Endometrial Hyperplasia and Neoplasia: Definition, Diagnosis, and Management Principles
Authors
INTRODUCTION
By definition, adenocarcinoma of the endometrium is an invasive disease, invading either the endometrial stroma or the underlying myometrium of extrauterine tissues. Most endometrial carcinomas maintain endometrioid differentiation; these also can contain areas of mucinous or squamous differentiation. Other nonendometrioid subtypes seen in routine practice include clear cell carcinoma, papillary serous carcinoma, and other rare variants. According to the US Gynecologic Oncology Group histologic grading system,1 grade 1, well-differentiated carcinoma, consists of a neoplasm with less than 5% of solid cancer; grade 2, moderately differentiated carcinoma, contains 6–50% solid cancer; and grade 3, poorly differentiated carcinoma, contains more than 50% of solid tumor.
Tumor grading is of greater independent prognostic value for endometrioid endometrial adenocarcinoma and its related types (i.e., endometrial, secretory, mucinous, squamous) than for papillary serous and clear cell adenocarcinomas. Papillary serous and clear cell cancers do not show the grade-dependent changes in aggressiveness seen with the endometrioid tumors; instead, as a group they are consistently aggressive. Division of endometrial adenocarcinomas into the clinicopathologic classes of endometrioid and nonendometrioid types is paralleled further by differences in epidemiologic risk factors and precursor lesions.2,3 Type I, or endometrioid, endometrial adenocarcinomas are more frequent in women taking exogenous estrogen and often are preceded by precursor lesions, which is the subject of this discussion.
It has traditionally been suggested that endometrioid endometrial adenocarcinoma is preceded by endometrial hyperplasia (EH).4 EH previously was considered a continuum of morphologic changes often beginning with simple glandular/stromal overgrowth (simple hyperplasia) and ending with complex, highly atypical histologic and cytologic proliferations, variously referred to as atypical adenomatous hyperplasia, dysplasia, or carcinoma in situ. The figure most often cited in the literature for progression of atypical adenomatous hyperplasia to carcinomas was 30% at 10 years.4
This hyperplasia model, as defined by the World Health Organization (WHO), was developed primarily by pathologists as a morphologic classification into four classes of hyperplasia, composed of complex or simple architecture combined variously with presence or absence of cytologic atypia. These do not cleanly correspond to four distinctive biologic categories, nor are there comparable numbers of clinical interventions individually matched to each hyperplasia subtype. A contraction of the number of categories to three was suggested by merging all atypical hyperplasia (AH) groups into one diagnostic category (atypical endometrial hyperplasia) which contained the highest endometrial cancer risk.5 Pathologist scoring of presence or absence of cytologic atypia is notoriously unreliable, and has become a major limitation of this approach.6 In a Gynecologic Oncology Group study only 38% of community diagnosed atypical endometrial hyperplasia were confirmed as atypical hyperplasia upon central review by a panel of gynecologic pathologists.7 A further complication of this contraction of diagnostic groups by cytologic atypia alone is the de-emphasis on architectural features which remain of value in stratifying high from low risk subgroups.
New data that have emerged in the last decade have changed the underlying assumptions upon which endometrial precancer diagnosis is constructed.8 The assumption of gradual evolution of endometrial histologic patterns across hyperplastic groups is incorrect. Endocrine induced endometrial changes, such as those conferred by unopposed estrogens, do produce a field-wide effect that gradually changes the histologic pattern as a function of time and dose. This can be described as a dynamically changing histotype, which early on has the appearance of a disordered proliferative endometrium, and with subsequent remodeling assumes a variable gland density that we prefer to designate as the benign endometrial hyperplasia sequence. Bona fide premalignant lesions, however, are of an entirely different character. Precancerous lesions of the endometrium originate focally as a result of clonal outgrowth of genetically mutated glands which have a differing cytologic and architectural pattern relative to the background.9 Their morphology is discontinuous from that of the background endometrium itself, and can only be recognized through a combination of newly defined histologic features which define the entity of endometrial intraepithelial neoplasia (EIN).10 This is more completely described in the next section.
EIN is not synonymous with carcinoma but indicates a lesion that may regress, persist, or progress to invasion. Approximately one third of women diagnosed with EIN will have a concurrent carcinoma diagnosed within the first year, and the long term cancer risk is 45 times increased beyond benign endometrial hyperplasia.11, 12
Morphologically, an altered relationship between glands and stroma distinguishes carcinoma from EIN. Even when present in the patient, myoinvasion is rarely evident in an endometrial curettage or biopsy, which rarely succeeds in sampling the underlying myometrium. For this reason, distinction between EIN and adenocarcinoma must commonly be performed in isolated endometrial samples devoid of myometrium. Within the endometrial compartment itself, examination of stromal quality and character in the region of a glandular lesion is not a reliable indicator of whether the stroma has been invaded. EIN lesions are made up of aggregates of individual glands which may have some branch points, but lack the complex folded sheets that produce a maze of interconnected lumens or villoglandular architecture in some carcinomas. The architectural pattern of the glands is an indicator of an altered interaction between glands and stroma. Functional changes which correspond to malignant behavior in vivo include loss of anchorage dependent growth. The histologic equivalent of this feature is growth of epithelial cells without a requirement for contact with a basement membrane. This is evident histologically by areas of solid epithelial growth without lumen formation or a cribriform pattern of multiple gland lumens within a single gland. The presence of myoinvasion, or any one of the above described patterns (solid, cribriform, villoglandular, maze-like), is diagnostic of adenocarcinoma.13
THE BENIGN ENDOMETRIAL HYPERPLASIA SEQUENCE AND EIN
A primary objective of endometrial diagnosis and therapy is distinction between primary hormonal abnormalities having a secondary endometrial effect (the benign endometrial hyperplasia sequence) and intrinsically abnormal neoplastic endometrial glands prone to malignant transformation (EIN). Unopposed estrogen creates field-wide changes in the endometrium, including cyst formation, randomly scattered tubal metaplasia, and remodeling of glands. In contrast, EIN is a clonal proliferation of abnormal endometrial glands which arises at a point in space and spreads peripherally, eventually involving the entire endometrial compartment in approximately a quarter of women at the time of initial EIN diagnosis. The diagnostic, nomenclature, and therapeutic distinctions between these processes are projected into the EIN diagnosis schema which is described below. This is intended to replace, rather than supplement, older classification using the 1994 WHO hyperplasia standards. The application of new diagnostic criteria which were not part of the 1994 WHO descriptions prevents an absolute concordance between the old and new systems. Below we review the expanded evidence base for revised criteria, and summarize diagnostic implemetation strategies.
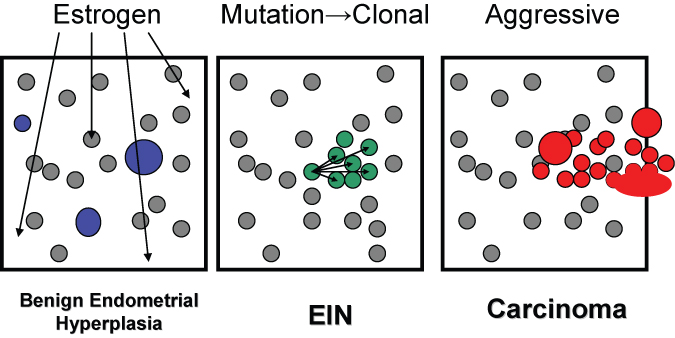 Fig. 1. Topography of hormonal and neoplastic endometrial disease. The diffuse field-wide endometrial effects of unopposed estrogens in benign endometrial hyperplasia are randomly scattered throughout the endometrial compartment and include cysts, and locally variable gland density. EIN lesions arise through local proliferation of genetically mutated glands which are characterized by an altered cytology and gland area exceeding stromal area. Adenocarcinoma has a similar clonal origin (often within a pre-existing EIN lesion) but with solid, cribriform, or maze-like architecture. With time, EIN and adenocarcinoma lesions can expand to occupy the entire endometrial compartment and thus no longer retain their earlier localizing character.
Fig. 1. Topography of hormonal and neoplastic endometrial disease. The diffuse field-wide endometrial effects of unopposed estrogens in benign endometrial hyperplasia are randomly scattered throughout the endometrial compartment and include cysts, and locally variable gland density. EIN lesions arise through local proliferation of genetically mutated glands which are characterized by an altered cytology and gland area exceeding stromal area. Adenocarcinoma has a similar clonal origin (often within a pre-existing EIN lesion) but with solid, cribriform, or maze-like architecture. With time, EIN and adenocarcinoma lesions can expand to occupy the entire endometrial compartment and thus no longer retain their earlier localizing character.
Endometrial precancers first were identified as premalignant lesions by virtue of their temporal and spatial association with cancer in large patient series. Of all women with atypical EH, 25% have an adenocarcinoma at hysterectomy.14 Although these strategies generally have been successful in defining broad classes of morphologic lesions most likely to be associated with cancer, clinical outcomes are highly insensitive in detection of precancers. A low precancer-to-cancer progression efficiency predicts that most premalignant lesions will never display a malignant end point. Further difficulty in standardizing diagnosis of endometrial precancers comes from poor reproducibility by pathologists of histopathologic criteria used for lesion classification.15 This situation has spurred development of novel diagnostic strategies applicable to lesional tissues of individual patients that are capable of accurately discriminating between biologic precancers and non-precancers. Even if such a laboratory approach were impractical for everyday use, it would constitute a powerful tool for critical evaluation and refinement of current histologic diagnostic practices.
