF, Kronquist K, Kwan K, Hsieh L, Greene G, Nimer SD 1995 Isolation of the human peroxisome proliferator activated receptor gamma cDNA: expression in hematopoietic cells and chromosomal mapping. Gene Expr 4:281-299 |
|
Lambe KG, Tugwood JD 1996 A human peroxisome-proliferator-activated receptor-gamma is activated by inducers of adipogenesis, including thiazolidinedione drugs. Eur J Biochem 239:1-7 |
|
Auboeuf D, Rieusset J, Fajas L, Vallier P, Frering V, Riou JP, Staels B, Auwerx J, Laville M, Vidal H 1997 Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes 46:1319-1327 |
|
Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruchart JC, Deeb S, Vidal-Puig A, Flier J, Briggs MR, Staels B, Vidal H, Auwerx J 1997 The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem 272:18779-18789 |
|
Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM 1997 Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A 94:4318-4323 |
|
Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA 1997 Peroxisome proliferator-activated receptors a and g are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem 272:3406-3410 |
|
Costet P, Christiane Legendre JM, Alan Edgar, Pierre Galtier,, Pineau T 1998 Peroxisome proliferator-activated receptor -Isoform Deficiency leads to progressive Dyslipidemia with Sexually Dimorphic Obesity and steatosis. 273:29577-29585 |
|
Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D, Koeffler HP 1998 Ligands for peroxisome proliferator-activated receptor gamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci USA 95:8806-8811 |
|
Poynter ME, Daynes RA 1998 Peroxisome Proliferator-activated Receptor Activation Modulates Cellular Redox Status, Represses Nuclear Factor-B Signaling, and Reduces Inflammatory Cytokine Production in Aging. J Biol Chem 273:32833-32841 |
|
Uppenberg J, Svensson C, Jaki M, Bertilsson G, Jendeberg L, Berkenstam A 1998 Crystal structure of the ligand binding domain of the human nuclear receptor PPARgamma. J Biol Chem 273:31108-31112 |
|
Feltkamp D, Wiebel FF, Alberti S, Gustafsson JA 1999 Identification of a novel DNA binding site for nuclear orphan receptor OR1. J Biol Chem 274:10421-10429 |
|
Kliewer SA, Lehmann JM, Milburn MV, Willson TM 1999 The PPARs and PXRs: nuclear xenobiotic receptors that define novel hormone signaling pathways. Recent Prog Horm Res 54:345-367; discussion 367-348 |
|
Suh N, Wang Y, Williams CR, Risingsong R, Gilmer T, Willson TM, Sporn MB 1999 A new ligand for the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), GW7845, inhibits rat mammary carcinogenesis. Cancer Res 59:5671-5673 |
|
Zhu Y, Qi C, Jain S, Le Beau MM, Espinosa R, 3rd, Atkins GB, Lazar MA, Yeldandi AV, Rao MS, Reddy JK 1999 Amplification and overexpression of peroxisome proliferator-activated receptor binding protein (PBP/PPARBP) gene in breast cancer. Proc Natl Acad Sci U S A 96:10848-10853 |
|
Jiang WG, Redfern A, Bryce RP, Mansel RE 2000 Peroxisome proliferator activated receptor-gamma (PPAR-gamma) mediates the action of gamma linolenic acid in breast cancer cells. Prostaglandins Leukot Essent Fatty Acids 62:119-127 |
|
Lee H, Shi W, Tontonoz P, Wang S, Subbanagounder G, Hedrick CC, Hama S, Borromeo C, Evans RM, Berliner JA, Nagy L 2000 Role for peroxisome proliferator-activated receptor alpha in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circ Res 87:516-521. |
|
Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, Bonacchi A, Caporale R, Laffi G, Pinzani M, Gentilini P 2000 Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology 119:466-478 |
|
Suchanek KM, May FJ, Robinson JA, Lee WJ, Holman NA, Monteith GR, Roberts-Thomson SJ 2002 Peroxisome proliferator-activated receptor alpha in the human breast cancer cell lines MCF-7 and MDA-MB-231. Mol Carcinog 34:165-171 |
|
Wick M, Hurteau G, Dessev C, Chan D, Geraci MW, Winn RA, Heasley LE, Nemenoff RA 2002 Peroxisome Proliferator-Activated Receptor-gamma Is a Target of Nonsteroidal Anti-Inflammatory Drugs Mediating Cyclooxygenase-Independent Inhibition of Lung Cancer Cell Growth. Mol Pharmacol 62:1207-1214 |
|
Jalouli M, Carlsson L, Ameen C, Linden D, Ljungberg A, Michalik L, Eden S, Wahli W, Oscarsson J 2003 Sex Difference in Hepatic Peroxisome Proliferator-Activated Receptor alpha Expression: Influence of Pituitary and Gonadal Hormones. Endocrinology 144:101-109. |
|
Jiang WG, Douglas-Jones A, Mansel RE 2003 Expression of peroxisome-proliferator activated receptor-gamma (PPARgamma) and the PPARgamma co-activator, PGC-1, in human breast cancer correlates with clinical outcomes. Int J Cancer 106:752-757 |
|
Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A 2003 The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha). J Biol Chem 278:9013-9018 |
|
Desvergne B, Michalik L, Wahli W 2004 Be fit or be sick: peroxisome proliferator-activated receptors are down the road. Mol Endocrinol 18:1321-1332 |
|
Qin C, Morrow D, Stewart J, Spencer K, Porter W, Smith R, 3rd, Phillips T, Abdelrahim M, Samudio I, Safe S 2004 A new class of peroxisome proliferator-activated receptor gamma (PPARgamma) agonists that inhibit growth of breast cancer cells: 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes. Mol Cancer Ther 3:247-260 |
|
Watkins G, Douglas-Jones A, Mansel RE, Jiang WG 2004 The localisation and reduction of nuclear staining of PPARgamma and PGC-1 in human breast cancer. Oncol Rep 12:483-488 |
|
Xu J, Chang V, Joseph SB, Trujillo C, Bassilian S, Saad MF, Lee WN, Kurland IJ 2004 Peroxisomal proliferator-activated receptor alpha deficiency diminishes insulin-responsiveness of gluconeogenic/glycolytic/pentose gene expression and substrate cycle flux. Endocrinology 145:1087-1095 |
|
Suzuki T, Hayashi S, Miki Y, Nakamura Y, Moriya T, Sugawara A, Ishida T, Ohuchi N, Sasano H 2006 Peroxisome proliferator-activated receptor {gamma} in human breast carcinoma: a modulator of estrogenic actions. Endocr Relat Cancer 13:233-250 |
|
Ulrich S, Loitsch SM, Rau O, von Knethen A, Brune B, Schubert-Zsilavecz M, Stein JM 2006 Peroxisome Proliferator-Activated Receptor {gamma} as a Molecular Target of Resveratrol-Induced Modulation of Polyamine Metabolism. Cancer Res 66:7348-7354 |
|
Shearer BG, Billin AN 2007 The next generation of PPAR drugs: Do we have the tools to find them? Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1771:1082-1093 |
|
Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM 1994 Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A 91:7355-7359 |
|
Choi H-S, Chung M, Tzameli I, Simha D, Lee YK, Seol W, Moore DD 1997 Differential transactivation by two isoforms of the orphan nuclear hormone receptor CAR. J Biol Chem 272:23565-23571 |
|
Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, Collins JL, Kliewer SA 2000 Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem 275:15122-15127 |
|
Yamamoto Y, Moore R, Goldsworthy TL, Negishi M, Maronpot RR 2004 The Orphan Nuclear Receptor Constitutive Active/Androstane Receptor Is Essential for Liver Tumor Promotion by Phenobarbital in Mice. Cancer Res 64:7197-7200 |
|
Zhang Z, Burch PE, Cooney AJ, Lanz RB, Pereira FA, Wu J, Gibbs RA, Weinstock G, Wheeler DA 2004 Genomic analysis of the nuclear receptor family: new insights into structure, regulation, and evolution from the rat genome. Genome Res 14:580-590 |
|
Koike C, Moore R, Negishi M 2005 Localization of the nuclear receptor CAR at the cell membrane of mouse liver. FEBS Letters 579:6733-6736 |
|
Wang L-H, Tsai SY, Cook RG, Beattie WG, Tsai M-J, O'Malley BW 1989 COUP transcription factor is a member of the steroid receptor superfamily. Nature 340:163-166 |
|
Qiu Y, Krishnan V, Zeng Z, Gilbert DJ, Copeland NG, Gibson L, Yang-Feng T, Jenkins NA, Tsai MJ, Tsai SY 1995 Isolation, characterization, and chromosomal localization of mouse and human COUP-TF I and II genes. Genomics 29:240-246 |
|
Muscatelli F, Strom TM, Walker AP, Zanaria E, Recan D, Meindl A, Bardoni B, Guioli S, Zehetner G, Rabl W, Schwara HP, Kaplan J-C, Camerino G, Meitinger T, Monaco AP 1994 Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature 372:672-676 |
|
Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, Moser C, Walker AP, McCabe ER, et al. 1994 An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 372:635-641 |
|
Bae D, Schaefer M, Partan B, Muglia L 1996 Characterization of the mouse DAX-1 gene reveals evolutionary conservation of a unique amino-terminal motif and widespread expression in mouse tissue. Endocrinology 137:3921-3927 |
|
Nachtigal MW, Hirokawa Y, Enyeart-VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA 1998 Wilms' tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 93:445-454 |
|
Kawabe K, Shikayama T, Tsuboi H, Oka S, Oba K, Yanase T, Nawata H, Morohashi K 1999 Dax-1 as one of the target genes of Ad4BP/SF-1. Mol Endocrinol 13:1267-1284 |
|
Zhang H, Thomsen JS, Johansson L, Gustafsson JA, Treuter E 2000 DAX-1 Functions as an LXXLL-containing corepressor for activated estrogen receptors. J Biol Chem 275:39855-39859 |
|
Zhou J, Oakley RH, Cidlowski JA 2008 DAX-1 (Dosage-Sensitive Sex Reversal-Adrenal Hypoplasia Congenita Critical Region on the X-Chromosome, Gene 1) Selectively Inhibits Transactivation But Not Transrepression Mediated by the Glucocorticoid Receptor in a LXXLL-Dependent Manner. Mol Endocrinol 22:1521-1534 |
|
Miyajima N, Kadowaki Y, Fukushige S, Shimizu S, Semba K, Yamanashi Y, Matsubara K, Toyoshima K, Yamamoto T 1988 Identification of two novel members of erbA superfamily by molecular cloning: the gene products of the two are highly related to each other. Nucleic Acids Res 16:11057-11074 |
|
Yang N, Shigeta H, Shi H, Teng CT 1996 Estrogen-related receptor, hERR1, modulates estrogen receptor-mediated response of human lactoferrin gene promoter. J Biol Chem 271:5795-5804 |
|
Bonnelye E, Vanacker JM, Dittmar T, Begue A, Desbiens X, Denhardt DT, Aubin JE, Laudet V, Fournier B 1997 The ERR-1 orphan receptor is a transcriptional activator expressed during bone development. Mol Endocrinol 11:905-916 |
|
Bonnelye E, Vanacker JM, Spruyt N, Alric S, Fournier B, Desbiens X, Laudet V 1997 Expression of the estrogen-related receptor 1 (ERR-1) orphan receptor during mouse development. Mech Dev 65:71-85 |
|
Shigeta H, Zuo W, Yang N, DiAugustine R, Teng CT 1997 The mouse estrogen receptor-related orphan receptor alpha 1: molecular cloning and estrogen responsiveness. J Mol Endocrinol 19:299-309 |
|
Giguere V 2002 To ERR in the estrogen pathway. Trends Endocrinol Metab 13:220-225. |
|
Trapp T, Holsboer F 1996 Nuclear orphan receptor as a repressor of glucocorticoid receptor transcriptional activity. J Biol Chem 271:9879-9882 |
|
Hong H, Yang L, Stallcup MR 1999 Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J Biol Chem 274:22618-22626 |
|
Koh SS, Chen D, Lee YH, Stallcup MR 2001 Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J Biol Chem 276:1089-1098 |
|
Yan ZH, Medvedev A, Hirose T, Gotoh H, Jetten AM 1997 Characterization of the response element and DNA binding properties of the nuclear orphan receptor germ cell nuclear factor/retinoid receptor-related testis-associated receptor. J Biol Chem 272:10565-10572 |
|
Greschik H, Jean-Marie Wurtz PH, Fabian Köhler, , Moras D, Schüle R 1999 Characterization of the DNA-Binding and Dimerization Properties of the Nuclear Orphan Receptor germ Cell Nuclear Factor. Mol Cell Biol 19:690-703. |
|
Cooney AJ, Lee CT, Lin SC, Tsai SY, Tsai MJ 2001 Physiological function of the orphans GCNF and COUP-TF. Trends Endocrinol Metab 12:247-251. |
|
Kaestner KH, Hiemisch H, Luckow B, Schutz G 1994 The HNF-3 gene family of transcription factors in mice: gene structure, cDNA sequence, and mRNA distribution. Genomics 20:377-385 |
|
Jiang G, Sladek FM 1997 The DNA Binding Domain of Hepatocyte Nuclear Factor 4 MediatesCooperative, Specific Binding to DNA and Heterodimerization withthe Retinoid X Receptor J Biol Chem 272:1218-1225 |
|
Drewes T, Senkel S, Holewa B, Ryffel GU 1996 Human hepatocyte nuclear factor 4 isoforms are encoded by distinct and differentially expressed genes. Mol Cell Biol 16:925-931 |
|
Wong M, Ramayya MS, Chrousos GP, Driggers PH, Parker KL 1996 Cloning and sequence analysis of the human gene encoding steroidogenic factor 1. J Mol Endocrinol 17:139-147 |
|
Nakai A, Kartha S, Sakurai A, Toback FG, DeGroot LJ 1990 A human early response gene homologous to murine nur77 and rat NGFI-B, and related to the nuclear receptor superfamily. Mol Endocrinol 4:1438-1443 |
|
Hirose T, DA OB, Jetten AM 1995 RTR: a new member of the nuclear receptor superfamily that is highly expressed in murine testis. Gene 152:247-251 |
|
Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, Hashimoto S 2002 A transcription factor response element for gene expression during circadian night. Nature 418:534-539. |
|
Greiner EF, Kirfel J, Greschik H, Dörflinger U, Becker P, Mercep A, Schüle R 1996 Functional analysis of retinoid Z receptor , a brain-specific nuclear orphan receptor. Proc Natl Acad Sci 93:10105-10110 |
|
Seol W, Choi HS, Moore DD 1996 An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science 272:1336-1339 |
|
Seol W, Mahon MJ, Lee YK, Moore DD 1996 Two receptor interacting domains in the nuclear hormone receptor corepressor RIP13/N-CoR. Mol Endocrinol 10:1646-1655 |
|
Seol W, Chung M, Moore DD 1997 Novel receptor interaction and repression domains in the orphan receptor SHP. Mol Cell Biol 17:7126-7131 |
|
Johansson L, Thomsen JS, Damdimopoulos AE, Spyrou G, Gustafsson J-Å, Treuter E 1999 The orphan nuclear receptor SHP inhibits agonist-dependent transcriptional activity of estrogen receptors ERalpha and ERbeta. J Biol Chem 274: 345-353 |
|
Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA 2000 A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6:517-526 |
|
Johansson L, Bavner A, Thomsen JS, Farnegardh M, Gustafsson JA, Treuter E 2000 The orphan nuclear receptor SHP utilizes conserved LXXLL-related motifs for interactions with ligand-activated estrogen receptors. Mol Cell Biol 20:1124-1133 |
|
Lee YK, Dell H, Dowhan DH, Hadzopoulou-Cladaras M, Moore DD 2000 The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression. Mol Cell Biol 20:187-195 |
|
Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, Karpen SJ 2001 The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology 121:140-147. |
