Clinical Anatomy of the Uterus, Fallopian Tubes, and Ovaries
Authors
INTRODUCTION
THE UTERUS
The uterus varies considerably in size, shape and weight depending on the status of parturition and estrogenic stimulation. The uterus is a fibromuscular organ that can be divided into the upper muscular uterine corpus and the lower fibrous cervix, which extends into the vagina. The upper part of the uterus above the insertion of the fallopian tubes is called the fundus. The narrow portion situated between corpus and cervix is known as the isthmus and lies approximately at the level of the course of the uterine artery and the internal os of the cervix. The endometrial cavity lies within the uterine corpus and is surrounded by a thick, muscular wall.
The musculature of the uterus is in several layers. There is an outer longitudinal layer (stratum supra-vasculare) continuing into the fallopian tubes and round ligaments. The vascular layer (stratum vasculare) consists of many interlacing spiral groups of smooth muscles and contains many blood vessels. An inner layer consists of muscle fibers arranged both longitudinally and obliquely.
The cervix, which protrudes into the vagina, is generally 2–3 cm long. The intravaginal portion of the cervix, known as the portio vaginalis, ordinarily is covered with nonkeritinizing squamous epithelium with a number of mucus-secreting glands (Fig. 2). The external os is the opening of the cervix within the vagina. Above the external os lies the fusiform endocervical canal, approximately 2 cm long and lined with columnar epithelium and endocervical glands. The intersection where the squamous epithelium of the exocervix and columnar epithelium of the endocervical canal meet, the squamocolumnar junction, is geographically variable and dependent on hormonal stimulation. It is this dynamic interface, the transformation zone, that is most vulnerable to the development of squamous neoplasia. In early childhood, during pregnancy, or with oral contraceptive use, columnar epithelium may extend from the endocervical canal onto the exocervix, a condition known as eversion or ectopy. After menopause, the transformation zone usually recedes entirely into the endocervical canal.
At the upper end of the endocervical canal at the junction with the uterine cavity is the internal os. The endocervical canal in the nullipara is lined by mucosa arranged in a series of folds. A vertical fold is present on the anterior and posterior cervical walls; from these, oblique folds radiate. These folds have been called the arbor vitae uteri or plicae palmatae. It was formerly thought that tubular glands descend vertically from the surface and divide into many branches forming compound racemose glands; however, secondary changes caused by the intense growth activity of the columnar cells result in the formation of tunnels, secondary clefts, and exophytic processes.
The endometrial cavity lies above the internal cervical os. It is roughly triangular in shape and measures approximately 3.5 cm in length. Ordinarily, the anterior and posterior walls of the uterus lie in apposition so that little if any actual cavity is present. At each cornu or horn of the uterus, the cavity of the uterus becomes continuous with the lumen of a fallopian tube. Peritoneum covers most of the corpus of the uterus and the posterior cervix and is known as the serosa. Laterally, the broad ligament, a double layer of peritoneum covering the neurovascular supply to the uterus, inserts into the cervix and corpus. Anteriorly, the bladder lies over the isthmic and cervical region of the uterus.
The “positions” of the uterus are of considerable interest but of much less importance in gynecologic practice than 50 years ago. The most common position of the uterus in a nulligravid female is in moderate anteflexion or bent slightly anteriorly, and the uterus as a whole is inclined toward the symphysis in ante version against the bladder, adapting its position as the latter organ distends or empties (Fig. 3 and Fig. 4). In a variable number of women, the uterus is retroverted or inclined posteriorly or retroflexed toward the sacrum. Quite a few disabilities were attributed to these “malpositions” in the past including dysmenorrhea, functional uterine bleeding, backache, dyspareunia, and leukorrhea. Many normal uteri are in mid position, with the axis of uterus being almost parallel to the spine.
The peritoneum covers the uterus and is separated from the uterine musculature by a thin layer of periuterine fascia, which is a continuation and extension of the transversalis fascia. This mobile fascial layer is areolar tissue and is easily separated except for a midline seam or raphe between the uterus and bladder anteriorly and between uterus and peritoneum posteriorly at the level of the isthmus. Posteriorly it sweeps down over the posterior vaginal wall and the cul-de-sac.