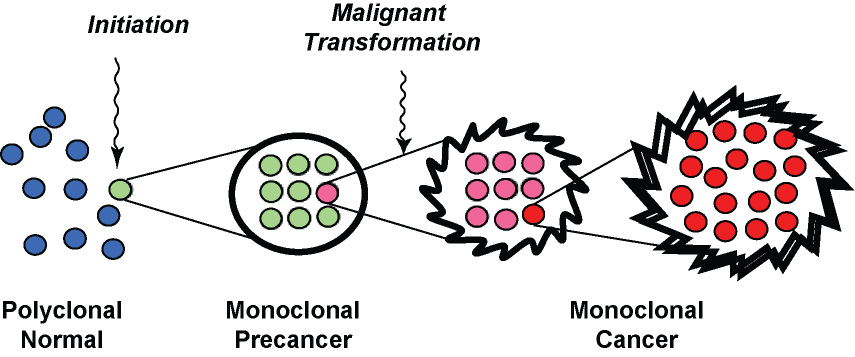 Fig. 2. A pathologist's view of multistep carcinogenesis. Initiation of carcinogenesis is accompanied by clonal expansion of a mutated cell, which subseqently undergoes additional mutation to generate new subclones with malignant behavior. The clonal expansion of mutated cells at each step is what generates a sufficient burden of abnormal glands to be seen by a pathologist.
Fig. 2. A pathologist's view of multistep carcinogenesis. Initiation of carcinogenesis is accompanied by clonal expansion of a mutated cell, which subseqently undergoes additional mutation to generate new subclones with malignant behavior. The clonal expansion of mutated cells at each step is what generates a sufficient burden of abnormal glands to be seen by a pathologist.
Monoclonal growth and mutation of tumor-suppressor genes are measurable features of the premalignant phase of endometrial tumorigenesis that can be directly ascertained in paraffin-embedded tissues and correlated with histology on a case-by-case basis. The idea that endometrial precancers are monoclonal proliferative products of a single transformed cell is based on a multistep model of tumorigenesis16 in which progression is driven by sequentially acquired mutations manifest as altered morphology and increasing aggressiveness. Although initial stages may not show an invasive phenotype, it is anticipated that premalignant lesions have sufficient growth advantage relative to their source tissues that they expand monoclonally. This expansion has now been shown to be the case for putative endometrial precancers using a variety of polymerase chain reaction-based molecular genetic methodologies applied to DNA isolated from targeted regions of paraffin sections: nonrandom X chromosome inactivation,17 clonal propagation of altered microsatellites in microsatellite-unstable tissues,18 and clonal propagation of acquired mutations of tumor-suppressor genes such as K-ras18 and PTEN.19 Monoclonal growth seems to be one of the seminal qualities of premalignant tissues at a variety of sites, including the oral mucosa, cervix, skin, stomach, and vulva.
Early stages of carcinogenesis are characterized by incremental growth advantages, which are necessarily small in relation to normal tissues and exquisitely sensitive to environmental modification. Hormonally mediated selection20 of latent transformed clones is one mechanism that might link genetic and endocrine events in genesis of this disease. This selection may occur through changes in precancer clone proliferation rates or remodeling of adjacent normal tissues. In the case of precancers confined to the functionalis, persistence is enhanced by absence of regular shedding (anovulation). Shedding is also a key part of progestin therapy for precancers because patients who have biopsies before a withdrawal bleed often have persistent lesions, albeit with an altered cytology. For this reason, repeat biopsy for confirmation of postprogestin precancer ablation is best accomplished after a withdrawal bleed to realize the full benefit of shedding and to avoid the confounding effects of progestins on histopathology interpretation.
Multiple marker systems (X inactivation, novel microsatellites) used together are approximately 80% sensitive in detection of monoclonal precancers from paraffin sections, a significant improvement over the 25% sensitivity of precancer detection realized when using a clinical standard of progression to carcinoma. In cases that do have an associated carcinoma, conservation of acquired genetic changes between matched premalignant and malignant tissues has provided a highly specific basis to conclude evolution from the former to the latter. Detailed lineage reconstruction, including hierarchical ordering of steps from precancer to cancer, has been accomplished in cases in which the repertoire of informative genetic markers is sufficiently rich.18 High cost and technical complexity place a molecular genetic laboratory standard of precancer diagnosis beyond the reach of a routine diagnostic setting.
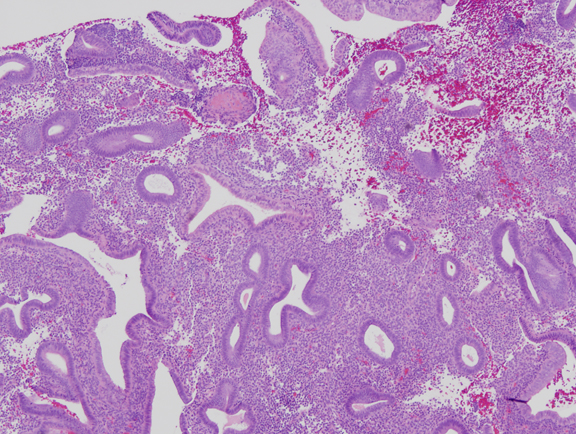 Fig. 3. Mild estrogen effect. Disordered proliferative endometrium has scattered cystically dilated glands but a low gland density overall. Randomly distributed glands may have tubal metaplasia, and fibrin thrombi can cause microinfarcts with symptomatic bleeding.
Fig. 3. Mild estrogen effect. Disordered proliferative endometrium has scattered cystically dilated glands but a low gland density overall. Randomly distributed glands may have tubal metaplasia, and fibrin thrombi can cause microinfarcts with symptomatic bleeding.
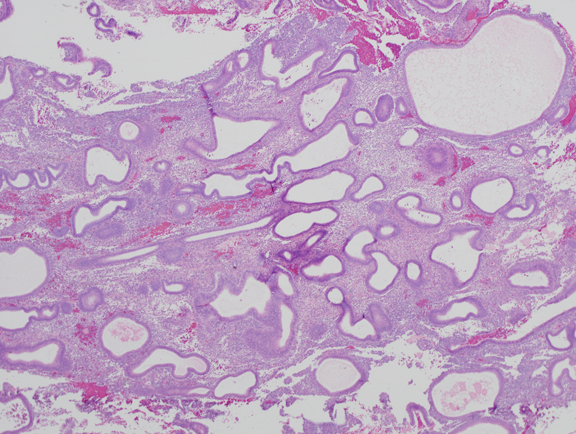 Fig. 4. Moderate estrogen effect. Benign endometrial hyperplasia. With additional duration of estrogen exposure, gland remodeling and expansion can lead to a higher gland density overall. Although the same underlying process as the less severe disordered proliferative endometrium, this is an example of benign endometrial hyperplasia. Crowded glands have not undergone any coordinated cytological change.
Fig. 4. Moderate estrogen effect. Benign endometrial hyperplasia. With additional duration of estrogen exposure, gland remodeling and expansion can lead to a higher gland density overall. Although the same underlying process as the less severe disordered proliferative endometrium, this is an example of benign endometrial hyperplasia. Crowded glands have not undergone any coordinated cytological change.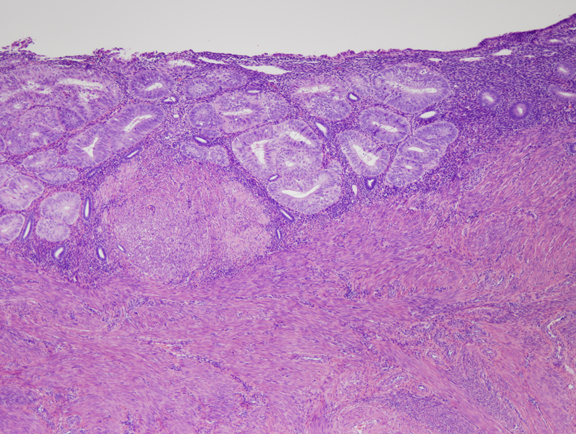 Fig. 5. A. Localized EIN lesion. A tight cluster of cytologically altered glands comprises the EIN lesion in this oriented section. Residual normal glands are visible on the right aspect of the endometrium.
Fig. 5. A. Localized EIN lesion. A tight cluster of cytologically altered glands comprises the EIN lesion in this oriented section. Residual normal glands are visible on the right aspect of the endometrium.