|
Kassam A, Capone JP, Rachubinski RA 2001 The short heterodimer partner receptor differentially modulates peroxisome proliferator-activated receptor alpha-mediated transcription from the peroxisome proliferator-response elements of the genes encoding the peroxisomal beta-oxidation enzymes acyl-CoA oxidase and hydratase-dehydrogenase. Mol Cell Endocrinol 176:49-56. |
|
Klinge CM, Jernigan SC, Risinger KE, Lee JE, Tyulmenkov VV, Falkner KC, Prough RA 2001 Short heterodimer partner (SHP) orphan nuclear receptor inhibits the transcriptional activity of aryl hydrocarbon receptor (AHR)/AHR nuclear translocator (ARNT). Arch Biochem Biophys 390:64-70. |
|
Brendel C, Schoonjans K, Botrugno OA, Treuter E, Auwerx J 2002 The small heterodimer partner interacts with the liver X receptor alpha and represses its transcriptional activity. Mol Endocrinol 16:2065-2076. |
|
Sanyal S, Kim J-Y, Kim H-J, Takeda J, Lee Y-K, Moore DD, Choi H-S 2002 Differential regulation of the orphan nuclear receptor Small Heterodimer Partner (SHP) gene promoter by orphan nuclear receptor ERR isoforms. J Biol Chem 277:1739-1748 |
|
Wang L, Han Y, Kim CS, Lee YK, Moore DD 2003 Resistance of SHP-null mice to bile acid-induced liver damage. J Biol Chem 278:44475-44481 |
|
Kim H-J, Kim J-Y, Kim J-Y, Park S-K, Seo J-H, Kim JB, Lee I-K, Kim K-S, Choi H-S 2004 Differential Regulation of Human and Mouse Orphan Nuclear Receptor Small Heterodimer Partner Promoter by Sterol Regulatory Element Binding Protein-1. J Biol Chem 279:28122-28131 |
|
Kim JY, Chu K, Kim HJ, Seong HA, Park KC, Sanyal S, Takeda J, Ha H, Shong M, Tsai MJ, Choi HS 2004 Orphan Nuclear Receptor Small Heterodimer Partner, a Novel Corepressor for a Basic Helix-Loop-Helix Transcription Factor BETA2/NeuroD. Mol Endocrinol 18:776-790 |
|
Kim JY, Kim HJ, Kim KT, Park YY, Seong HA, Park KC, Lee IK, Ha H, Shong M, Park SC, Choi HS 2004 Orphan nuclear receptor small heterodimer partner represses hepatocyte nuclear factor 3/Foxa transactivation via inhibition of its DNA binding. Mol Endocrinol 18:2880-2894 |
|
Suh JH, Huang J, Park Y-Y, Seong H-A, Kim D, Shong M, Ha H, Lee I-K, Lee K, Wang L, Choi H-S 2006 Orphan Nuclear Receptor Small Heterodimer Partner Inhibits Transforming Growth Factor-beta Signaling by Repressing SMAD3 Transactivation. J Biol Chem 281:39169-39178 |
|
Swales KE, Korbonits M, Carpenter R, Walsh DT, Warner TD, Bishop-Bailey D 2006 The Farnesoid X Receptor Is Expressed in Breast Cancer and Regulates Apoptosis and Aromatase Expression. Cancer Res 66:10120-10126 |
|
Hirose T, Fujimoto W, Yamaai T, Kin KH, Matsuura H, Jetten AM 1994 TAK 1: molecular cloning and characterization of a new member of the nuclear receptor superfamily. Mol Endocrinol 8:1667-1680 |
|
Wang S, Lai K, Moy FJ, Bhat A, Hartman HB, Evans MJ 2006 The Nuclear Hormone Receptor Farnesoid X Receptor (FXR) Is Activated by Androsterone. Endocrinology 147:4025-4033 |
|
Suh JM, Yu C-T, Tang K, Tanaka T, Kodama T, Tsai M-J, Tsai SY 2006 The Expression Profiles of Nuclear Receptors in the Developing and Adult Kidney. Mol Endocrinol 20:3412-3420 |
|
Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ 1995 LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev 9:1033-1045 |
|
Peet DJ, Janowski BA, Mangelsdorf DJ 1998 The LXRs: a new class of oxysterol receptors. Curr Opin Genet Dev 8:571-575 |
|
Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, Mangelsdorf DJ 1999 Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc Natl Acad Sci U S A 96:266-271 |
|
Edwards PA, Kennedy MA, Mak PA 2002 LXRs; oxysterol-activated nuclear receptors that regulate genes controlling lipid homeostasis. Vascul Pharmacol 38:249-256. |
|
Redinger RN 2003 The coming of age of our understanding of the enterohepatic circulation of bile salts. Am J Surg 185:168-172. |
|
Nilsson M, Stulnig TM, Lin C-Y, Yeo AL, Nowotny P, Liu ET, Steffensen KR 2007 Liver X Receptors Regulate Adrenal Steroidogenesis and Hypothalamic-Pituitary-Adrenal Feedback. Mol Endocrinol 21:126-137 |
|
Tabb MM, Blumberg B 2006 New Modes of Action for Endocrine-Disrupting Chemicals. Mol Endocrinol 20:475-482 |
|
Blumberg B, Evans RM 1998 Orphan nuclear receptors-new ligands and new possibilities. Genes Dev 12:3149-3155 |
|
Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, Tomkinson NC, LeCluyse EL, Lambert MH, Willson TM, Kliewer SA, Moore JT 2000 The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol 14:27-39 |
|
Moore JT, Kliewer SA 2000 Use of the nuclear receptor PXR to predict drug interactions. Toxicology 153:1-10 |
|
Gardner-Stephen D, Heydel JM, Goyal A, Lu Y, Xie W, Lindblom T, Mackenzie P, Radominska-Pandya A 2004 Human PXR variants and their differential effects on the regulation of human UDP-glucuronosyltransferase gene expression. Drug Metab Dispos 32:340-347 |
|
Chen Y, Tang Y, Wang M-T, Zeng S, Nie D 2007 Human Pregnane X Receptor and Resistance to Chemotherapy in Prostate Cancer. Cancer Res 67:10361-10367 |
|
Ekins S, Reschly EJ, Hagey LR, Krasowski MD 2008 Evolution of pharmacologic specificity in the pregnane X receptor. BMC Evol Biol 8:103 |
|
Greenblatt J 1997 RNA polymerase II holoenzyme and transcriptional regulation. Curr Opin Cell Biol 9:310-319 |
|
Sauer F, Tjian R 1997 Mechanisms of transcriptional activation: differences and similarities between yeast, Drosophila, and man. Curr Opin Genet Dev 7:176-181 |
|
Tsai M-J, O’Malley BW 1994 Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451-483 |
|
Baptista HA, Avellar MCW, Araujo RC, Pesquero JL, Schanstra JP, Bascands JL, Esteve JP, Paiva ACM, Bader M, Pesquero JB 2002 Transcriptional Regulation of the Rat Bradykinin B2 Receptor Gene: Identification of a Silencer Element. Mol Pharmacol 62:1344-1355 |
|
Lekstrom-Himes J, Xanthopoulos KG 1998 Biological role of the CCAAT/Enhancer-binding protein family of transcription factors. J Biol Chem 273:28545-28548 |
|
Kadonaga JT, Carner KR, Masiarz FR, Tjian R 1987 Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell 51:1079-1090 |
|
Powell E, Xu W 2008 Intermolecular interactions identify ligand-selective activity of estrogen receptor alpha/beta dimers. Proceedings of the National Academy of Sciences 105:19012-19017 |
|
Monroe DG, Secreto FJ, Subramaniam M, Getz BJ, Khosla S, Spelsberg TC 2005 Estrogen Receptor {alpha} and {beta} Heterodimers Exert Unique Effects on Estrogen- and Tamoxifen-Dependent Gene Expression in Human U2OS Osteosarcoma Cells. Mol Endocrinol 19:1555-1568 |
|
Beato M, Chalepakis G, Schauer M, Slater EP 1989 DNA regulatory elements for steroid hormones. J Steroid Biochem 32:737-747 |
|
Kinyamu HK, Fryer CJ, Horwitz KB, Archer TK 2000 The mouse mammary tumor virus promoter adopts distinct chromatin structures in human breast cancer cells with and without glucocorticoid receptor. J Biol Chem 275:20061-20068 |
|
Archer TK, Fryer CJ, Lee H-L, Zaniewski E, Liang T, Mymryk JS 1995 Steroid hormone receptor status defines the MMTV promoter chromatin structure in vivo. J Steroid Biochem Molec Biol 53:421-429 |
|
Jantzen HM, Strahle U, Gloss B, Stewart F, Schmid W, Boshart M, Miksicek R, Schutz G 1987 Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell 49:29-38 |
|
Cole TJ, Blendy JA, Schmid W, Strahle U, Schutz G 1993 Expression of the mouse glucocorticoid receptor and its role during development. J Steroid Biochem Mol Biol 47:49-53 |
|
De Martino MU, Bhattachryya N, Alesci S, Ichijo T, Chrousos GP, Kino T 2004 The Glucocorticoid Receptor and the Orphan Nuclear Receptor Chicken Ovalbumin Upstream Promoter-Transcription Factor II Interact with and Mutually Affect Each Other's Transcriptional Activities: Implications for Intermediary Metabolism. Mol Endocrinol 18:820-833 |
|
Feige JN, Auwerx J 2007 Transcriptional coregulators in the control of energy homeostasis. Trends in Cell Biology 17:292-301 |
|
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P 2005 Nutrient control of glucose homeostasis through a complex of PGC-1[alpha] and SIRT1. Nature 434:113-118 |
|
Drouin J, Sun YL, Chamberland M, Gauthier Y, De Lean A, Nemer M, Schmidt TJ 1993 Novel glucocorticoid receptor complex with DNA element of the hormone- repressed POMC gene. Embo J 12:145-156 |
|
Zhang G, Zhang L, Duff GW 1997 A negative regulatory region containing a glucocorticosteroid response element (nGRE) in the human interleukin-1beta gene. DNA Cell Biol 16:145-152 |
|
Chandran UR, Attardi B, Friedman R, Zheng ZW, Roberts JL, DeFranco DB 1996 Glucocorticoid repression of the mouse gonadotropin-releasing hormone gene is mediated by promoter elements that are recognized by heteromeric complexes containing glucocorticoid receptor. J Biol Chem 271:20412-20420 |
|
Subramaniam N, Cairns W, Okret S 1997 Studies on the mechanism of glucocorticoid-mediated repression from a negative glucocorticoid response element from the bovine prolactin gene. DNA Cell Biol 16:153-163 |
|
Klein-Hitpass L, Ryffel GU, Heitlinger E, Cato AC 1988 A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res 16:647-663 |
|
Klock G, Strahle U, Schutz G 1987 Oestrogen and glucocorticoid responsive elements are closely related but distinct. Nature 329:734-736 |
|
Klinge CM, Bambara RA, Hilf R 1992 Antiestrogen-liganded estrogen receptor interaction with estrogen responsive element DNA in vitro. J Steroid Biochem Molec Biol 43:249-262 |
|
Klinge CM, Peale FV, Jr., Hilf R, Bambara RA, Zain S 1992 Cooperative estrogen receptor interaction with consensus or variant estrogen responsive elements in vitro. Cancer Res 52:1073-1081 |
|
Anolik JH, Klinge CM, Bambara RA, Hilf R 1993 Differential impact of flanking sequences on estradiol- versus 4-hydroxytamoxifen-liganded estrogen receptor binding to estrogen responsive element DNA. J Steroid Biochem Molec Biol 46:713-730 |
|
Anolik JH, Klinge CM, Hilf R, Bambara RA 1995 Cooperative binding of estrogen receptor to DNA depends on spacing of binding sites, flanking sequence, and ligand. Biochemistry 34:2511-2520 |
|
Anolik JH, Klinge CM, Brolly CL, Bambara RA, Hilf R 1996 Stability of the ligand of estrogen response element-bound estrogen receptor depends on flanking sequences and cellular factors. J Steroid Biochem Molec Biol 59:413-429 |
|
Driscoll MD, Klinge CM, Hilf R, Bambara RA 1996 Footprint analysis of estrogen receptor binding to adjacent estrogen response elements. J Steroid Biochem Molec Biol 58:45-61 |
|
Driscoll MD, Sathya G, Muyan M, Klinge CM, Hilf R, Bambara RA 1998 Sequence requirements for estrogen receptor binding to estrogen response elements. J Biol Chem 273:29321-29330 |
|
Klinge CM 2001 Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 29:2905-2919. |
|
Walker P, Germond JE, Brown-Luedi M, Givel F, Wahli W 1984 Sequence homologies in the region preceding the transcription initiation site of the liver estrogen-responsive vitellogenin and apo-VLDL II genes. Nucleic Acids Res 12:8611-8626 |
|
Seiler-Tuyns A, Walker P, Martinez E, Merillat AM, Givel F, Wahli W 1986 Identification of estrogen-responsive DNA sequences by transient expression experiments in a human breast cancer cell line. Nucleic Acids Res 14:8755-8770 |
|
Wahli W, Martinez E, Corthesy B, Cardinaux JR 1989 Cis- and trans- acting elements of the estrogen-regulated vitellogenin gene B1 of Xenopus laevis. J Steroid Biochem 34:17-32 |
|
Brown AM, Jeltsch JM, Roberts M, Chambon P 1984 Activation of pS2 gene transcription is a primary response to estrogen in the human breast cancer cell line MCF-7. Proc Natl Acad Sci USA 81:6344-6348 |
|
Jeltsch JM, Roberts M, Schatz C, Garnier JM, Brown AM, Chambon P 1987 Structure of the human oestrogen-responsive gene pS2. Nucleic Acids Res 15:1401-1414 |
|
Berry M, Nunez AM, Chambon P 1989 Estrogen responsive element of the human pS2 gene is an imperfectly palindromic sequence. Proc Natl Acad Sci USA 86:1218-1222 |
|
Nunez AM, Berry M, Imler JL, Chambon P 1989 The 5' flanking region of the pS2 gene contains a complex enhancer region responsive to oestrogens, epidermal growth factor, a tumour promoter (TPA), the c-Ha-ras oncoprotein and the c-jun protein. EMBO J 8:823-829 |
|
Giamarchi C, Solanas M, Chailleux C, Augereau P, Vignon F, Rochefort H, Richard-Foy H 1999 Chromatin structure of the regulatory regions of pS2 and cathepsin D genes in hormone-dependent and -independent breast cancer cell lines. Oncogene 18:533-541. |
|
Richard S, Zingg HH 1990 The human oxytocin gene promoter is regulated by estrogens. J Biol Chem 265:6098-6103 |
|
Weisz A, Bresciani F 1988 Estrogen induces expression of c-fos and c-myc protooncogenes in rat uterus. Mol Endocrinol 2:816-824 |
|
El-Ashry D, Chrysogelos SA, Lippman ME, Kern FG 1996 Estrogen induction of TGF- is mediated by an estrogen response element composed of two imperfect palindromes. J Steroid Biochem Molec Biol 59:261-269 |
|
Berwaer M, Martial JA, Davis JR 1994 Characterization of an up-stream promoter directing extrapituitary expression of the human prolactin gene. Mol Endocrinol 8:635-642 |
|
Kraus WL, Montano MM, Katzenellenbogen BS 1994 Identification of multiple, widely spaced estrogen-responsive regions in the rat progesterone receptor gene. Mol Endocrinol 8:952-969 |
|
Augereau P, Miralles F, Cavailles V, Gaudelet C, Parker M, Rochefort H 1994 Characterization of the proximal estrogen-responsive element of human cathepsin D gene. Mol Endocrinol 8:693-703 |
|
Tan-Wong SM, French JD, Proudfoot NJ, Brown MA 2008 Dynamic interactions between the promoter and terminator regions of the mammalian BRCA1 gene. Proceedings of the National Academy of Sciences 105:5160-5165 |
|
Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M 2008 FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 132:958-970 |
|
Eeckhoute J, Keeton EK, Lupien M, Krum SA, Carroll JS, Brown M 2007 Positive Cross-Regulatory Loop Ties GATA-3 to Estrogen Receptor {alpha} Expression in Breast Cancer. Cancer Res 67:6477-6483 |
|
Eeckhoute J, Carroll JS, Geistlinger TR, Torres-Arzayus MI, Brown M 2006 A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev 20:2513-2526 |
|
Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M 2006 Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289-1297 |
|
Carroll JS, Brown M 2006 Estrogen Receptor Target Gene: An Evolving Concept. Mol Endocrinol 20:1707-1714 |