The blood supply of the uterus is derived chiefly from the uterine arteries (Fig. 5). These arise from the hypogastric artery and swing toward the uterus, which they reach at approximately the level of the internal os (Fig. 6 and Fig. 7). Here the uterine arteries divide, the descending limb coursing downward along the cervix and lateral wall of the vagina. The ascending limb passes upward alongside the uterus and continues below the fallopian tube. Frequent anterior and posterior branches go to vagina, cervix, and uterus.
The ovarian artery, which ordinarily arises from the aorta, passes along the ovary, dividing into a number of branches. At several places in the broad ligament there are anastomotic connections between the tubal branch of the uterine artery and the ovarian artery. A branch of the uterine artery nourishes the round ligament. The veins generally accompany the arteries.
Using injection and microradiographic and histologic techniques to study the vascular anatomy of the uterus, Farrer-Brown et al.1 showed that the uterine arteries run a tortuous course between the two layers of the broad ligament along the lateral side of the uterus and turn laterally at the junction of the uterus and fallopian tube, run toward the hilum of the ovary, and terminate by joining the ovarian arteries. In the broad ligament each uterine artery supplies lateral branches that immediately enter the uterus and give off tortuous anterior and posterior arcuate divisions, which run circumferentially in the myometrium approximately at the junction of its outer and middle thirds. In the midline the terminal branches of both arcuate arteries anastomose with those of the contralateral side.
Each arcuate artery throughout its course gives off numerous branches running both centrifugally towards the serosa and centripetally towards the endometrium. The arteries to the serosa at first are directed radially and then frequently became more circumferential. There is a plexus of small arterial radicals with a radial distribution located immediately below the serosa. The inner two-thirds of the myometrium is supplied by tortuous radial branches of the arcuate arteries. They provide numerous branches terminating in a capillary network which surrounds groups of muscle fibers. An abrupt change in the density of the arterial pattern occurs at the junction of the basal layer of the endometrium with the subjacent myometrium. The endometrial vessels are relatively sparse in comparison with those of the myometrium at all stages of the menstrual cycle.
The uterus is partially supported by three pairs of ligaments. The paired round ligaments extend from the anterosuperior surface of the uterus through the internal inguinal rings and through the inguinal canals to end in the labia majors. They are composed of muscle fibers, connective tissue, blood vessels, nerves, and lymphatics. The round ligaments stretch with relative ease, particularly in pregnancy. The uterosacral ligaments are condensations of endopelvic fascia that arise from the posterior wall of the uterus at the level of the internal cervical os. They fan out in the retroperitoneal layer and attach broadly at the second, third, and fourth segments of the sacrum. They are predominately composed of smooth muscle but also contain connective tissue, blood vessels, lymphatics, and parasympathetic nerve fibers.2 The paired cardinal (Mackenrodt's) or transverse cervical ligaments arise from the anterior and posterior marginal walls of the cervix and fan out laterally to insert into the fascia overlying the obturator muscles and the levator ani muscles. The cardinal ligaments form the base of the broad ligament. They are composed of perivascular connective tissue and nerves that surround the uterine artery and veins. The cardinal and uterosacral ligament complex is collectively called the parametrium.
The broad ligament is formed by folds of peritoneum covering the fallopian tubes, the infundibulopelvic vessels, and the hilus of the ovary. It contains a number of structures: fallopian tube, round ligament, ovarian ligament, uterine and ovarian blood vessels, nerves, lymphatics, and mesonephric remnants. Below the infundibulopelvic structures, the anterior and posterior leaves of peritoneum lie in apposition, leaving a clear space below the tube with its tubal branch of the uterine artery. This avascular area is useful to the surgeon in isolating the adnexal structures and in avoiding blood vessels while performing tubal ligations.