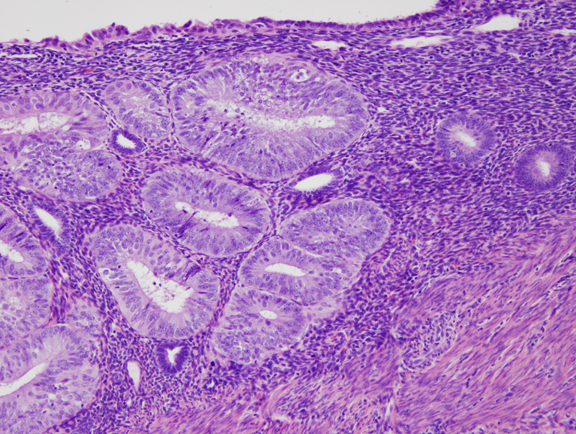 Fig. 5. B. Detail of localized EIN lesion shown in Figure 5A. Note the change in cytology between the neoplastic glands (left) and background endometrium (right).
Fig. 5. B. Detail of localized EIN lesion shown in Figure 5A. Note the change in cytology between the neoplastic glands (left) and background endometrium (right).
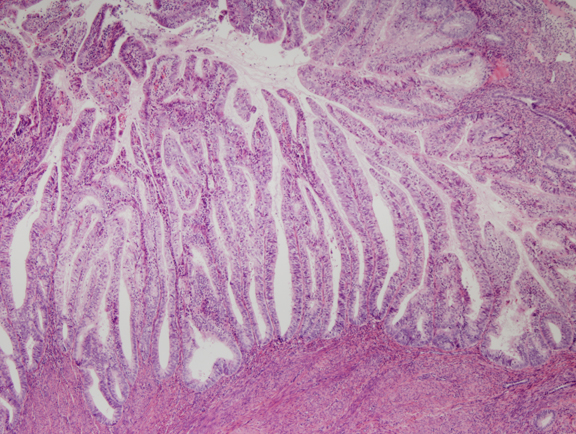 Fig. 6. Endometrial adenocarcinoma, endometrioid type, well differentiated. Unlike the EIN lesion above, the glands are no longer visible individually, but are arranged as folded villoglandular sheets.
Fig. 6. Endometrial adenocarcinoma, endometrioid type, well differentiated. Unlike the EIN lesion above, the glands are no longer visible individually, but are arranged as folded villoglandular sheets.
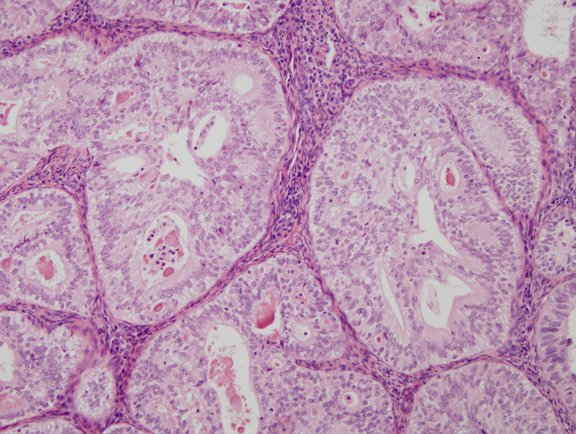 Fig. 7. Cribriform glands in well differentiated endometrial carcinoma.
Fig. 7. Cribriform glands in well differentiated endometrial carcinoma.
Careful histopathologic study of genotypically ascertained endometrial precancers explains prior problems in diagnosis and provides specific directions for improvement.21 Close correlations between histopathology and genotype are possible by isolating DNA from delineated regions of a paraffin section, which is also available as a serially sectioned stained slide. Epithelial differentiation of monoclonal precancers is usually endometrioid, but foci of squamous, mucinous, and tubal differentiation may be present. Changes in the hormonal environment, such as progesterone administration, may reduce the degree of cytologic atypia. Although most genetic precancers are diagnosed as atypical EH, poor reproducibility15 of this diagnosis compromises consistent management and raises the possibility that existing diagnostic criteria are inadequate. A particular void in precancer diagnosis has been absence of informative architectural criteria. Computerized morphometric analysis of monoclonal endometrial precancers,21 using algorithms that previously were shown to predict relevant clinical outcomes of concurrent22 or future23 endometrial adenocarcinoma, has broken this stalemate. When cytologically altered endometrial glands become so crowded that they comprise more than half of the sectioned surface, they predict monoclonality with a sensitivity and specificity at least equal to the clinical judgment of experienced subspeciality gynecologic pathologists.21 The absolute appearance of EIN cytology varies greatly between individual examples, and not all have the appearance of rounded nuclei with prominent nucleoli that is the classic definition of atypia in this tissue. What is consistent, however, is that the crowded glands of an EIN lesion always have an altered cytology relative to the background endometrium in the same patient. A relative internal, rather than absolute fixed, standard for recognition of altered cytology is the common feature of premalignant endometrial disease and EIN. Size is an important consideration in evaluation of a localizing lesion, such as an emergent EIN. A threshold of clinical relevance is when the crowded focus (areas with gland area exceeding stromal area) of cytologicially altered glands reaches a maximum dimension greater than 1 mm within a single fragment. Smaller lesions are not necessarily associated with heightened cancer risk, and should not be diagnosed as EIN.
Implementation of revised diagnostic criteria is a complicated process which cannot be completely described here. Detailed methods for routine histopathologic diagnosis of EIN are available in several dedicated gynecologic pathology texts2425 and online at www.endometrium.org.
The term endometrial intraepithelial neoplasia accurately describes endometrial precancers because monoclonal origin from a single transformed cell is the pathognomonic feature of all neoplasms. The superb performance of computerized morphometric analysis in classifying all genetically21 and clinically23 defined precancers into one group reaffirms the feasibility of using routine hematoxylin and eosin–stained tissue sections to define a singular category of precancers. Computerized morphometric analysis is inaccesible to most practice environments, but practicing pathologists may emulate its performance26 by applying the diagnostic criteria outlined in Table 1.
Table 1. Functional classification of estrogen effect, premalignant and malignant endometrial disease27
Nomenclature | Criteria | Functional Category | Management |
Benign endometrial hyperplasia (BEH) | 1. Irregular glands with cysts | Estrogen effect | Hormonal therapy |
| and | |||
2. More glands than stroma | |||
and | |||
3. No cytologic change | |||
| and | |||
| 4. Field-wide changes | |||
Endometrial intraepithelial neoplasia (EIN) | 1. Cytologic change | Precancer | Hormonal or surgical therapy |
and | |||
2. Gland exceeds stromal area | |||
| and | |||
| 3. Size >1 mm in single fragment | |||
| and | |||
| 4. Exclusion of mimics | |||
| and | |||
| 5. Exclusion of carcinoma | |||
Adenocarcinoma | 1. Myoinvasion | Cancer | Surgical and/or radiotherapy |
or | |||
2. Solid epithelium | |||
or | |||
3. Maze-like glands | |||
| or | |||
| 4. Cribriform architecture |
Classification of endometrial disease based on lesion biology aspires to place all precancers into a single group (EIN) and in contrast with mutually exclusive entities corresponding to different management options. Introduction of refined precancer diagnostic criteria, such as volume percentage stroma (VPS)22, 28 (that function of the sectioned tissue occupied by stroma), may improve histopathologic resolution between benign anovulatory (BEH) and premalignant (EIN) disease.
There are now several large clinical outcome studies outlining the clinical outcomes of patients diagnosed with EIN. There is a high rate of concurrent occult carcinoma in women diagnosed with EIN by biopsy. In a large GOG trial in which 153 women with EIN underwent hysterectomy, 36% (56) were found to have carcinoma.29 This is concordant with a retrospective pathology review study11 in which 39% of women diagnosed with EIN developed adenocarcinoma within the first year. In addition to this high rate of concurrent adenocarcinoma which is not evident from the initial EIN-containing biopsy, there is a 45-fold increased risk of developing carcinoma after the first year.11
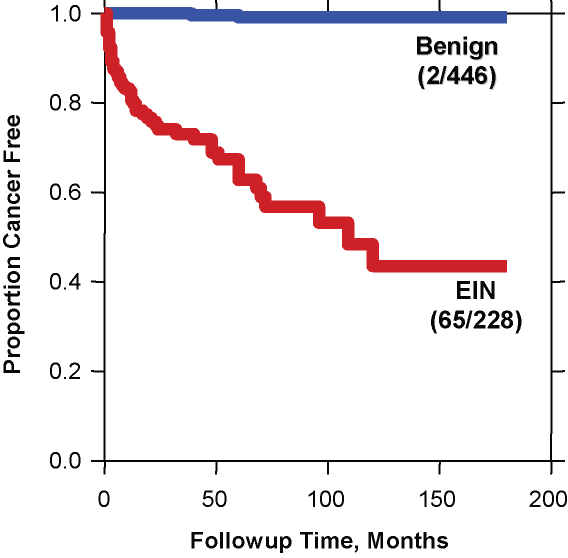
Fig. 8. Cancer outcomes of EIN. A total of 674 patients with various forms of endometrial hyperplasia were reclassified as EIN (red) or benign endometrial hyperplasia (blue) and followed for a median of over 5 years. The curve shows the proportion of each group which remained cancer free during follow up. Note the very low cancer incidence (2/446 cases) in the benign hyperplasia group compared to that of the EIN group (65/228). Although many cancers occurred shortly after EIN diagnosis and thus could be construed as concurrent, progression to carcinoma continued for several years thereafter. (Modified from Baak et al. Cancer 2005; 103(11): 2304.)