|
Liu Y, Gao H, Marstrand TT, Strom A, Valen E, Sandelin A, Gustafsson J-A, Dahlman-Wright K 2008 The genome landscape of ER{alpha}- and ER{beta}-binding DNA regions. Proceedings of the National Academy of Sciences:0712085105 |
|
Kwon Y-S, Garcia-Bassets I, Hutt KR, Cheng CS, Jin M, Liu D, Benner C, Wang D, Ye Z, Bibikova M, Fan J-B, Duan L, Glass CK, Rosenfeld MG, Fu X-D 2007 Sensitive ChIP-DSL technology reveals an extensive estrogen receptor {alpha}-binding program on human gene promoters. PNAS 104:4852-4857 |
|
Umesono K, Murakami KK, Thompson CC, Evans RM 1991 Direct repeats as selective response elements for thyroid hormone, retinoic acid, and Vitamin D3 receptors. Cell 65:1255-1266 |
|
Mangelsdorf DJ, Evans RM 1995 The RXR heterodimers and orphan receptors. Cell 83:841-850 |
|
Thompson EB 1995 Steroid hormones. Membrane transporters of steroid hormones. Curr Biol 5:730-732 |
|
Danzo BJ, Joseph DR 1994 Structure-function relationships of rat androgen--binding protein/human sex-hormone binding globulin: the effect of mutagenesis on steroid- binding parameters. Endocrinology 135:157-167 |
|
Hammond GL, Bocchinfuso WP 1995 Sex hormone-binding globulin/androgen-binding protein: steroid-binding and dimerization domains. J Steroid Biochem Mol Biol 53:543-552 |
|
Joseph DR 1994 Structure, function, and regulation of androgen-binding protein/sex hormone-binding globulin. Vitam Horm 49:197-280 |
|
Westphal U 1986 Steroid-protein interactions II. Monogr Endocrinol 27:1-603 |
|
Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA 1991 Sex hormone-binding globulin: anatomy and physiology of a new regulatory system. J Steroid Biochem Mol Biol 40:813-820 |
|
Porto CS, Lazari MF, Abreu LC, Bardin CW, Gunsalus GL 1995 Receptors for androgen-binding proteins: internalization and intracellular signalling. J Steroid Biochem Mol Biol 53:561-565 |
|
Fortunati N, Raineri M, Cignetti A, Hammond GL, Frairia R 1998 Control of the membrane sex hormone-binding globulin-receptor (SHBG-R) in MCF-7 cells: effect of locally produced SHBG. Steroids 63:282-284 |
|
Gorski J, Williams D, Giannopoulos G, Stancel G 1973 The continuing evolution of an estrogen-receptor model. Adv Exp Med Biol 36:1-14 |
|
Gorski J, Williams D, Giannopoulos G, Stancel G 1973 The continuing evolution of an estrogen-receptor model. Adv Exp Med Biol 36:1-14 |
|
Talwar GP, Segal SJ, Evans A, Davidson OW 1964 The binding of estradiol in the uterus: A mechanism for derepression of RNA synthesis. Proc Natl Acad Sci USA 52:1059-1066 |
|
Monje P, Boland R 2002 Expression and cellular localization of naturally occurring beta estrogen receptors in uterine and mammary cell lines. J Cell Biochem 86:136-144 |
|
Cammarata PR, Chu S, Moor A, Wang Z, Yang S-H, Simpkins JW 2004 Subcellular distribution of native estrogen receptor [alpha] and [beta] subtypes in cultured human lens epithelial cells. Experimental Eye Research 78:861-871 |
|
Chen JQ, Delannoy M, Cooke C, Yager JD 2004 Mitochondrial localization of ERalpha and ERbeta in human MCF7 cells. Am J Physiol Endocrinol Metab 286:E1011-1022 |
|
Chen JQ, Eshete M, Alworth WL, Yager JD 2004 Binding of MCF-7 cell mitochondrial proteins and recombinant human estrogen receptors alpha and beta to human mitochondrial DNA estrogen response elements. J Cell Biochem 93:358 |
|
Jin-Qiang C, Yager JD 2004 Estrogen's Effects on Mitochondrial Gene Expression: Mechanisms and Potential Contributions to Estrogen Carcinogenesis. Ann NY Acad Sci 1028:258-272 |
|
Nilsen J, Brinton RD 2004 Mitochondria as therapeutic targets of estrogen action in the central nervous system. Curr Drug Targets CNS Neurol Disord 3:297-313 |
|
Yang S-H, Liu R, Perez EJ, Wen Y, Stevens SM, Jr., Valencia T, Brun-Zinkernagel A-M, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW 2004 Mitochondrial localization of estrogen receptor {beta}. PNAS 101:4130-4135 |
|
Cammarata PR, Flynn J, Gottipati S, Chu S, Dimitrijevich S, Younes M, Skliris G, Murphy LC 2005 Differential expression and comparative subcellular localization of estrogen receptor beta isoforms in virally transformed and normal cultured human lens epithelial cells. Experimental Eye Research 81:165-175 |
|
Chen J-Q, Yager JD, Russo J 2005 Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1746:1-17 |
|
Levin ER 2005 Integration of the Extranuclear and Nuclear Actions of Estrogen. Mol Endocrinol 19:1951-1959 |
|
Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER 2005 A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science 307:1625-1630 |
|
Simpkins JW, Wang J, Wang X, Perez E, Prokai L, Dykens JA 2005 Mitochondria play a central role in estrogen-induced neuroprotection. Curr Drug Targets CNS Neurol Disord 4:69-83 |
|
Solakidi S, Psarra AM, Sekeris CE 2005 Differential subcellular distribution of estrogen receptor isoforms: Localization of ERalpha in the nucleoli and ERbeta in the mitochondria of human osteosarcoma SaOS-2 and hepatocarcinoma HepG2 cell lines. Biochim Biophys Acta 1745:382-392 |
|
Solakidi S, Psarra A-MG, Nikolaropoulos S, Sekeris CE 2005 Estrogen receptors {alpha} and {beta} (ER{alpha} and ER{beta}) and androgen receptor (AR) in human sperm: localization of ER{beta} and AR in mitochondria of the midpiece. Hum Reprod 20:3481-3487 |
|
Pedram A, Razandi M, Wallace DC, Levin ER 2006 Functional Estrogen Receptors in the Mitochondria of Breast Cancer Cells. Mol Biol Cell 17:2125-2137 |
|
Gavrilova-Jordan LP, Price TM 2007 Actions of steroids in mitochondria. Semin Reprod Med 25:154-164 |
|
Jonsson D, Nilsson J, Odenlund M, Bratthall G, Broman J, Ekblad E, Lydrup M-L, Nilsson B-O 2007 Demonstration of mitochondrial oestrogen receptor [beta] and oestrogen-induced attenuation of cytochrome c oxidase subunit I expression in human periodontal ligament cells. Archives of Oral Biology 52:669-676 |
|
Zeng Q, Chen G, Vlantis A, Tse G, van Hasselt C 2008 The contributions of oestrogen receptor isoforms to the development of papillary and anaplastic thyroid carcinomas. J Pathol 214:425-433 |
|
Chen J-Q, Russo PA, Cooke C, Russo IH, Russo J 2007 ER[beta] shifts from mitochondria to nucleus during estrogen-induced neoplastic transformation of human breast epithelial cells and is involved in estrogen-induced synthesis of mitochondrial respiratory chain proteins. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1773:1732-1746 |
|
Gehring U 1998 Steroid hormone receptors and heat shock proteins. Vitam Horm 54:167-205 |
|
Dittmar KD, Demady DR, Stancato LF, Krishna P, Pratt WB 1997 Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery: The role of p23 is to stabilize receptor.hsp90 heterocomplexes formed by hsp90.p60.hsp70. J Biol Chem 272:21213-21220 |
|
Hutchison KA, Dittmar KD, Stancato LF, Pratt WB 1996 Ability of various members of the hsp70 family of chaperones to promote assembly of the glucocorticoid receptor into a functional heterocomplex with hsp90. J Steroid Biochem Molec Biol 58:251-258 |
|
King WJ, Greene GL 1984 Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature 307:745-747 |
|
Perrot-Applanat M, Groyer-Picard MT, Logeat F, Milgrom E 1986 Ultrastructural localization of the progesterone receptor by an immunogold method: effect of hormone administration. J Cell Biol 102:1191-1199 |
|
Husmann DA, Wilson CM, McPhaul MJ, Tilley WD, Wilson JD 1990 Antipeptide antibodies to two distinct regions of the androgen receptor localize the receptor protein to the nuclei of target cells in the rat and human prostate. Endocrinology 126:2359-2368 |
|
Smith DF, Toft DO 2008 Minireview: The Intersection of Steroid Receptors with Molecular Chaperones: Observations and Questions. Mol Endocrinol 22:2229-2240 |
|
Nover L, Scharf KD 1997 Heat stress proteins and transcription factors. Cell Mol Life Sci 53:80-103 |
|
Pratt WB, Morishima Y, Osawa Y 2008 The Hsp90 Chaperone Machinery Regulates Signaling by Modulating Ligand Binding Clefts. J Biol Chem 283:22885-22889 |
|
Pratt WB, Toft DO 1997 Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev 18:306-360 |
|
Johnson J, Corbisier R, Stensgard B, Toft DJ 1996 The involvement of p23, hsp90, and immunophilins in the assembly of progesterone receptor complexes. J Steroid Biochem Molec Biol 56:31-37 |
|
Naar AM, Beaurang PA, Robinson KM, Oliner JD, Avizonis D, Scheek S, Zwicker J, Kadonaga JT, Tjian R 1998 Chromatin, TAFs, and a novel multiprotein coactivator are required for synergistic activation by Sp1 and SREBP-1a in vitro. Genes Dev 12:3020-3031 |
|
Zwijsen RML, Buckle RS, Hijmans EM, Loomans CJM, Bernards R 1998 Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D. Genes Dev 12:3488-3498 |
|
Watson CS, Alyea RA, Jeng YJ, Kochukov MY 2007 Nongenomic actions of low concentration estrogens and xenoestrogens on multiple tissues. Molecular and Cellular Endocrinology 274:1-7 |
|
Klinge CM 2008 Estrogenic control of mitochondrial function and biogenesis. J Cell Biochem 105:1342-1351 |
|
Segnitz B, Gehring U 1995 Subunit structure of the nonactivated human estrogen receptor. Proc Natl Acad Sci USA 92:2179-2183 |
|
Lee HS, Aumais J, White JH 1996 Hormone-dependent transactivation by estrogen receptor chimeras that do not interact with hsp90. Evidence for transcriptional repressors. J Biol Chem 271:25727-25730 |
|
Landel CC, Kushner PJ, Greene GL 1994 The interaction of human estrogen receptor with DNA is modulated by receptor-associated proteins. Mol Endocrinol 8:1407-1419 |
|
Gething M-J, Sambrook J 1992 Protein folding in the cell. Nature 355:33-45 |
|
Xiao N, DeFranco DB 1997 Overexpression of unliganded steroid receptors activates endogenous heat shock factor. Mol Endocrinol 11:1365 - 1374 |
|
Klinge CM, Brolly CL, Bambara RA, Hilf R 1997 Hsp70 is not required for high affinity binding of purified calf uterine estrogen receptor to estrogen response element DNA in vitro. J Steroid Biochem Molec Biol 63:283-301 |
|
Alarid ET 2006 Lives and Times of Nuclear Receptors. Mol Endocrinol 20:1972-1981 |
|
Guiochon-Mantel A, Delabre K, Lescop P, Milgrom E 1996 Intracellular traffic of steroid hormone receptors. J Steroid Biochem Molec Biol 56:1-6 |
|
DeFranco DB 1997 Subnuclear trafficking of steroid receptors. Biochem Soc Trans 25:592-597 |
|
Elbi C, Walker DA, Romero G, Sullivan WP, Toft DO, Hager GL, DeFranco DB 2004 Molecular chaperones function as steroid receptor nuclear mobility factors. Proc Natl Acad Sci U S A 101:2876-2881 |
|
Ruh MF, Chrivia JC, Cox LK, Ruh TS 2004 The interaction of the estrogen receptor with mononucleosomes. Mol Cell Endocrinol 214:71-79 |
|
Stenoien DL, Patel K, Mancini MG, Dutertre M, Smith CL, O'Malley BW, Mancini MA 2001 FRAP reveals that mobility of oestrogen receptor-alpha is ligand- and proteasome-dependent. Nat Cell Biol 3:15-23. |
|
Matsuda K, Ochiai I, Nishi M, Kawata M 2002 Colocalization and Ligand-Dependent Discrete Distribution of the Estrogen Receptor (ER)alpha and ERbeta. Mol Endocrinol 16:2215-2230. |
|
Zhu XG, Hanover JA, Hager GL, Cheng SY 1998 Hormone-induced translocation of thyroid hormone receptors in living cells visualized using a receptor green fluorescent protein chimera. J Biol Chem 273:27058-27063 |
|
Rosenfeld MG, Glass CK 2001 Coregulator Codes of Transcriptional Regulation by Nuclear Receptors. J Biol Chem 276:36865-36868 |
|
Rosenfeld MG, Lunyak VV, Glass CK 2006 Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev 20:1405-1428 |
|
Huang EY, Zhang J, Miska EA, Guenther MG, Kouzarides T, Lazar MA 2000 Nuclear receptor corepressors partner with class II histone deacetylases in a Sin3-independent repression pathway. Genes Dev 14:45-54 |
|
Carson MA, Tsai MJ, Conneely OM, Maxwell BL, Clark JH, Dobson AD, Elbrecht A, Toft DO, Schrader WT, BW OM 1987 Structure-function properties of the chicken progesterone receptor A synthesized from complementary deoxyribonucleic acid. Mol Endocrinol 1:791-801 |
|
Hollenberg SM, Giguere V, Segui P, Evans RM 1987 Colocalization of DNA-binding and transcriptional activation functions in the human glucocorticoid receptor. Cell 49:39-46 |
|
Lannigan DA 2003 Estrogen receptor phosphorylation. Steroids 68:1-9. |
|
Weigel NL, Moore NL 2007 Steroid Receptor Phosphorylation: A Key Modulator of Multiple Receptor Functions. Mol Endocrinol 21:2311-2319 |
|
Weigel NL, Zhang Y 1998 Ligand -independent activation of steroid hormone receptors. J Mol Med 76:469-479 |
|
Eickelberg O, Roth M, Lorx R, Bruce V, Rudiger J, Malcolm Johnson M, Block L-H 1999 Ligand-independent activation of the glucocorticoid receptor by beta 2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J Biol Chem 274:1005-1010 |
|
Massaad C, Houard N, Lombes M, Barouki R 1999 Modulation of human mineralocorticoid receptor function by protein kinase A. Mol Endocrinol 13:57-65 |
|
Metivier R, Stark A, Flouriot G, Hubner MR, Brand H, Penot G, Manu D, Denger S, Reid G, Kos M, Russell RB, Kah O, Pakdel F, Gannon F 2002 A dynamic structural model for estrogen receptor-alpha activation by ligands, emphasizing the role of interactions between distant A and E domains. Mol Cell 10:1019-1032. |
|
Reid G, Hubner MR, Metivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F 2003 Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell 11:695-707 |
|
Metivier R, Penot G, Carmouche RP, Hubner MR, Reid G, Denger S, Manu D, Brand H, Kos M, Benes V, Gannon F 2004 Transcriptional complexes engaged by apo-estrogen receptor-{alpha} isoforms have divergent outcomes. EMBO J 23:3653-3666 |
|
Huang J, Li X, Maguire CA, Hilf R, Bambara RA, Muyan M 2005 Binding of Estrogen Receptor {beta} to Estrogen Response Element in Situ Is Independent of Estradiol and Impaired by Its Amino Terminus. Mol Endocrinol 19:2696-2712 |
|
Leong H, Sloan JR, Nash PD, Greene GL 2005 Recruitment of Histone Deacetylase 4 to the N-Terminal Region of Estrogen Receptor {alpha}. Mol Endocrinol 19:2930-2942 |
|
Wei X, Xu H, Kufe D 2006 MUC1 Oncoprotein Stabilizes and Activates Estrogen Receptor [alpha]. Molecular Cell 21:295-305 |
|
Trauernicht AM, Kim SJ, Kim NH, Boyer TG 2007 Modulation of Estrogen Receptor {alpha} Protein Level and Survival Function by DBC-1. Mol Endocrinol 21:1526-1536 |
|
Wu HM, Crothers DM 1984 The locus of sequence-directed and protein-induced DNA bending. Nature 308:509-513 |
|