The endometrium lines the uterine cavity and is considered to have three layers: the pars basalis, the zona spongiosa, and the superficial zona compacta. The straight branches of the radial arteries of the uterus terminate in capillaries in the basal layer, while the spiral or coiled branches penetrate to the surface epithelium, where they give rise to superficial capillaries. Sinus-like dilatations of the capillaries in the superficial layer are called “lakes.” These vascular lakes and capillaries are drained by small veins.
The endometrium varies greatly depending on the phase of the menstrual cycle. Proliferation of the endometrium occurs under the influence of estrogen; maturation occurs under the influence of progesterone. The uterine endometrial cycle can be divided into three phases: the follicular or proliferative phase, the luteal or secretory phase, and the menstrual phase. The follicular, or proliferative phase, spans from the end of the menstruation until ovulation. Increasing levels of estrogen induce proliferation of the functionalis from stem cells of the basalis, proliferation of endometrial glands, and proliferation of stromal connective tissue. Endometrial glands are elongated with narrow lumens and their epithelial cells contain some glycogen. Glycogen, however, is not secreted during the follicular phase. Spiral arteries elongate and span the length of the endometrium.
After formation of the corpus luteum, the endometrial glands grow, become tortuous, and secrete. The luteal, or secretory, phase begins at ovulation and lasts until the menstrual phase of the next cycle (Fig. 8). At the beginning of the luteal phase, progesterone induces the endometrial glands to secrete glycogen, mucus, and other substances. These glands become tortuous and have large lumens due to increased secretory activity. The spiral arteries extend into the superficial layer of the endometrium. The spiral capillaries develop a terminal network of superficial capillaries. These changes result in the formation of a predeciduum prepared for the arrival of the trophoblast.
In the absence of fertilization by day 23 of the menstrual cycle, the corpus luteum begins to degenerate and ovarian hormone levels decrease. As estrogen and progesterone levels decrease, the endometrium undergoes involution. During days 25–26 of the menstrual cycle, endothelin and thromboxin begin to mediate vasoconstriction of the spiral arteries. The resulting ischemia may cause menstrual cramps. By day 28 of the menstrual cycle, intense vasoconstriction and subsequent ischemia cause mass apoptosis of the functionalis, with associated bleeding. The menstrual phase begins as the spiral arteries rupture secondary to ischemia, releasing blood into the uterus, and the apoptosed endometrium is sloughed off (Fig. 9). During this period, the functionalis is completely shed. Arterial and venous blood, remnants of endometrial stroma and glands, leukocytes, and red blood cells are all present in the menstrual flow.
Data on the lymphatic vessels of the uterus have been coordinated by Reynolds.3 The entire uterus has a rich capillary bed as extensive as the blood capillary system. The lymphatic capillary bed is arranged in four zones: (1) the lower uterine segment with its rich supply of fine capillaries, (2) the subserosa of the corpus with a few lymphatics, (3) a deep subserosal network, and (4) a plentiful supply in the muscularis proper. These vessels increase greatly in number and size during pregnancy. The collecting system of the uterine lymphatics is formed from anastomoses of a lateral-uterine descending network of lymph vessels which unites with collecting vessels from the utero-ovarian pedicle and the external iliac area. Lymphatic drainage of the uterus and upper two-thirds of the vagina is primarily to the obturator and internal and external iliac nodes.
FALLOPIAN TUBES
The fallopian tubes are bilateral muscular structures of paramesonephric duct origin. They are from 7 to 12 cm in length and usually less than 1 cm in diameter. The tubes or oviducts have a lumen that varies considerably in diameter. It is extremely narrow, being less than 1 mm at its opening into the uterine cavity. It is wider in the isthmus (Fig. 10) (2.5 mm) and in the ampulla (Fig. 11) is approximately 6 mm in diameter. The tube begins in the uterine cavity at the cornu and penetrates the myometrium (intramural or interstitial portion). The second portion is the relatively straight and narrow portion of the tube which emerges from the uterus posterior to and a little above the origin of the round ligament. The lumen of the narrow isthmus is relatively simple, with a few longitudinal folds. This portion of its tube is 2 or 3 cm long. There are three layers of musculature: the inner longitudinal, the middle circular layer, and the outer longitudinal layer. There is some evidence that the isthmus may act as a sphincter.