The relatively low risk of benign endometrial hyperplasia compared to EIN is evident in a study of over 600 women with various "endometrial hyperplasias" which were stratified as EIN vs. benign (usually estrogen effect, or benign endometrial hyperplasia sequence). The results shown in the figure indicate a very high negative predictive value for absence of cancer outcomes in women with benign endometrial hyperplasia.
Clinical management of EIN is similar to that previously applied to a diagnosis of atypical endometrial hyperplasia. In the US this is usually hysterectomy. There is a clinical need for nonsurgical alternative therapies in women who wish to retain fertility, or are poor surgical candidates, but there is a paucity of clinical trail data on the subject. High dose progestin therapy can succeed in ablating some EIN lesions, but, because of the high concurrent cancer rate and unpredictable response, must be accompanied by careful clinical surveillance.
POPULATION AT RISK
Most patients (75%) with endometrial cancer present with postmenopausal bleeding; however, only 10% of women with postmenopausal bleeding have endometrial carcinoma.28 The remaining women with postmenopausal bleeding have atrophic or inactive endometrium or benign endometrial conditions such as polyps. The clinical predictive model proposed by Feldman and colleagues30 (i.e., 70 years of age or older, diabetes, nulliparity, and postmenopausal status) is not predictive enough to distinguish between women with perimenopausal or postmenopausal bleeding at low versus high endometrial carcinoma risk.31 The traditional risk indicators associated with EIN/carcinoma are shown in Table 2. Most of the indicators are estrogen related, either from endogenous or exogenous sources. In women with these risk factors, the relative risk of developing carcinoma is 1.2–35.32 Experience also suggests that a significant proportion of patients fail to have these risk indicators but develop endometrial carcinoma. In these cases, either the disease may not be hormone related, or hyperestrogenism is metabolically inapparent. In a literature review, 74% of patients with adenocarcinoma of the endometrium were not obese, 58% were not nulliparous, 22% experienced menopause before age 49 years, and 43–89% were not exposed to hormone replacement therapy (HRT).33 It seems that the only constant endometrial carcinoma risk indicator is age. In women aged 65 years or older, endometrial cancer is generally aggressive and has a high mortality rate (75%) compared with that (15%) in the younger age group with hormone-related cancer.34
Table 2. Risk indicators for endometrial cancer and precursors
Age ≥60 years
Obesity (with upper body fat pattern)*
Estrogen-only replacement therapy
Previous breast cancer
Tamoxifen therapy for breast cancer
Chronic liver disease
Infertility
Low parity
Chronic anovulation (polycystic ovarian disease, estrogen secreting ovarian stroma or tumors)
*With or without diabetes and hypertension.
SCREENING AND DIAGNOSIS
In the case of endometrial carcinoma, the current consensus among experts in the field of periodic health examinations is not to recommend screening for endometrial cancer and its precursors because there is no scientific evidence to support such examinations in menopausal and postmenopausal women.35 The arguments against screening for endometrial carcinoma are as follows:
- Although endometrial carcinoma is common, morbidity rates are low, comparatively less important in number than breast carcinoma, colon carcinoma, lung carcinoma, leukemia, lymphoma, brain carcinoma, pancreas carcinoma, and ovary carcinoma.
- Based on the incidence of endometrial carcinoma in asymptomatic women, it would take about 1000 procedures to detect a single case of either a carcinoma or its precursor, atypical hyperplasia (AH).36, 37
- The techniques available for diagnosing endometrial disease in asymptomatic women suffer from pitfalls in interpretation or instrumentation. One is the difficulty in interpreting relatively inexpensive cytologic material;38 the other is that office biopsy aspiration techniques are relatively expensive and uncomfortable to painful, and tissue insufficient for diagnosis rates may be 25%.
- No controlled randomized trials have been done to evaluate the effectiveness of screening in endometrial carcinoma. Even in high-risk menopausal women, screening would detect only 50% of all cases of endometrial carcinoma.39
- Most patients with disease eventually become symptomatic (i.e., presenting with abnormal uterine bleeding, yet have early clinical stage disease at the time of surgical diagnosis and treatment). This contention is supported by the excellent 5-year survival rates of patients with stage I endometrial carcinoma (i.e., 80–91%).40 The clinicopathologic and epidemiologic data suggest that about 80% of endometrial carcinomas are slow growing with a favorable course,34 and earlier treatment of asymptomatic carcinomas would be no more effective than treatment given when symptoms appear.
- Elderly people are difficult to enroll into screening programs, and the dropout rate is relatively high. This is particularly true if painful techniques are used for endometrial evaluation.
- The incidence of endometrial carcinoma and its precursors is low in women aged younger than 50 years and in women receiving combination-type HRT (estrogen/progestins).32
Screening for endometrial carcinoma or its precursor, EIN, in asymptomatic, postmenopausal women is presently not recommended because of the low incidence of endometrial carcinoma in this group of women, estimated to be 1.7 cases per 1000 women per year, and the low prevalence, in the order of 1 per 1000 women.36 In the Postmenopausal Estrogen/Progestin Intervention (PEPI) trial, no patients developed endometrial carcinoma while on daily estrogen-only replacement therapy, 0.625 mg, during a follow-up of 36 months versus 1% of women who developed endometrial carcinoma on placebo.41 The average age of women with EIN is 52 years, which is about 8 years earlier than the average age of 60 for endometrioid endometrial adenocarcinoma in the same patient population.42 The interval for progression from EIN to adenocarcinoma can be more directly estimated in individual patients who undergo protracted surveillance following an EIN diagnosis. Once patients with concurrent adenocarcinoma are excluded (defined as cancer found within the first year of follow-up), the average interval to diagnosis of adenocarcinoma is 4 years.11
Who should be screened?
Women receiving unopposed estrogens need endometrial sampling once every 2 years (relative risk increases only after 2 years of estrogen use), particularly if endometrial hyperstimulation has been documented previously and has not been treated by short-term administration of progestins. Also, if the informed, high-risk individual requests an endometrial evaluation before or during HRT or at any time during her periodic health examinations, she should not be deprived of an office-based investigative procedure to rule out endometrial pathology. An endometrial evaluation also should be performed in women at high risk for endometrial carcinoma, such as women with history of Lynch II syndrome.43
The term diagnosis, as opposed to screening, refers to the application of a test to women presenting with symptoms (most commonly abnormal uterine spotting or bleeding) that presumably are related to endometrial carcinoma or its precursors. A study addressed the optimal evaluation strategy for patients with a first period of postmenopausal bleeding at various risks for endometrial carcinoma and AH.44 Among four options—office endometrial biopsy, dilation and curettage (D&C), hysterectomy, and observation alone (unless bleeding recurred)—office biopsy with the Vabra technique was the most cost-effective initial means, costing less than $41,000 US per year of life saved for patients with a 10% risk of having endometrial carcinoma or AH. For patients at 5% risk, the cost of endometrial biopsy increased, however, to $66,000 US per year of additional life saved for 60-year-old patients. Neither D&C nor hysterectomy was as cost-effective as office biopsy as an initial diagnostic evaluation procedure in patients with any risk for carcinoma/AH and abnormal uterine bleeding. Based on this decision-analytic model, the patient’s age and the risk for endometrial carcinoma/AH seem to be important determinants for the use of a given endometrial evaluation technique.
Screening and diagnostic techniques
At present, seven methods exist for assessing the endometrium: cervical/vaginal cytology, endometrial cytology, endometrial biopsy, transvaginal ultrasonography (TVUS), magnetic resonance imaging, hysteroscopy, and D&C.
CERVICAL/VAGINAL CYTOLOGY
The main drawbacks of this method are that it detects mainly advanced endometrial carcinoma and has a high false-negative rate (80%) in postmenopausal, asymptomatic endometrial carcinoma patients. In one study, the odds ratio of endometrial carcinoma in symptomatic postmenopausal women was three times greater in the presence of histiocytes with phagocytosis of acute inflammatory and red blood cells compared with controls.45 Histiocytes alone failed to predict either endometrial carcinoma or hyperplasia. Endometrial cells on cervical smears carried a fourfold odds ratio for EH. Vaginal cytology may detect recurrent cancer in women treated for endometrial carcinoma. Because the risk of recurrence of endometrial carcinoma (11–17%) and adjunct radiotherapy complications (70%) are greatest during the first 3 postoperative years and because most patients with recurrence are symptomatic and only few survive their recurrent disease, annual follow-up examination that includes vaginal cytology is sufficient.40
It is generally accepted that the best yield is obtained with tests that directly sample the endometrial lining.38 Numerous endometrial cell samplers are available commercially. Most of them obtain cellular samples either by brushing or by aspirating the superficial endometrial mucosa (Table 3). All endometrial cell samplers have been used under experimental conditions; the results in detection rates do not represent detection rates at large. Nevertheless, if cytologic atypia is the only feature to look for, endometrial cytology may be highly accurate in distinguishing carcinoma from normal or hyperplasia without cytologic atypia (Fig. 9). In one study, endometrial cytology using plastic brushes yielded 79% sensitivity, 95.4% specificity, and 80.5% negative predictive value.46 If the smear contains normal endometrial cells, the patient may have either a normal or a hyperplastic uterine lining. Often, hyperplasia without cytologic atypia is indistinguishable from normal proliferative endometrium. Because this form of hyperplasia is not a carcinoma precursor, however, the patients with symptoms such as uterine bleeding can be treated conservatively. Most cytologic laboratories lack expertise for distinguishing cytologic atypia related to neoplasia from atypia associated with degeneration or repair. As a result, false-positive rates may be too high to justify the routine use of cytology for endometrial disease. Also, the screening of an endometrial smear is time-consuming, and interpretation is difficult because of the complexity of endometrial gland cell morphology. Many carcinoma mimics lead to false-positive results.37, 38
Table 3. Accuracy of endometrial cytologic methods for detecting neoplasia*
Method | Diagnostic Accuracy†(%) | Unsatisfactory Specimen†(%) |
Brushing (Endo-Pap, Gynecyte, Endocyte, Endoscan) | 91–100 | 4–10 |
Aspiration (Isaacs cell sampler, Gravlee-jet washer) | 96–100 | 10–12 |
*Histologically verified.