Kerppola TK, Curran T 1997 The transcription activation domains of Fos and Jun induce DNA bending through electrostatic interactions. EMBO J 16:2907-2916 |
|
Schultz JR, Loven MA, Melvin VM, Edwards DP, Nardulli AM 2002 Differential modulation of DNA conformation by estrogen receptors alpha and beta. J Biol Chem 277:8702-8707. |
|
Kapanidis AN, Margeat E, Ho SO, Kortkhonjia E, Weiss S, Ebright RH 2006 Initial Transcription by RNA Polymerase Proceeds Through a DNA-Scrunching Mechanism. Science 314:1144-1147 |
|
Starr D, Hoopes B, Hawley D 1995 DNA bending is an important component of site-specific recognition by the TATA binding protein. J Mol Biol 250:434-446 |
|
Sabbah M, Ricousse SL, Redeuilh G, Baulieu EE 1992 Estrogen receptor-induced bending of the Xenopus vitellogenin A2 gene hormone response element. Biochem Biophys Res Comm 185: 944-952 |
|
Nardulli AM, Greene. GL, Shapiro DJ 1993 Human estrogen receptor bound to an estrogen response element bends DNA. Mol Endocrinol 7:331-340 |
|
Petz LN, Nardulli AM, Kim J, Horwitz KB, Freedman LP, Shapiro DJ 1997 DNA bending is induced by binding of the glucocorticoid receptor DNA binding domain and progesterone receptors to their response element. J Steroid Biochem Mol Biol 60:31-41 |
|
Prendergast P, Pan Z, Edwards DP 1996 Progesterone receptor-induced bending of its target DNA: distinct effects of the A and B receptor forms. Mol Endocrinol 10:393-407 |
|
Shulemovich K, Dimaculangan DD, Katz D, Lazar MA 1995 DNA bending by thyroid hormone receptor: influence of half-site spacing and RXR. Nucleic Acids Res 23:811-818 |
|
Nunez E, Kwon Y-S, Hutt KR, Hu Q, Cardamone MD, Ohgi KA, Garcia-Bassets I, Rose DW, Glass CK, Rosenfeld MG, Fu X-D 2008 Nuclear Receptor-Enhanced Transcription Requires Motor- and LSD1-Dependent Gene Networking in Interchromatin Granules. Cell 132:996-1010 |
|
Onate S, Prendergast P, Wagner JP, Nissen M, Reeves R, Pettijohn DE, Edwards DP 1994 The DNA-bending protein HMG-I enhances progesterone receptor binding to its target DNA sequences. Mol Cell Biol 14:3376-3391 |
|
Boonyaratanakornkit V, Melvin V, Prendergast P, Altmann M, Ronfani L, Bianchi ME, Taraseviciene L, Nordeen SK, Allegretto E, A,, Edwards DP 1998 High-Mobility Group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells. Mol Cell Biol 18:4471-4487 |
|
Bjorklund S, Kim Y-J 1996 Mediator of transcriptional regulation. Trends Biochem Sci 21:335-337 |
|
Kaiser K, Meisterernst M 1996 The human general co-factors. Trends Biochem Sci 21:342-345 |
|
Green MR 2000 TBP-associated factors (TAFIIs): multiple, selective transcriptional mediators in common complexes. Trends Biochem Sci 25:59-63 |
|
Malik S, Roeder RG 2005 Dynamic regulation of pol II transcription by the mammalian Mediator complex. Trends in Biochemical Sciences 30:256-263 |
|
Denissov S, van Driel M, Voit R, Hekkelman M, Hulsen T, Hernandez N, Grummt I, Wehrens R, Stunnenberg H 2007 Identification of novel functional TBP-binding sites and general factor repertoires. Embo J 26:944-954 |
|
Hirose Y, Ohkuma Y 2007 Phosphorylation of the C-terminal Domain of RNA Polymerase II Plays Central Roles in the Integrated Events of Eucaryotic Gene Expression. J Biochem 141:601-608 |
|
Nagy Z, Tora L 2007 Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene 26:5341-5357 |
|
Verrijzer CP, Tjian R 1996 TAFs mediate transcriptional activation and promoter selectivity. Trends Biochem Sci 21:338-342 |
|
Pfahl M 1993 Nuclear receptor/AP-1 interaction. Endocrine Rev 14:651-658 |
|
Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S-C, Heyman RA, Rose DW, Glass CK, Rosenfeld MG 1996 A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403-414 |
|
Scholz A, Truss M, Beato M 1998 Hormone-induced recruitment of Sp1 mediates estrogen activation of the rabbit uteroglobin gene in endometrial epithelium. J Biol Chem 273:4360-4366 |
|
Klein-Hitpass L, Schwerk C, Kahmann S, Vassen L 1998 Targets of activated steroid hormone receptors: basal transcription factors and receptor interacting proteins. J Mol Med 76:490-496 |
|
Ing NH, Beekman JM, Tsai SY, Tsai MJ, O'Malley BW 1992 Members of the steroid hormone receptor superfamily interact with TFIIB (S300-II). J Biol Chem 267: 17617-17623 |
|
Sadovsky Y, Webb P, Lopez G, Baxter JD, Fitzpatrick PM, Gizang-Ginsberg E, Cavailles V, Parker MG, Kushner PJ 1995 Transcriptional activators differ in their responses to over-expression of TATA-box-binding protein. Mol Cell Biol 15:1554-1563 |
|
Jacq X, Brou C, Lutz Y, Davidson I, Chambon P, Tora L 1994 Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell 79:107-117 |
|
Meyer ME, Gronemeyer H, Turcotte B, Bocquel MT, Tasset D, Chambon P 1989 Steroid hormone receptors compete for factors that mediate their enhancer function. Cell 57:433-442 |
|
Lonard DM, Nawaz Z 2001 Coactivators and corepressors. In: Nuclear Receptors and Genetic Disease: Academic Press |
|
Lonard DM, O'Malley BW 2006 The Expanding Cosmos of Nuclear Receptor Coactivators. Cell 125:411-414 |
|
Lonard DM, O'Malley BW 2007 Nuclear Receptor Coregulators: Judges, Juries, and Executioners of Cellular Regulation. Molecular Cell 27:691-700 |
|
Yoshinaga SK, Peterson CL, Herskowitz I, Yamamoto KR 1992 Roles of SWI1, SWI2, and SWI3 proteins for transcriptional enhancement by steroid receptors. Science 258:1598-1604 |
|
Ding XF, Anderson CM, Ma H, Hong H, Uht RM, Kushner PJ, Stallcup MR 1998 Nuclear receptor-binding sites of coactivators glucocorticoid receptor interacting protein 1 (GRIP1) and steroid receptor coactivator 1 (SRC-1): multiple motifs with different binding specificities. Mol Endocrinol 12:302-313 |
|
Eng FCS, Barsalou A, Akutsu N, Mercier I, Zechel C, Made S, White JH 1998 Different classes of coactivators recognize distinct but overlapping binding sites on the estrogen receptor ligand binding domain. J Biol Chem 273:28371-28377 |
|
Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai S, Tsai MJ, O'Malley BW 1997 Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389:194-198 |
|
Dallas PB, Cheney IW, Liao D-W, Bowrin V, Byam W, Pacchione S, Kobayashi R, Yaciuk P, Moran E 1998 p300/CREB binding protein-related protein p270 is a component of mammalian SWI/SNF complexes. Mol Cell Biol 18:3596-3603 |
|
Struhl K 1998 Histone acetylation and transcriptional regulatory mechanisms. Genes Dev 12:599-606 |
|
Roth SY, Denu JM, Allis CD 2001 HISTONE ACETYLTRANSFERASES. Annu Rev Biochem 70:81-120 |
|
Lee KK, Workman JL 2007 Histone acetyltransferase complexes: one size doesn't fit all. Nat Rev Mol Cell Biol 8:284-295 |
|
Ruthenburg AJ, Li H, Patel DJ, David Allis C 2007 Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol 8:983-994 |
|
Sternglanz R 1996 Histone acetylation: A gateway to transcriptional activation. Trends In Biochem Sci 21:357-358 |
|
Shibata H, Spencer TE, Onate SA, Jenster G, Tsai SY, Tsai MJ, O'Malley BW 1997 Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res 52:141-164 |
|
Zhou G, Cummings R, Li Y, Mitra S, Wilkinson HA, Elbrecht A, Hermes JD, Schaeffer JM, Smith RG, Moller DE 1998 Nuclear receptors have distinct affinities for coactivators: characterization by fluorescence resonance energy transfer. Mol Endocrinol 12:1594-1604 |
|
Smith CL, O'Malley BW 2004 Coregulator Function: A Key to Understanding Tissue Specificity of Selective Receptor Modulators. Endocr Rev 25:45-71 |
|
Misiti S, Schomburg L, Yen PM, Chin WW 1998 Expression and hormonal regulation of coactivator and corepressor genes. Endocrinology 139:2493-2500 |
|
Folkers GE, van der Burg B, van der Saag PT 1998 Promoter architecture, cofactors, and orphan receptors contribute to cell-specific activation of the retinoic acid receptor beta2 promoter. J Biol Chem 273:32200-32212 |
|
Klinge CM 2000 Estrogen receptor interaction with co-activators and co-repressors. Steroids 65:227-251 |
|
Gronemeyer H, Laudet V 1995 Transcription factors 3: nuclear receptors. Protein Profile 2:1173-1308 |
|
Chen H, Lin RJ, Schiltz L, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM 1997 Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90:569-580 |
|
Leo C, Li H, Chen JD 2000 Differential mechanisms of nuclear receptor regulation by receptor-associated coactivator 3. J Biol Chem 275:5976-5982 |
|
Nardulli AM, Greene GL, BW OM, Katzenellenbogen BS 1988 Regulation of progesterone receptor messenger ribonucleic acid and protein levels in MCF-7 cells by estradiol: analysis of estrogen's effect on progesterone receptor synthesis and degradation. Endocrinology 122:935-944 |
|
Yeh S, Miyamoto H, Shima H, Chang C 1998 From estrogen to androgen receptor: a new pathway for sex hormones in prostate. Proc Natl Acad Sci U S A 95:5527-5532 |
|
Yeh S, Chang C 1997 Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc Natl Acad Sci U S A 93:5517-5521 |
|
Alen P, Claessens F, Schoenmakers E, Swinnen JV, Verhoeven G, Rombauts W, Peeters B 1999 Interaction of the putative androgen receptor-specific coactivator ARA70/ELE1alpha with multiple steroid receptors and identification of an internally deleted ELE1beta isoform. Mol Endocrinol 13:117-128 |
|
Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S-C, Heyman RA, Rose DW, Glass CK, Rosenfeld MG 1996 A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403-414 |
|
Dallas PB, Cheney IW, Liao D-W, Bowrin V, Byam W, Pacchione S, Kobayashi R, Yaciuk P, Moran E 1998 p300/CREB binding protein-related protein p270 is a component of mammalian SWI/SNF complexes. Mol Cell Biol 18:3596-3603 |
|
Zhou G, Cummings R, Li Y, Mitra S, Wilkinson HA, Elbrecht A, Hermes JD, Schaeffer JM, Smith RG, Moller DE 1998 Nuclear receptors have distinct affinities for coactivators: characterization by fluorescence resonance energy transfer. Mol Endocrinol 12:1594-1604 |
|
Fronsdal K, Engedal N, Slagsvold T, Saatcioglu F 1998 CREB binding protein is a coactivator for the androgen receptor and mediates cross-talk with AP-1. J Biol Chem 273:31853-31859 |
|
Hanstein B, Eckner R, DiRenzo J, Halachmi S, Liu H, Servy B, Kurodawa R, Brown M 1996 P300 is a component of an estrogen receptor coactivator complex. Proc Natl Acad Sci (USA) 93:11540-11545 |
|
Chakravarti D, LaMorte VJ, Nelson MC, Nakajima T, Schulman IG, Juguilon H, Montminy M, Evans RM 1996 Role of CBP/P300 in nuclear receptor signalling. Nature 383:99-103 |
|
Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y 1996 The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87:953-959 |
|
Ogryzko VV, Kotani T, Zhang X, Schlitz RL, Howard T, Yang XJ, Howard BH, Qin J, Nakatani Y 1998 Histone-like TAFs within the PCAF histone acetylase complex. Cell 94:35-44 |
|
Yang X-J, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y 1996 A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature 382:319-324 |
|
Wang JC, Stafford JM, Granner DK 1998 SRC-1 and GRIP1 coactivate transcription with hepatocyte nuclear factor 4. J Biol Chem 273:30847-30850 |
|
Halachmi S, Marden E, Martin G, MacKay H, Abbondanza C, Brown M 1994 Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science 264:1455-1458 |
|
Cavailles V, Dauvois S, Danielian PS, Parker MGI 1994 Interaction of proteins with transcriptionally active estrogen receptors. Proc Natl Acad Sci USA 91:10009-10013 |
|
Cavailles V, Dauvois S, L’Horst F, Lopez G, Hoare S, Kushner PJ, Parker MG 1995 Nuclear factor RIP 140 modulates transcriptional activation by the estrogen receptor. EMBO J 14:3741-3751 |
|
L'Horset F, Dauvois S, Heery DM, Cavailles V, Parker MG 1996 RIP-140 interacts with multiple nuclear receptors by means of two distinct sites. Mol Cell Biol 16:6029-6036 |
|
Treuter E, Albrektsen T, Johansson L, Leers J, Gustafsson JA 1998 A regulatory role for RIP140 in nuclear receptor activation. Mol Endocrinol 12:864-881 |
|
Hanstein B, Liu H, Yancisin MC, Brown M 1999 Functional analysis of a novel estrogen receptor-b isoform. Mol Endocrinol 13:129-137 |
|
Onate SA, Boonyaratanakornkit V, Spencer TE, Tsai SY, Tsai M-J, Edwards DP, O'Malley BW 1998 The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J Biol Chem 273:12101-12108 |
|
Smith CL, Nawaz Z, O'Malley BW 1997 Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol 11:657-666 |
|
Onate SA, Tsai SY, Tsai M-J, O’Malley BW 1995 Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354-1357 |
|
Yao T-P, Ku G, Shou B, Scully R, Livingston DM 1996 The nuclear hormone receptor coactivator SRC-1 is a specific target of p300. Proc Natl Acad Sci USA 93:10026-10031 |
|
Yoshinaga SK, Peterson CL, Herskowitz I, Yamamoto KR 1992 Roles of SWI1, SWI2, and SWI3 proteins for transcriptional enhancement by steroid receptors. Science 258:1598-1604 |
|
Muchardt C, Yaniv M 1993 A human homologue of Saccharomyces cerevisiae SNF2/SWI2 and Drosophila brm genes potentiates transcriptional activation by the glucocorticoid receptor. EMBO J 12:4279-4290 |
|
Chiba H, Muramatsu M, Nomota A, Kato H 1994 Two human homologues of Saccharomyces cerevisiae SW12/SNF2 and Drosophila brahma are transcriptional coactivators cooperating with the estrogen receptor and the retinoid acid receptor. Nucl Acids Res 22:1815-1820 |
|
Le Douarin B, You J, Nielsen AL, Chambon P, Losson R 1998 TIF1a: A possible link between KRAB zinc finger proteins and nuclear receptors. J Steroid Biochem Molec Biol 65:43-50 |
|
Le Douarin B, Zechel C, Garnier J-M, Lutz Y, Tora L, Peirrat B, Heery D, Gronemeyer H, Chambon P, Losson R 1995 The N-terminal part of TIF-1, a putative mediator of the ligand-dependent activation function (AF-2) of nuclear receptors, is fused to B-raf in the oncogenic protein T18. EMBO J 14: 2020-2033 |
|
Le Douarin B, vom Baur E, Zechel C, Heery D, Heine M, Vivat V, Gronemeyer H, Losson R, Chambon P 1996 Ligand-dependent interaction of nuclear receptors with potential transcriptional intermediary factors (mediators). Philos Trans R Soc Lond B Biol Sci 351:569-578 |
|
Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V 1997 Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor b. Mol Endocrinol 11:353-365 |
|
vom Baur E, Zechel C, Heery D, Heine MJS, Garnier JM, Vivat V, LeDouarin B, Gronemeyer H, Chambon P, Losson R 1996 Differential ligand-dependent interactions between the AF-2 activating domain of nuclear receptors and the putative transcriptional intermediary factors mSUG1 and TIF1. EMBO J 15:110-124 |