The ampulla is the largest and longest portion of the tube, approximately 5 cm or more in length. The lumen enlarges from 1 or 2 mm near the isthmus to over a centimeter at the distal portion. The mucosa has multiple longitudinal folds. The ampulla is the portion usually involved in gonorrheal salpingitis and tubo-ovarian abscesses and is the site of most ectopic pregnancies.
At the distal end of the tube is the trumpet shaped infundibulum. The tube ends in a number of fimbriae or frond-like projections; the largest of these is ordinarily in contact with the ovary and is known as the ovarian fimbria. The peritoneal cavity in the female is connected with the exterior of the body through the patent distal end of the tube by way of the uterus and vagina. The ovum must enter through the open end of a tube if fertilization is to occur in the ampullary portion, where sperm have collected by migrating “upstream” against the current. This opening is of considerable clinical importance as blood, ascending infections, or pus can pass out of the tube to invade the abdominal cavity, with resultant pain, endometriosis, or pelvic infection.
The epithelial lining of the tube has been studied extensively by light and electron microscopy. On light microscopic examination, four types of cells can be readily seen. Secretory cells or nonciliated cells have a heavily granular cytoplasm and an oval nucleus. The ciliated cells have fine granular cytoplasms and are relatively square with large round nuclei. The intercalary or “placed-between” cells are long narrow cells with dark nuclei causing them to be called “peg cells.” The fourth type of cells are the small “indifferent” cells with large dark nuclei.
Pauerstein4 has reviewed and summarized the numerous studies on tubal ultrastructure. Two basic cell types have been described, ciliated and secretory. The ciliated cells have a clear cytoplasm with vesicular reticulum. Microvilli are seen extending from the luminal edge of the cell. The cilia themselves have two central filaments and nine double, lateral filaments. Secretory cells have a dark cytoplasm with fine granules. Darker secretory granules are prominent, with irregularly distributed endoplasmic reticulum. The tubal epithelium is responsive to the estrogen and progesterone levels during the menstrual cycle, pregnancy, and the menopause. The proliferative phase is characterized by elevated epithelium with ciliated and secretory cells of equal height. The luteal phase shows lower ciliated cells with higher and more prominent cytoplasm, sometimes with rupture and extrusion of the cytoplasm into the lumen. During menstruation and post-menstruation, cells are lower and smaller. During pregnancy, tubal epithelium remains low. There is considerable variation in postmenopausal changes in the tubal epithelium. Apparently significant secretory activity ceases, but the onset of atrophy is variable and deciliation may not occur until years after the menopause.
The principal blood supply of the tube is from the upper end of the uterine artery, which bifurcates and sends a large branch or ramus below the tube to anastomose with the ovarian artery. The proximal two-thirds of the tube is chiefly supplied by the uterine artery. The arterial supply is quite variable and there may be three branches (medial, intermediate, and lateral) or a branch from the uterine and another from the ovarian artery. Anastomoses between uterine and ovarian arteries in the mesosalpinx are variable but always present.
The venous system accompanies the arterial distribution. Capillary networks are to be found in subserosal, muscularis, and mucosal layers. The arrangement varies in different portions of the tube, but the venous plexuses become confluent in the subserosal layer. The lymphatic drainage runs along the upper edge of the broad ligament to the lymphatic network below the hilus of the ovary. From here the flow from uterus, tube, and ovary drains to the para-aortic or lumbar nodes.