†Including carcinoma and atypical hyperplasia.
†In postmenopausal women.
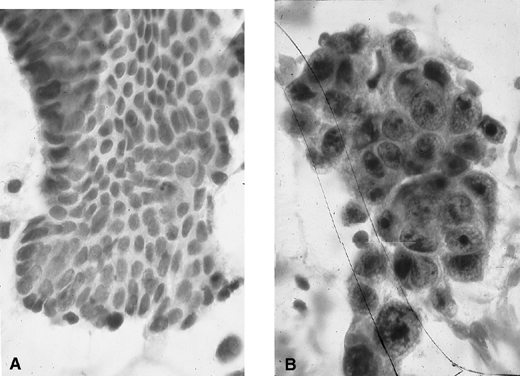 Fig. 9. Endometrial cytology obtained by endometrial aspiration. A. Hyperplastic endometrial cells with regular cytologic pattern are indistinguishable from normal endometrial cells of the proliferative phase of the cycle. B. Atypical endometrial cells with pleomorphic, hyperchromatic nuclei and macronucleoli. This obese patient was asymptomatic and had a FIGO stage 1A well-differentiated invasive adenocarcinoma.
Fig. 9. Endometrial cytology obtained by endometrial aspiration. A. Hyperplastic endometrial cells with regular cytologic pattern are indistinguishable from normal endometrial cells of the proliferative phase of the cycle. B. Atypical endometrial cells with pleomorphic, hyperchromatic nuclei and macronucleoli. This obese patient was asymptomatic and had a FIGO stage 1A well-differentiated invasive adenocarcinoma.
HISTOLOGIC METHODS
At present, histologic sampling is the best means to diagnose either asymptomatic or symptomatic (abnormal uterine bleeding) endometrial neoplasia. Plastic disposable or metal reusable devices using brushing, aspiration biopsy, suction curettage, or stroke biopsy have been used with similarly high diagnostic accuracy (Table 4).38 The pitfalls of histologic methods lie in their relatively high cost and degree of discomfort. The latter leads to low compliance rates for repeat testing. Conventional curettage is much too costly yet not 100% foolproof as far as diagnostic accuracy is concerned.47 According to current experience including our own, the endometrial devices that seem to be the most cost-effective and are associated with the least discomfort for patients are the endometrial aspirators.38 In cases in which tissue is not obtained with one of the low-vacuum, suction-type aspirators, particularly in an elderly postmenopausal woman whose endometrium is more often than not atrophic, aspirators with a powerful vacuum suction force (e.g., Vabra aspirator; Tis-u-Trap; or sharp-bladed, four-stroke biopsy curette) provide diagnostic tissues.
Table 4. Accuracy of endometrial histologic methods for diagnosing neoplasia
| Method | Diagnostic Accuracy* (%) | Tissue Insufficienct for Diagnosis† (%) |
| Aspiration | ||
| Pipelle | 95–98 | 9 |
| Endocell | 95–98 | 11 |
| Vabra | 95–98 | 26 |
| Isaacs cell sampler | 95–98 | 30 |
| Pistolette | 95–98 | 13 |
| Brushing | ||
| Endoscann | 100 | 36 |
| Biopsy | ||
| Kevorkian | 97 | 10 |
| Others |
*Including carcinoma and endometrial intraepithelial neoplasia.
†In postmenopausal women.
Although some physicians had success in using endometrial brushes such as the Gynecyte (Looper Surgical, Inc; European version of Endocyte) for cytologic sampling of the endometrium, most prefer to sample the endometrium for histology. The instrument used most frequently is the endometrial Pipelle (Sepal, Boston, MA) (Figs. 10 and 11) and, when appropriate, the Kevorkian curette (EuroMed, Redmond, WA) (see Fig. 11) for histologic sampling in asymptomatic and symptomatic women at risk for endometrial carcinoma and its precursors.38 In about 10% of postmenopausal women, the endometrial cavity is difficult or impossible to penetrate because of severe stenosis of the external/internal os or because of internal os spasm. In these cases, placing the patient on sequential cyclic therapy with conjugated estrogens (Premarin) (0.625 mg for 25 days) and medroxyprogesterone (Provera) (5 mg for 11–12 days) for 3 consecutive months often results in adequate dilation of the external/internal os to allow penetration of the endometrial cavity. Another alternative is to perform TVUS and assess the thickness of the endometrium (see Transvaginal Ultrasonography below). Finally, traction of the uterus with the endocervical Emmett’s tenaculum or a skin (Iris) hook (see Fig. 10) is of considerable help for entering the endometrial cavity in the office. If an endometrial aspirator of the Pipelle type is used, it is important to move and rotate the cannula under negative action suction force within the endometrial cavity at least six times to sample the greatest surface area of the endometrium. In a comparison of the Pipelle versus the Vabra aspirator, the percentage of endometrial surface mucosa sampled with the Pipelle was 4.2% versus 42% with the Vabra aspirator and 60% with D&C under general anesthesia.48 The difference in percentages of area sampled is likely due to the comparatively greater suction force of the Vabra than the Pipelle device.
 Fig. 10. Endometrial aspirators and curette. Pipelle, Z-sampler (BEI/Zinnanti, Chatsworth, CA), Uterobrush (Medscand USA, Hollywood, FL), Endocell, and Kevorkian curette without baskets (top). Emmet tenaculum and iris hook (Cooper Surgical, Shelton, CT) for uterine traction (bottom).
Fig. 10. Endometrial aspirators and curette. Pipelle, Z-sampler (BEI/Zinnanti, Chatsworth, CA), Uterobrush (Medscand USA, Hollywood, FL), Endocell, and Kevorkian curette without baskets (top). Emmet tenaculum and iris hook (Cooper Surgical, Shelton, CT) for uterine traction (bottom).
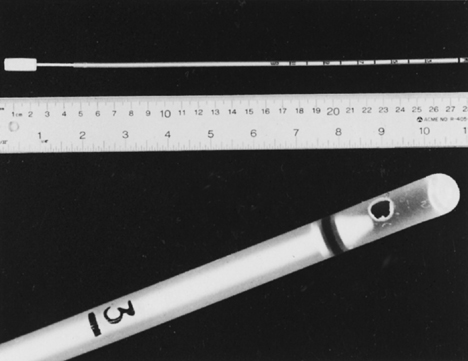 Fig. 11. Endometrial Pipelle. Flexible polypropylene suction cannula with a 24-cm-long, 3-mm-wide outer sheath and a fine-caliber piston (top). Detailed view of the 2.5-mm distal side port through which the specimen is aspirated (bottom).
Fig. 11. Endometrial Pipelle. Flexible polypropylene suction cannula with a 24-cm-long, 3-mm-wide outer sheath and a fine-caliber piston (top). Detailed view of the 2.5-mm distal side port through which the specimen is aspirated (bottom).
Sampling for histology: a step-by-step guide
- Bimanually examine the uterus to determine its position.
- Clean the cervix and vagina with acetic acid or other aseptic solution.
- Insert an Emmett’s tenaculum or iris hook (see Fig. 10) into the outer one third of the endocervical canal and pull gently to obtain traction of the uterus.
- Insert an endometrial aspirator into the endocervical canal (see Figs. 10 and 11). When the aspirator is at the lower uterine segment level, push and rotate it to facilitate entering the endometrial cavity.
- When it is at the fundus, pull the plunger back rapidly and completely in the cannula to create a high negative pressure gradient.
- Move the cannula back and forth 6–12 times in the endometrial cavity and rotate it at the same time.
- Remove the cannula with the plunger pulled back (retain suction) from the endometrial cavity. Empty the material on a lens paper by pushing the plunger forward and place it in 10% buffered formalin tissue fixative.
If little or no tissue is obtained, the procedure can be repeated once. If still no tissue has been obtained, endometrial sampling can be performed using either the Vabra or other powerful aspirators or a metal curette (see Fig. 10). If still no tissue is obtained and the uterus is small, one can assume endometrial atrophy or fibrous pedunculated polyps are present. Transvaginal sonography or hysteroscopy, if the patient is symptomatic, may be performed.