|
Owen-Hughes T, Utley RT, Cote J, Peterson CL, Workman JL 1996 Persistent site-specific remodeling of a nucleosome array by transient action of the SWI/SNF complex. Science 273:513-516 |
|
Voegel JJ, Meine MJS, Zechel C, Chambon P, Gronemeyer H 1996 TIF2, a 160kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J 15:3667-3675 |
|
Hong H, Kohli K, Garagedian MJ, Stallcup MR 1997 GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol Cell Biol 17:2735-2744 |
|
Voegel JJ, Heine MJ, Tini M, Vivat V, Chambon P, Gronemeyer H 1998 The coactivator TIF2 contains three nuclear receptor-binding motifs and mediates transactivation through CBP binding-dependent and -independent pathways. EMBO J 17:507-519 |
|
Norris JD, Fan D, Stallcup MR, McDonnell DP 1998 Enhancement of estrogen receptor transcriptional activity by the coactivator GRIP-1 highlights the role of activation function 2 in determining estrogen receptor pharmacology. J Biol Chem 273: 6679-6688 |
|
Saatcioglu F, Lopez G, West BL, Zandi E, Feng W, Lu H, Esmaili A, Apriletti JW, Kushner PJ, Baxter JD, Karin M 1997 Mutations in the conserved C-terminal sequence in thyroid hormone receptor dissociate hormone-dependent activation from interference with AP-1 activity. Mol Cell Biol 17:4687-4695 |
|
Walfish PG, Yoganathan T, Yang YF, Hong H, Butt TR, Stallcup MR 1997 Yeast hormone response element assays detect and characterize GRIP1 coactivator-dependent activation of transcription by thyroid and retinoid nuclear receptors. Proc Natl Acad Sci U S A 94:3697-3702 |
|
Gruvberger S, Ringner M, Chen Y, Panavally S, Saal LH, Borg A, Ferno M, Peterson C, Meltzer PS 2001 Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res 61:5979-5984. |
|
Lobenhofer EK, Bennett L, Cable PL, Li L, Bushel PR, Afshari CA 2002 Regulation of DNA replication fork genes by 17beta-estradiol. Mol Endocrinol 16:1215-1229. |
|
Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS 2003 Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144:4562-4574 |
|
Frasor J, Stossi F, Danes JM, Komm B, Lyttle CR, Katzenellenbogen BS 2004 Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res 64:1522-1533 |
|
Kian Tee M, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC 2004 Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol Biol Cell 15:1262-1272 |
|
Stossi F, Barnett DH, Frasor J, Komm B, Lyttle CR, Katzenellenbogen BS 2004 Transcriptional Profiling of Estrogen-Regulated Gene Expression via Estrogen Receptor (ER) {alpha} or ER{beta} in Human Osteosarcoma Cells: Distinct and Common Target Genes for These Receptors. Endocrinology 145:3473-3486 |
|
Labhart P, Karmakar S, Salicru EM, Egan BS, Alexiadis V, O'Malley BW, Smith CL 2005 Identification of target genes in breast cancer cells directly regulated by the SRC-3/AIB1 coactivator. PNAS 102:1339-1344 |
|
O'Donnell AJM, Macleod KG, Burns DJ, Smyth JF, Langdon SP 2005 Estrogen receptor-{alpha} mediates gene expression changes and growth response in ovarian cancer cells exposed to estrogen. Endocr Relat Cancer 12:851-866 |
|
Monroe DG, Getz BJ, Johnsen SA, Riggs BL, Khosla S, Spelsberg TC 2003 Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERalpha or ERbeta. J Cell Biochem 90:315-326 |
|
Monroe DG, Secreto FJ, Hawse JR, Subramaniam M, Khosla S, Spelsberg TC 2006 Estrogen Receptor Isoform-specific Regulation of the Retinoblastoma-binding Protein 1 (RBBP1) Gene: ROLES OF AF1 AND ENHANCER ELEMENTS. J Biol Chem 281:28596-28604 |
|
Cvoro A, Tatomer D, Tee M-K, Zogovic T, Harris HA, Leitman DC 2008 Selective Estrogen Receptor- Agonists Repress Transcription of Proinflammatory Genes. J Immunol 180:630-636 |
|
Gottlicher M, Heck S, Herrlich P 1998 Transcriptional cross-talk, the second mode of steroid hormone receptor action. J Mol Med 76:480-489 |
|
Maroder M, Farina AR, Vacca A, Felli MP, Meco D, Screpanti I, Frati L, Gulino A 1993 Cell-specific bifunctional role of Jun oncogene family members on glucocorticoid receptor-dependent transcription. Mol Endocrinol 7:570-584 |
|
Diamond MI, Miner JN, Yoshinaga SK, Yamamoto KR 1990 Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science 249:1266-1272 |
|
Cheung E, Acevedo ML, Cole PA, Kraus WL 2005 Altered pharmacology and distinct coactivator usage for estrogen receptor-dependent transcription through activating protein-1. PNAS 102:559-564 |
|
Webb P, Lopez GN, Uht RM, Kushner PJ 1995 Tamoxifen activation of the estrogen receptor/AP-1 pathway. Mol Endocrinol 9:443-456 |
|
Tzukerman M, Zhang XK, Pfahl M 1991 Inhibition of estrogen receptor activity by the tumor promoter 12-O- tetradeconylphorbol-13-acetate: a molecular analysis. Mol Endocrinol 5:1983-1992 |
|
Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS 1997 Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 277:1508-1510 |
|
Safe S, Kim K 2008 Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol 41:263-275 |
|
Krishnan V, Wang X, Safe S 1994 Estrogen receptor-SP1 complexes mediate estrogen-induced cathepsin D gene expression in MCF-7 human breast cancer cells. J Biol Chem 269:15912-15917 |
|
Rishi AK, Gerald TM, Shao ZM, Li XS, Baumann RG, Dawson MI, Fontana JA 1996 Regulation of the human retinoic acid receptor alpha gene in the estrogen receptor negative human breast carcinoma cell lines SKBR-3 and MDA-MB-435. Cancer Res 56:5246-5252 |
|
Stoner M, Wormke M, Saville B, Samudio I, Qin C, Abdelrahim M, Safe S 2004 Estrogen regulation of vascular endothelial growth factor gene expression in ZR-75 breast cancer cells through interaction of estrogen receptor alpha and SP proteins. Oncogene 23:1052-1063 |
|
Duan R, Porter W, Safe S 1998 Estrogen-induced c-fos protooncogene expression in MCF-7 human breast cancer cells: Role of estrogen receptor Sp1 complex formation. Endocrinology 139:1981-1990 |
|
Porter W, Saville B, Hoivik D, Safe S 1997 Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol Endocrinol 11:1569-1580 |
|
Chu S, Nishi Y, Yanase T, Nawata H, Fuller PJ 2004 Transrepression of Estrogen Receptor {beta} Signaling by Nuclear Factor-{kappa}B in Ovarian Granulosa Cells. Mol Endocrinol 18:1919-1928 |
|
Fronsdal K, Engedal N, Slagsvold T, Saatcioglu F 1998 CREB binding protein is a coactivator for the androgen receptor and mediates cross-talk with AP-1. J Biol Chem 273:31853-31859 |
|
Sheppard KA, Phelps KM, Williams AJ, Thanos D, Glass CK, Rosenfeld MG, Gerritsen ME, Tucker Collins T 1998 Nuclear integration of glucocorticoid receptor and nuclear factor-kappaB signaling by CREB-binding protein and steroid receptor coactivator-1. J Biol Chem 273:29291-29294 |
|
Gurevich I, Flores AM, Aneskievich BJ 2007 Corepressors of agonist-bound nuclear receptors. Toxicology and Applied Pharmacology 223:288-298 |
|
Wagner BL, Norris JD, Knotts TS, Weigel NL, McDonnell DP 1998 The nuclear corepressors NCoR and SMRT are key regulators of both ligand- and 8-bromo-cyclic AMP-dependent transcriptional activity of the human progesterone receptor. Mol Cell Biol 18:1369-1378 |
|
Naughton C, MacLeod K, Kuske B, Clarke R, Cameron DA, Langdon SP 2007 Progressive Loss of Estrogen Receptor {alpha} Cofactor Recruitment in Endocrine Resistance. Mol Endocrinol 21:2615-2626 |
|
Lavinsky R, Jepsen K, Heinzel T, Torchia J, Mullen TM, Schiff R, Del-Rio AL, Ricote M NS, Gemsch J, Hilsenbeck SG, Osborne CK, Glass CK, Rosenfeld MG, Rose DW 1998 Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc Natl Acad Sci USA 95:2920-2925 |
|
Smith CL, Nawaz Z, O'Malley BW 1997 Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol 11:657-666 |
|
Chen JD, Evans RM 1995 A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:454-457 |
|
Klinge CM, Silver BF, Driscoll MD, Sathya G, Bambara RA, Hilf R 1997 COUP-TF interacts with estrogen receptor, binds to estrogen response elements and half-sites, and modulates estrogen-induced gene expression. J Biol Chem 272:31465-31474 |
|
Shang Y, Brown M 2002 Molecular determinants for the tissue specificity of SERMs. Science 295:2465-2468. |
|
Onate SA, Boonyaratanakornkit V, Spencer TE, Tsai SY, Tsai M-J, Edwards DP, O'Malley BW 1998 The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J Biol Chem 273:12101-12108 |
|
Le Douarin B, You J, Nielsen AL, Chambon P, Losson R 1998 TIF1a: A possible link between KRAB zinc finger proteins and nuclear receptors. J Steroid Biochem Molec Biol 65:43-50 |
|
Smith CL, Nawaz Z, O'Malley BW 1997 Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol 11:657-666 |
|
Lavinsky R, Jepsen K, Heinzel T, Torchia J, Mullen TM, Schiff R, Del-Rio AL, Ricote M NS, Gemsch J, Hilsenbeck SG, Osborne CK, Glass CK, Rosenfeld MG, Rose DW 1998 Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc Natl Acad Sci USA 95:2920-2925 |
|
Jackson DA 1997 Chromatin domains and nuclear compartments: establishing sites of gene expression in eukaryotic nuclei. Mol Biol Rep 24:209-220 |
|
Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M 2000 Multiple actions of steroid hormones--a focus on rapid, nongenomic effects. Pharmacol Rev 52:513-556. |
|
Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR 2000 Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-Kinase-Akt pathway in human endothelial cells. Circ Res 87:677-682 |
|
Mendelsohn ME 2000 Mechanisms of estrogen action in the cardiovascular system. The Journal of Steroid Biochemistry and Molecular Biology 74:337-343 |
|
Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B 2000 Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc Natl Acad Sci U S A 97:11603-11608 |
|
Chambliss KL, Shaul PW 2002 Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev 23:665-686. |
|
Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW 2002 ERbeta has nongenomic action in caveolae. Mol Endocrinol 16:938-946. |
|
Watson CS, Campbell CH, Gametchu B 2002 The dynamic and elusive membrane estrogen receptor-alpha. Steroids 67:429-437. |
|
Li L, Haynes MP, Bender JR 2003 Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A 100:4807-4812. |
|
Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER 2003 Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol 23:1633-1646. |
|
Razandi M, Pedram A, Park ST, Levin ER 2003 Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem 278:2701-2712. |
|
Watson CS, Bulayeva NN, Wozniak AL, Finnerty CC 2005 Signaling from the membrane via membrane estrogen receptor-[alpha]: Estrogens, xenoestrogens, and phytoestrogens. Steroids 70:364-371 |
|
Jacob J, Sebastian KS, Devassy S, Priyadarsini L, Farook MF, Shameem A, Mathew D, Sreeja S, Thampan RV 2006 Membrane estrogen receptors: Genomic actions and post transcriptional regulation. Molecular and Cellular Endocrinology 246:34-41 |
|
Pedram A, Razandi M, Levin ER 2006 Nature of Functional Estrogen Receptors at the Plasma Membrane. Mol Endocrinol 20:1996-2009 |
|
Song RXD, Santen RJ 2006 Membrane Initiated Estrogen Signaling in Breast Cancer. Biol Reprod 75:9-16 |
|
Watson CS, Alyea RA, Hawkins BE, Thomas ML, Cunningham KA, Jakubas AA 2006 Estradiol effects on the dopamine transporter - protein levels, subcellular location, and function. J Mol Signal 1:5 |
|
Lopez-Tarruella S, Schiff R 2007 The Dynamics of Estrogen Receptor Status in Breast Cancer: Re-shaping the Paradigm. Clin Cancer Res 13:6921-6925 |
|
Pedram A, Razandi M, Sainson RCA, Kim JK, Hughes CC, Levin ER 2007 A Conserved Mechanism for Steroid Receptor Translocation to the Plasma Membrane. J Biol Chem 282:22278-22288 |
|
Vasudevan N, Pfaff DW 2007 Membrane-Initiated Actions of Estrogens in Neuroendocrinology: Emerging Principles. Endocr Rev 28:1-19 |
|
Gametchu B 1987 Glucocorticoid receptor-like antigen in lymphoma cell membranes: correlation to cell lysis. Science 236:456-461 |
|
Gametchu B, Watson CS, Pasko D 1991 Size and steroid-binding characterization of membrane-associated glucocorticoid receptor in S-49 lymphoma cells. Steroids 56:402-410 |
|
Heinlein CA, Chang C 2002 The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol 16:2181-2187. |
|
Lutz LB, Jamnongjit M, Yang WH, Jahani D, Gill A, Hammes SR 2003 Selective modulation of genomic and nongenomic androgen responses by androgen receptor ligands. Mol Endocrinol 17:1106-1116. |
|
Jones RD, English KM, Jones TH, Channer KS 2004 Testosterone-induced coronary vasodilatation occurs via a non-genomic mechanism: evidence of a direct calcium antagonism action. Clin Sci (Lond) 107:149-158 |
|
Alimirah F, Chen J, Basrawala Z, Xin H, Choubey D 2006 DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: Implications for the androgen receptor functions and regulation. FEBS Letters 580:2294-2300 |
|
Cheng J, Watkins SC, Walker WH 2007 Testosterone Activates Mitogen-Activated Protein Kinase via Src Kinase and the Epidermal Growth Factor Receptor in Sertoli Cells. Endocrinology 148:2066-2074 |
|
Gatson JW, Singh M 2007 Activation of a Membrane-Associated Androgen Receptor Promotes Cell Death in Primary Cortical Astrocytes. Endocrinology 148:2458-2464 |
|
Mendoza C, Tesarik J 1993 A plasma-membrane progesterone receptor in human sperm is switched on by increasing intracellular free calcium. FEBS Lett 330:57-60 |
|
Zhu Y, Rice CD, Pang Y, Pace M, Thomas P 2003 From the Cover: Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. PNAS 100:2231-2236 |
|
Zhu Y, Rice CD, Pang Y, Pace M, Thomas P 2003 Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A 100:2231-2236 |
|
Thomas P, Pang Y, Zhu Y, Detweiler C, Doughty K 2004 Multiple rapid progestin actions and progestin membrane receptor subtypes in fish. Steroids 69:567-573 |
|
Thomas P, Pang Y, Filardo EJ, Dong J 2005 Identity of an Estrogen Membrane Receptor Coupled to a G Protein in Human Breast Cancer Cells. Endocrinology 146:624-632 |
|
Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P 2006 Progesterone Signaling in Human Myometrium through Two Novel Membrane G Protein-Coupled Receptors: Potential Role in Functional Progesterone Withdrawal at Term. Mol Endocrinol 20:1519-1534 |
|
Thomas P, Dong J 2006 Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. The Journal of Steroid Biochemistry and Molecular Biology 102:175-179 |
|
Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P 2007 Activation of the Novel Estrogen Receptor G Protein-Coupled Receptor 30 (GPR30) at the Plasma Membrane. Endocrinology 148:3236-3245 |
|