The tube is provided with both sympathetic and parasympathetic innervation. Sympathetic fibers from T10 through L2 reach the inferior mesenteric plexus. Postganglionic fibers then pass to the oviduct. The fibers from the inferior mesenteric plexus pass to the cervicovaginal plexus, which in turn sends fibers to the isthmus and part of the ampulla. Some sympathetic fibers from T10 and T11 reach the celiac plexus and provide postganglionic fibers to the ovarian plexus, which supplies the distal ampulla and fimbriae. The parasympathetic supply is by vagal fibers from the ovarian plexus supplying the distal portion of the tube. Part of the isthmus receives its parasympathetic supply from S2, S3, and S4 via the pelvic nerve and the pelvic plexuses. The sympathetic innervation of the female pelvis is depicted in Fig. 12.
THE OVARIES
In the early embryo, differentiation of gonadal tissue occurs anterior to the mesonephros and along the entire medial aspect of the urogenital ridge. The cranial portions of the gonadal ridge degenerate, leaving an indifferent genital gland near the mesonephros. Primitive germ cells originate in the epithelial lining of the dorsal part of the hindgut. They migrate to the gonad and are seen as radial strands extending into the mesenchymal tissue. The migrating cells consist of primordial egg cells and prospective granulosa cells (Fig. 13).
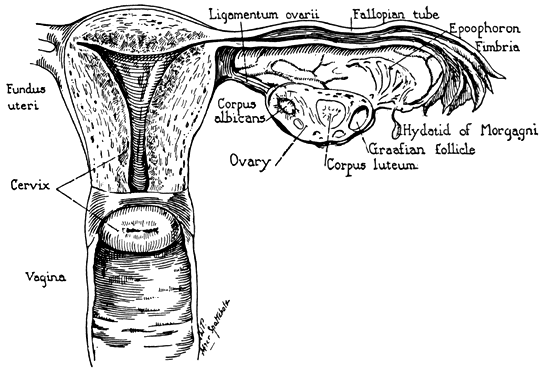
The glistening white ovaries are generally oval in shape but may vary in size, position, and appearance, depending on the age and the reproductive activities of the individual. The ovaries of a normal adult woman are 2.5–5 cm long, 1.5–3 cm thick, and 0.7–1.5 cm wide, with a weight of 3–8 g.2 The ovaries contain 1–2 million oocytes at birth. A woman will release up to 300 ova, on average, during her lifetime. Histologically the ovary is divided into the outer cortex and the inner medulla. The cortex consists of a cellular connective tissue stroma in which the ovarian follicles are embedded. The medulla is composed of loose connective tissue which contains blood vessels and nerves. The cortex is surrounded by a single layer of cuboidal epithelium called the germinal epithelium.5 Figure 14 shows a photomicrograph of a normal adult ovary.
In the nullipara, the ovary typically lies in the ovarian fossa, a depression in the pelvic wall below the external iliac vessels and in front of the ureter. A mesovarium attaches the ovary to the posterior wall of the broad ligament, while the posterior margin is free. The peritoneum does not cover the ovary proper, which is covered by germinal epithelium.
At either end the ovary is supported by ligaments. At the tubal pole the ovary is attached to the suspensory ligament, a fold of peritoneum which forms a mesentery for the ovary and contains the ovarian vessels. This suspensory ligament is often called the infundibulopelvic ligament. At the other pole is the uteroovarian ligament.
The hilus is the base of the ovary; at this point the ovarian blood vessels enter. The ovarian arteries arise from the abdominal aorta just below the renal arteries. They pass downward across the pelvic brim, cross the external iliac artery, and traverse the infundibulopelvic fold of peritoneum. Branches go to the ureter, round ligament, and tube and anastomose with the uterine artery.
As the ovarian artery passes through the mesovarium, it separates into multiple branches that enter the ovarian hilus. Each of these arteries divides into two medullary branches which cross the ovary. Cortical branches arise from the medullary branches and supply the cortex and follicles. Two prominent veins enter the hilus and, in general, follow the arterial pattern.
At the hilus venous drainage forms a pampiniform plexus, which consolidates to form the ovarian vein. On the right side the ovarian vein drains into the inferior vena cava, while the left ovarian vein drains into the left renal vein. The ovarian as well as the uterine blood supply frequently is anomalous.