In current practice, staging of endometrial carcinoma is surgical (hysterectomy, bilateral salpingo-oophorectomy, and pelvic node biopsy)49 and includes the histologic assessment of invasion of the endocervical mucosa (International Federation of Gynecology and Obstetrics [FIGO] stage IIA) versus the stroma (FIGO stage IIB) in the hysterectomy specimen. As a result, the preoperative evaluation of the endocervical canal is no longer necessary. The exception to the rule is a younger, premenopausal woman in whom a fractional sampling of the uterus can determine whether the patient has an endocervical or an endometrial primary tumor.50, 51
TRANSVAGINAL ULTRASONOGRAPHY
TVUS can visualize the endometrium on a monitor when a 5-MHz probe is placed against the vaginal fornix. The thickness of the endometrium can be measured with precision because the endometriomyometrial junction has a distinct halo-like appearance (Fig. 12). TVUS is highly sensitive but also has high false-positive rates (low specificity) for identifying endometrial carcinoma. Studies suggested that specificity may be improved without jeopardizing sensitivity rates if the cutoff values were based on length of time since menopause.46 When the endometrial thickness is 4 mm for women less than 5 years since menopause and 3 mm for women more than 5 years since menopause, TVUS had a 97.4% sensitivity, 75.7% specificity, and 99.7% negative predictive value. With respect to TVUS, the cutoff points for a minimum thickness have varied from one country to another. However, there is consensus that the mean double endometrial thickness in cases of endometrial hyperplasia/carcinoma is significantly greater than that in patients without such lesions. In the USA, the recommended lower limit of finding endometrial cancer is 4 or 5 mm, and at 3 mm, it is not necessary to perform endometrial biopsy.50, 51
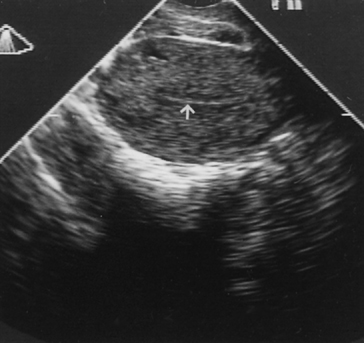 Fig. 12. Transvaginal ultrasonography of the uterus. Note the atrophic endometrial lining (arrow).
Fig. 12. Transvaginal ultrasonography of the uterus. Note the atrophic endometrial lining (arrow).
MAGNETIC RESONANCE IMAGING
At present, magnetic resonance imaging and computed tomography have been proved to be useful for obtaining preoperative data on the extent and depth of myometrial invasion by endometrial carcinoma rather than in the primary diagnosis of endometrial carcinoma and its precursors.52 Its role in the primary diagnosis of endometrial cancer and its precursors remain to be determined.
HYSTEROSCOPY
The value of hysteroscopy in the diagnosis and directed biopsy of a variety of intracavitary or endometrial lesions in women with postmenopausal bleeding has been extensively documented. If insufficient tissue is obtained on suction curettage, or if a patient continues to have abnormal bleeding, a formal D&C is often recommended, despite the fact that its superiority over office procedures in the diagnosis of cancer has not been established.47
Absolute indications for hysteroscopy have not been established. When available, however, hysteroscopy is indicated in any woman with abnormal uterine bleeding in whom an intrauterine abnormality is suspected (Fig. 13). Other indications include recurrent miscarriages, infertility caused by endometrial pathology, removal of an impacted intrauterine device, and suspected submucous leiomyomas before abdominal myomectomy. Hysteroscopy is contraindicated in the presence of active infection and intrauterine pregnancy. Active bleeding is a relative contraindication to office hysteroscopy only because blood interferes with vision if carbon dioxide is used as a distending medium. In patients who have severe medical problems, it is prudent to perform hysteroscopy in an outpatient setting where full monitoring and resuscitation facilities are available.
 Fig. 13. Hysteroscope with light source (left) and insufflator (right).
Fig. 13. Hysteroscope with light source (left) and insufflator (right).
DILATION AND CURETTAGE
D&C essentially has been replaced by office-based endometrial biopsy using flexible aspiration devices. The latter is more cost-effective than D&C, and the diagnostic yield in symptomatic and asymptomatic women is similar to D&C with sensitivity and specificity rates of 90% and 95%.38 Cervical stenosis prevents successful endometrial sampling in about 10% of cases.
FALSE-NEGATIVE HISTOLOGY
Even direct sampling of the endometrium for histology may fail to detect adenocarcinoma. In several studies, D&C under general anesthesia missed 10% of endometrial carcinomas.38, 47 This is not surprising for, as was stated earlier, only 60% of mean surface area is sampled with D&C versus 40% for Vabra curettage and 4% for endometrial biopsy with the Pipelle endometrial aspirator.48 Others found four of 86 (4.6%) women with postmenopausal bleeding with endometrial carcinoma who had either a negative endometrial biopsy result or D&C within 2 years before cancer diagnosis.53 In another study from Australia,54 the false-negative rate of endometrial biopsy of focal adenocarcinomas of the endometrium was 47%.
TREATMENT OF BENIGN ENDOMETRIAL HYPERPLASIA AND EIN
Management depends on whether the underlying disease is primarily hormonal (benign endometrial hyperplasia) or intrinsic premalignant disease (EIN). The choice of surgical or hormonal therapy depends on the histopathologic diagnosis, the reproductive status of the woman, whether the patient is on estrogen-only replacement therapy, and her general health. In general, EIN is managed using algorithms that have been previously developed for treatment of atypical endometrial hyperplasia, and benign endometrial hyperplasia using those previously employed for non-atypical hyperplasias. The high cancer risk conferred by an EIN diagnosis, including a 36% incidence of occult carcinoma of which one third are myoinvasive, must be carefully considered in deciding upon appropriate therapy.55 Although some cases of EIN/early intramucosal adenocarcinoma respond to exogenous progestogens, ovulation inducers, or both, in most cases the lesions tend to recur within a few months to a few years after delivery of the newborn. Medical hormone therapy is also given to women whose general health is unsuitable to withstand surgery.
Benign endometrial hyperplasia responds well to medroxyprogesterone acetate (MPA), 10 mg orally, or micronized progesterone, 300 mg orally, once a day for 14 days per month for 3 months. Such cyclic regimens lead to withdrawal bleeding; a biopsy specimen is obtained at the end of the progestin therapy at 3–4 months. Complete responders should be maintained on cyclic progesterone therapy or, if appropriate, combined cyclic or continuous HRT. If a partial response is obtained, another 3-month trial with MPA, 10 mg orally four times per day, or megestrol acetate, 80 mg, for 3 months may be carried out. Nonresponders and patients with intractable breakthrough bleeding may have transabdominal hysterectomy. Progestin therapy for premenopausal women with EIN calls for larger doses of MPA, 100 mg orally daily; megestrol acetate, 160 mg; or 1 g/week of MPA intramuscularly for 12 weeks. In recent years, another means to treat endometrial hyperplasia with or without atypia has been the medicated intrauterine device (IUD) such as the LNG- (levonorgestrel) releasing intrauterine system (Mirena®, Bayer Healthcare Pharmaceuticals, Inc. Wayne, NJ, USA). Several studies have shown complete response (reversal of endometrial hyperplasia to progestational-type endometrium) ranging from 25 to over 90%. In general, EH responds better (90–100%) than EIN (67–88%) to intrauterine LNG.56, 57, 58 In addition to the powerful progestational effect of Mirena on the endometrium, adverse events (side effects) that are commonly experienced by patients with oral progestational therapy are considerably reduced. This is because the systemic absorption of LNG is considerably reduced compared to oral progestational therapy.56, 57, 58, 59 The biopsy specimen should show progestational-type endometrium with marked stromal decidualization. Careful follow up surveillence, including repeated biopsy at approximately 6 month intervals until several are free of disease is advised to ensure complete ablation. Induction of ovulation should follow the progestational therapy.
Surgery (i.e., transabdominal hysterectomy) with or without bilateral salpingo-oophorectomy is recommended for women who have persistent benign endometrial hyperplasia but are symptomatic (abnormal uterine bleeding) and women in the postreproductive age group with EIN. Surgery is justified in this group in the face of 25–35% progression rates to invasion and an 80% failure rate to respond to progestational therapy.60 Women who develop benign endometrial hyperplasia during estrogen-alone replacement therapy may benefit from the addition of progestins into their replacement regimen. The rare patient (1%) who develops benign endometrial hyperplasia while on combined cyclic or continuous HRT41 may benefit from either higher doses of combined HRT or simply switching to a progestin-only replacement therapy for 3 months to attempt reverting the hyperplastic endometrium to normal.
It has been shown that duration of progestin administration is crucial for inhibiting endometrial mitotic activity; this is important because control of endometrial growth is primarily related to control of epithelial mitotic activity. Inhibition of endometrial mitotic activity is noted after 11 days of progestin treatment.61 The most frequent hormone preparations used for medical treatment of evaluated hyperplasia with or without atypia are presented in Table 5.
Table 5. Treatment regimens by type of hormone, dosage, and duration*
Endometrial Histology | ||
Hormone Preparations | Benign Endometrial Hyperplasia | EIN |
Medroxyprogesterone acetate | 10 mg PO × 14 days/month | 100 mg PO or 1000 mg/week IM |
Micronized progesterone | 300 mg PO × 14 days/month | 300 mg/day PO |
Megestrol acetate | 80 mg PO × 14 days/month | 160 mg/day PO |
| LNG-IUD | 20 μg/day x 6 months to 2 years | |
IM, intramuscularly; PO, orally; LNG, levonorgestrel.