Teng Y, Girvan AC, Casson LK, Pierce WM, Jr., Qian M, Thomas SD, Bates PJ 2007 AS1411 Alters the Localization of a Complex Containing Protein Arginine Methyltransferase 5 and Nucleolin. Cancer Res 67:10491-10500 |
|
Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, Zhu Y, Tubbs C 2007 Steroid and G Protein Binding Characteristics of the Seatrout and Human Progestin Membrane Receptor {alpha} Subtypes and Their Evolutionary Origins. Endocrinology 148:705-718 |
|
Scherrer LC, Pratt WB 1992 Association of the transformed glucocorticoid receptor with a cytoskeletal protein complex. J Steroid Biochem Mol Biol 41:719-721 |
|
Koukouritaki SB, Margioris AN, Gravanis A, Hartig R, Stournaras C 1997 Dexamethasone induces rapid actin assembly in human endometrial cells without affecting its synthesis. J Cell Biochem 65:492-500 |
|
Demonacos CV, Karayanni N, Hatzoglou E, Tsiriyiotis C, Spandidos DA, Sekeris CE 1996 Mitochondrial genes as sites of primary action of steroid hormones. Steroids 61:226-232 |
|
Sionov RV, Cohen O, Kfir S, Zilberman Y, Yefenof E 2006 Role of mitochondrial glucocorticoid receptor in glucocorticoid-induced apoptosis. J Exp Med 203:189-201 |
|
Joëls M, Karst H, DeRijk R, de Kloet ER 2008 The coming out of the brain mineralocorticoid receptor. Trends in Neurosciences 31:1-7 |
|
Baulieu EE, Robel P 1995 Non-genomic mechanisms of action of steroid hormones. Ciba Found Symp 191:24-37 |
|
Baulieu EE, Schumacher M 1997 Neurosteroids, with special reference to the effect of progesterone on myelination in peripheral nerves. Mult Scler 3:105-112 |
|
Maller JL 2003 Signal transduction. Fishing at the cell surface. Science 300:594-595 |
|
Hawkins MB, Thomas P 2004 The unusual binding properties of the third distinct teleost estrogen receptor subtype ERbetaa are accompanied by highly conserved amino acid changes in the ligand binding domain. Endocrinology 145:2968-2977 |
|
Braun AM, Thomas P 2004 Biochemical characterization of a membrane androgen receptor in the ovary of the atlantic croaker (Micropogonias undulatus). Biol Reprod 71:146-155 |
|
Josefsberg Ben-Yehoshua L, Lewellyn AL, Thomas P, Maller JL 2007 The Role of Xenopus Membrane Progesterone Receptor {beta} in Mediating the Effect of Progesterone on Oocyte Maturation. Mol Endocrinol 21:664-673 |
|
Thomas P 2008 Characteristics of membrane progestin receptor alpha (mPR[alpha]) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Frontiers in Neuroendocrinology 29:292-312 |
|
Krietsch T, Fernandes MS, Kero J, Losel R, Heyens M, Lam EWF, Huhtaniemi I, Brosens JJ, Gellersen B 2006 Human Homologs of the Putative G Protein-Coupled Membrane Progestin Receptors (mPR{alpha}, {beta}, and {gamma}) Localize to the Endoplasmic Reticulum and Are Not Activated by Progesterone. Mol Endocrinol 20:3146-3164 |
|
Martinez S, Pasten P, Suarez K, Garcia A, Nualart F, Montecino M, Hinrichs MV, Olate J 2007 Classical Xenopus laevis progesterone receptor associates to the plasma membrane through its ligand-binding domain. J Cell Physiol 211:560-567 |
|
Revelli A, Massobrio M, Tesarik J 1998 Nongenomic actions of steroid hormones in reproductive tissues. Endocrine Rev 19:3-17 |
|
Meyer C, Schmid R, Schmieding K, Falkenstein E, Wehling M 1998 Characterization of high affinity progesterone-binding membrane proteins by anti-peptide antiserum. Steroids 63:111-116 |
|
Lange CA, Richer JK, Shen T, Horwitz KB 1998 Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation Of mitogen-activated protein kinase pathways. J Biol Chem 273:31308-31316 |
|
Richer JK, Lange CA, Manning NG, Owen G, Powell R, Horwitz KB 1998 Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem 273:31317-31326 |
|
Qiu M, Lange CA 2003 MAP kinases couple multiple functions of human progesterone receptors: degradation, transcriptional synergy, and nuclear association. J Steroid Biochem Mol Biol 85:147-157 |
|
Lange CA 2004 Making sense of cross-talk between steroid hormone receptors and intracellular signaling pathways: who will have the last word? Mol Endocrinol 18:269-278 |
|
Watson CS, Lange CA 2005 Steadying the boat: integrating mechanisms of membrane and nuclear-steroid-receptor signalling. EMBO Rep 6:116-119 |
|
Faivre E, Skildum A, Pierson-Mullany L, Lange CA 2005 Integration of progesterone receptor mediated rapid signaling and nuclear actions in breast cancer cell models: Role of mitogen-activated protein kinases and cell cycle regulators. Steroids 70:418-426 |
|
Hawkinson JE, Kimbrough CL, Belelli D, Lambert JJ, Purdy RH, Lan NC 1994 Correlation of neuroactive steroid modulation of [35S]t- butylbicyclophosphorothionate and [3H]flunitrazepam binding and gamma- aminobutyric acidA receptor function. Mol Pharmacol 46:977-985 |
|
Kim JK, Levin ER 2006 Estrogen signaling in the cardiovascular system. Nucl Recept Signal 4:e013 |
|
Pappas TC, Gametchu B, Watson CS 1995 Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J 9:404-410 |
|
Watson CS, Pappas TC, Gametchu B 1995 The other estrogen receptor in the plasma membrane: implications for the actions of environmental estrogens. Environ Health Perspect 103 Suppl 7:41-50 |
|
Bulayeva NN, Gametchu B, Watson CS 2004 Quantitative measurement of estrogen-induced ERK 1 and 2 activation via multiple membrane-initiated signaling pathways. Steroids 69:181-192 |
|
Bulayeva NN, Watson CS 2004 Xenoestrogen-induced ERK-1 and ERK-2 activation via multiple membrane-initiated signaling pathways. Environ Health Perspect 112:1481-1487 |
|
Bulayeva NN, Wozniak A, Lash LL, Watson CS 2004 Mechanisms of membrane estrogen receptor-{alpha}-mediated rapid stimulation of Ca2+ levels and prolactin release in a pituitary cell line. Am J Physiol Endocrinol Metab:00349.02004 |
|
Steinsapir J 1992 Microsomal steroid receptors in target tissues. Receptor 2:45-76 |
|
Toran-Allerand CD 2004 Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology 145:1069-1074 |
|
Farhat MY, Lavigne MC, Ramwell PW 1996 The vascular protective effects of estrogen. FASEB J 10:615-624 |
|
Farhat MY, Abi-Younes S, Dingaan B, Vargas R, Ramwell PW 1996 Estradiol increases cyclic adenosine monophosphate in rat pulmonary vascular smooth muscle cells by a nongenomic mechanism. J Pharmacol Exp Ther 276:652-657 |
|
Mueck AO, Seeger H, Lippert TH 1996 Calcium antagonistic effect of natural and synthetic estrogens-- investigations on a nongenomic mechanism of direct vascular action. Int J Clin Pharmacol Ther 34:424-426 |
|
Simoncini T, Varone G, Fornari L, Mannella P, Luisi M, Labrie F, Genazzani AR 2002 Genomic and nongenomic mechanisms of nitric oxide synthesis induction in human endothelial cells by a fourth-generation selective estrogen receptor modulator. Endocrinology 143:2052-2061. |
|
Kim KH, Bender JR 2005 Rapid, estrogen receptor-mediated signaling: why is the endothelium so special? Sci STKE 2005:pe28 |
|
Warner M, Gustafsson J-A 2006 Nongenomic effects of estrogen: Why all the uncertainty? Steroids 71:91-95 |
|
Li L, Hisamoto K, Kim KH, Haynes MP, Bauer PM, Sanjay A, Collinge M, Baron R, Sessa WC, Bender JR 2007 Variant estrogen receptor c-Src molecular interdependence and c-Src structural requirements for endothelial NO synthase activation. Proceedings of the National Academy of Sciences 104:16468-16473 |
|
Subah Packer C 2007 Estrogen protection, oxidized LDL, endothelial dysfunction and vasorelaxation in cardiovascular disease: New insights into a complex issue. Cardiovascular Research 73:6-7 |
|
Tesarik J, Mendoza C 1997 Direct non-genomic effects of follicular steroids on maturing human oocytes: oestrogen versus androgen antagonism. Hum ReprodUpdate 3:95-100 |
|
Razandi M, Pedram A, Greene GL, Levin ER 1999 Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ER and ER expressed in Chinese hamster ovary cells. Mol Endocrinol 13:307-319 |
|
Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER 2004 Plasma Membrane Estrogen Receptors Exist and Functions as Dimers. Mol Endocrinol 18:2854-2865 |
|
Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M 2005 Palmitoylation-dependent Estrogen Receptor {alpha} Membrane Localization: Regulation by 17{beta}-Estradiol. Mol Biol Cell 16:231-237 |
|
Improta-Brears T, Whorton AR, Codazzi F, York JD, Meyer T, McDonnell DP 1999 Estrogen-induced activation of mitogen-activated protein kinase requires mobilization of intracellular calcium. Proc Natl Acad Sci USA 96:4686-4691 |
|
Kauser K, Rubanyi GM 1997 Potential cellular signaling mechanisms mediating upregulation of endothelial nitric oxide production by estrogen. J Vasc Res 34:229-236 |
|
Graber R, Sumida C, Vallette G, Nunez EA 1993 Rapid and long-term effects of 17 beta-estradiol on PIP2-phospholipase C-specific activity of MCF-7 cells. Cell Signal 5:181-186 |
|
Stoica GE, Franke TF, Wellstein A, Czubayko F, List HJ, Reiter R, Morgan E, Martin MB, Stoica A 2003 Estradiol Rapidly Activates Akt via the ErbB2 Signaling Pathway. Mol Endocrinol 17:818-830. |
|
Moriarty K, Kim KH, Bender JR 2006 Estrogen Receptor-Mediated Rapid Signaling. Endocrinology 147:5557-5563 |
|
Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ 2004 The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor {alpha} to the plasma membrane. PNAS 101:2076-2081 |
|
Greger JG, Guo Y, Henderson R, Ross JF, Cheskis BJ 2006 Characterization of MNAR expression. Steroids 71:317-322 |
|
Cheskis BJ 2004 Regulation of cell signalling cascades by steroid hormones. J Cell Biochem 93:20-27 |
|
Barletta F, Wong CW, McNally C, Komm BS, Katzenellenbogen B, Cheskis BJ 2004 Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol Endocrinol 18:1096-1108 |
|
Hisamoto K, Bender JR 2005 Vascular cell signaling by membrane estrogen receptors. Steroids 70:382-387 |
|
Flototto T, Niederacher D, Hohmann D, Heimerzheim T, Dall P, Djahansouzi S, Bender HG, Hanstein B 2004 Molecular mechanism of estrogen receptor (ER)[alpha]-specific, estradiol-dependent expression of the progesterone receptor (PR) B-isoform. The Journal of Steroid Biochemistry and Molecular Biology 88:131-142 |
|
Lim KT, Cosgrave N, Hill AD, Young LS 2006 Nongenomic oestrogen signalling in oestrogen receptor negative breast cancer cells: a role for the angiotensin II receptor AT1. Breast Cancer Res 8:R33 |
|
Filardo EJ 2002 Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol 80:231-238. |
|
Belcher SM, Le HH, Spurling L, Wong JK 2005 Rapid Estrogenic Regulation of Extracellular Signal- Regulated Kinase 1/2 Signaling in Cerebellar Granule Cells Involves a G Protein- and Protein Kinase A-Dependent Mechanism and Intracellular Activation of Protein Phosphatase 2A. Endocrinology 146:5397-5406 |
|
Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y 2006 G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochemical and Biophysical Research Communications 346:904-910 |
|
Vivacqua A, Bonofiglio D, Albanito L, Madeo A, Rago V, Carpino A, Musti AM, Picard D, Ando S, Maggiolini M 2006 17beta-Estradiol, Genistein, and 4-Hydroxytamoxifen Induce the Proliferation of Thyroid Cancer Cells through the G Protein-Coupled Receptor GPR30. Mol Pharmacol 70:1414-1423 |
|
Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M 2006 The G Protein-Coupled Receptor GPR30 Mediates the Proliferative Effects Induced by 17{beta}-Estradiol and Hydroxytamoxifen in Endometrial Cancer Cells. Mol Endocrinol 20:631-646 |
|
Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M 2007 G Protein-Coupled Receptor 30 (GPR30) Mediates Gene Expression Changes and Growth Response to 17{beta}-Estradiol and Selective GPR30 Ligand G-1 in Ovarian Cancer Cells. Cancer Res 67:1859-1866 |
|
Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, Steinhoff MM, Sabo E 2006 Distribution of GPR30, a Seven Membrane-Spanning Estrogen Receptor, in Primary Breast Cancer and its Association with Clinicopathologic Determinants of Tumor Progression. Clin Cancer Res 12:6359-6366 |
|
Filardo EJ, Quinn JA, Sabo E 2008 Association of the membrane estrogen receptor, GPR30, with breast tumor metastasis and transactivation of the epidermal growth factor receptor. Steroids 73:870-873 |
|
Herynk MH, Fuqua SAW 2004 Estrogen Receptor Mutations in Human Disease. Endocr Rev 25:869-898 |
|
Gronemeyer H, Laudet V 1995 Transcription factors 3: nuclear receptors. Protein Profile 2:1173-1308 |
|
Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P 1990 Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603-1614 |
|
Valley CC, Solodin NM, Powers GL, Ellison SJ, Alarid ET 2008 Temporal variation in estrogen receptor-{alpha} protein turnover in the presence of estrogen. J Mol Endocrinol 40:23-34 |
|
Syms AJ, Norris JS, Panko WB, Smith RG 1985 Mechanism of androgen-receptor augmentation. Analysis of receptor synthesis and degradation by the density-shift technique. J Biol Chem 260:455-461 |
|
Nardulli AM, Greene GL, BW OM, Katzenellenbogen BS 1988 Regulation of progesterone receptor messenger ribonucleic acid and protein levels in MCF-7 cells by estradiol: analysis of estrogen's effect on progesterone receptor synthesis and degradation. Endocrinology 122:935-944 |
|
McIntyre WR, Samuels HH 1985 Triamcinolone acetonide regulates glucocorticoid-receptor levels by decreasing the half-life of the activated nuclear-receptor form. J Biol Chem 260:418-427 |
|
Hiipakka RA, Liao S 1988 Steroid receptor recycling and interaction of receptor with RNA. Am J Clin Oncol 11 Suppl 2:S18-22 |
|
Horwitz KB, McGuire WL 1978 Nuclear mechanisms of estrogen action. Effects of estradiol and anti- estrogens on estrogen receptors and nuclear receptor processing. J Biol Chem 253:8185-8191 |
|
Lee JH, Kim J, Shapiro DJ 1995 Regulation of Xenopus laevis estrogen receptor gene expression is mediated by an estrogen response element in the protein coding region. DNA Cell Biol 14:419-430 |
|
Clemens JW, Robker RL, Kraus WL, Katzenellenbogen BS, Richards JS 1998 Hormone induction of progesterone receptor (PR) messenger ribonucleic acid and activation of PR promoter regions in ovarian granulosa cells: evidence for a role of cyclic adenosine 3',5'-monophosphate but not estradiol. Mol Endocrinol 12:1201-1214 |
|
Okulicz WC, Evans RW, Leavitt WW 1981 Progesterone regulation of the occupied form of nuclear estrogen receptor. Science 213:1503-1505 |
|