The nerve supply derives from a sympathetic plexus accompanying the vessels of the infundibulopelvic ligaments.6 The plexus arises at the level of the tenth thoracic segment, but fibers from renal and aortic plexuses as well as from the mesenteric and celiac ganglia are present.
Hilus cells, which are nonencapsulated nests of large vacuolated cells, frequently are found in the hilus of the ovary. These cells are similar to the interstitial or Leydig cells of the testis.
Any discussion of the ovary should include those portions of the mesonephric (wolffian) tubules and duct that persist in the adult female as vestigial structures between the peritoneal layers of the broad ligament. The epoophoron lies in the mesosalpinx between the tube and the ovary. It usually consists of 8–20 small tubules which join a common duct at right angles. Ordinarily the longitudinal duct has blind ends, but it may be prolonged as Gartner's duct. Mesonephric duct vestiges known as Gartner's duct cysts may be found alongside the uterus, cervix, or vagina. Vestiges of the mesonephric tubules also may be present as clear pedunculated cysts below the fimbria of the tube.
Medial to the epoophoron lies the paroophoron, a rudimentary organ with a few scattered tubules. It likewise is of mesonephric origin. These mesonephric vestiges are of clinical importance, since they occasionally give rise to cysts which require surgical excision.
Ovulation
In the female embryo, primitive germ cells migrate from the epithelial lining of the hindgut and invade the subjacent layer of mesenchyma in the sexually undifferentiated gonad. These cells form radial cords and consist of primordial egg cells and cellular masses of prospective granulosa cells. As the fetus develops, the germinal cords become segregated into islands, each containing several germinal cells. At birth the full-term infant already has developing and degenerating follicles.
A primordial follicle consists of an oocyte with a layer of follicular cells surrounding it. When such a follicle is to undergo ovulation, marked changes occur in the egg and its follicular cells (Fig. 15). The ovum reaches its mature size as the antrum appears in the follicle. Concurrent with the growth of the oocyte, the granulosa cells proliferate and a multi-layered structure develops. An outer connective tissue sheath derived from the ovarian stroma is formed. This sheath is called the theca and subsequently divides into the theca externa and theca interna.
At first the developing follicle sinks deeper into the cortex, but as it increases in size it again returns to the surface. A theca cone develops, its axis pointing to the surface. At the same time the zona pellucida, a clear zone around the ovum, forms. An antrum or cell-free area containing follicular fluid develops. Surrounding the oocyte is a cluster of granulosa cells resembling a small mound, upon which the oocyte rests; this is called the cumulus oophorus (Fig. 16). As ovulation approaches, the follicle bulges and the wall thins. The basic mechanism which precipitates ovulation has not been determined; it is obviously hormone related.
Following rupture of the follicle and extrusion of the ovum, bleeding occurs at the rupture site and a blood clot forms. This is called the corpus hemorrhagicum. Granulosa cells grow into this clot, and the resulting mass of cells is known as the corpus luteum (Fig. 17).
REFERENCES
Farrer-Brown G, Beilby JOW, Tarbit MH: Blood supply of the uterus. J Obstet Gynaecol Br Commonw 77: 673, 1970 |
|
DeLancey JOL: Principles of anatomy and perioperative considerations. In: Rock JA, Thompson JD, eds. TeLinde's Operative Gynecology, 8th edn. Philadelphia, Lippincott-Ravens, 1997:77 |
|
Reynolds SRM: Physiology of the Uterus. New York, Hafner, 1965 |
|
Pauerstein CJ: The fallopian tube – a reappraisal. Philadelphia, Lea & Febiger, 1974 |
|
Kleeman SD, Silva WA: Gynecologic anatomy. In: Sokol AI, Sokol ER, eds. The Requisites in Obstetrics and Gynecology: General Gynecology. Philadelphia, Mosby-Elsevier, 2007:87 |
|
Neilson D, Jones GS, Woodruff JD, Goldberg G: The innervation of the ovary. Obstet Gynecol Surv 35: 889, 1970 |