*All regimens are given for 3 months.
CONTROVERSIAL ISSUES
Should women who are to be placed on the combined variant of HRT receive endometrial evaluation, and if so, should endometrial biopsy or TVUS be used? There may be two factors in favor of assessing the endometrium in these women, preferably by obtaining histologic material. There is growing evidence of discovering preexisting endometrial carcinoma in women on either combined cyclic or combined continuous HRT.62, 63, 64, 65 It is important to avoid legal responsibilities in subjecting a patient with preexisting endometrial carcinoma to HRT. Although in most of these cases the endometrial carcinoma is early-stage FIGO IA/B, well-differentiated FIGO grade I with excellent prognosis, some are advanced carcinomas with poor prognostic factors. Also, some patients are asymptomatic, and it is possible at least theoretically that earlier detection could lead to better survival than later diagnosis of disease.
Screening for endometrial pathology with TVUS has been suggested in women receiving long-term tamoxifen therapy.66 Others caution that endometrial TVUS may have high false-positive rates because tamoxifen exerts an echogenic and sonolucent effect in the endometrial stroma and myometrium, masquerading as hyperplasia or carcinoma.67 Ultrasonographic studies found no evidence of increased prevalence of endometrial pathology in a series of 108 women on tamoxifen therapy who had biopsies.68 The various endometrial changes in postmenopausal breast carcinoma patients on tamoxifen treatment include endometrial polyps of the adenomatous type, hyperplasia, carcinoma, and sarcoma.69
PROGESTERONE CHALLENGE TEST
Historically, Padwick and colleagues70 introduced the progesterone challenge test into clinical practice in Europe, particularly in the United Kingdom, to the extent that bleeding on or after the 11th day of progestin administration was considered assurance of effective endometrial protection. This study was based on only 96 women, however. In a more recent study of 413 postmenopausal women on combined HRT using progestins for 10 days, there was no correlation between endometrial histology including hyperplasia and timing of onset of bleeding.71 In our experience of 25 years in practice and clinical research, although many women with an estrogenized endometrium including hyperplasia may experience withdrawal bleeding, others do not.72
CONCLUSIONS
Invasive carcinoma of the endometrium is preceded by EIN, which by genetic markers is monoclonal and morphologically is identified by significant cytologic change relative to the same patient's background endometrium within a region of glands in which gland area exceeds stromal area. Benign endometrial hyperplasia is not a carcinoma precursor lesion, but rather it is an endometrial response to an abnormal hormonal environment of unopposed estrogens.
Although endometrial carcinoma and its precursors are significant because of their morbidity, mortality resulting from carcinoma is low. As a result, mass screening for asymptomatic endometrial carcinoma and its precursors is not cost-effective and is not recommended. Nevertheless, if screening for endometrial carcinoma is desired in a private practice, it should focus on women aged 55 years old and older and women with high carcinoma risk indicators.
Cytologic sampling of the endometrium directly is limited to communities in which cytologic expertise is available. The most often used method to evaluate the endometrium is histology; to reduce cost, it should be carried out in the office, and the device used should employ vacuum suction force, be disposable, and be of low cost. TVUS seems to be a potentially useful alternative to histology for screening and diagnosing endometrial carcinoma and hyperplasia. Hysteroscopy is the diagnostic method of choice for patients in whom office biopsy and TVUS failed to provide a definite diagnosis. Patients with EH without cytologic atypia and patients with atypia who desire to conceive should have progestational therapy. Patients with atypia or intractable uterine bleeding without atypia benefit from hysteroscopy.
REFERENCES
Mittal KR, Schwartz PE, Barwick KW: Architectural (FIGO) grading, and other prognostic indicators in stage 1 endometrial adenocarcinoma with identification of high-risk and low-risk groups. Cancer 61:538, 1988 |
|
Bokhman J: Two pathogenic types of endometrial carcinoma. Gynecol Oncol 15:10, 1983 |
|
Deligdisch L, Holinka C: Endometrial carcinoma: Two diseases? Cancer Detect Prev 10:237, 1987 |
|
Gusberg SB: Precursors of corpus cancer. In Gusberg SB, Shingleton H, Deppe G (eds): Female Genital Cancer. pp 337, 378 New York, Churchill Livingstone, 1988 |
|
Kurman RJ, Kaminski PF, Norris HJ: The behavior of endometrial hyperplasia. A long-term study of "untreated" hyperplasia in 170 patients. Cancer 56(2):403, 1985 |
|
Kendall BS, Ronnett BM, Isacson C, Cho KR, Hedrick L, et al. Reproducibility of the diagnosis of endometrial hyperplasia, atypical hyperplasia and well-differentiated carcinoma. Am J Surg Pathol 25(8):1012, 1998. |
|
Zaino RJ, Kauderer J, Trimble CL et al: Reproducibility of the diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer 106(4):804, 2006. |
|
Mutter GL, Zaino RJ, Baak JP, Bentley RC, Robboy SJ. Benign endometrial hyperplasia sequence and endometrial intraepithelial neoplasia. Int J Gynecol Pathol 26(2):103, 2007. |
|
Mutter GL, Baak JP, Crum CP et al: Endometrial precancer diagnosis by histopathology, clonal analysis, and computerized morphometry. J Pathol 190(4):462, 2000. |
|
Mutter GL. Endometrial intraepithelial neoplasia (EIN): will it bring order to chaos? The Endometrial Collaborative Group. Gynecol Oncol 76(3):287, 2000. |
|
Baak JP, Mutter GL, Robboy S et al: The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer 103(11):2304, 2005. |
|
Mutter GL, Kauderer J, Baak JP et al: Biopsy histomorphometry predicts uterine myoinvasion by endometrial carcinoma: a Gynecologic Oncology Group study. Hum Pathol. 39(6):866, 2008 |
|
Mutter GL: Histopathology of genetically defined endometrial precancers. Int J Gynecol Pathol. 19(4):301, 2000. |
|
Winkler B, Alvarez S, Richart RM, et al: Pitfalls in the diagnosis of endometrial neoplasia. Obstet Gynecol 64:185, 1984 |
|
Kendall BS, Ronnett BM, Isaacson C, et al: Reproducibility of the diagnosis of endometrial hyperplasia, atypical hyperplasia, and well-differentiated carcinoma. Am J Surg Pathol 22:1012, 1998 |
|
Vogelstein B, Kinzler KW: The multistep nature of cancer. Trends Genet 9:138, 1993 |
|
Mutter GL, Chaponot M, Fletcher J: A PCR assay for non-random X chromosome inactivation identifies monoclonal endometrial cancers and precancers. Am J Pathol 146:501, 1995 |
|
Mutter GL, Boynton KA, Faquin WC, et al: Allelotype mapping of unstable microsatellites establishes direct lineage continuity between endometrial precancers and cancer. Cancer Res 56:4483, 1996 |
|
Maxwell G, Risinger J, Gumbs C, et al: Mutation of the PTEN tumor suppressor gene in endometrial hyperplasia. Cancer Res 58:2500, 1998 |
|
Tate JE, Mutter GL, Boynton KA, Crum CP: Monoclonal origin of vulvar intraepithelial neoplasia and some vulvar hyperplasias. Am J Pathol 150:315, 1997 |
|
Mutter GL, Baak JPA, Crum CP, et al: A multidisciplinary approach to diagnosis of endometrial precancers: Clonal analysis, histopathology, and computerized morphometry. Am J Pathol 190:462, 2000 |
|
Dunton C, Baak J, Palazzo J, et al: Use of computerized morphometric analyses of endometrial hyperplasias in the prediction of coexistent cancer. Am J Obstet Gynecol 174:1518, 1996 |
|
Baak JPA, Nauta J, Wisse-Brekelmans E, Bezemer P: Architectural and nuclear morphometrical features together are more important prognosticators in endometrial hyperplasia than nuclear morphometrical features alone. J Pathol 154:335, 1988 |
|
Mutter GL, Zaino RJ, Baak JPA, Bentley RC, Robboy SJ. Benign endometrial hyperplasia and EIN. In: Robboy SJ, Mutter GL, Prat J, Bentley R, Russell P, Anderson MC, editors. Robboy's Pathology of the Female Reproductive Tract. New York, New York: Elsevier, 2009: pp 367-391 |
|
Mutter GL, Duska L, Crum CP. Endometrial Intraepithelial Neoplasia. In: Crum CP, Lee K, editors. Diagnostic Gynecologic and Obstetric Pathology (1st Edition). Philadelphia: Saunders, 2006 |