Okret S, Dong Y, Bronnegard M, Gustafsson JA 1991 Regulation of glucocorticoid receptor expression. Biochimie 73:51-59 |
|
Couse JF, Korach KS 1998 Exploring the role of sex steroids through studies of receptor deficient mice. J Mol Med 76:497-511 |
|
Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS 1995 Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol 9:1441-1454 |
|
Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O 1993 Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162-11166 |
|
Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson J-A, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA 95:15677-15682 |
|
Couse JF, Korach KS 1999 Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358-417 |
|
Couse JF, Curtis Hewitt S, Korach KS 2000 Receptor null mice reveal contrasting roles for estrogen receptor alpha and beta in reproductive tissues. J Steroid Biochem Mol Biol 74:287-296. |
|
Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS, Eddy EM, Migliaccio S, Snedeker SM, Lubahn DB, Schomberg DW, Smith EP 1996 Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog Horm Res 51:159-186; discussion 186-158 |
|
Korach KS 2000 Estrogen receptor knock-out mice: molecular and endocrine phenotypes. J Soc Gynecol Investig:S16-17 |
|
Korach KS, Emmen JMA, Walker VR, Hewitt SC, Yates M, Hall JM, Swope DL, Harrell JC, Couse JF 2003 Update on animal models developed for analyses of estrogen receptor biological activity. The Journal of Steroid Biochemistry and Molecular Biology 86:387-391 |
|
Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, Clegg DJ, Kaplitt MG, Ogawa S 2007 Silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. PNAS 104:2501-2506 |
|
Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly M, Rudling M, Lindberg MK, Warner M, Angelin B, Gustafsson JA 2000 Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun 278:640-645 |
|
Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB 1997 A role for oestrogens in the male reproductive system. Nature 390:509-512 |
|
Ogawa S, Taylor JA, Lubahn DB, Korach KS, Pfaff DW 1996 Reversal of sex roles in genetic female mice by disruption of estrogen receptor gene. Neuroendocrinology 64:467-470 |
|
Ogawa S, Lubahn DB, Korach KS, Pfaff DW 1997 Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA 94:1476-1481 |
|
Pfaff DW 1997 Hormones, genes, and behavior. Proceedings of the National Academy of Sciences 94:14213-14216 |
|
Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS 1998 Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc Natl Acad Sci USA 95:13941-13946 |
|
Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW 2000 Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO). Proc Natl Acad Sci U S A 97:14737-14741 |
|
Iafrati MD, Karas RH, Aronovitz M, Kim S, Sullivan TR, Jr., Lubahn DB, O. Donnell TFJ, Korach KS, Mendelsohn ME 1997 Estrogen inhibits the vascular injury response in estrogen receptor alpha-deficient mice. Nat Med 3:545-548 |
|
Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS 1994 Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331:1056-1061 |
|
Gustafsson J, Warner M 2000 Estrogen receptor beta in the breast: role in estrogen responsiveness and development of breast cancer. J Steroid Biochem Mol Biol 74:245-248. |
|
Krezel W, Dupont S, Krust A, Chambon P, Chapman PF 2001 Increased anxiety and synaptic plasticity in estrogen receptor beta -deficient mice. Proc Natl Acad Sci U S A 98:12278-12282. |
|
Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA 2002 Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc Natl Acad Sci U S A 99:3996-4001. |
|
Cho NL, Javid SH, Carothers AM, Redston M, Bertagnolli MM 2007 Estrogen Receptors {alpha} and {beta} Are Inhibitory Modifiers of Apc-Dependent Tumorigenesis in the Proximal Colon of Min/+ Mice. Cancer Res 67:2366-2372 |
|
Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CAJ, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266-2278 |
|
Chappell PE, Lydon JP, Conneely OM, O'Malley BW, Levine JE 1997 Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology 138:4147-4152 |
|
Reichardt HM, Kaestner KH, Wessely O, Gass P, Schmid W, Schutz G 1998 Analysis of glucocorticoid signalling by gene targeting. J Steroid Biochem Mol Biol 65:111-115 |
|
Tronche F, Kellendonk C, Reichardt HM, Schutz G 1998 Genetic dissection of glucocorticoid receptor function in mice. Curr Opin Genet Dev 8:532-538 |
|
Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G 1998 DNA binding of the glucocorticoid receptor is not essential for survival. Cell 93 1:531-554 |
|
Berger S, Bleich M, Schmid W, Cole TJ, Peters J, Watanabe H, Kriz W, Warth R, Greger R, Schutz G 1998 Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci U S A 95:9424-9429 |
|
Hubert C, Gasc J-M, Berger S, Günther Schütz G, Corvol P 1999 Effects of mineralocorticoid receptor gene disruption on the components of the renin-angiotensin system in 8-day-old mice. Mol Endocrinol 13:297-306 |
|
He WW, Lindzey JK, Prescott JL, Kumar MV, Tindall DJ 1994 The androgen receptor in the testicular feminized (Tfm) mouse may be a product of internal translation initiation. Receptor 4:121-134 |
|
Lindzey J, Kumar MV, Grossman M, Young C, Tindall DJ 1994 Molecular mechanisms of androgen action. Vitam Horm 49:383-432 |
|
Yeh S, Tsai M-Y, Xu Q, Mu X-M, Lardy H, Huang K-E, Lin H, Yeh S-D, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung M-C, Zhang S, Gan L, Chang C 2002 Generation and characterization of androgen receptor knockout (ARKO) mice: An in vivo model for the study of androgen functions in selective tissues. Proceedings of the National Academy of Sciences 99:13498-13503 |
|
Altuwaijri S, Lee DK, Chuang KH, Ting HJ, Yang Z, Xu Q, Tsai MY, Yeh S, Hanchett LA, Chang HC, Chang C 2004 Androgen receptor regulates expression of skeletal muscle-specific proteins and muscle cell types. Endocrine 25:27-32 |
|
Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, Nagashima Y, Chang YJ, Hu YC, Tsai MY, Yeh S, Messing EM, Chang C 2007 Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst 99:558-568 |
|
Wu C-T, Altuwaijri S, Ricke WA, Huang S-P, Yeh S, Zhang C, Niu Y, Tsai M-Y, Chang C 2007 Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proceedings of the National Academy of Sciences 104:12679-12684 |
|
Rao CV 1988 Cell membrane receptors. In: Sciarra J ed. Gynecology and Obstetrics. Philadelphia: Harper and Row |
|
Clark GM, McGuire WL 1988 Steroid receptors and other prognostic factors in primary breast cancer. Semin Oncol 15:20-25 |
|
Chamness GC, Zava DT, McGuire WL 1978 Methods for assessing the binding of steroid hormones in nuclei. Methods Cell Biol 17:325-338 |
|
Syne JS, Markaverich BM, Clark JH, Panko WB 1982 Estrogen binding sites in the nucleus of normal and malignant human tissue: optimization of an exchange assay for the measurement of specific binding. Cancer Res 42:4443-4448 |
|
Pavlik EJ, Coulson PB 1976 Hydroxylapatite "batch" assay for estrogen receptor: Increased sensitivity over present receptor assays. J Steroid Biochem 7:357-368 |
|
Pasic R, Djulbegovic B, Wittliff JL 1990 Comparison of sex steroid receptor determinations in human breast cancer by enzyme immunoassay and radioligand binding. J Clin Lab Anal 4:430-436 |
|
Jordan VC, Morrow M, de Lima GR, Facina G, Shida JY, Chein MB, Tanaka P, Dardes RC, Gebrim LH, Schafer JM, Bentrem DJ, Takei H, Gajdos C, Badve S, Pappas SG 2003 Re: Trends in use of adjuvant multi-agent chemotherapy and tamoxifen for breast cancer in the United States: 1975-1999. J Natl Cancer Inst 95:683-684; author reply 684-685. |
|
Scatchard G 1949 The attraction of proteins for small molecules and ions. Ann New York Acad Sci 51:660-672 |
|
Yeh S, Miyamoto H, Shima H, Chang C 1998 From estrogen to androgen receptor: a new pathway for sex hormones in prostate. Proc Natl Acad Sci U S A 95:5527-5532 |
|
Sambrook J, Fritsch EF, Maniatis T 1989 Molecular Cloning. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press |
|
He WW, Fischer LM, Sun S, Bilhartz DL, Zhu XP, Young CY, Kelley DB, Tindall DJ 1990 Molecular cloning of androgen receptors from divergent species with a polymerase chain reaction technique: complete cDNA sequence of the mouse androgen receptor and isolation of androgen receptor cDNA probes from dog, guinea pig and clawed frog. Biochem Biophys Res Commun 171:697-704 |
|
Kumar V, Green S, Stack G, Berry M, Jin JR, Chambon P 1987 Functional domains of the human estrogen receptor. Cell 51:941-951 |
|
Reese JC, Katzenellenbogen BS 1991 Mutagenesis of cysteines in the hormone binding domain of the human estrogen receptor. Alterations in binding and transcriptional activation by covalently and reversibly attaching ligands. J Biol Chem 266:10880-10887 |
|
Klinge CM, Traish AM, Bambara RA, Hilf R 1996 Dissociation of 4-hydroxytamoxifen, but not estradiol or tamoxifen aziridine, from the estrogen receptor when the receptor binds estrogen response element DNA. J Steroid Biochem Molec Biol 57:51-66 |
|
Perissi V, Staszewski LM, McInerney EM, Kurokawa R, Krones A, Rose DW, Lambert MH, Milburn MV, Glass CK, Rosenfeld MG 1999 Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev 13:3198-3208. |
|
Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M 2000 Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843-852 |
|
Krieg SA, Krieg AJ, Shapiro DJ 2001 A unique downstream estrogen responsive unit mediates estrogen induction of proteinase Inhibitor-9, a cellular inhibitor of IL-1beta- converting enzyme (Caspase 1). Mol Endocrinol 15:1971-1982. |
|
Planas-Silva MD, Shang Y, Donaher JL, Brown M, Weinberg RA 2001 AIB1 enhances estrogen-dependent induction of cyclin d1 expression. Cancer Res 61:3858-3862. |
|
Shao W, Halachmi S, Brown M 2002 ERAP140, a conserved tissue-specific nuclear receptor coactivator. Mol Cell Biol 22:3358-3372. |
|
Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, Gannon F 2003 Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751-763 |
|
Lin CY, Strom A, Vega VB, Kong SL, Yeo AL, Thomsen JS, Chan WC, Doray B, Bangarusamy DK, Ramasamy A, Vergara LA, Tang S, Chong A, Bajic VB, Miller LD, Gustafsson JA, Liu ET 2004 Discovery of estrogen receptor alpha target genes and response elements in breast tumor cells. Genome Biol 5:R66 |
|
Mishra SK, Balasenthil S, Nguyen D, Vadlamudi RK 2004 Cloning and functional characterization of PELP1/MNAR promoter. Gene 330:115-122 |
|
Petz LN, Ziegler YS, Schultz JR, Kim H, Kemper JK, Nardulli AM 2004 Differential regulation of the human progesterone receptor gene through an estrogen response element half site and Sp1 sites. J Steroid Biochem Mol Biol 88:113-122 |
|
Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, Barch MJ, Klinge CM 2008 Estradiol stimulates transcription of Nuclear Respiratory Factor-1 and increases mitochondrial biogenesis. Mol Endocrinol 22:609-622 |
|
Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, Barch MJ, Klinge CM 2008 Estradiol stimulates transcription of Nuclear Respiratory Factor-1 and increases mitochondrial biogenesis. Mol Endocrinol 22:609-622 |
|
Lanzino M, De Amicis F, McPhaul MJ, Marsico S, Panno ML, Ando S 2005 Endogenous Coactivator ARA70 Interacts with Estrogen Receptor {alpha} (ER{alpha}) and Modulates the Functional ER{alpha}/Androgen Receptor Interplay in MCF-7 Cells. J Biol Chem 280:20421-20430 |
|
Nettles KW, Gil G, Nowak J, Metivier R, Sharma VB, Greene GL 2008 CBP Is a Dosage-Dependent Regulator of Nuclear Factor-{kappa}B Suppression by the Estrogen Receptor. Mol Endocrinol 22:263-272 |
|
Ruegg J, Swedenborg E, Wahlstrom D, Escande A, Balaguer P, Pettersson K, Pongratz I 2008 The Transcription Factor Aryl Hydrocarbon Receptor Nuclear Translocator Functions as an Estrogen Receptor {beta}-Selective Coactivator, and Its Recruitment to Alternative Pathways Mediates Antiestrogenic Effects of Dioxin. Mol Endocrinol 22:304-316 |
|
Theulaz I, Hipskind R, ten Heggeler-Bordier B, Green S, Kumar V, Chambon P, Wahli W 1988 Expression of human estrogen receptor mutants in Xenopus oocytes: correlation between transcriptional activity and ability to form protein-DNA complexes. EMBO J 7:1653-1660 |
|
Li X, Zhao X, Fang Y, Jiang X, Duong T, Fan C, Huang CC, Kain SR 1998 Generation of destabilized green fluorescent protein as a transcription reporter. J Biol Chem 273:34970-34975 |
|
Zhang X, Chen HZ, Rovin BH 2003 Unexpected sensitivity of synthetic Renilla luciferase control vectors to treatment with a cyclopentenone prostaglandin. Biotechniques 35:1144-1146, 1148 |
|
Ekena K, Katzenellenbogen JA, Katzenellenbogen BS 1998 Determinants of ligand specificity of estrogen receptor-: Estrogen versus androgen discrimination. J Biol Chem 273:693-699 |
|
Genazzani AR, Lucchesi A, Stomati M, Catarsi S, Genazzani AD, Criscuolo M, Petraglia F 1997 Effects of sex steroid hormones on the neuroendocrine system. Eur J Contracept Reprod Health Care 2:63-69 |
|
Spindler KD 1997 Interactions between steroid hormones and the nervous system. Neurotoxicology 18:745-754 |
|
Fink G, Sumner BE, McQueen JK, Wilson H, Rosie R 1998 Sex steroid control of mood, mental state and memory. Clin Exp Pharmacol Physiol 25:764-775 |
|
Cyr M, Calon F, Morissette M, Grandbois M, Di Paolo T, Callier S 2000 Drugs with estrogen-like potency and brain activity: potential therapeutic application for the CNS. Curr Pharm Des 6:1287-1312 |
|
Weickert CS, Miranda-Angulo AL, Wong J, Perlman WR, Ward SE, Radhakrishna V, Straub RE, Weinberger DR, Kleinman JE 2008 Variants in the Estrogen Receptor Alpha Gene and its mRNA Contribute to Risk for Schizophrenia. Hum Mol Genet:ddn130 |
|
Emilien G, Beyreuther K, Masters CL, Maloteaux JM 2000 Prospects for pharmacological intervention in Alzheimer disease. Arch Neurol 57:454-459 |
|
Palacios S, Cifuentes I, Menendez C, von Helde S 2000 The central nervous system and HRT. Int J Fertil Womens Med 45:13-21 |
|
Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA 2002 Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med 33:562-571 |
|
Ahlemeyer B, Krieglstein J 2003 Pharmacological studies supporting the therapeutic use of Ginkgo biloba extract for Alzheimer's disease. Pharmacopsychiatry 36 Suppl 1:S8-14 |
|