|
Hecht JL, Ince TA, Baak JP et al: Prediction of endometrial carcinoma by subjective endometrial intraepithelialneoplasia diagnosis. Mod Pathol. 18(3):324, 2005 |
|
Mutter GL, Zaino RJ, Baak JP et al: Benign endometrial hyperplasia sequence and endometrial intraepithelialneoplasia. Int J Gynecol Pathol. 26(2):103, 2007 |
|
Karlsson B, Granberg S, Wikland M, et al: Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding: A Nordic multicenter study. Am J Obstet Gynecol 172:1488, 1995 |
|
Mutter GL, Kauderer J, Baak JP et al: Biopsy histomorphometry predicts uterine myoinvasion by endometrial carcinoma: aGynecologic Oncology Group study. Hum Pathol. 39(6):866, 2008 |
|
Feldman S, Cook EF, Harlow BL, Berkowitz RS: Predicting endometrial cancer among older women who present with abnormal vaginal bleeding. Gynecol Oncol 56:376, 1995 |
|
Weber AM, Belinson JL, Piedmonte MR: Risk factors for endometrial hyperplasia and cancer among women with abnormal bleeding. Obstet Gynecol 93:594, 1999 |
|
Ferenczy A: Endometrial carcinoma and its precursors in relation to hormone replacement therapy. In Lorrain J, Plouffe L Jr, Ravnikar V, et al (eds): Comprehensive Management of Menopause. pp 254, 268 New York, Springer-Verlag, 1994 |
|
Richardson GS, MacLaughlin DT (eds): Hormonal Biology of Endometrial Cancer. A Series of Workshops on the Biology of Human Cancer. Report No. 8. Geneva, International Union Against Cancer, 1978 |
|
Homesley HD, Zaino R: Endometrial cancer: Prognostic factors. Semin Oncol 21:71, 1994 |
|
Pritchart KI: Screening for endometrial cancer: Is it effective? Ann Intern Med 110:177, 1989 |
|
Archer DF, McIntyre-Seltman K, Wilborn WW Jr, et al: Endometrial morphology in asymptomatic postmenopausal women. Am J Obstet Gynecol 165:317, 1991 |
|
Koss LG, Schriber K, Oberlander SG, et al: Detection of endometrial carcinoma and hyperplasia in asymptomatic women. Obstet Gynecol 64:1, 1984 |
|
Ferenczy A: Methods for detecting endometrial carcinoma and its precursors. In Buchsbaum HJ, Sciarra JJ (eds): Gynecology and Obstetrics. pp 1, 8 Vol 4:New York, Harper & Row, 1982 |
|
MacMahon B: Risk factors for endometrial cancer. Gynecol Oncol 2:122, 1974 |
|
Burke TW: How should we monitor women treated for endometrial carcinoma? Gynecol Oncol 65:377, 1997 |
|
The Writing Group for the PEPI Trial: Effects of hormone replacement therapy on endometrial histology in postmenopausal women: The Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. JAMA 275:370, 1996 |
|
Hecht JL, Ince TA, Baak JP et al: Prediction of endometrial carcinoma by subjective endometrial intraepithelialneoplasia diagnosis. Mod Pathol 18(3):324, 2005 |
|
Hakala T, Mecklin J-P, Forss M, et al: Endometrial carcinoma in the cancer family syndrome. Cancer 68:1656, 1991 |
|
Feldman S, Berkowitz RS, Tosteson ANA: Cost-effectiveness of strategies to evaluate postmenopausal bleeding. Obstet Gynecol 81:968, 1993 |
|
Nguyen TN, Bourdeau J-L, Ferenczy A, Franco EL: Clinical significance of histiocytes in the detection of endometrial adenocarcinoma and hyperplasia. Diagn Cytopathol 19:89, 1998 |
|
Tsuda H, Kawabata M, Yamamoto K, et al: Prospective study to compare endometrial cytology and transvaginal ultrasonography for identification of endometrial malignancies. Gynecol Oncol 65:383, 1997 |
|
Grimes DA: Diagnostic dilation and curettage: A reappraisal. Obstet Gynecol 142:1, 1982 |
|
Rodriguez GC, Yaqub N, King ME: A comparison of the Pipelle device and the Vabra aspirator as measured by endometrial denudation in hysterectomy specimens: The Pipelle device samples significantly less of the endometrial surface than the Vabra aspirator. Am J Obstet Gynecol 168:55, 1993 |
|
Homesley HD: Revised 1988 International Federation of Gynecology and Obstetrics staging systems for endometrial and vulvar cancer: An assessment. Clin Obstet Gynecol 35:89, 1992 |
|
Tabor A, Watt HC, Wald NJ. Endometrial thickness as a test for endometrial cancer in women with postmenopausal vaginal bleeding. Obstet Gynecol 99:663, 2002 |
|
Goldstein S, Nachtigall M, Snyder J, Nachtigall L. Endometrial assessment by vaginal ultrasonography before endometrial sampling in patients with postmenopausal bleeding. Am J Obstet Gynecol 163:119, 1990 |
|
Takahashi S, Murakami T, Narumi Y, et al: Preoperative staging of endometrial carcinoma: Diagnostic effect of T2-weighted fast spin-echo MR imaging. Radiology 206:539, 1998 |
|
Feldman S, Shapter A, Welch WR, Berkowitz RS: Two-year follow-up of 263 patients with post/perimenopausal vaginal bleeding and negative initial biopsy. Gynecol Oncol 55:56, 1994 |
|
Quinn MA, Kneale BJ, Fortune DW: Endometrial carcinoma in premenopausal women: A clinicopathological study. Gynecol Oncol 20:298, 1985 |
|
Mutter GL, Kauderer J, Baak JP et al: Biopsy histomorphometry predicts uterine myoinvasion by endometrial carcinoma: a Gynecologic Oncology Group study. Hum Pathol. 39(6):866, 2008 |
|
Haimovitch S, Checa MA, Mancebo G, Fusté P, Carreras R. Treatment of endometrial hyperplasia without atypia in peri- and postmenopausal women with a levonorgestrel intrauterine device. Menopause 15(5):1002, 2008 |
|
Varma R, Soneja H, Bhatia K, Ganesan R, Rollason T, Clark TJ, Gupta JK. The effectiveness of a levonorgestrel-releasing intrauterine device system (LNG-IUS) in the treatment od endometrial hyperplasia-a long term follow-up study. Eur J Obstet Gyaecol Reprod Biol 139(2):169, 2008 |
|
Kresowik J, Ryan GL, Van Voorhis BJ. Progression of atypical endometrial hyperplasia to adenocarcinoma despite intrauterine progesterone treatment with the levonorgestrel-releasing intrauterine system. Obstet Gynecol 111(2 Pt 2):547, 2008 |
|
Orbo A, Arnes M, Hancke C, Vereide AB, Pettersen I, Larsen K. Treatment results of endometrial hyperplasia after prospective D-score classification: a follow-up study comparing effect of LNG-IUD and oral progestins versus observation only. Gynecol Oncol 111(1):68, 2008 |
|
Ferenczy A, Gelfand MM: The biologic significance of cytologic atypia in progestogen-treated endometrial hyperplasia. Am J Obstet Gynecol 160:126, 1989 |
|
Moyer DL, de Lignieres B, Driguez P, Pez J-P: Prevention of endometrial hyperplasia by progesterone during long-term estradiol replacement: Influence of bleeding pattern and secretory changes. Fertil Steril 59:992, 1993 |
|
McGonigle KF, Karlan BY, Barbuto DA, et al: Development of endometrial cancer in women on estrogen and progestin hormone replacement therapy. Gynecol Oncol 55:126, 1994 |
|
Shipley CF III, Smith ST, Dennis EJ III, et al: Evaluation of pretreatment transvaginal ultrasonography in the management of patients with endometrial carcinoma. Am J Obstet Gynecol 167:406, 1992 |
|
Comerci JT, Fields AL, Runowicz CD, Goldberg GL: Continuous low-dose combined hormone replacement therapy and the risk of endometrial cancer. Gynecol Oncol 64:425, 1997 |
|
Dallenback-Hellweg G, Hahn U: Mucinous and clear cell adenocarcinomas of the endometrium in patients receiving antiestrogens (Tamoxifen) and gestagens. Int J Gynecol Pathol 14:7, 1995 |
|
Jordan VC, Morrow M: Should clinicians be concerned about the carcinogenic potential of tamoxifen? Eur J Cancer 30:1714, 1994 |
|
Goldstein SR: Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol 170:447, 1994 |
|
Cecchini S, Ciatto S, Bonardi R, et al: Screening by ultrasonography for endometrial carcinoma in postmenopausal breast cancer patients under adjuvant tamoxifen. Gynecol Oncol 60:409, 1996 |
|
Kennedy MM, Baigrie CF, Manek S: Tamoxifen and the endometrium: Review of 102 cases and comparison with HRT-related and non-HRT-related endometrial pathology. Int J Gynecol Pathol 18:130, 1999 |
|
Padwick ML, Pryse-Davies J, Whitehead MI: A simple method for determining the optimal dosage of progestin in postmenopausal women receiving estrogens. N Engl J Med 315:930, 1986 |
|
Sturdee DW, Barlow DH, Ulrich LG: Is the time of withdrawal bleeding a guide to endometrial safety during sequential oestrogen-progestagen replacement therapy? Lancet 344:979, 1994 |
|
Gelfand MM, Ferenczy A: A prospective 1-year study of estrogen and progestin in postmenopausal women: Effects on the endometrium. Obstet Gynecol 74:398, 1989 |