Marin R, Guerra B, Hernandez-Jimenez JG, Kang XL, Fraser JD, Lopez FJ, Alonso R 2003 Estradiol prevents amyloid-beta peptide-induced cell death in a cholinergic cell line via modulation of a classical estrogen receptor. Neuroscience 121:917-926 |
|
Singh M, Dykens JA, Simpkins JW 2006 Novel Mechanisms for Estrogen-Induced Neuroprotection. Experimental Biology and Medicine 231:514-521 |
|
Casadesus G, Rolston RK, Webber KM, Atwood CS, Bowen RL, Perry G, Smith MA 2008 Menopause, estrogen, and gonadotropins in Alzheimer's disease. Adv Clin Chem 45:139-153 |
|
Asarian L, Geary N 2006 Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci 361:1251-1263 |
|
Lindheim SR, Legro RS, Bernstein L, Stanczyk FZ, Vijod MA, Presser SC, Lobo RA 1992 Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. Am J Obstet Gynecol 167:1831-1836 |
|
Lindheim SR, Legro RS, Morris RS, Wong IL, Tran DQ, Vijod MA, Stanczyk FZ, Lobo RA 1994 The effect of progestins on behavioral stress responses in postmenopausal women. J Soc Gynecol Investig 1:79-83 |
|
Fletcher SW, Colditz GA 2002 Failure of estrogen plus progestin therapy for prevention. JAMA 288:366-368. |
|
Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD 2002 Postmenopausal hormone replacement therapy: scientific review. Jama 288:872-881. |
|
Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S 2004 Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291:1701-1712 |
|
Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB 1997 Estrogen receptors are essential for female sexual receptivity. Endocrinology 138:507-510 |
|
Frye CA, Vongher JM 2001 Progesterone and 3alpha,5alpha-THP enhance sexual receptivity in mice. Behav Neurosci 115:1118-1128 |
|
Mani SK, Blaustein JD, O'Malley BW 1997 Progesterone receptor function from a behavioral perspective. Horm Behav 31:244-255 |
|
Mong J, Easton A, Kow L-M, Pfaff D 2003 Neural, hormonal and genetic mechanisms for the activation of brain and behavior. European Journal of Pharmacology 480:229-231 |
|
Mong JA, Pfaff DW 2003 Hormonal and genetic influences underlying arousal as it drives sex and aggression in animal and human brains. Neurobiology of Aging 24:S83-S88 |
|
Mong JA, Pfaff DW 2004 Hormonal symphony: steroid orchestration of gene modules for sociosexual behaviors. Mol Psychiatry 9:550-556 |
|
Ogawa S, Gordan JD, Taylor J, Lubahn D, Korach K, Pfaff DW 1996 Reproductive functions illustrating direct and indirect effects of genes on behavior. Horm Behav 30:487-494 |
|
Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW 1998 Roles of estrogen receptor-alpha gene expression in reproduction- related behaviors in female mice. Endocrinology 139:5070-5081 |
|
Woolley SC, O'Malley B, Lydon J, Crews D 2006 Genotype differences in behavior and tyrosine hydroxylase expression between wild-type and progesterone receptor knockout mice. Behavioural Brain Research 167:197-204 |
|
Phelps SM, Lydon JP, BW Om, Crews D 1998 Regulation of male sexual behavior by progesterone receptor, sexual experience, and androgen. Horm Behav 34:294-302 |
|
Roper WG 1996 The etiology of male homosexuality. Med Hypotheses 46:85-88 |
|
James WH 2005 Biological and psychosocial determinants of male and female human sexual orientation. J Biosoc Sci 37:555-567 |
|
McFadden D, Pasanen EG 1998 Comparison of the auditory systems of heterosexuals and homosexuals: click-evoked otoacoustic emissions. Proc Natl Acad Sci U S A 95:2709-2713 |
|
Loehlin JC, McFadden D 2003 Otoacoustic emissions, auditory evoked potentials, and traits related to sex and sexual orientation. Arch Sex Behav 32:115-127 |
|
Adkins-Regan E, Mansukhani V, Thompson R, Yang S 1997 Organizational actions of sex hormones on sexual partner preference. Brain Res Bull 44:497-502 |
|
Gooren L 2006 The biology of human psychosexual differentiation. Hormones and Behavior 50:589-601 |
|
Cohen-Kettenis PT, van Goozen SH, Doorn CD, Gooren LJ 1998 Cognitive ability and cerebral lateralisation in transsexuals. Psychoneuroendocrinology 23:631-641 |
|
Schneider HJ, Pickel J, Stalla GK 2006 Typical female 2nd-4th finger length (2D:4D) ratios in male-to-female transsexuals--possible implications for prenatal androgen exposure. Psychoneuroendocrinology 31:265-269 |
|
Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM 1998 Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol 19:323-362 |
|
Matsumoto T, Honda S-i, Harada N 2003 Neurological effects of aromatase deficiency in the mouse. The Journal of Steroid Biochemistry and Molecular Biology 86:357-365 |
|
Sternbach H 1998 Age-associated testosterone decline in men: clinical issues for psychiatry. Am J Psychiatry 155:1310-1318 |
|
Davis SR 1998 The clinical use of androgens in female sexual disorders. J Sex Marital Ther 24:153-163 |
|
Schover LR 2008 Androgen therapy for loss of desire in women: is the benefit worth the breast cancer risk? Fertility and Sterility 90:129-140 |
|
Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM 1988 Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 240:327-330 |
|
Murono K, Mendonca BB, Arnhold IJ, Rigon AC, Migeon CJ, Brown TR 1995 Human androgen insensitivity due to point mutations encoding amino acid substitutions in the androgen receptor steroid-binding domain. Hum Mutat 6:152-162 |
|
Hughes IA, Deeb A 2006 Androgen resistance. Best Pract Res Clin Endocrinol Metab 20:577-598 |
|
Lubahn DB, Brown TR, Simental JA, Higgs HN, Migeon CJ, Wilson EM, French FS 1989 Sequence of the intron/exon junctions of the coding region of the human androgen receptor gene and identification of a point mutation in a family with complete androgen insensitivity. Proc Natl Acad Sci U S A 86:9534-9538 |
|
Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW 1998 The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res 13:325-349 |
|
Liberman UA 2007 Vitamin D-resistant diseases. J Bone Miner Res 22 Suppl 2:V105-107 |
|
Murphy LC, Dotzlaw H, Leygue E, Coutts A, Watson P 1998 The pathophysiological role of estrogen receptor variants in human breast cancer. J Steroid Biochem Mol Biol 65:175-180 |
|
McGuire WL, Clark GM, Dressler LG, Owens MA 1986 Role of steroid hormone receptors as prognostic factors in primary breast cancer. NCI Monogr:19-23 |
|
Speirs V, Kerin MJ, Newton CJ, Walton DS, Green AR, Desai SB, Atkin SL 1999 Evidence for transcriptional activation of ERalpha by IL-1beta in breast cancer cells. Int J Oncol 15:1251-1254. |
|
Jordan VC 2004 Selective estrogen receptor modulation: Concept and consequences in cancer. Cancer Cell 5:207-213 |
|
Jordan VC, O'Malley BW 2007 Selective Estrogen-Receptor Modulators and Antihormonal Resistance in Breast Cancer. J Clin Oncol 25:5815-5824 |
|
Jordan VC, Pappas SG 2003 Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. J Med Chem 46:1081-1111. |
|
Jordan VC, Pappas SG 2003 Is tamoxifen the Rosetta stone for breast cancer? J Natl Cancer Inst 95:338-340. |
|
Nilsson M, Dahlman-Wright K, Gustafsson JA 2004 Nuclear receptors in disease: the oestrogen receptors. Essays Biochem 40:157-167 |
|
Fukuda K, Mori M, Uchiyama M, Iwai K, Iwasaka T, Sugimori H 1998 Prognostic significance of progesterone receptor immunohistochemistry in endometrial carcinoma. Gynecol Oncol 69:220-225 |
|
Hempling RE, Piver MS, Eltabbakh GH, Recio FO 1998 Progesterone receptor status is a significant prognostic variable of progression-free survival in advanced epithelial ovarian cancer. Am J Clin Oncol 21:447-451 |
|
Charmandari E, Kino T, Ichijo T, Chrousos GP 2008 Generalized Glucocorticoid Resistance: Clinical Aspects, Molecular Mechanisms, and Implications of a Rare Genetic Disorder. J Clin Endocrinol Metab 93:1563-1572 |
|
Arai K, Chrousos GP 1995 Syndromes of glucocorticoid and mineralocorticoid resistance. Steroids 60:173-179 |
|
DeRijk R, Sternberg EM 1997 Corticosteroid resistance and disease. Ann Med 29:79-82 |
|
Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP 2003 Tissue glucocorticoid resistance/hypersensitivity syndromes. The Journal of Steroid Biochemistry and Molecular Biology 85:457-467 |
|
Lane SJ, Lee TH 1997 Mechanisms of corticosteroid resistance in asthmatic patients. Int Arch Allergy Immunol 113:193-195 |
|
Adcock IM, Barnes PJ 2008 Molecular mechanisms of corticosteroid resistance. Chest 134:394-401 |
|
Kuhnle U, Hinkel GK, Akkurt HI, Krozowski Z 1995 Familial pseudohypoaldosteronism: a review on the heterogeneity of the syndrome. Steroids 60:157-160 |
|
Fuller PJ 1995 Aldosterone and its mechanism of action: more questions than answers. Aust N Z J Med 25:800-807 |
|
Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT 1995 Estrogens, the immune response and autoimmunity. Clin Exp Rheumatol 13:217-226 |
|
Kelly RH, Vertosick FT, Jr. 1986 Systemic lupus erythematosus: a role for anti-receptor antibodies? Med Hypotheses 20:95-101 |
|
Feldman M 1987 Steroid receptor antibodies in autoimmune disorders. Biochem Biophys Res Commun 145:1342-1348 |
|
Suenaga R, Mitamura K, Evans MJ, Abdou NI 1996 Binding affinity and quantity of estrogen receptor in peripheral blood monocytes of patients with systemic lupus erythematosus. Lupus 5:227-231 |
|
Cohen-Solal JF, Jeganathan V, Hill L, Kawabata D, Rodriguez-Pinto D, Grimaldi C, Diamond B 2008 Hormonal regulation of B-cell function and systemic lupus erythematosus. Lupus 17:528-532 |
|
Suenaga R, Evans MJ, Mitamura K, Rider V, Abdou NI 1998 Peripheral blood T cells and monocytes and B cell lines derived from patients with lupus express estrogen receptor transcripts similar to those of normal cells. J Rheumatol 25:1305-1312 |
|
Davis DL, Bradlow HL, Wolff M, Woodruff T, Hoel DG, Anton-Culver H 1993 Medical hypothesis: Xenoestrogens as preventable causes of breast cancer. Environ Health Persp 101:372-377 |
|
Salehi F, Turner MC, Phillips KP, Wigle DT, Krewski D, Aronson KJ 2008 Review of the etiology of breast cancer with special attention to organochlorines as potential endocrine disruptors. J Toxicol Environ Health B Crit Rev 11:276-300 |
|
Feldman D 1997 Editorial: estrogens from plastic- Are we being exposed? Endocrinology 138:1777-1779 |
|
Wolff MS, Collman GW, Barrett JC, Huff J 1996 Breast cancer and environmental risk factors: Epidemiological and experimental findings. Annu Rev Pharmacol Toxicol 36:573-596 |
|
Wolff MS, Weston A 1997 Breast cancer risk and environmental exposures. Environ Health Perspect 105:891-896 |
|
Cecconi S, Paro R, Rossi G, Macchiarelli G 2007 The effects of the endocrine disruptors dithiocarbamates on the mammalian ovary with particular regard to mancozeb. Curr Pharm Des 13:2989-3004 |
|
Ho S-M, Tang W-Y, Belmonte de Frausto J, Prins GS 2006 Developmental Exposure to Estradiol and Bisphenol A Increases Susceptibility to Prostate Carcinogenesis and Epigenetically Regulates Phosphodiesterase Type 4 Variant 4. Cancer Res 66:5624-5632 |
|
Harding AK, Daston GP, Boyd GR, Lucier GW, Safe SH, Stewart J, Tillitt DE, Van Der Kraak G 2006 Endocrine disrupting chemicals research program of the U.S. Environmental Protection Agency: summary of a peer-review report. Environ Health Perspect 114:1276-1282 |
|
Waring RH, Harris RM 2005 Endocrine disrupters: A human risk? Molecular and Cellular Endocrinology 244:2-9 |
|
Feldman D, Krishnan A 1995 Estrogens in unexpected places: possible implications for researchers and consumers. Environ Health Persp 103(Suppl. 7) |
|
Giger W, Brunner PH, Schaffner C 1984 4-nonylphenol in sewage sludge: Accumulation of toxic metabolites from nonionic surfactants. Science 225:623-625 |
|
Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N 1995 Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspectives 104:608-612 |
|
Olea N, Pulgar R, Perez P, Olea-Serrano F, Rivas A, Novillo-Fertrell A, Pedraza V, Soto AM, Sonnenschein C 1996 Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect 104:298-305 |
|
Foster WG 2008 Environmental Estrogens and Endocrine Disruption: Importance of Comparative Endocrinology. Endocrinology 149:4267-4268 |
|
Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM 2007 In vitro molecular mechanisms of bisphenol A action. Reproductive Toxicology 24:178-198 |
|
Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM 2007 Exposure to Environmentally Relevant Doses of the Xenoestrogen Bisphenol-A Alters Development of the Fetal Mouse Mammary Gland. Endocrinology 148:116-127 |
|
Liu X, Matsushima A, Okada H, Tokunaga T, Isozaki K, Shimohigashi Y 2007 Receptor binding characteristics of the endocrine disruptor bisphenol A for the human nuclear estrogen-related receptor gamma: Chief and corroborative hydrogen bonds of the bisphenol A phenol-hydroxyl group with Arg316 and Glu275 residues. FEBS Journal 274:6340-6351 |
|
Keri RA, Ho S-M, Hunt PA, Knudsen KE, Soto AM, Prins GS 2007 An evaluation of evidence for the carcinogenic activity of bisphenol A. Reproductive Toxicology 24:240-252 |
|
Welshons WV, Nagel SC, vom Saal FS 2006 Large Effects from Small Exposures. III. Endocrine Mechanisms Mediating Effects of Bisphenol A at Levels of Human Exposure. Endocrinology 147:s56-69 |
|
vom Saal FS, Welshons WV 2006 Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environmental Research 100:50-76 |
|
Savabieasfahani M, Kannan K, Astapova O, Evans NP, Padmanabhan V 2006 Developmental Programming: Differential Effects of Prenatal Exposure to Bisphenol-A or Methoxychlor on Reproductive Function. Endocrinology 147:5956-5966 |
|
Lupulescu A 1993 Estrogen use and cancer risk: A review. Exp Clin Endocrinol 101:204-214 |
|
Tiezzi DG, Fernandez SV, Russo J 2007 Epithelial mesenchymal transition during the neoplastic transformation of human breast epithelial cells by estrogen. Int J Oncol 31:823-827 |
|
Huang Y, Fernandez SV, Goodwin S, Russo PA, Russo IH, Sutter TR, Russo J 2007 Epithelial to Mesenchymal Transition in Human Breast Epithelial Cells Transformed by 17 -Estradiol. Cancer Res 67:11147-11157 |
|
Russo J, Fernandez SV, Russo PA, Fernbaugh R, Sheriff FS, Lareef HM, Garber J, Russo IH 2006 17-Beta-estradiol induces transformation and tumorigenesis in human breast epithelial cells. FASEB J 20:1622-1634 |
|
Russo J, Hu YF, Tahin Q, Mihaila D, Slater C, Lareef MH, Russo IH 2001 Carcinogenicity of estrogens in human breast epithelial cells. Apmis 109:39-52. |
|
Routledge EJ, Sumpter JP 1997 Structural features of alkylphenolic chemicals associated with estrogenic activity. J Biol Chem 272:3280-3288 |